Abstract
The cellular products obtained following electrofusion (EF) of dendritic cells (DC) and tumour cells have shown promise as cancer vaccines. The immunogenicity of these preparations has been attributed to the presence of small numbers of DC-tumour hybrids and the contribution of the non-hybrid tumour cells present has received little attention. In this report, we investigated the effect of the EF process on the immunogenicity of allogeneic human cells, in particular the colorectal cell line, SW620. EF conditions were optimised to yield the maximum number of DC-SW620 hybrids co-expressing tumour associated antigen (TAA) and DC associated antigens. Exposure of SW620 to EF induced significant increases (P<0.05) in apoptosis and necrosis. Pre-exposure of SW620 to the EF buffer alone [0.3 M glucose, 0.1 mM Ca(CH3COO)2 and 0.5 mM Mg(CH3COO)2] resulted in significant increases in TAA uptake by DC during co-culture (P<0.05). DC phenotype was, however, not altered by exposure to EF treated tumour cells. In co-cultures of PBMC responders with SW620, the levels of IFNγ release and cytotoxic activity were significantly increased (P<0.05) by pre-exposure of the SW620 to EF. Pre-exposure of allogeneic non-T cells, the colorectal cell line Lovo and a breast cancer cell line (MCF7) to EF also significantly (P<0.05) increased the levels of IFNγ release by responding PBMC. These results demonstrate that the EF process itself can increase the immunogenicity of at least some human cell types independently of hybrid formation. These findings suggest that EF protocols should be evaluated with regard to the possibility that DC-tumour hybrids may not contribute all, or even most, of the immunostimulatory capacity present in preparations of EF treated cells.
Keywords: Electrofusion, Hybrid, Tumour, Colorectal, Dendritic cell
Introduction
Dendritic cells (DC) are potent inducers of anti-tumour responses and are being used as vehicles for the delivery of tumour associated antigen (TAA) in immunotherapy protocols. Although numerous approaches have been employed for the loading of DC with TAA, attention has focussed on the use DC–tumour cell hybrids, which in theory co-express all TAAs in concert with the functional attributes of DC [1–3]. Studies in a diverse range of animal tumour models have shown that vaccination with hybrid preparations not only stimulates protective immune responses [3–11], but is also one of the few immunotherapeutic approaches, which is effective in eliminating established metastatic disease [3–5, 10, 12–17]. Promising results have also been obtained with human DC–tumour hybrid preparations in both in vitro studies [18–26] and clinical trials [27–31] although it is clear that further optimisation of this approach is needed.
Chemical fusion using polyethlene glycol (PEG) is the traditional method of cell fusion, but is plagued by variable efficiency with different cell types, toxicity and poor reproducibility [2]. Electrofusion (EF), which involves the suspension of cells in a buffer of high-sugar concentration and subsequent electropulsing, is increasingly being used as a more effective method [1, 4, 7, 9, 16–18, 23, 24, 27, 29]. It is clear that purified DC-tumour hybrids can induce immune responses [11, 13, 25] and the immunogenicity of EF preparations has been commonly attributed to the presence of these hybrids. However, DC-tumour hybrids make up only a small percentage of the EF preparations used in the vast majority of studies. In addition, the majority of EF studies have used simple mixtures of DC and tumour cells as a comparative control. Although these controls allow the immunogenicity of the entire EF preparation to be evaluated, they do not allow any conclusions regarding the relative contributions of the hybrid and non-hybrid components to be made. Exposure to high-sugar concentration is known to cause a number of changes in cell structure and function [32, 33] and electropulsing is additionally associated with a marked loss in cell viability [1]. Although these events may potentially alter the immunogenicity of the non-hybrid tumour cells, which make up a considerable proportion of EF preparations this issue has not been addressed in the majority of EF studies. Results from two in vivo studies do, however, suggest that EF treated tumour cells have increased immunogenicity [4, 9]. It is therefore possible that the effectiveness of EF preparations arises, at least in part, from changes the EF process induces in non-hybrid tumour cells and the effect these changes have on the nature and/or magnitude of subsequent immune responses to the tumour cells.
Understanding the contributions that the different components of EF products make towards the induction of anti-tumour responses is critical for the further optimisation of EF protocols for use in immunotherapy. In this study, we have therefore investigated the potential role that the non-hybrid tumour cell component of EF preparations may have in modulating both DC and immune effector cell responses. The EF process involves both exposure to fusion buffer and electropulsing and the relative contribution of these components to observed effects was also analysed.
Materials and methods
Cell preparation
The adherent human colon cancer cell lines, SW620 and Lovo together with the breast cancer cell line MCF7, were obtained from the ATCC (Rockville, MD, USA) and grown in media [RPMI 1640 (Gibco BRL, Auckland, NZ) supplemented with 10% heat inactivated foetal calf serum [(FCS), Life technologies, Auckland, NZ], 100 U/ml penicillin and 50 μg/ml streptomycin (Sigma, Castle Hill, NSW, Australia). Cells were removed from the flask by brief incubation with 0.05% trypsin/0.02% EDTA solution (Sigma). In a number of experiments, the cell lines were pre-treated to inhibit proliferation by culture (37°C, 30 min) in culture media supplemented with mitomycin C (5 μg/ml).
Blood was obtained from volunteer donors with appropriate informed consent, according to Ethical Committee guidelines. Peripheral blood mononuclear cells (PBMC) were isolated over a Ficoll-Hypaque density-gradient (Ficoll-Hypaque, Amersham Pharmacia Biotech, Uppsala, Sweden) and resuspended in media. The non-T cells were prepared from PBMC by immunomagnetic depletion of CD3+ T cells. Briefly, PBMC were labelled with CD3 mAb (OKT3), then following incubation with goat anti-mouse Ig Dynabeads (Dynal ASA, Oslo, Norway) labelled cells were removed by placement on a magnet. Adherent and non-adherent PBMC fractions were prepared by incubation (37°C, 5%CO2) of PBMC (4×106/ml in 10% FCS media) for 1 h in plastic tissue culture flasks (Nunc, Rosklide, Denmark). The non-adherent PBMC (na-PBMC) were then harvested and after washing of the flasks with PBS, Monocyte derived DC (MoDC) were generated from the adherent fraction of PBMC by day 5–6 culture in recombinant GM-CSF (Roche, Auckland, NZ) and IL-4 (R&D Systems, Minneapolis, USA) as described previously [34]. In a number of experiments, DC were matured by the addition of LPS (1 μg/ml, Sigma) on days 5 or 6 and cells were harvested the following day.
Necrotic tumour cells were generated by suspending cells in PBS and then exposing them to four rapid cycles of freezing (−80°C) and thawing (37°C) as described previously [35].
Cell labelling, antibodies and flow cytometry
To quantitate the DC/SW620 fusion hybrids, the commercial fluorescent cell tracker dyes DiO and DiI (Vybrant, Molecular Probes, Eugene, Oregon, USA) were used to label the cell membranes of DC and tumour cells. Cells were stained according to the manufacturer’s instruction. Briefly, cells were resuspended in HBSS (Gibco BRL), dye was added to a final concentration of 5 μM and the cells incubated (10 min, 37°C, in the dark). After washing (×2), the efficiency of staining was analysed on a FACS Vantage flow cytometer using Cellquest software (Becton Dickinson, San Jose, CA, USA).
To quantitate hybrid formation in homogeneous cell populations, the populations were divided in two and separately labelled with different dyes as above.
To evaluate the phenotype of the DC/SW620 fusion hybrids, cells were incubated with a panel of antibodies post-fusion and analysed by FACS. W6/32 (class I), L243 (HLA-DR) and G28/5 (CD40) were produced from hybridomas obtained from ATCC. CD80, CD83 and CD86 mAb were purchased from Coulter Immunotech (Marseilles, France). Phycoerythrin (PE)- and fluorescein isothiocyante (FITC)- conjugated sheep anti-mouse Ig (PE-SAM and FITC-SAM, respectively) were purchased from Silenus (Boronia, Victoria, Australia). FITC-conjugated HLA-DR was obtained from Becton Dickinson. Isotype-matched monoclonal antibodies were used as negative controls. Labelling was carried out on ice as described previously [34].
Electrofusion
Cells were electrofused using a modification of a previously described method [1]. Briefly, immediately prior to EF cells were washed (×2) in fusion buffer (0.3 M glucose, 0.1 mM calcium acetate and 0.5 mM magnesium acetate), then mixed at a 1:1 ratio and resuspended in fusion buffer (1×107 cells/ml). Electrofusion was performed in 0.4 cm EF cuvettes (Biorad, Richmond, CA, USA). One aspect of the cuvette had been coated with paraffin wax, which served to polarise the fusion chamber. Cells (0.8 ml) were first di-electrophoretically aligned by application of a direct current of 25 V supplied for 10 s. In a second step, a single pulse of 500 V/cm at 25 ufd was applied to fuse the aligned cells using a Gene Pulsar (Biorad). The cuvette was left to stand for 10 min before cells were removed, washed in PBS (×2) and resuspended in phenol red free DMEM (Gibco BRL) supplemented with 10% FCS. Cells were left to rest for a further 2 h to complete the fusion before total viable cell yield was established by trypan blue staining. Cells were then analysed for dual positive hybrids by two-colour flow cytometry. EF controls included cells that had been washed in fusion buffer but not electropulsed (“mock-fused” cells) and cells that had been washed and incubated in media for equivalent periods of time (“untreated” cells).
Histochemical staining
Cytospin cell preparations were fixed and stained with May Grunwald Giemsa stain and analysed by microscopy.
Detection of apoptosis and necrosis
Apoptosis and/or necrosis was determined by Annexin-V-FITC and propidium iodide (PI) staining using a commercial kit (Nexins Research, distributed by DAKO, CA, USA) according to the manufacture’s protocol. Briefly, 1×106 cells were pelleted and annexin-FITC was added for 5 min on ice. Subsequently, 2 μl of PI (1 mg/ml) was added and samples were analysed by Flow Cytometry. Apoptosis is defined as Ann+/PI−, necrosis as Ann+/PI+ and live cells as Ann−/PI−.
Tumour antigen uptake assay
SW620 cells were mitomycin C treated and DiI labelled as described above. The tumour cells were then either untreated; mock fused or fused as described above and co-cultured in media plus 10%FCS with immature DC (day 5 or 6) at a 1:1 ratio. After 18 h, cells were labelled with the panel of mAbs described above and analysed by two-colour flow cytometry. Internalisation of tumour associated fluorescence by immature DC was defined as the percentage of live DC that were also DiI positive. The mean fluorescent intensity (MFI) was used in the analysis to determine the amount of tumour material taken up by the DC.
IFNγ release assay
The ability of allogeneic non-T stimulator populations (either untreated, mock fused or EF) to induce IFNγ release by PBMC responders was determined in a co-culture assay. Equivalent numbers (100 μl at 1×106/ml in culture media) of stimulators and PBMC were co-cultured in 96-well round bottom plates for 72 h prior to the removal of the supernatants for analysis of IFNγ levels by ELISA.
The ability of SW620, Lovo or MCF-7 stimulator populations to induce IFNγ release by non-adherent PBMC (na-PBMC) responders was determined using a modification of a previously described method [18]. Untreated, mock fused and EF preparations of mitomycin C treated stimulators were pre-cultured (37°C, 5% CO2) for 6 h in IL-12 (R&D systems, 10 ng/ml) before washing and their addition (100 μl at 1×106/ml) to responders in a 96-well round bottom plate. The responders used were na-PBMC, prepared as described above that had been pre-treated by culture (16 h, 37°C, 5% CO2) at 2×106/ml in culture media supplemented with IL-15 (R&D systems, 10 ng/ml). After washing to remove residual IL-15, responder cells were added (100 μl at 2×106/ml) to the stimulator populations to give a final responder: stimulator ratio of 2:1. Co-cultures were incubated 16 h prior to the removal of supernatant for the analysis of IFNγ levels by ELISA
IFNγ ELISA
Flat bottom 96-well microtitre plates (Maxisorp, Nunc, Rosklide, Denmark) were coated (1 h, 37°C) with 2 μg/ml anti-human IFNγ monoclonal antibody (R&D Systems) and washed four times with PBS/0.05% Tween 20. After blocking (5% sucrose, 1% BSA) and incubation with sample (2 h, 37°C), wells were sequentially incubated (1 h, 37°C) with biotinylated goat anti-human IFNγ (200 ng/ml in 0.1% g BSA, 0.05% Tween 20 in PBS), and streptavidin-HRP (1 in 10,000 in 1% BSA, (DAKO)). Plates were washed and developed by adding TMB+Substrate-Chromogen (DAKO) for 20 min. The absorbance of each well was measured at 450 nm on a multiplate reader. Standard curves were generated using serial dilutions of IFNγ (Boehringer Ingelheim).
Cytotoxicity assay
The ability of untreated, mock fused and EF SW620 populations to induce a cytotoxic response in na-PBMC responders was determined using a modification of a previously described method [18]. Responders used were na-PBMC that had been pre-treated by culture (16 h, 37°C, 5% CO2) in media supplemented with IL-15 (10 ng/ml). Untreated, mock fused and EF preparations of mitomycin C treated SW620 (8×105/ml) were pre-cultured (37°C, 5% CO2) for 6 h in IL-12 (10 ng/ml) washed and co-cultured (37°C, 5% CO2) with responders (8×106/ml) at a responder: stimulator ratio of 10:1 in 24-well plates. After 3 days of co-culture IL-2 (Roche) was added to a final concentration of 10 U/ml and co-culture continued for a further 3 days. The cells were then harvested and used as effectors in a calcein-AM release assay [36]. Doubling dilutions of the effectors (100 μl/well) were added to a 96-well round bottom plate prior to addition of targets. SW620 target cells (1×106/ml) were pre-labelled with fluorescent dye by incubation (30 min, 37°C) in medium supplemented with 15 μg/ml calcein-AM (Molecular Probes). Labelled targets were washed, suspended at 5×104/ml in media and 100 μl added per well. After incubation (4 h, 37°C, 5% CO2) supernatant was recovered and transferred to 96-well black walled plates. Samples were then measured using a spectrofluorometer (excitation filter: 485 nm, band pass filter: 530 nm) and fluorescence intensity expressed as arbitrary fluorescence units. Spontaneous cell death represents fluorescence release from targets cultured in media alone and maximum release is the fluorescence release from targets lysed in 2% NP-40. Specific lysis was calculated according to the formula [test release-spontaneous release]/[maximum release−spontaneous release]×100.
Statistical analysis
Data were expressed as means±SEM. Statistical significance was determined by the Student’s t-test for paired samples. Unless otherwise stated the statistical significance of normalised data was evaluated using confidence intervals. Differences were considered significant when P <0.05.
Results
Efficiency of EF
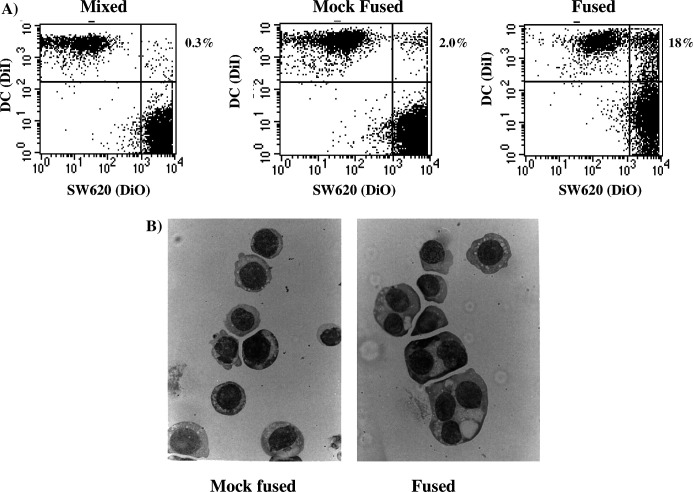
An EF protocol based on previously described methods was developed using the colorectal cell line SW620 as a human tumour model. The efficiency of EF was assessed by FACS analysis. Cells were loaded with either green (DiO) or red (DiI) fluorescent membrane dyes, suspended in glucose buffer and electropulsed using a range of electrical conditions. Following fusion, the number of dual colour hybrid cells were determined by FACS (Fig. 1a). As the mixing of cells and/or their suspension in fusion buffer may influence hybrid formation, independently of the effects due to electropulsing, “untreated” cells and “mock-fused” cells were also analysed. In all experiments untreated controls did not contain hybrid cells, whereas mock-fused controls contained between 2% and 4% hybrids (Fig. 1a). Hybrid formation was therefore scored as the percentage of hybrids in electrofused mixtures minus the percentage observed in the corresponding mock-fused control. A distinct hybrid population was clearly observed in all EF preparations. In accordance with previous reports, the unfused DC population in these preparations also showed a small increase in tumour cell associated fluorescence possibly due to an association/uptake of tumour cell fragments. To remove the possibility that the observed fusion products were the result of physical clumping, as opposed to fusion, the fusion products were treated with 1 μg/ml Dnase and 5 mM EDTA and repeatedly forced through a 29-gauge syringe. Fusion products were not dissociated following this procedure, nor did culturing samples overnight at 37°C alter the number of hybrids detected (data not shown). To further confirm DC/SW620 fusion, MGG staining was carried out on mock-fused and fused cells (Fig. 1b). Although preparations of mock-fused cells were mononuclear the preparation of EF cells contained many bi or multi-nucleated cells indicating the presence of true hybrids. Because of the presence of both homogeneous and heterogeneous fusion products, the number of fused cells observed following MGG staining was three fold more than that observed following FACS analysis.
Fig. 1.
Analysis of EF efficiency. SW620 tumour cells and DC were labelled with DiO and DiI fluorescent membrane dyes, respectively. Following staining cells were either mixed only, mock fused or fused prior to either FACS analysis or cytoplasmic/MGG staining. a Data are shown as dot plots and the percent of hybrids present in the upper right quadrant indicated. b MGG staining of cytospin preparations. Data are from a representative experiment of fourteen performed
As there was an inverse correlation between cell viability and percent hybrid yield, EF conditions were optimised (using PBMCs and the tumour cell line), to give the maximum yield of viable hybrid cells (data not shown). In all experiments, untreated and mock-fused controls had high levels of viable cells (>95%). Increasing the DC: tumour ratio to 3:1 did not increase the percentage of fused cells (data not shown).
Electrofusion using different combinations of cells as fusion partners demonstrated considerable differences in both the viability and hybrid yield (Table 1). Following homogeneous EF SW620 cells had significantly lower viability (P=0.024) than PBMC, although the overall hybrid yields were not significantly different. Heterogeneous EF of SW620 and PBMC resulted in similar hybrid yields to those obtained following homogeneous EF of SW620. EF of DC and SW620 also consistently resulted in the formation of hybrids (6–23%) and hybrid yields were similar to those obtained following PBMC: SW620 EF.
Table 1.
Efficiency of EF
| Cell:cell | % Viable cellsa | % Hybridsb | % Hybrid yieldc |
|---|---|---|---|
| PBMC:PBMC (n=16) | 71.9±3.8 (41–95) | 16.4±1.3 (7.4–28) | 11.3±1.0 (5.6–18.7) |
| SW620:SW620 (n=6) | 56.5±4.4 (49–69) | 16.2±1.1 (13–20) | 9.0±0.5 (6.9–10.1) |
| SW620:PBMC (n=6) | 58.2±4.5 (44–69) | 12.3±1.4 (7.4–16) | 7.2±1.0 (4.4–10.6) |
| SW620:DC (n=14) | 51.1±2.8 (30–68) | 12.8±1.4 (6–23) | 5.6±0.5 (4.2–9.0) |
Data shown are mean±SEM, with range shown in brackets
aViability was determined by trypan blue exclusion
bPercent hybrids were determined by flow cytometric analysis of cell preparations that contained 1:1 mixtures of populations (either identical or different cell types) that had been separately labelled with either DiI or DiO dyes
cPercent hybrid yield was defined as the number of viable hybrid cells (%viable×% hybrids/100)
Polyethylene glycol was also trialled as a means of fusing cells. Although fusion of PBMC using PEG consistently resulted in hybrid yields of 30%, little or no hybrid formation was observed following PEG fusion’s using SW620 (n=4, data not shown).
Phenotypic analysis of DC-tumour hybrid cells
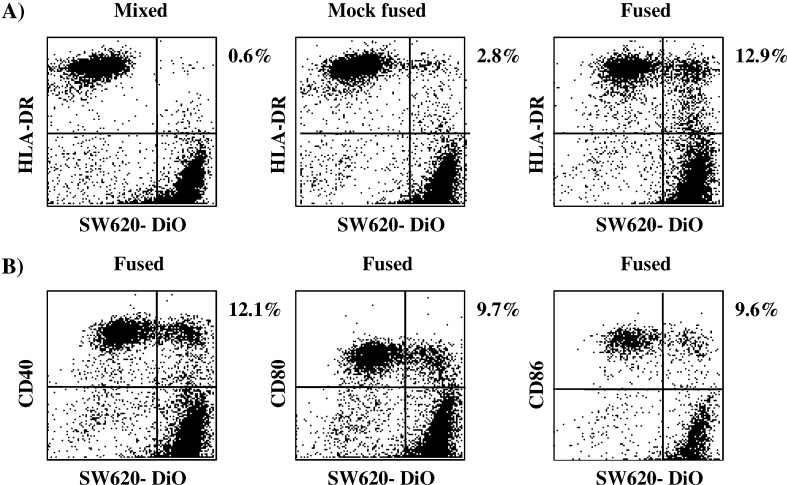
To determine whether the DC-tumour hybrids retained expression of molecules important in the induction of immune responses, immunolabelling of fusion products was performed. The fluorescent DiO marker was used to label the tumour cells prior to EF and DC-associated antigens were detected post-fusion with PE-conjugated antibodies. Samples were analysed for dual fluorescence by flow cytometry. As demonstrated in Fig. 2, DC/tumour hybrids could be clearly distinguished as a population of cells that co-expressed both tumour associated fluorescence and class II, CD40, CD80 and CD86 antigens. Similar levels of hybrid formation were observed using all four antigens for detection. The hybrids were also positive for MHC class I, which is expressed by both DC and SW620 tumour cells (data not shown). Estimations of hybrid formation obtained using monoclonal antibody labelling post-fusion were similar to those obtained using cells labelled pre-fusion with dyes.
Fig. 2.
Phenotypic analysis of DC-tumour hybrids. Dye labelled SW620 tumour cells (DiO) and unlabelled DC were mixed and either untreated, mock fused or fused prior to antibody labelling and analysis by FACS. a Untreated, mock fused and fused preparations labelled with HLA-DR. b EF preparations labelled with CD40, CD80 and CD86. Data are shown as dot plots and the percent of hybrids present in the upper right quadrant indicated. Data are from a representative experiment of six performed
Effect of tumour EF on apoptosis and necrosis
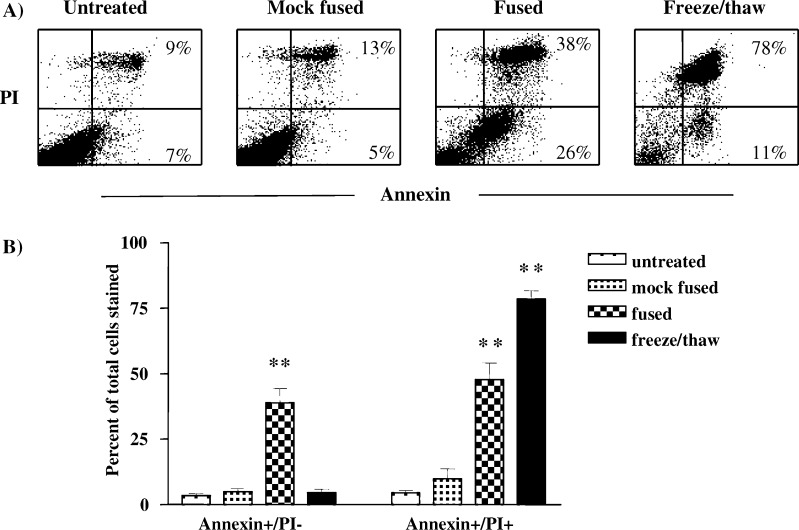
Trypan blue staining of EF SW620 preparations demonstrated that EF induced a significant loss of cell viability. The relative levels of apoptotic and necrotic SW620 cells was, therefore, determined by staining with Annexin-V-FITC (Ann) and Propidium Iodide (PI) in combination with flow cytometry (Fig. 3). Although untreated and mock-fused tumour cells contained only small numbers of Ann+/PI+ (necrotic) and Ann+/PI− (apoptotic) cells, EF induced a significant increase in both populations (n=5, P=0.0079). In contrast, although freeze/thawing induced a significant increase in the Ann+/PI+ population (P=0.0079), only a small non-significant increase in Ann+/PI− numbers was observed. Culture of treated tumour cells overnight did not alter the relative ratios of Ann+/PI+, Ann−/PI− or Ann+/PI− (data not shown). Electrofusion of another colon cancer cell line, Lovo, induced similar levels of apoptosis and necrosis as demonstrated by Annexin-V-FITC/PI staining (n=4, data not shown).
Fig. 3.
Effect of EF on apoptosis/necrosis. SW620 tumour cells were either untreated, mock fused, fused or freeze thawed prior to staining with Annexin-V-FITC versus PI and analysed by FACS. a Dot plots of Annexin-V-FITC versus PI staining following each treatment. Figures indicate the percentage of cells present in the upper right (Ann+/PI+) and lower right (Ann+/PI−) quadrants. Data are from a representative experiment of five performed. b Bar graph of the percentages of Ann+/PI− and Ann+/PI+ cells present following each treatment. Data are from five separate experiments and are shown as mean percent±SEM. ** indicates P<0.001 following comparison with untreated cells
Effect of tumour cell EF on internalisation by DC
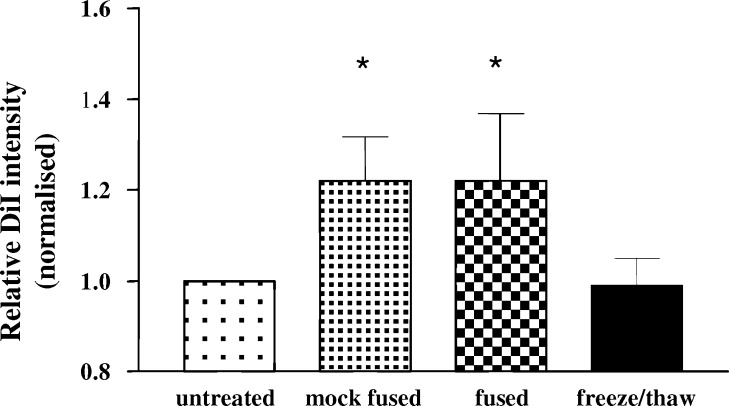
To determine whether exposure of tumour cells to EF altered the subsequent uptake of tumour material by immature DC, uptake experiments were performed. Tumour cells were labelled with DiI and following exposure to different treatments, were co-cultured with DC at a 1:1 ratio. After an overnight incubation, the cells were labelled with HLA-DR and the level of tumour cell uptake by DC was determined by enumeration of dual DiI+/DR+ staining by FACS analysis. Within 18 h of co-culture, tumour cell associated fluorescence could be detected in at least 70% of the DC present, irrespective of whether the tumour cells had been untreated, mock fused, fused or freeze/thawed (data not shown). Although the number of DC which were positive for tumour associated fluorescence was not affected by any treatment, the different treatments did affect the amount of tumour material taken up by the DC, as measured by the MFI of the tumour associated fluorescence (DiI) (Fig. 4). Both MF and EF significantly increased (P<0.05) the level of uptake by DC above that observed in mixed only preparations. In contrast only small, non-significant changes in the level of DiI uptake were observed following co-culture of DC with freeze/thawed tumour cells (0.85–1.32 MFI fold increase).
Fig. 4.
Uptake of tumour cells by DC. Dye labelled SW620 tumour cells (DiI) were either untreated, mock fused, fused or freeze/thawed prior to co-culture (18 h) with DC. Following co-culture, DC were labelled with HLA-DR and the MFI of the DC associated DiI fluorescence was determined by FACS analysis. Results are expressed as the change normalised relative to DC co-cultured with untreated tumour cells (1.0). Data are from five separate experiments and are shown as mean±SEM. * indicates P<0.05 following comparison with untreated cells
The uptake experiments used in this study measure total levels of tumour cell uptake and do not discriminate between material absorbed to the surface of the DC and material which the DC have fully internalised.
Effect of tumour EF on DC phenotype
To examine whether changes occurred in the phenotype of immature DC following co-culture with treated tumour cells, DC expression of maturation-associated antigens (CD40, CD80 and CD86) was analysed. DC expression of CD40 was high following culture in media alone and co-culture with either untreated or treated tumour cells did not further increase CD40 levels. However, in all experiments, co-culture of DC with both untreated and treated tumour cells resulted in significantly increased (P<0.05) expression of CD80 and CD86 antigens relative to levels on DC cultured in media alone. These changes in cell surface antigens were similar to those observed when DC were matured by culture with LPS (data not shown).
Effect of EF on PBMC responses
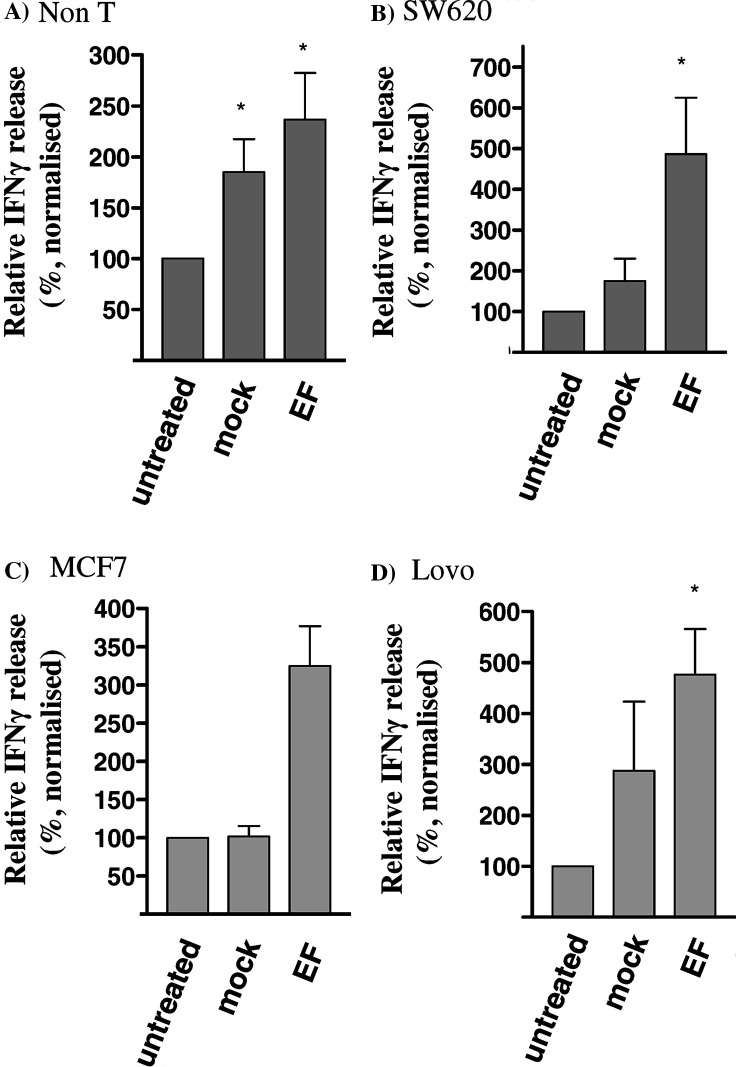
The effect that EF of stimulator populations had on the subsequent functional responses of PBMC populations was analysed. Allogeneic non-T cells were used as a model of strongly immunogenic stimulator cells and their ability to stimulate IFNγ release was analysed (Fig. 5a). The levels of IFNγ release in the presence of ‘untreated’ non-T stimulator cells ranged from 0.2–4.6 ng/ml (mean=2.24 ng/ml). Significantly increased (P<0.05) levels of IFNγ release were observed in cultures containing non-T cells pre-treated by exposure to either mock fusion (relative release, mean=185%, 95% CI=102–268%) or EF (relative release, mean=237%, 95% CI=119–354%).
Fig. 5.
Effect of EF on IFNγ release. IFNγ release was determined following co-culture of allogeneic PBMC responders with either untreated, mock fused or fused preparations of a Non-T cells b SW620 c MCF7 or d Lovo. Results are expressed as the percent change normalised relative to the levels in responders+untreated stimulators (100%). IFNγ levels in cultures containing untreated stimulators were in the range 0.2–4.6 ng/ml using non-T cells, 0.41–1.2 ng/ml using Lovo, 0.21–0.84 ng/ml using MCF-7 and 0.01–0.37 using SW620. Data from separate experiments using non-T cell (n=6), SW620 (n=10), Lovo (n=5) and MCF-7 (n=3) stimulators are shown as mean±SEM. * indicates P<0.05 following comparison with untreated cells
In experiments using SW620 as the stimulator, neither mock fusion nor EF induced significant increases in IFNγ release by PBMC (n=3, data not shown). Previous studies have reported that pre-treatment of responders with IL-15 and pre-treatment of stimulators with IL-12 enhances responses to EF preparations. Responses to SW620 were therefore evaluated in co-cultures using cells pre-treated with these cytokines (Fig. 5b). In this system EF treatment of SW620 resulted in significantly higher levels of IFNγ release than those observed using untreated SW620 (relative release, mean=486%, 95% CI=173–800%). In contrast, mock fusion of SW620 did not significantly increase IFNγ release (relative release, mean=175%, 95% CI=46–304%). Similar results were obtained in experiments using the colorectal cell line Lovo (n=5, Fig. 5c) and the breast cancer cell line MCF7 (n=3, Fig. 5d) as the stimulator populations. EF treatment resulted in significantly higher (P<0.05) levels of IFNγ release in response to both LOVO (relative release, mean=476%, 95% CI=228–724%) and MCF-7 (relative release, mean=325%, 95% CI=102–548%). Mock fusion of these cell lines did not significantly increase IFNγ release. However, considerable variation in the effect of mock fusion on the cell line Lovo was observed and in 2/5 experiments mock fusion and EF induced similar increases.
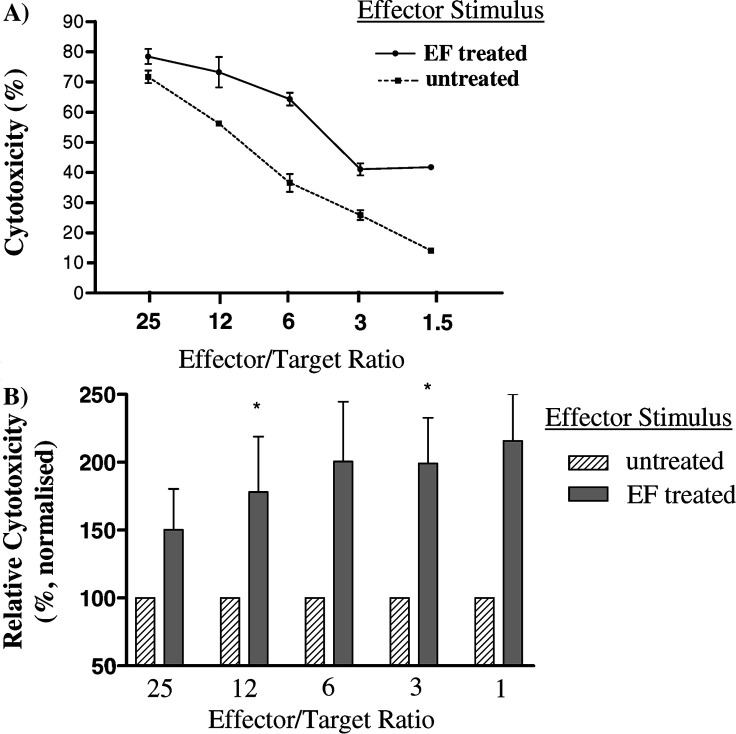
The effect that EF treatment of SW620 had on the development of subsequent cytotoxic responses by PBMC was evaluated in killing assays (Fig. 6). Relative to untreated SW620, the EF treated cells induced increased cytotoxic activity in the PBMC cultures from all four normal donors analysed. The level of increased killing observed was between 110% and 330% over the range of E:T ratios analysed and was significant at a number of these ratios.
Fig. 6.
Effect of EF on cytotoxicity. Effectors were generated by 6 day culture of Na-PBMC with either untreated or EF SW620 and then tested for cytotoxicity against SW620 targets. a Data from a representative experiment of four performed are presented as mean cytotoxicity±SEM at a range of effector:target ratios b Pooled data from the four separate experiments are presented as the relative cytotoxicity of the two effector populations. Relative cytotoxicities are expressed as a percentage normalised relative to the cytotoxicity of the Na-PBMC+untreated SW620 effectors (defined as 100%). The level of specific cytotoxicity in Na-PBMC+untreated SW620 effectors ranged between 41–81% at a Effector:Target ratio (E:T)=25:1 and 14–25% at a E:T=1.5:1. Data are shown as mean±SEM. Data was analysed by paired t-test:* P<0.05
Discussion
The cellular products obtained following EF of DC and tumour cells have shown promise as cancer vaccines. The immunogenicity of these preparations has been attributed to the presence of DC-tumour hybrids but the possibility that the EF conditions may alter tumour immunogenicity independently of hybrid formation has received little attention. We therefore investigated the effect of the EF process on the immunogenicity of a human colorectal cell line. We report that exposure of the cell line to the EF process resulted in increased uptake of TAA by responding DC and stimulated increased immune responses by allogeneic PBMC responders as measured by IFNγ release and cytotoxic activity.
In common with other reports we found that EF, but not PEG-fusion, provided an effective means of generating DC/tumour cell hybrids that co-express both TAA and DC associated antigens. Although concerns have been raised regarding the use of FACS analysis in the determination of hybrid yields, we found a good correlation between the yields estimated by FACS analysis and those estimated following morphology-based enumeration of multi-nucleated cell formation. The yields of hybrids obtained were similar to those reported in studies of other tumour cell types and emphasises the broad applicability of this approach [1, 4, 7, 9, 16–18, 23, 24, 27, 29]. Although apoptotic and/or necrotic tumour cells are reported to have enhanced immunogenicity [35, 37, 38], the relative level of these populations in EF preparations has not been previously reported. In the current study, we demonstrate that EF preparations contain significant populations of both apoptotic and late apoptotic/necrotic cells. Therefore, although our EF preparations were, in agreement with previous reports [1], 28–63% viable by trypan blue exclusion, only 6–21% were viable as defined by an absence of annexin V staining. The presence of large numbers of apoptotic and necrotic tumour cells may increase the immunogenicity of EF preparations. Conversely, the low number of viable DC present may reduce the effectiveness of EF preparations as vaccines as it has been reported that apoptotic or necrotic DC vaccines do not induce immune responses in vivo [39].
The development of anti-tumour responses requires that DC interact with tumour cells in a manner that enables the DC to not only uptake TAA, but also provides the inflammatory signals required to induce DC maturation. It has been reported that DC can actively acquire antigen from both viable and non-viable cells [10, 35, 38, 40]. The results of the current study confirm that the majority of DC actively acquire TAA from tumour cells during co-culture and that the pre-exposure of tumour cells to the EF process significantly increases the level of TAA uptake. The observation that both mock fusion and EF resulted in similar levels of TAA uptake demonstrates that it is exposure to the EF buffer rather than electrical pulses that induce this effect. Interestingly, similar levels of DC maturation, as measured phenotypically, were induced by both LPS and tumour cells irrespective of tumour cell pre-treatment.
Numerous in vivo and in vitro studies have analysed the immunostimulatory capability of the cellular product obtained following EF of tumour and DC. However, the majority of these studies have used tumour cells alone and tumour cells mixed with DC as their control preparations and so do not allow the relative immunogenicity of the hybrid and non-hybrid components to be determined. It is well established that exposure of tumour cells to stress and/or induction of tumour apoptosis/necrosis can significantly increase tumour cell immunogenicity [35, 37, 38]. The EF process involves considerable cellular stress in the form of high-sugar concentrations and electrical pulses and also results in considerable apoptosis and necrosis. Therefore, there is a strong rationale for investigating whether EF can increase tumour cell immunogenicity. The results of the current study clearly demonstrate that exposure of a colorectal cell line to the EF process significantly increases subsequent IFNγ release and cytotoxic activity by responding mononuclear cells. The observation that EF of allogeneic non-T stimulators, an additional colorectal cell line and a breast cancer cell line also induces increased IFNγ release demonstrates that the EF effect is not restricted to a single cell type. Two recent in vivo studies using murine tumour models have used EF treated tumour cells as control immunogens and similarly found that they stimulate increased immune responses relative to naive tumour cells [4, 9]. The exact nature of the EF induced cellular changes that result in increased cell immunogenicity is presently unknown but may include the induction of stress related proteins such as heat shock proteins. The results obtained in the current study suggest that exposure to EF buffer alone induces some changes in cellular immunogenicity and that subsequent exposure to electrical pulses results in further increases. The use in this study of allogeneic stimulators precludes the determination of whether these changes directly increase responses to both tumour specific antigen and alloantigen. Further experiments using autologous assays will be required to address this issue. Many of the current EF based immunotherapy protocols use allogeneic DC or tumour cells as part of the EF preparation as allogeneic responses are thought to provide help for the induction of tumour specific responses [26, 31, 41]. Therefore, even if the direct effects of the EF process on immunogenicity observed in this study are restricted to alloantigen these effects may still indirectly increase the magnitude of tumour specific responses and are therefore still of relevance to EF protocols.
The detection in this study of increased IFNγ release in response to EF treated tumour cells required that the assay used EF treated tumours cells that were pre-incubated with IL-12 and PBMC responders that were pre-incubated with IL-15. Although the relative importance of these cytokine treatments was not determined this observation provides strong evidence that either one or both of these cytokines can significantly enhance the immunogenicity of EF treated tumour cells. The importance of these cytokines in enhancing anti-tumour responses is well established and previous studies have also reported that these cytokines can enhance the immunogenecity of DC-tumour hybrids [14, 17, 18]. It is possible that the EF process may increase tumour cell responses to IL-12 and/or that IL-15 exposure increases APC and/or effector cell responses to EF treated tumours. Further studies are therefore required to optimise the use of these cytokines in EF based immunotherapy protocols.
It is unclear, which effector populations are stimulated either directly or indirectly by exposure to EF treated cells. The stimulation of increased activity by the responder DC population is one potential mechanism by which EF treated cells may increase adaptive and innate immune response. Another possibility is that EF treated cells may directly stimulate increased activity by cells of the innate immune system such as NK cells. Immunotherapy studies have generally focussed on the ability of vaccines to induce TAA specific CD8+ CTL responses, but increasing evidence suggests that cells of the innate system play a critical role in tumour rejection and that NK activation is involved in the development of T cell responses [42, 43]. Although the ability of EF preparations to stimulate CD8 responses is well established, their ability to stimulate cells of the innate system has received little study. However, in a neuroblastoma model, the depletion of both CD8 and NK populations abrogated the in vivo anti-tumour responses induced by an EF preparation [7].
It is now well accepted that DC loaded with TAA can induce powerful anti-tumour responses, but the optimal method of DC loading has not been established. Simple co-culture of DC and tumour cells has been reported to result in an immunogen that can induce strong anti-tumour responses [9, 10]. Many current protocols pre-treat tumour cells prior to co-culture with DC as apoptotic, necrotic or heat shocked tumour cells have all been reported to have increased immunogenicity [37, 38]. The formation of DC-tumour hybrids provides a potentially powerful alternative means of combining DC with TAA and consequently PEG mediated DC-tumour fusion has been widely used in immunotherapy protocols. The functional activity of these preparations has been attributed to the presence of hybrids. However, the majority of PEG fusion protocols subsequently culture the fused preparations in vitro before use, and few of these studies have directly analysed what advantage, if any, fusion offers over the simple co-culture of DC and tumour cells. Interestingly, two of the few studies, which directly compared “PEG fused” with “co-cultured only” preparations of DC and tumour cells, reported that both methods generated equally effective tumour vaccines in vivo [9, 10]. Similarly, with respect to EF protocols, it has not yet been established that the EF of DC and tumour provides a more effective vaccine than that which would be obtained by co-culturing DC with EF treated tumour cells. The results of our current study demonstrate that the EF process can increase the immunogenicity of at least some cell types and therefore co-culture of EF treated tumour with DC may result in an effective vaccine. One significant advantage that co-culture may provide over direct DC-tumour EF is that the DC present would be predominantly viable, which would increase their effectiveness in stimulating in vivo responses [39]. In addition, there is some evidence that hybrid cells can rapidly suppress or lose expression of TAA following their formation [12, 44] and therefore, DC loaded with TAA by co-culture may provide a more effective and longer lived TAA source.
Although the presence of DC within the vaccine preparation is considered critical to the effectiveness of EF based immunotherapy, the relative importance of vaccine derived versus recipient DC in inducing anti-tumour responses has not yet been established. The possibility that the immunogenicity of EF treated tumour cells is mediated primarily by their interaction with recipient DC cannot be presently excluded and raises the possibility that the presence/viability of vaccine derived DC may not be a major factor in determining EF vaccine effectiveness. The majority of EF products are irradiated prior to in vivo administration and the possibility that irradiation and other processing steps may modulate the immunogenicity of EF products requires further study.
The data presented in this study demonstrate that the EF process can increase the immunogenicity of at least some human cell types, independently of hybrid formation. This has considerable implications with respect to the optimisation of EF protocols as to date studies have concentrated predominantly on maximising hybrid yields. Understanding the relative contributions that hybrid and non-hybrid cells make to the effectiveness of EF preparations is critical for the development of more effective immunotherapy protocols. Further studies are therefore required to determine whether current EF protocols provide the most effective means of utilising the EF process in the generation of cancer vaccines.
Acknowledgements
This work was supported by the Canterbury Medical Research Foundation, the Robert McClelland Trust, the New Zealand Lottery Grants Board, the Cancer Society (Canterbury/Westland) and the Bone Marrow Transplant Trust.
Footnotes
BH and GR contributed equally as senior authors
References
- 1.Scott-Taylor TH, Pettengell R, Clarke I, Stuhler G, La Barthe MC, Walden P, Dalgleish AG. Human tumour and dendritic cell hybrids generated by electrofusion: potential for cancer vaccines. Biochim Biophys Acta. 2000;1500:265–279. doi: 10.1016/s0925-4439(99)00108-8. [DOI] [PubMed] [Google Scholar]
- 2.Shu S, Cohen P. Tumor-dendritic cell fusion technology and immunotherapy strategies. J Immunother. 2001;24:99–100. [PubMed] [Google Scholar]
- 3.Gong J, Chen D, Kashiwaba M, Kufe D. Induction of antitumour activity by immunization with fusions of dendritic and carcinoma cells. Nat Med. 1997;3:558–561. doi: 10.1038/nm0597-558. [DOI] [PubMed] [Google Scholar]
- 4.Siders WM, Vergilis KL, Johnson C, Shields J, Kaplan JM. Induction of specific antitumor immunity in the mouse with the electrofusion product of tumor cells and dendritic cells. Mol Ther. 2003;7:498–505. doi: 10.1016/S1525-0016(03)00044-3. [DOI] [PubMed] [Google Scholar]
- 5.Zhang JK, Li J, Zhang J, Chen HB, Chen SB. Antitumor immunopreventive and immunotherapeutic effect in mice induced by hybrid vaccine of dendritic cells and hepatocarcinoma in vivo. World J Gastroenterol. 2003;9:479–484. doi: 10.3748/wjg.v9.i3.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang J, Saffold S, Cao X, Krauss J, Chen W. Eliciting T cell immunity against poorly immunogenic tumors by immunization with dendritic cell-tumor fusion vaccines. J Immunol. 1998;161:5516–5524. [PubMed] [Google Scholar]
- 7.Orentas RJ, Schauer D, Bin Q, Johnson BD. Electrofusion of a weakly immunogenic neuroblastoma with dendritic cells produces a tumor vaccine. Cell Immunol. 2001;213:4–13. doi: 10.1006/cimm.2001.1864. [DOI] [PubMed] [Google Scholar]
- 8.Xia J, Tanaka Y, Koido S, Liu C, Mukherjee P, Gendler SJ, Gong J. Prevention of spontaneous breast carcinoma by prophylactic vaccination with dendritic/tumor fusion cells. J Immunol. 2003;170:1980–1986. doi: 10.4049/jimmunol.170.4.1980. [DOI] [PubMed] [Google Scholar]
- 9.Lindner M, Schirrmacher V. Tumour cell-dendritic cell fusion for cancer immunotherapy: comparison of therapeutic efficiency of polyethylen-glycol versus electro-fusion protocols. Eur J Clin Invest. 2002;32:207–217. doi: 10.1046/j.1365-2362.2002.00968.x. [DOI] [PubMed] [Google Scholar]
- 10.Celluzzi CM, Falo LD., Jr Physical interaction between dendritic cells and tumor cells results in an immunogen that induces protective and therapeutic tumor rejection. J Immunol. 1998;160:3081–3085. [PubMed] [Google Scholar]
- 11.Li J, Holmes LM, Franek KJ, Burgin KE, Wagner TE, Wei Y. Purified hybrid cells from dendritic cell and tumor cell fusions are superior activators of antitumor immunity. Cancer Immunol Immunother. 2001;50:456–462. doi: 10.1007/s002620100218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Phan V, Errington F, Cheong SC, Kottke T, Gough M, Altmann S, Brandenburger A, Emery S, Strome S, Bateman A, Bonnotte B, Melcher A, Vile R. A new genetic method to generate and isolate small, short-lived but highly potent dendritic cell-tumor cell hybrid vaccines. Nat Med. 2003;9:1215–1219. doi: 10.1038/nm923. [DOI] [PubMed] [Google Scholar]
- 13.Lespagnard L, Mettens P, Verheyden AM, Tasiaux N, Thielemans K, van Meirvenne S, Geldhof A, De Baetselier P, Urbain J, Leo O, Moser M. Dendritic cells fused with mastocytoma cells elicit therapeutic antitumor immunity. Int J Cancer. 1998;76:250–258. doi: 10.1002/(SICI)1097-0215(19980413)76:2<250::AID-IJC13>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 14.Gong J, Koido S, Chen D, Tanaka Y, Huang L, Avigan D, Anderson K, Ohno T, Kufe D. Immunization against murine multiple myeloma with fusions of dendritic and plasmacytoma cells is potentiated by interleukin 12. Blood. 2002;99:2512–2517. doi: 10.1182/blood.V99.7.2512. [DOI] [PubMed] [Google Scholar]
- 15.Gong J, Chen D, Kashiwaba M, Li Y, Chen L, Takeuchi H, Qu H, Rowse GJ, Gendler SJ, Kufe D. Reversal of tolerance to human MUC1 antigen in MUC1 transgenic mice immunized with fusions of dendritic and carcinoma cells. P Natl Acad Sci USA. 1998;95:6279–6283. doi: 10.1073/pnas.95.11.6279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hayashi T, Tanaka H, Tanaka J, Wang R, Averbook BJ, Cohen PA, Shu S. Immunogenicity and therapeutic efficacy of dendritic-tumor hybrid cells generated by electrofusion. Clin Immunol. 2002;104:14–20. doi: 10.1006/clim.2002.5224. [DOI] [PubMed] [Google Scholar]
- 17.Tanaka H, Shimizu K, Hayashi T, Shu S. Therapeutic immune response induced by electrofusion of dendritic and tumor cells. Cell Immunol. 2002;220:1–12. doi: 10.1016/S0008-8749(03)00009-1. [DOI] [PubMed] [Google Scholar]
- 18.Goddard RV, Prentice AG, Copplestone JA, Kaminski ER. In vitro dendritic cell-induced T cell responses to B cell chronic lymphocytic leukaemia enhanced by IL-15 and dendritic cell-B-CLL electrofusion hybrids. Clin Exp Immunol. 2003;131:82–89. doi: 10.1046/j.1365-2249.2003.02047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gong J, Avigan D, Chen D, Wu Z, Koido S, Kashiwaba M, Kufe D. Activation of antitumor cytotoxic T lymphocytes by fusions of human dendritic cells and breast carcinoma cells. P Natl Acad Sci USA. 2000;97:2715–2718. doi: 10.1073/pnas.050587197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gong J, Nikrui N, Chen D, Koido S, Wu Z, Tanaka Y, Cannistra S, Avigan D, Kufe D. Fusions of human ovarian carcinoma cells with autologous or allogeneic dendritic cells induce antitumor immunity. The J Immunol. 2000;165:1705–1711. doi: 10.4049/jimmunol.165.3.1705. [DOI] [PubMed] [Google Scholar]
- 21.Soruri A, Fayyazi A, Neumann C, Schlott T, Jung T, Matthes C, Zwirner J, Riggert J, Peters JH. Ex vivo generation of human anti-melanoma autologous cytolytic T cells by dentritic cell/melanoma cell hybridomas. Cancer Immunol Immunother. 2001;50:307–314. doi: 10.1007/s002620100198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chan RC, Xie H, Zhao GP, Xie Y. Dendritomas formed by fusion of mature dendritic cells with allogenic human hepatocellular carcinoma cells activate autologous cytotoxic T lymphocytes. Immunol Lett. 2002;83:101–109. doi: 10.1016/S0165-2478(02)00078-0. [DOI] [PubMed] [Google Scholar]
- 23.Gottfried E, Krieg R, Eichelberg C, Andreesen R, Mackensen A, Krause SW. Characterization of cells prepared by dendritic cell-tumor cell fusion. Cancer Immun. 2002;2:15. [PubMed] [Google Scholar]
- 24.Parkhurst MR, DePan C, Riley JP, Rosenberg SA, Shu S. Hybrids of dendritic cells and tumor cells generated by electrofusion simultaneously present immunodominant epitopes from multiple human tumor-associated antigens in the context of MHC class I and class II molecules. J Immunol. 2003;170:5317–5325. doi: 10.4049/jimmunol.170.10.5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holmes LM, Li J, Sticca RP, Wagner TE, Wei Y. A rapid, novel strategy to induce tumor cell-specific cytotoxic T lymphocyte responses using instant dendritomas. J Immunother. 2001;24:122–129. [PubMed] [Google Scholar]
- 26.Trevor KT, Cover C, Ruiz YW, Akporiaye ET, Hersh EM, Landais D, Taylor RR, King AD, Walters RE. Generation of dendritic cell-tumor cell hybrids by electrofusion for clinical vaccine application. Cancer Immunol Immunother. 2004;53:705–714. doi: 10.1007/s00262-004-0512-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trefzer U, Weingart G, Chen Y, Herberth G, Adrian K, Winter H, Audring H, Guo Y, Sterry W, Walden P. Hybrid cell vaccination for cancer immune therapy: first clinical trial with metastatic melanoma. Int J Cancer. 2000;85:618–626. doi: 10.1002/(SICI)1097-0215(20000301)85:5<618::AID-IJC4>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 28.Krause SW, Neumann C, Soruri A, Mayer S, Peters JH, Andreesen R. The treatment of patients with disseminated malignant melanoma by vaccination with autologous cell hybrids of tumor cells and dendritic cells. J Immunother. 2002;25:421–428. doi: 10.1097/00002371-200209000-00006. [DOI] [PubMed] [Google Scholar]
- 29.Kugler A, Stuhler G, Walden P, Zoller G, Zobywalski A, Brossart P, Trefzer U, Ullrich S, Muller CA, Becker V, Gross AJ, Hemmerlein B, Kanz L, Muller GA, Ringert RH. Regression of human metastatic renal cell carcinoma after vaccination with tumor cell-dendritic cell hybrids. Nat Med. 2000;6:332–336. doi: 10.1038/73193. [DOI] [PubMed] [Google Scholar]
- 30.Marten A, Renoth S, Heinicke T, Albers P, Pauli A, Mey U, Caspari R, Flieger D, Hanfland P, Von Ruecker A, Eis-Hubinger AM, Muller S, Schwaner I, Lohmann U, Heylmann G, Sauerbruch T, Schmidt-Wolf IG. Allogeneic dendritic cells fused with tumor cells: preclinical results and outcome of a clinical phase I/II trial in patients with metastatic renal cell carcinoma. Hum Gene Ther. 2003;14:483–494. doi: 10.1089/104303403321467243. [DOI] [PubMed] [Google Scholar]
- 31.Trefzer U, Herberth G, Wohlan K, Milling A, Thiemann M, Sherev T, Sparbier K, Sterry W, Walden P. Vaccination with hybrids of tumor and dendritic cells induces tumor-specific T-cell and clinical responses in melanoma stage III and IV patients. Int J Cancer. 2004;110:730–740. doi: 10.1002/ijc.20191. [DOI] [PubMed] [Google Scholar]
- 32.Miley HE, Sheader EA, Brown PD, Best L. Glucose-induced swelling in rat pancreatic beta-cells. J Physiol. 1997;504:191–198. doi: 10.1111/j.1469-7793.1997.00191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cortizo AM, Paladini A, Diaz GB, Garcia ME, Gagliardino JJ. Changes induced by glucose in the plasma membrane properties of pancreatic islets. Mol Cell Endocrinol. 1990;71:49–54. doi: 10.1016/0303-7207(90)90074-I. [DOI] [PubMed] [Google Scholar]
- 34.Hock BD, Fearnley DB, Boyce A, McLellan AD, Sorg RV, Summers KL, Hart DNJ. Human dendritic cells express a 95 kDa activation/differentiation antigen defined by CMRF-56. Tissue Antigens. 1999;53:320–334. doi: 10.1034/j.1399-0039.1999.530402.x. [DOI] [PubMed] [Google Scholar]
- 35.Kotera Y, Shimizu K, Mule JJ. Comparative analysis of necrotic and apoptotic tumor cells as a source of antigen(s) in dendritic cell-based immunization. Cancer Res. 2001;61:8105–8109. [PubMed] [Google Scholar]
- 36.Neri S, Mariani E, Meneghetti A, Cattini L, Facchini A. Calcein-acetyoxymethyl cytotoxicity assay: standardization of a method allowing additional analyses on recovered effector cells and supernatants. Clin Diagn Lab Immunol. 2001;8:1131–1135. doi: 10.1128/CDLI.8.6.1131-1135.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Larsson M, Fonteneau JF, Bhardwaj N. Dendritic cells resurrect antigens from dead cells. Trends Immunol. 2001;22:141–148. doi: 10.1016/S1471-4906(01)01860-9. [DOI] [PubMed] [Google Scholar]
- 38.Feng H, Zeng Y, Graner MW, Katsanis E. Stressed apoptotic tumor cells stimulate dendritic cells and induce specific cytotoxic T cells. Blood. 2002;100:4108–4115. doi: 10.1182/blood-2002-05-1389. [DOI] [PubMed] [Google Scholar]
- 39.Kleindienst P, Brocker T. Endogenous dendritic cells are required for amplification of T cell responses induced by dendritic cell vaccines in vivo. J Immunol. 2003;170:2817–2823. doi: 10.4049/jimmunol.170.6.2817. [DOI] [PubMed] [Google Scholar]
- 40.Harshyne LA, Watkins SC, Gambotto A, Barratt-Boyes SM. Dendritic cells acquire antigens from live cells for cross-presentation to CTL. J Immunol. 2001;166:3717–3723. doi: 10.4049/jimmunol.166.6.3717. [DOI] [PubMed] [Google Scholar]
- 41.Newton DA, Acierno PM, Metts MC, Baron PL, Brescia FJ, Gattoni-Celli S. Semiallogeneic cancer vaccines formulated with granulocyte-macrophage colony-stimulating factor for patients with metastatic gastrointestinal adenocarcinomas: a pilot phase I study. J Immunother. 2001;24:19–26. doi: 10.1097/00002371-200101000-00003. [DOI] [PubMed] [Google Scholar]
- 42.Mattes J, Hulett M, Xie W, Hogan S, Rothenberg ME, Foster P, Parish C. Immunotherapy of cytotoxic T cell-resistant tumors by T helper 2 cells: an eotaxin and STAT6-dependent process. J Exp Med. 2003;197:387–393. doi: 10.1084/jem.20021683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Westwood JA, Kelly JM, Tanner JE, Kershaw MH, Smyth MJ, Hayakawa Y. Cutting Edge: Novel priming of tumor-specific immunity by NKG2D-triggered NK cell-mediated tumor rejection and Th1-independent CD4+ T cell pathway. J Immunol. 2004;172:757–761. doi: 10.4049/jimmunol.172.2.757. [DOI] [PubMed] [Google Scholar]
- 44.Tsujimoto H, Nishizuka S, Redpath LJ, Stanbridge EJ. Examination of the oncogenic potential of H19 gene in HeLa x normal human fibroblast hybrid cells. Int J Oncol. 2001;19:89–95. doi: 10.3892/ijo.19.1.89. [DOI] [PubMed] [Google Scholar]