Abstract
With the objective of evaluating leukocyte orchestration in situ, serial blood samples and tumour tissue core needle biopsies were obtained at baseline and repeated after 1 month of therapy, among 49 consecutive single-institution patients with metastatic renal cell carcinoma (mRCC). Patients were treated with outpatient low-dose subcutaneous interleukin 2 (IL-2) and interferon α (IFN-α) alone (n=23) or in combination with histamine dihydrochloride (n=26). Objective responses were achieved in ten of 49 patients (20%) with an overall median survival of 14 months and an estimated 1- to 4-year survival rate of 57, 35, 24 and 22%, respectively. Toxicity was mild to moderate with no treatment-related deaths. High numbers of blood monocytes and neutrophils were significantly correlated to short survival. By contrast, high numbers of intratumoural CD3+, CD4+, CD8+ and CD57+ lymphocytes were positively correlated to objective response and/or long-term survival. Intratumoural lymphocytes showed low ζ expression, whereas blood lymphocytes showed almost normal levels of ζ expression. Neutrophils, the most frequent peripheral blood leukocyte subset, were scarce within the tumour tissue. Intratumoural eosinophils were not observed. In progressing patients, both the absolute number and the relative composition of leukocyte subsets in blood and tumour tissue remained unaffected by cytokine therapy. However, in responding patients, cytokine therapy was followed by an absolute and relative increase in T cells in blood as well as tumour tissue, an absolute and relative reduction in neutrophils in peripheral blood and a relative reduction of intratumoural macrophages. Histamine did not influence levels of intratumoural or blood leukocyte numbers, ζ-chain expression or cytotoxicity. In conclusion, the present regimen of outpatient low-dose subcutaneous IL-2 and IFN-α in mRCC should attract interest based on response, survival and toxicity. In responding patients, cytokine therapy was followed by substantial changes in the blood and tumour tissue leukocyte composition, correlated to response and survival. No discernable differences in immunologic parameters studied could be detected between histamine- and nonhistamine-treated patients.
Keywords: Histamine, Interferon α, Interleukin 2, Lymphocytes, Macrophages, Neutrophils, Renal cell carcinoma
Introduction
Interleukin 2 (IL-2) and interferon α (IFN-α) based immunotherapy for cancer is a targeted therapy that uses the patient’s own immune cells to attack and destroy tumours. IL-2 is a remarkable activator of immune cells with antitumour properties such as T cells and natural killer (NK) cells, and does not have any direct impact on tumour cells [1]. However, at baseline and following cytokine therapy, the interrelationship between specific (acquired) immune cells, i.e. T and B lymphocytes, and nonspecific (innate) immune cells, i.e. neutrophils, eosinophils, macrophages and NK cells, is incompletely understood.
The interaction between lymphocytic and phagocytic cells may be one explanation of why cytokine therapy only benefits a subgroup of treated patients. Data from experimental studies favour the theory that circulating monocytes or neutrophils interact with circulating T and NK cells and/or enter tumours and then secrete reactive oxygen compounds, especially hydrogen peroxide [2–5]. T cells and especially NK cells are considerably susceptible to oxidative stress, and consequently undergo apoptosis [6, 7]. A marker of oxidative stress is reduced expression of the transduction molecule–associated ζ chain on T and NK cells [8–10]. However, histamine dihydrochloride abrogates the inhibitory signal by inhibiting formation and release of hydrogen peroxide in phagocytes [2, 11, 12]. Moreover, histamine synergizes with IL-2 and IFN-α to activate CD4+ and CD8+ T cells and NK cells [7]. A synergistically increased antitumour efficacy of IFN-α [13, 14] and IL-2 [13, 15, 16] have been demonstrated in mice when concomitantly administered histamine. However, direct evidence supporting this hypothesis is still lacking, although a randomised melanoma phase III study of IL-2 with or without histamine showed a survival benefit with the addition of histamine for patients with liver metastases [17].
The aims of the present study were to provide an overview of the leukocyte orchestration in blood and tumour tissue at baseline and following IL-2–based immunotherapy in mRCC and to assess in situ a potential histamine effect on the leukocyte subsets.
Materials and methods
Patients and treatment
The study was carried out on 49 consecutive patients with inoperable mRCC treated in our institution from February 1999 to August 2000. Of these, 26 patients were enrolled between February 1999 and February 2000 in a multicentre prospective phase II trial of s.c. IL-2, IFN-α and histamine dihydrochloride [18]. The remaining 23 patients were treated as standard treatment in our institution with the same schedule, but without histamine. The local ethics committee approved the study and informed consent was obtained from all patients.
The treatment plan consisted of 1 priming week of daily IFN-α and up to nine treatment cycles of 4 weeks with IFN-α (human leukocyte IFN-α, Interferon Alfanative; BioNative, Sweden, for the phase II trial; otherwise, IntronA; Schering-Plough, Farum, Denmark) 3.0 mIU as a fixed dose s.c. once daily, 7 days per week; IL-2 (aldesleukin, rIL-2, Proleukin; Chiron, Amsterdam, The Netherlands), 2.4 mIU/m2 s.c. two times daily, 5 days per week, weeks 1 and 2 every cycle; and histamine dihydrochloride (Ceplene; Maxim, San Diego, CA, USA) 1.0 mg in 1.0 ml by a 20-min slow s.c. injection, two times daily, 5 days per week throughout the study.
Patients were evaluated for response every 3rd month until progressive disease was observed. Responses were reconfirmed after at least 4 weeks. Standard criteria (World Health Organization) were used for classifying response. No patients were lost to follow-up. All patients had clear cell carcinoma except two, who had papillary renal cell carcihoma (RCC) and collecting duct RCC. These two patients achieved stable disease and progressive disease, respectively. Table 1 lists baseline patient characteristics. Patients’ characteristics and Memorial Sloan-Kettering Cancer Center (MSKCC) prognostic criteria were similar between patients treated with or without histamine (data not shown).
Table 1.
Baseline patient characteristics (n=49). Risk factors: Karnofsky PS <80; LDH≥1.5 times upper limit of normal; hemoglobin < lower limit of normal; corrected s-Ca >10 mg/dl or ion-Ca > upper limit of normal and no prior nephrectomy. No risk factors, favorable prognosis; 1–2 risk factors, intermediate prognosis; and >2 risk factors, poor prognosis
| Numbers | Percentage | ||
|---|---|---|---|
| Patients evaluable for response | 46 | 94 | |
| Median age, years (range) | 56 (19–74) | ||
| Male | 36 | 74 | |
| Karnofsky performance status | |||
| 100 | 16 | 33 | |
| 90 | 16 | 33 | |
| 80 | 7 | 14 | |
| 70 | 10 | 20 | |
| Metastasis-free interval ≤ 1 year | 38 | 78 | |
| Prior nephrectomy | 20 | 41 | |
| Number of disease organ sites | |||
| 1 | 4 | 8 | |
| 2 | 13 | 27 | |
| 3 or more | 32 | 65 | |
| Most common sites of disease | |||
| Primary kidney tumour | 29 | 59 | |
| Local recurrence kidney bed | 7 | 14 | |
| Lung/pleura | 33 | 67 | |
| Lung metastasis alone | 1 | 2 | |
| Lymph node | 27 | 55 | |
| Liver | 12 | 25 | |
| Bone | 19 | 39 | |
| MSKCC prognostic criteriaa | |||
| Favourable prognosis | 3 | 6 | |
| Intermediate prognosis | 25 | 51 | |
| Poor prognosis | 21 | 43 | |
aMemorial Sloan-Kettering Cancer Center [19]
Blood and tumour samples
Of the 49 patients, 45 gave written informed consent for consecutive blood samples and core needle biopsies. The local ethics committee had approved the study. Two patients did not complete one course of therapy because of toxicity and were not evaluable for objective response. These two patients were excluded from all further blood/tumour tissue analyses. Two patients were excluded from the core needle biopsy analysis as they had only fine needle biopsies performed. Three patients had no biopsies performed for safety reasons because tumours were not accessible for core needle biopsies (metastases located in lung, bone, mediastinal lymph node or retrocrural lymph node close to aorta). No complete responding patients had accessible tumours for core needle biopsies. One patient had only a baseline biopsy but no blood samples performed (withdrawal of consent). Two patients had no baseline blood samples performed due to logistic reasons, but had baseline biopsies and on-treatment biopsies plus blood tests performed. Thus, a total of 40 patients had evaluable blood samples for NK activity and flow cytometry analyses and 38 patients had evaluable core needle tumour biopsies.
Blood samples and core needle biopsies were obtained at baseline and after 1 month of immunotherapy on day 1 in the 5th treatment week (i.e. after one cycle). A total of 72 blood samples from 40 patients were evaluated (Table 2). Core needle biopsies (18-G cutting needle) were collected by standard ultrasound-guided procedures [20]. On-treatment biopsy was obtained from the same tumour as baseline. A total of 76 core needle biopsies in 38 patients were performed at different tumour sites (kidney, n=43; abdominal soft tissue, n=10; liver, n=8; pleura, n=4; muscle, n=4; kidney bed, n=3 subcutis, n=2 and lymph node, n=2). There were four nonevaluable patients for the tumour tissue analyses, two because of insufficient tumour tissue in the biopsies and two because of necrosis in all biopsies at both biopsy time points. Eight patients had only a baseline biopsy performed (withdrawal of consent). Thirteen of the 76 biopsies (17%) were excluded because of necrosis and the two lymph node biopsies were excluded because of possibility of contamination from nontumour related immune cells. Thus, 61 core needle biopsies in 34 patients were evaluable (Table 2). Differential blood cell counts were determined by routine Coulter Stks analyses. Intratumoural eosinophils were visualized by carbol chromotrope [21]. Results from blood and tumour tissue lymphocyte subsets of 23 patients treated with IL-2, IFN-α and histamine have partly been published elsewhere [22].
Table 2.
Number of serial blood samples and tumour tissue core needle biopsies
| IL-2, IFN- α and histamine (n=26) | IL-2, IFN-α (n=23) | Total (n=49) | ||||
|---|---|---|---|---|---|---|
| Week 0 | Week 5 | Week 0 | Week 5 | Week 0 | Week 5 | |
| Serial blood samples | ||||||
| Number blood samples for flow cytometry | 21 | 23 | 19 | 9 | 40 | 32 |
| Number differential white blood cell countsa | 24 | 24 | 22 | 21 | 46 | 45 |
| Tumour tissue core needle biopsies | ||||||
| Number of performed biopsies/number of patients | 25/22 | 23/20 | 18/16 | 10/9 | 43/38 | 33/29 |
| Number of evaluable biopsies/number of patients | 20/19 | 19/18 | 15/15 | 7/7 | 35/34 | 26/25 |
aBlood samples obtained from all patients evaluable for treatment response
Preparation of PBMCs, cytotoxicity assay and flow cytometry
Peripheral blood mononuclear cells (PBMCs) were isolated from lithium-heparinized whole blood samples by Ficoll-Paque (Pharmacia Biotech, Uppsala, Sweden) gradient separation, washed twice and cryopreserved at −135°C until use. Cytolytic activity was determined by standard 4-h 51 Cr-release assay against K562 target, as previously reported in detail [22].
Cell surface phenotypes were determined by flow cytometry using a Coulter XL-2 flow cytometer (Coulter Electronics, Miami, FL, USA). Data were analysed using the Flow-Jo software (Treestar, San Carlos, CA, USA). Direct fluorochrome-conjugated antibodies (FITC or PE) were purchased from DAKO, Glostrup, Denmark (CD3, CD4, CD8, CD20) and Becton Dickinson, Denmark (CD56, CD57). Intracellular ζ expression was investigated on permeabilized cells, using 2H2D9 (TIA-2) PE-conjugated MoAb, Ramcon, Denmark. Relevant isotype controls were purchased from DAKO.
Immunohistochemistry
Sections (2-μm) of formalin-fixed, paraffin-embedded biopsy samples were mounted on ChemMate slides (cat. no. S2024; DAKO, Glostrup, Denmark), dried 1 hour at 60°C, deparaffinized and rehydrated. After endogenous peroxidase blocking (0.5% hydrogen peroxidase in water for 30 min), antigens were retrieved by microwave oven heating (3×5 min at 850 W in Tris/EGTA retrieval buffer [pH 9.0]). The tissue sections were incubated for 1 h with the following antibodies from DAKO: CD3 (A0452 1:100), CD8 (M7103 1:100), CD20 (M0755 1:500) and CD79-α (M7050 1:50); NoveCastra: CD4 (NCL-CD4-1F6 1:50); Macro (NCL-MACRO 1:80); Pharmingen: CD57 (33251A 1:500) and CD66b (33731A 1:100); and Ramcon: ζ (IM2549 1:20). As second layer, peroxidase-conjugated EnVision (K4000/K4002; DAKO, Glostrup, Denmark) was used for 30 min of incubation. Staining was visualized with diaminobenzidine tetrahydrochloride solution, counterstained in Mayer’s hematoxylin and mounted with Aqutex (64912-50; Kebo-lab, Denmark). All staining was performed in a TechMate automate machine (DAKO, Glostrup, Denmark). As positive control, a normal lymph node, tonsil or tuberculosis lesion was used. As negative control, substitution of primary antibody with PBS and isotype controls from Pharmingen/DAKO was used.
Measuring intratumoural immune cells
A stereologic examination was performed using a morphometric system consisting of an Olympus AH-3 microscope with a motorized stage, controlled by a computer for manual interactive counting on the computer screen [23]. The software used was CAST-GRID version 2.0, developed by Olympus, Denmark. Each microscopic field of vision was projected onto the computer screen with a video camera, and the computer generated an unbiased counting frame in which the measurements were performed. On the projected image of the section, the tumour area was circled. Necrosis, artefacts and fibrous areas were omitted. The first field of vision was chosen at random; thereafter, the computer sampled systematically the following fields of vision within the entire circled area. Using a ×40 objective, a number of 40 fields (4951 μm2 each) were counted, if possible. The entire core needle biopsy sample was assessed. Only a cell with staining restricted to the plasma membrane, a visible nucleus and located within the counting frame was counted as positive. The mean number of cells/mm2 tumour tissue was assessed for each patient. Staining was analysed blinded for one observer. For testing the reproducibility, CD8 sections were ranked according to their number of immune cells and every sixth case was selected and counted blinded by a senior histopathologist (N.M.). A high level of reproducibility (Spearman ρ=0.89, p=0.001) was found.
Statistics
Overall survival was measured from 1st day of treatment until death or last follow-up evaluation. The relationship between leukocyte subsets and objective response or treatment was evaluated using the Mann-Whitney U-test. The cumulated survival rate was analysed by Kaplan-Meier, and the log-rank test was used to analyse survival differences among subgroups of patients. Differences in patient characteristics and response rates were analysed by Fisher exact test. Dichotomy of the patients was done at the median value for each evaluated parameter, except for the analyses of intratumoural lymphocyte subsets in which the cutoff levels were predefined from our previous study [22]. All reported p values were two-sided. The median follow-up period was 45.5 months (range 38.2–52.8). Data were updated 30 October 2003. Statistical analyses were performed using SPSS version 11.0.
Results
Clinical treatment results
Forty-six patients were evaluable for response and 49 for toxicity. The three nonevaluable patients received less than one treatment cycle, two because of toxicity and one because of patient request. Another patient also received less than one treatment cycle, which, however, was caused by progressive disease, and this patient was included in the response evaluation. Based on intention-to-treat analysis, a total of ten patients, i.e. 20% (95% confidence interval [CI], 9–32%) responded to treatment. Three patients had a complete response (CR) and seven, a partial response (PR). Responses were noted in the primary kidney tumour as well as in metastases in the lung, pleura, lymph node, liver, bone, soft tissue, subcutaneous tissue and kidney bed. Fifteen patients (31%) had stable disease (SD). A total of seven patients (14%) had no evidence of disease (NED) and were alive at 38.2+ to 52.8+ months after immunotherapy alone or immunotherapy plus surgical resection of residual tumours. Median survival was 14.0 months (95% CI, 8.1–19.9 months; range 1.3–52.8+ months). The Kaplan-Meier plot is given in Fig. 1, demonstrating an estimated 4-year survival of 22%. At the time of analysis, 39 patients had died (censoring rate of 20.4%). In general, toxic effects were minor to moderate. There were no treatment-related deaths.
Fig. 1.
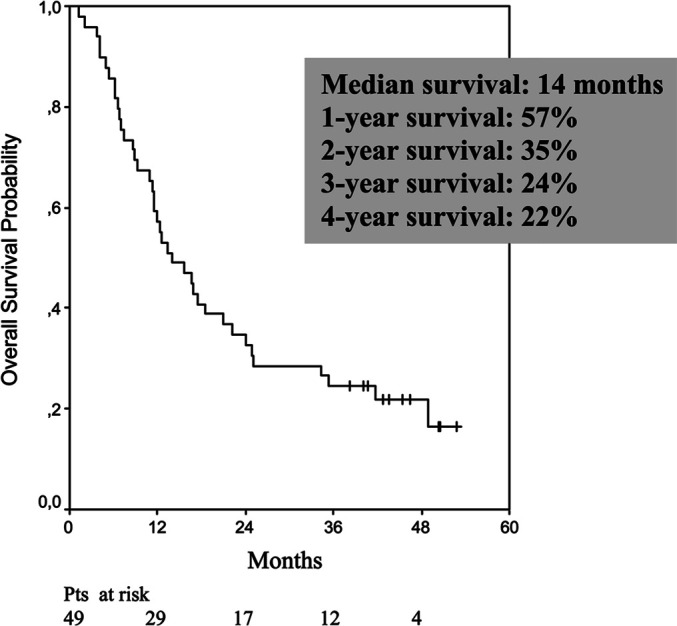
Kaplan-Meier survival estimates for all 49 patients. Tick marks represent ten censored patients
Correlation between absolute number of peripheral blood leukocyte subsets and objective response/survival
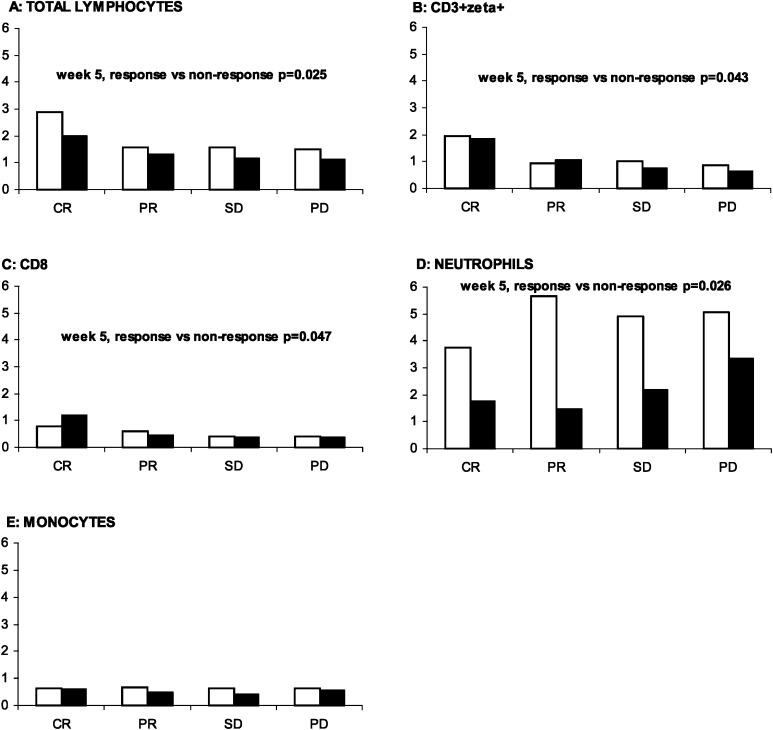
Baseline and week 5 absolute number of total leukocyte subsets defined by differential count and absolute number of lymphocyte subsets defined by flow cytometry were examined and compared in responding (CR + PR) and nonresponding (SD + progressive disease [PD]) patients. At baseline, no significant differences were demonstrated. After 1 month of treatment, the absolute number of total lymphocytes (p=0.025), CD8+ cells (p=0.047) and CD3+ζ+ cells (p=0.043) was significantly higher in responding patients compared with nonresponding patients. By contrast, the number of neutrophils was significantly lower (p=0.026) in responding patients compared with nonresponding patients (Fig. 2.) No significant differences between responding and nonresponding patients were seen in the absolute number of CD3+ Tcells, CD4+ T cells, CD20+ B cells, CD56+ NK cells, CD56dim, CD56bright, CD56+ζ+, CD57+ cells or monocytes.
Fig. 2A–E.
Peripheral blood leukocytes as predictors of response at baseline (white bars) and after 1 month of interleukin 2 (IL-2) based immunotherapy (black bars). Median values for patients with complete response (CR), partial response (PR), stable disease (SD) and progressive disease (PD) are shown for A total lymphocytes, B CD3+ζ+ T cells, C CD8+ T cells, D neutrophils and E monocytes. Vertical and horizontal axes, 109 cells/l and response to immunotherapy, respectively
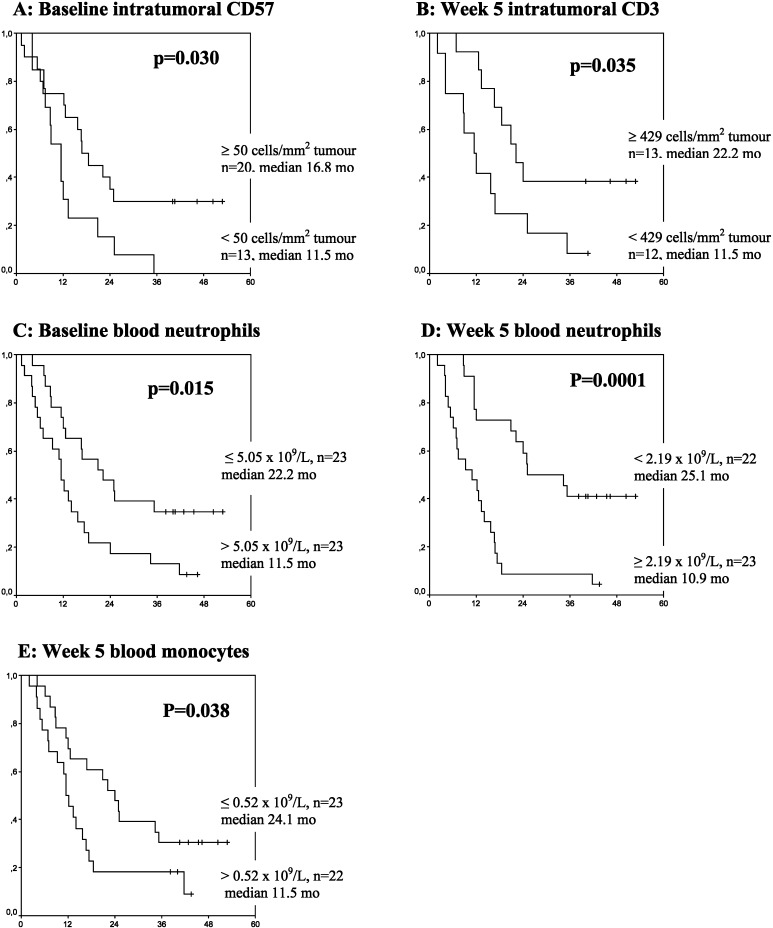
Baseline and on-treatment absolute number of peripheral blood leukocytes and lymphocyte subsets were also examined and compared with survival. Whereas no significant correlation to survival was demonstrated for total lymphocytes or lymphocyte subsets, a significant negative correlation was demonstrated between baseline neutrophils (p=0.015), week 5 neutrophils (p=0.0001), week 5 monocytes (p=0.038) and survival (Fig. 3C–E).
Fig. 3A–E.
Intratumoural lymphocyte subsets as positive prognostic factors for survival and peripheral blood neutrophils/monocytes as negative prognostic factors for survival. Kaplan-Meier plots concerning survival according to A baseline intratumoural CD57, B week 5 intratumoural CD3, C baseline blood neutrophils, D week 5 blood neutrophils and E week 5 blood monocytes in patients with metastatic renal cell carcinoma (mRCC) treated with IL-2–based immunotherapy. Dichotomy of the patients was done at the median value for neutrophils and monocytes. For the intratumoural lymphocyte subsets, the cutoff levels were predefined from our previous study [22]. Vertical and horizontal axes, the survival probability and months of follow-up, respectively
Correlation between absolute number of intratumoural leukocyte subsets and objective response / survival
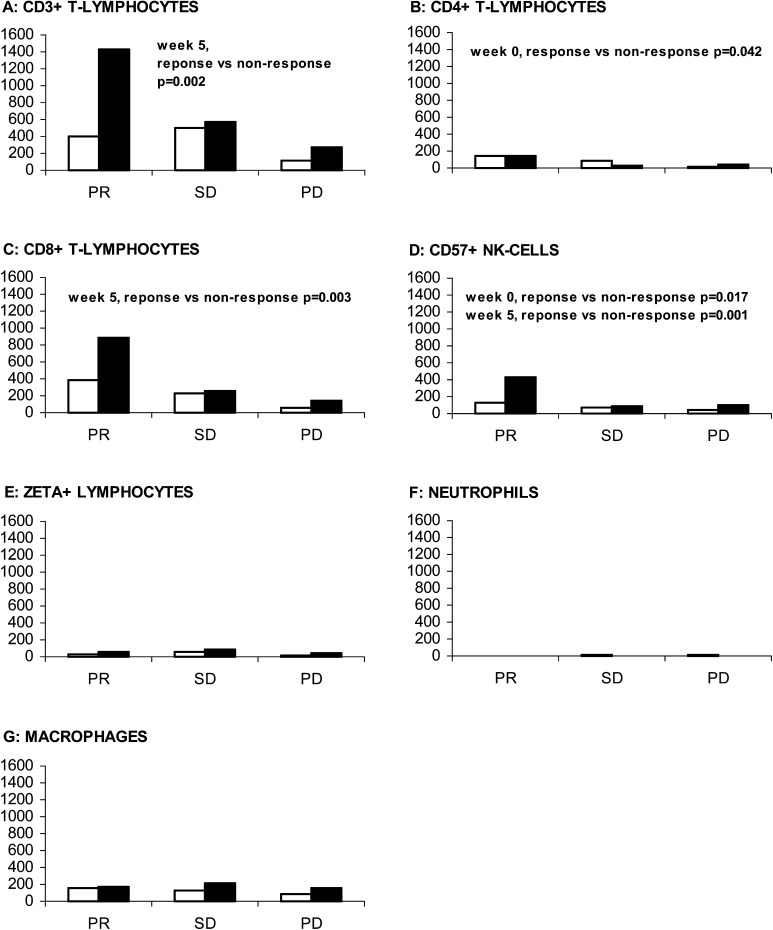
Baseline and on-treatment intratumoural leukocyte subsets defined by immunohistochemistry were evaluated and correlated to objective response. At baseline, the number of CD4+ and CD57+ cells/mm2 tumour tissue was significantly higher (p=0.042 and p=0.017, respectively) in responding patients compared with nonresponding patients (Fig. 4). After 1 month of therapy, the number of CD3+, CD8+ and CD57+ cells/mm2 tumour tissue were significantly higher (p=0.002, p=0.003 and p=0.001, respectively) in responding patients compared with nonresponding patients. A close cell-to-cell contact between intratumoural T cells and tumour cells was noted during immunotherapy (Fig. 5). The numbers of intratumoural B cells, plasma cells, neutrophils, macrophages and ζ+ lymphocytes were low, both at baseline and week 5, and were not significantly correlated to objective response (Fig. 4). In progressing patients, the numbers of intratumoural leukocyte subsets were low, both at baseline and at week 5 (Fig. 4). Eosinophils were not present within the tumour tissue.
Fig. 4A–G.
Intratumoural immune cells as predictors of response in patients with mRCC at baseline (white bars) and after 1 month of IL-2–based immunotherapy (black bars). A CD3+ T cells, B CD4+ T cells, C CD8+ T cells, D CD57+ NK cells, E ζ+ lymphocytes, F neutrophils and G macrophages. Vertical and horizontal axes, response to therapy and median number of cells/mm2 tumour tissue, respectively
Fig. 5.
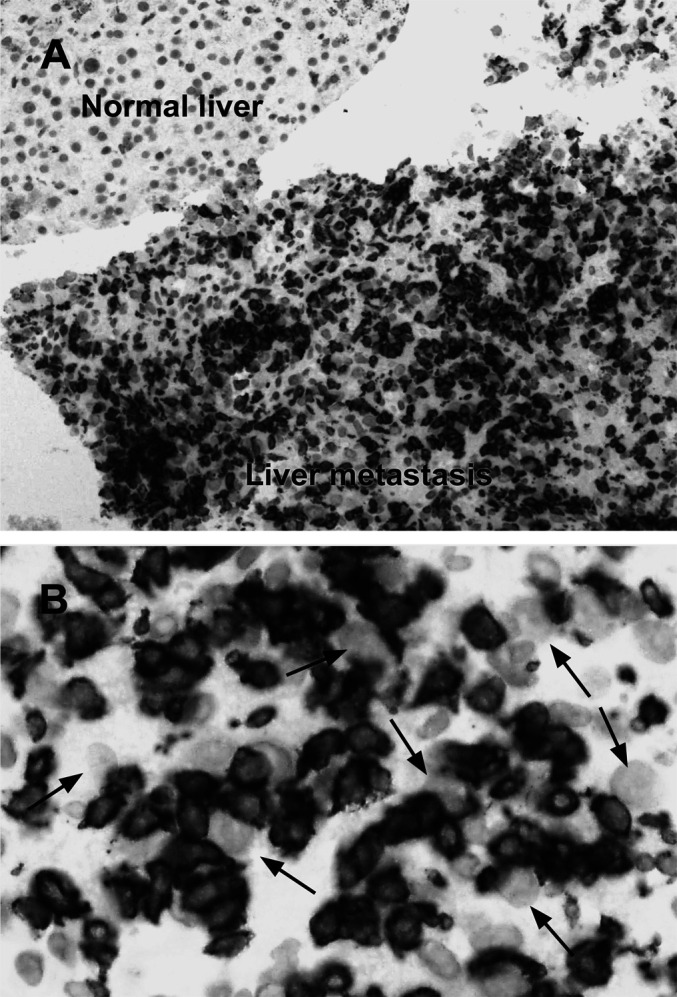
A Controlled CD8+ infiltration restricted to tumour tissue only. Liver metastasis core needle biopsy performed after 1 month of immunotherapy with IL-2 and IFN-α in a responding patient. The tumour subsequently totally disappeared (×10). B Same biopsy at higher magnification (×40) demonstrating closely cell-to-cell contact between tumour cells (arrows) and CD8+ T cells (brown membrane stained cells)
Baseline and on-treatment absolute numbers of intratumoural leukocyte subsets were examined and compared with survival. Baseline intratumoural CD57 and CD3 at week 5 was positively correlated to survival (p=0.030 and p=0.035, respectively; Fig. 3A, B), whereas intratumoural CD4, CD8, CD20, CD79α, ζ+ lymphocytes, neutrophils or macrophages were not correlated to survival.
Relative composition of leukocyte subsets in peripheral blood and tumour tissue
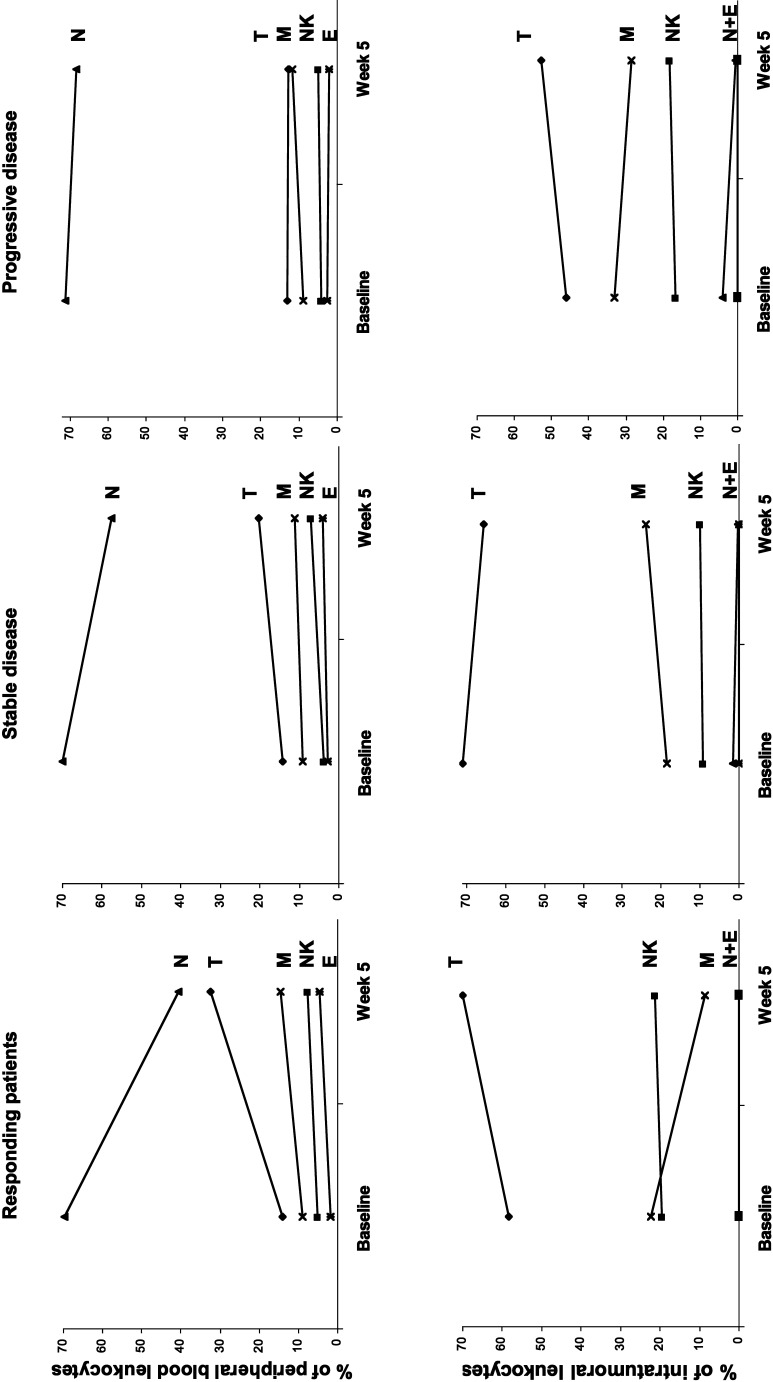
The relative composition of the five most frequently occurring leukocyte subsets in peripheral blood and tumour tissue was analysed. Neutrophils, the most frequent peripheral blood leukocyte subset, were, however, scarce within the tumour tissue (Fig. 6.) In progressing patients, the relative composition of leukocyte subsets in blood and tumour tissue remained unaffected by cytokine therapy. However, in responding patients, cytokine therapy was followed by a relative increase in T cells in blood and tumour tissue, a relative reduction in neutrophils in peripheral blood and a relative reduction of intratumoural macrophages (Fig. 6). The changes in patients with stable disease were modest.
Fig. 6.
The relative composition of the five most frequently occurring leukocyte subsets in peripheral blood and tumour tissue at baseline and after one cycle of therapy at week 5 in patients with response, stable disease and progressive disease. N neutrophils, M macrophages, T T cells, NK NK cells and E eosinophils
No effect of histamine on peripheral blood and intratumoural leukocyte number or function
We evaluated the influence of histamine on the absolute number of peripheral blood and intratumoural leukocyte subsets, levels of ζ-chain expression and NK-cell cytotoxicity. When numbers of blood and intratumoural leukocyte subsets in responding and nonresponding patients at baseline or during therapy were compared between histamine- and nonhistamine-treated patients, no statistically significant differences were noted. Moreover, histamine did not significantly affect ζ-chain expression in peripheral blood or intratumoural lymphocytes. Finally, histamine treatment did not significantly affect cytotoxicity of peripheral blood mononuclear cells (data not shown).
Discussion
The present study documents the efficacy and safety of outpatient low-dose subcutaneous IL-2 and IFN-α in patients with metastatic renal cell carcinoma. Despite the fact that only 6% of patients had a favourable prognosis according to MSKCC prognostic criteria [19] (Table 1), we observed objective responses in 20% of patients, an overall median survival of 14 months and an estimated 1- to 4-year survival rate of 57, 35, 24 and 22%, respectively. This seems consistent with results previously obtained with intermediate to high doses of i.v. IL-2 based immunotherapy [24, 25] and is in accordance with a recently published randomised 3-arm study of high-dose versus low-dose IL-2 [26]. It should be noted that 14% of the patients achieved NED and were alive at 38+ to 52+ months after immunotherapy alone or immunotherapy plus subsequent surgery of residual tumour. This combined treatment approach has also been reported by others [27] and indicates that resection of residual tumour(s) in patients with major tumour regression may result in a significant disease-free survival in patients with mRCC.
Our blood and tumour tissue analyses demonstrate that lymphocytes localise to sites of tumour in responding patients and that CD4+, CD8+ and CD57+ lymphocyte subsets are requisite for the response to IL-2–based immunotherapy as well as long-term survival. This is in accordance with our previous finding based on a smaller patient number [22] and with findings in metastatic malignant melanoma treated with IL-2 [28, 29] and IFN-α [30, 31]. Moreover, not only is treatment response apparently associated with an accumulation of lymphocyte subsets within tumour tissue, but a relative reduction in intratumoural macrophages and an absolute and relative reduction in peripheral blood neutrophils are also requisite. Elevated baseline neutrophil count as a prognostic factor for short-term survival and a predictive factor for rapid progression has also been demonstrated by others [32]. It should be noted that blood and tumour tissue leukocyte subsets in patients with progressive disease remain unaffected by cytokine therapy, both in absolute number and in the relatively composition. Thus, the blood and tumour tissue analyses at baseline and at week 5 reflect the subsequent clinical outcome. However, only 25 of 49 (51%) treated patients had evaluable biopsies after 1 month of treatment. Therefore, our results have to be confirmed in larger studies.
Subgroup comparison of blood and tumour effects of histamine versus no histamine should be done with great caution. Despite patient characteristics and the distribution of prognostic factors being comparable, the subgroup numbers were very small, and thus only large differences would be apparent. To control for potential bias, either known or unknown, a randomised trial is mandatory. However, a large randomised controlled study powered to detect a meaningful clinical benefit to the patients was not justified by the disappointing results obtained in the multicentre phase II trial [18]. Therefore, the present evaluation was done, searching for a potential histamine effect in situ, which was not necessarily translated to an objective clinical effect. Thus, data from the blood and tumour tissue analyses may serve to substantiate the conclusion reached in the phase II trial by itself. We are presently investigating whether histamine may improve efficacy with higher doses of IL-2 in a randomised phase II trial in mRCC. Moreover, it should be noted that a large randomised melanoma phase III study of IL-2 with or without histamine showed a survival benefit with the addition of histamine for patients with liver metastases [17]. A confirmatory large international phase III study of melanoma patients has recently finished accrual.
Three of our blood and tumour tissue observations support the oxidative stress hypothesis. First, high absolute numbers of blood monocytes and neutrophils were correlated to short-term survival. High absolute numbers of blood neutrophils were also significantly correlated to nonresponse. Second, intratumoural lymphocytes showed low ζ expression (<10%), whereas blood lymphocytes showed almost normal levels of ζ expression (approximately 90%). Third, CD3+ζ+ T cells, in contrast to CD3+ ζ− T cells, were significantly positively correlated to objective response. Thus, the oxidative stress hypothesis cannot be excluded. Moreover, regarding tumours as complex tissues in which mutant cancer cells have conscripted and subverted normal cell types to serve as active collaborators in their neoplastic agenda is a new paradigm based on molecular cancer research [33, 34]. Nevertheless, there was no indication that histamine did terminate the suppression of reactive oxygen species.
In summary, the present regimen of outpatient low-dose subcutaneous IL-2 and IFN-α in mRCC should attract interest based on response, survival and toxicity. In responding patients, cytokine therapy was followed by substantial changes in the blood and tumour tissue leukocyte composition, correlated to response and survival. However, in this small nonrandomised study, no discernable differences in immunologic parameters studied could be detected between histamine and nonhistamine treated patients.
Acknowledgements
We thank Dr Peter Hokland for providing access to the flow cytometer; Dr Theis Bacher for providing access to the gamma counter; Tom Nordfeld for running the Techmate automate immunohistochemistry machine; Karin Vestergaard for sectioning the biopsies; Anja Balmer and Bettina Grumsen for help with cytotoxicity and flow cytometry assays; Dr Ulrik Baandrup for help with eosinophil staining. Staff members at Department of Oncology are acknowledged for their careful management of the patients.
This study was supported by grants from the Danish Research Council, Radiumstationens Forskningsfond, Max and Inger Woerzner Foundation, Gerda and Aage Haench’s Foundation, Preben and Anna Simonsens Foundation, Agnes Niebuhr Anderssons Foundation, Kristian Kjaer Foundation, The Beckett Foundation, Hans and Nora Burchard’s Foundation, Agnes and Poul Friis Foundation, Erland Richard Frederiksen Foundation, Jacob Madsen and Olga Madsen Foundation, Johannes Fogh-Nielsen Foundation, Jens C. Christoffersen Foundation, the Danish Cancer Society (MH) and the Danish Medical Association Research Fund.
References
- 1.Rosenberg Nature. 2001;411:380. doi: 10.1038/35077246. [DOI] [PubMed] [Google Scholar]
- 2.Hellstrand J Immunol. 1994;153:4940. [PubMed] [Google Scholar]
- 3.Hellstrand Semin Oncol. 2002;29:35. doi: 10.1053/sonc.2002.33081. [DOI] [PubMed] [Google Scholar]
- 4.Saio J Immunol. 2001;167:5583. doi: 10.4049/jimmunol.167.10.5583. [DOI] [PubMed] [Google Scholar]
- 5.Kiessling Cancer Immunol Immunother. 1999;48:353. doi: 10.1007/s002620050586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hansson J Immunol. 1996;156:42. [Google Scholar]
- 7.Hansson J Interferon Cytokine Res. 1999;19:1135. doi: 10.1089/107999099313073. [DOI] [PubMed] [Google Scholar]
- 8.Kono Eur J Immunol. 1996;26:1308. doi: 10.1002/eji.1830260620. [DOI] [PubMed] [Google Scholar]
- 9.Tartour Int J Cancer. 1995;63:205. doi: 10.1002/ijc.2910630210. [DOI] [PubMed] [Google Scholar]
- 10.Finke Cancer Res. 1993;53:5613. [PubMed] [Google Scholar]
- 11.Hellstrand J Immunol. 1986;137:656. [PubMed] [Google Scholar]
- 12.Hellstrand Scand J Immunol. 1991;34:741. doi: 10.1111/j.1365-3083.1991.tb01599.x. [DOI] [PubMed] [Google Scholar]
- 13.Asea Scand J Immunol. 1996;43:9. doi: 10.1046/j.1365-3083.1996.d01-14.x. [DOI] [PubMed] [Google Scholar]
- 14.Hellstrand J Interferon Res. 1992;12:199. doi: 10.1089/jir.1992.12.199. [DOI] [PubMed] [Google Scholar]
- 15.Hellstrand Int Arch Allergy Appl Immunol. 1990;92:379. doi: 10.1159/000235169. [DOI] [PubMed] [Google Scholar]
- 16.Hellstrand J Immunol. 1990;145:4365. [PubMed] [Google Scholar]
- 17.Agarwala J Clin Oncol. 2002;20:125. [Google Scholar]
- 18.Donskov Ann Oncol. 2002;13:441. doi: 10.1093/annonc/mdf049. [DOI] [PubMed] [Google Scholar]
- 19.Motzer J Clin Oncol. 1999;17:2530. doi: 10.1200/JCO.1999.17.8.2530. [DOI] [PubMed] [Google Scholar]
- 20.Jennings Lancet. 1989;1:1369. doi: 10.1016/S0140-6736(89)92813-4. [DOI] [PubMed] [Google Scholar]
- 21.Horobin RW, Kiernan JA (2002) Conn’s biological stains: a handbook of dyes, stains and fluorochromes for use in biology and medicine, 10th edn. BIOS Scientific, Oxford, pp 112–113
- 22.Donskov Br J Cancer. 2002;87:194. doi: 10.1038/sj.bjc.6600437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gundersen APMIS. 1988;96:379. doi: 10.1111/j.1699-0463.1988.tb05320.x. [DOI] [PubMed] [Google Scholar]
- 24.Fisher Cancer J Sci Am. 2000;6:S55. [PubMed] [Google Scholar]
- 25.Negrier N Engl J Med. 1998;338:1272. doi: 10.1056/NEJM199804303381805. [DOI] [PubMed] [Google Scholar]
- 26.Yang J Clin Oncol. 2003;21:3127. doi: 10.1200/JCO.2003.02.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fyfe J Clin Oncol. 1995;13:688. doi: 10.1200/JCO.1995.13.3.688. [DOI] [PubMed] [Google Scholar]
- 28.Rubin Cancer Res. 1989;49:7086. [PubMed] [Google Scholar]
- 29.Cohen Am J Pathol. 1987;129:208. [PMC free article] [PubMed] [Google Scholar]
- 30.Hakansson Br J Cancer. 1996;74:670. doi: 10.1038/bjc.1996.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hakansson Br J Cancer. 2001;85:1871. doi: 10.1054/bjoc.2001.2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Negrier Ann Oncol. 2002;13:1460. doi: 10.1093/annonc/mdf257. [DOI] [PubMed] [Google Scholar]
- 33.Coussens LM, Werb Z (2001) Inflammatory cells and cancer: think different! J Exp Med 193:F23–F26 [DOI] [PMC free article] [PubMed]
- 34.Hanahan Cell. 2000;100:57. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]