Abstract
Background: Monoclonal antibodies (MAbs) can be used to detect, image and treat cancers. This study aimed to characterise the binding of BLCA-38 MAbs to human prostate cancer cell lines, human prostate cancer biopsy samples and normal tissues to enable future targeted studies. Methods: BLCA-38 antigen expression on cancer lines was determined by flow cytometry; that on patient specimens from normal tissues and cancers was tested by immunohistochemistry using fresh frozen tissues or paraffin-embedded tissues that had undergone antigen retrieval. Results: Cell surface BLCA-38 antigen expression was seen on DU-145, PC-3, PC-3 M and PC-3 M-MM2 prostate cancer lines, but LNCaP, MDA PCa 2a or MDA PCa 2b lines were negative. Other human lines, including 8/12 bladder cancer and A431 vulval epidermoid cells, but not breast cancer lines, expressed BLCA-38 antigen. Staining occurred in glandular epithelial cells in the majority of frozen, and paraffin-embedded prostate cancer tissues and was occasionally seen in prostatic intraepithelial neoplasia (PIN). No staining was observed in normal cadaver tissues or in benign areas from various other cancer tissues. Conclusions: The BLCA-38 antibody binds to the majority of human prostate cancers but not to normal cells, and has potential for targeting novel therapies in patients with this disease.
Keywords: Flow cytometry, Human prostate cancer biopsies, Immunohistochemistry, Monoclonal antibodies, Prostate cancer cell lines
Introduction
Polyclonal antibodies were first used clinically to image disease some 40 years ago. Although successful, their lack of specificity and our poor understanding of their target antigens limited their usefulness. Since hybridoma technology has been developed [1], MAbs can be generated in large quantities for use in detection, imaging and therapy of cancers. We described a series of MAbs raised against bladder cancer and their use in the detection of bladder cancer cells in urine [2]. We have recently found that one of these, BLCA-38 MAb, binds to human prostate cancer cell lines. Prostate cancer is the most common male cancer and second highest cause of cancer mortality in men in Western society. Tumour-specific or prostate tissue–specific MAbs may be useful in early detection, imaging and treatment [3–5]. Antibodies may be radiolabeled for imaging [4, 5] or conjugated with toxins, drugs or radionuclides for immunotherapy [6]. The use of MAbs for targeting genitourinary cancers has recently been reviewed [7].
BLCA-38 is an IgG1 murine MAb [2] raised against the human bladder cancer cell line, UCRU-BL-17CL [8, 9]. Western blotting of membrane preparations of BLCA-38-positive DU-145 cells has shown that the BLCA-38 MAb recognises a cell surface glycoprotein of around 30 kDa (unpublished data). However, the antigen has been extremely difficult to define by 2-D gel electrophoresis, and further work is in progress. BLCA-38 MAb has previously been shown to effect excellent targeting to human bladder cancer xenografts in nude mice, and a single dose of 250 μCi samarium 153–labeled BLCA-38 provided sustained growth delay of subcutaneous (s.c.) bladder cancer xenografts [10]. This paper describes the binding of BLCA-38 to human prostate cancers either in paraffin-fixed or frozen tissues, and its lack of binding to other tissues, suggesting that this antibody may have future use in imaging or in targeted therapy for prostate cancer.
Materials and methods
Human cancer cell lines
Several human cancer cell lines were studied: (1) Human prostate cancer cell lines: PZ-HPV-7 prostate cells, LNCaP [11], DU145 [12] and PC-3 [13] from American Type Culture Collection (ATCC, Rockville, MD, USA); LNCaP-C4 and LNCaP-C4–2 [14–16] from OnCor (Oklahoma City, OK, USA); LNCaP-LN3 [17], PC3-M and PC3-M-MM2 [17] from Dr C. Pettaway (M.D. Anderson Cancer Center, Houston, TX, USA); MDA PCa 2a and MDA PCa 2b [18] from Dr Nora Navone (MD Anderson, TX, USA); LAPC4 cells [19] from Dr C. Sawyers (Molecular Biology Institute, University of California, Los Angeles, CA, USA); and TSU-PR-1 [20] (now shown to be a bladder cancer line [21]) from W.W. Heston (Cleveland Clinic, Cleveland, OH, USA); (2) Human bladder cancer lines: UCRU-BL-17CL/0/X1 (abbreviated BL-17CL), cloned lines derived from UCRU-BL-17/2 (abbreviated BL17/2) and UCRU-BL-13/0 (abbreviated BL-13) from our laboratory [22–24]; J82 [25], T24 [26] and 5637 [27] from ATCC. (3) Human breast cancer cell lines: SKBR-3 [28], MCF-7 M [29] and MDA-MB-134 [30] from Elizabeth Musgrove (Garvan Institute of Medical Research, NSW, Australia); and (4) The human vulval epidermoid cell line, A431 [31] from ATCC. All were maintained at 37°C in an atmosphere of 5% CO2 in special conditions as described by ATCC or those who developed the cell lines.
Human specimens
With approval from the institutional human ethics committee and written informed consent of relatives, post mortem tissues were obtained from two male cadavers aged 26 and 56 years, who had died from overdose or an industrial accident, respectively. Tissues were partly fresh frozen in the cryopreservative OCT (Tissue-Tek, Torrance, CA, USA), or formalin-fixed and paraffin-embedded. Fresh prostate cancer tissues were obtained through PJC from men, mean age 62.8 years (range 26–94 years), undergoing radical prostatectomy (17 patients) or transurethral resection of the prostate (TURP, 3 patients), within 30 min of surgery. Punch biopsies (~4-mm diameter) were removed from tumour-containing areas in the posterior region of the prostate and cut longitudinally for fixation in 10% neutral buffered formalin and paraffin-embedding, or for fresh freezing in OCT (stored at −80°C). Cryostat sections (5 μm) were mounted on glass slides precoated with 0.5% gelatin and air-dried overnight at RT. A reference slide was stained with hematoxylin and eosin (H & E); others were stored at −20°C until used. Three punch biopsies from fresh frozen radical prostatectomies were examined for BLCA-38 expression by J. Pedersen, Melbourne Pathology. Additional paraffin blocks of radical prostatectomy specimens included two from A. Lochhead (Southern Pathology, Wollongong, NSW, Australia), eight from W. Delprado (Douglass Hanly Moir Pathology, Sydney, NSW, Australia) and three from C. Busch (University of Tromso, Tromso, Norway). For comparison, two sections showing benign hyperplasia of the prostate (BPH), two containing prostatic intraepithelial neoplasia (PIN) and one containing normal prostate from Prof. Busch (Norway) were examined by Dr E. Mortensen (University of Tromso, Norway). Needle biopsies containing paraffin-embedded normal prostate (three from A. Lochhead) or frozen normal prostate (one from W. Delprado) were examined independently by the pathologists for BLCA-38 staining. Dr Pedersen, from Melbourne Pathology, also independently examined microscopically normal or benign tissue within paraffin-embedded biopsy specimens for BLCA-38 expression using the methodology described below: prostate (12), normal small bowel mucosa (5), large bowel (5), urinary bladder (15), gastric epithelium (1), breast epithelium (5), gallbladder (5) and oesophagus (5).
Animal models and tumour xenografts
Male 6–8-week-old athymic nude mice, BALB/c (nu/nu), were obtained from Animal Resources Centre (ARC), Western Australia. The mice were housed and maintained in laminar flow cabinets under specific pathogen-free conditions in facilities approved by the University of New South Wales (UNSW) Animal Care and Ethics Committee (ACEC) and in accordance with their regulations and standards. The ethical guidelines that were followed meet the standards required by the UK coordinating committee on cancer research guidelines [32].
To establish s.c. tumours, DU-145 or PC-3 human prostate cancer cell lines (1×106 cells/injection) were implanted s.c. in the shoulder region of nude mice. Mice were euthanased when the xenografts reached ~8×8 mm in diameter, and the tumours and metastatic lymph nodes (from PC-3 cells) excised for analysis.
Monoclonal antibodies
BLCA-38, and two negative control IgG1 MAbs, K-1–21, reactive against human free kappa light chains [33], and A2, reactive against the variable region of the MOPC315 antibody, were used. The K-1–21 was from Dr R. Raison (then Department of Cellular Immunology, University of Sydney, Australia), and the A2 from Dr A. Collins (School of Microbiology and Immunology, UNSW, Sydney, Australia). Hybridoma supernatants were purified as described [2].
Flow cytometry
Cultured cells were harvested with 2 mM ethylenediaminetetraacetic acid (EDTA, ICN Bioradicals, OH, USA) in Dulbecco’s phosphate-buffered saline (PBS), pH 7.2, washed in PBS, and prepared for flow cytometric analysis as previously described [34]. This was performed using a fluorescence-activated cell scanner (FACScan 440; Becton Dickinson, Mountain View, CA, USA) with a 5-W argon ion laser tuned to 488 nm at 200 nW. The degree of fluorescence for each cell line tested was expressed as the geometric mean of fluorescence intensity, as calculated from a CellQuest programme.
Cytospins
Cells (50,000 in 100 μl PBS) were centrifuged onto slides at 7,000 rpm (1,250 g) for 5 min using a cytocentrifuge (Shandon Southern, Waterloo, NSW, Australia).
Fixation
Human tissue biopsy samples and post mortem tissues were immediately snap frozen in OCT embedding medium or fixed in 10% buffered formalin and processed overnight in Tissue-Tek VIP (Sakura Finetek, USA Inc, Torrance, CA, USA) before paraffin embedding. Superfrost Plus slides (Menzel-Glaser, Germany) were used to minimise tissue loss. Cytospins and frozen sections were air dried for 1 h at RT, fixed in cold acetone for 3 min (or in acetone at RT for 10 min) and washed twice in PBS for 5 min. The slides were quenched, stained and counterstained.
Antigen retrieval
To overcome cross-linking by formalin fixation, paraffin-embedded tissue sections were pretreated using high-temperature microwave irradiation. After deparaffinisation, slides were immersed in 0.01 M citrate solution, pH 6, heated to boiling in a 1,200 W microwave oven at maximum power for 3 min then at 95°C for another 10 min, then cooled at RT for 15 min.
Immunohistochemistry
Detection of BLCA-38 expression by indirect immunofluorescent, immunoperoxidase and the alkaline phosphatase anti–alkaline phosphatase (APAAP) [35] method was performed. Two- [34] and three-step methods (modified from [36]) were used for staining. The three-step method was slightly more sensitive, but less specific than the two-step method. (results not shown). For mouse tissues, the mouse-on-mouse (MOM) kit (Vector Labs, Burlingame, CA, USA), used as per the manufacturer’s instructions, was necessary to overcome the presence of mouse IgG in the tissues. DU-145 prostate cancer cell pellets were used as a positive control. Negative controls were treated with irrelevant isotype–matched primary antibody or with no primary antibody. Immunoreactivities were graded based on staining intensity above that seen on the negative control: ± (equivocal, focal, weak), 1+ (weak), 2+ (moderate) or 3+ (strong). For cytospins, BLCA-38 was used at 5 μg/ml, whereas for tissue samples, 5–10 μg/ml was used on fresh frozen OCT-embedded and 5–15 μg/ml on formalin-fixed, paraffin-embedded biopsy sections. Anti–high molecular weight cytokeratin, 34βE12 (Dakopatts, Glostrup, Denmark) was used at 1:250 dilution. All primary antibodies were diluted in 2% bovine serum albumin in PBS (BSA/PBS).
Results
Reactivity of BLCA-38 with cancer cell lines
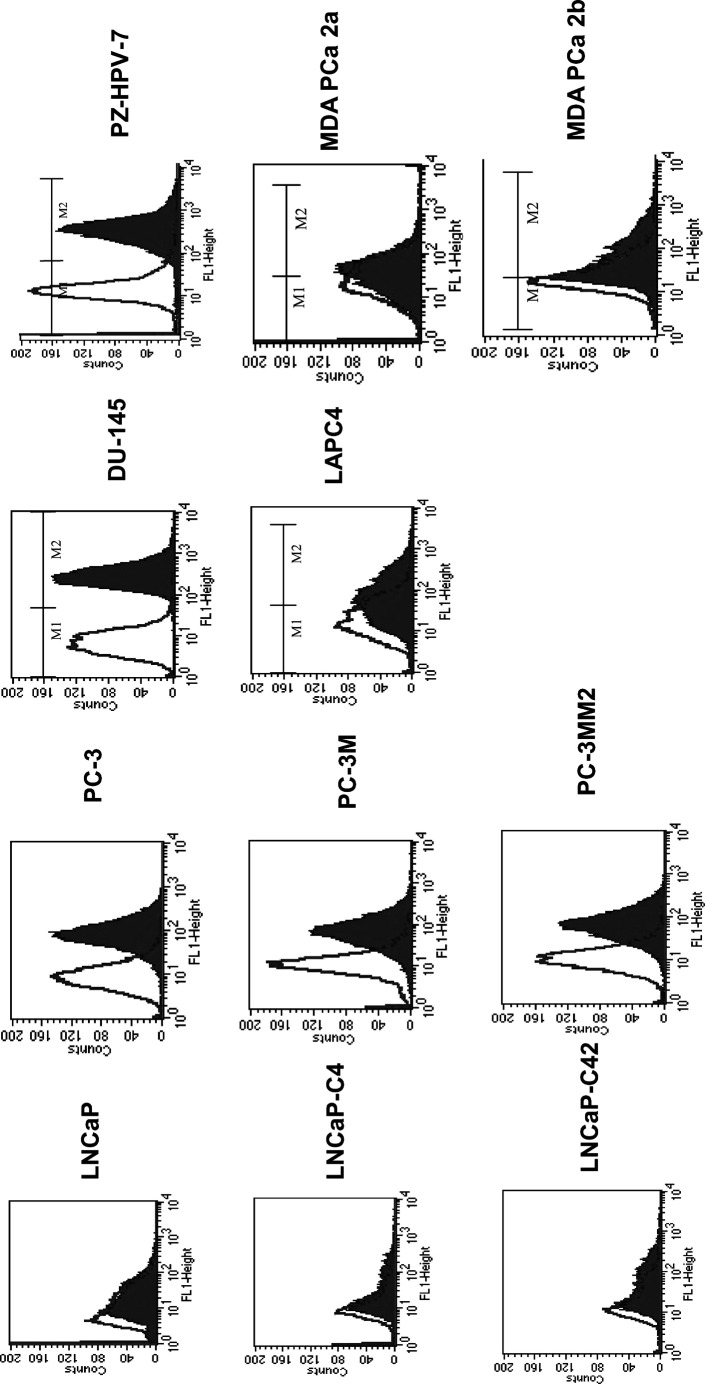
BLCA-38 hybridoma supernatant was tested against human cancer cell lines by flow cytometry (Table 1). Staining was considered positive when the geometric mean fluorescence intensity (CellQuest software) was 25 or over. BLCA-38 showed strong reactivity with some human prostate cancer cell lines (Fig. 1), especially androgen-independent lines (DU-145, PC-3 and sublines) but not with androgen-sensitive cell lines or with human breast cancer cell lines SKBR-3, MDA-MD-134 or MCF-7 M. BLCA-38 was strongly reactive against A431 vulval epidermoid cells, and the bladder lines J82, T24 and, to a lesser extent, 5637, but not against higher passage numbers of BL17/2 or cloned lines, B11, C1 or C10, derived from BL17/2, even though BLCA-38 was originally prepared by immunisation of BALB/c mice with UCRU-BL17CL line [2]. Positive staining of prostate cancer cells was also seen when the cells were permeabilised by treatment with cold methanol and vortexing prior to flow cytometric staining (data not shown), indicating that some form of the antigen was present in the cytoplasm. Flow cytometric data (Table 1, Fig. 1) were confirmed by immunoperoxidase staining of selected cell lines, using plasma clots of cell pellets that were formalin-fixed and paraffin-embedded. DU-145 and J82 cells showed predominantly membranous staining, with some cytoplasmic staining, while LNCaP and LNCaP-C4–2 cells were negative. We have previously reported that BLCA-38 reacted with 5/5 human ovarian, 3/4 human colonic cancer lines, and 2/5 melanoma lines, but had no reactivity against T lymphoid lines (0/3), B lymphoid lines (0/10), leukaemic lines (0/2 tested) or human peripheral white blood cells [37]. BLCA-38 reactivity was found to depend on how the target cells had been grown in tissue culture. We have found that when BL17/2 human bladder cancer cells were allowed to become confluent in tissue culture before passage, BLCA-38 expression declined, in some cases to zero, and the antigen was not reexpressed when these cells were recultured. However, earlier passage cells that had not been cultured at confluence were positive and remained so when appropriately cultured.
Table 1.
Flow cytometric reactivity of BLCA-38 against human cancer cell lines. AI Androgen independent, AS androgen sensitive
| Lines tested | Hormone sensitivity | Reactivitya | Lines (number positive/number tested) |
|---|---|---|---|
| Prostate cancer lines | 2/5 lineages | ||
| LNCaP | AS | − | 0/4 lines |
| LNCaP-C4 | AI | − | |
| LNCaP-C4–2 | AI | − | |
| LNCaP-LN3 | AS | − | |
| MDA PCa 2a | AS | − | 0/2 lines |
| MDA PCa2b | − | ||
| PC-3 | AI | + | 3/3 lines |
| PC-3 M | AI | + | |
| PC-3MM2 | AI | + | |
| DU-145 | AI | + | 1/1 lines |
| TSU-PR-1 | AI | − | 0/1 lines |
| Breast cancer lines | 0/3 lineages | ||
| SKBR-3 | − | ||
| MCF-7 M | − | ||
| MDA-MB-134 | − | ||
| Vulval epidermoid cells | + | 1/1 lines | |
| A431 | |||
| Bladder cancer lines | 5/5 lineages | ||
| 8/12 lines | |||
| J82 | + | ||
| T24 | + | ||
| 5637 | + | ||
| UCRU-BL-17/0/X1 | + | ||
| UCRU-BL-17/2 | − | ||
| Clones from BL-17/2 | |||
| B8 | + | ||
| B11 | − | ||
| C1 | − | ||
| C3 | + | ||
| C10 | − | ||
| UCRU-BL-13 | + | ||
aGeometric mean fluorescence intensity ≥25 taken as positive, <25 taken as negative
Fig. 1.
Flow cytometric profiles of human cancer cell lines stained for membrane expression of BLCA-38. X-axis log scale of green fluorescence units, Y-axis number of events. The clear histogram represents staining obtained with secondary antibody only; solid histogram represents staining using BLCA-38 against the named cell lines grown in culture
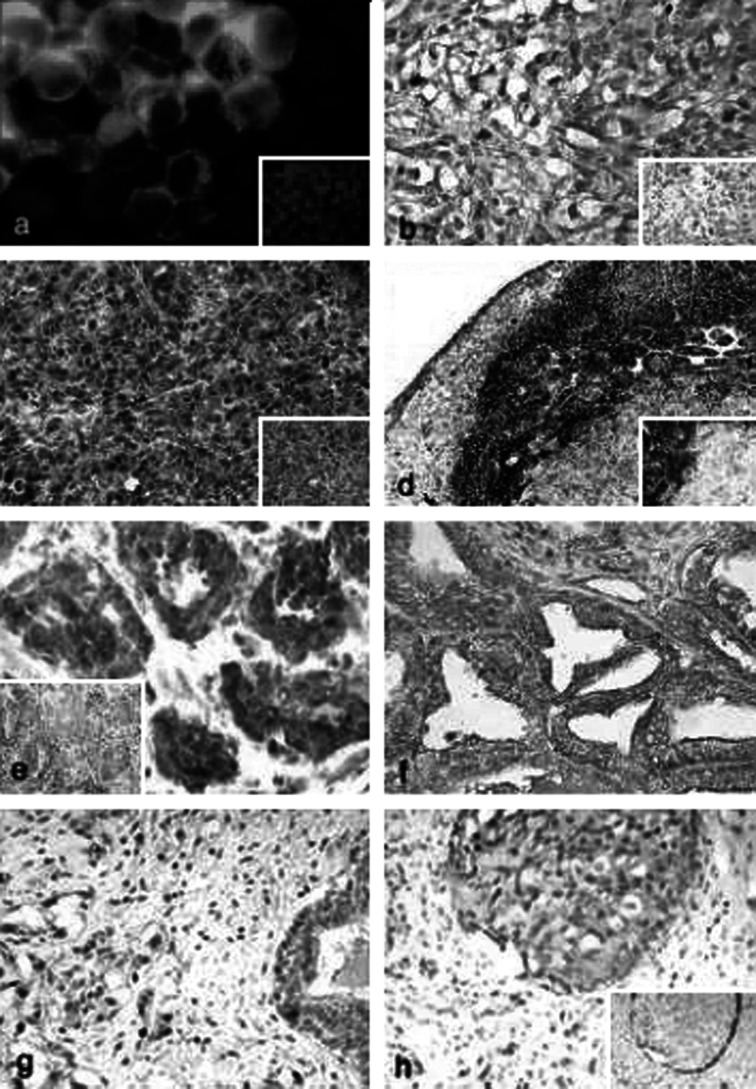
BLCA-38 reacted with cell surface antigens on the positive cell lines (Figs. 1 and 2a). This reactivity was maintained when cell lines were grown as xenografts in nude mice and was seen at the cell surface and in the cytoplasm. Strong staining was observed both in primary tumours (Fig. 2b, c) and in lymph node metastases (Fig. 2d) of human prostate cancer cell lines grown s.c. in nude mice.
Fig. 2a-h.
Indirect immunohistochemical staining of BLCA-38 expression in prostate cancer cell lines, xenografts in nude mice and human prostate tumour biopsies. Strong membrane and cytoplasmic staining were seen in a cytospins of the positive cell line DU-145, by green immunofluorescence; b paraffin sections of the DU-145 xenograft, by immunoperoxidase/DAB; c PC-3 xenografts grown s.c. detected by immunoalkaline phosphatase/fast red; d PC-3 lymph node metastases detected by immunoalkaline phosphatase/fast red. Inserts show negative controls, a stained with irrelevant isotype–matched MAbs or b,c,d procedure controls where no primary antibody was used. e Human prostate cancers of Gleason score 6 (frozen section), insert shows normal prostate (frozen section). f PIN in human prostate cancer specimen (formalin-fixed, paraffin-embedded). g Human prostate cancers of Gleason score 9 (formalin-fixed, paraffin-embedded). h Positive staining in intraductal carcinoma of the prostate, but with no staining of the basal cells, that stain positively for cytokeratin 34βE12 (insert, formalin-fixed, paraffin-embedded). Original magnification of images: a,b,e–h (also inserts) at ×40; c,d (also inserts) at ×20
Immunoreactivity of BLCA-38 with normal human tissues
Immunohistochemical staining of post mortem tissues was performed. At 5 μg/ml, there was no detectable BLCA-38 antigen expression in the post mortem tissues examined from adrenal (1 patient), bladder (2), blood (in the lung), cerebellum (1), cerebrum (1), heart (2), kidney (2), liver (1), lung (2), lymph node (1), muscle (1), oesophagus (1), pancreas (1), pituitary (1), prostate (2), skin (1), spleen (1), stomach (1), testis (1), thymus (1) or thyroid/parathyroid (1), but at 10 μg/ml of BLCA-38, there was some nonspecific cytoplasmic and background staining in stromal cells. Staining was weak and confined to the cytoplasm.
Microscopically benign tissues in biopsy samples were examined for BLCA-38 reactivity in our own laboratory (Lab 1) and in that of anatomical pathologist J. Pedersen (Melbourne Pathology, Victoria, Australia) (Lab 2), using the same methodology. No BLCA-38 immunoreactivity was observed with tissues derived from paraffin-embedded normal or benign adrenal (1), gall bladder (5), urinary bladder (15), small (5) and large bowel (5), breast epithelial cells (5 patients), gastric epithelium (1) or oesophagus (5). However, paraffin-embedded needle biopsy samples of prostate and frozen sections from three radical prostatectomies showed limited/equivocal staining (considered to be +/−) in benign and normal areas in Lab 2, while a frozen normal prostate from a cystectomy showed equivocal staining for BLCA-38 in Lab 1. As this staining was less than 1+, it is shown as negative in Table 2. Examination of larger numbers of normal prostate tissues would clarify if there were any staining of significance in normal prostate.
Table 2.
BLCA-38 immunoreactivity in human prostate tumour biopsy samples: intensity of staining and percentage positivity. Intensity of staining was scored from + (weak) to ++ (moderate) to +++ (strong)
| Tissue | Number of biopsies | Frozen tissues | Number of biopsies | Formalin-fixed, paraffin-embedded tissues | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| − | + | ++ | +++ | ≥2+ | % | − | + | ++ | +++ | ≥2+ | % | |||
| Normal (in cancer tissue) | 7 | 6 | 1 | 0/7 | 0 | 13 | 8 | 5 | 0 | 0 | ||||
| Benign (BPH) | 2 | 2 | 0 | 0 | ||||||||||
| Benign (BPH, in cancer tissues) | 3 | 1 | 2 | 0/3 | 0 | 21 | 11 | 10 | 0/21 | 0 | ||||
| PIN | 2 | 1 | 1 | 0/2 | 0 | |||||||||
| PIN (in cancer tissues) | 3 | 2 | 1 | 1/3 | 33 | 21 | 11 | 6 | 3 | 1 | 4/21 | 21 | ||
| Cancer: Gleason score 6 | 6 | 1 | 1 | 4 | 4/6 | 67 | 22 | 3 | 5 | 14 | 14/22 | 64 | ||
| Gleason score 7 | 13 | 4 | 6 | 10/13 | 77 | 7 | 3 | 4 | 4/7 | 57 | ||||
| Gleason score 8 | 3 | 2 | 2/3 | 67 | ||||||||||
| Gleason score 9 | 4 | 3 | 2 | 2 | 4/4 | 100 | 1 | 1 | 1/1 | 100 | ||||
| Overall BLCA-38 reactivity | 18/23 | 78 | 21/33 | 64 | ||||||||||
Immunoreactivity of BLCA-38 with human prostate cancer biopsy samples
Clear membranous and cytoplasmic staining were observed on tissues that had been frozen (Table 2, Fig. 2e), and although the number of cases examined was small, there was a trend indicating an increased intensity of BLCA-38 staining with increasing Gleason score (Fig. 2e, g). There was no BLCA-38 expression in a normal prostate. Some PIN lesions were BLCA-38 reactive (Fig. 2f), indicating that this antibody can detect early as well as later lesions. Antigen retrieval of formalin-fixed, paraffin-embedded tissue tended to lead to occasional nonspecific nuclear staining in addition to membrane and cytoplasmic staining, and for this reason, more weight has been placed on the results from frozen tissues. Where needle biopsies of tissues were used, the amount of fixation required was probably less, and less spurious results were obtained in paraffin-embedded tissues. Thus, in paraffin-embedded tissues, BLCA-38 staining of cancer tissues was clearly positive (Table 2), whereas normal tissues in the same section were negative (Fig. 2e, insert). In lower grade prostate cancer, BLCA-38 expression was seen in luminal epithelial cells (Fig. 2h), whereas basal cells, which were positive for high molecular weight cytokeratin (34βE12), were negative for BLCA-38 (Fig. 2h, insert). This allowed for discrimination of intraductal carcinoma of the prostate by the BLCA-38 antibody. Stromal cells were generally negative.
Overall, 18/23 (78%) frozen specimens of prostate cancers showed positive staining for BLCA-38, whereas, of 33 specimens that were formalin-fixed and paraffin-embedded, only 21 (64%) expressed BLCA-38 antigen, indicating that some antigenicity was masked by this fixation procedure. Specimens from the same patient that were frozen or paraffin-embedded and antigen retrieved showed generally comparable staining (data not shown).
Discussion
This study shows that the MAb BLCA-38 can bind to various human cancer cell lines of epithelial origin (Table 1). More importantly, BLCA-38 MAb stains 78% of frozen prostate cancer specimens, yet does not stain normal tissues from post mortem or from needle biopsies of benign tissues, indicating that it has potential for imaging and therapeutic targeting of prostate cancer in patients with this disease. BLCA-38 binding is best visualised using fresh frozen specimens, but with appropriate antigen retrieval, data can be obtained on formalin-fixed, paraffin-embedded tissues, although with decreased sensitivity (64% positive) and with occasional nuclear staining, considered to be an artefact of antigen retrieval. Because of this, we believe that frozen tissues are preferable to formalin-fixed, paraffin-embedded tissues for accurate BLCA-38 staining. BLCA-38 binds to some PIN (Fig. 2a), but there was a trend to increased staining for BLCA-38 with increasing Gleason score (Table 2), indicating that the antigen concerned is expressed early in prostate cancer, but may be more highly expressed on the surface of later stage cancers. Future studies will use frozen tissues to examine BLCA-38 expression after tumours become androgen-independent, or following androgen ablation therapy. BLCA-38 expression was clearly positive in androgen-independent prostate cancer cell lines (DU-145 and PC-3 and sublines), but not in the androgen-sensitive lines, LNCaP or MDA PCa 2a or b (Table 1). The binding to luminal cells (Fig. 2e) is perhaps at odds with the lack of binding on LNCaP cells that might be expected to present a luminal phenotype. While studies of cell lines are useful for indicating the reactivity of an antibody, the real test is in tissue samples. Expression of BLCA-38 on metastatic prostate cancer in patients has not yet been studied, but expression on lymph node metastasis of the PC-3 cell line in nude mice (Fig. 2d) indicates that reactivity is likely to be maintained in metastatic deposits. In collaborative studies with D. Hewish of the CSIRO in Victoria (unpublished data), we have shown that the BLCA-38 antibody is not internalised after binding to relevant tissues. As yet, the nature of the BLCA-38 antigen, a glycoprotein, has not been fully characterised, but the antigenic epitope is also expressed internally as shown by flow cytometric analysis of permeabilised cells. The fact that BLCA-38 is not internalised does not affect its ability to deliver a toxic payload when that is radioactive [10] or when the toxin it delivers is independent of internalisation [8].
While several MAbs have been generated against CaP cell lines and also against prostatic tumour tissue obtained from biopsy, the characterisation and identification of the antigens recognised by these MAbs has proven difficult due to problems in solubilisation and purification of their antigens. This is the case for the BLCA-38 antigen, a glycoprotein of approximately 30 kDa (unpublished data). At this time, we have not used the SEREX technique [38], which may be useful to determine the nature of the BLCA-38 antigen. Despite these difficulties, some target prostate cancer antigens have been identified to the extent of their molecular weight, isoelectric point and glycosylation. The best known MAb for prostate cancer imaging and treatment is a second generation MAb, J591, that reacts with the external domain of the prostate-specific membrane antigen (PSMA) now known as folate hydrolase 1 antigen (FOLH1) [39]. As recently reported at the AUA conference by Trabulsi et al. [40, 41], therapy using J591 has already shown promise in phase I and phase II clinical trials.
Other MAbs that show increasing reactivity with prostate cancers of increasing grade or stage include αPro3 [42], an IgG2 MAb raised against PC-3 cells that binds to an antigen (175 kDa in nonreduced form, 54 kDa when reduced) expressed on well to poorly differentiated prostate cancers; D83.21 [43], an IgM MAb that recognises a glycoprotein with multiple subunits and that binds to metastatic prostate cancer and to primary prostate tumour tissue, with stronger staining of poorly differentiated and undifferentiated tissue compared with well-differentiated tissue; TURP-27, an IgG3k MAb that binds to lung metastases and primary prostate cancer [44]; and P25.48, an IgG3 MAb, and P25.91, an IgG2a MAb [3], both generated against a cell suspension of moderately to poorly differentiated prostate carcinoma specimens, that react with poorly and moderately differentiated prostatic tumour tissue, but not with well-differentiated tissue. Other anti–prostate cancer MAbs that have been described to have more reactivity against well-differentiated than against poorly differentiated prostate cancers are not detailed here.
For therapeutic uses, the efficacy of MAbs may be further enhanced through conjugation with radioisotopes, drugs, prodrugs, or toxins [45]. We have shown that BLCA-38 labeled with bismuth 123 (213Bi), an alpha emitter, when used in a cocktail with other prostate cancer–reactive 213Bi-labeled MAbs and proteins, is cytotoxic to PC-3 and DU-145 cells in vitro [46]. In addition, the genetic modification or “humanisation” of MAbs, thereby minimising generation of any human antimurine antibody (HAMA) responses following administration, further complements MAb utility [47]. More recently, the use of engineered dimers and trimers (or diabodies and triabodies) has been shown to improve tissue uptake for both imaging and therapeutic studies. The advantages of the use of diabodies, triabodies and tetrabodies for cancer targeting have been reviewed [37].
Given that anti-PSMA antibodies that have been extensively used for imaging do not bind to all prostate tumours, it is possible that combinations of antibodies, including BLCA-38, would increase targeting ability, as shown by our in vitro studies [46]. We have also shown that BLCA-38 provides good targeting of DU145 xenografts in vivo [8, 48]. Thus our studies indicate that further investigations using the BLCA-38 MAb, possibly in combination with other prostate cancer antibodies, for imaging or therapeutic targeting of prostate cancer are warranted.
Acknowledgements
We wish to thank the pathologists, Prof. Christer Busch (University of Tromso, Tromso, Norway), Drs Alistair Lochhead (Sutherland Pathology, Wollongong, NSW, Australia), John Pedersen (Melbourne Pathology, Victoria, Australia) and Warick Delprado (Douglass Hanly Moir, Sydney, Australia) for providing human specimens suitable for study, and Dr Angus Collins (Douglass Hanly Moir, Sydney, Australia) for help with interpretation of Gleason scores in the prostate cancer biopsy samples. We also wish to thank the above-mentioned pathologists and Dr Elin Mortensen (University of Tromso, Tromso, Norway) for help with reading the intensity of some BLCA-38 staining in our laboratory.
References
- 1.Kohler Nature. 1975;256:495. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- 2.Walker J Urol. 1989;142:1578. doi: 10.1016/s0022-5347(17)39172-3. [DOI] [PubMed] [Google Scholar]
- 3.Bazinet Cancer Res. 1988;48:6938. [PubMed] [Google Scholar]
- 4.Leroy M, Teillac P, Rain JD, et al. Radioimmunodetection of lymph node invasion in prostatic cancer: the use of iodine-123(123I)-labeled monoclonal-prostatic acid phosphatase 227A F(ab)2 antibody fragments in vivo. Cancer (Phila) 1989;64:1. doi: 10.1002/1097-0142(19890701)64:1<1::aid-cncr2820640102>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 5.Lopes Cancer Res. 1990;50:6423. [PubMed] [Google Scholar]
- 6.DeNardo Curr Opin Immunol. 1999;11:563. doi: 10.1016/S0952-7915(99)00017-5. [DOI] [PubMed] [Google Scholar]
- 7.Palapattu J Urol. 2002;168:2615. doi: 10.1016/S0022-5347(05)64230-9. [DOI] [PubMed] [Google Scholar]
- 8.Russell PJ, Hewish D, Carter T, Sterling-Levis K, Ow K, Hattarki M, Doughty L, Guthrie R, Shapira D, Molloy PL, Werkmeister JA, Kortt A (2004) Cytotoxic properties of immunoconjugates containing melittin-like peptide 101 against prostate cancer: in vitro and in vivo studies. Cancer Immunol Immunother (in press) [DOI] [PMC free article] [PubMed]
- 9.Russell Urol Res. 1988;16:79. doi: 10.1007/BF00261960. [DOI] [PubMed] [Google Scholar]
- 10.Lightfoot Antibody Immunoconjugates Radiopharm. 1991;4:319. [Google Scholar]
- 11.Horoszewicz Prog Clin Biol Res. 1980;37:115. [PubMed] [Google Scholar]
- 12.Mickey Cancer Res. 1977;37:4049. [PubMed] [Google Scholar]
- 13.Kaighn Invest Urol. 1979;17:16. [PubMed] [Google Scholar]
- 14.Gleave Cancer Res. 1991;51:3753. [PubMed] [Google Scholar]
- 15.Thalmann Cancer Res. 1994;54:2577. [PubMed] [Google Scholar]
- 16.Wu Int J Cancer. 1994;57:406. [Google Scholar]
- 17.Pettaway Clin Cancer Res. 1996;2:1627. [PubMed] [Google Scholar]
- 18.Navone Clin Cancer Res. 1997;3:2493. [PubMed] [Google Scholar]
- 19.Klein Nat Med. 1977;3:402. [Google Scholar]
- 20.Iizumi J Urol. 1987;137:1304. doi: 10.1016/s0022-5347(17)44488-0. [DOI] [PubMed] [Google Scholar]
- 21.van Cancer Res. 2001;61:6340. [PubMed] [Google Scholar]
- 22.Brown Br J Cancer. 1990;61:369. [Google Scholar]
- 23.Russell Int J Cancer. 1988;41:74. doi: 10.1002/ijc.2910410115. [DOI] [PubMed] [Google Scholar]
- 24.Russell Int J Cancer. 1989;44:276. doi: 10.1002/ijc.2910440216. [DOI] [PubMed] [Google Scholar]
- 25.O‘Toole Br J Cancer. 1978;38:64. doi: 10.1038/bjc.1978.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bubenik Int J Cancer. 1970;5:310. doi: 10.1002/ijc.2910050303. [DOI] [PubMed] [Google Scholar]
- 27.Zinzar Exp Hematol. 1985;13:574. [PubMed] [Google Scholar]
- 28.Lundy J Surg Res. 1977;22:654. doi: 10.1016/0022-4804(77)90105-6. [DOI] [PubMed] [Google Scholar]
- 29.Koga Cancer Res. 1990;50:4849. [PubMed] [Google Scholar]
- 30.Rizk Acta Cytol. 1982;26:714. [PubMed] [Google Scholar]
- 31.Haigler Proc Natl Acad Sci U S A. 1978;75:3317. doi: 10.1073/pnas.75.7.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Workman Br J Cancer. 1998;77:1. doi: 10.1038/bjc.1998.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walker Eur J Nucl Med. 1986;12:461. [Google Scholar]
- 34.Li Prostate Cancer Prostatic Dis. 2002;5:36. doi: 10.1038/sj.pcan.4500543. [DOI] [PubMed] [Google Scholar]
- 35.Cordell J Histochem Cytochem. 1984;32:219. doi: 10.1177/32.2.6198355. [DOI] [PubMed] [Google Scholar]
- 36.Ow Urol Oncol. 1995;1:144. doi: 10.1016/1078-1439(95)00059-3. [DOI] [PubMed] [Google Scholar]
- 37.Todorovska J Immunol Methods. 2001;248:47. doi: 10.1016/S0022-1759(00)00342-2. [DOI] [PubMed] [Google Scholar]
- 38.Fossa A, Alsoe L, Crameri R, Funderud S, Gaudernack G, Smeland EB (2004) Serological cloning of cancer/testis antigens expressed in prostate cancer using cDNA phage surface display. Cancer Immunol Immunother (in press) [DOI] [PMC free article] [PubMed]
- 39.Liu Cancer Res. 1997;57:3629. [PubMed] [Google Scholar]
- 40.Trabulsi EJ, Yao D, Joyce MA, Milowksy M, Kostakoglu L, Vallabhajosula D Nanus D, Goldsmith S, Bander NH (2003) Phase I radioimmunotherapy (RIT) trials of monoclonal antibody (MAB) J591 to the extracellular domain of prostate specific membrane antigen (PSMAEXT) radiolabeled with yttrium 90 (90Y) or lutetium 177 (177Lu). J Urol 169[Suppl 4] [DOI] [PubMed]
- 41.Trabulsi EJ, Yao D, Kostakoglu L, Vallabhajosula S, Hoyce MA, Milowksy M, Nanus DM, Goldsmith SJ, Bander NH (2003) Targeting metastatic prostate cancer with radiolabeled J591 monoclonal antibody (MAB) specific for the extracellular domain of prostate specific membrane antigen. J Urol 169[Suppl 4] [DOI] [PubMed]
- 42.Ware Cancer Res. 1982;42:1215. [PubMed] [Google Scholar]
- 43.Starling Cancer Res. 1985;45:804. [PubMed] [Google Scholar]
- 44.Lipford Cancer Res. 1991;51:2296. [PubMed] [Google Scholar]
- 45.Kingsley EA, Russell PJ (1999) Radioimmunotherapy of superficial bladder cancer. In: Riva P (ed) Therapy of malignancies with radioconjugated monoclonal antibodies: present possibilities and future prospectives. Harwood Academic Publishers, The Netherlands, pp 411–441
- 46.Li Int J Radiation Oncology Biol Phys. 2004;59:in. [Google Scholar]
- 47.He J Immunol. 1998;160:1029. [PubMed] [Google Scholar]
- 48.Carter Cancer Immunol Immunother. 2004;53:533. doi: 10.1007/s00262-003-0460-1. [DOI] [PMC free article] [PubMed] [Google Scholar]