Abstract
This review focuses on the use of radiolabeled antibodies in the therapy of cancer, termed radioimmunotherapy (RAIT). Basic problems concerned with the choice of antibody, radionuclide, and physiology of the tumor and host are discussed, followed by a review of the pertinent clinical publications of various radioantibody constructs in the treatment of hematopoietic and solid tumors of diverse histopathology, grade, and stage, and in different clinical settings. Factors such as dose rate delivered, tumor size, and radiosensitivity play a major role in determining therapeutic response, while target-to-nontarget ratios and, particularly, circulating radioactivity to the bone marrow determine the principal dose-limiting toxicities. RAIT appears to be gaining a place in the therapy of hematopoietic neoplasms, such as non-Hodgkin's lymphoma: several agents are advancing in clinical trials toward registration, and one has recently been approved by the FDA. Although RAIT of solid tumors has shown less progress, use of pretargeting strategies, such as an affinity-enhancement system consisting of bispecific antibodies separating targeting from delivery of the radiotherapeutic, appears to enhance tumor-to-nontumor ratios, and may increase radiation doses to tumors more selectively than directly labeled antibodies.
Keywords: Antibodies, Cancer, Radioimmunotherapy, Therapy
Introduction
Although more than 20 years have elapsed since the introduction of radioimmunodetection (RAID) and radioimmunotherapy (RAIT), involving the use of isotopes conjugated to monoclonal antibodies (mAbs) for imaging and therapy, respectively [53, 54, 56], it has only been in the last few years that this technology has begun to gain a role in the treatment of cancer because of its success in lymphoma patients. This article is intended to review the current status of cancer therapy with radiolabeled antibodies, and to complement and extend other recent reviews [46, 47, 49, 50, 60, 66, 77, 92, 133]. It is now appreciated that (1) many radionuclides and antibodies have potential applicability for this therapy, (2) antibody accretion remains the major limitation in delivering effective tumor radiation doses, and (3) multiple administrations and combinations with other treatment modalities will prove necessary, especially in the therapy of solid tumors. Whereas RAIT of hematopoietic neoplasms is becoming established as a new future therapy modality [32, 40, 43, 48, 67, 103, 104, 127], solid tumors have been less responsive, although targeting minimal or micrometastatic disease appears at present to be the most optimal approach in solid tumor therapy. The challenge of treating solid tumors has stimulated a number of approaches to improve the radiation dose delivered and to achieve a more uniform distribution of ionizing radiation, the ultimate goal being the delivery of tumoricidal doses while sparing normal tissues.
Basic challenges
Numerous reviews have discussed the dependence of RAIT on three basic factors: the antibody, the radionuclide, and the target tumor and host [12, 14, 29, 34, 52, 58, 115, 117, 118, 129]. The most important variables affecting tumor response include cumulative radiation dose delivered to the tumor(s), dose rate, penetration, and tumor radiosensitivity. Tumor uptake and penetration have been the most challenging limitations, since accretion has been at such low levels as 0.001–0.01% of the injected dose of radiolabeled antibody per gram of tumor, resulting in tumor doses usually of <1,500 cGy. The dose-limiting organ has been the bone marrow, which appears to have a dose limitation of about 150–200 cGy. Hence, improvements in accretion need to be achieved before the radiation doses needed for efficacy are achieved, and here various approaches have been pursued, such as the use of agents that increase tumor vascularization (flow) and permeation, antibody combinations, repeated applications, and pretargeting methodologies, to mention a few [5, 16, 19, 20, 21, 29, 30, 31, 34, 42, 47, 52, 57, 91, 99, 114, 129].
The selection of appropriate antibodies seems to be sufficient for the principal cancer types, and the earlier limitation of using immunogenic murine antibodies has been overcome with the introduction of chimeric, humanized, and fully human antibodies. The relation of different antibody forms to tumor uptake and residence time is summarized in Table 1, showing that the smaller the molecule, the faster and lower the uptake, as well as the shorter the residence time in tumor. This suggests that larger, intact immunoglobulin molecules may still be the optimal targeting agent in RAIT. However, initial dose rates appear to be much higher with monovalent fragments, such as Fab, as compared to whole IgG, despite lower absorbed tumor doses at equitoxic dosing; this has also resulted, in animal models, in effective control of tumor growth because of high intratumoral dose rates [8, 10].
Table 1.
Targeting properties of different forms of antibodiesa (reproduced from [50], with permission)
| IgG | F(ab')2 | Fab' | Diabody | scFv | |
|---|---|---|---|---|---|
| Physical | |||||
| Molecular wt. | 150 k | 100 k | 50 k | 40 k | 20 k |
| Biological | |||||
| Immune effector function | Yes | No | No | No | No |
| Biol. T 1/2 blood | 2–3 days | 1–2 days | 4 h | <4 h | 1 h |
| Target organ | Liver | Liver | Kidney | Kidney | Kidney |
| Tumor binding | |||||
| Uptake (1= highest to 4= lowest) | 1 | 2 | 3 | 3 | 4 |
| Duration (1= longest to 4= shortest) | 1 | 2 | 3 | 3 | 4 |
| Optimal accretion time | Days | Day | Hours | Hours | Hour |
aKindly provided by R.M. Sharkey, Belleville, N.J.
Even the selection of radionuclides available for RAIT has expanded in recent years, such that several useful candidates are available and have shown clinical promise (Table 2). The suitability of a radionuclide resides in its physical and chemical properties, its stability after conjugation to antibody, and the nature of its radiation (high or low linear energy transfer). The choice is also influenced by the clinical setting, such as tumor location, size, morphology, physiology, and radiosensitivity, as well as the kinetics of the antibody. For example, a large tumor would be more amenable to therapy with a deep-penetrating beta-emitter, and radionuclides with shorter path-lengths of penetration, with higher LET, would be more suitable for smaller or minimal disease. The need for a radiolabeling procedure to be simple, efficient, reproducible, and affordable is self-evident, but these criteria are not necessarily fulfilled by some of the radionuclides reported in some clinical trials. Table 2 lists many of the radionuclides that have been studied clinically, but at present only 131I and 90Y appear to fulfill the requirements for commercial development. Nevertheless, necessity and success with any radionuclide will ultimately result in improved availability and cost, as market conditions come into play when new therapies are needed for life-threatening diseases. Recently, much interest was provoked by the report showing that actinium-225, with a 10-day half-life, coupled to internalizing antibodies, showed in vitro and in vivo (in mice) destruction of human tumor cells by alpha-irradiation [88].
Table 2.
Radionuclides of current interest in RAIT (reproduced from [5], with permission)
| Isotope | Half-life (h) | Emission (for therapy) | Maximum energy (keV) | Maximum particle range (mm) |
|---|---|---|---|---|
| Iodine-131 (131I) | 193 | Beta | 610 | 2.0 |
| Yttrium-90 (90Y) | 64 | Beta | 2,280 | 12.0 |
| Lutetium-177 (177Lu) | 161 | Beta | 496 | 1.5 |
| Copper-67 (67Cu) | 62 | Beta | 577 | 1.8 |
| Rhenium-186 (186Re) | 91 | Beta | 1,080 | 5.0 |
| Rhenium-188 (188Re) | 17 | Beta | 2,120 | 11.0 |
| Bismuth-212 (212Bi) | 1 | Alpha | 8,780 | 0.09 |
| Bismuth-213 (213Bi) | 0.77 | Alpha | >6,000 | <0.1 |
| Astatine-211 (211At) | 7.2 | Alpha | 7,450 | 0.08 |
The excellent targeting of tumors with diverse pancarcinoma antibodies and the relatively good radiosensitivity of certain neoplasms encourage the pursuit of cancer radioimmunotherapy. However, there are still too few such studies reported, and none has yet progressed beyond phase I or II trials. This may be due both to the nature of the patient population being studied and to development requirements involving the radiolabeled antibodies. As in most phase I studies, usually patients with advanced disease are entered, and these have been heavily pretreated, resulting in unfavorable patients for assessing dose response or potential efficacy. Whereas small doses of antibody fragments, which are virtually nonimmunogenic, can be used for immunoscintigraphy, higher protein doses of intact immunoglobulins used in a therapy setting can result in immune responses, especially with repeated doses, if murine antibodies are used. Therefore, there has been an effort to use chimeric, humanized (CDR-grafted), and now even fully human immunoglobulins, and these appear to be well tolerated. More potent beta-emitting radionuclides, such as 90Y, have also been used increasingly as stable chelation methods have developed [60]. The few clinical trials of RAIT thus far published have focused mostly on CEA, MUC1, TAG-72, L6, and Thomsen-Friedenreich (Tn) antibodies, and have been early studies by single groups of investigators.
Optimization of RAIT
Strategies to improve RAIT reduce to five basic goals: (a) enhance antibody uptake and distribution in tumor by increasing tumor vascular permeability and flow, using smaller molecules, and possibly exploiting pretargeting strategies; (b) decrease nontargeted antibody in the blood by in vivo clearance or ex vivo adsorption mechanisms, as well as the pretargeting approaches, (c) protect normal organs from radiotoxicity, e.g., by using hematopoietic growth factors and peripheral blood stem cell reconstitution, and by blocking readsorption of antibody fragments by the kidneys with cationic amino acids, amino sugars, and their polymers; (d) decrease the immunoglobulin's immunogenicity by humanization or use of human antibodies, or immunosuppressing the host; and (e) improve the radiation dose and dose rate in tumor without concomitantly increasing cumulative radiation in normal organs, which is accomplished by many of the other strategies and perhaps also by adjusting the antibody dose and the dose schedule (e.g., dose fractionation) of the radiopharmaceutical. Since these topics have been covered in other publications [5, 16, 20, 21, 29, 30, 34, 42, 47, 52, 57, 91, 99, 114, 129], they will be addressed here only briefly and mostly in reference to clinical studies.
Dose fractionation
Regardless of which antibody or radionuclide is used in RAIT, consideration of how this is administered is now receiving considerable attention. Should the radioimmunoconjugate be given in a bolus as a single, maximally tolerated dose, or should it be administered in smaller, fractionated doses, as now practiced with external beam radiation therapy? The convention of administering external beam radiotherapy by fractionated doses has suggested that the same improved effects could be achieved with fractionated RAIT. One possible reason for such an improvement is that fractionation of the amounts of radionuclide delivered may contribute to a more uniform distribution of radiation dose throughout the tumor, with different tumor regions radiated with each dose, especially if tumor size and cell numbers decrease with the first injections, making the remaining viable cells more amenable to subsequent cytotoxic irradiation. The evidence and rationale supporting the advantage of fractionated RAIT suggest that fractionated RAIT provides more uniform distribution of antibody and radiation dose, reduced toxicity, increased maximal tolerated dose and tumor radiation, and prolongation of tumor response [36, 91, 114]. Disadvantages of fractionated RAIT have also been listed, such as lower radiation dose rate, a more complicated therapy schedule, increased cost, and a potential delay in tumor regression. In a clinical study comparing dose fractionation with single doses of radiolabeled antibody, it was found that the former produced a modest increase in the therapeutic window, because bone marrow suppression was statistically significantly less than with fractionated RAIT [114]. Such dose fractionation, however, requires the use of a nonimmunogenic antibody, such as human or humanized (CDR-grafted) forms, since fractionated small doses of foreign immunoglobulin, such as murine antibodies, would result in human anti-murine antibodies that affect the kinetics of the radiolabeled antibody with subsequent doses [114].
Combination RAIT and chemotherapy
Most efforts to improve RAIT have focused on increasing the uptake of the radioimmunoconjugate, improving its penetration and distribution within the tumor, and enhancing tumor to nontumor localization ratios, so as to afford a more selective tumor targeting. Since these have been reviewed elsewhere, as discussed above, they will not be discussed again here. Even more than external beam irradiation, RAIT is potentially used optimally in combined therapy modalities, since the carrier antibody can also deliver other therapeutic agents, radiosensitizers, and vascularization and biological response modifiers. Since the foremost dose-limiting toxicity of RAIT is bone marrow suppression, hematopoietic cytokines and autologous blood stem cell grafting can be combined with RAIT in order to overcome myelosuppression.
The combination of radiolabeled antibodies with drugs has received increased interest and investigation in recent years. Studies have been concerned with choice of antibody and radionuclide, drug, tumor type, sequence of the application of the two modalities, and relative doses of each. It is still too early to make generalizations, but current evidence suggests the beginning with the radiolabeled antibody may be more effective than initiating the drug first, followed by RAIT [42]. But it is not established yet whether this is true for all tumor systems and all RAIT and drug choices. It seems that the more radiosensitizing the drug, the better the combination performs, but less radiosensitizing agents have not been studied as much. Since combined modality RAIT is, in my view, a rational improvement over either modality alone, some of the interesting studies published will be discussed further. Most preclinical experiments involving xenografted human tumors in nude mice have shown evidence of improved therapeutic efficacy for the combination, but there is a paucity of data supporting any particular schedule for the two modalities. Except for the study by DeNardo et al. [42], few investigators have reported on the effect of scheduling of the two modalities, and how dose scheduling may be influenced by the tumor and its clinical setting. It has even been found that external beam irradiation can improve therapeutic efficacy when combined with radiolabeled CEA antibodies [27, 28]. In experimental systems, RAIT has also been found to affect tumor vascularization and vascular permeability, as well as hypoxia [16, 20, 21], which may be one mechanism explaining how subsequent therapies, including drugs, could be affected.
The few clinical studies reported on the combination of chemotherapy with RAIT indicate an acceptable toxicity and some antitumor activity, but as yet no additive or synergistic effects have been shown in a randomized trial. Another problem in such trials is the generally poor condition of the study subjects, most of whom have large, progressing tumors that have relapsed after prior chemotherapy. This is why patient selection, emphasizing more limited disease, is essential for the true assessment of RAIT alone or RAIT in combination with other modalities.
Pre-targeting strategies
In order to increase the tumor-to-background ratios in antibody targeting, several promising pretargeting strategies separating antibody targeting from radionuclide delivery are being developed. These methods are intended to minimize the systemic radiation resulting from prolonged circulation of antibodies directly conjugated with isotopes, so that delivery of the radionuclide is accomplished only after most antibody has cleared from normal tissues. In general, a nonradioactive antibody containing a second recognition site, such as to a radiolabeled small molecule (or a hapten), is injected. At the time of maximal tumor accretion and circulatory clearance of the antibody, the relevant hapten bearing the radionuclide is injected as a second step. The radiolabeled hapten binds to the second recognition site of the tumor-localizing antibody, whereas unbound hapten is rapidly cleared from the body. Compared to directly labeled antibodies, these methods achieve higher tumor-to-blood and tumor-to-normal tissue ratios, but timing and the doses given are critical [4, 5, 31, 57, 58, 98, 99, 119].
One approach has been the noncovalent interaction between avidin or streptavidin and biotin, which have a high binding affinity, in the order of K a=1015 M −1 [65]. Avidin or streptavidin conjugated to antibody is targeted first, followed by the administration of radiolabeled biotin. Conversely, the targeting antibody can be biotinylated and, after being injected, avidin or streptavidin is administered in order to bind to the antibody at the tumor. The final step involves injection of radiolabeled biotin, which attaches to avidin at the tumor. Modifications of both approaches are being made, including the use of clearing agents that reduce the amounts of the targeting antibody at nontumor sites [4]. Thus, these can involve two or multistep procedures, all intended to increase tumor-to-nontumor ratios. However, both endogenous biotin and the immunogenicity of avidin and streptavidin can be problematic [31, 57, 58, 98, 99, 119].
Despite impressive results in animal studies of biotin/avidin methods [4], clinical trials have been less encouraging. A phase I clinical dose-escalation study found that nonmyeloablative doses exceeding 200 mCi of 90Y could be tolerated, with radioactivity in the tumor equaling that achieved with conventional RAIT [25, 26, 75]. However, untoward side-effects, particularly intestinal toxicity, limited further escalation and resulted in these particular studies being abandoned. Using another system involving CD20 antibody for the treatment of non-Hodgkin's lymphoma, doses up to 50 mCi/m2 of 90Y-DOTA biotin resulted in evidence of tumor regression [131]. Since this hematopoietic neoplasm is very radiosensitive, and has responded well to virtually all forms of RAIT, it is not clear whether this result represents a demonstration of the advantage of this pretargeting method or of the optimal results obtained with radiosensitive lymphomas. Nevertheless, in a preclinical study comparing conventionally labeled CD20 mAb with pretargeted RAIT with 90Y, higher tumor-to-nontumor tissue ratios and markedly better therapeutic results were obtained with the pretargeting approach [105].
Paganelli and co-workers have pioneered a three-step pretargeting method that involves biotinylated antibody given in the first step, the administration of streptavidin or avidin to clear circulating antibody and to couple to biotinylated antibody localized at the tumor as the second step, and finally the administration of radiolabeled biotin [94, 98, 99]. In a phase I/II clinical trial of high-grade glioma, 48 patients with residual disease or recurrence were treated with 90Y-DOTA biotin at 60–80 mCi/m2. The primary biotinylated antibody was an anti-tenascin mAb. An objective response of 25% and stable disease in 52% of the patients were reported. The mean absorbed dose to tumor, at the maximal tolerated dose, was 1,200 cGy per cycle. In some cases, the duration of response was more than 1 year, which is an encouraging finding in this tumor type [98]. A different pretargeting approach to increase the radiation dose delivered to tumor, as compared to blood and other normal tissues, has involved re-engineering of the targeting antibody molecule as a bispecific antibody (bsAb). This is made chemically or recombinantly from monovalent antibody fragments that target two different antigens, one at the tumor and the other a hapten chelate. After the bispecific antibody localizes in the tumor and clears from normal tissues, the second agent that binds selectively to the second arm of the antibody and delivers the radioactivity to the tumor is administered [5, 31]. A diagram of this method, termed the "affinity enhancement system" (AES), is presented in Fig. 1. In the most extensive work with this approach, the targeting antibody is against carcinoembryonic antigen (CEA) and the second arm recognizes a DTPA-indium chelate [5]. After the localization step, a bivalent hapten, which is a peptide incorporating two DTPA-indium moieties as well as tyrosine carrying a diagnostic or therapeutic radionuclide, is given. The novelty appears to include the use of a bivalent hapten, which forms a stable complex with two molecules of pretargeted bsAb at the tumor. Timing of the second injection of the radiolabeled hapten is important, in order to achieve high tumor-to-background ratios when there is little or no bsAb circulating in the blood. Excellent tumor imaging has been obtained with this AES method in several clinical trials [5, 6, 31, 78, 113]. Figure 2 is an image of a metastatic CEA-expressing carcinoma targeted by the radiolabeled hapten given as a second step in this AES system, showing excellent targeting against virtually no background radioactivity, as compared to the results when the bsAb is labeled. This involved a half-humanized (anti-CEA), half-murine (anti-hapten) bispecific antibody (Pentacea, IBC Pharmaceuticals, LLC, Morris Plains, N.J.) [31].
Fig. 1.
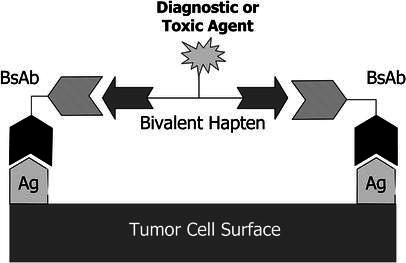
Schematic diagram of bispecific antibody pretargeting system, using a bivalent hapten for binding to two arms of a tumor-localizing bispecific antibody. (Reproduced with permission from [31])
Fig. 2.
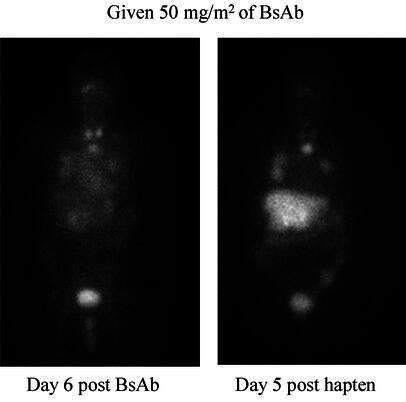
Comparison of targeting with an 131I-labeled anti-CEA bsAb (left) to that achieved with the 131I-labeled hapten, 6 and 5 days after injection, respectively, in a patient with a CEA-producing cancer who had lesions in the chest and liver. Some normal thyroid and urinary bladder activity is also seen. (Reproduced from [31] with permission)
Using a different bsAb, excellent targeting results have been reported in a preclinical model of human renal carcinoma. With the G250 bispecific antibody and pretargeting, Boerman et al. [22] showed that tumor to blood ratios increased up to values as high as 3,500 at 72 h post radiochelate injection. At 20 h post injection, about 50% of the whole-body activity was localized in the tumor. Therapy experiments in animal models have also confirmed the efficacy of AES, including substantial cures of xenografted human colon carcinoma [45]. This AES approach appears to hold much promise for RAIT, but may also be applicable to a more selective and enhanced delivery of drugs to tumors.
The AES pretargeting technology, using an anti-CEA bispecific antibody and a 131I-labeled hapten given 4 days later, has been studied in the treatment of patients with disseminated small cell lung cancer [128]. Doses of 1.48–6.66 GBq (40–180 mCi) of 131I were administered, with hematological rescue with autologous stem cells being done at doses above 150 mCi. Tumor targeting was excellent, and the estimated tumor doses in six patients were 2.6–32.2 cGy/mCi. Among the 12 patients evaluated, two partial responses, one stabilization and nine progressions were observed, with efficacy and toxicity being dose-related.
Clinical results
Hematopoietic tumors
Various antibodies, labels, and treatment strategies have been studied, and most have shown evidence of efficacy in hematopoietic tumors, particularly non-Hodgkin's lymphoma (NHL). For example, antibodies against CD20, CD22, CD37, and HLA-DR antigens have been used with either 131I, 90Y, or, rarely, other radionuclides, such as 186Re or 67Cu. Most initial studies showed favorable results with indolent forms of NHL, but more recent trials have also shown efficacy in aggressive NHL. Four agents have been studied most often in NHL: 131I-B1, 90Y-2B8, 131I/90Y-LL2, and 131I-Lym-1. A number of excellent reviews of progress in the RAIT of lymphomas have appeared in the past [32, 40, 43, 48, 67, 103, 104, 127].
The antitumor activity of RAIT is primarily due to the associated radioactivity of the radiolabel attached to the antibody, which emits continuous, exponentially decreasing, low-dose-rate irradiation with a heterogeneous dose deposition. In some cases, as is evidenced in lymphoma, the antibody itself may contribute to tumor destruction. There may also be an immune response of the host to tumor antigens released after antibody- or isotope-mediated cell destruction, as has been suggested in NHL treatment [41]. In summary, important considerations in the efficacy of RAIT include the nature of the antibody (specificity, affinity, avidity, dose, immunoreactivity, mechanism of action of naked antibody), the radiolabel (emission properties, half-life, stability of radioconjugate), the antigen targeted (location, modulation, stability, density, expression), and the nature of the target neoplasm (radiosensitivity, location, size, vascularization, immunogenicity, proliferative rate), as well as such factors as heterogeneity of dose deposition, dose rate effects and status of the host's bone marrow and normal organ functions following other forms of cytotoxic therapy. These considerations are illustrated well in the RAIT of NHL, but are also relevant to other neoplasms.
Non-Hodgkin's lymphoma
Bexxar (131I-tositumomab; Corixa Corp., Seattle, Wash.) and Zevalin (90Y-ibritumomab tiuxetan, IDEC-Y2B8; IDEC Pharmaceuticals, San Diego, Calif.) are the two most advanced RAIT products under regulatory review for NHL therapy. Both are murine antibodies directed against the CD20 antigen expressed on the surface of normal and malignant B lymphocytes. Bexxar is conjugated with 131I, whereas Zevalin is labeled with 90Y. Bexxar is used as an IgG2a murine mAb with cold murine antibody added, whereas Zevalin has the murine antibody labeled and cold human/mouse chimeric rituximab (Rituxan, IDEC/Genentech) added to the product. Bexxar is given with a patient-specific dosimetric pretherapy study, whereas Zevalin has been developed so that this pretherapy dosimetry is not needed, and is administered on a body weight basis. Both products, however, require a pretherapy cold antibody dosing in order to improve tumor targeting, which involves a 1-h infusion of 450 mg of unlabeled antibody with Bexxar and a much longer infusion (4–6 h) of 450 mg of rituximab with Zevalin. Hence, Bexxar involves three injections and three imaging sessions and Zevalin requires only two injections, unless the early imaging with 111In becomes required as a pretherapy step. Nevertheless, the time involved in treating a patient with these radiolabeled antibodies is even shorter than with nonradioactive rituximab, which is administered weekly over 4–8 weeks. Both products have shown higher and more durable responses than naked antibodies, but they also have dose-limiting toxicity, predominantly myelotoxicity. Infusional adverse reactions are minimal for Bexxar, as compared to Zevalin, and both show minimal nonhematological toxicities, with no hair loss or mucositis and generally minimal nausea. Because of the usually high release of 131I from Bexxar, thyroid blockage is required, yet may pose a complication of hypothyroidism even with such blockage. Some patients have shown myelodysplasia after long-term follow-up following Bexxar, but they were also heavily pretreated with chemotherapy, which could have contributed to this complication.
Antibody responses to the injected antibody can have adverse consequences, including anaphylaxis. When murine antibodies are administered, a human anti-mouse antibody (HAMA) response is usual, but this is diminished in patients with NHL who have had prior chemotherapy. When they are chemotherapy-naïve, the HAMA response can be considerable, such as ~60% for Bexxar [130]. Also of concern is that HAMA can alter murine-based immunoassays for analytes that may be important for patient management, as discussed elsewhere [63]. But most critical may be the altered biodistribution and targeting that would preclude readministration of the foreign protein. In fact, if HAMA is present, administration of a chimeric antibody or even humanized antibody may enhance the HAMA response (personal observations).
With the new Nuclear Regulatory Commission's regulations for 131I providing that patients may be released if the total effective dose equivalent to another individual from exposure to a treated patient is <500 mrem [121], Bexxar can be given in most (but not all) states of the U.S. on an outpatient basis, while Zevalin and other products using pure beta-emitters, such as 90Y, can be used throughout the U.S. on an outpatient basis. Both products have been studied predominately as a single-cycle therapy.
A phase III clinical trial of Bexxar in follicular, low-grade and transformed low-grade NHL who were heavily pretreated and chemotherapy resistant has been completed [72], and gave a response rate of 65%, compared with 28% for the last chemotherapy. A complete response (CR) rate of 30% was reported, with a median remission duration of almost 5 years [72].
Bexxar has also been studied in a phase II trial in previously untreated low-grade or transformed NHL patients [130]. Seventy-four of the 76 patients (97%) had an objective response, with 63% achieving a CR. None of the patients required hematological supportive therapy, but HAMA was observed in 64% of the patients. It was found that less heavily pretreated patients responded more favorably to RAIT, at least with Bexxar and probably also with other such agents.
Kaminski and associates recently summarized their experience with Bexxar in 53 chemotherapy-relapsed/refractory NHL patients [73]. They determined the maximal total-body dose for patients not requiring autologous stem cell transplantation (ASCT) as 75 cGy and post-ASCT as 45 cGy. Forty-two of 59 patients (71%) responded; 20 (34%) had CRs. Thirty-five (83%) of 42 with low-grade or transformed NHL responded versus 7 (41%) of 17 de novo intermediate-grade NHL patients (P=0.005). The median progression-free survival was 12 months for all 42 responders, and 20.3 months for those with CR. Seven patients were still in CR 3–5.7 years later. Sixteen were retreated after progression; nine responded and five had a CR. Ten patients (17%) had HAMA elevations. Long-term, five patients developed elevated TSH levels, five were diagnosed with myelodysplasia and three with solid tumors.
Press and associates [106, 108], using myeloablative doses of the B1 antibody of Bexxar labeled with 131I and combined with peripheral blood stem cell transplantation, showed that very high objective responses of 86%, with 79% CRs, could be achieved; 39% of the patients survived free of any recurrences for 5–10 years without any further therapy. This was extended in a study of 29 patients receiving therapeutic infusions of 280–785 mCi (10.4–29.0 GBq) of 131I-murine B1 [80]. Fourteen of 29 patients remained in unmaintained remissions ranging from 27+ to 87+ months after RAIT. The estimated overall and progression-free survival rates were 68% and 42%, respectively, with a median follow-up time of 42 months [80]. Nonhematological dose-limiting toxicity was reversible cardiopulmonary insufficiency, which occurred in two patients at RAIT doses that delivered ≥27 Gy to the lungs. Late toxicity has been uncommon, except for elevated TSH levels found in about 60% of the subjects. Two patients developed second malignancies, but none have developed myelodysplasia [86].
When the Seattle group extended RAIT at myeloablative doses to include chemotherapy with etoposide and cyclophosphamide (followed by ASCT), an overall survival rate of 83% and a progression-free survival of 68% were observed after a median follow-up of 2 years [109]. These results compared favorably with those of a nonrandomized control group of patients treated at the same institution with the same doses of the drugs, but who received total-body irradiation instead of the radiolabeled antibody (overall survival 53% and progression-free survival of 36%). Of the 52 patients treated, four died of opportunistic infections. In another study by this group, using 131I-CD20 murine mAb (tositumomab) at a median dose of 510 mCi (18.87 GBq), combined with high-dose etoposide (30–60 mg/kg), cyclophosphamide (60–100 mg/kg), and infusion of cryopreserved autologous stem cells, the respective complete and overall response rates were 91% and 100% among 11 patients with conventionally measurable mantle cell lymphoma that relapsed or was refractory to treatment [59]. Fifteen of the 16 treated are alive, with 12 having no progression of disease at 6–57 months from transplantation and 16–97 months from diagnosis. Overall survival at 3 years from transplantation is estimated at 93%, and progression-free survival is estimated at 61%. These appear to be the best results in this lymphoma type, which is a radiation-sensitive malignancy.
90Y-Zevalin (yttrium-90 ibritumomab tiuxetan, IDEC-Y2B8) was studied in a phase I/II dosimetry trial in relapsed/refractory NHL patients by Wiseman et al. [134]. Patients received 111In-Zevalin on day 0 followed by a therapeutic dose of 90Y-Zevalin on day 7, in a dose-escalation (7.4–15 MBq/kg, or 0.2–0.4 mCi/kg) mode. Both doses were preceded by an infusion of the chimeric, unlabeled rituximab antibody. Median estimated radiation absorbed dose was 3.4 Gy to liver, 2.6 Gy to lungs, and 0.38 Gy to kidneys, with the median estimated tumor radiation absorbed dose being 17 Gy. Thus, Zevalin administered at nonmyeloablative MTDs resulted in acceptable absorbed doses to normal organs.
Results of a prospective, randomized trial of Zevalin in 143 patients with relapsed/refractory low-grade, follicular or transformed NHL, compared to a standard course of rituximab, showed an overall objective response rate of 80% for the Zevalin group versus 56% in the group who received unlabeled rituximab (P=0.002), with a 30% CR rate for Zevalin versus 16% CR for rituximab (P=0.04) [136]. These investigators also determined that Zevalin given at nonmyeloablative doses of 0.4 mCi/kg (15 MBq/kg) delivers acceptable radiation absorbed doses to normal organs without the need for pretherapy-based dosimetry with 111In-labeled Zevalin.
Another study was conducted to evaluate the response rate to Zevalin in follicular NHL patients who were refractory to rituximab (defined as those who failed to achieve an objective response or had progression of disease within 6 months of the most recent course of rituximab given weekly ×4 at 375 mg/m2). In the analysis of the 54 patients, an overall objective response of 74% and a CR rate of 15% were achieved according to the International Workshop standards [32], or an objective response rate of 59% and a CR of 4% by the prior IDEC criteria [138]. Duration of response was significantly longer (7.7+ mos. vs 4 mos.) for Zevalin as compared to prior rituximab (P<0.01). In an analysis of 211 patients receiving Zevalin, it was reported that 1.4% developed HAMA and one patient (0.5%) developed human anti-chimeric antibody (HACA) [137]. Zevalin was the first radioimmunoconjugate to achieve regulatory approval, being licensed by the FDA in February of 2002 for the treatment of patients with follicular and transformed NHL.
A third antibody for NHL, targeting a different antigen, CD22, is also emerging as potentially a third radiotherapeutic [55], or as a second unlabeled product [81]. This CD22 antibody, first named EPB-2 and subsequently LL2, was first developed as a murine form [100]. It was then shown, by labeling the Fab' fragment with 99mTc (LymphoScan, Immunomedics, Inc., Morris Plains, N.J.), to target all forms, stages, and sites of NHL, with only normal spleen showing accretion of the antibody [7, 96]. Subsequently, the murine antibody was humanized, or CDR-grafted onto human framework regions of IgG (hLL2 or epratuzumab; Immunomedics, Inc.) to reduce the murine component to less than 10%, thus having more human components than the chimeric rituximab anti-CD20 antibody. The LL2 mAb has been determined to target the surface CD22 antigen and then internalize rapidly into the cell [120]. Later resumption of synthesis and expression of CD22 permits binding of the antibody and further internal processing. This internalization has enabled the attachment of radiometals for a higher residence time and, in turn, dose delivered, in the tumor [116]. One of the interesting observations of the first RAIT trial in NHL with the murine LL2 labeled with 131I was the apparent efficacy of very low doses of radiation [55], confirmed also in further studies by us [61], as well as by Vose et al. [126] and Linden et al. [84]. Subsequent studies with a 90Y form of hLL2 indicated antitumor activity at the first dose levels of a dose-escalation study, including patients who failed prior high-dose chemotherapy [62]. A clinical trial comparing the dosimetry and pharmacokinetics of hLL2 labeled with 131I or 90Y in patients with NHL showed the advantage of the 90Y label with this antibody [70]. At present, a phase I/II study with myeloablative doses of 90Y-hLL2 in patients with predominantly aggressive NHL, including those who had prior high-dose chemotherapy, is being conducted [62]. It is noteworthy that the 90Y-labeled hLL2 is given as a single injection with a protein dose of about 100 mg in these studies, without the need for a prior predosing to improve its biodistribution. 111In-hLL2 is given in advance for targeting and dosimetry purposes, but it is not anticipated that 90Y-hLL2 will need individualized patient dosimetry [70], as it is not required for Zevalin [37, 134, 135, 136]. Another difference between hLL2 and the other radiolabeled antibodies used for NHL therapy is that this antibody has the humanized form labeled, whereas Zevalin and Bexxar have murine antibodies radiolabeled, thus involving the administration of a murine antibody with its potential immunogenicity and prospect of precluding repeated administrations.
The hLL2 antibody labeled with 90Y is also being studied in a dose fractionation schedule, beginning with two doses given once weekly and expanded up to four weekly doses. Initial results show responses at the schedule of two to three weekly doses [83]. Another phase I trial is in progress with hLL2 labeled with 186Re, which also allows simultaneous imaging and therapy (like 131I), and is showing antitumor activity at the initial doses [102]. Finally, comparing myeloablative and conventional doses of 131I-labeled CD20 (chimeric rituximab) and hLL2 antibodies in a small series of NHL patients, Behr et al. reported superior results with the high, myeloablative doses [15]. These various reports indicate that chimeric CD20 and humanized CD22 mAbs can be effective in NHL with diverse radiolabels, such as 131I, 90Y, and 186Re, but it is premature to determine which label and dose schedule will prove best for the treatment of NHL, and how it will be incorporated in a management paradigm. A preclinical study of rituximab labeled with the alpha-emitter 211At also supports its potential use with this radiolabel [3]. In addition to antibodies against CD20 and CD22, a recent experimental study suggests that radiolabeled CD19 antibodies may also be of value in the RAIT of NHL [87].
A fourth radiolabeled antibody product under development for the RAIT of NHL is Lym-1 (Oncolym, Peregrine Pharmaceuticals, Inc., Fullerton, Calif.), which targets the HLA-DR10β subunit expressed on most malignant B cells [82]. DeNardo and associates, whose work forms the basis of virtually all conclusions concerning the role of this antibody, have shown that it is useful for the treatment of NHL when labeled with 131I or 67Cu [35, 36, 39, 82]. In a low-dose trial of 131I-Lym-1, 17 of 30 (57%) patients had durable responses, including three CRs. A maximum tolerated dose trial of this agent yielded responses in 11 of 21 (52%), including seven CRs [35]. Thrombocytopenia was the only dose-limiting toxicity. 67Cu used as the radiolabel provides both imaging and a β-emitting therapeutic, and responses were shown in 7 of 12 (58%) NHL patients treated [97]. Since Lym-1 is a murine antibody, these investigators studied the HAMA response in their patients, and found a 28% response among 43 patients treated with multiple doses of the antibody, but with no evidence of anaphylactoid or related complications [38]. However, HAMA activity interrupted therapy in 6 of the 43 patients (14%). It is interesting that the median survival for the HAMA-positive patients was longer (18 months) than for those who did not develop HAMA (9 months). These authors have speculated that HAMA may contribute to the antitumor responses [41].
The various trials of RAIT in NHL lead to the following tentative general conclusions [48]:
Durable and major responses can be achieved, even following relapse to chemotherapy and with bulky tumors.
Low radiation doses can achieve objective tumor responses.
Administration of unlabeled antibody may improve biodistribution of the labeled antibodies, either as a predose or concomitantly.
High-dose therapy combined with autologous bone marrow or peripheral stem cell transplantation can result in higher overall response rates of longer duration than the application of nonmyeloablative doses.
Patients with low involvement of disease in the bone marrow, with a low tumor burden and without an enlarged spleen respond more favorably.
mAbs with radiometals, such as 90Y, show better tumor dosimetry than 131I-labeled antibodies, and the former do not appear to require the pretherapy dosimetry essential for 131I-labeled antibodies.
When combined with certain chemotherapeutic agents and autologous stem cell transplants, RAIT may be more effective than any single modality.
RAIT appears to be more effective than use of the same antibody unlabeled.
Long-term side-effects may include hypothyroidism (with 131I products), myelodysplasia, and, possibly, secondary neoplasms.
In general, fewer studies have been pursued with RAIT in malignancies other than NHL, but efficacy in Hodgkin's disease, T-cell leukemia, and acute myelocytic leukemia has been reported, as reviewed elsewhere [50, 67].
Solid tumors
The more radioresistant solid tumors have not been as responsive to RAIT as the hematopoietic neoplasms, and for this reason a number of strategies to improve results are being pursued. Clinically, the major interests have been colorectal, ovarian, breast, medullary thyroid, and brain cancers, with some early studies being reported also in urinary bladder cancer, prostate carcinoma, and other tumors, as summarized elsewhere [50]. Many different radionuclides, antibody forms, and methods to increase antibody accretion and penetration are under investigation, and in fact a number of approaches appear to be promising. On the other hand, methods to prevent or alleviate dose-limiting side-effects, such as myelosuppression, are also of interest because they potentially enable the administration of higher radiation doses. However, at this moment no radiolabeled antibody has yet shown sufficient antitumor activity in advanced metastatic disease of any solid tumor type to suggest that it represents a new therapy modality. Nevertheless, recent results in the therapy of small-volume or micrometastatic disease, such as in colorectal and ovarian cancers, suggest that these neoplasms, in the minimal disease setting, may be the best first opportunity for systemic RAIT under current limitations of the technology.
The principal antibodies being studied are against CEA, TAG-72, MUC1, and other glycoproteins [e.g., Le(y)], tenascin, and prostate-specific membrane antigen [50]. The majority are being used as directly labeled intact IgG immunoglobulins, labeled with either 131I or 90Y, and being either chimeric or humanized forms. Most have been studied as a single-dose therapy, but there is increasing evidence that a fractionated dose schedule is more efficacious [91, 114]. As mentioned earlier and as shown by a number of reviews of the progress of RAIT in solid tumors [46, 47, 49, 50, 60, 66, 77, 92, 133], advances have not been as impressive as in the hematopoietic malignancies, and at this time the role of RAIT in any single neoplasm is still not established. The recent excellent discussion of clinical RAIT by Knox and Meredith [77] has catalogued a number of studies in solid tumors, so this discussion will only select representative reports of interest. It should be noted that almost all of the trials reported are phase I–II dose-escalation studies, so that suboptimal doses were used in many cases. Indeed, the antibodies and their forms, doses of the antibodies and the radionuclides administered, stage of the disease studied, and radiation absorbed doses accreted in the tumors have varied considerably among the clinical trials. Most investigations have involved a single dose of 131I- or 90Y-labeled antibody, mainly at low but occasionally at myeloablative doses requiring hematopoietic support. The majority of trials have involved advanced cancer patients who had failed other forms of therapy, thus constituting a difficult patient populations in terms of therapeutic response. Although complete responses are rare, partial and minor responses, and durable disease stabilization, have been observed, suggesting that optimization of RAIT in future clinical trials could improve the prospects of RAIT in solid tumors. Examples of solid tumor RAIT will be given for central nervous system and colorectal cancers. The majority of human solid tumors express CEA, so that antibodies to this antigen have been studied in colorectal, pancreas, lung, breast, and medullary thyroid cancers [13]. In the study by Behr et al. [13], tumor doses were found to be inversely related to the tumor mass, and ranged between 2 and 218 cGy/mCi; doses between 44 and 268 mCi of 131I-NP-4 murine anti-CEA antibody (Immunomedics, Inc.) were administered. Modest antitumor effects were seen in 12 of 35 assessable patients, comprising one partial response, four minor/mixed responses, and seven with stabilization of previously rapidly progressing disease. The authors proposed that small tumors are more suitable for RAIT, and that bulky tumors will probably require myeloablative doses. More modest progress is being made in the RAIT of breast cancer [51], although it seems that this would be an optimal tumor type for this technology, since radiation has a role in the management of this disease. A review of results in diverse solid tumor types has appeared elsewhere [50].
Brain and other CNS cancers
Results from numerous studies have shown that the best efficacy has been achieved by locoregional administration or by systemic administration for the treatment of small tumors or minimal disease. Brain and central nervous system (CNS) tumors are particularly good candidates for locoregional therapy. Using anti-tenascin antibodies labeled initially with 131I and more recently with 90Y, Riva et al. [111] injected these into the tumor bed after surgery of malignant gliomas, and reported impressive growth control. The median survival time for patients with glioblastoma was prolonged to 25 months for the 131I-labeled antibody and 31 months for the 90Y group. In many cases, significant tumor shrinkage was observed. Compared with the 131I-labeled antibody, the 90Y-radioimmunoconjugate showed more favorable effects in bulky lesions, and had fewer radioprotection problems. Employing another anti-tenascin antibody labeled with 131I and injected directly into surgically created resection cavities of patients with malignant gliomas, average absorbed doses in the tumor cavities were 41 Gy [1]. In yet another study with a different 131I-anti-tenescin antibody (81C6) given at a dose of 120 mCi in the intraresection cavity of patients with newly diagnosed glioma, 11 of 33 patients were alive at a median follow-up of 93 weeks, showing an increase as compared to historical controls treated with conventional radiotherapy and chemotherapy [110]. Nine patients (27%) developed reversible hematologic toxicity, and treatment-related neurologic toxicity was reported in five patients (15%). These results encourage conducting a randomized trial with this agent.
Systemic or intra-arterial RAIT has also been explored for brain tumors with 131I- and 125I-labeled antibodies, and these have provided evidence of objective responses without significant toxicities [23, 71]. Both in studies involving established disease and in investigations of its use as an adjuvant therapy, 125I-anti-epidermal growth factor receptor antibody 425 has been shown to be active in the treatment of patients with primary glioblastoma multiforme, the results having included a 20% objective response rate [23, 24, 93]. The intra-arterial route of administration did not appear to offer any advantage over i.v. infusions, as was also found by others [139].
In addition to using radiolabeled 3F8 antibody in neuroblastoma therapy, Cheung and co-workers have studied this RAIT for leptomeningeal cancer by an intraventricular administration route, with estimated radiation doses of 14.9–56 cGy/mCi to the cerebrospinal fluid and less than 2 cGy/mCi to blood and other organs outside the CNS [79]. Intrathecal RAIT has also been applied to patients with medulloblastoma and neuroblastoma, resulting in objective and durable responses in some of the patients, such as in 5 of 11 recurrent neuroblastoma patients, and a CR was noted in 3 of 15 recurrent primitive neuroectodermal tumor patients [74, 110].
Colorectal cancer
Experimental studies have shown that radiolabeled CEA antibodies can be curative of minimal metastatic disease [8, 10, 18, 19, 117]. Also, clinical findings have observed that the highest radiation doses delivered to tumors are inversely proportional to tumor size [14]. Similar calculations and predictions were made by Sgouros [115]. Clinical studies with humanized CEA antibodies labeled with 131I confirmed these animal studies, since patients with colorectal cancer metastases of small volume, after unsuccessful chemotherapy, showed encouraging responses [9, 11]. In an ongoing trial of RAIT with humanized anti-CEA MN-14 IgG (Immunomedics, Inc) in an adjuvant setting following resection of metastatic colorectal cancer, seven of nine patients showed no relapse at up to 36 months, in contrast to 67% in a control group at the same institution [9].
Early studies with 131I-labeled CEA and B72.3 murine antibodies in colorectal cancer showed modest antitumor effects at nonmyeloablative doses. Four of 15 patients showed an objective response with B72.3 and other antibodies [112], while CEA antibodies showed antitumor effects in 12/35 patients with colorectal and other CEA-expressing cancers [13]. Studies with diverse CEA antibodies have also shown modest therapeutic responses with nonmyeloablative doses of 131I-labeled antibodies [12, 42, 68].
Buchegger and associates have suggested, in early clinical studies [27, 28], that RAIT in close association with external beam irradiation is more efficient in an adjuvant setting after surgery. Clinically, six patients with limited liver metastases from colorectal cancer were treated with RAIT using 20 mCi 131I-labeled anti-CEA antibody F(ab')2 fragments combined with fractionated external beam radiation of 20 Gy to the entire liver. Spontaneously reversible bone marrow toxicities of grades 3 and 4 and reversible liver toxicity of grades 1–3 were observed. Three of the patients showed stable disease and one had a partial response, while two had disease progression.
A phase II RAIT trial with 131I-CC49, which is the second-generation murine B72.3 pancarcinoma antibody, reported no objective tumor responses at the MTD dose of 75 mCi/m2 [94, 95]. Twelve of 13 patients developed HAMA at 6–8 weeks post infusion. High-dose RAIT with autologous stem cell replacement was then undertaken with 131I-labeled murine mAb CC49 in 15 patients with gastrointestinal cancers in a dose-escalation study from 50 to 300 mCi/m2 [124]. Tumor localization was excellent, the percent injected dose per kg of tumor ranged from 0.2 to 2.1, and the absorbed dose in metastatic tumor sites ranged from 630 to 3,300 cGy. These authors then tested the same antibody labeled with 90Y [125], and found a heterogeneous liver and splenic uptake, photopenic lesions in the liver for metastases, and generally poor uptake of the antibody in metastases. The absorbed tumor doses ranged from 180 to 3,000 cGy, but tumor to normal liver dose ratios were less than 1. No objective responses were observed. Doses up to 0.5 mCi/kg could be administered with reversible grade IV myelotoxicity.
Another target for colorectal cancer RAIT is the A33 antigen, which is a transmembrane glycoprotein of the immunoglobulin superfamily [64]. A study of 23 patients who failed prior chemotherapy were treated with escalating doses of 131I-A33 murine mAb, and the MTD was found to be 75 mCi/m2 in these heavily pretreated patients [132]. The antibody showed variable uptake in the normal bowel, and no objective responses.
The NR-LU-10 pancarcinoma antibody (NeoRx Corp) was also studied in colorectal cancer patients by the pretargeting scheme using a streptavidin conjugate of the antibody [75]. Twenty-five patients were treated with a single dose of 110 mCi/m2 of 90Y-DOTA-biotin, 24 h after a clearing agent was given to remove the NR-LU-10/streptavidin. Diarrhea was the most frequent grade 4 nonhematological toxicity. A modest overall response rate of 8% was reported, with four patients having stable disease with freedom from progression of 10–20 weeks. These results do not confirm the promising preclinical studies with the same reagents and technology [4].
Pretherapy dosimetry
Methods for estimating tumor and organ doses prior to RAIT have been derived from external beam radiation calculations but appear to be less accurate for RAIT, thus provoking some controversy on the role of this technology for treatment planning. In contrast to external beam therapy, there are fewer sample points and an inhomogeneous dose distribution; there can be a wide dose variability for different lesions in the same patient [77, 122].
Dose estimations for RAIT are made on the basis of calculating the volume of tumors and normal organs, the cumulative radioactivity estimated as accreted in the organs and tumors, and the pharmacokinetics of the radioactivity given with the antibody. Various methods have been used to gain these data, including serial gamma camera imaging and biopsy [44, 61, 70, 80, 89, 90, 94, 95, 122, 135], for most organs and tumors, but the bone marrow dose estimates have been based upon blood pharmacokinetics and/or imaging of bone in areas of active marrow, such as the spine or sacrum [122]. When a therapeutic isotope has a gamma imaging energy, then it can be used in tracer doses for pretherapy dose estimates. In the case of pure beta emitters, such as 90Y, a surrogate gamma-imaging isotope, such as 111In, is used to predict the therapeutic dose. Tracer studies often predict the doses obtained from subsequent RAIT well, but variations, even in the same patient, can be experienced [44, 90, 94].
A major problem with pretherapy dosimetry has been a failure to achieve a consistent dose-response relationship for RAIT. For example, tumor doses in patients with lymphoma show a tenfold range, from 0.5 to 5.4 mGy/MBq, and have had a variable correlation between the estimated dose delivered and the response [122]. But at the extremes there is evidence for a relationship between estimated tumor doses and response rates [89, 135]. Nevertheless, RAIT appears to achieve responses at dose estimates which are far lower than those calculated for external beam therapy [63, 123]. Normal organ doses from RAIT have ranged from 0.2 to 2.2 mGy/MBq, with considerable variability between patients [122]. When very high doses of RAIT are given, such as in the myeloablative studies performed by the Seattle group, secondary organ toxicity involved cardiopulmonary complications in patients who received more than 27 Gy to the lungs [107]. Thus, it appears that the low-dose-rate irradiation given by RAIT is tolerated relatively well by normal organs [122]. An inverse relationship between tumor size and dose delivered has also been observed [14], indicating that small-volume tumors and micrometastases may be the best targets for current RAIT methods. Indeed, this is supported by both experimental [8, 10, 18, 117], and clinical studies [9, 11]. In a comprehensive evaluation of 119 tumors in 93 patients given the 131I-NP-4 and -MN-14 anti-CEA murine mAbs, it was reported that an inverse logarithmic relationship exists between tumor size and antibody uptake [12]. The most important factor determining the radiation dose to the tumor was found to be the absolute tumor uptake of the radiolabel, and the second most important factor was the biological half-life of the antibody in the tumor. Different antibody affinities did not appear to affect tumor uptake. At comparable masses, colorectal and medullary thyroid cancers had significantly higher uptake of antibody, as well as tumor-to-red marrow dose ratios, than other cancer types. Thus, it appears that tumor uptake of the antibody is the most important dose-determining factor, so that both colorectal and medullary thyroid cancers seem to be good targets for CEA antibodies used in RAIT.
It is well known from external beam irradiation that higher dose rates result in higher therapeutic efficacy [2, 85], but this has not been investigated well with internal emitters [10]. Recent studies in experimental models [8, 10] have begun addressing this issue, and it seems that dose rate effects are very important, not only at the comparatively high levels experienced with external beam therapy but also in the lower ranges associated with internal emitters and RAIT.
Based on the observation that during the recovery period after anticancer myelosuppressive therapy, hematopoietic progenitor cells become mitotically active in order to replenish the bone marrow compartment, and remain hyperproliferative even after normalization of blood counts of leukocytes and platelets, Blumenthal et al. [17] conducted a retrospective study of the blood levels of several hematopoietic cytokines following a single dose of RAIT with CEA antibodies in CEA-expressing solid tumors. It was found that the plasma level of Flt3-ligand could predict excessive platelet toxicity caused by additional cytotoxic therapy. This encouraging report suggests that the measurement of this hematopoietic cytokine may be a reliable surrogate marker of the status of the bone marrow following cytotoxic therapy, thus perhaps predicting how aggressive a therapy, whether RAIT or chemotherapy, may be undertaken in any individual patient. In a more recent study, the use of Flt3-L blood levels to adjust bone marrow dose estimates in RAIT-treated patients with solid tumors was demonstrated [123]. Studies of this kind may be more clinically relevant than current bone marrow dosimetry methods.
Conclusion
RAIT of cancer has had a more than 20-year history, and during this time profound advances have been achieved in the development of tumor-seeking, humanized antibodies, in radiochemistry with diverse radionuclides, in the mitigation of dose-limiting myelosuppression, and in the improved targeting and delivery of radiation doses to tumors of different types and in diverse locations. Hematopoietic neoplasms have shown the best responses to RAIT, despite the delivery of relatively low doses of radiation; this has resulted in several radiolabeled antibodies advancing toward commercialization for the treatment of NHL. These results are due, perhaps, to good vascularization, high antigen density on a more homogenous tumor cell population, and possibly the involvement of concomitant apoptotic and immune mechanisms. In contrast, solid tumors fail to receive the radiation doses required to achieve similar responses. Although RAIT of solid tumors represents the principal challenge of the future, it is already apparent that use of this modality in a minimal disease setting, in locoregional applications, in combination modalities, in fractionated dose schedules, and in pretargeting strategies shows sufficient promise to justify continued optimism for its future role in the management of cancer.
Acknowledgements
The author's research has been supported in part by grants from the National Institutes of Health (PO1 CA79857 and Outstanding Investigator Grant CA39841). He also serves as a Director and officer of Immunomedics, Inc., a biopharmaceutical company involved in the development of radioimmunoconjugate products.
Footnotes
This article forms part of the Symposium in Writing on "Antibodies in Cancer Immunotherapy", published in this issue (Vol. 52, No. 2) of the journal.
This paper is dedicated to the memory of my friend and colleague, Professor Wolfgang Becker, with whom I enjoyed a long and stimulating collaboration on clinical studies of radioimmunotherapy, and who educated me in the area of infection/inflammation imaging with radiolabeled antibodies.
This article forms part of the Symposium in Writing on "Antibodies in cancer immunotherapy," published in vol 52 of the journal.
References
- 1.Akabani Int J Radiat Oncol Biol Phys. 2000;46:947. doi: 10.1016/S0360-3016(99)00500-3. [DOI] [PubMed] [Google Scholar]
- 2.Amdur Int J Radiat Oncol Biol Phys. 1994;30:83. [Google Scholar]
- 3.Aurlien Br J Cancer. 2000;83:1375. doi: 10.1054/bjoc.2000.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Axworthy Proc Natl Acad Sci U S A. 2000;97:1802. doi: 10.1073/pnas.97.4.1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barbet Cancer Biother Radiopharm. 1999;14:153. doi: 10.1089/cbr.1999.14.153. [DOI] [PubMed] [Google Scholar]
- 6.Bardies J Nucl Med. 1996;37:1853. [PubMed] [Google Scholar]
- 7.Baum Cancer. 1994;73:896. doi: 10.1002/1097-0142(19940201)73:3+<896::aid-cncr2820731322>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 8.Behr Clin Cancer Res. 2000;6:4900. [PubMed] [Google Scholar]
- 9.Behr Cancer. 2002;94:1373. doi: 10.1002/cncr.10308. [DOI] [PubMed] [Google Scholar]
- 10.Behr Int J Cancer. 1998;77:787. doi: 10.1002/(SICI)1097-0215(19980831)77:5<787::AID-IJC19>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 11.Behr Clin Cancer Res. 1999;5:3232s. [PubMed] [Google Scholar]
- 12.Behr J Nucl Med. 1997;38:409. [PubMed] [Google Scholar]
- 13.Behr J Nucl Med. 1997;38:858. [PubMed] [Google Scholar]
- 14.Behr Cancer Res. 1996;56:1805. [PubMed] [Google Scholar]
- 15.Behr Clin Cancer Res. 1999;5:3304s. [PubMed] [Google Scholar]
- 16.Blumenthal Int J Cancer. 1995;61:557. doi: 10.1002/ijc.2910610421. [DOI] [PubMed] [Google Scholar]
- 17.Blumenthal Cancer. 2000;88:333. doi: 10.1002/(SICI)1097-0142(20000115)88:2<333::AID-CNCR13>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 18.Blumenthal Cancer Res. 1992;52:6036. [PubMed] [Google Scholar]
- 19.Blumenthal Int J Cancer. 1989;44:292. doi: 10.1002/ijc.2910440218. [DOI] [PubMed] [Google Scholar]
- 20.Blumenthal Tumour Biol. 1997;18:367. doi: 10.1159/000218051. [DOI] [PubMed] [Google Scholar]
- 21.Blumenthal Int J Cancer. 2001;94:564. doi: 10.1002/ijc.1500. [DOI] [PubMed] [Google Scholar]
- 22.Boerman Cancer Res. 1999;59:4400. [PubMed] [Google Scholar]
- 23.Brady J Neurosurg Sci. 1990;34:243. [PubMed] [Google Scholar]
- 24.Brady Antib Immunoconjug Radiopharm. 1990;3:169. [Google Scholar]
- 25.Breitz Cancer Biother Radiopharm. 1999;14:381. doi: 10.1089/cbr.1999.14.381. [DOI] [PubMed] [Google Scholar]
- 26.Breitz J Nucl Med. 2000;41:131. [Google Scholar]
- 27.Buchegger Anticancer Res. 2000;20:1889. [PubMed] [Google Scholar]
- 28.Buchegger Ann N Y Acad Sci. 2000;910:263. doi: 10.1111/j.1749-6632.2000.tb06714.x. [DOI] [PubMed] [Google Scholar]
- 29.Buchsbaum Semin Radiat Oncol. 2000;10:156. doi: 10.1016/s1053-4296(00)80052-1. [DOI] [PubMed] [Google Scholar]
- 30.Burke Cancer. 2002;94:1320. doi: 10.1002/cncr.10303. [DOI] [PubMed] [Google Scholar]
- 31.Chang Mol Cancer Ther. 2002;1:553. [PubMed] [Google Scholar]
- 32.Cheson Blood. 2003;101:391. doi: 10.1182/blood-2002-06-1793. [DOI] [PubMed] [Google Scholar]
- 33.Cheson J Clin Oncol. 1999;17:1244. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 34.DeNardo GL, DeNardo SJ (1995) Overview of obstacles and opportunities for radioimmunotherapy of cancer. In: Goldenberg DM (ed) Cancer therapy with radiolabeled antibodies. CRC Press, Boca Raton, Fla.
- 35.DeNardo J Clin Oncol. 1998;16:3246. doi: 10.1200/JCO.1998.16.10.3246. [DOI] [PubMed] [Google Scholar]
- 36.DeNardo Cancer Biother Radiopharm. 1998;13:239. doi: 10.1089/cbr.1998.13.239. [DOI] [PubMed] [Google Scholar]
- 37.DeNardo Crit Rev Oncol Hematol. 2001;39:203. doi: 10.1016/S1040-8428(01)00109-3. [DOI] [PubMed] [Google Scholar]
- 38.DeNardo Int J Biol Markers. 1995;10:67. [Google Scholar]
- 39.DeNardo Clin Cancer Res. 1999;5:533. [PubMed] [Google Scholar]
- 40.DeNardo Curr Opin Immunol. 1999;11:563. doi: 10.1016/s0952-7915(99)00017-5. [DOI] [PubMed] [Google Scholar]
- 41.DeNardo Cancer Biother Radiopharm. 1998;13:1. doi: 10.1089/cbr.1998.13.1. [DOI] [PubMed] [Google Scholar]
- 42.DeNardo Proc Natl Acad Sci U S A. 1997;94:4000. doi: 10.1073/pnas.94.8.4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dillman J Clin Oncol. 2002;20:3545. doi: 10.1200/JCO.2002.02.126. [DOI] [PubMed] [Google Scholar]
- 44.Eary J Nucl Med. 1990;31:1257. [PubMed] [Google Scholar]
- 45.Gautherot J Nucl Med. 2000;41:480. [Google Scholar]
- 46.Goldenberg Am J Med. 1993;94:297. doi: 10.1016/0002-9343(93)90062-t. [DOI] [PubMed] [Google Scholar]
- 47.Goldenberg DM (ed) (1995) Cancer therapy with radiolabeled antibodies. CRC Press, Boca Raton, Fla.
- 48.Goldenberg Crit Rev Oncol Hematol. 2001;39:195. doi: 10.1016/s1040-8428(01)00108-1. [DOI] [PubMed] [Google Scholar]
- 49.Goldenberg DM (2001) Radioimmunotherapy. In: Freeman LM (ed) Nuclear medicine annual 2001. Lippincott, Williams & Wilkins, Philadelphia
- 50.Goldenberg J Nucl Med. 2002;43:693. [PubMed] [Google Scholar]
- 51.Goldenberg Semin Breast Dis. 2002;5:142. [Google Scholar]
- 52.Goldenberg Semin Cancer Biol. 1990;1:217. [PubMed] [Google Scholar]
- 53.Goldenberg N Engl J Med. 1978;298:1384. doi: 10.1056/NEJM197806222982503. [DOI] [PubMed] [Google Scholar]
- 54.Goldenberg Cancer Res. 1981;41:4354. [PubMed] [Google Scholar]
- 55.Goldenberg J Clin Oncol. 1991;9:548. [Google Scholar]
- 56.Goldenberg Cancer Res. 1980;40:2984. [PubMed] [Google Scholar]
- 57.Goodwin Strategies for antibody. 1991;targeting:427. [Google Scholar]
- 58.Goodwin J Nucl Med. 1998;29:226. [Google Scholar]
- 59.Gopal Blood. 2002;99:3158. doi: 10.1182/blood.v99.9.3158. [DOI] [PubMed] [Google Scholar]
- 60.Govindan Pharm Sci Techn Today. 2000;3:90. doi: 10.1016/S1461-5347(00)00241-8. [DOI] [PubMed] [Google Scholar]
- 61.Griffith J Nucl Med. 1992;33:2020. [Google Scholar]
- 62.Hajjar J Nucl Med. 2001;42:157P. [Google Scholar]
- 63.Hansen J Clin Immunoassay. 1993;16:294. [Google Scholar]
- 64.Heath Proc Natl Acad Sci U S A. 1997;94:469. doi: 10.1073/pnas.94.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hnatowich Nucl Med Commun. 1994;15:575. doi: 10.1097/00006231-199408000-00001. [DOI] [PubMed] [Google Scholar]
- 66.Illidge Curr Pharm Des. 2000;6:1399. doi: 10.2174/1381612003399257. [DOI] [PubMed] [Google Scholar]
- 67.Jurcic Clin Cancer Res. 1995;1:1439. [PubMed] [Google Scholar]
- 68.Juweid J Nucl Med. 1996;37:1504. [PubMed] [Google Scholar]
- 69.Juweid Cancer Res. 1995;55:5899s. [PubMed] [Google Scholar]
- 70.Juweid Clin Cancer Res. 1999;5:3292s. [PubMed] [Google Scholar]
- 71.Kalofonos J Nucl Med. 1989;30:1636. [PubMed] [Google Scholar]
- 72.Kaminski Blood. 1998;92:1296. [PubMed] [Google Scholar]
- 73.Kaminski Cancer Res. 2000;96:1259. [Google Scholar]
- 74.Kemshead Eur J Cancer. 1992;28:511. doi: 10.1016/s0959-8049(05)80090-5. [DOI] [PubMed] [Google Scholar]
- 75.Knox Clin Cancer Res. 2000;6:406. [Google Scholar]
- 76.Knox Radiother Oncol. 1992;23:111. doi: 10.1016/0167-8140(92)90342-r. [DOI] [PubMed] [Google Scholar]
- 77.Knox Semin Radiat Oncol. 2000;10:73. doi: 10.1016/s1053-4296(00)80045-4. [DOI] [PubMed] [Google Scholar]
- 78.Kraeber-Bodere J Nucl Med. 2001;42:123P. [Google Scholar]
- 79.Kramer Med Pediatr Oncol. 2000;35:716. doi: 10.1002/1096-911X(20001201)35:6<716::AID-MPO51>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 80.Larson J Nucl Med. 1992;33:2020. [Google Scholar]
- 81.Leonard Proc Am Soc Clin Oncol. 2000;19:17a. [Google Scholar]
- 82.Lewis Hybridoma. 1995;14:115. doi: 10.1089/hyb.1995.14.115. [DOI] [PubMed] [Google Scholar]
- 83.Linden Cancer Biother Radiopharm. 2000;15:413. [Google Scholar]
- 84.Linden Clin Cancer Res. 1999;5:3287s. [PubMed] [Google Scholar]
- 85.Ling Br J Radiol. 1984;57:723. doi: 10.1259/0007-1285-57-680-723. [DOI] [PubMed] [Google Scholar]
- 86.Liu J Clin Oncol. 1998;16:3270. doi: 10.1200/JCO.1998.16.10.3270. [DOI] [PubMed] [Google Scholar]
- 87.Ma Leukemia. 2002;16:60. doi: 10.1038/sj.leu.2402320. [DOI] [PubMed] [Google Scholar]
- 88.McDevitt Science. 2000;294:1537. doi: 10.1126/science.1064126. [DOI] [PubMed] [Google Scholar]
- 89.Meredith RF, Buchsbaum DJ, Knox SJ (2000) Radionuclide dosimetry and radioimmunotherapy of cancer. In: Abrams AR, Fritzberg AR (eds) Radioimmunotherapy of cancer. Marcel Dekker, New York
- 90.Meredith Antib Immunoconj Radiopharm. 1993;6:1. [Google Scholar]
- 91.Meredith J Nucl Med. 1992;33:1648. [PubMed] [Google Scholar]
- 92.Meredith RF, LoBuglio AF, Spencer EB. Recent progress in radioimmunotherapy for cancer. Oncology (Huntingt) 1997;11:979–987. [PubMed] [Google Scholar]
- 93.Miyamoto Radiat Oncol Invest. 1995;3:126. [Google Scholar]
- 94.Murray JL, Macey DJ, Kasi LP, Rieger P, Cunningham J, Bhadkamkar V, Zhang HZ, Schlom J, Rosenblum MG, Podoloff DA (1994) Phase II radioimmunotherapy trial with131I-CC49 in colorectal cancer. Cancer (Suppl) 73:1057–1066 [DOI] [PubMed]
- 95.Murray JL (1995) Radioimmunotherapy of colorectal cancer. In: Goldenberg DM (ed) Cancer therapy with radiolabeled antibodies. CRC, Boca Raton, Fla.
- 96.Murthy Eur J Nucl Med. 1992;19:394. doi: 10.1007/BF00177365. [DOI] [PubMed] [Google Scholar]
- 97.O'Donnell Clin Cancer Res. 1999;5:3330s. [PubMed] [Google Scholar]
- 98.Paganelli Eur J Nucl Med. 1999;26:348. [Google Scholar]
- 99.Paganelli Int J Biol Markers. 1993;8:155. doi: 10.1177/172460089300800304. [DOI] [PubMed] [Google Scholar]
- 100.Pawlak-Byczkowska Cancer Res. 1989;49:4568. [PubMed] [Google Scholar]
- 101.Pizer Antib Immunoconj Radiopharm. 1991;4:753. [Google Scholar]
- 102.Postema Cancer Biother Radiopharm. 2000;15:407. [Google Scholar]
- 103.Postema Eur J Nucl Med. 2001;28:1725. doi: 10.1007/s002590100570. [DOI] [PubMed] [Google Scholar]
- 104.Press Semin Oncol. 1999;26:58. [PubMed] [Google Scholar]
- 105.Press Blood. 2001;98:2535. [Google Scholar]
- 106.Press OW, Eary JF, Appelbaum FR, Bernstein ID (1995) Treatment of relapsed B cell lymphomas with high-dose radioimmunotherapy and bone marrow transplantation. In: Goldenberg DM (ed) Cancer therapy with radiolabeled antibodies. CRC, Boca Raton, Fla.
- 107.Press N Engl J Med. 1993;329:1219. doi: 10.1056/NEJM199310213291702. [DOI] [PubMed] [Google Scholar]
- 108.Press Lancet. 1995;346:336. doi: 10.1016/s0140-6736(95)92225-3. [DOI] [PubMed] [Google Scholar]
- 109.Press Blood. 2000;96:2934. [PubMed] [Google Scholar]
- 110.Reardon J Clin Oncol. 2002;20:1389. doi: 10.1200/JCO.20.5.1389. [DOI] [Google Scholar]
- 111.Riva Eur J Nucl Med. 2000;27:601. doi: 10.1007/s002590050549. [DOI] [PubMed] [Google Scholar]
- 112.Riva Nucl Med Biol. 1991;18:109. [Google Scholar]
- 113.Rouvier Horm Res. 1997;47:163. doi: 10.1159/000185460. [DOI] [PubMed] [Google Scholar]
- 114.Schlom J Natl Cancer Inst. 1990;82:763. doi: 10.1093/jnci/82.9.763. [DOI] [PubMed] [Google Scholar]
- 115.Sgouros J Nucl Med. 1995;36:1910. [PubMed] [Google Scholar]
- 116.Sharkey Cancer Immunol Immunother. 1997;44:179. doi: 10.1007/s002620050371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sharkey Int J Cancer. 1997;72:477. doi: 10.1002/(sici)1097-0215(19970729)72:3<477::aid-ijc16>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 118.Sharkey Cancer Res. 1990;50:964s. [PubMed] [Google Scholar]
- 119.Sharkey Bioconjug Chem. 1997;8:595. doi: 10.1021/bc970101v. [DOI] [PubMed] [Google Scholar]
- 120.Shih Int J Cancer. 1994;56:538. doi: 10.1002/ijc.2910560413. [DOI] [PubMed] [Google Scholar]
- 121.Siegel JA. Revised Nuclear Regulatory Commission's regulations for release of patients administered radioactive materials: outpatient iodine 131 anti-B1 therapy. J Nucl Med [Supp] 1998;39:28–33. [PubMed] [Google Scholar]
- 122.Siegel Med Phys. 1999;20:579. doi: 10.1118/1.597052. [DOI] [PubMed] [Google Scholar]
- 123.Siegel J Nucl Med. 2003;44:67. [Google Scholar]
- 124.Tempero J Clin Oncol. 1997;15:1518. doi: 10.1200/JCO.1997.15.4.1518. [DOI] [PubMed] [Google Scholar]
- 125.Tempero Clin Cancer Res. 2000;6:3095. [PubMed] [Google Scholar]
- 126.Vose Leuk Lymphoma. 2000;38:91. doi: 10.3109/10428190009060322. [DOI] [PubMed] [Google Scholar]
- 127.Vose Semin Hematol. 1999;36:15. [PubMed] [Google Scholar]
- 128.Vuillez Clin Cancer Res. 1999;5:3259s. [PubMed] [Google Scholar]
- 129.Wahl RL (1994) Experimental radioimmunotherapy. A brief overview. Cancer (Supp) 73:989–992 [DOI] [PubMed]
- 130.Wahl J Nucl Med. 2000;41:78. [Google Scholar]
- 131.Weiden Cancer Biother Radiopharm. 2000;15:15. doi: 10.1089/cbr.2000.15.15. [DOI] [PubMed] [Google Scholar]
- 132.Welt J Clin Oncol. 1994;12:1561. doi: 10.1200/JCO.1994.12.8.1561. [DOI] [PubMed] [Google Scholar]
- 133.Wilder J Clin Oncol. 1996;14:1383. doi: 10.1200/JCO.1996.14.4.1383. [DOI] [PubMed] [Google Scholar]
- 134.Wiseman Eur J Nucl Med. 2000;27:766. doi: 10.1007/s002590000276. [DOI] [PubMed] [Google Scholar]
- 135.Wiseman J Nucl Med. 1999;40:64. [Google Scholar]
- 136.Witzig J Clin Oncol. 2002;20:2453. [Google Scholar]
- 137.Witzig Part. 2000;1:731a. [Google Scholar]
- 138.Witzig Blood. 2000;96:507a. [Google Scholar]
- 139.Zalutsky Cancer Res. 1990;50:4105. [PubMed] [Google Scholar]


