Abstract
Human melanoma is hardly ever curable at an advanced stage, but overwhelming evidence from untreated or vaccinated patients indicates that this tumor is highly antigenic and frequently immunogenic. Here, we review recent results indicating that CD8+ T cell–mediated antitumor immunity is activated at the systemic and tumor level in the early clinical stages (AJCC stages I and II) and continues to be promoted, in a fraction of patients, even in metastatic disease (stages III and IV). This evidence was obtained by looking at frequency, differentiation phenotype, and function of antitumor T cells in periphery and tumor site of melanoma patients. On the other hand, the paradox of immunity in spite of poor clinical evolution of the disease, points toward a model of concurrent evolution of immunity and tumor escape. As melanoma progresses to metastatic disease, powerful mechanisms of tumor evasion from immune recognition, and of immunosuppression, are activated, thus tilting the balance between immunity and escape in favor of tumor resistance to host defense. Nevertheless, recent developments in our understanding of regulation of T cell–mediated immunity can provide clues to the prospects for improved immunotherapy approaches. By integrating the information from basic research in immunology, from murine tumor models, and from trials of immunotherapy, we discuss how the most relevant steps of the antitumor response should be manipulated with greater efficacy by future clinical trials.
Keywords: Melanoma, T cell–mediated immunity, Tumor escape, Tumor progression
Introduction
Human melanoma has been considered a paradigm of immunogenicity among human tumors, as well as of powerful mechanisms of tumor escape. In fact, in a fraction of patients, a high frequency of T cells directed to several different tumor-associated antigens (TAAs) has been documented in both peripheral blood and tumor site, providing support to the notion of tumor immunogenicity. On the other hand, almost all of the classical or newly discovered mechanisms of tumor escape from immune surveillance have been shown to be active at some point during melanoma progression. In addition, evidence for development of immunity to tumor antigens can be observed even in patients with a very poor clinical evolution of the disease. This apparent paradox, revealed in untreated patients, matches results from a number of clinical trials of immunotherapy, where it has been shown that boosting of T cell–mediated immunity to melanoma can be achieved through vaccination, while the clinical response is either missing, minor or, generally, not long lasting, even when significant. Thus, the evidence from a large set of studies looking at development of immunity in untreated, or in vaccinated, melanoma patients points toward a common conclusion: antitumor immunity does evolve along with tumor progression, at least in a fraction of patients, or can be boosted by immunotherapy even in advanced disease. However, when melanoma has progressed to metastatic disease, the balance between immunity and tumor escape has inevitably been lost in favor of tumor resistance to cell-death mechanisms and of negative regulation of immune response. Here, we review the evidence supporting such a model of concurrent evolution of immunity and tumor escape in this disease, with emphasis on regulation of CD8+ T cell–mediated response to melanocyte-lineage antigens. In addition, we address the contrasting role of CD4+ T cells in antitumor immunity and discuss how recent progress on regulation of T cell–mediated antitumor immunity, coupled to an improved immunological characterization of patients, may lead to the development of more effective immunotherapy treatments.
T cell–mediated immunity evolves along with tumor progression
A quick review of the salient facts about the clinical evolution of melanoma may provide an efficient reminder as to the nature of the task posed by the attempt to control this disease in an advanced stage, when it becomes hardly ever curable. The surgical excision of thin (<1.0 mm) primary lesions without ulceration (AJCC stage IA) is associated with a 10-year survival of 86±0.9% [1, 2], but survival decreases with increasing thickness of the primary tumor, since this in turn signals for increased probability of lymph node metastases. Ten-year survival in patients with >4-mm primary lesions without ulceration (stage IIB) is reduced to ~50% [1, 2] and decreases further with development of regional lymph node metastases (stage III). With the appearance of distant and visceral metastases (stage IV), 5-year survival drops to less than 10% [1]. By looking at the striking and progressive drop in survival from stage I to IV, one could be brought to conclude that the immune response against tumor growth is beginning to lose its war—assuming that one is being fought—from the very beginning of the natural history of the disease. However, early evidence that this may not be true came from the seminal observations by Clark et al. [3], indicating that tumor-infiltrating lymphocytes represent a significant prognostic indicator of survival in stage I “thin” VGP melanomas. Later, a positive role of tumor-infiltrating lymphocytes on disease-free survival has been shown even by looking at their presence as a “brisk” infiltrate in the neoplastic tissue of lymph node metastases of stage III patients [4]. This evidence has suggested that the immune response might counteract tumor growth not only in early stages, but also in metastatic disease. Interestingly, similar data on the role of intratumoral lymphocytes have been obtained more recently in other human tumors, as indicated by the relationship between T-cell infiltration of neoplastic tissue and patient survival documented in colorectal, esophageal, and ovarian cancer [5–7]. On the other hand, these findings do not provide direct evidence for a role of antigen-specific T cells recognizing TAAs. However, throughout most of the past decade, this gap in knowledge has been largely removed thanks to the molecular identification of a large set of TAAs expressed on melanoma and other human tumors, and recognized by patients’ T cells [8]. As far as melanoma is concerned, the improved knowledge on the nature of the TAAs has allowed several investigators to show the presence of tumor-specific T cells in both peripheral circulation and tumor site of a significant fraction of patients, even in metastatic disease [9]. This evidence has provided strong support to the notion that immunity to melanoma antigens may not necessarily be suppressed in advanced clinical stages.
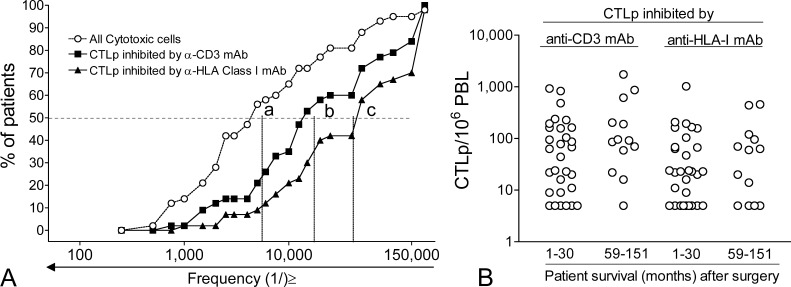
The possible relationship between the presence of circulating tumor-specific T cells and patient survival has been initially addressed by us by limiting-dilution analysis (LDA) for tumor-specific CTL precursors (CTLps) directed to autologous tumor, in a series of stage III and IV melanoma patients. The cytolytic assay at the end of the LDA was modified by us to increase its efficiency [10]. Such a modified LDA assay was validated by testing for frequency of antigen-specific T cells present in peptide-specific T cell lines in comparison with frequency determination by tetramer staining [10]. Although CTLp frequency by LDA may be underestimated due to the requirement for extensive T-lymphocyte proliferation [11], nevertheless, compared to other assays, the LDA approach has the significant advantage of detecting CTLps directed to all possible TAAs expressed by each patient’s tumor. This provides an estimate of the overall circulating immune repertoire. The results of such LDA analysis in 43 metastatic patients for the frequency of CTLps against autologous HLA class I+ tumors were classified by cumulative frequency plots (Fig. 1A), showing the distribution of cytotoxic cells and CTLp frequencies in the population of patients. As a positive control of tumor susceptibility to cell-mediated cytotoxicity, we even evaluated the frequency of all cytolytic cells (at least 1/5,500 PBLs in 50% of the patients) found against autologous tumor and defined only on the basis of significant tumor lysis. CTLp frequencies, defined by significant inhibition with anti-CD3 mAb (at least 1/17,000 PBLs in 50% of the patients), or by anti-HLA class I mAb (at least 1/41,500s PBL in 50% of the patients), ranged from the lowest limit of detection (<1/200,000) to as high as 1/1,000 PBLs in a few patients (Fig. 1A). Among the 43 patients, a subset consisted of long-term survivors (between 59 and 151 months after surgery for removal of metastases), while many other patients showed rapid progression and survival limited to less than 30 months. Thus, we could test whether the two subsets of patients showed different CTLp frequency distributions, since the LDA had been performed with a blood sample taken at the time of the surgical removal of metastases. Unfortunately, the comparison of CTLp frequencies in long-term subset versus short-term survivors did not reveal significant differences (Fig. 1B). Thus, analysis for CTLp frequency in peripheral blood indicates that circulating tumor-specific T cells are present, even at a very high frequency in some instances, in agreement with the notion of antitumor immunity being detected even at a late stage of disease. On the other hand, this approach does not predict subsequent clinical outcome. Clearly, it is to be pointed out that the distribution of values of CTLp frequencies found by LDA in the patients reflects not only the number of circulating tumor-specific T cells and their proliferative potential, but even the overall susceptibility of the autologous tumor to CTL-mediated lysis. This, in turn, is a complex trait resulting from the antigenic profile (number and level of expression of all possible MHC-TAA complexes, adhesion molecules, death receptor expression, etc.) and from the functional status of pathways regulating cell death in each tumor. Although we did not find a correlation between susceptibility of each tumor to cell-mediated lysis and CTLp frequency in the same patients; nevertheless, the antigenic profile of a melanoma and the function of the cell death pathways may contribute to explain why CTLp frequencies evaluated on autologous tumor may not correlate with clinical outcome.
Fig. 1A,B.
Frequency of cytotoxic T-cell precursors (CTLp) to autologous tumor in 43 metastatic melanoma patients. A LDA was performed as described previously in [34]. This LDA assay does not allow us to detect frequencies of cytolytic T cells <1/200,000. The resulting frequencies for all patients were plotted in a cumulative frequency graph as proportion of patients having cytotoxic cell frequencies equal or higher than a given value in a set of 23 frequency classes. By linear regression analysis, it was estimated that 50% of the patients had either ≤1/5,500 cytotoxic cells against the tumor (a), or ≤1/17,000 CTLp inhibited by anti-CD3 mAb (i.e., all true CTLp) (b), or ≤1/41,500 CTLp inhibited by anti-HLA-class I mAb (i.e., all CTLp restricted to HLA class I antigens) (c). B Comparison of CTLp frequencies in short-term survivors (1–30 months) and long-term survivors (59–151 months) after removal of lymph node metastases, at the time when CTLp had been evaluated; p>0.05 for both CTLp inhibited by anti-CD3 mAb and anti-HLA-class I mAb
More recently, the analysis of immunity to melanoma antigens has been greatly improved by the development of soluble tetramers of HLA molecules complexed with TAA peptides, for the identification and characterization of antigen-specific T cells [12]. Thanks to this new tool, it becomes possible to address the relationship between presence, number, tissue distribution, and function of tumor-specific T cells, on the one hand, and clinical stage or even patient survival, on the other. To address the issue of possible development of T cell–mediated immunity, along with tumor progression, tetramers of HLA-A*0201 were used to look for the peripheral frequency of T cells directed to melanocyte differentiation antigens (MDAs) in a panel of AJCC stage I to IV HLA-A*0201+ melanoma patients. A set of HLA-A*0201+ healthy donors was also investigated. The results indicated a significant increase in the frequency of T cells directed to gp100209–217 and Melan-A/MART-126–35 epitopes in stage I/II patients, compared with healthy donors [13]. Moreover, stage III/IV patients showed a much higher frequency, compared with stage I/II patients, for T cells directed to four different MDA epitopes from gp100154–162, gp100209-217, Melan-A/MART-126–35, and tyrosinase369–377 [13]. In addition, accumulation of functional memory T cells, directed to Melan-A/MART-126–35, was found at the tumor site in a fraction of the patients [13]. Increased peripheral precursor frequency and generation of memory cells at sites of antigen expression can be considered hallmarks of immunization [14]. Thus, these results indicated not only that immunity to melanoma antigens is activated already at the early clinical stages, but also that it continues to be promoted even when patients progress to metastatic disease. Noticeably, the MDA epitopes being investigated were normal proteins expressed in melanocytes, not only in melanoma, thus challenging the reasonable view that antitumor immunity against such determinants should be hampered at any stage of tumor progression, by tolerance mechanisms [15]. On the contrary, a possible interpretation of these findings is that the presence of neoplastic cells in the invaded lymph nodes, and/or higher MDA levels resulting from increasing tumor load, do represent an activating signal for the patient’s immune system. Interestingly, results from murine models [16] have indicated that the ability of tumors to migrate into secondary lymphoid organs and to intermingle with T cells in such tissues is a relevant requisite for activation of T cell–mediated antitumor response. This supports the possibility that early metastases to regional lymph nodes, a hallmark of melanoma progression, may in fact represent a significant trigger for activation of antitumor immunity. The relevance of metastatic disease and/or actual tumor load on peripheral immunity has received further support from the analysis of antigen-specific T-cell frequency in peripheral blood of disease-free, long-term survivors, after surgical removal of lymph node metastases. In these patients, in the absence of tumor load, we found a progressive decay of peripheral T-cell frequency to MDA, but not to influenza virus epitopes, over a follow-up time of 3–7 years [13]. On the basis of these data, it is reasonable to conclude that progression to metastatic disease is not synonymous with inhibition of immunity.
In spite of this evidence, the relationship of antitumor immunity (either in periphery or at tumor site) with tumor destruction, or even with clinical outcome, remains a paradox. In fact, in an initial study we addressed CD8+ T cell–mediated immunity to Melan-A/MART-126–35 peptide, by both peptide-specific LDA and tetramer analysis, in several metastatic melanoma patients [10]. We found a subset of patients with high frequency of peptide-specific T cells to this antigen and displaying a “memory” phenotype (at that time defined only by CD45RO expression) in both periphery and tumor site [10]. However, a detailed immunohistochemical analysis of the metastatic lymph nodes indicated frequent loss of antigen expression and, in most lesions, lack of tumor destruction. Furthermore, few areas of regression appeared void of surrounding or infiltrating T cells, but presented histological features associated with coagulative necrosis, suggesting that insufficient vascular supply, rather than an immune response, was responsible for damage to the neoplastic tissue [10]. Thus, evidence for peripheral immunity and for accumulation of tumor-specific T cells at tumor site may be uncoupled from evidence of immune-mediated tumor regression in the same patients.
More recently, the increasing knowledge on the processes of antigen-induced T-cell differentiation and activation has shifted the attention of several investigators away from the simple analysis of T-cell frequency in the periphery or at the tumor site of cancer patients. Thus, new emphasis is being placed on the actual differentiation stage and functional status of antitumor T cells found in distinct tissues of cancer patients. Thanks mainly to investigations on immune response to viral epitopes, the process driving antigen-induced CD8+ T-cell maturation has been understood in terms of changes in the expression of leukocyte common antigen isoforms (CD45RA and RO), chemokine receptors (e.g., CCR4, CCR5, CCR6, and CCR7), costimulatory molecules (e.g., CD27 and CD28), L-selectins (CD62L), and cytotoxic factors (e.g., perforin and granzymes) [17–19]. By looking at transient or stable changes in the expression of these markers, antigen- or even cytokine-induced CD8+ T-cell maturation has been defined as a linear sequence, where at each maturation step the cells can be identified by a specific phenotype. This process begins with the CCR7+CD45RA+ “naïve” (TN) stage. Maturation to memory stage then identifies two distinct subsets: antigen-experienced CCR7+CD45RA− “central memory” (TCM), and CCR7−CD45RA− “effector memory” (TEM) cells. A further step, characterized by a CCR7−CD45RA+ phenotype, defines cells as “terminally differentiated” (TTD) [18] or, more recently [20], as “TEMRA” (“CD45RA+ effector memory” cells). A distinct three-step model for CD8+ maturation, not fully agreeing with the former one, has been defined by looking at the changes of expression of CD27 and CD28 costimulatory molecules [17].
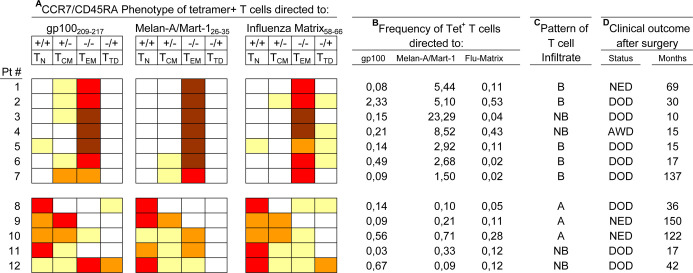
These models of CD8+ T-cell differentiation, coupled to tetramer analysis, were recently applied by us to T lymphocytes from invaded lymph nodes of metastatic patients. This approach allowed us to evaluate not only the presence and frequency, but also the differentiation status, of the tetramer+ T cells along the TN→TCM→TEM→TTD maturation pathway [13]. By this approach, we identified patients whose metastatic lesions contained a very high frequency (up to >20% of the CD8+) of Melan-A/MART-1–specific T cells with a predominant “effector memory” phenotype (i.e., mostly CCR7−CD45RA−; see patients 1–7, Fig. 2A, B). Interestingly, these cells were not functionally impaired, as documented by antigen-specific assays looking at IFN-γ production or proliferation, even without preactivation [13]. In this subset of patients, by immunohistochemistry, we found infiltrating CD3+CD8+ T cells within the neoplastic tissue of the invaded lymph node (Fig. 2C). However, although we did not find evidence for loss of HLA-A2 or Melan-A/MART-1 expression in the same tissues, the neoplastic lesions showed no evidence of tumor regression, by either necrosis or apoptosis (by histochemical analysis and TUNEL assays in tissue sections, see 34). Moreover, in the same subset of patients, even clinical outcome in most instances was not better than expected for this clinical stage (Fig. 2D). This suggested that tumor immunity, developing naturally in a fraction of patients, leads to a remarkable accumulation of highly differentiated tumor-specific T cells at the tumor site—a condition hardly achieved even by current vaccination approaches. In spite of this immune response, no protective effect against the subsequent clinical evolution could be observed.
Fig. 2A–D.
Differentiation phenotype and frequency of MDA- and influenza-specific CD8+ T cells in invaded lymph nodes of stage III melanoma patients. A CD8+ T cells from tumor-invaded lymph nodes, stained with tetramers to gp100209–217, Melan-A/MART-126–35 and influenza-matrix58–66, were characterized for the maturation phenotype by four-color flow cytometry, as described in [35]. Proportion of tetramer+ T cells showing each of the four possible phenotypes (T N naïve, T CM central memory, T EM effector memory, and T TD terminally differentiated) defined by staining for CCR7 vs CD45RA is shown with the following color code: 0–10% white, 11–30% yellow, 31–50% light orange, 51–80% red, 81–100% brown. B Frequency of the tetramer+ T cells (whose phenotype is shown in A) was expressed as percentage of Tet+ cells/CD8+ cells. C The same metastatic lesions characterized by tetramer analysis were evaluated for the pattern of T-cell infiltration. To this end, sections were stained, by immunohistochemistry, with mAbs to CD3 and CD8. The results were expressed by the brisk (B), nonbrisk (NB), absent (A) code, as defined in [10]. D Clinical outcome (status and follow-up, in months) of patients after surgical removal of metastatic lymph nodes: NED alive with no evidence of disease, DOD dead of disease, AWD alive with disease
Tumor escape mechanisms and tumor progression
The paradox of immunity in spite of poor clinical evolution of the disease, points toward the relevance of immune evasion mechanisms. Although a detailed analysis of such mechanisms is beyond the scope of this article, it is nevertheless to be kept in mind that most, if not all, of the known mechanisms of tumor escape and immune suppression by neoplastic cells have been shown to become active in metastatic melanoma. Thus, advanced melanoma has been a prototype for the description of classical mechanisms of escape and immune suppression. These include loss/down-regulation of HLA, of adhesion molecules and of TAA, production of immunosuppressive factors, killing of activated T cells or induction of TCR signaling defects, inhibition of proinflammatory signals, impaired maturation of DCs, and defective CD4+ T-cell help [21].
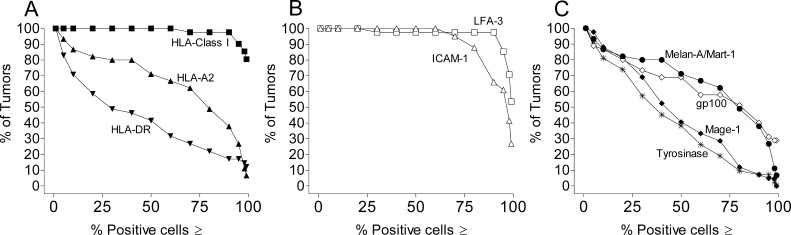
An example of one of the main mechanisms of tumor escape—i.e., the loss of HLA-restricting elements and/or the loss of TAA—can be documented by looking at the expression of several of such proteins in panels of melanoma cells. For example, in a panel of short-term metastatic melanoma cell lines, isolated in our laboratory from HLA-A2+ patients, we investigated the expression of HLA class I and II molecules, adhesion/costimulatory molecules and several TAAs. As shown in the cumulative frequency plots of Fig. 3, more than 90% of the tumors expressed adhesion molecules (e.g., ICAM-1 and LFA-3) on at least 75% of the cells. Similarly, HLA class I molecules (recognized by a mAb to a monomorphic determinant) were found always expressed at high levels in all tumors. In contrast, the HLA-A2 allele, a frequent restricting element for several melanoma-associated antigens recognized by CD8+ T cells, was not expressed on at least a fraction of the cells in several tumors. Similarly, reduced expression of four different TAAs (Melan-A/MART-1, gp100, tyrosinase, and MAGE-1) was frequently documented in many tumor lines (Fig. 3). These results match similar findings described by several authors even by looking at tissue sections [22].
Fig. 3A–C.
Expression of HLA class I, HLA-A2, HLA-DR, adhesion molecules, and TAA on 42 human melanoma cell lines isolated from invaded lymph nodes of HLA-A2+ patients. Expression of the indicated molecules was evaluated by flow cytometry after staining with specific mAbs [34, 35]. Cell permeabilization was performed to identify expression of Melan-A/MART-1, gp100, tyrosinase, and MAGE-1 in the cytoplasm. The distribution of the antigen expression in the panel of cell lines is described through cumulative frequency plots. In such plots the proportion of tumors showing a fraction of positive cells equal to or higher than an increasing value was plotted against a range of 14 frequency classes ranking from ≥1 to ≥99% positive cells
In addition to these classical examples of tumor escape mechanisms, new pathways of immune resistance are being continuously discovered in human melanoma, such as those based on tryptophan degradation, leading to impaired T-cell proliferation, increased resistance to cell death through triggering of CCR10, and inhibition of DC maturation through production of gangliosides [23–25]. Furthermore, this human tumor is even a prototype for the development of cell-surface and intracellular mechanisms of resistance to programmed cell death. This can result from down-regulation of death receptors, up-regulation of antiapoptotic inhibitors of caspase activation, or even by silencing of proapoptotic genes acting at several levels of the mitochondrial pathway of apoptosis [26]. These cellular mechanisms of resistance to apoptosis contribute to explaining the well-known chemoresistance of melanoma. These processes may be highly effective even in tumor defense against cell-killing induced by immune effectors, either by engagement of death receptors of the TNF family, or by release of cytolytic granules [27]. The resistance to CTL-mediated killing, mediated by expression of the serine protease inhibitor PI-9/SPI-6, which inactivates the cytotoxic factor granzyme B, is a significant example of such mechanisms [28].
Not surprisingly, many of the tumor resistance and immune-evasion mechanisms are linked to tumor progression, as exemplified by the well-known association between tumor stage and loss/down-modulation of HLA class I antigens and of HLA class I–processing molecules, or up-regulation of Fas-L [29–32]. Moreover, the development of tumor-escape mechanisms, along with melanoma progression, has another significant, but disappointing, implication for the clinical management of disease: the highly diverse landscape of immune evasion generally leads to coexistence of multiple mechanisms in the same lesion. This process may efficiently neutralize the concurrent activation of immunity, and the host response to tumor may even be a selective force in this scenario, as suggested by the “cancer immunoediting” theory [33]. In fact, according to this theory, put forward by R.D. Schreiber and colleagues [33], the immune system may contribute to defining the immunogenic phenotype of tumors that develop in immunocompetent hosts. The evolution of immune escape mechanisms in advanced disease, in spite of development of immunity, may contribute to explain the lack of relationship between evidence of immunity in peripheral blood or at the tumor site, and effective tumor destruction in the lesions. This, in turn, would even explain the lack of correlation between immunity and patients’ survival. The strength of these processes in neutralizing an existing antitumor immunity can be exemplified by a potential new mechanism of tumor escape found by looking at tissue sections of lymph node metastases from a large panel of melanoma patients. In these lesions, by immunohistochemistry, we evaluated not only the presence of CD4+ and CD8+ T cells infiltrating the neoplastic tissue, but also the maturation stage of the CD8+ T cells as defined by CCR7 and intracellular expression of cytolytic factors [13]. In most lesions, we found that CD4+ T cells did not infiltrate the neoplastic mass, although they were abundantly present in the peritumoral tissue. In contrast, several lesions contained infiltrating CD8+ T cells intermingling with the neoplastic tissue. In addition, in some of the patients, the infiltrating CD8+ T cells had lost expression of CCR7, indicating progression along their differentiation pathway to at least the TEM stage. However, a careful comparison of intratumoral and peritumoral CD8+ lymphocytes for the expression of cytolytic granules (perforin+ or granzyme B+) indicated a significantly higher expression of these factors in the CD8+ T cells surrounding the tumor tissue compared with those that infiltrated it [13]. Thus, apparently, the infiltration of CD8+ T cells, and their partial maturation to the CCR7− stage, was not inhibited in the invaded lymph nodes, while full maturation to cytotoxic factor+ cells was significantly hampered. These results might provide an explanation for the apparent paradox of a high number of differentiated tumor-specific T cells found at the tumor site in some of these patients, without evidence for tumor destruction in the same lesions [13]. The nature of the mechanism that may prevent full CD8+ T-cell maturation at the tumor site remains to be elucidated. However, lack of CD4+ T-cell help is a possible explanation, in agreement with the observed paucity of CD4+ T cells within the tumor tissue. On the other hand, additional mechanisms may be invoked. For example, neoplastic cells may express cell-surface receptors, or release soluble factors, that may influence the ability of T cells to differentiate into cytolytic effectors. Interestingly, with respect to these potential mechanisms, tumor cells would not be the only example of inhibition of T-cell differentiation, as shown by the blocking activity exerted on T cells by endothelial or stromal cells from bone marrow [34, 35].
CD4+ T cells as double-edged swords: “help” vs immune suppression
Development of powerful escape and resistance mechanisms, along with tumor progression, is not the only explanation for the failure of the immune system to control melanoma growth, in spite of its immunogenicity. Defective or altered regulation of the induction phase of the response could contribute to impair development of effectors (such as CTLs), independently from tumor escape mechanisms. This phase in the generation of immunity is mainly under the control of CD4+ T cells through either positive or negative effects. In fact, on the one hand, an effective CD4+-dependent, priming phase of antitumor immunity would clearly promote activation of both humoral and cell-mediated responses, while, on the other hand, tumor-specific CD4+ T suppressor cells may impair the response. As revealed by classical experiments performed in the 1980s by Greenberg et al. [36] and Greenberg [37], in murine models of chemoimmunotherapy, noncytolytic CD4+ T cells play a key role in the eradication of established tumors. These initial observations have been confirmed in different tumor models by several investigators throughout the past 2 decades, fostering the search for tumor antigen-specific CD4+ T cells in melanoma patients. Indeed, a large number of HLA class II–restricted epitopes have been identified on melanoma cells (see http://www.cancerimmunity.org/peptidedatabase/Tcellepitopes.htm for a complete listing of currently known HLA class II–restricted tumor antigens). In several instances, HLA class II–restricted epitopes have been found within the sequence of known HLA class I–restricted tumor antigens, indicating both a CD4+ and a CD8+-mediated response to the same protein expressed in melanoma cells. Moreover, as exemplified by the results obtained with the NY-ESO-1 protein, the same melanoma-associated antigen can be the target of an integrated immune response, regulated by TH1- and TH2-type CD4+ T cells and leading to development of both antibodies and CD8+ cytolytic T cells [38–40]. Thus, these results suggest that the CD4-dependent, helper arm of the immunity to melanoma antigens is not necessarily defective in all patients and that, in at least some instances, the same tumor antigen can be the target of an integrated immune response.
On the other hand, the CD4+ T-cell subset has recently gained new attention due to the definition of phenotype and function of CD4+ Treg cells with suppressive function [41–43]. These cells have been initially considered as a physiological mechanism to maintain peripheral tolerance to self-antigens [43]. However, the identification of antigen-specific T-suppressor cells directed to melanoma-associated antigens and able to inhibit antitumor immunity would open a new window on our understanding of mechanisms that may impair the ability of the immune system to control tumor growth in this disease. The recent identification of Treg cells, from tumor-infiltrating lymphocytes of cancer patients and directed to LAGE1 [44], a cancer-testis antigen expressed in several tumors including melanoma, represents a remarkable example of a new field of investigation that may ultimately lead to a better definition of the dual role of CD4+ T cells in the regulation of immunity to melanoma.
The prospects for improved immunotherapy approaches
Several small-scale phase I/II clinical trials of vaccination of metastatic melanoma patients have been performed over the past few years (see [45, 46] for review). These studies have enrolled more than 400 patients worldwide, but have been performed using a variety of approaches, including peptides ± adjuvants, or ± cytokines, or DCs loaded with tumor antigens, thus hampering a critical comparison of the clinical and immunological results. Nevertheless, an analysis of the clinical results of such trials indicate that objective responses could range from 0 up to 42% of enrolled patients in each study, but were below 20% in most instances, and often not long lasting, even when significant (see [45, 46] for details). In spite of the still unsatisfactory clinical results, several of these studies have shown, by immune monitoring of vaccinated patients, that the immunotherapy does boost immunity to the target antigens and/or to the autologous tumor, as indicated by increased frequency and maturation to effector stage of antigen-specific T cells in either peripheral blood or tumor site. A possible interpretation of these results is that the road taken to promote antitumor immunity by immunotherapy is effective in some of the patients, at least in terms of boosting the response. However, these clinical trials suggest also that the manipulation of the immune system through vaccination is not yet able to generate, in most patients, effector cells in number, specificity, functional status, homing properties, and survival characteristics, above the threshold needed to overcome existing tumor escape mechanism and promote antitumor responses even in presence of a significant tumor load. In fact, providing that a sufficiently high number of effector T cells are generated, then tumor burden and tumor escape mechanisms may not represent an absolute barrier. In support of this notion, remarkable clinical results have been recently achieved in patients with refractory metastatic melanoma by a combination of adoptive cell transfer and chemotherapy [47].
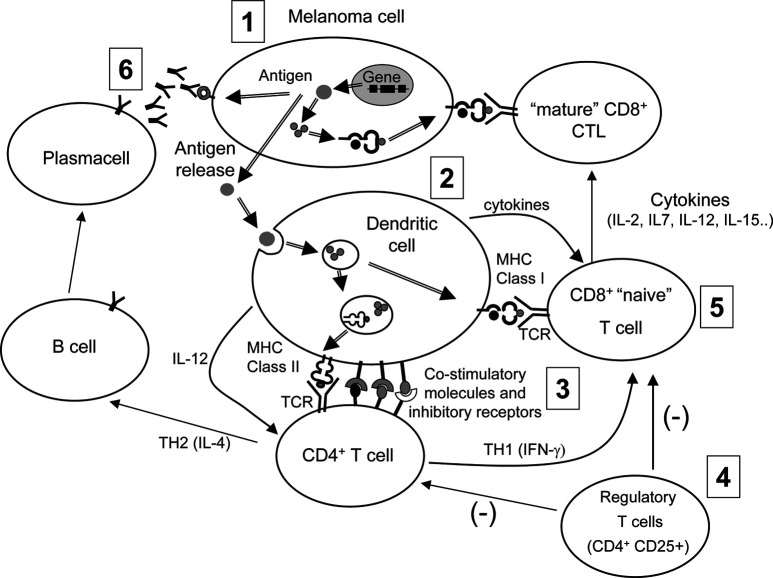
Thus, by integrating the information coming from the basic research in immunology, from murine models of immunotherapy, and from trials of vaccination or adoptive immunotherapy in melanoma patients, we are beginning to appreciate which phases and processes of immune response to melanoma should be manipulated with greater efficacy by future clinical attempts. By referring to a highly simplified view of immune response to melanoma, at least six critical steps can be identified (see Fig. 4). Each of these steps needs to be targeted with improved efficacy, compared to what has been already achieved. For example, selection of the antigen (step 1) needs to be reevaluated in the light of current information on differential immunogenicity [13] of distinct melanoma antigens (perhaps, not all tumor antigens are created equal). Thus, a careful immunological characterization of patients on the basis of not only antigen expression on their tumor, but even on number and functional status of their tumor-specific T cells (e.g., by tetramer analysis) could provide useful clues as to the most effective target antigen in each patient. Step 2, the generation and usage of DCs, is a field with very significant areas for improvement for the immunotherapy of melanoma. Our current knowledge regarding choice of DC progenitors, cocktail of cytokines for generating DCs, and, above all, the possibility of triggering Toll-like receptors for inducing full maturation to the highest immunostimulatory stages [48], are just a few examples of how the professional APCs could be exploited to induce better results as far as boosting antitumor immunity. The third step, the interface between T cells and DCs, is emerging as the most critical phase for inducing response, or even autoimmunity, rather than for contributing to maintenance of tolerance [49, 50]. Moreover, at this stage, bidirectional signals from costimulatory molecules and their receptors on DCs and T cells (e.g., B7-1/CD28 or CD40/CD40L), from inhibitory receptors (such as CTLA-4), and from cytokines, may regulate “intensity” (by promoting or inhibiting T-cell activation) and “direction” of the immune response (e.g., by skewing CD4+ T cells toward a TH1 or a TH2 functional program) [51]. The recent description of induction of autoimmunity, together with cancer regression, observed in vaccinated melanoma patients subjected to CTLA-4 blockade [52], exemplifies the clinical opportunity, as well as the risks, connected with the interference with the regulatory molecules involved in T-DC interaction. Step 4, the role of regulatory T cells, is an emerging field, and several lines of evidence suggest that such cells contribute to maintain peripheral tolerance to self-antigens, thus preventing autoimmunity, by cell contact-dependent and -independent mechanisms [41]. Nevertheless, these cells may even contribute to down-modulating responses to vaccines in neoplastic patients [42], and strategies to block or inhibit these cells may improve the outcome of immunotherapy approaches. Step 5, the generation of cytolytic effector cells, is a critical phase for both vaccination and adoptive immunotherapy attempts. This phase of the response could be significantly influenced by improving the priming of CD8+ T cells through T-cell interaction with mature DCs. At the same time it is becoming clear that several cytokines (such as those mentioned in Fig. 4) have a remarkable effect on either antigen-dependent or independent differentiation of CD8+ T cells to mature CTLs, as well as on their survival and homing properties [53, 54]. Although the use of cytokines in melanoma therapy is hampered by toxicity limits, a careful evaluation of the risks and benefits of these soluble mediators should nevertheless allow us to include them in vaccination trials, as it has been done in some instances. This would ease the generation of large numbers of tumor-specific effector cells and promote their survival. Last but not least, the generation of an effective antibody response to melanoma (step 6), should not be forgotten as a relevant issue in the attempt to control tumor growth, even in the context of immunotherapy approaches initially aimed at promoting a cellular, rather than a humoral response. The experience of investigators working with SEREX technology [55] has indicated that melanoma patients can produce antibodies to a wide set of gene products expressed in the neoplastic cells, suggesting that even a TH2-type response might not be detrimental to achieving tumor regression. This suggests that in principle, and as several murine models have clearly indicated, one of the most effective immunotherapy approaches would be one able to generate both a humoral and a cell-mediated response [56, 57].
Fig. 4.
A simplified view of immune response to melanoma. Antigen expression and release (step 1) leads to uptake by professional APCs (step 2). After maturation of DCs induced by proinflammatory signals and T-DC interaction, priming of CD4+ T cells can take place (step 3). At this stage, a bidirectional interaction between T cells and DCs, mediated by cell-surface regulatory molecules and cytokines, may determine activation versus inhibition of a T cell–mediated response and skewing of a CD4+ T cell developmental program toward either a TH2- (step 6) or a TH1-type response. Activation of a cell-mediated response is also under the negative regulatory action (−) of suppressor cells (step 4). Priming, clonal expansion, and functional maturation of CD8+ T cells (step 5) requires interaction with mature DCs, and with helper T cells. In addition, this step is regulated by several cytokines, which can promote maturation of CD8+ T cells to cytolytic effectors, even in an antigen-independent fashion
In conclusion, although the challenge remains daunting, we now have reason to believe that an integrated approach to the manipulation of antitumor immunity, attempting to influence simultaneously or sequentially most of the key steps of the immune response, is the way forward for melanoma immunotherapy. Such an approach, although remarkably difficult to implement in real clinical trials, may hold the promise for turning the paradox of tumor immunity in spite of poor clinical response into a contradiction of the past.
Acknowledgements
We are grateful to the melanoma patients for their generous participation in this study. This work was supported in part by grants from A.I.R.C., Milan; CNR, Rome; the Ministry of Health, Rome; and I.S.S. Rome.
Footnotes
This article forms part of the Symposium in Writing “Tumor escape from the immune response,” published in Vol. 53.
References
- 1.Balch J Clin Oncol. 2001;19:3635. doi: 10.1200/JCO.2001.19.16.3635. [DOI] [PubMed] [Google Scholar]
- 2.Balch J Clin Oncol. 2001;19:3622. doi: 10.1200/JCO.2001.19.16.3622. [DOI] [PubMed] [Google Scholar]
- 3.Clark J Natl Cancer Inst. 1989;81:1893. doi: 10.1093/jnci/81.24.1893. [DOI] [PubMed] [Google Scholar]
- 4.Mihm Lab Invest. 1996;74:43. [Google Scholar]
- 5.Naito Cancer Res. 1998;58:3491. [PubMed] [Google Scholar]
- 6.Schumacher Cancer Res. 2001;61:3932. [PubMed] [Google Scholar]
- 7.Zhang N Engl J Med. 2003;348:203. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 8.Renkvist Cancer Immunol Immunother. 2001;50:3. doi: 10.1007/s002620000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palmowski Immunol Rev. 2002;188:155. doi: 10.1034/j.1600-065X.2002.18814.x. [DOI] [PubMed] [Google Scholar]
- 10.Anichini J Exp Med. 1999;190:651. doi: 10.1084/jem.190.5.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McMichael J Exp Med. 1998;187:1367. doi: 10.1084/jem.187.9.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Altman Science. 1996;274:94. [PubMed] [Google Scholar]
- 13.Mortarini Cancer Res. 2003;63:2535. [PubMed] [Google Scholar]
- 14.Kaech Nat Rev Immunol. 2002;2:251. doi: 10.1038/nri778. [DOI] [PubMed] [Google Scholar]
- 15.Houghton Curr Opin Immunol. 2001;13:134. doi: 10.1016/s0952-7915(00)00195-3. [DOI] [PubMed] [Google Scholar]
- 16.Ochsenbein Nature. 2001;411:1058. doi: 10.1038/35082583. [DOI] [PubMed] [Google Scholar]
- 17.Appay Nat Med. 2002;8:379. doi: 10.1038/nm0402-379. [DOI] [PubMed] [Google Scholar]
- 18.Champagne Nature. 2001;410:106. doi: 10.1038/35065118. [DOI] [PubMed] [Google Scholar]
- 19.Willis J Immunol. 2002;168:5455. doi: 10.4049/jimmunol.168.11.5455. [DOI] [PubMed] [Google Scholar]
- 20.Geginat Blood. 2003;10:1182. [Google Scholar]
- 21.Marincola Trends Immunol. 2003;24:335. doi: 10.1016/s1471-4906(03)00116-9. [DOI] [PubMed] [Google Scholar]
- 22.Seliger Semin Cancer Biol. 2002;12:3. doi: 10.1006/scbi.2001.0404. [DOI] [PubMed] [Google Scholar]
- 23.Murakami J Exp Med. 2003;198:1337. doi: 10.1084/jem.20030593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peguet-Navarro J Immunol. 2003;170:3488. doi: 10.4049/jimmunol.170.7.3488. [DOI] [PubMed] [Google Scholar]
- 25.Uyttenhove Nat Med. 2003;9:1269. doi: 10.1038/nm934. [DOI] [PubMed] [Google Scholar]
- 26.Soengas Oncogene. 2003;22:3138. doi: 10.1038/sj.onc.1206454. [DOI] [PubMed] [Google Scholar]
- 27.Ivanov Oncogene. 2003;22:3152. doi: 10.1038/sj.onc.1206456. [DOI] [PubMed] [Google Scholar]
- 28.Medema Proc Natl Acad Sci U S A. 2001;98:11515. doi: 10.1073/pnas.201398198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dissemond Arch Dermatol Res. 2003;295:43. doi: 10.1007/s00403-003-0393-8. [DOI] [PubMed] [Google Scholar]
- 30.Kageshita Am J Pathol. 1999;154:745. doi: 10.1016/S0002-9440(10)65321-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Geertsen J Invest Dermatol. 1998;111:497. doi: 10.1046/j.1523-1747.1998.00305.x. [DOI] [PubMed] [Google Scholar]
- 32.Terheyden J Invest Dermatol. 1999;112:899. doi: 10.1046/j.1523-1747.1999.00607.x. [DOI] [PubMed] [Google Scholar]
- 33.Dunn Nat Immunol. 2002;3:991. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 34.Biedermann J Immunol. 1998;161:4679. [PubMed] [Google Scholar]
- 35.Di Blood. 2002;99:3838. doi: 10.1182/blood.V99.10.3838. [DOI] [PubMed] [Google Scholar]
- 36.Greenberg J Exp Med. 1981;154:952. [Google Scholar]
- 37.Greenberg Adv Immunol. 1991;49:281. doi: 10.1016/s0065-2776(08)60778-6. [DOI] [PubMed] [Google Scholar]
- 38.Gnjatic Proc Natl Acad Sci U S A. 2003;100:8862. doi: 10.1073/pnas.1133324100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jager J Exp Med. 1998;187:265. [Google Scholar]
- 40.Zarour Cancer Res. 2002;62:213. [PubMed] [Google Scholar]
- 41.Bach Nat Rev Immunol. 2003;3:189. doi: 10.1038/nri1026. [DOI] [PubMed] [Google Scholar]
- 42.Javia J Immunother. 2003;26:85. doi: 10.1097/00002371-200301000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shevach Nat Rev Immunol. 2002;2:389. doi: 10.1038/nri821. [DOI] [PubMed] [Google Scholar]
- 44.Wang Immunity. 2004;20:107. doi: 10.1016/S1074-7613(03)00359-5. [DOI] [PubMed] [Google Scholar]
- 45.Parmiani J Natl Cancer Inst. 2002;94:805. doi: 10.1093/jnci/94.11.805. [DOI] [PubMed] [Google Scholar]
- 46.Parmiani Anns Oncol. 2003;14:817. doi: 10.1093/annonc/mdg246. [DOI] [Google Scholar]
- 47.Dudley Science. 2002;298:850. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gordon Cell. 2002;111:927. doi: 10.1016/S0092-8674(02)01201-1. [DOI] [PubMed] [Google Scholar]
- 49.Abken Trends Immunol. 2002;23:240. doi: 10.1016/S1471-4906(02)02180-4. [DOI] [PubMed] [Google Scholar]
- 50.Appleman Immunol Rev. 2003;192:161. doi: 10.1034/j.1600-065X.2003.00009.x. [DOI] [PubMed] [Google Scholar]
- 51.Schwartz Nat Immunol. 2002;3:427. doi: 10.1038/ni0502-427. [DOI] [PubMed] [Google Scholar]
- 52.Phan Proc Natl Acad Sci U S A. 2003;100:8372. [Google Scholar]
- 53.Anichini J Immunol. 2003;171:2134. doi: 10.4049/jimmunol.171.4.2134. [DOI] [PubMed] [Google Scholar]
- 54.Schluns Nat Rev Immunol. 2003;3:269. doi: 10.1038/nri1052. [DOI] [PubMed] [Google Scholar]
- 55.Preuss Immunol Rev. 2002;188:43. doi: 10.1034/j.1600-065X.2002.18805.x. [DOI] [PubMed] [Google Scholar]
- 56.Dranoff J Clin Invest. 2003;111:1116. doi: 10.1172/JCI200318359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Waldmann Nat Med. 2003;9:269. doi: 10.1038/nm0303-269. [DOI] [PubMed] [Google Scholar]