Abstract
The mouse 4T1 mammary carcinoma is a BALB/c-derived tumor that spontaneously metastasizes and induces immune suppression. Although >95% of wild type BALB/c mice die from metastatic 4T1 tumor even if the primary mammary tumor is surgically removed, >65% of BALB/c mice with a deleted Signal Transducer Activator of Transcription 6 (STAT6) gene survive post-surgery. STAT6-deficiency also confers enhanced immunity against spontaneously developing breast cancer since NeuT+/− mice that are STAT6-deficient develop mammary tumors later and survive longer than NeuT+/− mice that are STAT6-competent. Rejection of metastastic disease and survival of STAT6-deficient mice after removal of primary tumor involve three mechanisms: (1) The generation of M1 type macrophages that produce nitric oxide and are tumoricidal; (2) A decrease to normal in the elevated levels of myeloid suppressor cells that accumulate during primary tumor growth; and (3) CD8+ tumor-specific T lymphocytes. STAT6-deficient, but not wild type BALB/c, mice generate nitric oxide producing macrophages because they lack the STAT6 transcription factor which is necessary for signaling through the type 2 IL-4Rα complex, and which induces the production of arginase instead of nitric oxide.
Keywords: Tumor-induced immune suppression, Immune surveillance, M1 macrophages, Metastatic breast cancer, Cell-mediated tumor immunity
Signal Transducer Activator of Transcription 6 deficient (STAT6−/−) mice have enhanced immunity to transplanted tumors
The STAT6 gene transmits IL-4 and IL-13 signals via the IL-4Rα and is required for the generation of CD4+ Th2 lymphocytes. As a result, STAT6−/− mice have their CD4+ T cells polarized towards a Type 1 phenotype [12]. Others [11, 32] and ourselves [22] have hypothesized that STAT6−/− mice might have enhanced immunity because they preferentially generate CD4+ Th1 cells that faciliate CD8+ -mediated tumor rejection. Studies conducted in multiple laboratories using three different transplanted tumors (mammary carcinoma, fibrosarcoma, and mastocytoma) demonstrated that STAT6−/− mice have heightened tumor immunity [10, 11, 22, 32]. Our studies used the BALB/c-derived mouse 4T1 mammary carcinoma [19]. This tumor closely models human breast cancer in its growth in the mammary gland, its pattern of disease progression, and its ability to metastasize to a variety of target organs (brain, bone marrow, liver, lungs, blood, lymph nodes) while the primary tumor is present, as well as after the primary tumor is surgically removed [25, 26]. Tumor resistance of STAT6−/− mice was particularly effective after primary mammary tumors were excised, with >65% of STAT6−/− mice surviving indefinitely, while >95% of wild type BALB/c mice died from metastatic disease [23]. Therefore, deletion of the STAT6 gene provides enhanced tumor immunity.
In a previous report, we described our earlier studies, demonstrating enhanced immunity to metastatic mammary carcinoma in STAT6−/− mice. Similar immunosurveillance was also observed in CD1−/− mice, which lack NKT cells, and therefore, are deficient in IL-13 [24]. In the present report, we define the mechanisms responsible for the increased immunosurveillance in STAT6−/− mice.
STAT6−/− mice have enhanced resistance to spontaneously arising mammary carcinoma
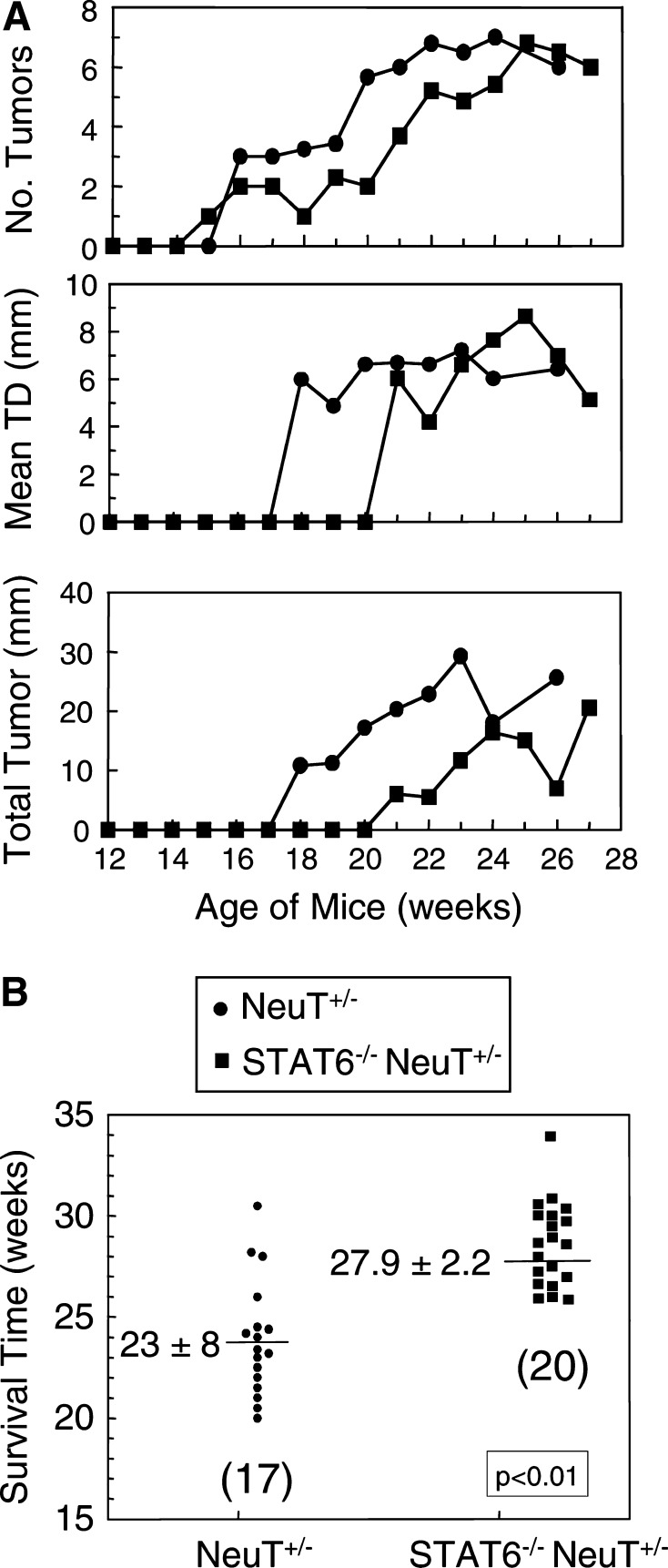
NeuT+/− mice are transgenic for the transforming rat her2/neu gene and spontaneously develop multifocal and metastatic mammary carcinoma, starting at approximately week 6–8 of age [3]. To determine if deletion of the STAT6 gene also protects against spontaneous cancer, neuT+/− males were crossed to STAT6−/− females and the female F1’s PCR screened and selected for neuT expression (neuT+/−). These heterozygotes (STAT6+/− neuT+/−) were then backcrossed to STAT6−/− females, and the offspring PCR screened for neuT expression and homozygous deletion of STAT6 (STAT6−/− neuT+/− mice). Female STAT6−/− neuT+/− mice were then observed weekly for a minimum of six months for mammary tumor development and survival. As seen in Fig. 1A, mammary tumor onset, diameter (TD) of individual tumors, and total tumor mass is delayed in STAT6−/− neuT+/− mice vs. STAT6-competent neuT+/− mice. Similarly, the survival time of STAT6−/− neuT+/− mice is statistically longer than that of neuT+/− mice by approximately one month (Fig. 1B). Therefore, deletion of the STAT6 gene facilitates rejection of metastatic disease, and also promotes survival of mice with spontaneous mammary carcinoma.
Fig. 1.
Deletion of the STAT6 gene delays tumor progression and extends survival time of mice that spontaneously develop mammary carcinoma. NeuT+/− mice, which spontaneously develop multifocal breast cancer, were crossed and backcrossed to STAT6−/− mice to obtain STAT6−/− neuT+/− mice. The STAT6−/− neuT+/− and neuT+/− mice were observed weekly for A the number of primary mammary tumors per mouse, the mean tumor diameter (TD) of individual tumors, and the sum of the diameters of all tumors per mouse; and B survival time
Myeloid-derived suppressor cells inhibit T cell activation and immunity in mice with large, primary mammary tumors
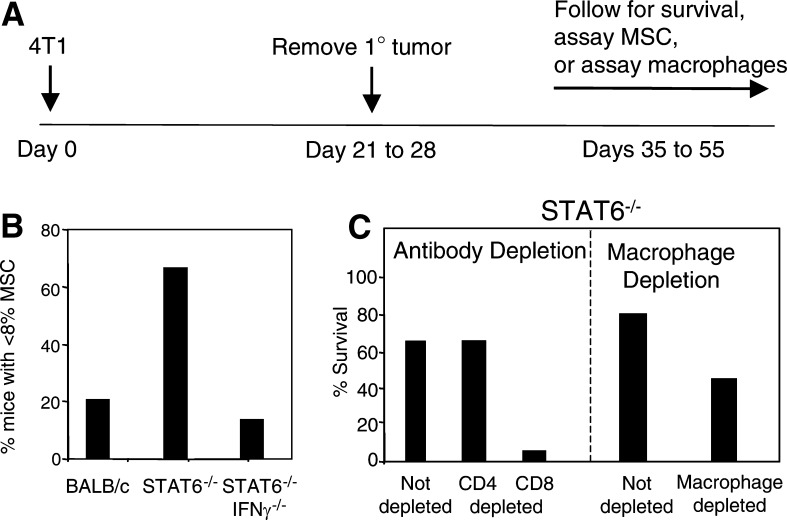
Myeloid-derived cells that suppress the immune system have been identified in many patients and experimental animals with tumors [1, 5, 9, 13, 15, 29]. These so-called myeloid suppressor cells (MSC) are immature myeloid cells that suppress the activation of CD4+ and CD8+ T lymphocytes and thereby inhibit immune surveillance [4, 9, 16, 18]. The accumulation of MSC in the spleen and blood of tumor-bearing individuals is associated with increased tumor burden. Since removal of primary 4T1 tumor partially restores immunocompetence [6], we have hypothesized that immunity in STAT6−/− mice with primary tumor is inhibited by the presence of MSC. To test this hypothesis, BALB/c and STAT6−/− mice were inoculated with 4T1 tumor in the mammary gland and their splenocytes tested by flow cytometry for the presence of Gr1+ CD11b+ MSC. In some groups, the primary tumor was surgically removed according to the schedule shown in Fig. 2A, and 10–12 days after surgery, the spleens were removed and tested for MSC. Mice that were never exposed to tumor, have less than 8% Gr1+ CD11b+ cells in their spleens. In contrast, BALB/c and STAT6−/− mice with primary 4T1 mammary carcinomas, have 30–60% Gr1+ CD11b+ splenocytes. Although these levels decline after surgery, 80% of BALB/c mice retain elevated levels of MSC, while only 33% of STAT6−/− mice have above normal levels of Gr1+ CD11b+ splenocytes (Fig. 2B). Therefore, the retention of high levels of MSC, after surgery, is associated with shortened survival, while a decrease to baseline levels of MSC is associated with resistance to metastatic disease
Fig. 2.
Resistance to metastatic mammary carcinoma requires M1 macrophages and CD8+ T cells and is counter-acted by myeloid suppressor cells (MSC). A Mice are inoculated in the mammary gland on day 0 with 7000 4T1 mammary carcinoma cells; primary tumors are surgically removed on day 21–28; and mice are either followed for survival or sacrificed 10 days after surgery and their spleens analyzed for MSC or their bone-marrow-derived macrophages assayed for arginase and iNOS activity. B BALB/c, STAT6−/−, and STAT6−/− IFNγ−/− mice were treated according to the schedule shown in Part A, and their splenocytes were analyzed by flow cytometry for Gr1+ CD11b+ MSC. Data are shown as percentage of mice that have normal levels of MSC (<8% of splenocytes are Gr1+ CD11b+). C STAT6−/− mice were treated according to the schedule in Part A and concomitantly depleted for CD4+ or CD8+ T cells (left hand panel), or depleted for phagocytic cells/macrophages (right-hand panel)
Although a reduction in MSC after removal of primary tumor is associated with resistance to metastatic disease, this alone is not sufficient for resistance since BALB/c mice treated with all trans retinoic acid [14] have greatly reduced levels of MSC, but still die from metastatic 4T1 [31]. This finding has led us to examine other effector mechanisms that might be responsible for resistance of STAT6−/− mice.
CD8+ T cells are required for immunity to metastatic disease in post-surgery STAT6−/− mice
In earlier studies, we noted that STAT6−/− mice have a modest immune response against primary tumor, and in vivo antibody depletion experiments demonstrated that this immunity was mediated by CD8+ T cells, and that CD4+ T cells were not involved [22]. The lack of involvement of CD4+ T cells was surprising and demonstrated that our original hypothesis that heightened immunity was due to polarization towards a type 1 CD4+ T cell response was incorrect. In addition, depletion of CD4+ CD25+ T regulatory cells had no impact on 4T1 tumor growth in BALB/c mice, demonstrating that regulatory T cells were also not involved [23].
Since immunity after removal of primary tumor is much more effective than immunity in mice with primary tumor in place, we have also monitored T cell activity in post-surgery STAT6−/− mice that are resistant to 4T1 metastatic disease. STAT6−/− and control BALB/c mice were inoculated in the mammary gland with 4T1 cells according to the schedule shown in Fig. 2A, and concomitantly in vivo depleted for CD4+ or CD8+ T cells using antibodies to CD4 and CD8 as previously described [22]. All BALB/c mice died by day 47, regardless of antibody treatment, and all CD8-depleted STAT6−/− mice died by day 66. In contrast, all of the CD4-depleted STAT6−/− mice survived (Fig. 2C, left-hand panel). Therefore, CD8+, but not CD4+, T cells are essential for immunity to metastatic disease in STAT6−/− mice.
Cytotoxic nitric oxide producing M1 macrophages are required for immunity to metastatic disease in post-surgery STAT6−/− mice
Macrophages can also be key players in tumor immunity. Macrophages polarized towards an M1 phenotype produce nitric oxide (NO) and are cytotoxic for tumor cells, whereas M2 macrophages produce arginase which facilitates tumor growth and progression [17, 20, 21]. Since earlier studies demonstrated that macrophages are involved in immune surveillance against the 4T1 tumor [27], we have examined the role of macrophages in STAT6−/− mice. To determine if macrophages are required for resistance to metastatic 4T1 tumor, STAT6−/− mice were inoculated with 4T1 cells and primary tumors removed and mice followed for survival according to the schedule shown in Fig. 2A. One group of mice was also treated with carrageenan, which depletes for phagocytic cells such as macrophages [31]. Macrophage/phagocytic cell depletion was monitored by measuring reduced susceptibility to lipopolysaccharide-induced toxic shock [27]. Seventy-five percent of the non-carrageenan treated STAT6−/− mice survived; whereas only 45% of the carrageenan-treated mice survived (Fig. 2C, right-hand panel). Mice in the carrageenan-treated group also developed more rapidly growing tumors than the mice in the non-carrageenan-treated group. Therefore, macrophages appear to be required for resistance to metastatic disease in STAT6−/− mice.
Since M1 macrophages are associated with tumor regression while M2 macrophages are associated with tumor progression, we have analyzed the phenotype of macrophages from tumor-bearing and post-surgery BALB/c and STAT6−/− mice. Although non-activated bone marrow-derived macrophages (BMDM) from either strain had no NO activity, lipopolysaccharide and IFNγ-activated macrophages from STAT6−/− mice made high levels of NO, while activated macrophages from BALB/c mice produced arginase [31]. Therefore, STAT6−/− mice produce M1 macrophages which are essential for resistance to established metastatic disease, while BALB/c mice which are not resistant, produce M2 macrophages.
STAT6−/− mice generate M1 macrophages because they cannot transmit IL-13 signals which polarize macrophages towards an M2 phenotype
The production of arginase, which is a characteristic of M2 macrophages, is induced by IL-4 and/or IL-13 when these cytokines bind to the IL-4Rα−/− and signal through the JAK3/STAT6 pathway [28, 33]. Since STAT6−/− mice are deficient for STAT6, this signaling pathway is inoperative in STAT6−/− mice. Hence, arginase production does not occur. In other studies, we have observed that IL-4Rα−/− mice, which also cannot transmit IL-4 and/or IL-13 signals because they lack the requisite receptor, make M1 macrophages that produce NO (Sinha and Ostrand-Rosenberg, unpublished results). Interestingly, although IL-4Rα−/− mice make M1 macrophages, they are not resistant to metastatic 4T1 tumor because they retain high levels of MSC after removal of primary tumor (Sinha and Ostrand-Rosenberg, unpublished results). Therefore, STAT6−/− mice have M1 macrophages because they lack the signaling machinery to stimulate arginase production; however, the generation of M1 macrophages without concomitant reduction in MSC is not sufficient for resistance to metastatic disease.
IFNγ is essential for resistance and is required for the reduction in MSC and may be required for the activation of M1 macrophages
IFNγ is a pleiotropic cytokine that affects a wide variety of genes and is instrumental in immune surveillance [7, 8, 30]. To determine if IFNγ is also required for resistance to metastatic disease in STAT6−/− mice, we have crossed STAT6−/− mice with BALB/c IFNγ−/− mice and intercrossed the F1’s to obtain double knockout STAT6−/− IFNγ−/− mice. The STAT6−/− IFNγ−/− mice were then inoculated with 4T1 in the mammary fat pad, primary tumors removed and mice followed for survival according to the schedule shown in Fig. 2A. Not surprisingly, the STAT6−/− IFNγ−/− mice have the same survival times as wild type BALB/c mice, indicating that IFNγ is essential for STAT6−/− resistance to metastatic disease [31]
Experiments tracking MSC in STAT6−/− mice demonstrate that the decrease to normal levels, after removal of primary tumor, is dependent on IFNγ because MSC levels remain highly elevated in post-surgery STAT6−/− IFNγ−/− mice (Fig. 2B). In addition to its role in reducing MSC, IFNγ may also drive M1 macrophage production in STAT6−/− mice since it is required in vitro to activate macrophages from STAT6−/− mice [31]. Therefore, IFNγ appears to be a critical regulatory molecule in the induction of resistance to metastatic disease and it mediates its effects by reducing MSC levels and activating M1 macrophages.
Concluding remarks
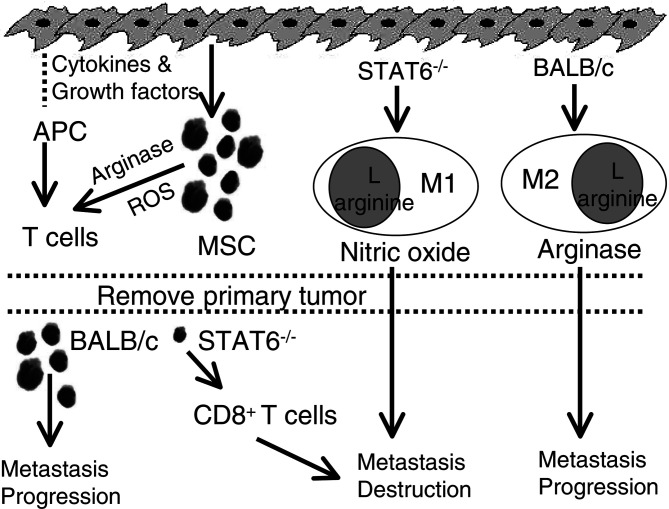
Figure 3 shows a schematic model of how M1 macrophages, MSC levels, and activated CD8+ T cells may interact to provide effective immune surveillance against metastatic disease. Under ideal conditions, tumor antigens of primary tumor cells would be processed and presented by professional antigen presenting cells (APC) and activate tumor-specific CD8+ T cells. However, many tumors, including the 4T1 mammary carcinoma, produce cytokines and/or growth factors that up-regulate Gr1+ CD11b+ MSC in both BALB/c and STAT6−/− mice. The MSC produce arginase and reactive oxygen species (ROS) which then inhibit T cell activation; thereby blocking immune surveillance and favoring tumor progression. Concomitantly, in BALB/c mice IL-4 and IL-13 induce the production of M2 macrophages which also promote tumor progression. In contrast, STAT6−/− mice generate M1 macrophages, because they lack the machinery to transmit IL-4 and/or IL-13 signals. Although the M1 macrophages are cytotoxic for tumor cells, they alone are insufficient for tumor rejection. When primary tumor is surgically removed, the quantities of tumor-produced cytokines and/or growth factors decrease and the levels of MSC decrease to baseline in STAT6−/− mice, permitting tumor-specific CD8+ T cells to differentiate. However, the level of MSC does not decrease sufficiently in BALB/c mice after surgery, so tumor-specific CD8+ T cells do not develop. The combination of activated, tumor-specific CD8+ T cells and M1 macrophages in STAT6−/− mice is then sufficient to mediate complete rejection of metastatic disease. Therefore, effective immune surveillance requires a decrease to baseline levels of MSC coupled with the activation of tumor-specific CD8+ T cells and cytotoxic M1 macrophages.
Fig. 3.
Proposed pathways for immunological resistance to metastatic mammary carcinoma in post-surgery mice. Resistance requires three mechanisms: (1) Reduction in tumor-induced myeloid suppressor cells (MSC); (2) Activation of tumor-specific CD8+ T lymphocytes; and (3) Activation of tumoricidal M1 macrophages. See text for detailed description
The increase in immune surveillance associated with deletion of the STAT6 gene could potentially be exploited for immunotherapy, and we are currently exploring the use of small interfering RNAs (siRNA or RNAi) [2] to down-regulate STAT6 in selected target cells as a strategy to promote immune surveillance. Although this approach is technically feasible, it is not clear as to ‘in which cells STAT6 should be deleted’. Our data suggest that enhanced immunity is associated with deletion of the STAT6 gene in MSC and macrophages. However, STAT6−/− mice are globally deleted for the STAT6 gene, so optimal immunity may require deletion of the STAT6 gene in these and/or other cells. Indeed, previous studies with bone marrow chimeras indicate that effective immune surveillance requires both hematopoietic and non-hematopoietic components [23]. Studies are in progress to further clarify the role of the STAT6 gene in inhibiting immune surveillance, to identify the relevant target cells, and to apply this information for cancer therapy.
Acknowledgements
We thank Ms. Cordula Davis for her help in monitoring tumor progression in the neuT+/− and STAT6−/− neuT+/− mice and Dr. Beth Pulaski for performing the carrageenan experiment. We appreciate the excellent care given to our mouse colony by Ms. Sandra Mason. These studies were supported by NIH grants R01 CA52527 and R01 CA84232, and by U.S. Army Breast Cancer Program Grant DAMD-17-01-1-0312.
Footnotes
This article is a symposium paper from the conference “Tumor Escape and Its Determinants”, held in Salzburg, Austria, on 10–13 October 2004
References
- 1.Almand B, Clark JI, Nikitina E, van Beynen J, English NR, Knight SC, Carbone DP, Gabrilovich DI. Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J Immunol. 2001;166:678. doi: 10.4049/jimmunol.166.1.678. [DOI] [PubMed] [Google Scholar]
- 2.Bantounas I, Phylactou LA, Uney JB. RNA interference and the use of small interfering RNA to study gene function in mammalian systems. J Mol Endocrinol. 2004;33:545. doi: 10.1677/jme.1.01582. [DOI] [PubMed] [Google Scholar]
- 3.Boggio K, Nicoletti G, Di Carlo E, Cavallo F, Landuzzi L, Melani C, Giovarelli M, Rossi I, Nanni P, De Giovanni C, Bouchard P, Wolf S, Modesti A, Musiani P, Lollini PL, Colombo MP, Forni G. Interleukin 12-mediated prevention of spontaneous mammary adenocarcinomas in two lines of Her-2/neu transgenic mice. J Exp Med. 1998;188:589. doi: 10.1084/jem.188.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bronte V, Apolloni E, Cabrelle A, Ronca R, Serafini P, Zamboni P, Restifo NP, Zanovello P. Identification of a CD11b(+)/Gr-1(+)/CD31(+) myeloid progenitor capable of activating or suppressing CD8(+) T cells. Blood. 2000;96:3838. [PMC free article] [PubMed] [Google Scholar]
- 5.Bronte V, Serafini P, Apolloni E, Zanovello P. Tumor-induced immune dysfunctions caused by myeloid suppressor cells. J Immunother. 2001;24:431. doi: 10.1097/00002371-200111000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Danna EA, Sinha P, Gilbert M, Clements VK, Pulaski BA, Ostrand-Rosenberg S. Surgical removal of primary tumor reverses tumor-induced immunosuppression despite the presence of metastatic disease. Cancer Res. 2004;64:2205. doi: 10.1158/0008-5472.can-03-2646. [DOI] [PubMed] [Google Scholar]
- 7.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 8.Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21:137. doi: 10.1016/j.immuni.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 9.Gabrilovich DI, Velders MP, Sotomayor EM, Kast WM. Mechanism of immune dysfunction in cancer mediated by immature Gr-1+ myeloid cells. J Immunol. 2001;166:5398. doi: 10.4049/jimmunol.166.9.5398. [DOI] [PubMed] [Google Scholar]
- 10.Jensen SM, Meijer SL, Kurt RA, Urba WJ, Hu HM, Fox BA. Regression of a mammary adenocarcinoma in STAT6−/− mice is dependent on the presence of STAT6-reactive T cells. J Immunol. 2003;170:2014. doi: 10.4049/jimmunol.170.4.2014. [DOI] [PubMed] [Google Scholar]
- 11.Kacha AK, Fallarino F, Markiewicz MA, Gajewski TF. Cutting edge: spontaneous rejection of poorly immunogenic P1.HTR tumors by Stat6-deficient mice. J Immunol. 2000;165:6024. doi: 10.4049/jimmunol.165.11.6024. [DOI] [PubMed] [Google Scholar]
- 12.Kaplan MH, Schindler U, Smiley ST, Grusby MJ. Stat6 is required for mediating responses to IL-4 and for development of Th2 cells. Immunity. 1996;4:313. doi: 10.1016/S1074-7613(00)80439-2. [DOI] [PubMed] [Google Scholar]
- 13.Kusmartsev S, Gabrilovich DI. Immature myeloid cells and cancer-associated immune suppression. Cancer Immunol Immunother. 2002;51:293. doi: 10.1007/s00262-002-0280-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kusmartsev S, Cheng F, Yu B, Nefedova Y, Sotomayor E, Lush R, Gabrilovich D. All-trans-retinoic acid eliminates immature myeloid cells from tumor-bearing mice and improves the effect of vaccination. Cancer Res. 2003;63:4441. [PubMed] [Google Scholar]
- 15.Kusmartsev S, Gabrilovich DI. Inhibition of myeloid cell differentiation in cancer: the role of reactive oxygen species. J Leukoc Biol. 2003;74:186. doi: 10.1189/jlb.0103010. [DOI] [PubMed] [Google Scholar]
- 16.Liu Y, Van Ginderachter JA, Brys L, De Baetselier P, Raes G, Geldhof AB. Nitric oxide-independent CTL suppression during tumor progression: association with arginase-producing (M2) myeloid cells. J Immunol. 2003;170:5064. doi: 10.4049/jimmunol.170.10.5064. [DOI] [PubMed] [Google Scholar]
- 17.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549. doi: 10.1016/S1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 18.Mazzoni A, Bronte V, Visintin A, Spitzer JH, Apolloni E, Serafini P, Zanovello P, Segal DM. Myeloid suppressor lines inhibit T cell responses by an NO-dependent mechanism. J Immunol. 2002;168:689. doi: 10.4049/jimmunol.168.2.689. [DOI] [PubMed] [Google Scholar]
- 19.Miller FR, Miller BE, Heppner GH. Characterization of metastatic heterogeneity among subpopulations of a single mouse mammary tumor: heterogeneity in phenotypic stability. Invasion Metastasis. 1983;3:22. [PubMed] [Google Scholar]
- 20.Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol. 2000;164:6166. doi: 10.4049/jimmunol.1701141. [DOI] [PubMed] [Google Scholar]
- 21.Mills CD. Macrophage arginine metabolism to ornithine/urea or nitric oxide/citrulline: a life or death issue. Crit Rev Immunol. 2001;21:399. [PubMed] [Google Scholar]
- 22.Ostrand-Rosenberg S, Grusby MJ, Clements VK. Cutting edge: STAT6-deficient mice have enhanced tumor immunity to primary and metastatic mammary carcinoma. J Immunol. 2000;165:6015. doi: 10.4049/jimmunol.165.11.6015. [DOI] [PubMed] [Google Scholar]
- 23.Ostrand-Rosenberg S, Clements VK, Terabe M, Park JM, Berzofsky JA, Dissanayake SK. Resistance to metastatic disease in STAT6-deficient mice requires hemopoietic and nonhemopoietic cells and is IFN-gamma dependent. J Immunol. 2002;169:5796. doi: 10.4049/jimmunol.169.10.5796. [DOI] [PubMed] [Google Scholar]
- 24.Ostrand-Rosenberg S, Sinha P, Clements V, Dissanayake SI, Miller S, Davis C, Danna E. Signal transducer and activator of transcription 6 (Stat6) and CD1: inhibitors of immunosurveillance against primary tumors and metastatic disease. Cancer Immunol Immunother. 2004;53:86. doi: 10.1007/s00262-003-0446-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pulaski BA, Clements VK, Pipeling MR, Ostrand-Rosenberg S. Immunotherapy with vaccines combining MHC class II/CD80+ tumor cells with interleukin-12 reduces established metastatic disease and stimulates immune effectors and monokine induced by interferon gamma. Cancer Immunol Immunother. 2000;49:34. doi: 10.1007/s002620050024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pulaski BA, Terman DS, Khan S, Muller E, Ostrand-Rosenberg S. Cooperativity of Staphylococcal aureus enterotoxin B superantigen, major histocompatibility complex class II, and CD80 for immunotherapy of advanced spontaneous metastases in a clinically relevant postoperative mouse breast cancer model. Cancer Res. 2000;60:2710. [PubMed] [Google Scholar]
- 27.Pulaski BA, Smyth MJ, Ostrand-Rosenberg S. Interferon-gamma-dependent phagocytic cells are a critical component of innate immunity against metastatic mammary carcinoma. Cancer Res. 2002;62:4406. [PubMed] [Google Scholar]
- 28.Rutschman R, Lang R, Hesse M, Ihle JN, Wynn TA, Murray PJ. Cutting edge: Stat6-dependent substrate depletion regulates nitric oxide production. J Immunol. 2001;166:2173. doi: 10.4049/jimmunol.166.4.2173. [DOI] [PubMed] [Google Scholar]
- 29.Serafini P, De Santo C, Marigo I, Cingarlini S, Dolcetti L, Gallina G, Zanovello P, Bronte V. Derangement of immune responses by myeloid suppressor cells. Cancer Immunol Immunother. 2004;53:64. doi: 10.1007/s00262-003-0443-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ, Schreiber RD. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- 31.Sinha P, Clements VK, Ostrand-Rosenberg S. Reduction of myeloid-derived suppressor cells and induction of m1 macrophages facilitate the rejection of established metastatic disease. J Immunol. 2005;174:636. doi: 10.4049/jimmunol.174.2.636. [DOI] [PubMed] [Google Scholar]
- 32.Terabe M, Matsui S, Noben-Trauth N, Chen H, Watson C, Donaldson DD, Carbone DP, Paul WE, Berzofsky JA. NKT cell-mediated repression of tumor immunosurveillance by IL-13 and the IL-4R-STAT6 pathway. Nat Immunol. 2000;1:515. doi: 10.1038/82771. [DOI] [PubMed] [Google Scholar]
- 33.Wei LH, Jacobs AT, Morris SM, Jr, Ignarro LJ. IL-4 and IL-13 upregulate arginase I expression by cAMP and JAK/STAT6 pathways in vascular smooth muscle cells. Am J Physiol Cell Physiol. 2000;279:C248. doi: 10.1152/ajpcell.2000.279.1.C248. [DOI] [PubMed] [Google Scholar]