Abstract
The coexistence of tumor progression with a tumor-specific immune response constitutes a major paradox of tumor immunity. During the last decade, the presence of cytotoxic T lymphocytes (CTLs) recognising melanoma-associated antigens has been unequivocally demonstrated in numerous different in vivo and in vitro models. However, most often these melanoma-specific T lymphocytes do not control tumor growth. Several mechanisms that involve changes in melanoma phenotype and/or in T-cell differentiation and function could explain the inability of the immune response to control melanoma. In the last few years it has been demonstrated that cellular cytotoxicity is the result of a balance between activating signals triggered by the TCR and costimulatory molecules and inhibitory signals triggered by inhibitory receptors expressed by the CTL. Because the final outcome of the immune response against melanoma depends on the balance between activating and inhibitory signals, the expression de novo on melanoma cells of ligands for inhibitory NKRs and the down-regulation of costimulatory molecules may favor the escape of tumor cells from immunosurveillance. In this paper we review how altered expression of molecules required for T-cell costimulation could result in impaired lysis of melanoma. The modulation of antimelanoma T-cell responses by a group of receptors originally described on NK cells (NK-associated receptors) but which are now known also to be expressed on a subset of cytolytic effector cells is reviewed. We hypothesize that the expression of ligands for NKRs on melanoma cells may contribute to T-cell–mediated immune responses against melanoma either enhancing or inhibiting activation and differentiation to effector cells. Blocking inhibitory receptors or increasing activating receptors could result in new strategies to improve T-cell–mediated rejection of melanoma.
Keywords: CD28, CD94/NKG2, Costimulation, KIR, NKR, Tumor escape, Tumor immunity
Introduction
Cytolytic lymphocytes play a pivotal role in protection from tumors. Several strategies have been developed to enhance tumor recognition, including induction of MHC molecules by cytokines. In the last decade, it has been reported that cytotoxic T-cell activation and effector function, upon recognition of specific peptides presented by MHC class I, may be regulated by the expression of natural killer (NK) cell–associated receptors (NKRs) specific for major histocompatibility complex (MHC) class I molecules [13]. NKRs were first identified on NK cells as a regulatory mechanism of cytotoxicity [47, 62, 63, 81, 88, 146]. Although most NKRs are not present on high percentages of T cells, a subpopulation of T cells that expresses those receptors and that corresponds to cells with a memory/effector phenotype has been identified [86]. The majority of T cells expressing NKRs are included within the CD8+CD28− T-cell subset, characterized by higher cytotoxic capacity, diminished proliferative response, shortened telomeres, and low levels of telomerase activity [116, 125]. The functional significance of the expression of ligands for NKRs and costimulatory molecules on melanoma cells and their correlation with their susceptibility to lysis by T lymphocytes and NK cells has not yet been fully established. In addition, the study of the role of several cytokines as modulators of NKRs and costimulatory molecule expression on both melanoma and lymphocytes may open new avenues for successful immunotherapy against melanoma.
Immune response against melanoma
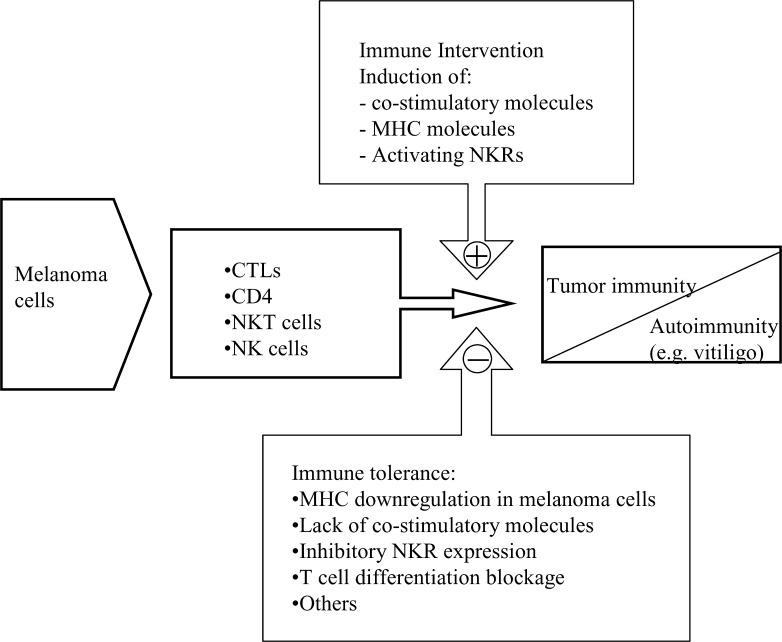
Different components of humoral and cellular immune responses are involved in the elimination of tumor cells, although apparently paradoxical, tumor immunity frequently coexists with tumor progression (Fig. 1). Even though cellular immune responses against tumors are generally mediated by T lymphocytes, the role of other components of the immune response such as NK cells, NKT cells, antibodies, or phagocytes in the elimination of melanoma cells has also been defined in different experimental models.
Fig. 1.
Immune responses against melanoma. Different cell types participate in the rejection of tumor cells leading to tumor immunity. A potential consequence of tumor immunity is autoimmunity. Immune intervention can enhance tumor immunity and overcome the immune tolerance status mediated by several escape mechanisms
Thus, antibodies against melanoma have been identified in many patients but their role in the antitumoral immune response has not been fully clarified. It has been reported that the induction of antibodies against melanoma antigens after vaccination may contribute to the therapeutic efficacy by a mechanism primarily involving antibody destruction of tumor cells by antibody-dependent cellular cytotoxicity (ADCC) through the engagement of Fc receptors [76]. Furthermore, antibody-coated tumor cells could also be killed by a process involving opsonization and phagocytosis. However, the role of macrophages in the immune response against melanoma is mainly related to their role as antigen-presenting cells and to their capacity to secrete several effector molecules such as tumor necrosis factor α (TNF-α) and interleukin (IL)-12 that may participate in melanoma rejection [54].
Natural killer (NK) cells are cytolytic cells that also contribute to the immune responses against tumors as they kill some cancer cells without prior sensitization and without a requirement for MHC restriction [95, 145]. NK cells are CD3-negative lymphocytes and, within NK cells, two subsets have been defined according to the level of expression of CD56. The CD56dim NK-cell subset is characterized by higher cytotoxic capacity than the CD56bright subset, which represents a minority subset and is characterized by the production of greater amounts of cytokines [24]. NK-cell cytotoxicity has been found to be diminished in patients with metastatic melanoma and could reflect the immunosuppressed state associated with advanced tumors [60]. NK cytotoxicity is controlled by the balance of activating and inhibitory signals mediated by different receptors some of which bind to MHC class I molecules on target cells [13, 47, 124, 146]. As will be discussed in this review, these receptors can play a significant role in tumor immunity because of their expression by a subset of cytotoxic T cells.
T-cell–mediated immune response against melanoma
Cellular immunity plays a pivotal role in the immune response against melanoma and an active T-cell–mediated immunity against melanoma-associated antigens has been demonstrated in different in vivo and in vitro models [102]. During the last decade, significant progress has been made in the identification and characterization of several MHC class I–restricted melanoma-associated antigens recognized by CTLs. These antigens belong to three main groups: cancer-testis–specific antigens (e.g., MAGE, BAGE, GAGE, PRAME, and NY-ESO-1), melanocyte differentiation antigens (e.g., tyrosinase, Melan-A/MART-1, gp100, TRP-1, and TRP-2), and mutated or aberrantly expressed antigens (e.g., MUM-1, CDK4, beta-catenin, gp100-in4, p15, and N-acetylglucosaminyl transferase V). CD4+ T cells can also recognize several MHC class II–restricted epitopes from melanoma antigens [46, 72, 128, 129, 131]. The discovery of new antigens recognized by T cells has allowed a better understanding of the cellular and molecular bases of the immune response against melanoma. Because many of these melanoma-associated antigens are self-proteins, T-cell effector function is frequently found to be weak or absent as a consequence of immune tolerance, which allows melanoma cells to escape from immune responses [35].
The development of MHC class I–peptide tetrameric complexes to study antigen-specific T cells ex vivo by flow cytometry has significantly increased our understanding of the immune response against melanoma antigens and has allowed the quantification and phenotypic characterization of specific CD8+ T cells [70, 94]. At the same time, the improvement of technology for the in vitro expansion of specific T cells allows the functional characterization of different T-cell subsets [17]. Such approaches have shown that different melanoma-associated antigens may stimulate a differential T-cell response with selective involvement of either CD4 or CD8 T cells [50].
Melanoma-specific T cells: differentiation from naïve to effector and memory cells
Differentiation of naïve CD8+ T cells toward effector/memory T cells is required for an effective immune response against melanoma. Frequently, CTLs from melanoma patients show a low cytotoxic capacity, and it has been postulated by several authors that this defect could be the consequence of an impaired maturation from memory/effector lymphocytes to terminally differentiated cytotoxic effector lymphocytes [2, 35, 70, 90].
T-cell differentiation from naïve to effector cytolytic T lymphocytes is an area of intense study by many investigators. Although no consensus on the transition from naïve to memory cells has yet been established, four different stages of maturation have been defined within CD8+ T cells according to their expression of CCR7 and CD45RA molecules [108]. Whereas CD8 naïve cells express both CCR7 and CD45RA, central memory cells are CCR7+CD45RA−, and effector memory cells CCR7−CD45RA−. A fourth stage of differentiation that constitutes terminally differentiated cytotoxic effector cells is defined by the lack of CCR7 expression, the reacquisition of CD45RA and large amounts of perforin [108]. The relationship between central memory and effector/memory T cells has been further analyzed in humans [7] and mice [130, 143]. In mice, a linear differentiation model has been proposed by Wherry et al. in which effector/memory cells are derived from effector cells, and central/memory cells are derived from effector/memory cells [143]. This model has not been confirmed in humans, and recent results by Baron et al. suggest that most effector memory and central memory cells are independently generated [7].
Different stages of T-cell maturation, from naïve (CD45RA+CCR7+) to terminally differentiated (CD45RA+CCR7−) CD8+ T cells, have been observed in melanoma-specific T cells obtained from patients [3, 56, 134]. Dunbar et al. [33] found that Melan-A–specific CD8+ T cells from melanoma patients may have a CCR7+CD45RA+ naïve phenotype associated with a defective immune response to Melan-A peptide ex vivo. In contrast, a group of melanoma patients with metastases were found to possess Melan-A–specific CD8+ T cells with a CCR7−CD45RO+CD45RA− effector memory phenotype and were able to respond to Melan-A peptide. Tyrosinase-specific T cells with an effector/memory phenotype have also been found in bone marrow from melanoma patients at similar or higher levels than in peripheral blood [71].
In melanoma patients, CD8+ T cells from tumor-free lymph nodes showed a naïve phenotype characterized mainly by the expression of CCR7 and CD45RA [3]. A similar phenotype was found in a large fraction of CD8+ T cells specific for melanocyte differentiation antigens or tumor-restricted antigens obtained from tumor-invaded lymph nodes. In contrast, preterminally differentiated CTLs (CCR7−CD45RA−CD27+CD28− perforin+) were observed in tumor-invaded lymph nodes from a small fraction of melanoma patients, but no evidence for tumor regression was found in these patients. This may suggest a lack of terminally differentiated CD8+ T cells [3, 90], but interestingly, in vitro treatment with IL-2 or IL-15 promoted differentiation to melanoma-specific effector T cells [3].
Antitumor activity of NKT cells
Recently, natural killer T (NKT) cells have been implicated in defense against tumors [26, 65, 66, 113]. NKT cells represent a novel T-cell lineage characterized by the restricted expression of an invariant TCR α chain encoded by Vα24/JαQ gene segments in humans and Vα14/Jα281 in mice. NKT cells constitute a small subpopulation of T cells (0.1–0.5% of peripheral blood lymphocytes) that have been frequently confused with NK cells because they share several phenotypic and functional characteristics [45]. Because NKT cells can express NKRs (e.g., CD161, CD56) it was also suggested that they could represent the subset of NKR+ cytotoxic T cells. However, detailed analysis of these cells has demonstrated that NKT and NKR+ cytotoxic T cells represent different T-cell subpopulations [29]. NKT cells react with CD1d and, after stimulation, produce high levels of IL-4 and interferon (INF)-γ [37]. The specific ligand of murine Vα14+ and human Vα24+ NKT cells is α-galactosylceramide (αGalCer) which is presented by the CD1d molecule [45]. Recently, a ganglioside named GD3 that is presented by CD1d molecules has been found to be expressed on several human tumors including melanoma. GD3 expression on normal tissues is low or absent. Furthermore, NKT cells from mice immunized with a human melanoma cell line expressing GD3 recognized human GD3 cross-presented by mouse APCs in a CD1d-restricted fashion, resulting in production of IL-4, IFN-γ, and IL-10 [144].
It has been observed that NKT cells exert protective immunity against melanoma in vivo and in vitro. In a murine model, dendritic cells pulsed with αGalCer have been shown to be effective against tumor development in vivo by means of activating Vα14 NKT cells and increasing their cytolytic capacity [64, 132]. Studies in vitro using human Vα24+ NKT cells from melanoma patients have shown that αGalCer-stimulated NKT cells did not display cytolytic activity against melanoma cell lines (autologous or allogeneic) but were able to suppress proliferation of melanoma cell lines cocultured with activated Vα24+ NKT cells. This effect was mediated by the induction of cytokine release (IFN-γ and to a lesser extent IL-12) rather than by direct cytotoxicity [66]. Together, these results indicate that αGalCer can induce antitumor effects that can be mediated by increased cytolytic activity or by cytokine release depending on the experimental model used.
Balance between activating and inhibitory signals
As mentioned above, the final outcome of T-cell–mediated immune responses against melanoma depends on a balance between activating and inhibitory signals. Activation of T lymphocytes requires two signals, the first mediated by T-cell receptor (TCR) recognition of the specific peptide in the context of an appropriate MHC molecule and the second mediated by costimulatory receptors on T cells that interact with their specific ligands on antigen-presenting cells or target cells. Interaction of CD28 on T cells with members of the B7 family (e.g., CD80 and CD86) on target cells delivers the second signal for an efficient T-cell activation after TCR-mediated recognition of the specific ligand [78]. In addition to CD28-B7, other receptors have recently been defined that also enhance T-cell responses and may be important in tumor immunity. Manipulation of the immune system through induction of costimulatory molecules could lead to enhanced immune responses against tumors [136].
NKRs expressed on T cells can mediate negative as well as positive signals, resulting in melanoma growth promotion or suppression, respectively. The role in tumor rejection of signals mediated by activating NKRs has not been characterized. In contrast, interaction of inhibitory NKRs on the effector cells with their ligands on tumor cells leads to inhibition of TCR-mediated cytotoxicity against melanoma [6, 137]. Modulation of this balance between activating and inhibitory signals invites a new strategy for enhancing effective immunity against melanoma.
Expression of costimulatory molecules by T cells from melanoma patients
Delivery of a costimulatory signal through engagement of CD28 on T cells by members of the B7 family (e.g., CD80 [B7.1], CD86 [B7.2]) on antigen-presenting cells is essential for full T-cell activation, potentially leading to tumor elimination. The outcome of T-cell costimulation through B7 molecules involves a combination of activating and inhibitory signals from CD28 and CTLA-4 molecules [20, 36, 112]. Although the expression of B7 family molecules in nonlymphoid tissues is mostly not constitutive, it is sometimes inducible by cytokines such as IFN-γ, IFN-α, IFN-β, or TNF-α [112]. It should be also considered that recognition of melanoma antigens by tumor-infiltrating lymphocytes induces the release of several cytokines such as granulocyte-macrophage colony-stimulating factor (GM-CSF), TNF-α, and IFN-γ that may have clinical relevance [49, 109].
Frequently, T-cell responses in melanoma patients are defective [70], and tumor-associated antigens expressed by melanoma cells are poorly immunogenic due, in part, to the lack of expression of costimulatory molecules by human melanoma cells [10, 19, 43, 48] that render melanoma-specific CTLs anergic in vivo [70]. Thus, it has been reported that the expression of CD80 and CD86 molecules in spontaneously regressing primary melanomas improved T-cell–mediated antitumor immunity, whereas these molecules were absent in metastatic melanoma and consequently favored the escape of melanoma cells from immune surveillance [30]. On the other hand, others have found no significant correlation between CD80 and CD86 expression on melanoma metastases and regression of the tumor, time to progression or survival [11].
It has also been reported that transfection of melanoma cells with the costimulatory molecules CD80 or CD86 improves immune responses mediated by both CD4+ and CD8+ T cells [14, 34, 74, 80]. Transfection of HLA-A*0201 melanoma cell lines with both the IL-2 and B7-1 (CD80) genes also resulted in an improved response by specific CTLs. Transfected melanoma cells displayed higher immunogenicity even with low antigen expression and were able to stimulate cytotoxicity and IFN-γ release by autologous and HLA-A*0201–compatible allogeneic T cells [79].
Although the CD28-CD80/CD86 and CTLA4-CD80/CD86 pathways of costimulatory and inhibitory signals are the best characterized, other new members of the B7/CD28 superfamily have recently been identified. One involves the molecule B7 h (also known as ICOSL, LICOS, GL50, B7RP-1, and B7H2) a member of the B7 family of costimulatory molecules, which interacts with inducible costimulatory molecule (ICOS). ICOS is expressed on activated T cells and it has been found that expression of B7 h on tumor cells enhanced CTL response in vivo [36, 75, 112, 139]. Recently, B7-H1 (PD-L1) has been identified as a new member of the B7 family of costimulatory molecules. B7-H1 molecules are involved in the negative regulation of the immune responses through engagement of the PD-1 (programmed death-1) receptor on activated T and B cells [18]. Interestingly, B7-H1 expression has been found in several tumors including melanomas. Furthermore, B7-H1 expression may be induced by IFN-γ treatment of tumor cell lines [32]. In vitro experiments showed that the interaction of B7-H1 with its ligand induces apoptosis of activated tumor-reactive T cells and may favor the growth of B7-1+ tumors in vivo [32]. The expression of B7-H1 on tumor cells can represent an escape mechanism from the T-cell immune response, and blockade of PD-1−B7-H1 interactions may provide new perspectives for tumor immunotherapy [55].
The human NK-like cell line YT-Indy constitutively expresses CD28 that triggers NK-mediated lysis of target cells expressing B7. By transfection of KIR2DL2 on YT-Indy cells we have analyzed the role of inhibitory NKRs on CD28-mediated lysis. Interaction of KIR2DL2 on YT-Indy cells with its ligand, HLA-Cw3, on target cells inhibited CD28-dependent cytotoxicity. This inhibition was blocked by the addition of anti-KIR2DL2 or anti-HLA class I mAb, whereas it was not affected by the addition of other control mAb [126]. CD28-mediated lysis of CD80-transfected melanoma cells by YT-Indy cells has also been observed and this effect was inhibited by interaction of the inhibitory receptor KIR2DL2 on YT-Indy with its ligand on melanoma cells (J.G. Casado et al., unpublished data).
Manipulation of costimulation through B7 molecule interactions with their different receptors on effector cells is therefore expected to provide a useful model not only to further our understanding of immune responses against melanoma but also for the development of new immunotherapy strategies.
Inhibitory and activating MHC class I–specific NK receptors on T cells in melanoma patients
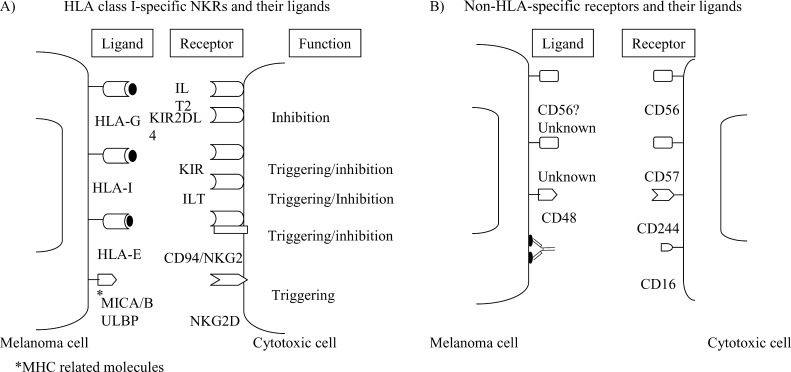
NKRs were firstly described in NK cells and in a subset of CTLs and play an important role in regulating cytotoxicity (for review, see [13]). In humans, three families of genes encode these HLA class I–specific receptors (Fig. 2A). The first family identified is called the killer cell immunoglobulin-like receptor (KIR) which consists of type I transmembrane molecules belonging to the immunoglobulin (Ig) superfamily, and interacts with HLA class I molecules to regulate NK- and T-cell function. KIRs with long cytoplasmic tails contain immunoreceptor tyrosine-based inhibitory motifs (ITIM) (e.g., KIR2DL and KIR3DL) and inhibit lysis of target cells expressing their MHC class I molecule ligands [87], and also inhibit cytokine production by the effector cells [27]. In contrast, KIR with short cytoplasmic tails (e.g., KIR2DS) can activate NK-cell cytotoxicity toward target cells expressing an appropriate MHC class I ligand [89] through interactions with the adaptor molecule DAP12, a molecule expressed as a homodimer that contains an immunoreceptor tyrosine-based activation motif (ITAM) [69]. A second group of receptors also belonging to the Ig superfamily are named immunoglobulin-like transcripts (ILTs) and are expressed predominantly in monocytes, NK cells, and also on a subpopulation T cells. KIR and ILT ligands include several classical HLA class I molecules and the nonclassical HLA-G molecule. C-type lectin family members constitute the third family of HLA-specific NK receptors and are composed of heterodimers of CD94 covalently associated with a member of the NKG2 family of molecules. The ligand for CD94/NKG2A (inhibitory) and CD94/NKG2C (activating) receptors is the nonclassical class I molecule HLA-E. The inhibitory members of this family contain ITIM in their cytoplasmic tails and the activating receptors associate with DAP12 [13]. It has been repeatedly demonstrated that KIR, ILT, and C-type lectin receptors (CD94:NKG2) can modulate T-cell cytotoxicity [86].
Fig. 2A,B.
Inhibitory and activating NKRs. Expression and function of human NKRs on cytotoxic cells and their potential ligands on melanoma cells. A HLA class I–specific receptors. B Non-HLA-specific receptors
Predominance of stimulatory signals mediated by activating NKRs has been observed in autoimmune diseases. Thus, CD4+ T cells from rheumatoid arthritis patients frequently lack the costimulatory molecule CD28 but have an increased expression of activating forms of KIRs (CD158j). These receptors were functional and enhanced IFN-γ production after T-cell receptor stimulation. These results suggest that activating KIRs in T cells function as costimulatory molecules and may contribute to the autoreactivity observed in rheumatoid arthritis [92, 114].
NKG2D is another C-type lectin-activating receptor that is expressed by both NK cells and CTLs in humans and by all NK cells and activated T cells in mice [103]. NKG2D recognizes MICA and MICB that are stress-induced cell-surface MHC class I–related molecules. Interestingly, MICA and MICB expression is frequently up-regulated on tumor cell lines and primary tumors of epithelial origin [110, 138], and shedding of MIC has been observed in epithelial tumors [39]. Expression of MIC has been recently reported in primary and metastatic melanomas, and NKG2D expression was also found in lymphocytes infiltrating the tumor [138]. NKG2D/MIC interaction induces endocytosis and degradation of NKG2D. Furthermore, defective expression of NKG2D has been linked to the presence of tumor-associated MIC that impairs NK- and T-cell–mediated immune responses and constitutes a new immune evasion mechanism [39]. NKG2D also recognizes a new family of MHC class I–related molecules named UL16-binding proteins (ULBPs). Four different ULBP proteins (ULBP1–4) have been identified so far and are characterized by their ability to bind to the cytomegalovirus (CMV) glycoprotein UL16 [25, 57, 119]. These results suggest that NKG2D/MIC interactions may be involved in NK-cell–mediated as well as T-cell–mediated cytotoxicity to melanoma cells [82, 99]. Furthermore, NKG2D-mediated NK-cell activation may be inhibited by the engagement of inhibitory NKRs [99].
The expression of classical MHC class I molecules on melanoma cells is required for TCR-mediated recognition by specific T cells and the down-regulation of HLA class I molecules in melanoma cells is an important tumor escape mechanism (for review, see Garrido et al. in this Symposium in Writing). Furthermore, although its in vivo relevance has not been defined, the engagement of MHC class I–specific activating receptors might enhance T-cell–mediated immune responses against melanoma cells.
In contrast, as discussed above, MHC class I molecules on melanoma cells can also be recognized by inhibitory receptors that would block T-cell activation and then favor tumor escape. The analysis on tumor cells of HLA-A, HLA-B, HLA-C, and nonclassical MHC molecules such as HLA-E and HLA-G that are recognized by KIR, CD94/NKG2, and ILT receptors, respectively, has been limited by the absence of monoclonal antibodies. Even though MHC class I down-regulation in tumor cells is a well-known escape mechanism to avoid T-cell recognition, frequently tumor cells are also resistant to NK-mediated lysis and the expression of nonclassical HLA molecules recognized by inhibitory receptors has been postulated to be involved in this process.
In a recent study, the expression of HLA-E molecules was analyzed in a panel of tumor cells. The expression of HLA-E on the cell surface of tumor cell lines correlated inversely with the expression of other HLA class I molecules and was related to the availability of β2-microglobulin. Thus, HLA-E expression could be induced in an HLA class I–negative melanoma cell line by transfection of the β2-microglobulin gene [77]. Therefore, in contrast with total loss of HLA class I molecules, partial loss of HLA-A, HLA-B, or HLA-C class I molecules (allelic or haplotype HLA loss) may at the same time inhibit CTL responses by deleting the restriction element and also inhibit NK-cell–mediated cytotoxicity by expressing HLA-E molecules. Furthermore, HLA-E expression on tumor cells could inhibit specific CTL-mediated cytotoxicity by interaction with inhibitory NKRs (CD94/NKG2A) [77].
An increased expression of NKRs, including HLA-specific receptors, on CD8+ T lymphocytes has been observed in several clinical conditions that involve chronic activation of the immune system (e.g., HIV infection, as well as tumor patients) [86, 116, 125]. Furthermore, the expression of NKRs in T cells has been associated with cells that have undergone a process of replicative senescence after multiple rounds of cell division probably as a consequence of chronic stimulation [15, 124].
Recently, the expression of several NKRs on T cells has been analyzed in melanoma patients. However, the proportion of NKR+ T cells showed great variation between individuals, and it was therefore difficult to establish significant differences compared with healthy donors [115, 116]. The functional capabilities of NKRs expressed on T cells from melanoma patients have been assessed against melanoma cells. Thus, HLA-specific inhibitory NKRs have been shown to inhibit lysis of melanoma cells mediated by specific CTLs transfected with the inhibitory receptor KIR3DL1 [6]. It has also been reported that melanoma-specific CTLs can express inhibitory NKRs in vivo that impair their cytolytic activity [51, 53, 98, 115].
Ikeda et al. found the expression of an inhibitory NKR (KIR2DL2) on melanoma-specific CTLs that was able to inhibit cytolysis upon interaction with its ligand (HLA-Cw7) on melanoma cells [53]. The expression of C-type lectin-like NKRs has been also analyzed in melanoma patients. Triggering of CD94/NKG2A inhibitory receptor by its ligand on melanoma cells induced inhibition of CTL-mediated lysis of melanoma cells [115]. Both inhibitory and activating forms of CD94/NKG2 receptors have been found in MART-1–specific T cells in different areas of a primary melanoma. Inhibitory CD94/NKG2-A receptors were found exclusively in the vitiligo-like areas, whereas both the inhibitory receptors and the activating CD94/NKG2-C isoforms were present within the tumor [98].
In vitro cultures of CD8+ T cells primed with melanocyte differentiation antigens, showed that a fraction of CD8+ T cells from healthy donors expressed both activating and inhibitory forms of NKRs, but only inhibitory receptors display functional activity inhibiting effector functions of CD8+ T cells [51]. Furthermore, phenotypic characterization of melanoma-specific CD8+ T lymphocytes expanded in vitro has allowed the identification of changes in several NKRs after stimulation [51, 117] (Casado et al. unpublished results).
We have previously discussed NKR expression as effector molecules that may trigger or inhibit CD8+ T-cell function [86, 124]. However, recent data also support the notion that NKR expression may correlate with the differentiation stage of T cells [147]. Although NKR expression is preferentially observed in the CD8+CD28− T-cell subset, we have found that other subsets of CD28+ T lymphocytes can also express NKRs [19], and we suggest that these subsets correspond to intermediate maturation stages from “naïve” to cytolytic effector cells. Thus, a differential expression of KIR and ILT2 receptors in CTLs that correlates with transition from effector to memory lymphocytes has been recently demonstrated [147]. KIR expression is associated with acquisition of a memory phenotype and resistance to apoptosis induced by antigenic activation. In contrast, ILT2 expression correlates with effector cytotoxic cells. Recently, KIR [133] and CD94/NKG2A [42] expression has also been associated with the survival of memory CD8+ T lymphocytes. The correlation of other NKRs with resistance to apoptosis remains to be clarified, and new research on the development of specific T cells will help to characterize the role of NKRs in the transition from immature to cytotoxic effector T cells during chronic activation of the immune system. Furthermore, characterization of phenotypic and functional changes in pathological conditions such as those of melanoma patients will allow us to identify new markers of T-cell differentiation as targets for possible immunotherapies.
Expression of non-MHC-specific NK receptors on T cells in melanoma patients
Several receptors, usually expressed on NK cells, recognizing ligands others than MHC class I molecules, are also expressed by a subpopulation of T cells and, to some extent, may contribute to cytotoxicity or other effector functions (Fig. 2B). One of these receptors is CD56, an isoform of the neuronal cell–adhesion molecule (NCAM), that is involved in homotypic and heterotypic binding of cytotoxic cells to target cells, although its precise function, either on NK cells or on T cells, remains elusive [68]. CD56 expression on NK cells defines two different subsets according to the cell-surface density of this marker, CD56dim and CD56bright NK cells, with differential phenotype and function [24, 96, 127]. In contrast to NK cells, CD56 expression on T cells is not constitutive. It has been observed that CD56+ T cells are almost absent at birth and in adults are found as oligoclonal expansions [101]. Cord blood CD3+ cells can acquire CD56 expression after culture with IL-15 and the expanded CD3+CD56+ T cells have a CD8+CD25+ IFN-γ+ phenotype, with 40% being γδ T cells [23]. Furthermore, the percentage of CD8+CD56+ T cells increased with age (DelaRosa, unpublished results) but is diminished in HIV infection [125]. CD8+CD56+ T cells generally contain high amounts of intracellular perforin and granzyme B, and CD56 expression on CD8+ T cells correlates with cytolytic activity [101]. The finding that CD8+CD28−DR− T cells have low telomerase activity and frequently express CD56 [118] further supports the idea that CD56+ T cells have an effector/memory phenotype. CD56 expression has been demonstrated in several tumors of the nervous system, melanomas, and other epithelial cancers suggesting that CD56 molecules may be involved in tumor biology [52, 105]. CD56-mediated homotypic adhesion between CD56+ tumor cell lines and NK cells has been shown [93, 122].
CD57 is an oligosaccharide found on many cell-surface glycoproteins that has been implicated in cell-to-cell and cell-to-extracellular matrix adhesion. CD57 is preferentially expressed in NK cells and in a subset of T cells. The expansion of the CD8+CD28−CD57+ T-cell subset is age-associated probably due to repeated antigenic stimulation [83]. Several studies report the association between viral infections (e.g., human cytomegalovirus) and increased numbers of CD8+CD57+ T cells [107, 142]. Furthermore, CD57 expression on virus-specific CD8+ T cells is related to a state of replicative senescence with low proliferative capacity and short telomeres [15]. CD57+ T cells are also frequently found in tumor patients, generally as a monoclonal or oligoclonal expansions, have a low rate of turnover and low expression of the apoptotic marker CD95 [120], and exert higher cytotoxic activity ex vivo than their CD57– counterparts in an anti-CD3-redirected assay [135]. It has also been found that most NKR+ T cells are included within the CD28−CD57+ subset [84]. This CD57+ T-cell subset comprises the majority of the CD56+ T cells previously described, which represent differentiated effector T cells [101]. Glycoproteins containing carbohydrate epitopes recognized by antibodies against CD57 can also be found in several tumors including melanoma. Moreover, CD57 expression on melanoma cells has been associated with metastatic potential and seems to have a function in intercellular adhesion [58, 123].
CD244 (2B4 or C1.7) is a cell-surface glycoprotein related to CD2 that belongs to the Ig-like superfamily and is involved in T- and NK-cell activation [21, 59]. CD244 is expressed on the surface of all human NK cells, a subset of CD8+ T cells, monocytes, and basophils. In NK cells, CD244 ligation can induce redirected lysis [21, 67]. It has been reported that CD244 expression on CD8+ T cells overlaps with activation markers such as granzyme B and perforin [117]. CD244 expression can be induced in vitro on CD8+ T cells by anti-CD3 cross-linking, and its expression constitutes a marker for activated/memory CD8+ T cells [100]. Furthermore, recent studies show that CD244 activation causes an increased expression of matrix metalloproteinase-2 and may induce degradation of extracellular matrices promoting lymphocyte invasion of tumors [21]. CD48, the ligand for CD244, is a ubiquitously expressed molecule in humans [16, 91]. Until now, expression of CD48 has not been reported in melanoma cells; however, it has recently been shown that CD244-CD48 interactions can occur directly between T cells and induce proliferative responses of neighboring T cells [61]. Furthermore, transfection of CD48 into poorly immunogenic melanoma cells enhances the immune response [73]. In melanoma patients, CD244 was found to be up-regulated in Melan-A–specific T cells, although its role in promoting T-cell effector function has not been established [117]. In addition, inhibitory receptors as KIR2DL1 or CD94/NKG2 can block CD244 activation [141].
CD16 is a low-affinity Fc receptor for IgG (FcγRIII) that mediates ADCC by binding to the Fc portion of antibodies. CD16 is mainly expressed in a subset of NK cells (CD56dim) and in a small subpopulation of CD3+ lymphocytes. Ligation of CD16 stimulates not only cytotoxicity but also cytokine production [1] and proliferation [140]. In animal models, CD16 has been involved in both passive and active immunization against melanoma [22].
CD161 (NKRP1) belongs to the C-type lectin family and is expressed by NK cells and a subpopulation of T cells. While the expression of the majority of NKRs on T cells is essentially restricted to CD8+ T cells, CD161 is expressed on both CD4 and CD8 T cells. Moreover, expression of CD161 is a characteristic of NKT cells [121, 124]. CD161 cross-linking triggers cytotoxicity. Although its ligand remains to be identified, it has been postulated that this receptor recognizes carbohydrate structures expressed on target cells [4].
Natural cytotoxicity receptors (NCRs) expressed in NK cells have recently been identified. These receptors belong to the Ig-superfamily but their ligands are not HLA class I molecules. NKp46, NKp44, and NKp30 were the first members identified and are characterized by their capacity to trigger natural cytotoxicity. NCRs associate with signal-transducing polypeptides containing ITAM. The expression of NCR seems to be restricted to NK cells and, so far, none of these receptors has been identified in T cells.
Several groups, including our own, have found that the expression of CD56, CD57, CD244, or CD16 in T cells is associated with an activated/effector phenotype [28, 38, 101, 116, 117]. NKR expression is mainly restricted to the CD8+CD28−CD56+ T-cell subset that represents the mature effector cytolytic subset responsible for the immune response against viral infection and tumors [116, 125].
Different studies show that several cytokines may modulate the expression of HLA-specific NKRs on T lymphocytes. Thus, TGF-β [12, 41], IL-10 [106], and IL-15 [85] have been implicated in the induction of CD94/NKG2A receptors on T cells. Thus, IL-15 produced by melanoma cells induces the expression of inhibitory NKRs on specific T cells and may constitute a new escape mechanism [8]. Recently, the induction of CD94/NKG2A receptors has also been observed after IL-12 treatment. Because IL-12 is frequently used for the in vitro induction of antigen-specific T cells, the expression of the inhibitory receptor CD94/NKG2A may have functional relevance [31]. In contrast, expression of the triggering receptor CD161 is also up-regulated by IL-12 [5].
Conclusions
A major paradox of tumor immunity is that tumor progression frequently, if not always, coexists with tumor-specific immune responses (Fig. 1). Numerous studies have been performed to investigate possible tumor escape mechanisms from immunosurveillance. Thus, MHC class I down-regulation by tumor cells represents a well-known major evasion mechanism. Expression of HLA class I has been found to be altered in human melanoma, including total absence of HLA class I, loss of expression of particular allotypes, or loss of heterozygosity (LOH) leading to an HLA haplotype loss [97, 104].
Total loss of HLA class I molecules would interfere with CTL recognition but would render melanoma cells susceptible to NK-cell–mediated cytotoxicity. A recent report correlates inversely the expression of HLA-E on the cell surface of tumor cell lines with the expression of other HLA class I molecules [77]. Thus, partial loss of HLA class I molecules (allelic or haplotype HLA loss) may inhibit CTL responses if the restriction element is lost and may favor the expression of HLA-E molecules that can be recognized by inhibitory NKRs (CD94/NKG2A) on T cells or NK cells.
The expression of surface molecules on melanoma cells, such as adhesion molecules (e.g., intercellular adhesion molecule 1 [ICAM-1]) has been associated with aggressive tumors and defective immune responses. ICAM-1 shedding by melanoma cells can inhibit conjugate formation between T-cell clones and the autologous melanoma cells and abrogated the MHC-restricted killing of the melanoma by T-cell clones. In addition, melanoma cells induced anergy of CD4+ T cells due to lack of a costimulatory signal mediated by B7/CD28 interactions [9].
Many of the antigens presented by melanoma cells are unaltered self-proteins and may induce autotolerance. Several strategies have been developed to overcome poor immunogenicity and tumor evasion of CTL-mediated destruction. It has been observed that induction of HLA class I expression on melanoma cells can restore specific CTL responses [111]. In contrast, increased HLA I expression in melanoma cells could induce the activation of HLA-specific inhibitory NKRs leading to inhibition of lysis both in NK cells and T cells. On the other hand, activating HLA-specific receptors could be expressed both in NK cells and T cells. The final balance between activating and inhibitory signals will generate an effective immune response or tumor progression.
Loss of costimulatory molecules [19, 43, 44] or defective function of TCR signaling molecules has been reported in melanoma patients [40, 148]. The induction of several costimulatory molecules has been also attempted in order to increase tumor immunity against melanoma cells. Transfection of melanoma cells with CD80/CD86 or CD48 molecules increases tumor recognition by CTLs. It is interesting to consider that NKRs can also inhibit CD28/CD80 and CD244/CD48 interactions [126, 141].
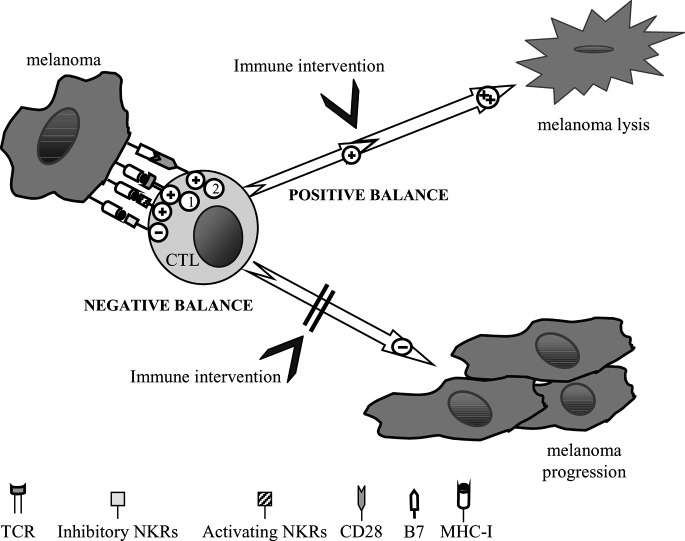
Studies on new antigenic determinants capable of generating adequate tumor immunity constitute a priority area in melanoma research. In this context, the expression on melanoma cells of ligands for inhibitory NKRs expressed on effector T cells may represent an escape mechanism used by these tumor cells. In contrast, the expression of ligands for activating or costimulatory receptors may contribute to the efficient recognition and killing of melanoma cells (Fig. 3). Blocking inhibitory receptor function and enhancing activating receptors offers a new strategy with therapeutic potential.
Fig. 3.
Inhibitory/activating balance. Different receptors participate in the immune response against melanoma mediated by CTLs. Positive signals mediated by TCR and costimulatory molecules may be enhanced by the expression of activating NKRs on CTLs and their ligands on melanoma. By contrast, inhibitory signals triggered by inhibitory NKRs after interaction with their ligands will allow melanoma escape from immunosurveillance
Acknowledgements
This work was supported by grants QLRT-2001-00668 (Outcome and Impact of Specific Treatment in European Research on Melanoma, OISTER) and QLK6-CT2002-02283 (T cells in Ageing, T-CIA) from the 5th Framework Program of the European Union, FIS01/0478, FIS03/1383 (to R.S.); FIS00/0853 (to R.T.) from the Ministry of Health; SAF2003-05184 (to R.T.) from the Ministry of Science and Technology; and 03/2 (to R.T.) from the “Consejería de Sanidad y Consumo” Junta de Extremadura (Spain).
Abbreviations
- CTL
cytotoxic T lymphocyte
- Ig
immunoglobulin
- ILT
immunoglobulin-like transcripts
- ITAM
immunoreceptor tyrosine-based activation motif
- ITIM
immunoreceptor tyrosine-based inhibition motif
- KIR
killer cell immunoglobulin-like receptors
- NK cell
natural killer cell
- NKR
natural killer cell–associated receptor
- TCR
T-cell receptor
Footnotes
This article forms part of the Symposium in Writing “Tumor escape from the immune response,” published in Vol. 53
References
- 1.Anegon J Exp Med. 1988;167:452. doi: 10.1084/jem.167.2.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anichini J Exp Med. 1999;190:651. doi: 10.1084/jem.190.5.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anichini J Immunol. 2003;171:2134. doi: 10.4049/jimmunol.171.4.2134. [DOI] [PubMed] [Google Scholar]
- 4.Arase J Exp Med. 1997;186:1957. doi: 10.1084/jem.186.12.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Azzoni J Immunol. 1998;161:3493. [PubMed] [Google Scholar]
- 6.Bakker J Immunol. 1998;160:5239. [PubMed] [Google Scholar]
- 7.Baron Immunity. 2003;18:193. [Google Scholar]
- 8.Barzegar Oncogene. 1998;16:2503. doi: 10.1038/sj.onc.1201775. [DOI] [PubMed] [Google Scholar]
- 9.Becker Recent Results Cancer Res. 1995;139:205. doi: 10.1007/978-3-642-78771-3_15. [DOI] [PubMed] [Google Scholar]
- 10.Becker Int Immunol. 1993;5:1501. doi: 10.1093/intimm/5.12.1501. [DOI] [PubMed] [Google Scholar]
- 11.Bernsen Br J Cancer. 2003;88:424. doi: 10.1038/sj.bjc.6600703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bertone Eur J Immunol. 1999;29:23. doi: 10.1002/(SICI)1521-4141(199901)29:01<23::AID-IMMU23>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 13.Borrego Mol Immunol. 2002;38:637. doi: 10.1016/s0161-5890(01)00107-9. [DOI] [PubMed] [Google Scholar]
- 14.Brady J Immunother. 2000;23:353. doi: 10.1097/00002371-200005000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Brenchley Blood. 2003;101:2711. doi: 10.1182/blood-2002-07-2103. [DOI] [PubMed] [Google Scholar]
- 16.Brown J Exp Med. 1998;188:2083. doi: 10.1084/jem.188.11.2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bullock J Immunol. 2001;167:5824. doi: 10.4049/jimmunol.167.10.5824. [DOI] [PubMed] [Google Scholar]
- 18.Carreno Annu Rev Immunol. 2002;20:29. doi: 10.1146/annurev.immunol.20.091101.091806. [DOI] [PubMed] [Google Scholar]
- 19.Casado JG, DelaRosa O, Peralbo E, Tarazona R, Solana R (2003) CD28 downregulation and expression of NK associated receptors on T cells in aging and situations of chronic activation of the immune system. In: Pawelec G (ed) Basic biology and clinical impact of immunosenescence. Elsevier, Amsterdam, p 123
- 20.Chambers Annu Rev Immunol. 2001;19:565. doi: 10.1146/annurev.immunol.19.1.565. [DOI] [PubMed] [Google Scholar]
- 21.Chuang Immunology. 2000;100:378. doi: 10.1046/j.1365-2567.2000.00031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clynes Proc Natl Acad Sci U S A. 1998;95:652. [Google Scholar]
- 23.Cookson Blood. 2003;102:2195. doi: 10.1182/blood-2003-01-0232. [DOI] [PubMed] [Google Scholar]
- 24.Cooper Trends Immunol. 2001;22:633. doi: 10.1016/s1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- 25.Cosman Immunity. 2001;14:123. [Google Scholar]
- 26.Cui Science. 1997;278:1623. doi: 10.1126/science.278.5343.1623. [DOI] [PubMed] [Google Scholar]
- 27.D’Andrea J Exp Med. 1996;184:789. doi: 10.1084/jem.184.2.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Hum Immunol. 2000;61:74. [Google Scholar]
- 29.DelaRosa Exp Gerontol. 2002;37:213. doi: 10.1016/s0531-5565(01)00186-3. [DOI] [PubMed] [Google Scholar]
- 30.Denfeld Int J Cancer. 1995;62:259. doi: 10.1002/ijc.2910620305. [DOI] [PubMed] [Google Scholar]
- 31.Derre J Immunol. 2002;168:4864. doi: 10.4049/jimmunol.168.10.4864. [DOI] [PubMed] [Google Scholar]
- 32.Dong Nat Med. 2002;8:793. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 33.Dunbar J Immunol. 2000;165:6644. doi: 10.4049/jimmunol.165.11.6644. [DOI] [PubMed] [Google Scholar]
- 34.Fenton J Immunother. 1998;21:95. [PubMed] [Google Scholar]
- 35.Ferrone Immunol Today. 2000;21:70. doi: 10.1016/s0167-5699(99)01569-8. [DOI] [PubMed] [Google Scholar]
- 36.Frauwirth J Clin Invest. 2002;109:295. doi: 10.1172/JCI14941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Godfrey Immunol Today. 2000;21:573. doi: 10.1016/s0167-5699(00)01735-7. [DOI] [PubMed] [Google Scholar]
- 38.Gritzapis Cancer Immunol Immunother. 2002;51:440. doi: 10.1007/s00262-002-0298-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Groh Nature. 2002;419:734. doi: 10.1038/nature01112. [DOI] [PubMed] [Google Scholar]
- 40.Guarini Blood. 1997;89:212. [PubMed] [Google Scholar]
- 41.Guerra Eur Cytokine Netw. 1999;10:357. [PubMed] [Google Scholar]
- 42.Gunturi J Immunol. 2003;170:1737. doi: 10.4049/jimmunol.170.4.1737. [DOI] [PubMed] [Google Scholar]
- 43.Hakansson Cancer Immunol Immunother. 1999;48:253. doi: 10.1007/s002620050573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hakansson Cancer Immunol Immunother. 2002;51:499. doi: 10.1007/s00262-002-0304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hammond Eur J Immunol. 1999;29:3768. doi: 10.1002/(SICI)1521-4141(199911)29:11<3768::AID-IMMU3768>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 46.Heinzel Cancer Immunol Immunother. 2001;49:671. doi: 10.1007/s002620000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Held Curr Opin Immunol. 2003;15:233. doi: 10.1016/s0952-7915(02)00031-6. [DOI] [PubMed] [Google Scholar]
- 48.Hersey Int J Cancer. 1994;58:527. doi: 10.1002/ijc.2910580413. [DOI] [PubMed] [Google Scholar]
- 49.Hom J Immunother. 1993;13:18. [Google Scholar]
- 50.Houghton Curr Opin Immunol. 2001;13:134. doi: 10.1016/s0952-7915(00)00195-3. [DOI] [PubMed] [Google Scholar]
- 51.Huard Eur J Immunol. 2000;30:1665. doi: 10.1002/1521-4141(200006)30:6<1665::AID-IMMU1665>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 52.Huttenbach J Cutan Pathol. 2002;29:562. doi: 10.1034/j.1600-0560.2002.290909.x. [DOI] [PubMed] [Google Scholar]
- 53.Ikeda Immunity. 1997;6:199. doi: 10.1016/s1074-7613(00)80426-4. [DOI] [PubMed] [Google Scholar]
- 54.Imaizumi Am J Physiol. 1999;277:L49. doi: 10.1152/ajplung.1999.277.1.L49. [DOI] [PubMed] [Google Scholar]
- 55.Iwai Proc Natl Acad Sci U S A. 2002;99:12293. doi: 10.1073/pnas.192461099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jager Int J Cancer. 2002;98:376. doi: 10.1002/ijc.10165. [DOI] [PubMed] [Google Scholar]
- 57.Jan Biochem Biophys Res Commun. 2003;305:129. [Google Scholar]
- 58.Jaques Biochem J. 1996;316:427. doi: 10.1042/bj3160427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Johnson In Vivo. 2000;14:625. [Google Scholar]
- 60.Jovic Tumori. 2001;87:324. doi: 10.1177/030089160108700509. [DOI] [PubMed] [Google Scholar]
- 61.Kambayashi J Immunol. 2001;167:6706. doi: 10.4049/jimmunol.167.12.6706. [DOI] [PubMed] [Google Scholar]
- 62.Karlhofer Nature. 1992;358:66. doi: 10.1038/358066a0. [DOI] [PubMed] [Google Scholar]
- 63.Karre Nature. 1986;319:675. doi: 10.1038/319675a0. [DOI] [PubMed] [Google Scholar]
- 64.Kawano Proc Natl Acad Sci U S A. 1998;95:5690. [Google Scholar]
- 65.Kawano Cancer Res. 1999;59:5102. [PubMed] [Google Scholar]
- 66.Kikuchi Br J Cancer. 2001;85:741. doi: 10.1054/bjoc.2001.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kubin Eur J Immunol. 1999;29:3466. doi: 10.1002/(SICI)1521-4141(199911)29:11<3466::AID-IMMU3466>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 68.Lanier J Immunol. 1991;146:4421. [PubMed] [Google Scholar]
- 69.Lanier Nature. 1998;391:703. doi: 10.1038/35642. [DOI] [PubMed] [Google Scholar]
- 70.Lee Nat Med. 1999;5:677. doi: 10.1038/9525. [DOI] [PubMed] [Google Scholar]
- 71.Letsch Cancer Res. 2003;63:5582. [PubMed] [Google Scholar]
- 72.Li Cancer Immunol Immunother. 1998;47:32. doi: 10.1007/s002620050501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li J Exp Med. 1996;183:639. doi: 10.1084/jem.183.2.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li J Immunol. 1994;153:421. [Google Scholar]
- 75.Liu J Exp Med. 2001;194:1339. doi: 10.1084/jem.194.9.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Maio Cancer Immunol Immunother. 2002;51:9. [Google Scholar]
- 77.Marin Immunogenetics. 2003;54:767. doi: 10.1007/s00251-002-0526-9. [DOI] [PubMed] [Google Scholar]
- 78.Marincola Trends Immunol. 2003;24:335. doi: 10.1016/s1471-4906(03)00116-9. [DOI] [PubMed] [Google Scholar]
- 79.Mazzocchi Cancer Immunol Immunother. 2001;50:199. doi: 10.1007/PL00006687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.McCarthy Cancer Immunol Immunother. 2000;49:85. doi: 10.1007/s002620050606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.McQueen Curr Opin Immunol. 2002;14:615. doi: 10.1016/s0952-7915(02)00380-1. [DOI] [PubMed] [Google Scholar]
- 82.Menier Int J Cancer. 2002;100:63. doi: 10.1002/ijc.10460. [DOI] [PubMed] [Google Scholar]
- 83.Merino Clin Exp Immunol. 1998;112:48. doi: 10.1046/j.1365-2249.1998.00551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mingari Proc Natl Acad Sci U S A. 1996;93:12433. [Google Scholar]
- 85.Mingari Proc Natl Acad Sci U S A. 1998;95:1172. [Google Scholar]
- 86.Mingari Hum Immunol. 2000;61:44. doi: 10.1016/s0198-8859(99)00158-5. [DOI] [PubMed] [Google Scholar]
- 87.Moretta Curr Opin Immunol. 1997;9:694. doi: 10.1016/s0952-7915(97)80051-9. [DOI] [PubMed] [Google Scholar]
- 88.Moretta J Exp Med. 1993;178:597. doi: 10.1084/jem.178.2.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Moretta J Exp Med. 1995;182:875. doi: 10.1084/jem.182.3.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mortarini Cancer Res. 2003;63:2535. [PubMed] [Google Scholar]
- 91.Nakajima Eur J Immunol. 1999;29:1676. doi: 10.1002/(SICI)1521-4141(199905)29:05<1676::AID-IMMU1676>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 92.Namekawa J Immunol. 2000;165:1138. doi: 10.4049/jimmunol.165.2.1138. [DOI] [PubMed] [Google Scholar]
- 93.Nitta J Exp Med. 1989;170:1757. doi: 10.1084/jem.170.5.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Palmowski Immunol Rev. 2002;188:155. doi: 10.1034/j.1600-065x.2002.18814.x. [DOI] [PubMed] [Google Scholar]
- 95.Park J Immunol. 2003;170:1197. [Google Scholar]
- 96.Parrish-Novak Nature. 2000;408:57. doi: 10.1038/35040504. [DOI] [PubMed] [Google Scholar]
- 97.Paschen Int J Cancer. 2003;103:759. doi: 10.1002/ijc.10906. [DOI] [PubMed] [Google Scholar]
- 98.Pedersen J Invest Dermatol. 2002;118:595. doi: 10.1046/j.1523-1747.2002.01698.x. [DOI] [PubMed] [Google Scholar]
- 99.Pende Eur J Immunol. 2001;31:1076. doi: 10.1002/1521-4141(200104)31:4<1076::aid-immu1076>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 100.Peritt J Immunol. 1999;162:7563. [PubMed] [Google Scholar]
- 101.Pittet J Immunol. 2000;164:1148. [Google Scholar]
- 102.Ramirez-Montagut Oncogene. 2003;22:3180. doi: 10.1038/sj.onc.1206462. [DOI] [PubMed] [Google Scholar]
- 103.Raulet Nat Rev Immunol. 2003;3:781. doi: 10.1038/nri1199. [DOI] [PubMed] [Google Scholar]
- 104.Real Cancer Immunol Immunother. 2001;49:621. doi: 10.1007/s002620000154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Reed Am J Pathol. 1999;155:549. doi: 10.1016/S0002-9440(10)65150-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Romero J Leukoc Biol. 2001;70:219. [PubMed] [Google Scholar]
- 107.Rowbottom Br J Haematol. 2000;110:315. doi: 10.1046/j.1365-2141.2000.02107.x. [DOI] [PubMed] [Google Scholar]
- 108.Sallusto Nature. 1999;401:708. [Google Scholar]
- 109.Schwartzentruber J Immunol. 1991;146:3674. [PubMed] [Google Scholar]
- 110.Seliger Trends Immunol. 2003;24:82. doi: 10.1016/s1471-4906(02)00039-x. [DOI] [PubMed] [Google Scholar]
- 111.Serrano Int J Cancer. 2001;94:243. doi: 10.1002/ijc.1452. [DOI] [PubMed] [Google Scholar]
- 112.Sharpe Nature Rev Immunol. 2002;2:116. doi: 10.1038/nri727. [DOI] [PubMed] [Google Scholar]
- 113.Smyth J Exp Med. 2000;191:661. doi: 10.1084/jem.191.4.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Snyder J Exp Med. 2003;197:437. doi: 10.1084/jem.20020383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Speiser J Exp Med. 1999;190:775. doi: 10.1084/jem.190.6.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Speiser Eur J Immunol. 1999;29:1990. doi: 10.1002/(SICI)1521-4141(199906)29:06<1990::AID-IMMU1990>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 117.Speiser J Immunol. 2001;167:6165. doi: 10.4049/jimmunol.167.11.6165. [DOI] [PubMed] [Google Scholar]
- 118.Speiser Eur J Immunol. 2001;31:459. doi: 10.1002/1521-4141(200102)31:2<459::aid-immu459>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 119.Sutherland J Immunol. 2002;168:671. doi: 10.4049/jimmunol.168.2.671. [DOI] [PubMed] [Google Scholar]
- 120.Sze Blood. 2001;98:2817. doi: 10.1182/blood.v98.9.2817. [DOI] [PubMed] [Google Scholar]
- 121.Takahashi J Immunol. 2000;164:4458. [Google Scholar]
- 122.Takasaki Cell Biol Int. 2000;24:101. doi: 10.1006/cbir.1999.0457. [DOI] [PubMed] [Google Scholar]
- 123.Tang Melanoma Res. 1996;6:411. doi: 10.1097/00008390-199612000-00002. [DOI] [PubMed] [Google Scholar]
- 124.Tarazona Mech Ageing Dev. 2000;121:77. doi: 10.1016/s0047-6374(00)00199-8. [DOI] [PubMed] [Google Scholar]
- 125.Tarazona AIDS. 2002;16:197. [Google Scholar]
- 126.Tarazona Mol Immunol. 2002;38:495. doi: 10.1016/s0161-5890(01)00092-x. [DOI] [PubMed] [Google Scholar]
- 127.Tarazona J Clin Immunol. 2002;22:176. doi: 10.1023/a:1015476114409. [DOI] [PubMed] [Google Scholar]
- 128.Topalian Proc Natl Acad Sci U S A. 1994;91:9461. doi: 10.1073/pnas.91.20.9461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Topalian J Exp Med. 1996;183:1965. doi: 10.1084/jem.183.5.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tough Trends Immunol. 2003;24:404. doi: 10.1016/s1471-4906(03)00169-8. [DOI] [PubMed] [Google Scholar]
- 131.Touloukian J Immunol. 2000;164:3535. [Google Scholar]
- 132.Toura J Immunol. 1999;163:2387. [PubMed] [Google Scholar]
- 133.Ugolini Nat Immunol. 2001;2:430. doi: 10.1038/87740. [DOI] [PubMed] [Google Scholar]
- 134.Valmori Cancer Res. 2002;62:1743. [PubMed] [Google Scholar]
- 135.Van Leukemia. 1998;12:1573. doi: 10.1038/sj.leu.2401146. [DOI] [PubMed] [Google Scholar]
- 136.Vesosky Cancer Immunol Immunother. 2003;52:663. doi: 10.1007/s00262-003-0424-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Vetter J Invest Dermatol. 2000;114:941. doi: 10.1046/j.1523-1747.2000.00958.x. [DOI] [PubMed] [Google Scholar]
- 138.Vetter J Invest Dermatol. 2002;118:600. doi: 10.1046/j.1523-1747.2002.01700.x. [DOI] [PubMed] [Google Scholar]
- 139.Wallin J Immunol. 2001;167:132. doi: 10.4049/jimmunol.167.1.132. [DOI] [PubMed] [Google Scholar]
- 140.Warren J Immunol. 1999;162:735. [PubMed] [Google Scholar]
- 141.Watzl J Immunol. 2000;165:3545. doi: 10.4049/jimmunol.165.7.3545. [DOI] [PubMed] [Google Scholar]
- 142.Weekes Immunology. 1999;98:443. doi: 10.1046/j.1365-2567.1999.00901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wherry Nat Immunol. 2003;4:225. [Google Scholar]
- 144.Wu J Exp Med. 2003;198:173. doi: 10.1084/jem.20030446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wu Adv Cancer Res. 2003;90:127. doi: 10.1016/s0065-230x(03)90004-2. [DOI] [PubMed] [Google Scholar]
- 146.Yokoyama Nat Rev Immunol. 2003;3:304. doi: 10.1038/nri1055. [DOI] [PubMed] [Google Scholar]
- 147.Young J Immunol. 2001;166:3933. doi: 10.4049/jimmunol.166.6.3933. [DOI] [PubMed] [Google Scholar]
- 148.Zea Clin Cancer Res. 1995;1:1327. [PubMed] [Google Scholar]