Abstract
The human CD44 gene encodes type 1 transmembrane glycoproteins involved in cell-cell and cell-matrix interactions. The structural heterogeneity of the gene products is caused primarily by alternative splicing of at least 10 out of 20 exons. Certain CD44 variant isoforms, in particular those containing CD44 variant domain 6 (CD44v6), have been implicated in tumourigenesis, tumour cell invasion and metastasis. Here we will give an overview of immunohistochemically determined CD44v6 expression in human malignancies (primary epithelial and nonepithelial tumours as well as metastases) and normal tissues, and review several examples of the clinical use of CD44v6-specific antibodies. In nonmalignant tissues, CD44v6 expression is essentially restricted to a subset of epithelia. Intense and homogeneous expression of CD44v6 was reported for the majority of squamous cell carcinomas and a proportion of adenocarcinomas of differing origin, but was rarely seen in nonepithelial tumours. This expression pattern has made CD44v6 an attractive target for antibody-guided therapy of various types of epithelium-derived cancers.
Keywords: Bivatuzumab, CD44v6 immunohistochemistry, Monoclonal antibody, Tumour therapy
Introduction
CD44 glycoproteins constitute one of five major families of cell adhesion molecules [92]. The structural heterogeneity of CD44 is caused by alternative splicing of at least 10 out of 20 exons of the CD44 gene which gives rise to CD44 variants with variable extracellular domains [106, 107]. In addition, also cell-specific posttranslational glycosylation with N- and O-linked oligosaccharides [74], decoration with chondroitin sulphate and keratosulphate, and phosphorylation [88] of the extracellular and cytoplasmic domain contribute to the diversity of CD44. CD44 is known as the principal cell surface receptor for the extracellular matrix glycosaminoglycan hyaluronate [1, 75]. Furthermore, CD44 isoforms decorated with heparan sulphate side chains bind growth factors and can promote growth factor receptor–mediated signalling [7, 57, 121]. The intracellular domain of CD44 interacts with certain cytoskeletal proteins, such as ankyrin, merlin, c-src, and ERMs (ezrin, radixin, moesin) [11, 83, 97]. The signal-transducing functions of CD44 glycoproteins may be of similar importance as their function in cell adhesion and have become a subject of intense research [52, 97].
Since its first description as a surface antigen of T lymphocytes and granulocytes [24], CD44 has been implicated in a diverse spectrum of physiological and pathophysiological processes, including embryogenesis, haematopoiesis, lymphocyte homing, inflammation and tumour progression. Of particular interest is the involvement of CD44 in oncogenic signalling, tumour growth and metastasis [12]. Differential expression of CD44 isoforms during tumour progression has been described for a variety of different tumours.
The variant isoform CD44v6 has attracted increasing interest since the demonstration 1 decade ago that the transfection of splice variant CD44v4–v7 conferred metastatic potential on cells of a nonmetastatic rat tumour cell line [44]. This raised questions about the significance of CD44v6 as a prognostic factor of tumour progression and the therapeutic potential of anti-CD44v6 monoclonal antibodies. Many structural and functional aspects of CD44 have been dealt with in a series of excellent reviews [10, 12, 75, 87, 96, 97]. We will focus on the expression of CD44v6 in human tumour cells and its significance as a target antigen for novel antibody-based treatment modalities.
CD44 family of proteins
Standard form of CD44 (CD44s)
CD44 proteins are encoded by a single gene which is located on the short arm of the human chromosome 11 and which comprises 20 exons [106, 107]. The smallest and most abundant isoform of CD44 is the so-called standard form (CD44s), also referred to as the hematopoietic isoform (CD44H). It lacks amino acid sequences encoded by any of the 10 variant exons (v1–v10). The human CD44s protein consists of 341 amino acids. The largest proportion (248 amino acids) is contained in the extracellular domain. The single transmembrane-spanning domain contains 23 amino acids and the highly conserved intracellular domain, 70 amino acids. CD44s has a predicted molecular mass of about 37 kDa, but extensive posttranslational glycosylation more than doubles the apparent molecular mass to 85–95 kDa. Further modification by heparan sulphate and chondroitin sulphate may result in another increase of molecular mass [87].
CD44 isoforms (CD44v)
Variations of the extracellular domain are generated by alternative splicing of 10 variant exons (v1–v10). In addition, the intracellular domain may be subject to alternative splicing, resulting in a short-tailed version and a long-tailed version, the latter being much more abundant [87]. By alternative use of the variant exons during the mRNA processing, theoretically more than 700 different CD44 isoforms can be generated [124]. To date, only a few (about 20) of these transcripts have been described in tissues and cell lines. Inclusion of the entire variable region would enlarge the amino acid sequence of CD44s by 381 amino acids, thereby introducing additional glycosylation sites. So far, the largest transcript encoding for 338 additional amino acids (v2–v10) was found in squamous epithelial cells [123]. Another isoform, epithelial CD44 (CD44E; also CD44v8–v10, Epican) is a 130-kDa protein which is preferentially expressed by certain tumour cell lines.
CD44 in cell-cell interactions
Cell adhesion and signalling
Through its extracellular domain, CD44 is able to bind hyaluronate, an important component of the extracellular matrix of many tissues and the basement membrane. Hyaluronate interacts with proteoglycans of the extracellular matrix and has been implicated in the regulation of cell-cell adhesion, cell motility, proliferation and differentiation [71]. Three mammalian hyaluronan synthase genes are described (HAS1, HAS2, HAS3). Hyaluronate is a large polysaccharide (molecular mass: several million daltons) composed of a repeating disaccharide formed from d-glucuronic acid and N-acetyl-D-glucosamine. This huge glycosaminoglycan molecule contains multiple binding sites for interaction with CD44 receptors on the cell surface. An effective interaction can only be accomplished by many closely arrayed CD44 receptors [76]. Accordingly, not only the types of receptor (CD44 isoform variants), but also the receptor density on the cell surface determines the extent and strength of the binding to the extracellular matrix.
Hyaluronate is known to activate intracellular signalling cascades involving a variety of protein tyrosine and serine/threonine kinases in several cell types of the haematopoietic and immune system. This obviously requires interactions with hyaluronate-binding proteins (hyaladherins) which mediate transmembrane signalling. For two hyaladherins, CD44 and RHAMM (CD168), involvement in the transduction of hyaluronate-associated signals has been reported [11, 131]. The cytoplasmic domain of CD44 isoforms has the potential to bind oncogenic signalling molecules, including Src and p185HER2 kinases. Furthermore, interactions with the Rho/Ras signalling and receptor-linked tyrosine kinase pathways have been suggested [11]. However, the molecular details of CD44-mediated signal transduction processes, particularly in tumour cells, are still poorly understood.
Growth factor and cytokine presentation
Soluble growth factors and cytokines present in the environment of tumours regulate a variety of tumour cell properties, such as proliferation, migration and tissue invasion [45]. A model for CD44-mediated growth factor presentation during tumour growth was presented [96]. Growth factors, which bind to heparin, e.g. epidermal growth factors, also bind heparan. Thus, CD44v proteins expressed on tumour cells can bind heparin-binding growth factors and present them to other high-affinity receptors on their own surface or on neighbouring tumour and stromal cells. Thereby, favourable conditions for tumour cell growth could be generated. This kind of growth factor presentation has previously been described for skin keratinocytes [7].
Tumour dissemination
A subfamily of CD44 splice variants encompassing the variant domain 6 (CD44v6) has been implicated in the metastatic potential of tumours [44, 102, 108, 132]. CD44 isoforms containing CD44v6 (isoforms v4–7 and v6–7) were found to confer metastatic potential on nonmetastatic tumour cell lines in a syngeneic rat tumour model [44]. Coinjection of a variant-specific monoclonal antibody with the metastatic cells led to retardation or even complete blockage of metastatic spread in vivo [108]. These findings prompted the generation of antibodies specific for human CD44v6 which were suitable to investigate protein expression in human tissue specimens.
Expression of CD44v6 in human tumours
During the past decade, a vast number of primary tumours and metastases from close to 10,000 patients have been screened for the expression of CD44v6 both on the RNA and protein level. The investigation of the differential expression of CD44 protein variants was enabled by the development of murine monoclonal antibodies specific for epitopes encoded by the different variant exons, including exon v6 [36, 50, 67]. The majority of these antibodies is suitable for the immunohistochemical detection of CD44 both on frozen and formalin-fixed, paraffin-embedded tissues. Several monoclonal antibodies specific for the domain encoded by exon v6 (42 amino acids) are available. These are designated 2F10, VFF4, VFF7, VFF18 (BIWA 1), U36, V6B3, HB-256 and Var 3.1 [36, 50, 67, 103]. The epitopes recognised by VFF18 and U36 have been mapped [50, 122].
In this section, we will give an representative overview of published CD44v6 protein expression frequencies determined in primary epithelial and nonepithelial tumours as well as in metastatic lesions thereof. In the majority of studies, expression of CD44v6 was analysed by immunohistochemistry using sections of routinely obtained, formalin-fixed and paraffin-embedded tissue specimen, which were deparaffinised and stained according to standard protocols, often applying microwave-assisted antigen retrieval. In the majority of investigations a sample was judged “positive” when at least 10% of the tumour cells of a given slide showed membranous staining for CD44v6 (an example is shown in Fig. 1A). Several authors, however, applied other threshold levels for positivity which range from 5% up to 50% of stained tumour cells (see Tables 1, 2 and 3). Several reports additionally qualified the intensity of staining, usually applying scoring systems from + to +++ (weak to strong). In some papers, weakly staining cells did not contribute to the fraction of positive tumour cells, whereas in others, all positive cells were counted.
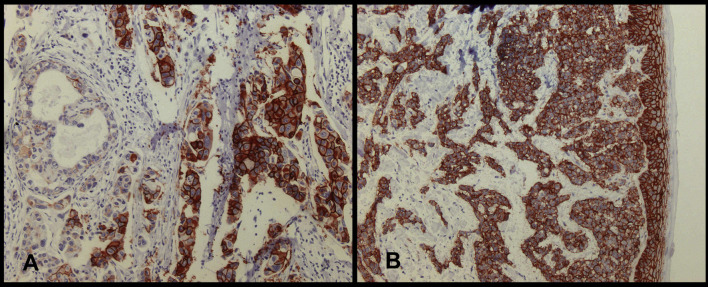
Fig. 1A, B.
Immunostaining of CD44v6 with mAb VFF18 in formalin-fixed specimens from patients with invasive ductal breast cancer. A In addition to distinct membrane staining, cytoplasmic staining can also be observed in part of the tumour cells. Note the heterogeneous staining of the tumour cells with about 30% of cells displaying no reactivity with the antibody. B CD44v6 expression in normal skin keratinocytes adjacent to the tumour. Counter stain haematoxylin, original magnification x200
Table 1.
Expression frequencies of CD44v6 in primary epithelial tumours
| Positive (%) | Samples | Cutoff | MAb | Reference | |
|---|---|---|---|---|---|
| Squamous cell carcinoma | |||||
| Head and neck | 100 | 34 of 34 | 10% | VFF18 | Heider et al. 1996 [50] |
| 99 | 274 of 277 | 10% | VFF18 | Van Hal et al. 1999 [123] | |
| 98 | 58 of 60 | 10% | VFF18 | Fabricius et al. 1997 [30] | |
| 98 | 54 of 55 | 50% | VFF7 | Herold-Mende et al. 1996 [51] | |
| 96 | 85 of 89 | 5% | 2F10 | Kanke et al. 2000 [63] | |
| 95 | 36 of 38 | 10% | 2F10 | Kunishi et al. 1997 [69] | |
| 66 | 37 of 56 | 10% | 2F10 | Fonseca et al. 2001 [34] | |
| 61 | 30 of 49 | 10% | Var3.1 | Soukka et al. 1997 [113] | |
| Oesophagus | 95 | 19 of 20 | 10% | VFF18 | Heider et al. 1996 [50] |
| 97 | 226 of 233 | 5% | 2F10 | Gotoda et al. 2000 [40] | |
| Lung | 93 | 38 of 41 | 25% | VFF18 | Mizera-Nyczak et al. 2001 [80] |
| 89 | 16 of 18 | 10% | VFF18 | Heider et al. 1996 [50] | |
| 75 | 9 of 12 | 20% | VFF18 | Fukuse et al. 1999 [37] | |
| 52 | 13 of 25 | 20% | VFF18 | Hirata et al. 1998 [53] | |
| 100 | 20 of 20 | 10% | VFF7 | Carbognani et al. 1998 [15] | |
| 83 | 10 of 12 | 0% | VFF7 | Miyoshi et al. 1997 [81] | |
| 49 | 72 of 146 | 66% | VFF7 | Pirinen et al. 2000 [95] | |
| 97 | 30 of 31 | 10% | 2F10 | Fosano et al. 1997 [35] | |
| 91 | 43 of 47 | 10% | 2F10 | Tran et al. 1997 [120] | |
| 82 | 23 of 28 | 10% | 2F10 | Wimmel et al. 1997 [129] | |
| 91 | 30 of 33 | 10% | V6B3 | Nguen et al. 2000 [90] | |
| Skin | 100 | 15 of 15 | 10% | VFF18 | Heider et al. 1996 [50] |
| 100 | 16 of 16 | 25% | VFF18 | Simon et al. 1996 [111] | |
| 100 | 24 of 24 | 10% | VFF7 | Seiter et al. 1996 [109] | |
| Cervix | 32 | 20 of 62 | 10% | VFF18 | Ayhan et al. 2001 [3] |
| 100 | 16 of 16 | 10% | VFF7 | Dall et al. 1994 [25] | |
| 73 | 26 of 35 | 10% | VFF7 | Kainz et al. 1996 [62] | |
| 67 | 20 of 30 | 10% | VFF7 | Kainz et al. 1995 [60] | |
| 67 | 70 of 105 | 10% | VFF7 | Kainz et al. 1995 [61] | |
| 51 | 92 of 180 | 10% | VFF7 | Speiser et al. 1999 [114] | |
| 73 | 32 of 44 | 50% | 2F10 | Callagy et al. 2000 [14] | |
| Vulva | 33 | 23 of 70 | 10% | VFF7 | Tempfer et al. 1998 [118] |
| Adenocarcinoma | |||||
| Breast | 80 | 374 of 465 | 10% | VFF18 | Foekens et al. 1999 [33] |
| 88 | 15 of 17 | 10% | VFF7 | Dall et al. 1995 [26] | |
| 70 | 73 of 103 | 50% | VFF7 | Sinn et al. 1995 [112] | |
| 51 | 28 of 55 | 10% | VFF7 | Schumacher et al. 1996 [105] | |
| 24 | 28 of 115 | 10% | VFF7 | Tempfer et al. 1996 [116] | |
| 80 | 87 of 109 | 10% | 2F10 | Morris et al. 2001 [82] | |
| 76 | 72 of 95 | 5% | 2F10 | Tokue et al. 1998 [119] | |
| 65 | 219 of 338 | 5% | 2F10 | Jansen et al 1998 [58] | |
| Barrett’s oesophagus | 10 | 4 of 41 | 50% | VFF7 | Boettger et al. 1998 [8] |
| 63 | 46 of 73 | 10% | 2F10 | Lagorce-Pages et al. 1998 [70] | |
| Lung | 95 | 19 of 20 | 25% | VFF18 | Mizera-Nyczak et al. 2001 [80] |
| 43 | 15 of 35 | 10% | VFF18 | Heider et al. 1996 [50] | |
| 19 | 8 of 43 | 20% | VFF18 | Hirata et al. 1998 [53] | |
| 10 | 6 of 58 | 66% | VFF7 | Pirinen et al. 2000 [95] | |
| 0 | 0of 12 | 10% | VFF7 | Carbognani et al. 1998 [15] | |
| 48 | 21 of 44 | 10% | 2F10 | Tran et al. 1997 [120] | |
| 10 | 2 of 21 | 10% | 2F10 | Fosano et al. 1997 [35] | |
| 36 | 13 of 36 | 10% | V6B3 | Nguen et al. 2000 [90] | |
| Gastric | 77 | 322 of 418 | 5% | VFF18 | Mueller et al. 1997 [84] |
| 68 | 15 of 22 | 10% | VFF18 | Heider et al. 1996 [50] | |
| 26 | 17 of 64 | – | VFF7 | Harn et al. 1995 [46] | |
| 62 | 26 of 42 | 10% | VFF4 | Heider et al. 1993 [47] | |
| 41 | 63 of 155 | 25% | 2F10 | Xin et al. 2001 [130] | |
| Pancreas | 100 | 24 of 24 | – | VFF18 | Gansauge et al. 1995 [38] |
| 100 | 16 of 16 | – | VFF7 | Satoh et al. 1997 [104] | |
| 50 | 21 of 42 | 5% | 2F10 | Gotoda et al. 1998 [39] | |
| 29 | 7 of 24 | 50% | 2F10 | Castella et al. 1996 [16] | |
| Colon/rectum | 100 | 37 of 37 | 10% | VFF18 | Mueller et al. 1998 [85] |
| 94 | 64 of 68 | 10% | VFF18 | Wielenga et al. 1998 [128] | |
| 86 | 54 of 63 | 10% | VFF18 | Ishida et al. 2000 [56] | |
| 91 | 31 of 34 | – | VFF7 | Coppola et al. 1998 [23] | |
| 40 | 28 of 69 | – | VFF7 | Nanashima et al. 1999 [86] | |
| 16 | 11 of 69 | 20% | VFF7 | Finke et al. 1995 [32] | |
| 6 | 10 of 180 | – | VFF7 | Koretz et al. 1995 [68] | |
| 86 | 37 of 43 | 5% | 2F10 | Clarke et al. 2000 [17] | |
| Endometrium (uterine) | 53 | 41 of 78 | 10% | VFF18 | Ayhan et al. 2001 [4] |
| 100 | 13 of 13 | – | VFF7 | Lin et al. 2001 [78] | |
| 48 | 13 of 27 | 10% | VFF7 | Katsura et al. 1998 [65] | |
| 22 | 35 of 156 | 10% | VFF7 | Tempfer et al. 1998 [117] | |
| 48 | 16 of 33 | 10% | 2F10 | Leblanc et al. 2001 [72] | |
| Prostate | 31 | 5 of 16 | 10% | VFF18 | Heider et al. 1996 [50] |
| 69 | 69 of 97 | 10% | VFF7 | Noordzij et al. 1997 [91] | |
| Other tumours | |||||
| Thyroid carcinoma | 100 | 11 of 11 | 10% | 2F10 | Figge et al. 1994 [31] |
| 85 | 68 of 78 | 10% | 2F10 | Gu et al. 1998 [43] | |
| Small cell lung cancer | 23 | 7 of 31 | 10% | VFF18 | Heider et al. 1996 [50] |
| Renal cell carcinoma | 18 | 5 of 27 | 10% | VFF18 | Heider et al. 1996 [49] |
| 3 | 2 of 66 | – | 2F10 | Paradis et al. 1999 [93] | |
| Urinary bladder | |||||
| Small cell | 7 | 3 of 46 | 5% | HB-256 | Iczkowski et al. 1999 [54] |
| Transitional | 60 | 13 of 21 | 5% | HB-256 | Iczkowski et al. 1999 [54] |
| Ovarian cancer | |||||
| Clear cell | 48 | 10 of 21 | 5% | – | Rodriguez-Rodriguez et al. 1998 [101] |
| Basal cell carcinoma | 100 | 37 of 37 | 10% | VFF7 | Seiter et al. 1996 [109] |
Table 2.
Expression frequencies of CD44v6 in metastases of epithelial tumours
| Positive (%) | Samples | Cutoff | MAb | Reference | |
|---|---|---|---|---|---|
| Head and neck (squamous cell) carcinoma) | |||||
| Lymph node | 99 | 80 of 81 | 10% | VFF18 | Heider et al. 1996 [50] |
| 93 | 27 of 29 | 50% | VFF7 | Herold-Mende et al. 1996 [51] | |
| 29 | 11 of 38 | 10% | 2F10 | Kunishi et al. 1997 [69] | |
| Liver | 100 | 4 of 4 | 10% | VFF18 | Heider et al. 1996 [50] |
| Lung Cancer | |||||
| Lymph node | 96 | 26 of 27 | 10% | VFF18 | Heider et al. 1996 [50] |
| 95 | 19 of 20 | 10% | 2F10 | Tran et al. 1997 [120] | |
| Breast adenocarcinoma | |||||
| Lymph node | 100 | 4 of 4 | 10% | VFF18 | Dall et al. 1995 [26] |
| 91 | 31 of 34 | 10% | VFF18 | Heider et al. 1996 [50] | |
| 100 | 17 of 17 | 50% | VFF7 | Sinn et al. 1995 [112] | |
| 92 | 33 of 36 | 10% | VFF7 | Tempfer et al. 1996 [116] | |
| Liver | 100 | 4 of 4 | 10% | VFF18 | Heider et al. 1996 [50] |
| Gastric Adenocarcinoma | |||||
| Lymph node | 60 | 6 of 10 | 10% | VFF18 | Heider et al. 1993 [47] |
| 37 | 16 of 43 | 10% | VFF18 | Heider et al. 1996 [50] | |
| 20 | 4 of 20 | – | VFF7 | Harn et al. 1995 [46] | |
| Liver | 100 | 4 of 4 | 10% | VFF18 | Heider et al. 1996 [50] |
| Pancreas adenocarcinoma | |||||
| Lymph node visceral | 72 | 13 of 18 | 50% | 2F10 | Castella et al. 1996 [16] |
| Colon adenocarcinoma | |||||
| Lymph node | 41 | 21 of 51 | 10% | VFF18 | Heider et al. 1996 [50] |
| Liver | 50 | 13 of 26 | 10% | VFF18 | Heider et al. 1996 [50] |
| Brain | 100 | 6 of 6 | 10% | VFF18 | Heider et al. 1996 [50] |
| Liver | 59 | 26 of 44 | 10% | VFF7 | Nanashima et al. 1999 [86] |
| Various | 17 | 6 of 35 | 20% | VFF7 | Finke et al. 1995 [32] |
| Ovarian cancer | |||||
| Various | 22 | 5 of 23 | 5% | – | Rodriguez-Rodriguez et al. 1998 [101] |
| Prostate adenocarcinoma | |||||
| Lymph node | 0 | 0 of 18 | 10% | VFF18 | Heider et al. 1996 [50] |
| 0 | 0 of 12 | 10% | VFF7 | Noordzij et al. 1997 [91] |
Table 3.
Expression frequencies of CD44v6 in nonepithelial tumours
| Positive (%) | Samples | Cutoff | MAb | Reference | |
|---|---|---|---|---|---|
| Various | |||||
| Glioma | 0 | 0 of 29 | 10% | VFF18 | Kaaijk et al. 1995 [59] |
| 0 | 0 of 84 | 10% | 2F10 | Ranuncolo et al. 2002 [98] | |
| Neuroblastoma | 0 | 0 of 55 | 10% | VFF7 | Gross et al. 1995 [42] |
| Schwannoma | 0 | 0 of 4 | 10% | VFF18 | Kaaijk et al. 1995 [59] |
| 40 | 2 of 5 | – | VFF18 | Sherman et al. 1995 [110] | |
| Melanoma | 0 | 0 of 16 | 10% | VFF18 | Simon et al. 1996 [111] |
| 0 | 0 of 19 | 10% | VFF18 | Manten-Horst et al. 1995 [79] | |
| Sinonasal | 0 | 0 of 14 | 10% | VFF18 | Regauer et al. 1999 [99] |
| Leukaemias and malignant lymphomas | |||||
| AML | 73 | 69 of 95 | 5% | VFF18 | Legras et al. 1998 [73] |
| 7 | 2 of 27 | 10% | VFF7 | Bendall et al. 2000 [6] | |
| 16 | 5 of 32 | 15% | VFF7 | Khaldoyanidi et al. 1996 [66] | |
| Non-Hodgkin’s lymphoma | 54 | 59 of 109 | 15% | VFF7 | Khaldoyanidi et al. 1996 [66] |
| 28 | 10 of 36 | 10% | VFF7 | Koopmann et al. 1993 [67] | |
| 57 | 32 of 56 | – | Var3.1 | Ristamäki et al. 1995 [100] | |
| Cutaneous lymphoma | 0 | 0 of 25 | 10%; | VFF7 | Wagner et al. 1998 [127] |
| 30 | 3 of 10 | 25% | V6B3 | Liang et al. 2002 [77] | |
| Multiple myeloma | 22 | 6 of 27 | 15% | VFF7 | Khaldoyanidi et al. 1996 [66] |
| Diffuse large B cell lymphoma | 31 | 13 of 42 | 10% | VFF18 | Inagaki et al. 1999 [55] |
| 16 | 38 of 236 | 10% | VFF18 | Drillenburg et al. 1999 [29] | |
| Hodgkin’s lymphoma | 28 | 8 of 29 | 10% | VFF18 | Beham-Schmid et al. 1998 [5] |
Squamous cell carcinomas
Squamous cell carcinomas (SCCs) represent a major group of epithelial tumours of the airway, the digestive and the genital tract as well as the skin. Irrespective of the tissue of origin, the overwhelming majority of primary SCCs, in samples from more than 1,000 patients, expressed CD44v6 (Table 1). Lymph node metastases associated with squamous cell carcinoma were found to express CD44v6 to the high extent seen in the primary tumours (see Table 2). The highest expression frequencies were found in tumours derived from head and neck, oesophagus, skin, and lung, where most authors reported frequencies exceeding 90%. In the majority of positive samples, more than 70% of the tumour cells within a given section expressed CD44v6. This frequent and homogeneous expression of CD44v6 makes SCC a suitable target for therapeutic approaches with CD44v6-specific antibodies.
In one report, only slightly more than half of the investigated tumours were found to express CD44v6; the majority of those cases were scored as weakly positive [113]. A possible explanation for this clearly lower rate of positivity compared with other studies may be the use of antibody Var3.1, which was generated after immunisation with a synthetic peptide corresponding to part of the human v6 domain. This antibody possibly recognises an epitope which is masked by posttranslational modification or secondary structures in part of the tumour cells, or may have low affinity to the antigen. In conclusion, mAb Var3.1 does not appear to be suitable to detect CD44v6 expression in tissue specimens.
In cervical squamous cell carcinomas the frequencies of CD44v6 expression ranged from 32% to 100% of the samples and thus showed higher variability than in other tumour types. The antibody used for most studies in cervical cancer, VFF7, has a lower affinity for CD44v6 than other antibodies, e.g. VFF4, VFF18 or 2F10 [50]. Parallel investigations with the VFF series of antibodies revealed that all three antibodies showed a qualitatively similar pattern of staining, whereas the percentage of stained tumour cells of serial sections decreased with the affinity of the antibodies [84, 128]. Interestingly, the highest frequency of CD44v6 expression was observed in a study which, in contrast to all other investigations, used fresh-frozen tumour samples [25]. Based on our own experience with these antibodies and published data we conclude that staining with antibody VFF7 may underestimate the expression of CD44v6, especially in formalin-fixed tumour samples, where proper antigen retrieval, e.g. by microwave heating, is mandatory for optimal staining results. Therefore either the use of fresh-frozen tumour samples or high-affinity antibodies (e.g. VFF18, 2F10) in combination with microwave or waterbath-assisted antigen retrieval is recommended.
Adenocarcinomas
The term “adenocarcinoma” describes the largest group of epithelial-derived cancers which comprises a variety of different histological subtypes and which contains tumours from many different locations. Data on CD44v6 expression are available for all major types of adenocarcinoma.
Breast adenocarcinomas were positive in 65% to 88% of the samples in six out of eight reports. The two studies reporting lower frequencies of CD44v6 expression used the lower-affinity mAb VFF7, which again suggests that VFF7-staining underestimates CD44v6 expression [33] (see also previous section). In several studies, the expression of CD44v6 in metastases of breast cancer patients was also investigated. In a total of 95 samples of mostly lymph node metastases, between 91% and 100% of the specimens were positive (Table 2). These data indicate a higher prevalence of CD44v6 in metastatic lesions of primary breast cancer than in their corresponding primaries, which led to the speculation that CD44v6 expression increases the risk of metastasis formation. In two large studies, however, no correlation between patient survival and CD44v6 expression could be found [33, 58]. To date, the use of CD44v6 as a prognosticator in breast cancer is not established.
In lung adenocarcinoma in general less than 50% of cases were found to express CD44v6, except in one study where 95% of cases were scored positive [80]. In this study the majority of cases was scored “moderately” positive, which means that between 25% and 75% of tumour cells expressed the antigen. Taken together, the data on lung adenocarcinoma show that there is more heterogeneity of CD44v6 expression in these tumours compared with lung tumours derived from squamous epithelium, which consistently expressed CD44v6.
Highly varying expression frequencies between different studies were reported for colorectal, pancreatic, and gastric adenocarcinomas, again attributable to the use of different antibodies and differently processed tumour material (Table 1) [85, 128]. Therefore the “real” expression frequencies of CD44v6 in these tumour types are more likely to be in the upper range of reported frequencies than in the lower one. In studies in which metastases were also investigated, a reduced expression frequency compared with primary tumours was observed [32, 46, 50, 86]. In addition, considerable intratumour heterogeneity of CD44v6 expression was also described in several of these studies (see e.g. [62, 68, 77, 100]).
Very little published data for prostate carcinoma is available (Table 1). In two studies, freqencies of 31% and 69%, respectively, were reported. In most cases the expression of CD44v6 was restricted to less than 25% of the total tumour cells, with a tendency to reduced expression in more advanced cancers. No CD44v6 was detected in 30 lymph node metastases (Table 2). Interestingly, in a recent publication it was reported that in advanced stages of prostate cancer the transcriptional repressor EZH2 is up-regulated, leading to silencing of many genes. CD44 appears to be one of the genes affected by EZH2 silencing [125].
In summary, expression of CD44v6 was detectable in all types of adenocarcinoma (Table 1). There was, however, a higher degree of variability in the frequency of expression as compared to CD44v6 expression in squamous cell carcinoma both between different types of adenocarcinomas and between different studies of the same type of tumour. The difference between studies in the same type of tumour is attributable to the use of different antibodies and/or tumour specimens (frozen versus formalin-fixed); especially the use of VFF7 on formalin-fixed material yields consistently lower expression frequencies. With respect to the use of CD44v6-specific antibodies for cancer therapy in adenocarcinomas, breast cancer appears to be an attractive indication due to high expression frequencies both in primary tumours and lymph node metastases, whereas colon and prostate adenocarcinomas are less favourable in this regard. Due to the observed intratumour and intertumour heterogeneity of CD44v6 expression, however, a patient selection based on a diagnostic test for CD44v6 expression appears to be mandatory for adenocarcinomas. Such a test would also allow us to treat selected patients with indications of lower expression frequencies of CD44v6.
Nonepithelial tumours
The reports on nonepithelial tumours cover a heterogeneous group of malignancies, including solid tumours and tumours of the haematopoietic and lymphatic systems (Table 3).
No or little cell surface expression of CD44v6 was observed in samples of malignant melanoma, neuroblastoma, glioma, schwannoma and cutaneous lymphomas. In haematopoietic malignancies the expression frequencies of CD44v6 range from less than 10% up to 73% of samples. In one study of acute myeloid leukaemia 73% of cases were reported to be positive. However, only a fraction of tumour cells of individual patients was positive (range 5–80%), and positivity was “weak” as indicated by a mean fluorescence intensity increase by threefold to fivefold (when measured by flow cytometry). In two other flow cytometry studies, only 7% and 16% of cases were scored as positive. Therefore positivity in acute myeloid leukaemia appears to be low and highly dependent on the cutoff levels applied in the flow cytometric measurement. Taken together, these data do not suggest the use of CD44v6-specific treatment modalities in nonepithelial tumours.
Expression of CD44v6 in normal tissues
In contrast to the standard form of CD44, which is almost ubiquitously expressed, splice variants containing variant exon v6 are highly restricted in their expression in normal tissues. CD44v6 expression can be detected only in a subset of epithelial tissues, such as squamous epithelia (e.g. skin, cervix, oral mucosa, oesophagus), myoepithelia (e.g. breast, prostate), or pneumocytes (type II) in the lung and bronchial epithelium [48]. The levels of expression vary from weak to strong with the highest intensities found in skin keratinocytes and other squamous epithelia (Fig. 1B). Considering the high variability in CD44v6 expression, it is not possible to draw general conclusions concerning CD44v6 “overexpression” or “down-regulation” in tumour tissues compared with their normal tissue of origin or with normal tissues in general. In many cases of tumours derived from squamous epithelia, the expression level is comparable to that in normal squamous epithelium. Since in certain tumour areas or individual samples of SCC, CD44v6 expression is weak or even absent (e.g. in keratinizing areas), some authors considered this as evidence for “down-regulation” in this tumour type [30, 34, 69, 103, 113]. In contrast, the term “overexpression” has been used for adenocarcinomas, since CD44v6 expression is mostly absent from the corresponding normal tissues of origin.
On resting haematopoietic cells including bone marrow stem/progenitor cells, no or little expression of CD44v6 was detected [48, 89]. In vitro experiments have shown transient expression of CD44v6-containing isoforms on peripheral human blood T and B lymphocytes upon stimulation with antigen or mitogens [41, 67]. However, immunohistochemical analysis from patients with atopic or allergic contact dermatitis revealed no expression of CD44v6 on skin-infiltrating lymphocytes [127]. Taken together, the limited expression of CD44v6 on epithelia and the absent or low expression on other tissue types including haematopoietic stem/progenitor cells decreases the likelihood of target antigen–related side effects caused by treatment with CD44v6-specific antibodies.
CD44v6-specific monoclonal antibodies in clinical trials
To determine the applicability of CD44v6-specific antibodies in cancer patients, several clinical phase I trials with either murine (U36, VFF18), chimerised (chU36) or humanised (BIWA 4) antibodies were initiated. In all these trials radiolabelled antibodies were used.
Radioimmunoscintigraphy with murine monoclonal antibody 99mTc-U36 in head and neck cancer patients
This study was undertaken to investigate the biodistribution of U36 labelled with technetium 99m in ten patients suspected of having neck lymph node metastases from a histologically proven squamous cell carcinoma of the head and neck, and who had been scheduled to undergo resection of the primary tumour and neck dissection [27]. Biodistribution was determined by radioimmunoscintigraphy and by measuring radioactivity in biopsies. All ten primary tumours were visualised by scintigraphy. Both diagnostic modalities were correct in 7 of 14 tumours with involvement of lymph nodes. The missed lymph node metastases in the study comprised micrometastases, small tumour–involved nodes (<9 mm), and tumour-involved nodes with considerable necrosis, keratinisation or fibrosis. There were no false-positive observations with U36. Biopsies from the surgical specimen showed high tumour uptake and favourable tumour to nontumour ratios. In an additional clinical phase I study the tumour-targeting properties of a rhenium 186 radioimmunoconjugate were superior to that of a radioiodinated antibody. Thus, for further radioimmunotherapy studies rhenium 186 was selected [28]. However, as human antimouse antibody (HAMA) responses were observed in one third of the patients [28], a human-mouse chimeric U36 antibody was constructed in order to reduce immunogenicity [13].
Radioimmunotherapy with chimeric 186Re-U36 antibody in head and neck cancer patients
This phase I study was conducted in 13 patients with SCC of the head and neck. The administration of chimeric 186Re-U36 was well tolerated, and favourable targeting of tumour lesions was seen in all patients. Dose-limiting myelotoxicity was observed in two patients treated at a dose of 1.5 GBq/m2, with thrombocytopenia being most prominent. The maximum tolerated dose was established at 1.0 GBq/m2 (transient CTC grade 3 thrombocytopenia in one patient). One patient showed stable disease for 6 months after treatment. Two patients with dose-limiting myelotoxicity showed a reduction in tumour size. The reduction was of short duration and not considered an objective response. Five patients experienced a human antichimeric antibody response, one of which was directed against a mouse epitope. The use of the chimeric monoclonal antibody U36 instead of its murine counterpart did not decrease the induction of antibody responses [18, 19, 20, 21].
Radioimmunoscintigraphy study with murine 99mTc-BIWA 1 in head and neck cancer patients
This radioimmunoscintigraphy study was undertaken to investigate the biodistribution and tumour-targeting properties of the murine monoclonal antibody BIWA 1 (VFF18). Twelve head and neck cancer patients undergoing surgery were included. Preoperatively, 99mTc-labelled BIWA 1 was administered. No drug-related adverse events were observed. Human antimouse antibody responses were observed in 11 patients. Besides activity uptake in the tumour region, minimal accumulation of activity was observed in normal tissues. Analysis of tissue biopsies revealed high uptake in tumours, comparable to that of the U36 antibody. BIWA 1 was considered a promising monoclonal antibody for targeting head and neck cancer with a potential for radioimmunotherapy. Due to the observed immunogenicity of BIWA 1, a humanised version was considered mandatory for further clinical development [115].
Radioimmunotherapy with humanised monoclonal antibody 186Re-bivatuzumab (BIWA 4) in head and neck cancer patients
To reduce immunogenicity, several fully humanised versions of BIWA 1 were generated by grafting of the complementarity-determining region. The humanised monoclonal antibody bivatuzumab was selected for further development after extensive biodistribution and therapy experiments in nude mice xenografted with human tumours [126]. As a prelude to radioimmunotherapy, the safety, tumour-targeting potential, pharmacokinetics and immunogenicity of 99mTc-labelled bivatuzumab was evaluated in patients undergoing surgery for primary head and neck cancer. Ten patients received 750 MBq of 99mTc-labelled bivatuzumab. Administration of the conjugate was well tolerated by all patients and no human antihuman antibody responses were observed. Radioimmunoscintigraphy showed targeting of primary tumours in eight out of ten patients [22].
In a recently completed phase I dose escalation radioimmunotherapy study, safety, maximum tolerated dose, pharmacokinetics, immunogenicity and therapeutic potential of 186Re-labelled bivatuzumab in 22 patients with inoperable recurrent or metastatic SCC of the head and neck was determined. Patients received a single dose of radiolabelled bivatuzumab, three patients were eligible to a second dose at least 3 months after the initial dose. All administrations were well tolerated and targeting of tumour lesions was confirmed. The only significant side effect was dose-limiting myelotoxicity consisting of thrombopenia and leukocytopenia (CTC grade 3/4). The maximum tolerated radiation dose for phase II studies with 186Re-bivatuzumab was established at 1.85 GBq/m2, at which level, dose-limiting myelotoxicity was seen in two out of six patients. Stable disease, varying between 6 and 21 weeks, was observed in three of six patients treated at the maximum tolerated dose level. Two patients showed formation of human antihuman antibodies without clinical symptoms. In comparison to the murine and chimeric monoclonal antibodies U36 and BIWA 1, humanised bivatuzumab appears to be significantly less immunogenic. The antitumour effects seen in incurable patients with bulky disease will promote the further evaluation of anti-CD44v6-based radioimmunotherapy in the adjuvant setting [9].
Conclusions
Antibodies are finally fulfilling their potential as anticancer therapeutics. Three decades after the invention of monoclonal antibodies, several antibody-based therapeutics have been registered for the treatment of cancer. A key feature for the success of tumour-targeting antibodies is the choice of an appropriate target antigen. Ideally, such an antigen is strongly and homogenously expressed on tumour tissues of a large proportion of patients, but not on normal tissues that may be damaged by immune effector functions or payload therapeutics of the antibody.
CD44v6 was shown in numerous studies to display a favourable pattern of expression. In normal tissues, expression is restricted to a subset of epithelia. In tumours, CD44v6 appears to be an ideal target antigen for the majority of SCCs and for a subset of adenocarcinomas. Especially in SCC, CD44v6 expression is high and homogeneous in most patients.
In addition to the target antigen, the targeting vehicle will also decide on the success of any treatment approach. Several versions of CD44v6-specific antibodies (murine, chimeric, humanised) have been tested in clinical trials. Whereas all antibodies showed excellent tumour targeting and favourable biodistribution, murine and chimeric versions of the antibodies evoked anti-antibody responses. Reduction of immunogenicity is essential for repeated cycles of antibody administration, a criterion which was achieved with humanised antibody bivatuzumab.
CD44v6 and bivatuzumab thus appear to be a suitable target/carrier system for antibody-guided tumour therapy. In addition to radioimmunotherapy studies, several clinical trials employing the novel cytotoxic immunoconjugate bivatuzumab mertansine are currently underway to translate successful results from preclinical studies into benefit for tumour patients (Fig. 2)[94].
Fig. 2.
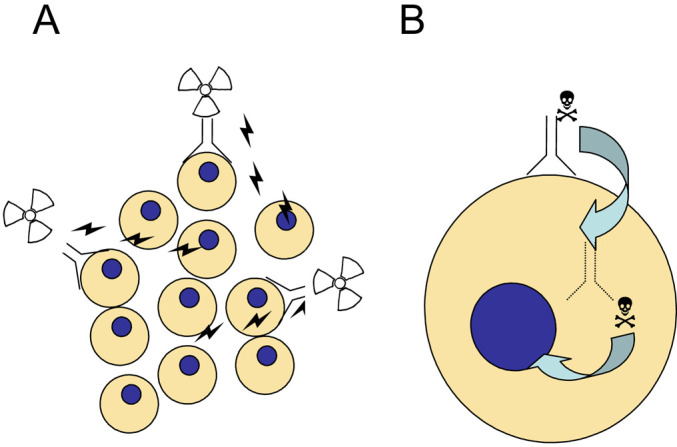
Scheme of CD44v6 antibody-based cancer therapy. A: Radioimmunotherapy with an anti-CD44v6 radioimmunoconjugate. The therapeutic radionuclide (e.g. 186Re) is coupled to the antibody via linker technology. The radioimmunoconjugate binds to CD44v6-positive tumour cells and may kill both antigen-positive and antigen-negative or inaccessible tumour cells. B: Antibody-drug conjugates may consist of the CD44v6-specific antibody covalently linked to a potent cytotoxic drug (e.g. a maytansinoid). After binding to CD44v6 on the cell surface the conjugate gets internalised. Inside the cell, the drug is released from the antibody and consequently can bind to its intracellular target, e.g. tubulin
Acknowledgements
The authors would like to thank Ms Cornelia Fehr for editorial assistance, and Drs Günther Adolf (Boehringer Ingelheim Austria) and Helmut Ponta (Institute of Toxicology and Genetics, University of Karlsruhe, Germany) for critical reading of the manuscript.
Abbreviations
- CD44
type 1 transmembrane glycoprotein, cell surface receptor for hyaluronate
- CD44s (CD44H)
standard form of CD44
- CD44v6
splice variant exon 6 of CD44
- CTC
common toxicity criteria
- 2F10, VFF4, VFF7, VFF18 (BIWA 1), U36, V6B3, HB-256, Var 3.1
monoclonal antibodies targeting the CD44v6 antigen
- SCC
squamous cell carcinoma
References
- 1.Aruffo Cell. 1990;61:1303. doi: 10.1016/0092-8674(90)90694-a. [DOI] [PubMed] [Google Scholar]
- 2.Aruffo J Clin Invest. 1996;98:2191. doi: 10.1172/JCI119026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ayhan Gynecol Oncol. 2001;83:569. doi: 10.1006/gyno.2001.6406. [DOI] [PubMed] [Google Scholar]
- 4.Ayhan Gynecol Oncol. 2001;80:355. doi: 10.1006/gyno.2000.6014. [DOI] [PubMed] [Google Scholar]
- 5.Beham-Schmid J Pathol. 1998;186:383. doi: 10.1002/(SICI)1096-9896(199812)186:4<383::AID-PATH202>3.3.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 6.Bendall Leukemia. 2001;14:1239. doi: 10.1038/sj.leu.2401830. [DOI] [Google Scholar]
- 7.Bennett J Cell Biol. 1995;128:687. doi: 10.1083/jcb.128.4.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boettger Cancer. 1998;83:1074. doi: 10.1002/(sici)1097-0142(19980915)83:6<1074::aid-cncr4>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 9.Börjesson Clin Cancer Res. 2003;9:3961. [Google Scholar]
- 10.Borland Immunology. 1998;93:139. doi: 10.1046/j.1365-2567.1998.00431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bourguignon J Mammary Gland Biol Neoplasia. 2001;6:287. doi: 10.1023/A:1011371523994. [DOI] [PubMed] [Google Scholar]
- 12.Bourguignon LYW, Zhu D, Zhu H. CD44 Isoform-cytoskeleton interaction in oncogenic signaling and tumor progression. Frontiers in Bioscience. 1998;3:637. doi: 10.2741/a308. [DOI] [PubMed] [Google Scholar]
- 13.Brakenhoff Cancer Immunol Immunother. 1995;40:191. doi: 10.1007/s002620050162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Callagy Gynec Oncol. 2000;76:73. doi: 10.1006/gyno.1999.5661. [DOI] [PubMed] [Google Scholar]
- 15.Carbognani P, Spaggiari L, Romani A, Solli P, Corradi A, Cantoni AM, Petronini PG, Borghetti AF, Rusca M, Bobbio P. Expression of human CD44v6 in non-small-cell lung cancer. Eur Surg Res. 1998;30:403. doi: 10.1159/000008605. [DOI] [PubMed] [Google Scholar]
- 16.Castella Virchows Arch. 1996;429:191. doi: 10.1007/BF00198333. [DOI] [PubMed] [Google Scholar]
- 17.Clarke J Gastrenterol Hepatol. 2000;15:1028. doi: 10.1046/j.1440-1746.2000.02285.x. [DOI] [Google Scholar]
- 18.Colnot J Nucl Med. 2000;41:1999. [Google Scholar]
- 19.Colnot Head Neck. 2001;23:559. doi: 10.1002/hed.1078. [DOI] [PubMed] [Google Scholar]
- 20.Colnot J Nucl Med 2001. 2001;42:1364. [PubMed] [Google Scholar]
- 21.Colnot Clin Cancer Res. 2002;8:3401. [PubMed] [Google Scholar]
- 22.Colnot Cancer Immunol Immunother. 2003;52:576. doi: 10.1007/s00262-003-0396-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coppola Hum Pathol. 1998;29:627. doi: 10.1016/s0046-8177(98)80014-2. [DOI] [PubMed] [Google Scholar]
- 24.Dalchau Eur J Immunol. 1980;10:737. doi: 10.1002/eji.1830101003. [DOI] [PubMed] [Google Scholar]
- 25.Dall Cancer Res. 1994;54:3337. [PubMed] [Google Scholar]
- 26.Dall Int J Cancer. 1995;60:471. doi: 10.1002/ijc.2910600408. [DOI] [PubMed] [Google Scholar]
- 27.de Clin Cancer Res. 1995;1:591. [Google Scholar]
- 28.de Br J Cancer. 1997;75:1049. doi: 10.1038/bjc.1997.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Drillenburg Leukemia. 1999;13:1448. doi: 10.1038/sj/leu/2401490. [DOI] [PubMed] [Google Scholar]
- 30.Fabricius Cancer J. 1997;10:325. [Google Scholar]
- 31.Figge Exp Mol Pathol. 1994;61:203. doi: 10.1006/exmp.1994.1037. [DOI] [PubMed] [Google Scholar]
- 32.Finke Lancet. 1995;345:583. doi: 10.1016/S0140-6736(95)90491-3. [DOI] [PubMed] [Google Scholar]
- 33.Foekens Int J Cancer. 1999;84:209. doi: 10.1002/(SICI)1097-0215(19990621)84:3<209::AID-IJC2>3.3.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 34.Fonseca J Surg Oncol. 2001;76:115. doi: 10.1002/1096-9098(200102)76:2<115::AID-JSO1021>3.3.CO;2-0. [DOI] [Google Scholar]
- 35.Fosano Cancer. 1997;80:34. doi: 10.1002/(SICI)1097-0142(19970701)80:1<34::AID-CNCR5>3.3.CO;2-Z. [DOI] [Google Scholar]
- 36.Fox Cancer Res. 1994;54:4539. [PubMed] [Google Scholar]
- 37.Fukuse Cancer. 1999;86:1174. doi: 10.1002/(SICI)1097-0142(19991001)86:7<1174::AID-CNCR11>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 38.Gansauge Cancer Res. 1995;55:5499. [PubMed] [Google Scholar]
- 39.Gotoda Jpn J Cancer Res. 1998;89:1033. doi: 10.1111/j.1349-7006.1998.tb00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gotoda Gut. 2000;46:14. doi: 10.1136/gut.46.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Griffioen Cell Adhesion Commun. 1994;2:195. doi: 10.3109/15419069409004437. [DOI] [PubMed] [Google Scholar]
- 42.Gross Eur J Cancer. 1995;31A:471. doi: 10.1016/0959-8049(95)00029-I. [DOI] [PubMed] [Google Scholar]
- 43.Gu Pathol Int. 1998;48:184. doi: 10.1111/j.1440-1827.1998.tb03891.x. [DOI] [PubMed] [Google Scholar]
- 44.Gunthert Cell. 1991;65:13. [Google Scholar]
- 45.Hamada Frontiers in Bioscience. 1998;3:657. [Google Scholar]
- 46.Harn Cancer. 1995;5:1065. doi: 10.1002/1097-0142(19950301)75:5<1065::aid-cncr2820750503>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 47.Heider Cancer Res. 1993;53:4197. [PubMed] [Google Scholar]
- 48.Heider Eur J Cancer. 1995;31A:2385. doi: 10.1016/0959-8049(95)00420-3. [DOI] [PubMed] [Google Scholar]
- 49.Heider Virchows Arch. 1996;428:267. doi: 10.1007/BF00196700. [DOI] [PubMed] [Google Scholar]
- 50.Heider Cancer Immunol Immunother. 1996;43:245. doi: 10.1007/s002620050329. [DOI] [PubMed] [Google Scholar]
- 51.Herold-Mende J Pathol. 1996;179:66. doi: 10.1002/(SICI)1096-9896(199605)179:1<66::AID-PATH544>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 52.Herrlich Ann N Y Acad Sci. 2000;910:106. [Google Scholar]
- 53.Hirata Cancer Res. 1998;58:1108. [PubMed] [Google Scholar]
- 54.Iczkowski Histopathology. 1999;35:150. doi: 10.1046/j.1365-2559.1999.00715.x. [DOI] [PubMed] [Google Scholar]
- 55.Inagaki Mod Pathol. 1999;12:546. [Google Scholar]
- 56.Ishida Jpn J Surg. 2000;30:28. [Google Scholar]
- 57.Jackson J Cell Biol. 1995;128:673. doi: 10.1083/jcb.128.4.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jansen Ann Oncol. 1998;9:109. [Google Scholar]
- 59.Kaaijk P, Troost D, Morsink F, Keehnen RM, Leenstra S, Bosch DA, Pals ST. Expression of CD44 splice variants in human primary brain tumors. J Neuro-Oncol. 1995;26:185. doi: 10.1007/BF01052621. [DOI] [PubMed] [Google Scholar]
- 60.Kainz Eur J Cancer. 1995;31A:1706. doi: 10.1016/0959-8049(95)00353-K. [DOI] [PubMed] [Google Scholar]
- 61.Kainz Gynecol Oncol. 1995;57:383. doi: 10.1006/gyno.1995.1159. [DOI] [PubMed] [Google Scholar]
- 62.Kainz Int J Cancer. 1996;69:170. doi: 10.1002/(SICI)1097-0215(19960621)69:3<170::AID-IJC3>3.3.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 63.Kanke Jpn J Cancer Res. 2000;91:410. doi: 10.1111/j.1349-7006.2000.tb00960.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kasper Amer J Resp Cell Mol Biol. 1995;13:648. [Google Scholar]
- 65.Katsura Gynecol Oncol. 1998;71:185. doi: 10.1006/gyno.1998.5169. [DOI] [PubMed] [Google Scholar]
- 66.Khaldoyanidi Leukemia Res. 1996;20:839. doi: 10.1016/S0145-2126(96)00048-3. [DOI] [PubMed] [Google Scholar]
- 67.Koopman J Exp Med. 1993;177:897. doi: 10.1084/jem.177.4.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Koretz Lancet. 1995;345:327. doi: 10.1016/s0140-6736(95)90320-8. [DOI] [PubMed] [Google Scholar]
- 69.Kunishi Int J Oral Maxillofac Surg. 1997;26:280. doi: 10.1016/s0901-5027(97)80869-7. [DOI] [PubMed] [Google Scholar]
- 70.Lagorce-Pages Histopathology. 1998;32:7. doi: 10.1046/j.1365-2559.1998.00316.x. [DOI] [PubMed] [Google Scholar]
- 71.Laurent Immunol Cell Biol. 1996;74:A1. doi: 10.1038/icb.1996.32. [DOI] [PubMed] [Google Scholar]
- 72.Leblanc Virchows Arch. 2001;438:78. doi: 10.1007/s004280000269. [DOI] [PubMed] [Google Scholar]
- 73.Legras Blood. 1998;91:3401. [PubMed] [Google Scholar]
- 74.Lesley Frontiers Biosci. 1998;3:616. [Google Scholar]
- 75.Lesley Adv Immunol. 1993;54:271. doi: 10.1016/s0065-2776(08)60537-4. [DOI] [PubMed] [Google Scholar]
- 76.Lesley J Biol Chem. 2000;275:26967. doi: 10.1074/jbc.M002527200. [DOI] [PubMed] [Google Scholar]
- 77.Liang J Cutan Pathol. 2002;29:459. doi: 10.1034/j.1600-0560.2002.290803.x. [DOI] [PubMed] [Google Scholar]
- 78.Lin J Korean Med Sci. 2001;16:317. doi: 10.3346/jkms.2001.16.3.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Manten-Horst Int J Cancer. 1995;64:182. doi: 10.1002/ijc.2910640307. [DOI] [PubMed] [Google Scholar]
- 80.Mizera-Nyczak Tumour Biol. 2001;22:45. doi: 10.1159/000030154. [DOI] [PubMed] [Google Scholar]
- 81.Miyoshi Clin Cancer Res. 1997;3:1289. [PubMed] [Google Scholar]
- 82.Morris EJSO. 2001;27:527. doi: 10.1053/ejso.2001.1167. [DOI] [PubMed] [Google Scholar]
- 83.Morrison Genes Dev. 2001;15:968. doi: 10.1101/gad.189601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mueller J Pathol. 1997;183:222. doi: 10.1002/(SICI)1096-9896(199710)183:2<222::AID-PATH923>3.0.CO;2-C. [DOI] [Google Scholar]
- 85.Mueller Virchows Arch. 1998;433:407. doi: 10.1007/s004280050267. [DOI] [PubMed] [Google Scholar]
- 86.Nanashima J Gastroenterol Hepatol. 1999;14:1004. doi: 10.1046/j.1440-1746.1999.01991.x. [DOI] [PubMed] [Google Scholar]
- 87.Naor Adv Cancer Res. 1997;71:241. doi: 10.1016/s0065-230x(08)60101-3. [DOI] [PubMed] [Google Scholar]
- 88.Neame EMBO J. 1992;11:4733. doi: 10.1002/j.1460-2075.1992.tb05578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Neu Bone Marrow Transplant. 1997;20:593. doi: 10.1038/sj.bmt.1700940. [DOI] [PubMed] [Google Scholar]
- 90.Nguen Neoplasma. 2000;47:400. [Google Scholar]
- 91.Noordzij Clin Cancer Res. 1997;3:805. [PubMed] [Google Scholar]
- 92.Ohene-Abuakwa Adv Exp Med Biol. 2000;465:115. doi: 10.1007/0-306-46817-4_11. [DOI] [PubMed] [Google Scholar]
- 93.Paradis J Urol. 1999;161:1984. doi: 10.1097/00005392-199906000-00079. [DOI] [PubMed] [Google Scholar]
- 94.Payne Cancer Cell. 2003;3:207. doi: 10.1016/s1535-6108(03)00057-6. [DOI] [PubMed] [Google Scholar]
- 95.Pirinen Hum Pathol. 2000;31:1088. doi: 10.1053/hupa.2000.16277. [DOI] [PubMed] [Google Scholar]
- 96.Ponta Int J Biochem Cell Biol. 1998;30:299. doi: 10.1016/S1357-2725(97)00152-0. [DOI] [PubMed] [Google Scholar]
- 97.Ponta Nature Rev Mol Cel Bio. 2003;4:33. doi: 10.1038/nrm1004. [DOI] [Google Scholar]
- 98.Ranuncolo J Surg Oncol. 2002;79:30. doi: 10.1002/jso.10045. [DOI] [PubMed] [Google Scholar]
- 99.Regauer J Pathol. 1999;187:184. doi: 10.1002/(SICI)1096-9896(199901)187:2<184::AID-PATH216>3.3.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 100.Ristamäki J Pathol. 1995;176:259. doi: 10.1002/path.1711760308. [DOI] [PubMed] [Google Scholar]
- 101.Rodriguez-Rodriguez Gynecol Oncol. 1998;71:223. doi: 10.1006/gyno.1998.5108. [DOI] [PubMed] [Google Scholar]
- 102.Rudy Cancer Res. 1993;53:1262. [PubMed] [Google Scholar]
- 103.Salmi J Cell Biol. 1993;122:431. doi: 10.1083/jcb.122.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Satoh Anticancer Res. 1997;17:215. [PubMed] [Google Scholar]
- 105.Schumacher Eur J Surg Oncol. 1996;22:259. doi: 10.1016/s0748-7983(96)80014-x. [DOI] [PubMed] [Google Scholar]
- 106.Screaton Proc Natl Acad Sci U S A. 1992;89:12160. doi: 10.1073/pnas.89.24.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Screaton J Biol Chem. 1993;268:12235. [PubMed] [Google Scholar]
- 108.Seiter J Exp Med. 1993;177:443. doi: 10.1084/jem.177.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Seiter Virchows Arch. 1996;428:141. doi: 10.1007/BF00200656. [DOI] [PubMed] [Google Scholar]
- 110.Sherman J Neurooncol. 1995;26:171. [Google Scholar]
- 111.Simon Eur J Cancer. 1996;32A:1394. doi: 10.1016/0959-8049(96)00196-7. [DOI] [PubMed] [Google Scholar]
- 112.Sinn Breast Cancer Res Treat. 1995;36:307. doi: 10.1007/BF00713402. [DOI] [PubMed] [Google Scholar]
- 113.Soukka Cancer Res. 1997;57:2281. [PubMed] [Google Scholar]
- 114.Speiser Int J Gyneol Cancer. 1999;9:160. doi: 10.1046/j.1525-1438.1999.09913.x. [DOI] [Google Scholar]
- 115.Stroomer Clin Cancer Res. 2000;6:3046. [PubMed] [Google Scholar]
- 116.Tempfer Eur J Cancer. 1996;32:2023. doi: 10.1016/0959-8049(96)00185-2. [DOI] [PubMed] [Google Scholar]
- 117.Tempfer Br J Cancer. 1998;77:1137. doi: 10.1038/bjc.1998.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tempfer Br J Cancer. 1998;78:1091. doi: 10.1038/bjc.1998.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tokue Jpn J Cancer Res. 1998;89:283. doi: 10.1111/j.1349-7006.1998.tb00560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tran Hum Pathol. 1997;28:809. doi: 10.1016/s0046-8177(97)90154-4. [DOI] [PubMed] [Google Scholar]
- 121.Van J Biol Chem. 1999;274:6499. doi: 10.1074/jbc.274.10.6499. [DOI] [PubMed] [Google Scholar]
- 122.Van Int J Cancer. 1996;68:520. doi: 10.1002/(SICI)1097-0215(19961115)68:4<520::AID-IJC19>3.3.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 123.Van Int J Cancer. 1999;82:837. doi: 10.1002/(SICI)1097-0215(19990909)82:6<837::AID-IJC12>3.3.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 124.Van PCR Methods Appl. 1993;3:100. doi: 10.1101/gr.3.2.100. [DOI] [PubMed] [Google Scholar]
- 125.Varambally Nature. 2002;419:624. doi: 10.1038/nature01075. [DOI] [PubMed] [Google Scholar]
- 126.Verel Int J Cancer. 2002;99:396. doi: 10.1002/ijc.10369. [DOI] [PubMed] [Google Scholar]
- 127.Wagner SN, Wagner C, Reinhold U, Funk R, Zoller M, Goos M. Predominant expression of CD44 splice variant v10 in malignant and reactive human lymphocytes. J Invest Dermatol. 1998;111:464. doi: 10.1046/j.1523-1747.1998.00302.x. [DOI] [PubMed] [Google Scholar]
- 128.Wielenga Scand J Gastrenterol. 1998;33:82. doi: 10.1080/00365529850166257. [DOI] [Google Scholar]
- 129.Wimmel Lung Cancer. 1997;16:151. doi: 10.1016/S0169-5002(96)00625-3. [DOI] [PubMed] [Google Scholar]
- 130.Xin Appl Immunohistochem Mol Morphol. 2001;9:138. doi: 10.1097/00022744-200106000-00006. [DOI] [PubMed] [Google Scholar]
- 131.Zhang JBC. 1998;273:11342. doi: 10.1074/jbc.273.18.11342. [DOI] [PubMed] [Google Scholar]
- 132.Zöller J Mol Med. 1995;73:425. doi: 10.1007/BF00202261. [DOI] [PubMed] [Google Scholar]