Abstract
Interferon α (IFN-α) has been widely used in the treatment of human solid and haematologic malignancies. Although the antitumour activity of IFN-α is well recognised at present, no major advances have been achieved in the last few years. Recent findings have provided new information on the molecular mechanisms of the antitumour activity of the cytokine. In fact, IFN-α appears to block cell proliferation, at least in part, through the induction of apoptotic effects. This cytokine can also regulate the progression of tumour cells through the different phases of the cell cycle inducing an increase of the expression of the cyclin-dependent kinase inhibitors p21 and p27. However, it must be considered that IFN-α is a physiologic molecule with ubiquitously expressed receptors that is likely to activate survival mechanisms in the cell. We have recently identified an epidermal growth factor (EGF) Ras-dependent protective response to the apoptosis induced by IFN-α in epidermoid cancer cells. The identification of tissue- and/or tumour-specific survival pathways and their selective targeting might provide a new approach to improve the efficacy of IFN-α–based treatment of human cancer. Moreover, new pegylated species of IFN-α are now available with a more favourable pharmacokinetic profile. We will review these achievements, and we will specifically address the topic of IFN-α–based molecularly targeted combinatory antitumour approaches.
Keywords: Combinatory treatment, Interferon α, Pegylated species, Survival pathways
Introduction
Interferon α (IFN-α) is the first cytokine produced by recombinant DNA technology for wide-spread clinical usage in the treatment of infectious as well as malignant diseases. The type I IFNs, α and β (IFN-α/β), comprise the products of multiple (>12) IFN-α genes and of a single IFN-β gene [63]. At the present, IFN-α is a licensed agent for haematologic as well as for solid tumours [38, 78, 79]. In clinical practice, IFN-α is used in the treatment of multiple myeloma (MM), chronic myeloid leukaemia (CML), non-Hodgkin’s lymphoma (NHL), renal cell carcinoma (RCC), epidermoid cervical cancer, head and neck tumours (H&NT), melanoma and medullary thyroid carcinoma (MTC). The effectiveness of IFN-α in these diseases is presently clearly recognized, but no major advances have been achieved in the last few years. In Table 1, we provide a general overview of IFN-α activity in human malignancies [2, 36, 42, 43, 54, 64].
Table 1.
IFN-α efficacy in human malignancies. RFS Relapse-free survival, OS overall survival, HR haematologic response, MS median survival, OBS observation, i.v. intravenous, s.c. subcutaneous
| Tumour type | Stage | Doses IFN-α | Clinical effects |
|---|---|---|---|
| Melanoma adjuvant | |||
| ECOG E1684 study [41] | IIb, III | 20 MU/m2 i.v. 5 days per week for 1 month | Benefit in RFS and OS at 6.9 years (p=0.0237) |
| IFN-α vs Obs | 10 MU/m2 s.c. three times a week for 3 years | ||
| ECOG E1690 study [42] | IIb, III | 20 MU/m2 i.v. 5 days per week for 1 month | Benefit in RFS at 5.1 years |
| IFN-α vs Obs | 10 MU/m2 s.c. three times a week for 48 weeks | ||
| ECOG E2696 study [43] | IIb, III | 20 MU/m2 i.v. 5 days per week for 1 month | Benefit in RFS and OS |
| IFN-α vs GMK vaccine | 10 MU/m2 s.c. three times a week for 3 years | ||
| Metastatic renal cell cancer | |||
| Council renal cancer collaborators [54] | IV | 5–10 MU for the first week then 10 MU three times per week for 4 weeks | Benefit in 1-year survival and MS |
| IFN-α vs medroxyprogesterone acetate | |||
| Pyrhonen et al. [64] | IV | 3 MU s.c. three times a week for 1 week | Benefit in MS |
| IFN-α + vinblastine (VLB) vs VBL alone | 18 MU s.c. three times a week and VLB | ||
| Chronic myeloid leukemia | |||
| The Italian Cooperative Study Group on chronic myeloid leukemia [36] | 3 MU for the first 2 weeks | Benefit in HR rate and OS (p=0.002) | |
| IFN-α vs hydroxyurea/busulfan | 6 MU for another 2 weeks | ||
| 9 MU thereafter | |||
| The UK Medical Research Council’s working parties for therapeutic trials in adult leukaemia [2] | 3 MU per day s.c. for 3 weeks after 6, 9, or 12 MU per day if tolerated and leukocyte count was good | Benefit in OS (p=0.0009) | |
| IFN-α vs hydroxyurea/busulfan | |||
| Multiple myeloma | |||
| Myeloma trialists’ collaborative group (meta-analysis) [58] | Benefit in RFS (p<0.00001) and OS (p=0.01) | ||
| IFN-α vs no IFN-α | |||
| Fritz et al. (meta-analysis) [30] | Benefit in RFS (p<0.01) and OS (p<0.01) | ||
| IFN-α vs no IFN-α | |||
As for the majority of biological agents, antitumour activity of IFN-α is somewhat difficult to evaluate, ranging from clear-cut tumour regression in some instances, to symptomatic relief in others. These latter effects are difficult to evaluate. Therefore, structured algorithms for clinical benefit assessment as well as quality-of life-questionnaires have been recently generated to measure the activity of biological agents. We have developed an algorithm able to provide unbiased and objective criteria for the definition of response to the therapeutic combination between IFN-α and lanreotide in MTC [76, 77].
A wide range of different schedules and doses have been used, up to the high-dose approach that resulted in an efficacious strategy in the adjuvant setting for high-risk patients with resected malignant melanoma and node metastases [42, 43]. However, high-dose IFN-α also produced important toxicity, and at least 40% of patients required dose reduction and/or recycling delay due to severe toxicity, such as granulocytopenia, liver toxicity, neurological disorders, and fatigue.
The optimal method of IFN-α administration is still far from being defined in the different tumour affections. A promising approach is its use in combination with other bioreagents. In fact, IFN-α has been concomitantly administered with somatostatin analogs in MTC [76, 77], with the anti-CD-20 MAb rituximab in NHL [66], and with retinoic acid in RCC [56] and cervical carcinomas [79]. The combination of outpatient subcutaneous (s.c.) IFN-α and interleukin 2 (IL-2), according to the Atzpodien regimen, produces long-term survival benefits in a subset of patients with metastatic RCC and has achieved wide clinical usage [3]. Although some of these combinations have been proven to be effective in the clinical setting and, in some instances, have been based on preclinical studies, the whole range of rationally designed therapeutic combinations remains mostly unexplored and has still not been translated into clinical practice.
In the last few years, major advances have been achieved in the identification of cellular targets of IFN-α action at the tumour cell level and different escape mechanisms have been recognised; at the same time, novel pharmaceutical preparations have been generated to provide a more favourable pharmacokinetic (PK) profile.
In this article, we will review these important achievements and discuss the potential new avenues for the rational use of IFN-α in the treatment of human malignancies.
Cellular and molecular basis of IFN-α-mediated antitumour effects
Interferon α modulates cancer cell growth and differentiation, and affects cellular communication and intracellular signalling. However, the way tumour cell growth is suppressed in vivo by IFN-α remains to be fully elucidated. The occurrence of apoptotic effects in tumour cells exposed to IFN-α has been widely described, but the induction of nonapoptotic cell death has also been observed [18].
For decades, direct and indirect antitumour effects of IFN-α have been postulated taking into account the presence of the specific type I IFN receptor at the tumour cell level. These effects induced by IFN-α can be translated in the direct tumour cell growth inhibition and/or cell death, and/or the stimulation of an antitumour immune response (indirect effects).
The up-regulated expression of MHC class I antigens has been generally considered to play a role in the antitumour activity of IFN-α. In fact, this effect might favour tumour cell recognition by specific cytolytic T cells as well as the activation of cytotoxic effectors of the innate cell-mediated immune response, i.e. natural killer (NK) cells. Indeed, very recent studies have demonstrated that the absence of signal transducer and activator of transcription (STAT) 1-dependent signalling (see below) makes transgenic mice STAT1−/− injected with STAT1+/+ melanoma cells unable to benefit from antitumour treatment with IFN-α [48]. The cytokine appears therefore to produce its activity in this cell system mainly through the activation of NK cells. An additional important finding has been provided by the E2690 laboratory corollary of intergroup adjuvant trial E1690 of high-dose IFN-α in high-risk resected melanoma. In fact, it is shown that important changes in immunologic parameters are induced by the treatment with IFN-α and are dependent upon the administered dosage of the cytokine [44]. Finally, an interesting recent hypothesis has been proposed on the role of IFN-α/β in producing an important link between innate and adaptive immune responses [5]. It is our opinion that the indirect “immunologic” effects of IFN-α remain a provocative issue, which should be addressed now in clinical studies taking advantage of the new information and technologies which are presently available for the study of antitumour immunity. Tumour microenvironmental antagonizing factors, such as reactive oxygen species, might explain the lack of IFN-α effects on NK antitumour activity in vivo and should be specifically evaluated [35].
An additional important mechanism of the IFN-α–mediated antitumour activity appears to rely on the interference with tumour-mediated angiogenesis as demonstrated by several observations from Folkman’s and Fidler’s research groups [29, 69].
In our opinion, all these hypotheses are still valid and provide wide fields for investigation. However, we think that they need to be integrated into a general view of microenvironmentally based activity of IFN-α. In fact, it must be considered that IFN-α is a physiologic molecule and specific receptors (see below) are ubiquitously expressed in human organisms [60]. It is conceivable that human cells have developed resistance mechanisms to avoid being killed by IFN-α and that such mechanisms should be operating also in the presence of pharmacological IFN-α concentrations. In this case the tumour cells will develop a stress response and probably up-regulate the resistance mechanisms due to specific survival signalling pathways. We have hypothesized, providing also experimental evidence, that the selective targeting of these pathways is a powerful strategy to potentiate the antitumour effects of IFN-α.
The IFN-α signalling
IFN-α signalling is mediated by the activation of the human type I IFN-α receptor that is composed, in the activated state, of two protein chains called IFNAR1 and IFNAR2c [19, 22, 51, 59, 61, 80, 83, 84].
The binding of IFN-α induces the assembly of these two chains and their phosphorylation in the intracellular domain by the Janus kinases Tyk2 and Jak1, which themselves become activated by tyrosine phosphorylation. The activated Janus kinases mediate the phosphorylation and activation of STAT1 and/or STAT2 proteins which retain transcriptional regulatory activity [20, 23]. Moreover, the phosphorylated STATs are released from the cytoplasmic region of the receptor subunits to form homodimers or heterodimers through reciprocal interaction between the phosphotyrosines on SH2 domains of different STAT proteins [46, 68, 72]. The heterodimer STAT1/STAT2 associates with another cytoplasmic protein, p48, resulting in the formation of a protein complex named interferon-stimulated gene factor 3 (ISGF3) that translocates to the nucleus and modulates target gene transcription by the binding to regulatory regions called interferon-stimulated response elements (ISRE) [18, 31].
The activated Jak1 can also stimulate STAT3 that acts as adapter to couple another signalling pathway to the type I IFN receptor: the phosphoinositide kinase-3 (PI3 K)-dependent pathway [83] (See below).
Other molecular targets of IFN-α receptor pathway are protein kinase C (PKC)-δ (a member of the protein kinase C family that is activated during the engagement of type I IFN receptor and associates with STAT1) [75] and the SH2-containing protein-tyrosine phosphatases 1 and 2 (SHP1 and SHP2). SHP1 participates in negative control of distinct components in the JAK/STAT pathway [25], and SHP2 is a positive regulator in mitogenic stimulation of ERK (extracellular signal-regulated kinase), which has a negative effect in JNK activation under cellular stress [89].
IFN-α regulates cell cycle and apoptosis
IFN-α induces apoptosis in tumour cell lines [65]. It must be considered that apoptosis is an active process that eliminates unnecessary or damaged cells. It is generally considered to involve different phases based on cellular events that have been only partially defined at the molecular level. Some of these are the positive regulation on death receptor signalling (see below), the activation of the cystein protease caspase cascade and the activation of the mitochondrial system through the stimulation of the proapoptotic proteins Bak and Bax [47].
Death receptors of the tumour necrosis factor α (TNF-α) family (TNF-α/TNF-αR, FasL/Fas, Apo1, TRAIL/TRAILR, Apo2) have been investigated as possible mediators of IFN-α induced proapoptotic activity [21, 52, 55].
FasL induction has been described to mediate the apoptotic activity of IFN-α in human basal cell carcinoma [8, 90] and in CML cells [69]. Moreover, Fas activation by TNF-α cross-linking has been described by the Talpaz group in human Burkitt lymphoma Daudi cells exposed to IFN-α [33], while type I IFN-β induces apoptosis in melanoma cells by selective induction of TNF-related apoptosis inducing the ligand/apoptosis-2 ligand (TRAIL/Apo2L) [17]. The cell and tissue specificity of the activation of such death receptors by IFN-α/β is another important topic of investigation. IFN-α can also contribute to boosting p53 responses to stress signal [73], or activate p38 and its downstream kinases [53].
Another mechanism of IFN-α antitumour effects is the regulation of cell cycle. More than 10 years ago it was demonstrated that the induction of RB/p105 expression in Daudi cells could be an important mechanism of IFN-α–mediated growth regulation [91]. More recently, it has been found that activated STAT1 induces an up-regulation of cyclin-dependent kinase inhibitors (CDKI) p21 and p27 and, consequently, reduces the phosphorylative status of RB/p105 [67].
Moreover, IFN-α has postgenomic effects that are based on the regulation of protein synthesis machinery and on the selective translation of proteins correlated to growth arrest and apoptosis. In this regard, IFN-α induces dsRNA-activated protein kinase (PKR). Identified PKR substrates are eukariotic initiation factor 2 (eIF2), NF-KB, IRF1, p53, STAT1, and NF-90. The modulation of these targets results in the control of cell growth, differentiation and apoptosis. In details, PKR activation regulates translational and transcriptional pathways (eIF-2a and NF-kB-dependent) resulting in the specific expression of selected proteins (Fas, p53, Bax and others) that trigger cell death by engaging the caspase pathway [32, 37]. The role of PKR in tumour cell growth regulation is, however, far from being elucidated even if higher enzyme levels have been associated with tumour progression in human melanoma and colon cancer [40].
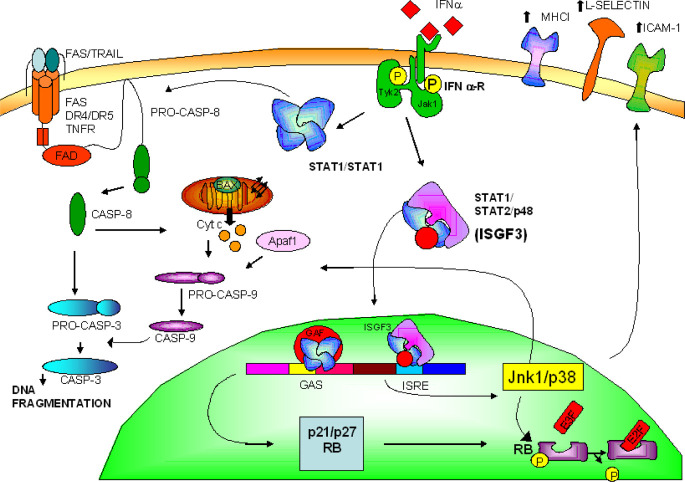
Another protein synthesis factor, the eIF-5A, is a molecular target of IFN-α [49]. In fact, we have demonstrated that IFN-α reduces the activity of this protein and that an inactivator of eIF-5A, GC7, synergizes with IFN-α in inducing apoptosis and tumour cell growth inhibition [14]. We have, moreover, demonstrated that IFN-α induces apoptosis in human epidermoid cancer cells by a stress signalling which targets JNK-1 and p38 mitogen-activated protein kinase [12, 13] (Fig. 1).
Fig. 1.
IFN-α regulates cell cycle and apoptosis. Activation of STAT pathways by the type I IFN/tyrosine kinases (Jak/Tyk-2) complex leads to induction of proapoptotic machinery through caspase cascade activation depending upon Fas/FasL up-regulation, Jnk1/p38 stimulation or still unidentified mechanisms; inhibition of cell cycle progression via p21/p27 increased expression, p105 hypophosphorylation and retardation of cell cycle progression
Escape mechanisms to the antitumour effects of IFN-α
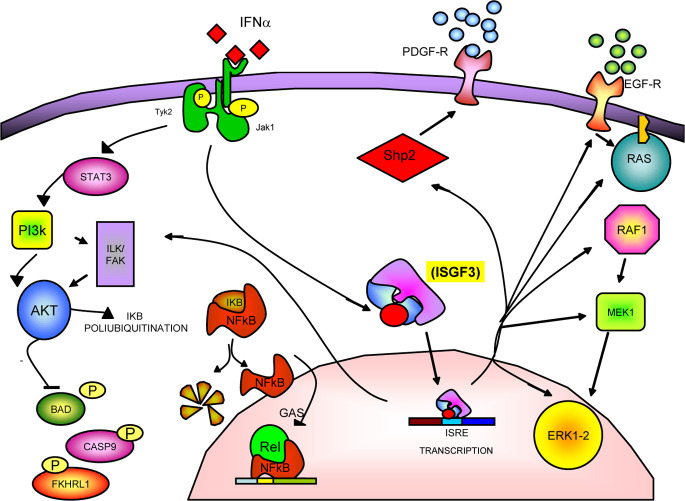
Interferon α induces different survival pathways that protect tumour cells from different stress-producing events (i.e. viral infections). One of these survival pathways is mediated by PI3 K and by several of its downstream targets [86]. In detail, after IFN-α binding, the IFN-αR1 subunit recruits and activates STAT3 protein. The activated STAT3 acts as adapter for PI3 K, forming a final complex composed of IFNAR1, STAT3, p85 and PI3 K [75]. One of the targets downstream to PI3 K is the serine-threonine kinase Akt, which is important for the generation of antiapoptotic signals such as the phosphorylation, and consequent inactivation, of caspase-9 [15], BAD [24], and Forkhead transcription factor FKHRL1 [6] (which modulates the Fas-L gene expression). Another effect induced by IFN-α is the induction of the ubiquitin cross-reactive protein (ISG 15) and of two ubiquitin-conjugating enzymes (UbcH5 and UbcH8) [62]. A molecular target of these enzymes is the inhibitor of kB (IkB) protein, a cytoplasmic protein that binds and inactivates NF-kB. IkB degration activates Rel/NFkB that triggers the transcription of genes involved in the apoptotic/antiapoptotic process. The involvement of NFkB in a survival pathway induced by IFN-α has recently been demonstrated by Pfeffer et al. [87]. You et al. [89] have studied other targets involved in the IFN-α–activated pathway, such as the Shp1 and Shp2 proteins. After IFN-α binding, Shp1 is associated to the IFN-α receptor, complexed to Jak1 and Tyk2. In this complex, it exercises a negative feedback by inhibiting the IFN-α–stimulated Jak/STAT pathways. Moreover, Shp2 has a positive effect on the mitogenic stimulation of ERK 1/2 and on the expression of platelet-derived growth factor receptor and, on the other hand, inhibits JNK under cellular stress [89]. Other Jak/STAT pathway inhibitors have recently been identified, such as the suppressors of cytokine signalling (SOCS)-1 and 2 [71]. These two proteins contain an SH2-domain and can inhibit Jak, thus playing a negative control on the Jak/STAT signalling. Other antiapoptotic factors are the protein inhibitor of activated STAT (PIAS) proteins. In detail, PIAS1 [50] inhibits the STAT-1 DNA binding activity and the consequent induction of gene expression. Moreover, Ki-Ras can inhibit the expression of IFN-α–regulated genes providing a novel mechanism of tumour cell escape to the cytokine [45].
Finally, we have demonstrated that IFN-α regulates another important survival pathway, which is mediated by the EGF-receptor (EGF-R). In fact, IFN-α is able to increase EGF-R expression and function [7, 11, 13] and to activate its downstream targets that include the Ras/MEK1/ERK1-2 pathway. This effect appears to protect the cells from apoptosis, behaving like a survival pathway. Selective inhibition of this pathway at different molecular levels always results in increased apoptosis in cells exposed to IFN-α. In fact, either the transfection of a dominant form of Ras RASN17 or the treatment of tumour cells with a specific MEK-1 inhibitor (PD098056) strongly potentiated the apoptosis induced by the cytokine [13] (Fig. 2).
Fig. 2.
Escape mechanisms to the antitumour effects of IFN-α. Survival and antiapoptotic signalling are induced by IFN-α through the activation of different pathways. In detail, the following have been reported: the hyperactivation of the EGF-mediated Ras/Raf/ERK1-2–dependent pathway; the induction of the Akt and NFkB-dependent pathways through the activation of the STAT3/PI3 K–mediated signalling; and the stimulation of Shp2 that determines an increased response to peptide growth factors
IFN-α pharmacokinetics and novel IFN-α species
The pharmacological characteristics of the IFN-α family are based on the impossibility of its oral administration due to proteolytic degradation by the gastrointestinal tract. Interferon α is commonly administered s.c. or i.m. which allows >80% absorption and the achievement of detectable serum concentrations [82]. These modalities of administration determine a transient serum distribution phase with maximal serum or plasma concentrations commonly recorded after 1–8 h, followed by a measurable concentration phase lasting 4–24 h after injection. A bioexponential decrease in serum concentration has been observed with intravenous delivery of IFN-α. PK analysis has demonstrated that a median maximum observed serum concentration (C max) of 2,630 IU/ml might be achieved at the end of the induction intravenous phase of high-dose interferon according to Kirkwood’s schedule and 149 IU/ml after s.c. administration. In the latter administration modality, 8 h are required to achieve C max. The terminal elimination half-life is 4–6 h for both modalities of IFN-α administration [44, 82].
The general picture arising from these data suggests that newer approaches for the achievement of protracted drug serum concentration are strictly required to enhance the IFN-α bioavailability. The PK profile which can be achieved by i.m./i.v. administration appears indeed to produce relevant side effects, while the concentrations achievable in the serum as well as in the target tumour tissues do not appear to be consistent with the concentration necessary to produce antitumour activity in the preclinical experimental systems.
A novel formulation that readily modifies the PK profile of IFN-α is the pegylated form. These pegylated species of IFN-α are now available for clinical use (Table 2) [9, 10, 57].
Table 2.
Novel IFN-α formulations
| IFN-α species | Molecular characteristics | Doses | Advantages vs conventional formulation |
|---|---|---|---|
| Pegylated IFN-α-2a (Pegasys) | 40-kDa branched polyethylene glycol (PEG) | 450-μg weekly administration | Allows protracted tumour cell exposure |
| Improves patient compliance | |||
| Reduces toxicity | |||
| Pegylated IFN-α-2b (Peg-Intron A) | 12-kDa monomethoxy PEG | 7.5 μg/kg weekly administration | Allows protracted tumour cell exposure |
| Improves patient compliance | |||
| Reduces toxicity |
One of these species is the polyethylene glycol (PEG) IFN-α2b (PEG-Intron; Schering-Plough, Kenilworth, NJ, USA) that has been produced by attaching a 12-kDa monomethoxy PEG polymer to the IFN-α2b protein. This new pegylated form at weekly doses of 7.5 μg/kg can allow the achievement of persistent serum concentrations of 9×103 pg/ml, and induces a tenfold increase of the serum half-life (t 1/2) (from approximately 4–40 h) [10]. It has also been demonstrated that this species retains its biologic activity as compared with unpegylated IFN-α2b. Most of the clinical studies with PEG-IFN were made in patients with chronic hepatitis C, CML, and in solid tumours such as melanoma and RCC. In these patients, the weekly administration of PEG-IFN appeared to improve patient compliance and to reduce the related toxicity [9]. Moreover, the most commonly reported adverse events in patients with solid tumours receiving weekly administration of PEG-IFN were nausea, anorexia, fatigue and rigors. However, the severity of these events was mild to moderate and rarely were grade 3 or 4 (according to WHO) events recorded.
Another new pegylated species of IFN-α2a (Pegasys; Hoffman-La Roche, Nutley, NJ, USA) has also been developed, and clinical studies with this new reagent have been performed in patients with solid tumours [57]. Pegasys has been constructed by the attachment of a 40-kDa branched PEG moiety (larger than the 12-kDa linear moiety of pegylated IFN-α2b) to IFN-α2a. With these species, the PK properties are improved and a favourable PK profile is readily achievable by weekly administration. After s.c. injection, serum concentration of PEG-IFN-α2a increases, becoming measurable at 6 h; maximum serum IFN-α concentrations are reached after 48 h and remain elevated for a long time [9, 10].
At present, pegylated IFN-α species appear to provide a theoretically more advantageous PK profile and a somewhat better tolerance, as compared to the conventional recombinant species, while the antitumour activity, although promising, needs to be evaluated in larger studies.
New approaches to IFN-α–containing therapeutic strategies based on recent pharmacodynamic and pharmacokinetic advances
The scenario of IFN-α use in human cancer is rapidly changing. New species of IFN-α, derived from molecular changes able to produce a more favourable PK profile, are now available and large-scale studies to define their precise role in antitumour treatment are eagerly awaited.
The molecular studies have allowed the identification of valuable targets for combinatory approaches which can be now designed on solid molecular rationales and appear ready to be translated from the bench to the bedside. The notion that IFN-α is a physiological moiety has suggested that tumour cells can indeed escape from the antiproliferative and proapoptotic effects induced by this cytokine through the hyperactivation of physiological survival pathways. This hypothesis has indeed been evaluated in different experimental systems. We and others have been able to demonstrate that the Ras/MEK1/ERK and the Akt/NFkB pathways can indeed mediate a survival response in the cells exposed to IFN-α. Moreover, the selective targeting of critical transducing molecules—such as EGFR, Ras oncoprotein, and MEK1/ERK—by the use of different bioreagents resulted in a strong synergistic activity in human epithelial cancer and melanoma cells. Interestingly, the antitumour effects of IFN-α could be potentiated by means of highly selective agents like the quinazolinic EGFR inhibitor Gefitinib (IRESSA; Astra Zeneca, UK) (Budillon et al., in preparation) or the dominant negative Ras N17, as well as by the use of less selective molecules like the farnesyl transferase inhibitors (FTI) R115777 (Tipifarnib; Zarnestra; Johnson & Johnson Pharmaceutical Research and Development, Raritan, NJ, USA) (Tagliaferri et al. AACR 2003). These results suggest that both single and multiple pathway inhibitors might be useful to increase the antitumour activity of IFN-α, reducing also its therapeutic doses. However, additional experimental work is required to fully address these important questions. The molecular bases for the rational combination of IFN-α with imatinib mesylate (Gleevec, Glivec, formerly STI571; Novartis, Basel, Switzerland) is an additional important area of investigation. It has been demonstrated that IFN-α and imatinib mesylate are synergistic in the growth inhibition of CML cell lines [74]. This latter combinatory approach (presently in clinical testing) might readily be improved by the recent identification of specific mechanims of resistance to imatinib mesylate at the BCR/ABL level, the fusion oncoprotein in CML cells [81]. Moreover, it has been demonstrated that the Rac1/p38 MAPK pathway mediates the growth-inhibitory effect of IFN-α in human BCR-ABL–expressing CML cells [53].
Another important issue to be considered is the relative resistance of human tumours to the antisignalling approaches based on small molecules or MAbs. This observation could be explained on the basis of pathway redundancy in human tumour cells characterized by the existence of multiple and often compensatory survival and proliferative signals. Therefore, it becomes even more urgent that we define new strategies to switch off these antiapoptotic pathways. In this view, IFN-α can be of help. In fact, the exposure of tumour cells to IFN-α can result in the activation of tumour- or tissue-specific survival responses based on a few transducers that assure the protection of tumour cells from the apoptosis and growth inhibition induced by the cytokine. In this way, tumour cells exposed to IFN-α become highly sensitive to specific signalling inhibitors (“target prioritization”) avoiding the need for a wide inhibition of multiple survival signals. Moreover, new gene expression and proteomic profiling technologies, which have been made available in the last few years or are in rapid development, might be of major help for the identification and dissection of such pathways and for the use of patient-customized approaches in the clinical setting [39].
The novel IFN-α species that can produce stable serum concentration appear to offer an added value for combinatory approaches, taking into account that most of the in vitro data have been achieved in cells continuously exposed (24–48 h) to IFN-α. Such exposure conditions cannot currently be achieved in vivo by the conventional i.v./i.m. routes due to the brief serum half-life of unpegylated IFN-α. In fact, serum concentrations of bioavailable cytokine equivalent to experimental conditions cannot easily be reached with conventional IFN-α pharmaceutical formulations, thus hampering the translational potential of such studies.
Finally, it has to be considered that the effects of IFN-α therapy are not only the inhibition of tumour cell growth and the improvement of survival, but also the control of clinical symptoms. There is now great interest in the potential clinical advantages produced by the treatment of highly symptomatic human tumours even in the absence of objective tumour regression. Therefore, emerging medical approaches must be evaluated for their impact on the functional, psychological and social health of individuals. Although tumour response, disease-free interval, time to progression, and overall survival remain the main endpoints of cancer clinical trials, quality of life has been included as a major parameter in the evaluation process.
In conclusion, new molecular-based rationales as well as new bioreagents in early clinical testing are now ready to expand the usage of IFN-α in the treatment of human malignancies. A translational approach, integrating preclinical and clinical studies, is strictly required for the achievement of such important goals.
References
- 1.Ahmad J Biol Chem. 1997;272:29991. doi: 10.1074/jbc.272.48.29991. [DOI] [PubMed] [Google Scholar]
- 2.Allan Lancet. 1995;345:1392. doi: 10.1016/s0140-6736(95)92596-1. [DOI] [PubMed] [Google Scholar]
- 3.Atzpodien Cancer. 2002;95:1045. doi: 10.1002/cncr.10783. [DOI] [PubMed] [Google Scholar]
- 4.Berg Clin Cancer Res. 1999;5:1671. [PubMed] [Google Scholar]
- 5.Biron Immunity. 2001;14:661. doi: 10.1016/S1074-7613(01)00154-6. [DOI] [PubMed] [Google Scholar]
- 6.Brunet Cell. 1999;96:857. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 7.Budillon Cancer Res. 1991;51:1294. [PubMed] [Google Scholar]
- 8.Buechner J Clin Invest. 1997;100:2691. doi: 10.1172/JCI119814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bukowski J Clin Oncol. 2002;20:3841. doi: 10.1200/JCO.2002.02.051. [DOI] [PubMed] [Google Scholar]
- 10.Bukowski Cancer. 2002;95:389. doi: 10.1002/cncr.10663. [DOI] [PubMed] [Google Scholar]
- 11.Caraglia Int J Cancer. 1995;61:342. doi: 10.1002/ijc.2910610312. [DOI] [PubMed] [Google Scholar]
- 12.Caraglia Cell Death. 1999;Differ:773. doi: 10.1038/sj.cdd.4400550. [DOI] [PubMed] [Google Scholar]
- 13.Caraglia Cell Death Differ. 2003;10:218. doi: 10.1038/sj.cdd.4401131. [DOI] [PubMed] [Google Scholar]
- 14.Caraglia M, Marra M, Giuberti G, D’Alessandro AM, Baldi A, Tassone P, Venuta S, Tagliaferri P, Abbruzzese A. The eukaryotic initiation factor 5A is involved in the regulation of proliferation and apoptosis induced by interferon-alpha and EGF in human cancer cells. J Biochem (Tokyo) 2003;133:757. doi: 10.1093/jb/mvg097. [DOI] [PubMed] [Google Scholar]
- 15.Cardone Science. 1998;282:1318. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- 16.Chang Leukemia. 2003;17:590. doi: 10.1038/sj.leu.2402824. [DOI] [PubMed] [Google Scholar]
- 17.Chawla-Sarkar Clin Cancer Res. 2001;7:1821. [PubMed] [Google Scholar]
- 18.Chawla-Sarkar Apoptosis. 2003;8:237. doi: 10.1023/A:1023668705040. [DOI] [PubMed] [Google Scholar]
- 19.Cohen Mol Cell Biol. 1995;15:4208. doi: 10.1128/mcb.15.8.4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Colamonici Mol Cell Biol. 1994;14:8133. doi: 10.1128/mcb.14.12.8133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Costelli Cell Death Differ. 2003;10:997. doi: 10.1038/sj.cdd.4401281. [DOI] [PubMed] [Google Scholar]
- 22.Croze Reconstitution of a high affinity binding site for type I interferons J Biol Chem. 1996;271:33165. doi: 10.1074/jbc.270.44.26033. [DOI] [PubMed] [Google Scholar]
- 23.Darnell Science. 1994;264:1415. [Google Scholar]
- 24.Datta Cell Oct. 1997;91:231. doi: 10.1016/S0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 25.David J Biol Chem. 1996;271:4585. doi: 10.1074/jbc.271.8.4134. [DOI] [PubMed] [Google Scholar]
- 26.Dijkers Mol Cell Biol. 2000;20:9138. doi: 10.1128/MCB.20.24.9138-9148.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dijkers Curr Biol. 2000;10:1201. doi: 10.1016/S0960-9822(00)00728-4. [DOI] [PubMed] [Google Scholar]
- 28.Dijkers J Cell Biol. 2002;156:531. doi: 10.1083/jcb.200108084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ezekowitz N Engl J Med. 1992;326:1456. doi: 10.1056/NEJM199205283262203. [DOI] [PubMed] [Google Scholar]
- 320.Fritz Ann Oncol. 2000;11:1427. doi: 10.1023/a:1026548226770. [DOI] [PubMed] [Google Scholar]
- 31.X Proc Natl Acad Sci U S A. 1990;87:8555. [Google Scholar]
- 32.Gil Apoptosis. 2000;5:107. doi: 10.1023/A:1009664109241. [DOI] [PubMed] [Google Scholar]
- 33.Gisslinger Blood. 2001;97:2791. doi: 10.1182/blood.V97.9.2791. [DOI] [PubMed] [Google Scholar]
- 34.Gray Cancer Control. 2002;9:16. doi: 10.1177/107327480200900103. [DOI] [PubMed] [Google Scholar]
- 35.Herberman Semin Oncol. 2002;29:27. doi: 10.1053/sonc.2002.33079. [DOI] [PubMed] [Google Scholar]
- 36.The N Engl J Med. 1994;330:820. doi: 10.1056/NEJM199403243301204. [DOI] [PubMed] [Google Scholar]
- 37.Jagus Int J Biochem Cell Biol. 1999;31:123. doi: 10.1016/S1357-2725(98)00136-8. [DOI] [PubMed] [Google Scholar]
- 38.Jonasch Interferon in oncological. 2001;practice:review. [Google Scholar]
- 39.Khan Pharmacogenomics. 2003;4:245. doi: 10.1517/phgs.4.3.245.22696. [DOI] [PubMed] [Google Scholar]
- 40.Kim Oncogene. 2002;21:8741. doi: 10.1038/sj.onc.1205987. [DOI] [PubMed] [Google Scholar]
- 41.Kirkwood J Clin Oncol. 1996;14:7. [Google Scholar]
- 42.Kirkwood J Clin Oncol. 2000;18:2444. [Google Scholar]
- 43.Kirkwood J Clin Oncol. 2001;19:1430. doi: 10.1200/JCO.2001.19.5.1430. [DOI] [PubMed] [Google Scholar]
- 44.Kirkwood Cancer. 2002;95:1101. doi: 10.1002/cncr.10775. [DOI] [PubMed] [Google Scholar]
- 45.Klampfer J Biol Chem. 2003;278:46278. doi: 10.1074/jbc.M304721200. [DOI] [PubMed] [Google Scholar]
- 46.Koch Science. 1991;252:668. doi: 10.1126/science.1708916. [DOI] [PubMed] [Google Scholar]
- 47.Lawen Bioessays. 2003;25:888. doi: 10.1002/bies.10329. [DOI] [PubMed] [Google Scholar]
- 48.Lesinski J Clin Invest. 2003;112:170. doi: 10.1172/JCI200316603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lipowsky EMBO J. 2000;19:4362. doi: 10.1093/emboj/19.16.4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu Proc Natl Acad Sci U S A. 1998;95:10626. doi: 10.1073/pnas.95.18.10626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lutfalla EMBO J. 1995;14:5100. doi: 10.1002/j.1460-2075.1995.tb00192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lyonel The Oncologist. 1999;4:332. [PubMed] [Google Scholar]
- 53.Mayer J Biol Chem. 2001;276:28570. doi: 10.1074/jbc.M011685200. [DOI] [PubMed] [Google Scholar]
- 54.Medical Lancet. 1999;353:14. doi: 10.1016/S0140-6736(98)03544-2. [DOI] [Google Scholar]
- 55.Mitsiades J Endocrinol. 2003;178:205. [Google Scholar]
- 56.Motzer J Clin Oncol. 1995;13:1950. doi: 10.1200/JCO.1995.13.8.1950. [DOI] [PubMed] [Google Scholar]
- 57.Motzer J Clin Oncol. 2001;19:1312. doi: 10.1200/JCO.2001.19.5.1312. [DOI] [PubMed] [Google Scholar]
- 58.The Br J Haematol. 2001;113:1020. doi: 10.1046/j.1365-2141.2001.02857.x. [DOI] [PubMed] [Google Scholar]
- 59.Nadeau J Biol Chem. 1999;274:4045. doi: 10.1074/jbc.274.7.4045. [DOI] [PubMed] [Google Scholar]
- 60.Navarro Mod Pathol. 1996;9:150. [Google Scholar]
- 61.Novick Cell. 1994;77:391. doi: 10.1016/0092-8674(94)90154-6. [DOI] [PubMed] [Google Scholar]
- 62.Nyman Eur J Biochem. 2000;267:4011. doi: 10.1046/j.1432-1327.2000.01433.x. [DOI] [PubMed] [Google Scholar]
- 63.Pestka Semin Oncol. 1997;24:S9. [Google Scholar]
- 64.Pyrhonen J Clin Oncol. 1999;17:2859. doi: 10.1200/JCO.1999.17.9.2859. [DOI] [PubMed] [Google Scholar]
- 65.Rodriguez-Villanueva Int J Cancer. 1995;61:110. doi: 10.1002/ijc.2910610119. [DOI] [PubMed] [Google Scholar]
- 66.Sacchi Haematologica. 2001;86:951. [Google Scholar]
- 67.Sangfelt Oncogene. 1999;18:2798. doi: 10.1038/sj.onc.1202609. [DOI] [PubMed] [Google Scholar]
- 68.Schindler Annu Rev Biochem. 1995;64:621. doi: 10.1146/annurev.bi.64.070195.003201. [DOI] [PubMed] [Google Scholar]
- 69.Selleri Blood. 1997;89:957. [PubMed] [Google Scholar]
- 70.Slaton Clin Cancer Res. 1999;5:2726. [PubMed] [Google Scholar]
- 71.Song J Biol Chem. 1998;273:35056. doi: 10.1074/jbc.273.52.35056. [DOI] [PubMed] [Google Scholar]
- 72.Stahl Science. 1995;267:1349. doi: 10.1126/science.7871433. [DOI] [PubMed] [Google Scholar]
- 73.Takaoka Nature. 2003;424:516. doi: 10.1038/nature01850. [DOI] [PubMed] [Google Scholar]
- 74.Thiesing Blood. 2000;96:3195. [PubMed] [Google Scholar]
- 75.Uddin J Biol Chem. 2002;277:14408. doi: 10.1074/jbc.M109671200. [DOI] [PubMed] [Google Scholar]
- 76.Vitale J Clin Endocrinol Metab. 2000;85:983. doi: 10.1210/jc.85.3.983. [DOI] [PubMed] [Google Scholar]
- 77.Vitale Cancer. 2001;91:1797. doi: 10.1002/1097-0142(20010501)91:9<1797::AID-CNCR1199>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 78.Wadler Oncologist. 1997;2:254. [PubMed] [Google Scholar]
- 79.Wadler Cancer. 1997;79:1574. doi: 10.1002/(SICI)1097-0142(19970415)79:8<1574::AID-CNCR20>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 80.Wagner J Biol Chem. 2002;277:1493. doi: 10.1074/jbc.M108928200. [DOI] [PubMed] [Google Scholar]
- 81.Weisberg Drug Resist Updat. 2003;6:231. doi: 10.1016/S1368-7646(03)00062-1. [DOI] [PubMed] [Google Scholar]
- 82.Wills Clin Pharmacokinet. 1990;19:390. doi: 10.2165/00003088-199019050-00003. [DOI] [PubMed] [Google Scholar]
- 83.Yan EMBO J. 1996;15:1064. [PMC free article] [PubMed] [Google Scholar]
- 84.Yan Mol Cell Biol. 1996;16:2074. doi: 10.1128/mcb.16.5.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yang J Biol Chem. 1995;270:11711. doi: 10.1074/jbc.270.20.11711. [DOI] [PubMed] [Google Scholar]
- 86.Yang Proc Nat Acad Sci U S A. 1998;95:5568. doi: 10.1073/pnas.95.10.5568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chuan-He J Biol Chem. 2001;276:13756. doi: 10.1074/jbc.M011006200. [DOI] [PubMed] [Google Scholar]
- 88.Yee Expert Rev Anticancer Ther. 2003;3:295. doi: 10.1586/14737140.3.3.295. [DOI] [PubMed] [Google Scholar]
- 89.You Mol Cell Biol. 1999;19:2416. doi: 10.1128/mcb.19.3.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zella J Immunol. 1999;163:3169. [PubMed] [Google Scholar]
- 91.Zhang Biochem Biophys Res Commun. 1994;200:522. doi: 10.1006/bbrc.1994.1479. [DOI] [PubMed] [Google Scholar]