Abstract
Recent data suggest that chemokines and chemokine receptors mediate leukocyte recruitment of all components of the antitumor response. This study aimed to phenotypically characterize the immune lymphocyte infiltrate in human renal cell carcinomas (RCCs) and at the invasive margin (tumor–host interface) and to define the association of these findings with established prognostic indicators. Tumor infiltrating lymphocytes (TILs) were obtained from 24 patients with RCC undergoing radical nephrectomy. Peripheral blood cells from 37 patients were also obtained before surgery. Our findings are consistent with the preferential recruitment of CD4+ Th1-polarized effector memory cells that express CXCR3/CCR5. These cells were the main component of TILs and expressed as CXCR3, CCR5, CD45RO, and CD95. Natural killer (NK) cells were found in significantly higher proportions in TILs of RCCs than in peripheral blood lymphocytes (PBLs) or in other tumors studied (colorectal and breast cancers), where these cells were found in small proportions. No differences in nuclear grade or other studied parameters were observed between the TILs and the lymphocytes present at the invasive margin, which showed a similar composition. However, differences were found according to the tumor stage. First, significantly fewer NK cells were observed in PBLs from metastatic patients. Second, a significantly lower proportion of CCR5/CXCR3/CD4+ cells and a higher proportion of CCR4/CD4+ cells were observed in metastatic patients, suggesting that preferential Th1-polarization may gradually change during the progression of renal cancer cells. Finally, the frequency of CD25/CD4+ cells was higher in metastatic patients. Although the sample of patients with metastasis was small, the overall results suggest a change in composition of the TILs that may potentially confer a selective advantage for tumor growth and may account for the suppression of an effective cytotoxic response.
Keywords: Chemokine receptors, NK cells, Renal cell carcinomas, Tumor infiltrating lymphocytes
Introduction
The central role of T cells in antitumor immunity is well established [14]. However, the fact that tumors grow in the presence of substantial lymphocytic infiltration suggests that these T cells are not capable of mounting an effective immune response. It is clear that tumors can develop strategies that lead to evasion from immune response in vivo and may explain the poor immunogenicity of tumor cells. Downregulation of HLA cell-surface expression on tumor cells is frequently observed and mediates negative effects on immune surveillance [4, 9, 16]. In many cases, tumors show selective loss of individual HLA-A or HLA-B alleles and complete loss of HLA class-I antigen expression [10], which was demonstrated to cause resistance to lysis by tumor-associated antigen (TAA)-specific, HLA class-I-restricted cytotoxic T lymphocytes (CTLs). Tumors can also fail to present antigens because of defects in the TAP or in the components of immunoproteasome genes [3, 10] . However, besides failure of the antitumor immune response due to alterations in antigen processing machinery or apoptosis, other studies also report defects in CTL effectiveness by direct suppressive mechanisms that modify the tumor environment and host immune responses [13]. In fact, an important set of experiments demonstrated that the stroma barrier inhibits the infiltration and the activation of T cells at the tumor [5, 11].
T lymphocytes that infiltrate human neoplasms were shown to be functionally defective, incompletely activated, depleted, or anergic [11, 14]. Tumors are also able to overcome immune surveillance by changing the polarity of effector cells, thereby downregulating the proliferation of tumor-specific cytotoxic T cells, or by altering the effector composition of immune cells within the tumor [16]. Leukocyte traffic is highly coordinated, and a breakdown of the underlying control mechanisms might contribute to immune suppression [12]. Chemokines are leukocyte attractants and also contribute to immune processes that do not directly involve leukocyte migration. Certain combinations of chemokine receptors define populations that are greatly enriched in major subsets of Th1 versus Th2 cells. In fact, the expression of CXCR3 and CCR5 correlates with polarized Th1 T-cell differentiation, whereas CCR4 is selectively expressed in polarized Th2 cell differentiation [2, 18]. The aim of this study was to characterize lymphoid infiltrates of renal cell carcinomas (RCCs). Among tumor-infiltrating lymphocytes (TILs) directly isolated from RCCs, we found substantial numbers of natural killer (NK) cells and polarized Th1 CD4+ cells. This situation changed in advanced tumors, where increased infiltration of CD4/CD25+ and CCR4/CD4+ cells was observed. Our results indicate a change in TH cell responses during the progression of renal cell cancers that may be a critical factor in determining the effectiveness of an antiself immune response.
Material and methods
Tissue samples
Thirty-seven patients with RCCs histopathologically classified as clear-cell type and who underwent nephrectomy between 2002 and 2003 at our hospital were studied. Peripheral blood lymphocytes (PBLs) obtained immediately before radical nephrectomy and TILs were both analyzed in 24 patients. Only PBLs were studied in the remaining 13 patients. Patient age ranged from 26 years to 84 years (mean 60.6 years), and the male:female ratio was 24:13. Resected specimens were fixed in formalin and paraffin embedded or cryopreserved for routine histopathologic diagnosis. Samples were serially sliced in three fragments along the long axis. The central fragment was sent for flow cytometry analysis. One of the lateral fragments was frozen using liquid N2, and slides of 4–8 μm were obtained in a cryostat, cutting the surface adjacent to the central fragment. These slides were hematoxylin-eosin stained and immediately examined under light microscope for assessment of tissue quality (presence of hemorrhage, necrosis or infarct areas, abscess areas, etc.) and sample accuracy (matching and cataloguing tumor and histologic type, interface presence, and some tissue without microscopic alterations). In each tumor, discrete regions were analyzed that included tumor–host tissue interface sectors. Fresh specimens were analyzed by the pathologist, and histologic slides were reviewed to confirm the status of the surgical margin [tumor–host interface (THI)] and the tumor. The identification in healthy renal tissue, interface, and tumor tissue was performed under light microscope, identifying tubular and glomerular structures of the healthy renal tissue. A fibrous reaction, the interface, was seen opposite the healthy tissue and was composed of stromal cells, inflammatory cells and fibrosis, and, finally, tumor tissue, where nests of tumor cells arranged in expansive or infiltrative growth pattern were observed among stromal tissue.
Both tissues (tumor and THI) were separated for extraction of lymphocyte infiltrate and TILs. Tumors were staged according to the TNM classification (Table 1), and the cancer cells were graded [1–4] according to the Fuhrman system. Five surgically resected cases of colorectal cancer were randomly selected from the Department of Surgery and histopathologically classified according to the WHO classification. The processing and analysis of the tumor samples were the same as for the RCC. Informed consent was obtained from each patient before the surgery, and the study was approved by the Ethical Committee of Virgen de las Nieves Hospital.
Table 1.
Patient characteristics (n=37)
| Stage (TNM) | Grade | Age/sex | Cell type |
|---|---|---|---|
| T1N0M0 | 1 | 47/M | Clear |
| T2N0M0 | 2 | 42/F | Clear |
| T3N0M0 | 2 | 58/F | Clear |
| T1N0M0 | 1 | 49/F | Clear |
| T3N0M0 | 3 | 61/M | Clear |
| T1N0M0 | 1 | 69/M | Clear |
| T1N0M0 | 2 | 77/F | Clear |
| T3N1M0 | 3 | 77/M | Clear |
| T2N1M0 | 3 | 63/M | Clear |
| T1N0M0 | 3 | 66/F | Clear |
| T3N0M1 | 4 | 63/M | Clear |
| T1N0M0 | 1 | 54/F | Clear |
| T3N0M0 | 4 | 59/M | Clear |
| T3N0M0 | 4 | 65/M | Clear |
| T2N0M0 | 1 | 44/M | Clear |
| T3N0M0 | 4 | 56/M | Clear |
| T3N1M1 | 4 | 43/M | Clear |
| T2N0M0 | 1 | 69/M | Clear |
| T2N0M0 | 2 | 69/M | Clear |
| T3bN0M0 | 3 | 63/M | Clear |
| T1N0M0 | 1 | 60/M | Clear |
| T1N0M0 | 2 | 68/M | Clear |
| T3bN0M0 | 2 | 45/M | Clear |
| T1N0M0 | 2 | 55/F | Clear |
| T3N1M1 | 3 | 57/F | Clear |
| T3bN0M0 | 3 | 77/M | Clear |
| T1N0M0 | 1 | 53/F | Clear |
| T2N0M0 | 1 | 65/F | Clear |
| T3aN0M0 | 2 | 84/M | Clear |
| T2N0M0 | 2 | 75/M | Clear |
| T2N1M1 | 2 | 60/M | Clear |
| T3aN0M0 | 4 | 66/F | Clear |
| T3aN0M0 | 1 | 75/F | Clear |
| T1N0M0 | 3 | 26/F | Clear |
| T2N0M0 | 4 | 48/M | Clear |
| T1N0M0 | 1 | 65/M | Clear |
| T2N0M0 | 2 | 70/M | Clear |
Patients treated by radical nephrectomy between 2002 and 2003. Mean age: 60.6 years (SD±12.1). Sex: 24 M (male) (65%), 13 F (female) (35%)
Flow cytometry analysis
In all the cases, multiparameter flow cytometry immunophenotyping studies were performed on EDTA-anticoagulated peripheral blood (PB) samples or TIL cell suspension samples, and the analysis was done on erythrocyte-lysed buffer using well-established stain, lysing, and wash procedures. One hundred microliters of whole blood were incubated with 20 μl of each antibody. After incubation, red blood cells were lysed (FACS Lysing Solution; Becton Dickinson Biosciences, San Jose, CA, USA), washed twice in PBS, and finally resuspended in this buffer.
Tissue samples were mechanically homogenized, and mononuclear cells were washed in PBS and then centrifuged. TILs were incubated with either anti-CD8 (PerCP) or anti-CD4 (PerCP) and anti-CXCR3 (PE) anti-CCR5 (PE), anti CCR4 PE, anti-CD45RO (PE), anti-CD62L (FITC), anti-CD25 (PE), and anti-CD95 (PE). We also used CD45 (FITC)/CD14 (PE) and CD3 (FITC)/CD16/56 (PE) combinations to identify lymphocytes and NK cells, respectively. NK cells were defined as CD3-negative and CD16/CD56-positive lymphocytes. Finally, we used an anti-CD19 (FITC) monoclonal antibody to identify B cells in PBLs and TILs. All monoclonal antibodies (mAbs) were purchased (BD Biosciences, San Jose, CA, USA). Phenotypic expression of the TILs was determined by three-color fluorescence. Cells (5×105) were resuspended in 50 μl of buffer (PBS, 2% fetal calf serum) and incubated with 10 μl of appropriate FITC-, PE-, and PercP-labeled mAbs for 30 min at 4°C. After incubation, the cells were washed twice and resuspended in 0.5 ml of PBS. FACSort flow cytometer and CellQUEST software (BD Biosciences) were used for data acquisition. In all the cases, lymphocyte cells were specifically identified on the basis of intermediate side scatter (SSC) and forward scatter (FSC) characteristics and intense reactivity for CD45. The settings for all of these parameters were optimized at the start of the study and maintained constant during all subsequent analyses. Absolute counts of CD3, CD4, CD8, NK, and B lymphocytes were determined using TruCOUNT tubes with Multiset software (BD Biosciences)
Statistical analysis
Data were expressed as means ± standard deviation. The Kolmogorov–Smirnov test was used to check the normal distribution of study variables. Parametric (Student’s t-test) and nonparametric (Mann–Whitney U) tests were used to compare TIL and PBL phenotypes with different clinical parameters. Linear regression analysis was applied to correlate the presence of NK cells in TILs with that in PBLs. Statistical significance was defined as P<0.05.
Results
Immunophenotype of tumor infiltrating lymphocytes in RCCs
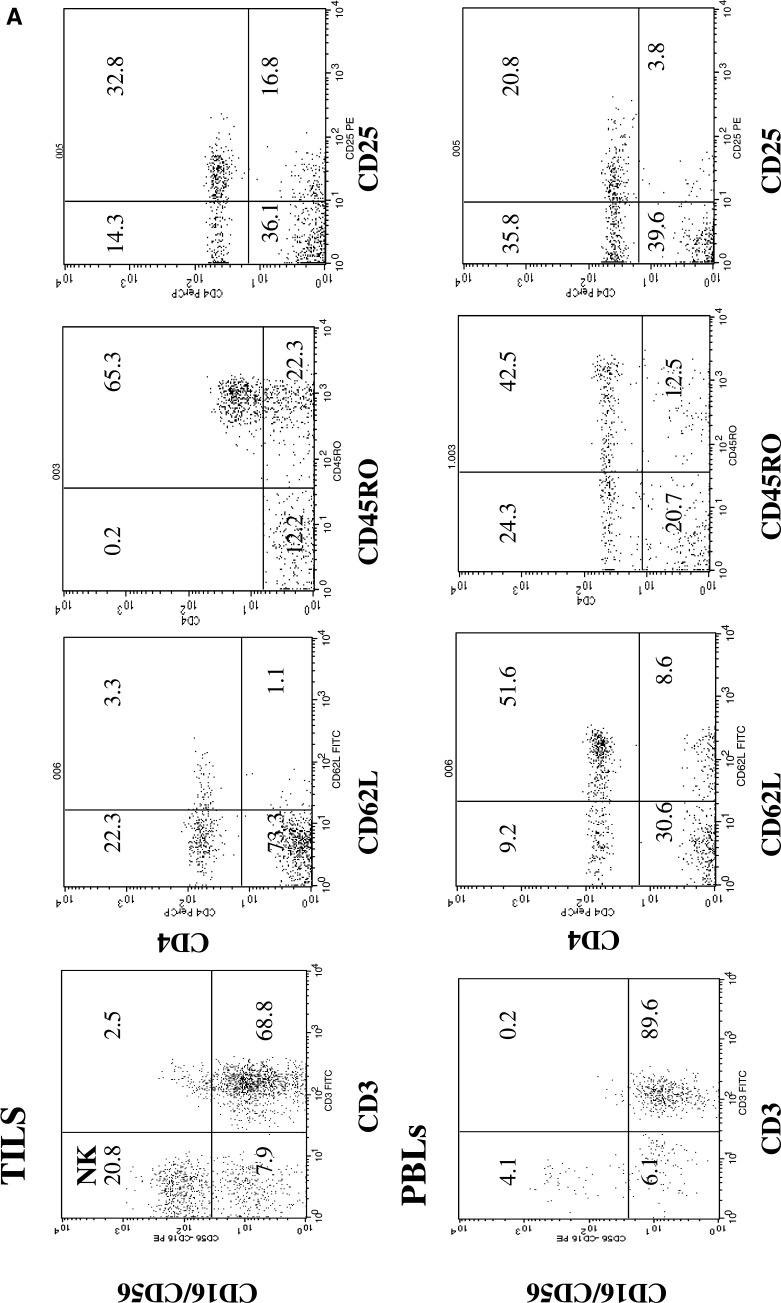
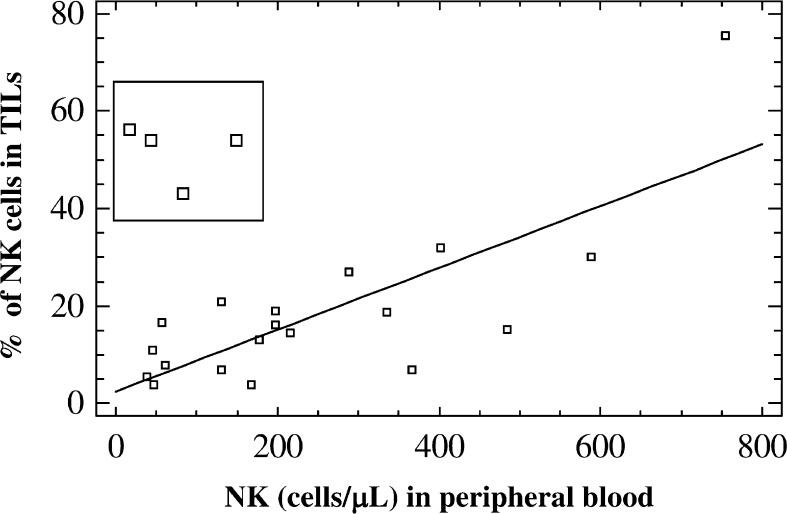
Three-color flow cytometry was used to accurately select lymphocyte subsets, especially subpopulations of CD4+, CD8+ and NK cells in TILs (Fig. 1a, b), and to study the local infiltrate in nontumor tissue surrounding the tumor. We found no differences in PBLs or in the composition of the tumor infiltrate according to the age or nuclear grading (data not shown). Table 2 shows the results of comparisons between PBLs and TILs and between TILs and lymphocytes derived from the THI in RCC tissue. The results are expressed as percentage of positive cells within the lymphocyte gate of the CD4 and CD8 subsets. There was a lower mean percentage of CD3 cells in TILs than in PBLs, although the difference was not significant (P=0.082). In contrast, there were significantly more NK cells in TILs than in PBLs (25.1 vs. 14.4, respectively; P=0.026). Using antibodies against CD16 and CD56 antigens, an elevated proportion of NK cells were identified in TILs of RCC. In all the present cases except four, a higher concentration of NK cells in PBLs correlated (P<0.001) with a higher percentage of NK cells in tumor tissue (Fig. 2); in the four exceptions, there was a very low number of NK cells in PBLs and a high proportion in TILs. We found no differences in the stage of tumor progression among these four patients (T2N0M0, T2N0M0, T3N0M0, and T2N1M1).
Fig. 1.
a Dot plot histograms of the flow cytometric analysis of CD16/CD56 (NK), CD62L/CD4, CD45RO/CD4, and CD25/CD4 in PB cells and TILs. These data demonstrate a high percentage of CD45RO+, CD62Llow T CD4 cells (memory cells), and NK cells in the TILs. b Dot plot histograms of the flow cytometric analysis of CXCR3/CD4, CCR5/CD4, CCR4/CD4, and CD95/CD4 cells in PB cells and TILs. The proportion of CCR5/CXCR3/CD4 cells was higher in TILs than in PBLs
Table 2.
Lymphocyte subpopulations in PBLs, THI, and intratumoral tissue (TILs) of renal cell cancer patients
| PBLs (n=37) | TILs (n=24) | P value (PBLs/TILs) | THI (n=24) | P value (TILs/THI) | |
|---|---|---|---|---|---|
| NK cells | 14.4±8.6 | 27.3±21.8 | 0.026 | 25.3±15.2 | 0.77 |
| T cells (CD3) | 73.2±12.6 | 65.9±16.8 | 0.082 | 65.2±18.8 | 0.87 |
| B cells (CD19) | 12.5±7.9 | 3.0±3.9 | 0.001 | 6.2±6.7 | 0.03 |
| CD4 T cells | 42.0±11.6 | 36.0±12.5 | 0.129 | 31.8±9.8 | 0.65 |
| CD8 T cells | 26.5±11.2 | 28.5±14.7 | 0.591 | 25.8±12.3 | 0.34 |
| CD4/CXCR3 | 42.7±12.7 | 76.0±20.3 | 0.0001 | 64.3±20.9 | 0.03 |
| CD4/CCR5 | 21.7±3.9 | 66.5±26.0 | 0.0001 | 61.6±16 | 0.27 |
| CD4/CCR4 | 25.9±9.7 | 22.9±21.1 | 0.577 | 19.1±13.3 | 0.92 |
| CD4/CD45RO | 35.2±2.0 | 88.9±12.7 | 0.0001 | 80.2±16.5 | 0.86 |
| CD4/CD62L | 49.3±2.8 | 10.9±12.2 | 0.0001 | 11.8±11.4 | 0.93 |
| CD4/CD95 | 66.0±8.0 | 90.3±8.7 | 0.0001 | 83.7±13 | 0.06 |
| CD4/CD25 | 51.8±11.9 | 32.4±15.2 | 0.0001 | 42.7±17.7 | 0.65 |
| CD8/45RO | 35.9±2 | 76.3±15.6 | 0.0001 | 78.9±14.7 | 0.86 |
| CD8/CD95 | 69.5±23.2 | 85.1±16.9 | 0.054 | 91.1±6.6 | 0.80 |
Data are expressed as mean ± standard deviation. The results are expressed as the percentage of positive cells within the lymphocyte gate (CD3, CD19, CD4, CD8) or the CD4 and CD8 cells (CCR5, CCR4, CXCR3, CD45RO, CD62L, CD95, CD25). NK cells were determined as CD3(-)/CD16/CD56(+) cells. The Mann–Whitney U test was used
n number of cases studied
Fig. 2.
Association between the concentration of NK cells in PB (cells/μl) and percentage of these cells in the TILs in 24 renal carcinomas. All but four patients demonstrated statistically significant correlation between these two parameters. Four exceptional cases showed a high NK cell number in TILs accompanied by a very low blood level of NK cells. Correlation coefficient=0.77 (P<0.001), calculated excluding the four cases indicated in the box
The mean percentage of infiltrating B cells was much lower in tumor specimens than in PBLs (3.0% vs. 12.5%, P<0.001). Furthermore, we found significant differences in the percentage of naïve and memory T cells based on CD45RO, CD62L, and CD95 expression. CD4+ T cells were CD95+, CD45RO+, and CD62Llow, a phenotype consistent with previous activation (Fig. 1a). In order to represent the differences in CD62L, we took account of the fact that this antigen is downmodulated, as is CD45RO. For this reason, the differences were established based on a threshold for cells with a strong expression of this antigen, considering cells with a low or absent expression to be negative.
The majority of the CD8 and CD4 tumor infiltrating cells had a memory phenotype with expression of CD45RO and downregulation of CD62L. There were significant differences between TILs and PBLs in mean percentage of CD4+/CD45RO (88.9 vs. 35.2; P<0.0001) and CD8+/CD45RO cells (76.3 vs. 35.9; P<0.0001). The CD4/CD8 ratio in PBL and TILs was 1.58 and 1.26, respectively.
Presence of NK cells in TILs of RCC differ from presence in other tumors
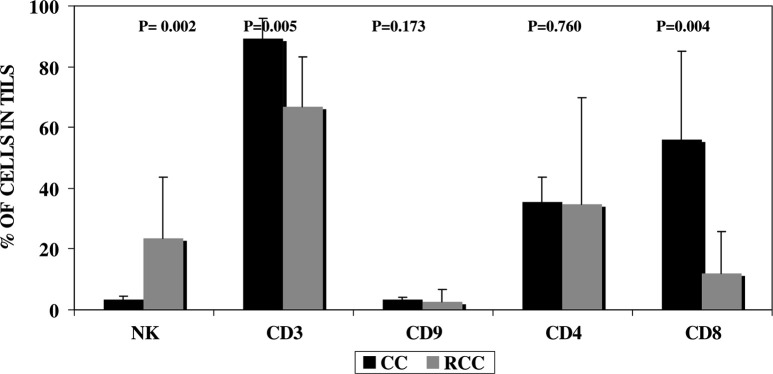
A completely different situation was observed in colorectal cancer (Fig. 3) and breast cancer (not shown), in which tumor-infiltrating NK cells were either not detectable or present only at a very small frequency. A significantly (P=0.002) lower percentage of NK cells and higher proportion of CD3+ T cells were observed in colon carcinomas, which also showed a markedly higher (P=0.004) proportion of CD8 cells compared with the RCCs (P=0.004; Fig. 3). There was a very low presence of B cells in both types of tumor.
Fig. 3.
Analysis of the intratumoral lymphocyte subsets in renal cell carcinoma and colon carcinoma. Results are expressed as ratio of the mean percentage of cells +/- standard deviation of 23 RCCs (grey bars) and five colon carcinomas (black bars). Statistical analyses were performed using the Mann–Whitney test
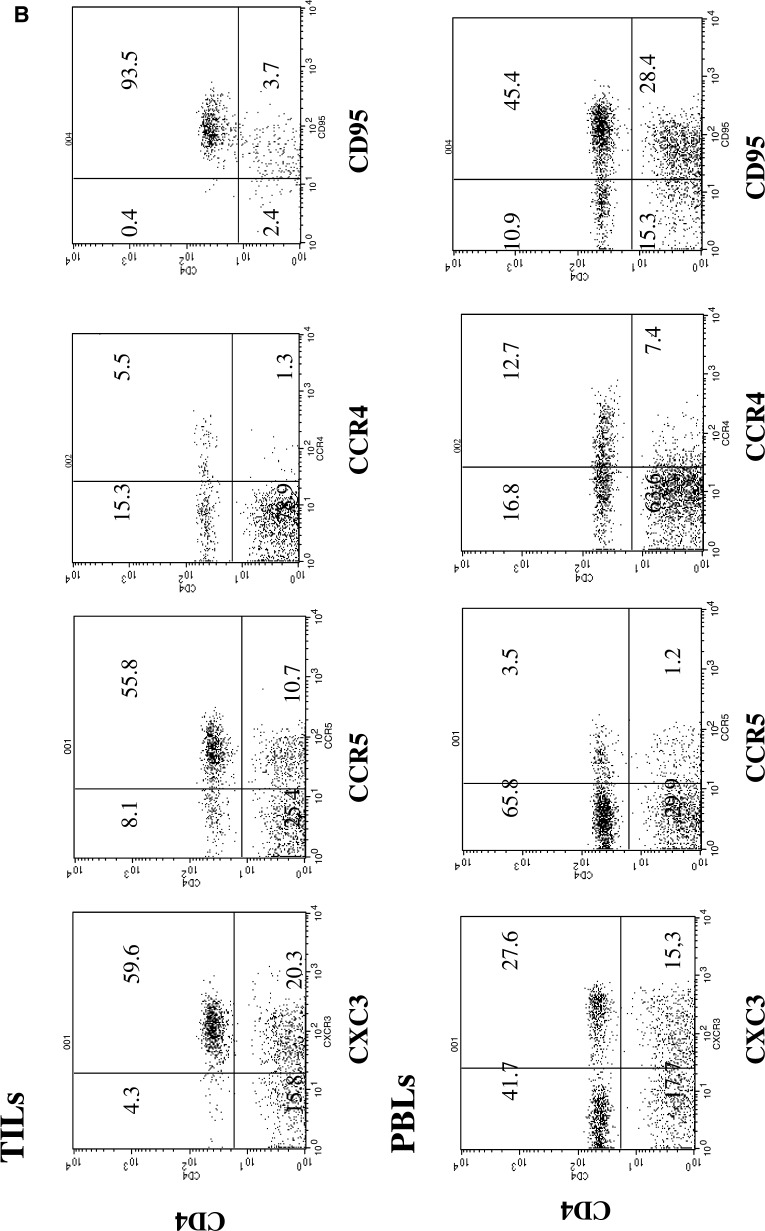
CXCR3/CCR5 T CD4 cells predominate in RCC TILs
Chemokines and their receptors control leukocyte migration at multiple levels, and the differential expression of chemokine receptors may largely dictate the migration and tissue homing of Th1s and Th2s. Our results demonstrate that the chemokine receptors CXCR3 and CCR5 are predominant markers in Th1-type peripheral CD4+ TIL cells (Fig. 1b). The proportion of CD4 cells expressing these markers was higher in TILs than in PBLs (P<0.0001; Table 2). Virtually all CD4+ T cells within the tumors expressed CXCR3 and CCR5, whereas fewer T cells expressed CCR4 (Fig 1b). Our results showed the predominance of type 1-polarized CD4+ “effector memory” cells in the tumor tissue.
When our results in TILs and PBLs were related to the tumor stage, the presence of NK cells and CD4/CXCR3+ and CD4/CCR5+ cells was observed to be lower in more advanced (M1) tumors than in T1 and T2 N0M0 tumors (Table 3). By contrast, the presence of CD4/CD25+ and CD4/CCR4+ was higher in M1 tumors than in T1 and T2N0M0 tumors (Table 3). When the lymphocyte subpopulations of T1-2 N0M0 and T3-4N0M0 tumors were compared with those of N+ and M+ tumors, the differences between the two groups of tumors were less evident. We observed a progressive fall in the percentage of CXCR3/CD4+ y CCR5/CD4+ cells and a progressive rise in CCR4/CD4+ cells. However, the differences did not reach statistical significance using the ANOVA test, probably because of the small sample size.
Table 3.
Average yield of lymphocyte subpopulations in renal cell cancer patients with different tumor stages
| TNM | TNM | TNM | T1-2N0M0/M1 | T3-4N0M0/M1 | |
|---|---|---|---|---|---|
| T1-2N0M0 | T3-4N0M0 | M1 | P value | P value | |
| PBLs (a) | n=20 | n=11 | n=4 | ||
| NK (b) | 256.7±246 | 238.9±.179 | 40.0±.7.1 | 0.001e | 0.011e |
| CD4/CXCR3 | 44.9±14 | 47.8±6.6 | 27.6±9.6 | 0.047e | 0.013e |
| CD4/CCR5 | 20.6±7.9 | 25.7±17 | 7.6±4.2 | 0.008d | 0.007d |
| CD4/CCR4 | 30.0±12 | 30.7±13 | 23.1±5 | 0.510e | 0.518e |
| CD4/CD25 | 54.3±11 | 48.6±14 | 43.3±10 | 0.092e | 0.569e |
| TILs | n=12 | n=8 | n=4 | ||
| NK(c) | 24.5±22 | 19.6±16 | 22.7±28 | 0.66d | 0.99e |
| CD4/CXCR3 | 88.1±8.7 | 69.1±16 | 59.8±33 | 0.017d | 0.61e |
| CD4/CCR5 | 78.8±1.6 | 60.1±28 | 44.8±42 | 0.024d | 0.414e |
| CD4/CCR4 | 14.6±8 | 27.1±24 | 37.5±34 | 0.047d | 0.683e |
| CD4/CD25 | 32.6±14 | 31.3±14 | 51.6±17 | 0.05d | 0.30e |
Data are expressed as mean ± standard deviation
n number of cases studied
aTwo cases, T3N1M0 and T2N1M0 were not included in the table
bMean of absolute NK cells count in PB (number of cells/μl)
cMean percentage of NK cells in TILs
dStudent’s t test
eMann–Whitney U test
Composition of infiltrate in the tumor–host interface and TILs
Comparison between TILs and lymphocytes present at invasive margin (THI) showed similar results in the distribution of most lymphocyte subsets, although there was a lower presence of B lymphocytes and a higher presence of CD4/CXCR3+ cells in TILs (Table 2).
Discussion
Tumor infiltrating lymphocytes are known to participate in the tumor–host reaction in various types of cancers. Regional changes in TILs in vivo may be significant for elucidating cancer–host immune interactions. In fact, the uncontrolled growth of tumor observed may be the consequence of poor local immune response. Therefore, a better characterization of tumor infiltrates is crucial to evaluate possible cancer–host immune interactions. In the present study, differences were observed in the composition of tumor infiltrate according to tumor stage, which is the most important prognostic factor in RCC, but not according to nuclear grading or patient age.
Natural killer cells are not found in great numbers in advanced human cancers, indicating that they do not usually effectively infiltrate the malignant tissue [1]. However, we observed substantial percentages of NK cells in TILs directly isolated from RCCs in most of the patients. On the other hand, NK cells were under-represented in some of the RCCs, indicating that the tumor-infiltrating capacity of NK cells varies among different patients. The factors involved in the presence of NK cells in tumors are not fully understood. In fact, NK cells were rarely observed among infiltrating tumor cells from breast (not shown) and colon carcinomas (Fig. 3). Interestingly, tumor infiltrating NK cells were positively correlated with the concentration of NK cells in PB in most of the RCCs studied (Fig. 2). However, these data do not explain the efficiency of NK cell infiltration in RCC compared with other tumors. Our results suggest that NK cell infiltration in renal cancer may also result from a local effect of the tumor environment. In fact, four patients showed a high percentage of NK cells in TILs, but a low concentration in PBLs. This may indicate that NK cells efficiently homed to malignant tissue in these patients, suggesting the possible participation of chemotactic signals from the RCC microenviroment. In this context, differences in NK infiltration among the present patients may have been influenced by the local chemokine production in the renal cancer. Thus, intratumoral expression of chemokines was recently reported to be positively correlated with the degree and type of lymphocyte infiltration in the tumor and inversely correlated with the microvessel density [6]. Further research is warranted on chemokine expression in RCC tissue and chemokine receptors on NK cells because of the role that chemokines may play in regulating cellular NK infiltration.
Natural killer cells have an inherent ability to recognize a wide variety of tumor cells. They are sensitive to inhibitory ligands and can recognize tumors that might evade T-cell killing because of altered HLA expression[4, 15]. NK-TILs can also lyse autologous RCC tumor cell lines in vitro, and this activity is correlated with low HLA class I surface expression [19]. The correlation between intratumoral NK cell infiltration and clinicopathologic features remains unclear. Patients with a high level of NK infiltration were found to have a better prognosis than those with a low level of NK cells in colon and lung carcinomas [5, 21]. In our study, NK cell levels were directly associated with tumor stage but not with nuclear grading, and a lower concentration of PB NK cells was observed in more advanced M1 tumors (Table 3).
With respect to T cells, CD4+ T cells expressing CXCR3 (and CCR5) in TILs were mostly CD45RO+, and they generally expressed low expression of CD62L (Fig. 1a). These antigens are primarily expressed on effector memory T cells, and the phenotype resembles that of T cells infiltrating inflammatory lesions, which are responsible for many cell-mediated cytotoxic functions [2, 18]. Our results clearly demonstrate a high proportion of this type of cells compared to the levels of CXCR3/CD4+ and CCR5/CD4+ cells in blood (P<0.001). These findings are consistent with the preferential recruitment of CD4+ Th1-polarized effector memory cells expressing CXCR3/CCR5 at tumor tissue. In this context, it was recently observed that NK cells are capable of inducing stable Th1-polarized effector memory cells [8]. The decreased presence of tumor-infiltrating CXCR3/CD4+, CCR5/CD4+, and NK cells was further reduced in the most advanced patients with distant metastasis (M1) in comparison with patients with T1 and T2 N0M0 tumors and the difference was statistically significant (Table 3). This may suggest a poor antitumor response in the former patients. In fact, a progressive change was observed from T1-2 N0M0 through T3-4N0M0 to M+, although differences among these three groups were not significant, probably because of the small sample size.
Patients with metastasis showed a higher mean percentage of tumor-infiltrating CD4/CD25+ and CD4/CCR4 cells. A lower proportion of polarized CD4+ Th1 and a higher proportion of CD4/CD25+ and CD4/CCR4 cells at tumor sites may have a profound effect on the inhibition of T cell responses against cancer [7, 22]. CD4+/CD25+ regulatory T cells also play an important role in the maintenance of immunological self-tolerance by suppressing immune responses against cancer [22, 23]. Suppression induced by these cells may be mediated through cytokines or cell–cell contacts or both and may limit autoaggressive responses and autoimmunity reactions. In fact, these cells may play a major natural regulatory function in controlling autoimmune disease by a mechanism that involves IL-10 [23]. In conclusion, we suggest that the greater presence of CD4/CCR4 and CD4/CD25+ regulatory T cells and the lesser presence of NK and CD4/CXCR3/CCR5 cells observed in metastatic patients may affect the overall activation status of TILs and may contribute to the inhibition of cytotoxic cell-mediated immune responses.
Acknowledgements
We thank Carmen Amezcua and Carmen Gonzalez for expert technical assistance. This work was partially supported by the Fondo de Investigaciones Sanitarias, (PI020175) the plan Andaluz de Investigación, Instituto de Salud Carlos III-Red de centros de Cancer-RTICCC-contract n° CO3/10 and the Plan Nacional, Spain.
References
- 1.Albertsson PA, Basse PH, Hokland M, Goldfarb RH, Nagelkerke JF, Nannmark U, Kuppen PJ. NK cells and the tumour microenvironment: implications for NK-cell function and anti-tumour activity. Trends Immunol. 2003;24:603. doi: 10.1016/j.it.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 2.Bonecchi R, Bianchi G, Bordignon PP, D’Ambrosio D, Lang R, Borsatti A, Sozzani S, Allavena P, Gray PA, Mantovani A, Sinigaglia F. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J Exp Med. 1998;187:129. doi: 10.1084/jem.187.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cabrera CM, Jimenez P, Cabrera T, Esparza C, Ruiz-Cabello F, Garrido F. Total loss of MHC class I in colorectal tumors can be explained by two molecular pathways: beta2-microglobulin inactivation in MSI-positive tumors and LMP7/TAP2 downregulation in MSI-negative tumors. Tissue Antigens. 2003;61:211. doi: 10.1034/j.1399-0039.2003.00020.x. [DOI] [PubMed] [Google Scholar]
- 4.Garrido F, Ruiz-Cabello F, Cabrera T, Perez-Villar JJ, Lopez-Botet M, Duggan-Keen M, Stern PL. Implications for immunosurveillance of altered HLA class I phenotypes in human tumours. Immunol Today. 1997;18:89. doi: 10.1016/S0167-5699(96)10075-X. [DOI] [PubMed] [Google Scholar]
- 5.Ishigami S, Natsugoe S, Tokuda K, Nakajo A, Che X, Iwashige H, Aridome K, Hokita S, Aikou T. Prognostic value of intratumoral natural killer cells in gastric carcinoma. Cancer. 2000;88:577. doi: 10.1002/(SICI)1097-0142(20000201)88:3<577::AID-CNCR13>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 6.Kondo T, Ito F, Nakazawa H, Horita S, Osaka Y, Toma H. High expression of chemokine gene as a favorable prognostic factor in renal cell carcinoma. J Urol. 2004;71:2171. doi: 10.1097/01.ju.0000127726.25609.87. [DOI] [PubMed] [Google Scholar]
- 7.Li J, Hu P, Khawli LA, Epstein AL. Complete regression of experimental solid tumors by combination LEC/chTNT-3 immunotherapy and CD25(+) T-cell depletion. Cancer Res. 2003;63:8384. [PubMed] [Google Scholar]
- 8.Mailliard RB, Son YI, Redlinger R, Coates PT, Giermasz A, Morel PA, Storkus WJ, Kalinski P. Dendritic cells mediate NK cell help for Th1 and CTL responses: two-signal requirement for the induction of NK cell helper function. J Immunol. 2003;171:2366. doi: 10.4049/jimmunol.171.5.2366. [DOI] [PubMed] [Google Scholar]
- 9.Maleno I, López Nevot MA, Seliger B, Garrido F. Low frequency of HLA haplotype loss asoociated with loss of heterozigosity in chromosome region 6p21 in clear renal cell carcinomas. Int J Cancer. 2004;109:636. doi: 10.1002/ijc.20000. [DOI] [PubMed] [Google Scholar]
- 10.Marincola FM, Jaffee EM, Hicklin DJ, Ferrone S. Escape of human solid tumors from T-cell recognition: molecular mechanisms and functional significance. Adv Immunol. 2000;74:181. doi: 10.1016/s0065-2776(08)60911-6. [DOI] [PubMed] [Google Scholar]
- 11.Mizoguchi H, O’Shea JJ, Longo DL, Loeffler CM, McVicar DW, Ochoa AC. Alterations in signal transduction molecules in T lymphocytes from tumor-bearing mice. Science. 1992;258:1795. doi: 10.1126/science.1465616. [DOI] [PubMed] [Google Scholar]
- 12.Moser B, Wolf M, Walz A, Loetscher P. Chemokines: multiple levels of leukocyte migration control. Trends Immunol. 2004;25:75. doi: 10.1016/j.it.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 13.Mukherjee P, Ginardi AR, Madsen CS, Tinder TL, Jacobs F, Parker J, Agrawal B, Longenecker BM, Gendler SJ. MUC1-specific CTLs are non-functional within a pancreatic tumor microenvironment. Glycoconj J. 2001;18:931. doi: 10.1023/A:1022260711583. [DOI] [PubMed] [Google Scholar]
- 14.Ochsenbein AF, Klenerman P, Karrer U, Ludewig B, Pericin M, Hengartner H, Zinkernagel RM. Immune surveillance against a solid tumor fails because of immunological ignorance. Proc Natl Acad Sci U S A. 1999;96:2233. doi: 10.1073/pnas.96.5.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pardoll D. Does the immune system see tumors as foreign or self? Annu Rev Immunol. 2003;21:807. doi: 10.1146/annurev.immunol.21.120601.141135. [DOI] [PubMed] [Google Scholar]
- 16.Paul S, Calmels B, Regulier E. Tumor-induced immunosuppression. Ann Biol Clin. 2002;60:143. [PubMed] [Google Scholar]
- 17.Ruiz-Cabello F, Cabrera T, Lopez-Nevot MA, Garrido F. Impaired surface antigen presentation in tumors: implications for T cell-based immunotherapy. Semin Cancer Biol. 2002;12:15. doi: 10.1006/scbi.2001.0406. [DOI] [PubMed] [Google Scholar]
- 18.Sallusto F, Lanzavecchia A, Mackay CR. Chemokines and chemokine receptors in T-cell priming and Th1/Th2-mediated responses. Immunol Today. 1998;19:568. doi: 10.1016/S0167-5699(98)01346-2. [DOI] [PubMed] [Google Scholar]
- 19.Schleypen JS, Von Geldern M, Weiss EH, Kotzias N, Rohrmann K, Schendel DJ, Falk CS, Pohla H. Renal cell carcinoma-infiltrating natural killer cells express differential repertoires of activating and inhibitory receptors and are inhibited by specific HLA class I allotypes. Int J Cancer. 2003;106:905. doi: 10.1002/ijc.11321. [DOI] [PubMed] [Google Scholar]
- 20.Schwartz M, Kipnis J. Autoimmunity on alert: naturally occurring regulatory CD4(+)CD25(+) T cells as part of the evolutionary compromise between a ’need’ and a ’risk’. Trends Immunol. 2002;23:530. doi: 10.1016/S1471-4906(02)02322-0. [DOI] [PubMed] [Google Scholar]
- 21.Villegas FR, Coca S, Villarrubia VG, Jimenez R, Chillon MJ, Jareno J, Zuil M, Callol L. Prognostic significance of tumor infiltrating natural killer cells subset CD57 in patients with squamous cell lung cancer. Lung Cancer. 2002;35:23. doi: 10.1016/S0169-5002(01)00292-6. [DOI] [PubMed] [Google Scholar]
- 22.Wang HY, Lee DA, Peng G, Guo Z, Li Y, Kiniwa Y, Shevach EM, Wang RF. Tumor-specific human CD4+ regulatory T cells and their ligands: implications for immunotherapy. Immunity. 2004;20:107. doi: 10.1016/S1074-7613(03)00359-5. [DOI] [PubMed] [Google Scholar]
- 23.Zhang X, Koldzic DN, Izikson L, Reddy J, Nazareno RF, Sakaguchi S, Kuchroo VK, Weiner HL. IL-10 is involved in the suppression of experimental autoimmune encephalomyelitis by CD25+CD4+ regulatory T cells. Int Immunol. 2004;16:249. doi: 10.1093/intimm/dxh029. [DOI] [PubMed] [Google Scholar]