Abstract
Infection, disease relapse, graft failure, and graft-versus-host disease (GVHD) are significant adverse events associated with allogeneic bone marrow transplantation. Donor natural killer (NK) cells may be an ideal cell type for prevention or treatment of all these adverse events. Therefore, we investigated the phenotype and function of human NK cells purified by using a clinical-scale immunomagnetic method. We found that the NK cell purification procedures did not adversely affect the expression of killer cell immunoglobulin-like receptors, adhesion molecules, intracellular cytokines, perforin, and granzyme B. Purified NK cells had extensive proliferative capacity and potent antitumor activity when assessed using an immunodeficient mouse model. While all mice transplanted with unpurified mononuclear cells developed GVHD, none of the mice transplanted with purified NK cells did. NK cells were highly susceptible to lysis by antithymocyte globulin (ATG), whereas G-CSF had a minimal effect on their natural cytotoxicity. These results support future clinical investigation of the use of purified NK cells for adoptive immunotherapy in the absence of ATG.
Keywords: Adoptive immunotherapy, Antithymocyte globulin, Bone marrow transplantation, Leukemia, Natural killer cell, Neuroblastoma
Introduction
Allogeneic bone marrow transplantation (BMT) is commonly used for the treatment of high-risk hematologic malignancies, and has been increasingly used in studies of solid tumors [1]. Donor leukocyte infusion (DLI) has been used for the prevention or treatment of common adverse events after BMT including graft rejection, infection, or relapse. However, the risk of T-cell–mediated graft-versus-host disease (GVHD) often outweighs the potential clinical benefits of DLI [2]. This is particularly problematic in major histocompatibility complex (MHC) nonidentical transplants, in which as few as 3×104 T cells may cause fatal GVHD [3, 4]. Like T cells, natural killer (NK) cells have been shown in animal studies to have potent antiviral [5], antifungal [6], engraftment-facilitating [7], and antitumor activity [8]. Indeed, clinical studies have demonstrated that the risk of relapse, for both lymphoid [9] and myeloid leukemia [9, 10], can be markedly reduced by donor NK cells targeting recipient leukemia cells that lack the appropriate MHC ligand for their inhibitory killer cell immunoglobulin-like receptors (KIRs). Unlike T cells, however, NK cells do not cause GVHD, even in MHC-mismatched transplantation [11]. In fact, NK cells may reduce the risk of GVHD by targeting recipient antigen-presenting cells [10, 12].
Because the results of these studies suggest that donor NK cells may be ideal for adoptive immunotherapy, we have developed a clinical-scale immunomagnetic method for automated, efficient, and rapid isolation of human NK cells [13]. This procedure depleted T cells for a median of 5.3 logs with a 48% recovery of NK cells. We hypothesized that adoptive transfer of these highly purified NK cells from the stem cell donor before NK cell engraftment may reduce the risk of graft rejection, infections, disease relapse, and GVHD. The aim of this study was to investigate the phenotypes, functions, and therapeutic uses of purified NK cells. We also assessed the effects of antithymocyte globulin (ATG) and granulocyte colony-stimulating factor (G-CSF) on human NK cells because these agents are commonly used in BMT and their effect on purified mature NK cells is unclear.
Materials and methods
Isolation of NK cells
Peripheral blood cells were obtained by leukapheresis from healthy adult volunteers after informed consent was obtained using an institutional review board–approved protocol. NK cells were purified by using a 2-step procedure as described previously [13]. Briefly, CD3+ cells were first depleted from the apheresis products on the CliniMACSplus device equipped with a 162-01 LS or 168-01 LS separation column and Depletion 2.1 software (Miltenyi Biotec, Auburn, CA, USA). CD56+ cells were then enriched from the CD3+ cell–depleted products on a CliniMACSplus device equipped with a 165-01 TS separation column and Enrichment 1.1 software.
Flow cytometry
The BD LSR system and CellQuest Pro software (BD Immunocytometry Systems, San Jose, CA, USA) were used. Surface or intracellular staining with flurochrome-conjugated antibodies was performed as suggested by the suppliers for CD45, CD158a (KIR2DL1), CD158b (KIR2DL2/L3), CD62L (Beckman Coulter, Miami, FL, USA), CD11a, CD56, perforin, (BD PharMingen, San Diego, CA, USA), CD158e1 (KIR3DL1), CD14 (BD Biosciences, San Jose, CA, USA), CD19 (Dako, Carpinteria, CA, USA), TNF-α (Serotec, Raleigh, NC, USA), IFN-γ (R&D Systems, Minneapolis, MN, USA), and granzyme B (Holzel Diagnostika, Cologne, Germany).
HLA and KIR typing
HLA-typing was performed using serologic and DNA methods as previously described [14]. Donor KIR repertoire was determined by genotyping using PCR-SSP and by flow cytometry using monoclonal antibodies [9].
Cytotoxicity and proliferation assays
The cytotoxicity of NK cells in vitro was determined by a standard europium-release assay, with or without prestimulation with IL-2 (1,000 IU/ml) [9]. The cytotoxicity of NK cells in vivo was studied by using a nonobese diabetic SCID mouse model. Control mice received only cancer cells (20×106) via tail vein injections; treated mice received cancer cells and NK cells in a low 1:1 cell ratio (20×106 each). The tumor burden in the mice at the time of sacrifice were compared by using the Wilcoxon rank-sum test. Target cells included SEM human lymphoid leukemia cells (kindly provided by Dr J. Greil, University of Tuebingen, Germany), and NB1691 human neuroblastoma cells (kindly provided by Dr A. Davidoff, St Jude Children’s Research Hospital, Memphis, TN, USA). Susceptibility of NK cells and T cells to lysis by ATG in the presence of 3% serum was measured as previously described [15]. NK cell proliferation was monitored by labeling the NK cells with carboxyfluorecein succinimidyl ester (CFSE) before injection into the mice.
GVHD assays
The potential of the cells in the leukapheresis products before and after NK cell enrichment to cause GVHD in vivo was tested by using a nonobese diabetic SCID mouse model [16]. Mice were killed when they developed signs of GVHD (weight loss, pallor, lethargy, ruffled fur, and humped back), and cells from their bone marrow and spleens were examined by flow cytometry to confirm extensive proliferation of human T cells in these organs [16].
Results
The phenotypes of purified NK cells were similar to those before purification
We first ascertained whether the cell-processing procedures would negatively alter the phenotypes or receptor repertoire of the NK cells. Samples of cells obtained before and after purification were analyzed by flow cytometry. Expression patterns of all of the following were not notably changed after the isolation procedures: KIRs (CD158a, CD158b, and CD158e1), adhesion molecules (CD11a and CD62L), intracellular cytokines (TNF-α and IFN-γ), perforin, and granzyme B (Fig. 1).
Fig. 1.
Representative histograms before (black lines) and after (gray lines) purification of NK cells in three independent experiments using cells from different donors. Gates were set on CD56+CD3− cells.
Purified NK cells had extensive proliferative ability and potent antitumor activity in vivo
To monitor the proliferative potential of purified NK cells in vivo, we labeled them with CFSE before cotransplantation with leukemia cells into the SCID mice. Within 5 days after transplantation, the NK cells had undergone approximately seven to eight cell divisions (Fig. 2a). The CFSE staining continued to diminish gradually over the following weeks.
Fig. 2.
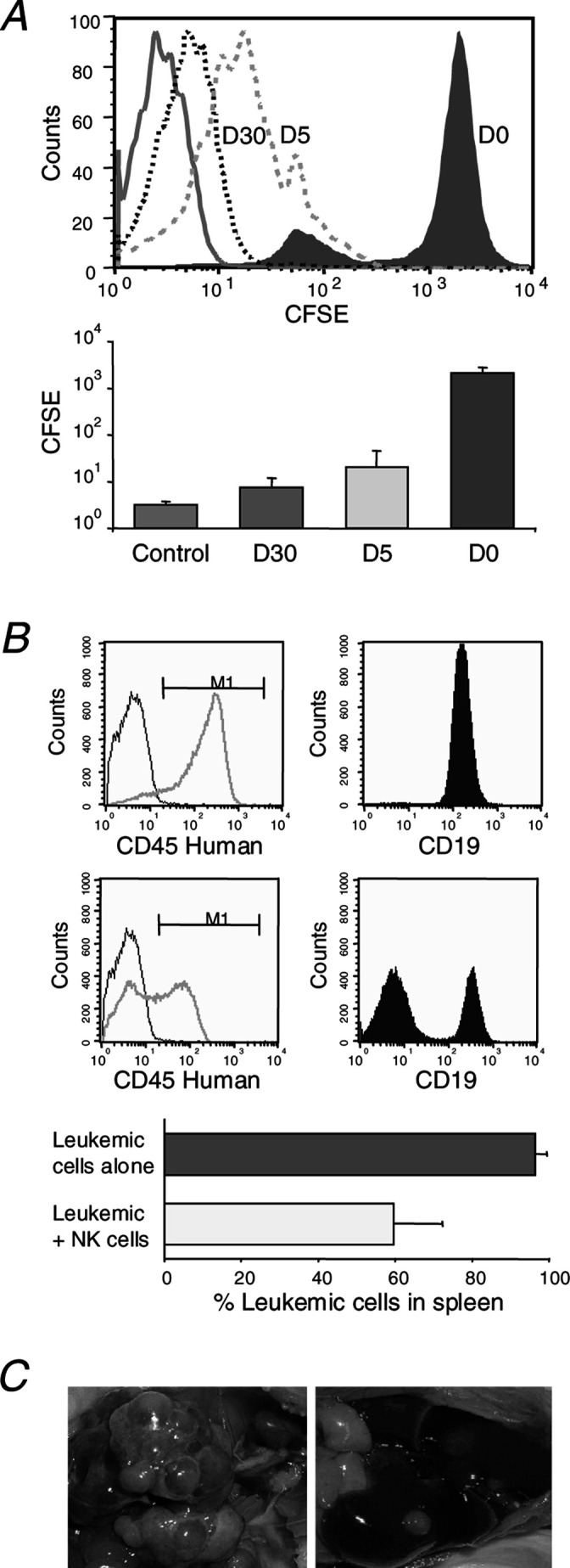
Proliferation and antitumor activity of NK cells in vivo. a The NK cells that were stained with CFSE on day 0 (D0) before injection had undergone multiple cell divisions by day 5 (D5) and day 30 (D30) after transplantation. Gates were set on CD56+ cells. Solid line in the histogram was unstained control. b Mice that were injected with human CD45+CD19+ SEM leukemic cells alone (upper panel) had higher numbers of leukemia cells in the spleen at 1 month after transplantation, as compared with mice injected with leukemic cells and purified NK cells (middle panel) in a very low effector to target cell ratio (1:1) (p=0.015 by Wilcoxon rank-sum test). Black lines in the histograms depict human CD45 staining of splenic cells from normal unmanipulated mice. Results shown in (a) and (b) are from two independent experiments on ten mice injected with cells from different donors. c Mice that were injected with NB1691 neuroblastoma cells alone had higher numbers of tumor nodules in the liver at 1 month after transplantation (left panel) compared with mice injected with neuroblastoma cells and purified NK cells (1:1 ratio) (right panel; p=0.046 by rank-sum test). Results shown in (c) are representative of two independent experiments on eight mice injected with cells from different donors.
We then examined the cytotoxicity of purified NK cells against malignant cells. NK cells from donors who were KIR2DL1-positive were used for the experiments against SEM leukemia cells (HLA-Cw03, HLA-Cw07) that lack the corresponding HLA-C ligand. Similarly, NK cells from KIR3DL1-positive donors were used against NB1691 neuroblastoma cells (HLA-B18, HLA-B50) that lack the corresponding HLA-B ligand. In all experiments, potent antitumor activity was observed in the mouse model using a low effector to target cell ratio (1:1) (Fig. 2b, c).
Purified NK cells did not cause GVHD in mice
No mice that were transplanted with purified NK cells developed signs of GVHD. In contrast, all mice that were transplanted with unpurified mononuclear cells developed GVHD (Fig. 3a); flow cytometric analysis confirmed extensive infiltration of human T cells in their bone marrow and spleen (Fig. 3b), a hallmark for multiorgan GVHD in this mouse model [16].
Fig. 3.
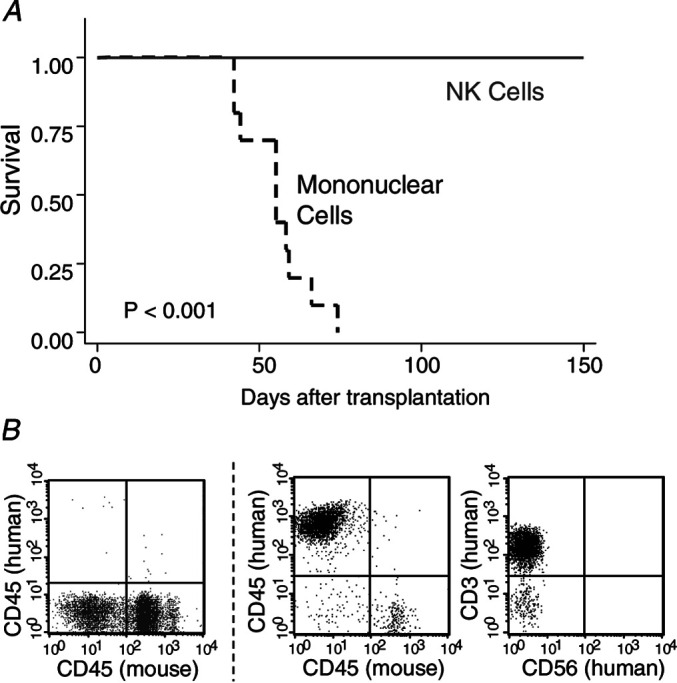
Graft-versus-host disease (GVHD)-free survival. a Mice were injected with either unpurified mononuclear cells (dashed line) or purified NK cells (solid line) from the same donor in each experiment. b Flow cytometric analysis of cells from the spleen of mice with GVHD (right panels) showed a large number of human CD45+CD3+CD56− T cells with minimal residual murine CD45+ leukocytes (in contrast to mice that received purified NK cells, left panel). Results were combined from two independent experiments on 20 mice with NK cells from different donors.
Effects of ATG and G-CSF on purified NK cells
Antithymocyte globulin (ATG) and G-CSF are commonly used in allogeneic BMT, and their effects on purified mature human NK cells are uncertain. We found that NK cells were highly susceptible to lysis by ATG, even more so than T cells from the same donors (Fig. 4a). G-CSF, on the other hand, had a minimal effect on the natural cytotoxicity or lymphokine-activated killer activity of the purified NK cells (Fig. 4b).
Fig. 4.
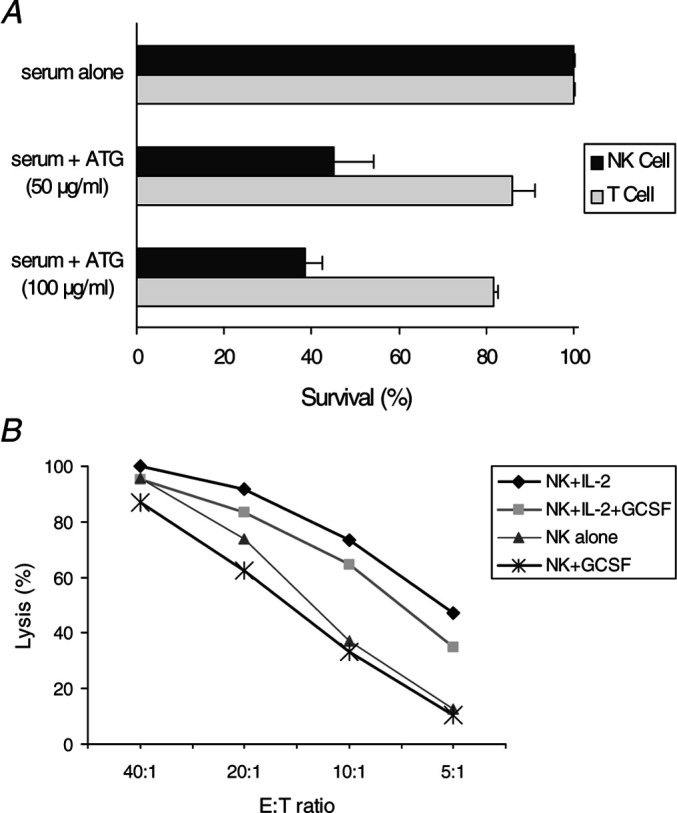
Effects of ATG and G-CSF on purified NK cells. a Purified NK cells were highly susceptible to ATG-mediated lysis. CliniMACS-purified T cells (obtained after step 1), and NK cells (obtained after step 2) from the same donor were incubated for 1 h in the presence of 3% serum with or without pharmacologic concentrations of ATG (50 or 100 μg/ml). b Incubation of purified NK cells for 24 h in the presence of a pharmacologic concentration of G-CSF (100 ng/ml) had a minimal effect on their cytotoxicity and lymphokine-activated killer activity toward K562 cells. Results were combined from three independent experiments with NK cells from different donors.
Discussion
Donor leukocyte infusion has been used for the prevention or treatment of graft rejection, primary disease relapse, and severe infections after allogeneic BMT [2]. However, its application is limited by complications such as T-cell–mediated acute and chronic GVHD and marrow aplasia. An alternative to the infusion of total lymphocyte populations is to infuse purified NK cells alone. Therefore, we developed a clinical-scale purification method for the isolation of highly purified human NK cells that yields minimal contamination with B and T cells [13]. This reduces significantly the risk of B-cell lymphoproliferative disease as well as T-cell alloreactivity because our prior study showed that T-cell alloreactivity dominates that of NK cells [11]. Here, we provide experimental data to further support future clinical investigation on the use of these purified NK cells for adoptive immunotherapy. We showed that the phenotypes of the purified NK cells did not differ from those before isolation. Proliferative capacity and potent antitumor effects of the purified NK cells were demonstrated using a mouse model. Unlike the mononuclear cells from the original apheresis products, purified NK cells did not cause GVHD. NK cells were highly susceptible to lysis by ATG, whereas G-CSF had a minimal effect on their natural cytotoxicity.
One of the major concerns regarding any ex vivo cell processing procedure is whether there is any negative impact on the cells of interest. We found that the phenotypes of the purified NK cells did not differ from those of the original apheresis products. Receptor repertoire study showed that the percentage of cells positive for each KIR was unchanged. There was also no evidence that the isolation procedure affected the expression of adhesion molecules, intracellular cytokines, or cytotoxic granules. These data provide assurance of a full repertoire of normal NK cells that will be important for infection control and prevention of graft rejection [17].
By in vitro assays, we have previously shown that the biologic functions of NK cells were preserved during cell processing [13]. Binding of the anti-CD56 antibody-conjugated microbeads on the NK cells did not affect the cytotoxic function of NK cells and their IL-2 receptor pathways. We extended these in vitro observations by demonstrating herein their extensive proliferative capacity and potent lytic function in vivo against malignant cells with incompatible KIR ligands. Because our purification procedures will not alter the KIR repertoire of donor NK cells and previous study has demonstrated that a perfect KIR-mismatch stem cell donor could be selected by evaluating the donor’s KIR repertoire before transplantation [9], these results suggest that the optimal donor who will donate both stem cells and NK cells may be selected in advance by a single KIR typing.
While all mice transplanted with unpurified mononuclear cells developed GVHD, none of the mice that were transplanted with purified NK cells did. One limitation of this study was that we did not use our mouse system to confirm that the isolated human NK cells can prevent GVHD mediated by graded numbers of T cells in an adoptive transfer system, and to determine the maximum numbers of T cells allowable in donor inoculums. In this regard, Murphy et al. [18] has previously demonstrated that as few as 3×107 IL-2–activated NK cells could promote allogeneic marrow engraftment and B-cell reconstitution, as well as completely prevent GVHD mediated by 1×106 T-cell–containing marrow cells that were mismatched at both class I and class II MHC loci. Furthermore, 2×107 IL-2–activated NK cells could successfully exert antitumor effects and delay the onset of GVHD, with an average of 20–30% being GVHD-free survivors after transplantation with as high as 1×107 MHC-disparate T-cell–containing marrow cells and 2×107 splenocytes [19].
In preparation for future clinical trials, we studied the effects of G-CSF and ATG because these agents are commonly used in BMT and their effect on purified mature NK cells is uncertain. Previous studies have demonstrated that G-CSF can impede the development of immature NK cells from their progenitors and reduce cytotoxicity of G-CSF–primed NK cells in peripheral blood stem cell grafts [20, 21]. However, we observed no direct suppressive effects of G-CSF on the natural cytotoxicity of mature NK cells from unprimed apheresis products of adult donors. This is in agreement with a recent randomized double-blinded study by the same investigators showing that G-CSF had no adverse impact on either NK cell frequency or function on day 28 through 1 year after transplantation [22]. In fact, the placebo group tended to have lower natural cytotoxicity than the G-CSF–treated group. Taken together, these data suggest that G-CSF is not contraindicated in patients who have received mature NK cell infusion.
The impact of ATG on NK cells is also uncertain, with studies showing enhanced, neutral, or suppressive effects depending on the clinical situation [23–25]. In addition, it is unclear whether the suppression observed in some studies was due to a direct effect on NK cells or to a bystander effect during ATG-mediated T-cell apoptosis (e.g., NK cell apoptosis secondary to cytokine release during T-cell apoptosis) [26, 27]. Our results from experiments using highly purified NK cells showed that ATG can cause lysis of NK cells directly; thus suggesting that concurrent use of ATG should be avoided during NK cell infusions, even in the first few weeks after BMT when the patients are lymphopenic in T cells.
In summary, we provided preclinical data to support the use of purified donor NK cells for adoptive immunotherapy and data concerning potential clinical interaction with G-CSF and ATG. We demonstrated that these donor NK cells had extensive proliferative capacity and potent antitumor activity but did not cause GVHD. Thus, in comparison to conventional DLI that includes the total lymphocyte population, the infusion of NK cells alone appears to have a more favorable benefit-to-risk ratio in the prevention or treatment of many significant adverse events after transplantation, including graft rejection, infections, GVHD, and recurrence of malignancy.
Acknowledgements
This work was supported in part by National Institutes of Health grant P30 CA21765-24 (to W.L.), by the Assisi Foundation of Memphis, and by the American Lebanese Syrian Associated Charities (ALSAC).
References
- 1.Little MT, Storb R. History of haematopoietic stem-cell transplantation. Nat Rev Cancer. 2002;2:231–238. doi: 10.1038/nrc748. [DOI] [PubMed] [Google Scholar]
- 2.Dazzi F, Goldman J. Donor lymphocyte infusions. Curr Opin Hematol. 1999;6:394–399. doi: 10.1097/00062752-199911000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Aversa F, Tabilio A, Velardi A, Cunningham I, Terenzi A, Falzetti F, Ruggeri L, Barbabietola G, Aristei C, Latini P, Reisner Y, Martelli MF. Treatment of high-risk acute leukemia with T-cell-depleted stem cells from related donors with one fully mismatched HLA haplotype. N Engl J Med. 1998;339:1186–1193. doi: 10.1056/NEJM199810223391702. [DOI] [PubMed] [Google Scholar]
- 4.Lewalle P, Triffet A, Delforge A, Crombez P, Selleslag D, De Muynck H, Bron D, Martiat P. Donor lymphocyte infusions in adult haploidentical transplant: a dose finding study. Bone Marrow Transplant. 2003;31:39–44. doi: 10.1038/sj.bmt.1703779. [DOI] [PubMed] [Google Scholar]
- 5.French AR, Yokoyama WM. Natural killer cells and viral infections. Curr Opin Immunol. 2003;15:45–51. doi: 10.1016/S095279150200002X. [DOI] [PubMed] [Google Scholar]
- 6.Morrison BE, Park SJ, Mooney JM, Mehrad B. Chemokine-mediated recruitment of NK cells is a critical host defense mechanism in invasive aspergillosis. J Clin Invest. 2003;112:1862–1870. doi: 10.1172/JCI200318125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murphy WJ, Koh CY, Raziuddin A, Bennett M, Longo DL. Immunobiology of natural killer cells and bone marrow transplantation. Immunol Rev. 2001;181:279–289. doi: 10.1034/j.1600-065X.2001.1810124.x. [DOI] [PubMed] [Google Scholar]
- 8.Smyth MJ, Hayakawa Y, Takeda K, Yagita H. New aspects of natural-killer-cell surveillance and therapy of cancer. Nat Rev Cancer. 2002;2:850–861. doi: 10.1038/nrc928. [DOI] [PubMed] [Google Scholar]
- 9.Leung W, Iyengar R, Turner V, Lang P, Bader P, Conn P, Niethammer D, Handgretinger R. Determinants of antileukemia effects of allogeneic natural killer cells. J Immunol. 2004;172:644–650. doi: 10.4049/jimmunol.172.1.644. [DOI] [PubMed] [Google Scholar]
- 10.Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A, Posati S, Rogaia D, Frassoni F, Aversa F, Martelli MF, Velardi A. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295:2097–2100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 11.Lowe EJ, Turner V, Handgretinger R, Horwitz EM, Benaim E, Hale GA, Woodard P, Leung W. T-cell alloreactivity dominates natural killer cell alloreactivity in minimally T-cell-depleted HLA-non-identical paediatric bone marrow transplantation. Br J Haematol. 2003;123:323–326. doi: 10.1046/j.1365-2141.2003.04604.x. [DOI] [PubMed] [Google Scholar]
- 12.Yamasaki S, Henzan H, Ohno Y, Yamanaka T, Iino T, Itou Y, Kuroiwa M, Maeda M, Kawano N, Kinukawa N, Miyamoto T, Nagafuji K, Shimoda K, Inaba S, Hayashi S, Taniguchi S, Shibuya T, Gondo H, Otsuka T, Harada M. Influence of transplanted dose of CD56+ cells on development of graft-versus-host disease in patients receiving G-CSF-mobilized peripheral blood progenitor cells from HLA-identical sibling donors. Bone Marrow Transplant. 2003;32:505–510. doi: 10.1038/sj.bmt.1704165. [DOI] [PubMed] [Google Scholar]
- 13.Iyengar R, Handgretinger R, Babarin A, Leimig T, Otto M, Geiger T, Holladay M, Houston J, Leung W. Purification of human natural killer cells using a clinical-scale immunomagnetic method. Cytotherapy. 2003;5:479–484. doi: 10.1080/14653240310003558. [DOI] [PubMed] [Google Scholar]
- 14.Leung WH, Turner V, Richardson SL, Benaim E, Hale G, Horwitz EM, Woodard P, Bowman LC. Effect of HLA class I or class II incompatibility in pediatric marrow transplantation from unrelated and related donors. Hum Immunol. 2001;62:399–407. doi: 10.1016/S0198-8859(01)00220-8. [DOI] [PubMed] [Google Scholar]
- 15.Naundorf S, Preithner S, Mayer P, Lippold S, Wolf A, Hanakam F, Fichtner I, Kufer P, Raum T, Riethmuller G, Baeuerle PA, Dreier T. In vitro and in vivo activity of MT201, a fully human monoclonal antibody for pancarcinoma treatment. Int J Cancer. 2002;100:101–110. doi: 10.1002/ijc.10443. [DOI] [PubMed] [Google Scholar]
- 16.Leung W, Ramirez M, Mukherjee G, Perlman EJ, Civin CI. Comparisons of alloreactive potential of clinical hematopoietic grafts. Transplantation. 1999;68:628–635. doi: 10.1097/00007890-199909150-00006. [DOI] [PubMed] [Google Scholar]
- 17.Raziuddin A, Longo DL, Bennett M, Winkler-Pickett R, Ortaldo JR, Murphy WJ. Increased bone marrow allograft rejection by depletion of NK cells expressing inhibitory Ly49 NK receptors for donor class I antigens. Blood. 2002;100:3026–3033. doi: 10.1182/blood.V100.8.3026. [DOI] [PubMed] [Google Scholar]
- 18.Murphy WJ, Bennett M, Kumar V, Longo DL. Donor-type activated natural killer cells promote marrow engraftment and B cell development during allogeneic bone marrow transplantation. J Immunol. 1992;148:2953–2960. [PubMed] [Google Scholar]
- 19.Asai O, Longo DL, Tian ZG, Hornung RL, Taub DD, Ruscetti FW, Murphy WJ. Suppression of graft-versus-host disease and amplification of graft-versus-tumor effects by activated natural killer cells after allogeneic bone marrow transplantation. J Clin Invest. 1998;101:1835–1842. doi: 10.1172/JCI1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller JS, Prosper F, McCullar V. Natural killer (NK) cells are functionally abnormal and NK cell progenitors are diminished in granulocyte colony-stimulating factor-mobilized peripheral blood progenitor cell collections. Blood. 1997;90:3098–3105. [PubMed] [Google Scholar]
- 21.Joshi SS, Lynch JC, Pavletic SZ, Tarantolo SR, Pirruccello SJ, Kessinger A, Bishop MR. Decreased immune functions of blood cells following mobilization with granulocyte colony-stimulating factor: association with donor characteristics. Blood. 2001;98:1963–1970. doi: 10.1182/blood.V98.6.1963. [DOI] [PubMed] [Google Scholar]
- 22.Joshi SS, Bishop MR, Lynch JC, Tarantolo SR, Abhyankar S, Bierman PJ, Vose JM, Geller RB, McGuirk J, Foran J, Bociek RG, Hadi A, Day SD, Armitage JO, Kessinger A, Pavletic ZS. Immunological and clinical effects of post-transplant G-CSF versus placebo in T-cell replete allogeneic blood transplant patients: results from a randomized double-blind study. Cytotherapy. 2003;5:542–552. doi: 10.1080/14653240310003648. [DOI] [PubMed] [Google Scholar]
- 23.Neudorf S, Jones M. The effects of antithymocyte globulin on natural killer cells. Exp Hematol. 1988;16:831–835. [PubMed] [Google Scholar]
- 24.Myint AA, Malkovska V, Morgan S, Luckit J, Wonke B, Gordon-Smith EC. Antilymphocyte globulin therapy enhances impaired function of natural killer cells and lymphokine activated killer cells in aplastic anaemia. Br J Haematol. 1990;75:578–584. doi: 10.1111/j.1365-2141.1990.tb07802.x. [DOI] [PubMed] [Google Scholar]
- 25.Meijer E, Bloem AC, Dekker AW, Verdonck LF. Effect of antithymocyte globulin on quantitative immune recovery and graft-versus-host disease after partially T-cell-depleted bone marrow transplantation: a comparison between recipients of matched related and matched unrelated donor grafts. Transplantation. 2003;75:1910–1913. doi: 10.1097/01.TP.0000065737.60591.9D. [DOI] [PubMed] [Google Scholar]
- 26.Preville X, Flacher M, LeMauff B, Beauchard S, Davelu P, Tiollier J, Revillard JP. Mechanisms involved in antithymocyte globulin immunosuppressive activity in a nonhuman primate model. Transplantation. 2001;71:460–468. doi: 10.1097/00007890-200102150-00021. [DOI] [PubMed] [Google Scholar]
- 27.Ross ME, Caligiuri MA. Cytokine-induced apoptosis of human natural killer cells identifies a novel mechanism to regulate the innate immune response. Blood. 1997;89:910–918. [PubMed] [Google Scholar]



