Abstract
Interferon-alpha (IFN-α) is used as an adjuvant therapy in patients with malignant melanoma and who have undergone surgical resection of high-risk lesions. Defective expression or activation of STAT1 or STAT2 has been shown to correlate with IFN-α or resistance in vitro; however, recent data from our laboratory suggest that the anti-tumor effects of IFN-α are dependent on STAT1 signaling within host immune cells. We measured STAT1 and STAT2 expression in 28 melanoma biopsies (8 cutaneous lesions; 1 lung metastasis; 19 nodal metastases) obtained from patients prior to the initiation of adjuvant IFN-α therapy. Disease recurrence following IFN-α treatment did not correlate with the staining intensity of either STAT1 (P=0.61) or STAT2 (P=0.52). Tumors with minimal STAT1 or STAT2 expression (<20% positive) were present in four patients with tumor-positive lymph nodes, who exhibited prolonged relapse-free survival (>44 months) following adjuvant therapy. Conversely, high levels of STAT1 were present in a patient who recurred during the course of IFN-α therapy. A case study of one patient who experienced recurrent disease during IFN-α treatment revealed that STAT1 levels were greater in the recurrent tumor when compared to the original lesion. These studies provide direct evidence to suggest that levels of STAT1 and STAT2 within the tumor do not influence a patient’s response to adjuvant IFN-α.
Keywords: Interferon-alpha, STAT1, STAT2, Malignant melanoma, Adjuvant therapy
Introduction
Interferon-alpha (IFN-α) has demonstrated clinical activity when administered at high doses to patients with metastatic melanoma [13–15, 17]. The utility of adjuvant IFN-α has also been explored in patients who underwent complete surgical excision of their tumor, yet were at high-risk for recurrent disease [7, 13–15]. Data obtained from trials utilizing IFN-α in the adjuvant setting yielded conflicting results. Kirkwood et al. [15] conducted a phase III trial in which patients were randomized to receive high-dose IFN-α [20 million units (MU)/m2/day i.v. for 1 month followed by 10 MU/m2 /thrice weekly s.c. for 48 weeks] or observation. The results of this trial were reported in 1996 and revealed increased overall survival and relapse-free survival in patients who received IFN-α. In contrast, a confirmatory trial comparing the efficacy of high-dose and low-dose IFN-α regimens (3 MU thrice weekly × 2 years) in a similar population of surgically-treated patients revealed no difference in overall survival for either schedule of IFN-α as compared to observation alone [12]. Despite these conflicting reports, and our current lack of knowledge regarding its mechanism of anti-tumor action, IFN-α remains the only FDA-approved adjuvant treatment for patients who underwent surgical resection of high-risk malignant melanoma lesions (primary tumors >4 mm or lymph node metastases) [17].
The receptor for IFN-α (IFNAR) is widely expressed on both tumor and host-immune cells [11]. Binding of IFN-α to its receptor activates the associated kinases, Janus kinase 1 (Jak1) and Tyrosine kinase 2 (Tyk2), which phosphorylate the cytoplasmic tails of the interferon receptor subunits on specific tyrosine residues. These phosphotyrosine residues provide docking sites for cytoplasmic transcription factors belonging to the signal transducer and activator of transcription (STAT) family of proteins. STAT proteins are phosphorylated following ligand binding [8] and form a DNA binding complex known as the interferon-stimulated gene factor 3 (ISGF3) which consists of STAT1α or STAT1β—an inactive form), STAT2 and a p48 DNA binding protein, known as interferon regulatory factor 9 (IRF9) [18]. ISGF3 initiates the transcription of IFN-responsive genes through its interaction with a shared promoter element, the interferon stimulated response element (ISRE) [6].
Interferon-alpha is known to exert direct anti-proliferative, pro-apoptotic and anti-angiogenic effects on melanoma cells in culture. We previously showed that ex vivo treatment of patient tumors with clinically relevant concentrations of IFN-α consistently activated STAT1 and STAT2 [3]. These observations suggested that IFN-α treatments exert a direct effect on tumor cells regardless of their ability to stimulate host tissues. More recently, it was also observed that some IFN-α-resistant human tumor cell lines exhibited defects in the expression of specific Jak-STAT intermediates (STAT1, STAT2 or IRF9) [22, 25, 28]. Interestingly, restoration of STAT1 expression in an IFN-α-resistant melanoma cell line led to the recovery of in vitro sensitivity to IFN-α [29]. These data supported the hypothesis that the clinical response to IFN-α may be mediated, in part, by a direct effect on the melanoma cell. In contrast, other evidence suggest that the immunomodulatory effects of IFN-α might be most critical to its anti-tumor actions. We recently showed that activation of STAT1 within host immune effectors and not melanoma cells is responsible for the anti-tumor effects of IFN-α in a murine model of malignant melanoma [1, 19]. Furthermore, a study by Chawla-Sarkar et al. reported that defects in expression or activation of STAT1 or STAT2 were infrequent in melanoma cell lines and tumor samples and did not correlate with IFN-resistance in vitro [5].
The current study investigated whether the expression of STAT1 and STAT2 within 28 retrospectively identified melanoma tumors correlated with clinical responsiveness to IFN-α adjuvant therapy. We found that the expression of STAT1 and STAT2 within patient melanoma tumors was not associated with either time to disease recurrence or overall survival (OS). Thus in the clinical setting, the expression of STAT1 and STAT2 within the tumor did not predict a patient’s ultimate response to adjuvant IFN-α. These data provide clinical evidence to support the hypothesis that other targets of IFN-α action, such as host immune effectors, or other tumor signaling pathways may be more important in determining the clinical response to this treatment modality.
Materials and Methods
Antibodies
Murine anti-human STAT1 antibody (N-terminus) was obtained from Becton Dickinson Transduction Laboratories (Franklin Lakes, NJ, USA). Rabbit anti-human STAT2 antibody was obtained from Biosource International (Camarillo, CA, USA). Murine IgG1 and rabbit IgG antibodies were used as isotype controls to assess background staining on negative control slides for STAT1 and STAT2, respectively.
Surgical specimens and patients
All studies were conducted in accordance with the rules and regulations of The Ohio State University Institutional Review Board (protocol# 2002H0089). Malignant melanoma surgical specimens were obtained through the Cooperative Human Tissue Network facility at the Ohio State University, Columbus, OH, USA. Tumor specimens that prompted the initiation of IFN-α adjuvant therapy were obtained from 28 patients with malignant melanoma and who had undergone a lymph node dissection (n=19), wide local resection of a primary tumor (n=8), or resection of an isolated visceral metastasis (n=1). All patients underwent surgical excision of their index lesion at the University Hospitals of the Ohio State University between 1991 and 1999. When possible, these specimens were selected to include tumor and adjacent non-malignant tissue. Paraffin embedded tumors were sectioned into 5-μM slices using a standard microtome and attached to a lysine-coated slide. A melanotic phenotype was confirmed by staining with hematoxylin and eosin (H & E) and immunohistochemistry for vimentin, S100 and HMB45. All specimens were obtained from index lesions prior to the initiation of IFN-α adjuvant therapy.
Immunostaining of paraffin sections
Sections were de-paraffinized twice in xylene for 10 min at room temperature and rehydrated by stepwise washes in decreasing ethanol/H2O ratio (100, 95, 70%) followed by soaking in dH2O. Endogenous peroxidase activity was blocked with dH2O containing 3% hydrogen peroxide for 5 min, followed by repeated rinses in dH2O. Antigen retrieval was achieved in Dako’s target retrieval solution (Dako S1699) by heating slides in a steamer at 94°C for 30 min and cooling at room temperature for 15 min. After rinsing in dH2O, slides were incubated for 60 min with a 1:2,000 dilution of murine anti-STAT1 (N-terminus) antibody or a 1:50 dilution of rabbit anti-human STAT2 antibody. Detection was achieved with the Isab+ system and DAB Chromogen (Dako, Carpinteria, CA, USA). Coverslips were applied following counterstaining with Mayer’s hematoxylin and dehydration. Tissue specimens were processed in a single batch to ensure a valid comparison between samples.
Analysis of STAT1 and STAT2 expression
Analysis of patient samples was performed in a blinded fashion by an experienced dermatopathologist. Three or more high power fields per tumor section were analyzed using a Nikon Eclipse E400 microscope (Tokyo, Japan). Care was taken to eliminate compromised areas that could bias the analysis, such as artifacts generated during processing or staining. Following the identification of malignant tissue by H & E staining, the percentage of cells positive for STAT1 or STAT2 was calculated. Location of positive staining (cytoplasmic vs. nuclear) and intensity of staining (strong, moderate, or weak) was also recorded. All antibodies were titrated prior to staining using control tissue from a single malignant melanoma patient who underwent a lymph node biopsy. Positive staining of lymphocytes for STAT1 and STAT2 within each lymph node was used to validate the specificity of the staining, and to determine the optimal dilution of antibody.
Patient data
A total of 28 specimens were obtained from tumors of retrospectively identified patients with histologically proven malignant melanoma. The Breslow thickness and Clark’s Level of each primary tumor was obtained from individual patient records. Due to the retrospective nature of this study, this information was not specified in the medical records of all patients. Information on Clark’s Level was available in only 21 out of 28 patients. Information on Breslow thickness was available in 25 out of 28 patients, as 3 out of 26 patients studied presented with a primary tumor in a lymph node. Patient characteristics including age, sex, race, primary tumor site, and date of relapse (if applicable) were recorded. Patients were staged according to general guidelines of the American Joint Committee on Cancer (AJCC) [23, 26]. All surgical procedures were performed prior to initiation of IFN-α adjuvant therapy.
Response criteria/clinical course definitions
The protocol for IFN-α adjuvant therapy has been previously described [15]. Briefly, this treatment consisted of 1 month of high-dose i.v. interferon-alpha 2b (IFN-α2b) administered at 20 MU/m2 /day, for 5 days each week, followed by s.c. administration of low-dose IFN-α2b (10 MU/m2 /day) 3 times weekly for 11 months. The number of months completed by each patient was recorded. Completion of the entire 12-month treatment regimen without decreasing the standard dosage of IFN-α2b (due to toxicity or non-compliance) was defined as a complete course of adjuvant therapy.
Statistical analysis
Disease recurrence from the start of IFN-α therapy was the primary outcome of interest. We investigated the association between recurrence and STAT1 scores, STAT2 scores, tumor involved lymph nodes, STAT1 location and STAT2 location via Fisher’s Exact test. STAT1 and STAT2 scores incorporate the percentage of cells positive for STAT1 or STAT2 and the intensity of staining into a five level score (1–5, where 5 is high). To compare tumor thickness between patients who recurred and those who did not, we used the Wilcoxon rank–sum test. To measure the strength of dependence between the measures of STAT1 and STAT2, the percentage of cells staining positive for STAT1 was compared with the percentage of cells staining positive for STAT2 using Spearman’s rank correlation. Estimates of the time to recurrence for patients were calculated via the Kaplan–Meier estimator [10]. All reported P-values were two-sided and considered significant at the 0.05 level. Due to the small number of patients involved in this study, the associations were limited to univariable analysis.
Results
Patient and specimen characteristics
The study cohort consisted of 28 retrospectively identified malignant melanoma patients with tumors that prompted initiation of IFN-α adjuvant therapy (Breslow thickness >4 mm, Clark’s Level III or greater, nodal involvement, isolated recurrences). Only patients for whom this specific tumor tissue was available were selected for this study. Patient demographics are summarized in Table 1. Of the specimens being examined in this study, eight consisted of primary tumors of the skin, one was an isolated lung metastasis, and 19 were lymph node metastases. All patients were Caucasian, 16 of the 28 patients were male and the median age was 53. The majority of primary tumors were located on the extremities (13 of 28) with the remaining tumors arising from the skin of the trunk or head/neck. Following surgical resection, patients were determined to be disease-free and subsequently underwent adjuvant therapy with IFN-α. Patients were monitored for recurrence and mortality from the time IFN-α adjuvant therapy was initiated (Table 2). Patients with recurrent disease are represented by single-letter nomenclature (i.e. A-R), while patients who remained disease-free are represented by two-letter nomenclature (i.e. AA-JJ).
Table 1.
Patient demographics
| Number of patients total | 28 |
|---|---|
| Sex (male/female) | 16/12 |
| Age | |
| Median | 53 |
| Range | 25–82 |
| Primary tumor site | |
| Head/neck | 5 |
| Trunk | 7 |
| Extremeties | 13 |
| Other | 3 |
| Tumor thickness | |
| Mean (mm) | 3.7 |
| Range (mm) | 0.78–8.5 |
| Clarks level | |
| III | 6 |
| IV | 14 |
| V | 3 |
| Unspecified | 5 |
| Nodal invovlement | |
| None | 7 |
| 1–3 | 13 |
| >4 | 4 |
| Undetermined | 4 |
Table 2.
Disease related survival of the study population
| Number of patients | Percentage | |
|---|---|---|
| Relapse | 18 | 64.3 |
| Site of recurrence | ||
| Regional | 18 | 64.3 |
| Distant | 0 | 0 |
| Mortality | ||
| Total | 14 | 50 |
| Disease related | 13 | 46.4 |
| Treatment related | 0 | 0 |
| Other causes | 1 | 3.6 |
Clinical response to adjuvant therapy
The main objective of this study was to determine whether intratumoral expression of STAT1 and STAT2 by melanoma cells correlated with the clinical outcome of patients receiving IFN-α in the adjuvant setting. Ten patients in the current study reported significant toxicity and did not receive a full course of IFN-α adjuvant therapy (Patients E, I, J, N, P, Q, CC, EE, FF, JJ), although all received at least 1 month of high-dose IFN-α. The average length of maintenance IFN-α administered in this group of ten patients was 5.7 months. Analysis of data from two clinical trials of IFN-α adjuvant therapy (E1684 and E1690) has suggested that the survival benefit associated with adjuvant IFN-α might be attributable to the i.v. induction phase in which nearly 40% of the annual dose is administered [4, 12, 15]. Clinical trials designed to test the efficacy of the 1 month intravenous regimen are ongoing (Intergroup E1697). Based on these data, we elected to include patients receiving at least 1 month of IFN-α2b adjuvant therapy in our analysis. Seven patients in the current study experienced progressive disease during the course of adjuvant IFN-α, resulting in cessation of immunotherapy (Patients C, G, H, K, L, O, R).
Immunohistochemical analysis of STAT1 expression
Expression of STAT1 in patient tumor tissue was evaluated by immunohistochemistry. Small quantities of tumor tissue were a limitation to acquiring complete data for some patients included in the current study. As a result, STAT1 staining was performed on tumors from 26 patients in the study population. Representative sections of each tumor were first processed for histopathology by H & E staining to confirm the presence of malignant tissue. Many sections involving lymph node metastases contained normal lymphocytes that served as an internal positive control for STAT1 staining. Representative immunohistochemical staining of STAT1 in tumors metastatic to the lymph nodes is shown in Fig. 1a–c. Widespread positivity for STAT1 was observed in a majority of lymphoid cells contained within tumor sections. The staining in both tumor and lymphoid cells was predominantly localized to cytoplasmic regions; however scattered nuclear positivity was also evident. Marked variability in STAT1 expression was observed across patient tumors. Only 3 of 26 patient tumors (Patient D, K, and CC) demonstrated no detectable STAT1 staining, while the remaining patient tumors expressed levels ranging from very low expression (positive staining in ≤5% of tumor cells) in 9 out of 26 of patient tumors to high expression (≥60% positive) in 11 out of 26 of patient tumors. Multiple sections from tumors with very low STAT1 expression were stained to confirm this phenotype. The location of STAT1 staining in tumors was variable (4 out of 26 tumors displayed staining that was predominantly cytoplasmic; 5 out of 26 tumors displayed staining that was predominantly nuclear; and in 17 out of 26 tumors STAT1 staining was observed in both the nucleus and the cytoplasm). We did not find any association between the location of STAT1 and whether or not a patient exhibited recurrent disease (P=0.94, Fisher’s Exact Test). Representative sections of tumors with cytoplasmic and nuclear staining STAT1 are shown in Fig. 2a–c.
Fig. 1.
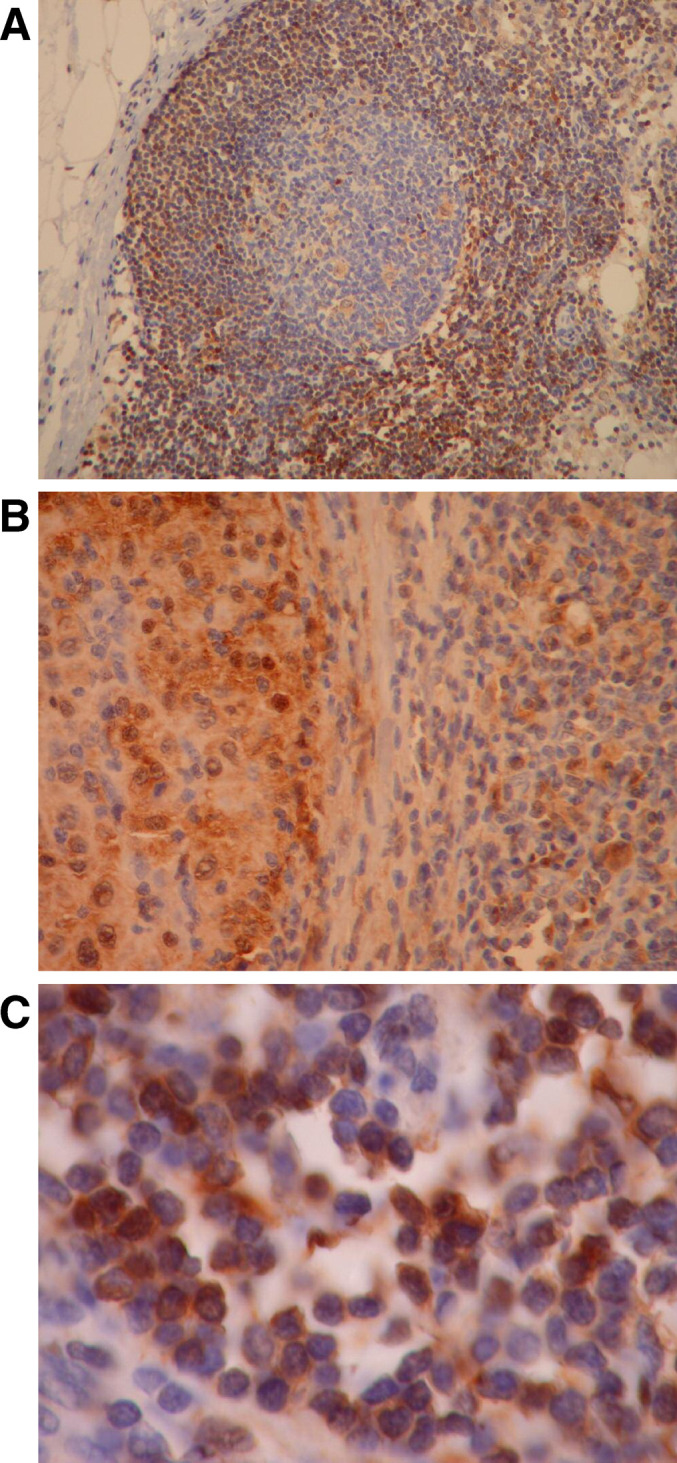
Representative STAT1 staining from a melanoma positive lymph node. Strong cytoplasmic and nuclear staining of STAT1 was observed in both melanoma cells and mononuclear cells present in the lymph nodes. a Representative tumor-infiltrated lymph node magnified at 200×, b Representative tumor-infiltrated lymph node magnified at 400×, (c) Representative mononuclear cells magnified at 800×
Fig. 2.
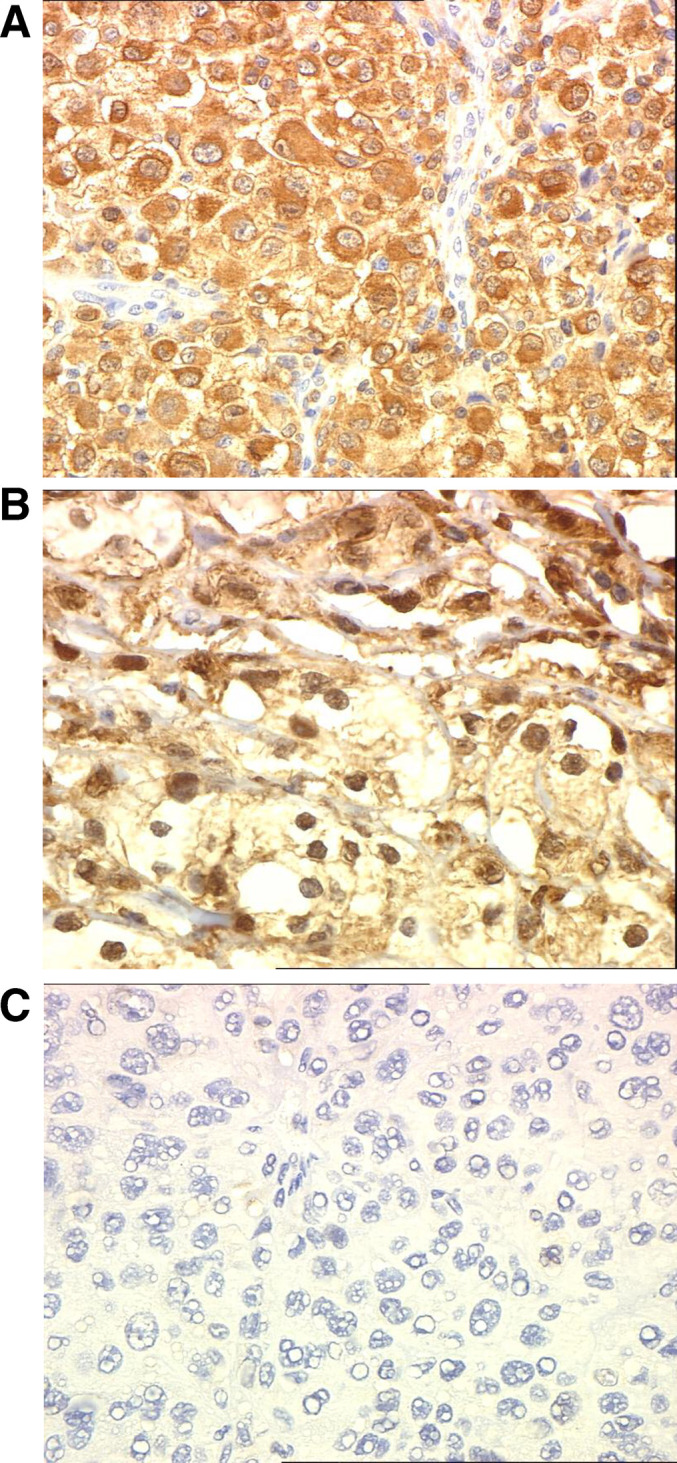
STAT1 staining. Representative STAT1 staining from malignant melanoma lesions showing a strong cytoplasmic staining b strong nuclear and cytoplasmic staining or c samples stained with a control murine IgG antibody (negative control). All sections shown are magnified at 400×
Immunohistochemical analysis of STAT2 expression
Expression of STAT2 was also analyzed and representative immunohistochemical staining is shown in Fig. 3a–c. With limited quantities of tumor tissue available from some patients, we were unable to determine STAT2 expression by immunohistochemistry in 4 of 28 patients in the study population. The level of STAT2 expression varied significantly across patient tumors (15–100% positive). On average, the overall expression was higher than that of STAT1 (Table 3). The location of STAT2 staining within tumors was variable (4 out of 24 tumors displayed predominantly nuclear staining; 7 out of 24 of tumors displayed predominantly cytoplasmic staining; and in 13 out of 24 of tumors STAT2 staining was observed in both the nucleus and the cytoplasm). We did not find any association between the location of STAT2 and whether or not a patient developed recurrent disease (P=0.85, Fisher’s Exact Test). Moreover, we did not find any association between the location of STAT1 and STAT2 staining overall (P=0.51). A summary of immunohistochemical data is presented in Table 4. The percentage of STAT1 staining was not correlated to the percentage of STAT2 staining (Spearman’s rank correlation=0.23, P=0.30).
Fig. 3.
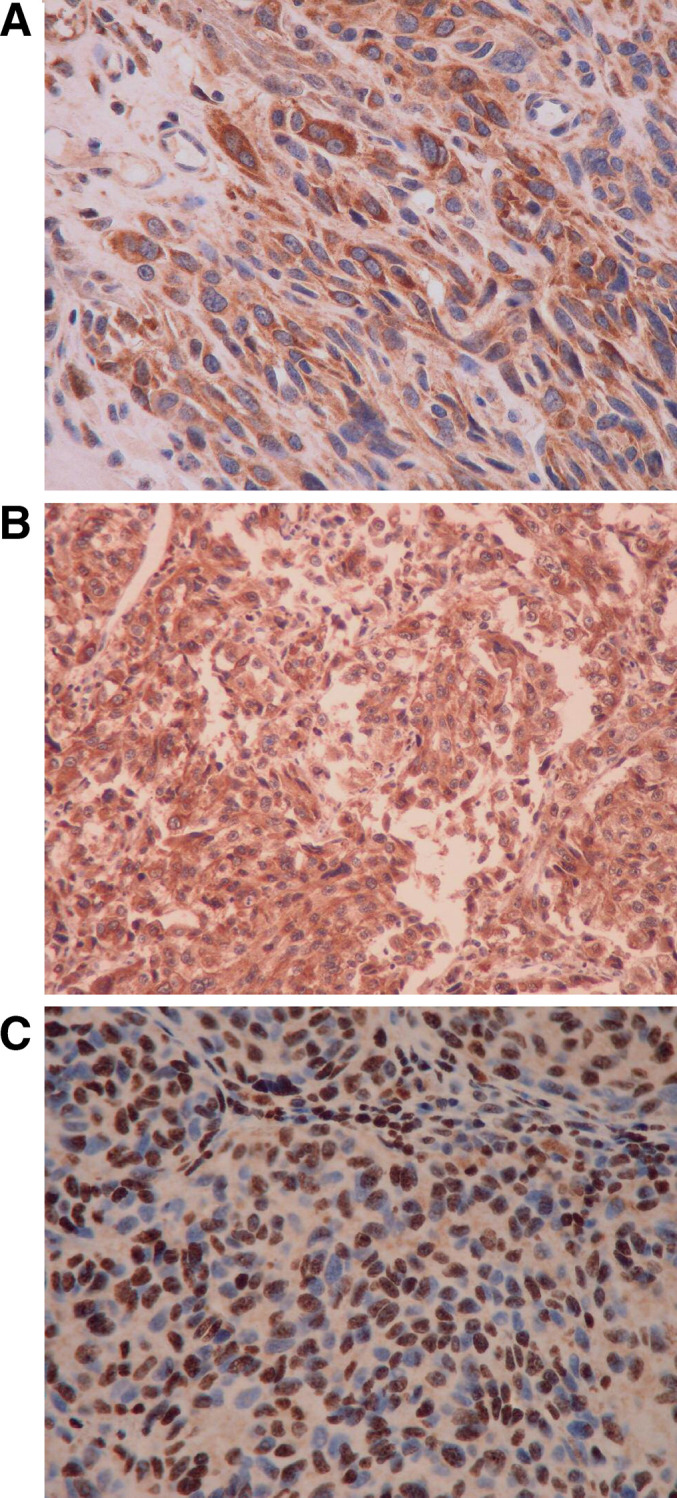
STAT2 Staining. Representative STAT2 staining from malignant melanoma lesions showing a, b strong cytoplasmic staining and c strong nuclear staining. All sections shown are magnified at 400×
Table 3.
Distribution of STAT1 and STAT2 staining within patient tumors
| Percentage positive | STAT1 | STAT2 |
|---|---|---|
| 0–20 | 9 | 2 |
| 21–40 | 6 | 4 |
| 41–60 | 4 | 4 |
| 61–80 | 5 | 10 |
| 81–100 | 2 | 4 |
Table 4.
Immunohistochemical analysis and clinical characteristics of the study population
| Patient | STAT1 level | STAT2 level | STAT1 location | STAT2 location | AJCC stage | Months completed | (Months post resection) | Clark’s level | Breslow thickness (mm) | LN positive | Primary tumor site | Site of recurrence |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | 4 | 4 | Cytoplasmic | Cytoplasmic | 3 | 12 | 19 | III | 0.78 | 12 of 14 | Back | Back |
| B | 3 | ND | Nuclear | ND | 1B | 12 | 52 | III | 1.15 | 0 of 19 | Back | Back |
| C | 1 | 2 | Cytoplasmic | Both | 2A | 9 | 9 | IV | 2.9 | 0 | Right leg | Right groin |
| D | 1 | ND | Both | ND | 4 | 12 | 20 | ND | 8.5 | 1 of 1 | Right arm | Right arm |
| Ea | 1 | 4 | Nuclear | Both | ND | 7 | 15 | ND | ND | 0 | Toe | Right foot |
| F | 3 | 5 | Both | Both | ND | 12 | 21 | IV | ND | 0 | Right leg | Right groin |
| G | 3 | 2 | Both | Both | 2B | 3 | 3 | IV | 3.9 | 0 of 18 | Left leg | Left hip |
| H | 4 | 3 | Both | Cytoplasmic | ND | 4 | 4 | V | 4 | ND | Forehead | Neck |
| Ia | 1 | ND | Both | ND | 3 | 11 | 44 | IV | 1.5 | 2 | Right temple | Neck |
| Ja | 2 | 4 | Both | Both | 4 | 4 | 39 | ND | ND | 1 of 33 | Right groin | Labia majora |
| K | 1 | 4 | None | Nuclear | 4 | 5 | 5 | IV | 8 | 25 of 40 | Right leg | Right leg |
| L | 2 | 1 | Both | Cytoplasmic | 4 | 6 | 6 | IV | 1.44 | 7 of 33 | Neck | Neck |
| M | 5 | 3 | Both | Both | 4 | 12 | 16 | V | 5 | 1 of 29 | Left leg | Left leg |
| Na | 1 | 4 | Cytoplasmic | Both | 4 | 9 | 20 | ND | 9 | 1 of 14 | Vulva | Lung |
| O | 2 | 3 | Nuclear | Nuclear | 4 | 5 | 5 | IV | 1 | 1 | Left finger | Left forearm |
| Pa | 2 | 3 | Both | Cytoplasmic | ND | 1 | 14 | III | 4.7 | ND | Foot | Leg |
| Qa | 2 | 5 | Both | Both | 4 | 10 | 13 | III | 2.3 | 6 of 8 | Right shoulder | Right shoulder |
| R | ND | 5 | ND | Both | 2C | 10 | 10 | III | 5.7 | 0 of 12 | Back | Back |
| AA | 4 | 5 | Cytoplasmic | Cytoplasmic | 4 | 12 | 52 mos. clear | ND | ND | 2 of 22 | Lymph node | Not applicable |
| BB | 4 | 4 | Both | Both | 4 | 12 | 59 mos. clear | IV | 1 | 1 of 21 | Left leg | |
| CCa | 1 | 2 | None | Cytoplasmic | 3 | 5 | 70 mos. clear | IV | 2.6 | 2 of 3 | Left leg | |
| DD | 2 | ND | Both | ND | 3 | 12 | 44 mos. clear | IV | 1 | 1 of 30 | Right leg | |
| EEa | 5 | 4 | Both | Both | ND | 4 | 45 mos. clear | IV–V | 5 | ND | Nasal dorsum | |
| FFa | 3 | 4 | Both | Cytoplasmic | ND | 4 | 65 mos. clear | IV | 2.2 | ND | Scalp | |
| GG | 1 | 1 | Nuclear | Both | 4 | ND | 61 mos. clear | IV | 2.6 | 1 of 41 | Back | |
| HH | 1 | 4 | Both | Nuclear | 3 | 12 | 51 mos. clear | IV | 8.5 | 2 of 25 | Back | |
| II | 4 | 4 | Nuclear | Both | 2B | 12 | 50 mos. clear | IV | 5 | 0 | Right shoulder | |
| JJa | ND | 2 | ND | Nuclear | 4 | 2 | 72 mos. clear | III | 1.2 | 2 of 2 | Right leg |
aDenotes toxicity
ND not determined
Expression of STAT1 and STAT2 within melanoma cells does not correlate with clinical response to IFN-α
Eleven of 26 patient tumors stained strongly (>60% positive) for STAT1, while 18 out of 24 patient tumors stained strongly for STAT2. However, disease recurrence following IFN-α2b treatment was not associated with staining intensity of either STAT1 or STAT2 P=0.61 for STAT1; P=0.52 for STAT2; Fisher’s Exact test). For patients with a low STAT1 score (1–3), the median time to recurrence was 20 months (95% CI=9–51 months). Five of the ten patients who did not recur during the study had low STAT1 scores. Four of the ten patients who did not recur were categorized as having high STAT1 scores, and one patient’s STAT1 level could not be determined. The median time to recurrence for patients with a high STAT1 score was at least 45 months, however at the last observation, half of the patients with high STAT1 scores were free of disease. Follow-up times for patients with high STAT1 scores ranged from 4–52 months and 370 months for those with low STAT1 scores. The median time to recurrence for patients with high STAT2 score (4 or 5) was 21 months and 9 months for those with a low STAT2 score (1, 2, 3). Follow-up times for patients with high and low STAT2 scores ranged from 5–65 months and 3–72 months respectively.
In patients who underwent excision of high-risk skin lesions, there were examples of prolonged RFS in the setting of STAT1-negative tumors (e.g., Patient CC, GG, HH) as well as rapid recurrence of tumors that expressed abundant levels of both STAT1 and STAT2 (e.g., patient A, M). Similar scenarios were observed in patients that received IFN-α2b following resection of nodal metastases. Patients HH, CC, and GG had very low STAT1 staining (5% STAT1 positive), yet remained disease free at 50, 70, and 61 months post-IFN-α2b therapy. Conversely, patient H had exhibited strong expression of both STAT1 and STAT2 within a large nodal metastasis and yet recurred after just 4 months of therapy. We also examined whether or not tumor thickness or ulceration were related to recurrence. No difference in thickness of the original tumor was observed between patients who recurred and those who did not (P=0.55; Wilcoxon rank-sum test). Ulceration was present in primary tumors from only 5 of 26 patients in the current study (Patients C, G, K, M, R). As might be expected, the five patients with an ulcerated primary tumor did exhibit disease recurrence. Although these data were interesting, the limited number of patients with ulcerated primary tumors prevented us from drawing meaningful statistical conclusions.
Case report
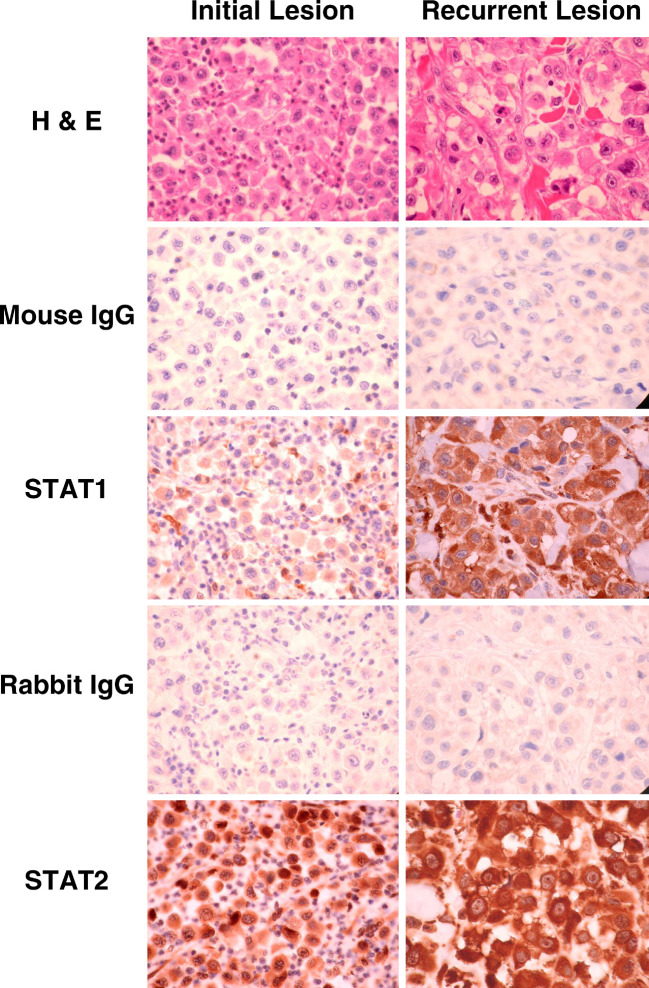
A 56-year-old male presented in November 2002 with nodal metastases in the right groin and a history of a melanoma (2 cm × 0.7 cm in width) of the right lower extremity, resected 4 months earlier. A benign pigmented skin lesion had also been resected 27 years earlier from the same area. A full inguinal lymph node dissection was performed. Three of 13 superficial nodes contained tumor (largest nodal metastasis was 6 cm in diameter). The deep inguinal nodes were negative for melanoma (0/23). Adjuvant therapy with IFN-α2b was initiated 2 months following lymph node dissection and continued for 6 months until the patient presented with tumor in the right lower extremity. Immunohistochemical staining for STAT1 and STAT2 expression in these tumor tissues was performed and the results are presented in Fig. 4. Only focal positivity for STAT1 was observed in the initial lesion (prior to IFN-α2b therapy), whereas STAT1 was highly expressed in the recurrent lesion (during IFN-α2b therapy). Of note, tumor tissue obtained from both lesions displayed robust expression of STAT2.
Fig. 4.
Immunohistochemical staining of malignant melanoma from a patient exhibiting recurrent disease during IFN-α2b adjuvant therapy. Tumor tissue was obtained prior to IFN-α2b adjuvant therapy (initial nodal lesion) and from the lesion that developed at the site of the wide local excision on the calf during IFN-α2b treatment. Sections from each tumor were stained by hematoxylin and eosin, or with anti-STAT1, -STAT2 or appropriate isotype control antibodies (mouse IgG or rabbit IgG). All panels represent tumor as seen at 600× magnification. Analysis indicated the presence of a polymorphonuclear cell (PMN) infiltrate in viable tumor (see H and E panels), consistent with inflammation and tumor cell necrosis. No substantial background staining was observed with isotype control antibodies while cytoplasmic staining was observed for STAT1 and STAT2 in all panels
Discussion
We examined STAT1 and STAT2 expression by immunohistochemistry in tumors obtained from melanoma patients prior to the initiation of adjuvant therapy with IFN-α2b. We found that neither the location nor the level of STAT1 or STAT2 within tumors correlated with time to disease recurrence in these patients. STAT1 levels did not correlate with the level of STAT2, and the expression of each protein was highly variable across tumors. Individual tumors appeared to consist of a heterogeneous cell population, likely reflecting a rapidly growing and changing tumor. These observations were not surprising as genetic instability is a hallmark of tumor transformation, particularly in the setting of malignant melanoma [22]. We identified a subset of patients with low STAT1 staining (<20%) who remained disease-free up to 50 months post-IFN-α2b adjuvant therapy. Finally, in a case study, we demonstrated that STAT1 levels were elevated (compared to the initial lesion) in a recurrent melanoma tumor that developed during IFN-α2b adjuvant therapy. These observations provide direct clinical evidence to suggest that low intratumoral expression of STAT1 and STAT2 do not correlate with IFN-α-resistance.
Defective Jak-STAT signaling, particularly in STAT1, has been implicated in IFN-resistance within melanoma cells in vitro. Furthermore, reconstitution of STAT1 has been shown to restore, at least in part, IFN-responsiveness to STAT1 deficient cell lines as measured in an anti-viral assay [29]. These findings suggested that decreased levels of STAT1 protein expression in the tumor might render an individual less responsive to IFN-α2b adjuvant therapy. In contrast to this hypothesis, the present study has demonstrated that intratumoral expression of STAT1 did not correlate with disease recurrence following adjuvant IFN-α2b. Defective phosphorylation of STAT1 at Tyr701 and Ser 727 in response to interferons has been documented in both melanoma cell lines and primary cultures derived from melanoma patients [16]. These functional abnormalities of melanoma cells could potentially lead to decreased expression of interferon-stimulated genes (ISGs) within the tumor cell. It is possible that attenuated phosphorylation events downstream of the IFNAR could be attributed to defects in other constituents of the Jak-STAT signaling pathway (Jak1, Tyk2). However, these hypotheses do not provide a rational explanation as to how patients with little or no expression of total STAT1 protein obtained clinical benefit from exogenous IFN-α. Certainly, STAT1-independent signaling pathways could theoretically be responsible for mediating the anti-tumor effects of IFN-α in melanoma cells, in which case the levels of Jak-STAT intermediates might not correlate with the clinical outcome.
Evidence is emerging that intratumoral expression of STAT1 is not the main determinant of clinical responsiveness to IFN-α. For example, a recent study by Chawla-Sarkar et al. reported no correlation between expression or activation of STAT1 and in vitro IFN-resistance in 30 patient samples and several melanoma cell lines. In addition, no significant differences were observed in the formation of gamma activated factor (GAF) or IFN-stimulated gene factor 3 (ISGF3) complexes or the induction of two ISGs (ISG-54 and IRF-1) in sensitive and IFN-α-resistant melanoma cell lines. The authors concluded that cellular resistance to IFN-α most likely results from defective quantitative or qualitative expression of specific ISGs that may affect the sensitivity of tumor cells to the pro-apoptotic effects of IFN-α [5]. Interestingly, Jackson et al. demonstrated by western blot that melanoma cell lines resistant to the anti-proliferative effects of IFN-α possessed all protein components of the Jak-STAT pathway. Furthermore, these IFN-resistant cell lines were shown to be capable of generating functional transcription factors as demonstrated by an electrophoretic mobility shift assay and a ribonuclease protection assay of known IFN-induced genes. In addition, these cell lines had intact antiviral and HLA upregulation responses [9]. Together, these data suggest that IFN-resistance within melanoma cell lines is likely mediated through cellular components of the tumor cell that function independently of STAT1, or perhaps through other cellular compartments. In contrast to these studies, the present report did not utilize melanoma cell lines, but instead examined the relationship between STAT1 or STAT2 within primary patient tumors and clinical responsiveness to IFN-α.
The ability of the host immune system to control the progression of melanoma and other IFN-α-responsive malignancies (Renal Cell Carcinoma, Chronic Myeloid Leukemia) has long been recognized [2]. For instance, spontaneous regressions of malignant melanoma lesions have been observed on rare occasions, and careful histologic evaluations have revealed that this process is mediated by activated lymphocytes [24]. Recent studies in our laboratory utilizing murine models of melanoma have demonstrated that defective STAT1 signaling within immune cells renders a host unresponsive to the anti-tumor effects of exogenously administered IFN-α [1, 19]. In contrast, IFN-α administration was found to significantly prolong the survival of wild type mice bearing STAT1-deficient melanoma tumors. Further experiments revealed that STAT1 signal transduction within the NK cell compartment was responsible for the anti-tumor actions of IFN-α [19]. In a separate study, the B16F1 melanoma cell line was transfected with a plasmid construct designed to overexpress STAT1. Interestingly, the survival of IFN-α-treated mice bearing this STAT1-overexpressing cell line was not significantly prolonged when compared to IFN-α-treated mice bearing wild type B16F1 tumors [1]. Most recently, we have developed a novel flow cytometric assay to monitor Jak-STAT signal transduction in peripheral blood mononuclear cells (PBMCs) of patients undergoing cytokine immunotherapy. Studies utilizing this method have determined that basal levels of phosphorylated STAT1 (at Tyr701) were significantly lower in PBMCs from patients with metastatic melanoma when compared to normal adult donors [20]. Together, these findings suggest that inherent signal transduction defects within immune cells might influence patient responsiveness to IFN-α. The current study supports these findings and further demonstrates that the clinical response to exogenous IFN-α may not correlate with the presence or absence of STAT1 within the tumor.
In the clinical setting, melanoma lesions that recur during a course of IFN-α-adjuvant therapy are commonly encountered. Although these tumors by definition are IFN-resistant, they may not necessarily be IFN-insensitive. In theory, if STAT1 or STAT2 expression were critically important for the IFN-α-resistant phenotype of tumor cells, then one might hypothesize that low or non-existent levels of these proteins would be evident in lesions that arise during a clinical course of IFN-α immunotherapy. However, data from our case study does not support this hypothesis, as STAT1 levels were markedly higher in a recurrent lesion that arose during IFN-α treatment when compared to the primary lesion. Although this data does not exclude a role for functional defects in either STAT1-phosphorylation or in the expression of specific IFN-regulated genes, it does support the idea that intratumoral expression of STAT1 or STAT2 is not predictive of a clinically responsive tumor. The presence of substantially higher levels of STAT1 within the lesion that originated during IFN-α immunotherapy was surprising. Although recent literature has demonstrated a role for constitutively active STAT proteins (namely STAT3) in promoting tumor growth [27, 30], other reports have associated STAT1 with the antiproliferative effects of IFN-α [21]. Therefore, it is open to question whether this observation is indicative of a growth advantage provided to the tumor by overexpression of STAT1 in an IFN-α-resistant lesion.
The current data support the hypothesis that the anti-tumor effects of exogenously administered IFN-α may proceed independently of the Jak-STAT signal transduction pathway within tumor cells [5, 19]. Observations from the present study suggest that factors other than the expression of STAT1 or STAT2 by tumor cells may directly impact patient survival in response to IFN-α adjuvant therapy.
Acknowledgements
The authors thank Patrick Roche and The Ohio State University Histology Core Facility for assistance in optimizing immunohistochemical staining. We also thank Judith Bowers for her help in the management of clinical data. This work was supported by National Institutes of Health (NIH) Grants CA84402, P30-CA16058, The Valvano Foundation for Cancer Research Award, and The Ohio State University Department of Surgery Clinical Science Seed Grant. GBL is a NRSA T32 fellow (5 T32 CA90223-02). The Tissue Procurement Shared Resource of the Comprehensive Cancer Center, The Ohio State University, Columbus, Ohio is supported in part by NIH Grant P30 CA16059.
Abbreviations
- IFN-α
Interferon-alpha
- IFN-α2b
Interferon-alpha 2b
- STAT
Signal transducer and activator of transcription
- H & E
Hematoxylin and eosin
References
- 1.Badgwell B, Lesinski GB, Magro C, Abood G, Skaf A, Carson WE. The anti-tumor effects of interferon-alpha are maintained in mice challenged with a STAT1-deficient murine melanoma cell line. J Surg Res. 2003;116:129. doi: 10.1016/j.jss.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 2.Belardelli F, Ferrantini M, Proietti E, Kirkwood JM. Interferon-alpha in tumor immunity and immunotherapy. Cytokine Growth Factor Rev. 2002;13:119. doi: 10.1016/S1359-6101(01)00022-3. [DOI] [PubMed] [Google Scholar]
- 3.Carson WE. Interferon-alpha-induced activation of signal transducer and activator of transcription proteins in malignant melanoma. Clin Cancer Res. 1998;4:2219. [PubMed] [Google Scholar]
- 4.Creagan ET, Dalton RJ, Ahmann DL, Jung SH, Morton RF, Langdon RM, Jr, Kugler J, Rodrigue LJ. Randomized, surgical adjuvant clinical trial of recombinant interferon alfa-2a in selected patients with malignant melanoma. J Clin Oncol. 1995;13:2776. doi: 10.1200/JCO.1995.13.11.2776. [DOI] [PubMed] [Google Scholar]
- 5.Chawla-Sarkar M, Leaman DW, Jacobs BS, Tuthill RJ, Chatterjee-Kishore M, Stark GR, Borden EC. Resistance to interferons in melanoma cells does not correlate with the expression or activation of signal transducer and activator of transcription 1 (stat1) J Interferon Cytokine Res. 2002;22:603. doi: 10.1089/10799900252982089. [DOI] [PubMed] [Google Scholar]
- 6.Darnell JE, Jr, Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264:1415. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- 7.Hancock BW, Wheatley K, Harris S, Ives N, Harrison G, Horsman JM, Middleton MR, Thatcher N, Lorigan PC, Marsden JR, Burrows L, Gore M. Adjuvant interferon in high-risk melanoma: the AIM HIGH Study–United Kingdom Coordinating Committee on Cancer Research randomized study of adjuvant low-dose extended-duration interferon Alfa-2a in high-risk resected malignant melanoma. J Clin Oncol. 2004;22:53. doi: 10.1200/JCO.2004.03.185. [DOI] [PubMed] [Google Scholar]
- 8.Haque SJ, Williams BR. Signal transduction in the interferon system. Semin Oncol. 1998;25:14. [PubMed] [Google Scholar]
- 9.Jackson DP, Watling D, Rogers NC, Banks RE, Kerr IM, Selby PJ, Patel PM. The JAK/STAT pathway is not sufficient to sustain the antiproliferative response in an interferon-resistant human melanoma cell line. Melanoma Res. 2003;13:219. doi: 10.1097/00008390-200306000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457. [Google Scholar]
- 11.Kim SH, Cohen B, Novick D, Rubinstein M. Mammalian type I interferon receptors consists of two subunits: IFNaR1 and IFNaR2. Gene. 1997;196:279. doi: 10.1016/S0378-1119(97)00240-0. [DOI] [PubMed] [Google Scholar]
- 12.Kirkwood JM, Ibrahim JG, Sondak VK, Richards J, Flaherty LE, Ernstoff MS, Smith TJ, Rao U, Steele M, Blum RH. High- and low-dose interferon alfa-2b in high-risk melanoma: first analysis of intergroup trial E1690/S9111/C9190. J Clin Oncol. 2000;18:2444. doi: 10.1200/JCO.2000.18.12.2444. [DOI] [PubMed] [Google Scholar]
- 13.Kirkwood JM, Ibrahim JG, Sosman JA, Sondak VK, Agarwala SS, Ernstoff MS, Rao U. High-dose interferon alfa-2b significantly prolongs relapse-free and overall survival compared with the GM2-KLH/QS-21 vaccine in patients with resected stage IIB-III melanoma: results of intergroup trial E1694/S9512/C509801. J Clin Oncol. 2001;19:2370. doi: 10.1200/JCO.2001.19.9.2370. [DOI] [PubMed] [Google Scholar]
- 14.Kirkwood JM, Manola J, Ibrahim J, Sondak V, Ernstoff MS, Rao U. A pooled analysis of eastern cooperative oncology group and intergroup trials of adjuvant high-dose interferon for melanoma. Clin Cancer Res. 2004;10:1670. doi: 10.1158/1078-0432.ccr-1103-3. [DOI] [PubMed] [Google Scholar]
- 15.Kirkwood JM, Strawderman MH, Ernstoff MS, Smith TJ, Borden EC, Blum RH. Interferon alfa-2b adjuvant therapy of high-risk resected cutaneous melanoma: the Eastern Cooperative Oncology Group Trial EST 1684. J Clin Oncol. 1996;14:7. doi: 10.1200/JCO.1996.14.1.7. [DOI] [PubMed] [Google Scholar]
- 16.Kovarik J, Boudny V, Kocak I, Lauerova L, Fait V, Vagundova M. Malignant melanoma associates with deficient IFN-induced STAT 1 phosphorylation. Int J Mol Med. 2003;12:335. [PubMed] [Google Scholar]
- 17.Lens MB, Dawes M. Interferon alfa therapy for malignant melanoma: a systematic review of randomized controlled trials. J Clin Oncol. 2002;20:1818. doi: 10.1200/JCO.2002.07.070. [DOI] [PubMed] [Google Scholar]
- 18.Leonard WJ, O’Shea JJ. Jaks and STATs: biological implications. Annu Rev Immunol. 1998;16:293. doi: 10.1146/annurev.immunol.16.1.293. [DOI] [PubMed] [Google Scholar]
- 19.Lesinski GB, Anghelina M, Zimmerer J, Bakalakos T, Badgwell B, Parihar R, Hu Y, Becknell B, Abood G, RayChaudhury A, Magro C, Durbin J, Carson WE. The anti-tumor effects of interferon-alpha are abrogated in a STAT1-deficient mouse. J Clin Invest. 2003;112:170. doi: 10.1172/JCI200316603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lesinski GB, Kondadasula SV, Crespin T, Shen L, Kendra K, Walker MJ, Carson WE. Multiparametric flow cytometric analysis of inter-patient variation in STAT1 phosphorylation following interferon alfa immunotherapy. J Natl Cancer Inst. 2004;96:1131. doi: 10.1093/jnci/djh252. [DOI] [PubMed] [Google Scholar]
- 21.Levy DE, Gilliland DG. Divergent roles of STAT1 and STAT5 in malignancy as revealed by gene disruptions in mice. Oncogene. 2000;19:2505. doi: 10.1038/sj.onc.1203480. [DOI] [PubMed] [Google Scholar]
- 22.Pansky A, Hildebrand P, Fasler-Kan E, Baselgia L, Ketterer S, Beglinger C, Heim MH. Defective Jak-STAT signal transduction pathway in melanoma cells resistant to growth inhibition by interferon-alpha. Int J Cancer. 2000;85:720. doi: 10.1002/(SICI)1097-0215(20000301)85:5<720::AID-IJC20>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 23.Rousseau DL, Jr, Ross MI, Johnson MM, Prieto VG, Lee JE, Mansfield PF, Gershenwald JE. Revised American Joint Committee on Cancer staging criteria accurately predict sentinel lymph node positivity in clinically node-negative melanoma patients. Ann Surg Oncol. 2003;10:569. doi: 10.1245/ASO.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 24.Schneeberger A, Koszik F, Stingl G. Immunologic host defense in melanoma: delineation of effector mechanisms involved and of strategies for the augmentation of their efficacy. J Invest Dermatol. 1995;105:110S. doi: 10.1111/1523-1747.ep12316635. [DOI] [PubMed] [Google Scholar]
- 25.Sun WH, Pabon C, Alsayed Y, Huang PP, Jandeska S, Uddin S, Platanias LC, Rosen ST. Interferon-alpha resistance in a cutaneous T-cell lymphoma cell line is associated with lack of STAT1 expression. Blood. 1998;91:570. [PubMed] [Google Scholar]
- 26.Thompson JA. The revised American Joint Committee on Cancer staging system for melanoma. Semin Oncol. 2002;29:361. doi: 10.1053/sonc.2002.34115. [DOI] [PubMed] [Google Scholar]
- 27.Wang T, Niu G, Kortylewski M, Burdelya L, Shain K, Zhang S, Bhattacharya R, Gabrilovich D, Heller R, Coppola D, Dalton W, Jove R, Pardoll D, Yu H. Regulation of the innate and adaptive immune responses by Stat-3 signaling in tumor cells. Nat Med. 2004;10:48. doi: 10.1038/nm976. [DOI] [PubMed] [Google Scholar]
- 28.Wong LH, Hatzinisiriou I, Devenish RJ, Ralph SJ. IFN-gamma priming up-regulates IFNstimulated gene factor 3 (ISGF3) components, augmenting responsiveness of IFNresistant melanoma cells to type I IFNs. J Immunol. 1998;160:5475. [PubMed] [Google Scholar]
- 29.Wong LH, Krauer KG, Hatzinisiriou I, Estcourt MJ, Hersey P, Tam ND, Edmondson S, Devenish RJ, Ralph SJ. Interferon-resistant human melanoma cells are deficient in ISGF3 components, STAT1, STAT2, and p48-ISGF3gamma. J Biol Chem. 1997;272:28779. doi: 10.1074/jbc.272.45.28779. [DOI] [PubMed] [Google Scholar]
- 30.Zhang YW, Wang LM, Jove R, Vande Woude GF. Requirement of Stat3 signaling for HGF/SF-Met mediated tumorigenesis. Oncogene. 2002;21:217. doi: 10.1038/sj.onc.1205004. [DOI] [PubMed] [Google Scholar]