Abstract
Objective: A majority of human cancers, including head and neck cancer (HNC), “overexpress” p53. Although T cells specific for wild-type (wt) sequence p53 peptides are detectable in the peripheral blood of patients with HNC, it is unknown whether such T cells accumulate in tumor-involved tissues. Also, the localization of “regulatory” T cells (Treg) to tumor sites in HNC has not been investigated to date. Methods: Tumor infiltrating lymphocytes (TIL), tumor-involved or non-involved lymph node lymphocytes (LNL) and peripheral blood mononuclear cells (PBMC) were obtained from 24 HLA-A2.1+ patients with HNC. Using tetramers and four-color flow cytometry, the frequency of Treg and CD3+CD8+ T cells specific for wt p53 epitopes as well as their functional attributes were determined. Results: The CD3+CD8+ tetramer+ cell frequency was significantly higher (P<0.001) in TIL than autologous PBMC as was the percentage of CD4+CD25+ T cells (P<0.003). TIL were enriched in FOXp3+, GITR+ and CTLA-4+ Treg. CD8+ TIL had low Ζ expression and produced little IFN-γ after ex vivo stimulation relative to autologous PBMC or PBMC from NC. Conclusions: Anti-wt p53 epitope-specific T cells and Treg preferentially localize to tumor sites in patients with HNC. However, despite enrichment in tumor peptide-specific T cells, the effector cell population (CD3+CD8+) in TIL or PBMC was unresponsive to activation in the tumor microenvironment enriched in Treg.
Keywords: TIL, Tetramers, wt p53 epitopes, CD4+CD25+ T cells, Regulatory T cells (Treg), Tumor escape
Introduction
Effective antitumor responses in individuals with cancer depend on the presence and function of immune cells that are able to recognize and eliminate tumor cells. Although such tumor antigen-specific immune T cells are known to be present in the peripheral circulation and tissues of patients with cancer, the tumor often evades their effects through various mechanisms referred to as “escape mechanisms” [17, 29]. An obvious consequence of tumor escape from immune surveillance is cancer progression and metastasis. Molecular events that result in a loss of p53 function [11, 12] characterize the majority of human malignancies [5, 20]. By a variety of mechanisms, wild-type (wt) sequence p53 peptides are processed and presented to the host immune cells either by the tumor or by dendritic cells (DC), because wt p53 peptide-specific T cells and, in some instances, p53-specific antibodies are detectable in patients with cancer [1, 7, 25]. We have recently reported that the frequency of tetramer+ CD8+ T cells specific for the HLA-A2.1-restricted wt p53264-272 peptide is significantly higher in the peripheral circulation of HLA-A2.1+ patients with head and neck cancer (HNC) than in normal donors [7]. Further, vaccination of patients with ovarian carcinoma with p53-based vaccines leads to an increased frequency of tetramer+ wt p53264-272 specific T lymphocytes in the peripheral circulation, as recently shown by us [6]. However, the presence of wt p53 peptide-specific T cells in the periphery has no apparent effect on tumor progression, and it has been suggested that these T cells either do not reach the tumor nor are not functional in the tumor microenvironment.
In this manuscript, we investigated these two possibilities by: (a) determining the frequency of wt p53264-272 peptide- or wt p53149-157 peptide-specific T cells in the circulation and at the tumor site in a cohort of HLA-A2.1+ patients with HNC; and (b) evaluating the extent of accumulation of CD4+CD25+ T cells at the tumor site in the same patients. Recent reports in the literature on suppressor activities of Treg in patients with tumors [2, 16, 31] provide a strong rationale for this experimental approach. The hypothesis tested in this study predicts that the accumulation of CD4+CCD25+ T cells, which are enriched in FOXp3+, GITR+ and CTLA-4+ Treg, at the tumor site could negatively impact antitumor functions of tumor-specific CD8+ effector lymphocytes.
Materials and methods
Patients and tumor tissues
Patients with HNC included in this study (n=24) were cared for at the Oral Cancer Center, University of Pittsburgh. All patients underwent surgery for the treatment of primary squamous cell carcinoma of the head and neck (SCCHN). Tumor tissues or lymph nodes were collected after the patients signed the IRB-approved informed consent. The tissues were processed and banked at the University of Pittsburgh Cancer Institute (UPCI) Tissue Procurement Facility. Samples of peripheral blood mononuclear cells (PBMC) were obtained from the same patients (#1–#17) and from an additional five patients prior to surgery. All patients were HLA-A2+, as previously determined by sero-phenotyping of their PBMC, using monoclonal antibodies (mAbs) BB7.2 and MA2.1 produced by hybridomas obtained from American Type Culture Collection (ATCC, Manassas, VA, USA). The age, sex and clinicopathologic characteristics of the patients are listed in Table 1.
Table 1.
Clinicopathologic characteristics of patients with SCCHN who donated PBMC and/or TIL for this study
| n | |
|---|---|
| Age (years) | |
| Mean range | 41–82 |
| Sex | |
| Male | 19 |
| Female | 5 |
| Total | 24 |
| Tumor site | |
| Larynx | 5 |
| Oral cavity | 10 |
| Pharynx | 4 |
| Hypopharynx | 3 |
| Other | 2 (1 skin and 1 unknown) |
| Tumor stage | |
| T1 | 1 |
| T2 | 5 |
| T3 | 8 |
| T4 | 8 |
| Unstaged | 2 |
| Nodal status | |
| N0 | 6 |
| N1 | 5 |
| N2 | 11 |
| unknown | 2 |
| Tumor differentiation | |
| Well | 3 |
| Moderate | 15 |
| Poor | 4 |
| Undifferentiated | 2 |
Collection of PBMC
Peripheral venous blood (20–30 ml) was drawn into heparinized tubes. The samples were hand-carried to the laboratory and immediately centrifuged on Ficoll-Hypaque. PBMC were recovered, washed in AIM-V medium (Invitrogen, Carlsbad, CA, USA) counted in trypan blue and either cryopreserved or immediately used for experiments.
Isolation of tumor infiltrating lymphocytes (TIL)
Tumor infiltrating lymphocytes (TIL) were isolated at the UPCI Tissue Bank according to a standard operating procedure (SOP) as previously described [3]. Briefly, after removal of fat, blood or necrotic areas, primary or metastatic solid human tumor tissue or tumor-involved and non-involved lymph nodes were washed in RPMI-1640 containing 50 μg/ml gentamycin (Invitrogen), cut into 1 mm3 pieces in a petri dish covered with RPMI-1640, washed again with RPMI-1640 and upon transfer to flasks, dissociated using 0.05% collagenase (type IV, Sigma, St. Louis, MO, USA) and 0.02% DNase (type I; Sigma) in RPMI-1640 supplemented with 5% (v/v) fetal calf serum and antibiotics (Invitrogen). Tissues were dissociated for up to 4 h using a magnetic stirrer at 37°C. The digest was then passed through 90 μm and 50 μm nylon mesh to remove clumps, and the filtrate was washed 2–3× in medium followed by centrifugation at 350× g for 10 min. To separate tumor cells from lymphocytes, the cell suspension was layered onto a discontinuous Ficoll-Paque gradient of 75% over 100% Ficoll-Paque in medium and centrifuged for 800× g for 20 min at RT. Lymphocytes were collected from the interphase between 75% and 100% Ficoll-Paque and washed twice before further use.
Isolation of LNL was performed as previously described [15].
Cryopreservation of TIL and PBMC
Peripheral blood mononuclear cells, TIL or LNL were cryopreserved at a cell concentration of up to 20×106 cells/ml in a freezing medium consisting of 90% (v/v) human AB serum (Nabi, Miami, FL, USA) plus 10% dimethylsulfoxide (DMSO; Fisher Scientific, Pittsburgh, PA, USA). Cells were cooled at a rate of 1°C per min to −80°C (Cryomed, Thermo Forma, Pittsburgh, PA, USA) and stored in liquid N2. Prior to use, cells were thawed at 37°C and immediately resuspended in an excess of warm AIM-V medium (Invitrogen). Cells were washed twice in medium, equilibrated in medium for 2–4 h, adjusted to the concentration of 5×106/ml and used for experiments.
Tetrameric peptide-MHC class I complex (tetramer) assay
The streptavidin-APC-labeled tetramers were made at the Tetramer Core Facility of the National Institute of Allergy and Infectious Disease (Atlanta, GA, USA). Tetramer specific for the wt sequence p53 peptides: wt p53264-272, wt p53149-157 and wt p5365-73 were used. Four-color flow cytometry-based assays were performed as previously described [9], using FACS (Becton Dickinson, San Jose, CA, USA). As a negative control, an irrelevant HLA-A2-restricted tetramer (e.g., HIV pol peptide ILKEPVHGV) obtained from Beckman Coulter (Miami, FL, USA) was used. We have previously established a lower limit of detection (LLD) for the assay based on the upper 99th percentile of tetramer+CD8+ T cells in HLA-A2*0201-negative individuals as the frequency of 0.01% or 1/7,600 cells [8]. This LLD was used in evaluating the results of all tetramer experiments.
Antibodies
All the mAbs used for surface staining of lymphocytes were purchased from Becton Dickinson (San Jose, CA, USA) and included mAbs to: CD3, CD4, CD8, CD14, CD25, and the respective isotypes used as negative controls. In addition, anti-IFN-γ mAb was purchased from Beckman Coulter, and anti-CD247 mAb from Santa Cruz, Biotechnology, Santa Cruz, CA, USA. Unlabeled polyclonal anti-FOXp3 and the secondary labeled Ab were purchased from Abcam Ltd., Cambridge, MA, USA. Carboxyfluorescein-conjugated mAb to glucocorticoid-induced TNF receptor (GITR) was purchased from R&D Systems (Minneapolis, MN, USA) and labeled anti-CTLA-4 Ab was from Beckman Coulter. Prior to use, all mAbs were titrated using normal resting or activated PBMC to establish optimal staining dilutions. Checkerboard titrations were first performed to determine optimal dilutions of primary and secondary Abs used for the indirect staining of FOXp3.
Staining and flow cytometry for regulatory T cells (Treg)
For flow cytometry, lymphocytes were stained as previously described by us [9, 10]. Briefly, cells were thawed and washed twice in prewarmed AIM-V medium and then washed again with 2 ml PBS + 0.1% (w/v) BSA + 0.1% (w/v) sodium azide (flow buffer). For phenotypic analysis of Treg, 5 μl aliquots of the labeled mAbs to CD3, CD4, CD8 and CD25 were first added to cell pellets (1×104 cells). Cells were incubated for 30 min at 4°C in the dark to obtain surface staining. Next, cells were washed twice with the flow buffer and fixed in 2.5% paraformaldehyde (PFA) in PBS for 10 min at room temperature in the dark. After another wash with the same buffer, cells were permeabilized with saponin (0.1% v/v in BSA) and washed with cold saponin solution. Next, Abs to FOXp3, GITR, CTLA-4 or isotype control Abs were added to the cells. Following a 25-min incubation at 4°C in the dark, cells were washed again with 0.1% saponin and then with the flow buffer. In case of FOXp3, the pretitered secondary Ab was then added for an additional 25-min incubation followed by washing in the flow buffer. Finally, the cells were fixed with PFA in PBS. Staining of cells for intracytoplasmic ζ and IFN-γ expression was performed as described above. All stained samples were immediately analyzed by flow cytometry. Multiparameter flow cytometry for T-cell subsets, ζ or IFN-γ expression in T cell was performed using Coulter Epics XL. The subsequent data analysis was performed with Expo 32 ADC (Beckman Coulter).
Flow cytometry for tetramers in PBMC, TIL and LNL
For tetramer staining, 15 μl aliquots of diluted tetramer stock (1/100) were directly added to cell pellets (2–5×106 cells). Cells were incubated for 30 min at room temperature in the dark. Next, 5 μl aliquots of the labeled mAbs were added for surface staining, and cells were incubated for 30 min at 4°C in the dark. Cells were washed twice with 2 ml flow buffer and fixed in 300 μl PBS without Ca2+/Mg2+ supplemented with 2% (w/v) paraformaldehyde in PBS and stored at 4°C in the dark. Samples were analyzed within 1 day of staining. Gating strategy consisted of backgating on CD3+CD8+ bright lymphocytes followed by acquisition of tetramer+ cells within this population. All tetramer data were acquired on a BD FACS-Calibur with dual laser capability, using Cell Quest software.
Immunohistochemistry for p53
For detection of p53 expression in the tumor, formalin-fixed, paraffin-embedded tumor tissues were sectioned (3–5 μm), air dried overnight at 37°C, deparaffinized, dehydrated, and stained with a mAb against p53, (DO-7, Dako, Carpinteria, CA, USA), which recognizes an epitope in the N-terminus between amino acid 35 and 45 and reacts with the wt and most mutant forms of p53 protein. The avidin–biotin–peroxidase method was used to visualize p53, according to instructions supplied by the manufacturer (Dako). The immunostained slides were evaluated for p53 accumulation by light microscopy. The tumor was considered p53 positive when >25% of the cells showed staining intensity of 1+ or higher on a scale of 0–4+. IgG isotype mAb, used at the same concentration as the primary mAb, served as negative controls.
Activation of TIL and PBMC
Lymphocytes (1–2×106 ml) were incubated in the presence of anti-CD3 mAb (OKT3, UPCI Pharmacy) at the final concentration of 5 μg/ml in AIM V medium supplemented with 10% (v/v) human serum (Nabi, Miami, FL, USA) in 12-well plates (2–4 ml/well) for 24 h at 37°C. Monensin (2 μM; Sigma) was added to each well for the last 4 h of culture. The cells were harvested, stained for surface markers using relevant mAbs washed, permeabilized, as described previously [21], and stained for intracellular expression IFN-γ. Flow cytometry was performed as described above.
Statistical analysis
Differences between TIL and PBMC obtained from the same patients were evaluated using paired Student’s t test. The P values <0.05 were considered significant.
Results
Specificity of tetramer binding to CD8+ T cells
Specificity of tetramer binding was determined by: (a) positive staining of peptide-specific vs. no staining of irrelevant T-cell lines, (b) double staining of a T-cell line with two differently labeled tetramers (a tetramer mix) to show that tetramer+ T cells are single-stained and not double-stained, and (c) a lack of staining of CD8+ T cells from HLA-A2 negative patients and healthy donors [7]. In addition, all p53-specific tetramers were pretitered using T-cell lines or T-cell clones specific for wt p53 peptides to determine optimal staining dilutions and distinguish positive from negative signals [9].
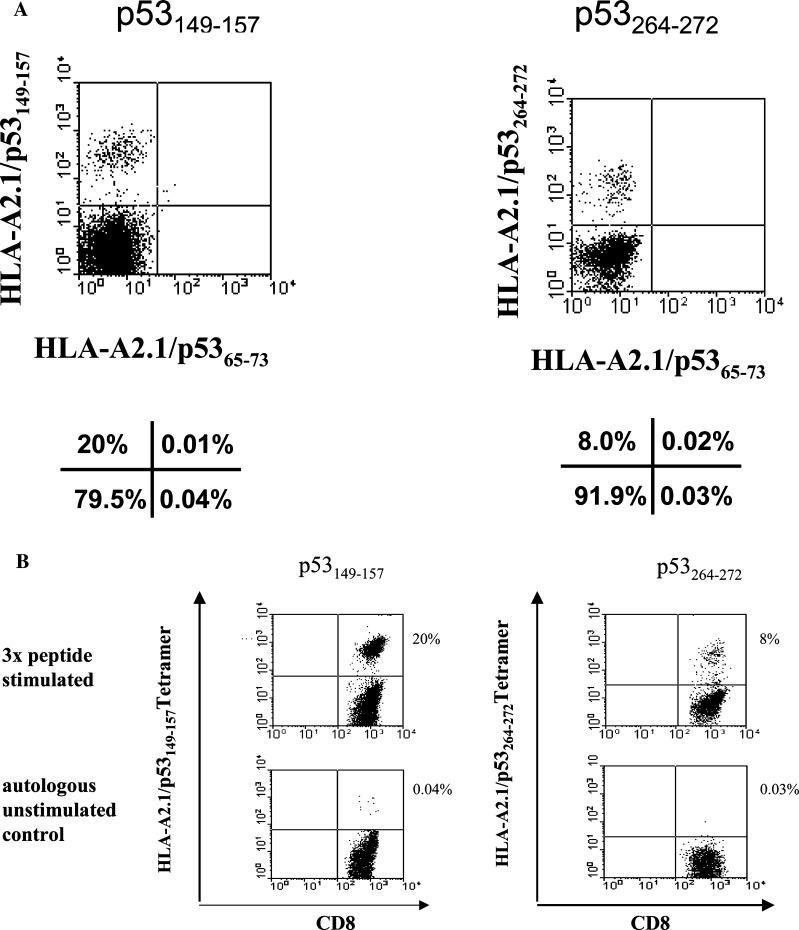
Binding of p53-specific tetramers to wt p53 epitope-specific T-cell lines was tested using T-cell lines generated by in vitro sensitization (IVS) of PBMC, obtained from HLA-A2.1+ patients with SCCHN with wt p53 epitopes pulsed on DC as previously described [7]. Figure 1a shows that these T-cell lines contained CD8+ T cells which were reactive with the relevant tetramers. The generated T-cell lines were then used to test the specificity of tetramers. As shown in Fig. 1b, a T-cell line generated by IVS in response to wt p53149-157 epitope only bound the relevant tetramer and not the irrelevant wt p5365-73 peptide-specific tetramer. Similarly, a T-cell line generated by IVS in response to wt p53264-272 epitope only bound the relevant tetramer and not the irrelevant wt p5365-73 tetramer. In these preliminary experiments, the available tetramer reagents were demonstrated to be sufficiently specific for testing of T lymphocytes obtained from patients with HNC.
Fig. 1.
Specificity of tetramer binding to T cells expressing TCR for wt p53 epitopes. In a, T-cell lines were generated using wt p53 peptides presented on DC obtained from PBMC of HLA-A2+ patients with SCCHN. These T-cell lines were simultaneously stained with two different p53 tetramers and were shown to react only with the tetramers specific for the wt p53 peptides used for priming. In b, the generated wt p53 peptide-specific CTL lines were stained with the relevant and irrelevant (e.g., wt p5365-73) tetramers. Only the relevant wt p53 peptide tetramers bound to CTL lines generated in cultures with wt p53149-157 or wt p53264-272 peptides. An irrelevant tetramer (wt p5365-73) did not bind to these CTL. The percentages of positive cells are indicated below or next to every dot plot
Frequency of tetramer+ T cells in TIL versus PBMC
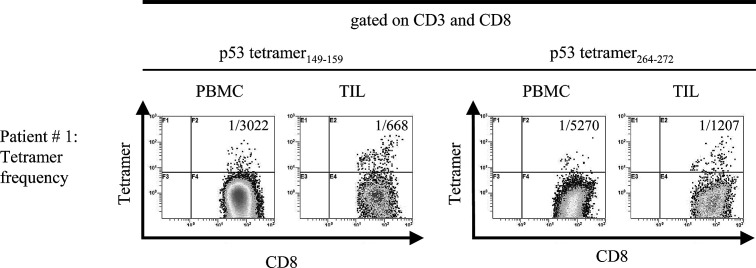
Using the validated tetramers, we determined the frequencies of CD3+CD8+ T cells specific for wt p53264-272 peptide or wt p53149-157 peptide in patients‘ PBMC as well as TIL by multicolor flow cytometry (Table 2). It is apparent that these frequencies are significantly higher in TIL than autologous PBMC in every case (Table 2). The PBMC frequency of T cells recognizing wt p53264-272 epitope is lower than that of T cells specific for wt p53149-157 epitope. In fact, taking the frequency value of 1/7,600 as a LLD for the wt p53 tetramer-based assays, as previously determined [8], only 6/10 (~50%) patients tested had detectable wt p53264-272 tetramer+CD8+ T cells in the peripheral circulation. In contrast, in 9/10 patients frequency values for circulating T cells specific for wt p53149-157 epitope were high (i.e., lower than 1/7,600). The data in Table 2 indicate that the frequency values for wt p53-peptide-specific CD8+ cells do not appear to be related to p53 overexpression in the tumor, as determined by immunohistochemistry. Overall, the frequency values for TIL with specificity for these epitopes are high, ranging from 1/87 to 1/2,175, an indication that the epitope-specific T lymphocytes accumulate at the tumor site. Consistent with the frequency values for PBMC, more CD8+ T cells specific for wt p53149-157 epitope than for wt p53264-272 epitope are present at the tumor site. Figure 2 presents representative flow cytometry data, illustrating a clear-cut enrichment in wt p53 epitope-specific CD8+ T cells among TIL as compared to autologous PBMC.
Table 2.
Reciprocal frequency values for tetramer+CD3+CD8+ T cells in tumor infiltrating lymphocytes (TIL) and autologous peripheral blood mononuclear cells (PBMC) in patients with SCCHN relative to p53 expression in the tumor
| Patient no. | CD3+CD8+ tetramer264-272+ | CD3+CD8+ tetramer149-157+ | p53 accumulation in the tumora | ||
|---|---|---|---|---|---|
| PBMC | TILb | PBMC | TILb | ||
| #1 | 5,270 | 1,207 | 3,022 | 668 | + |
| #2 | 10,000 | 1,503 | 10,000 | 776 | + |
| #3 | 4,460 | 2,175 | 2,863 | 87 | NA |
| #4 | 10,000 | 2,015 | 5,637 | NA | − |
| #6 | 8,963 | 240 | 3,211 | 456 | + |
| #7 | 8,129 | 1,660 | 2,521 | 1,355 | NA |
| #8 | 2,626 | 1,881 | 3,368 | 351 | + |
| #9 | 6,369 | 817 | 2,687 | 620 | + |
| #10 | 1,822 | 1,056 | 2,923 | 1,426 | − |
| #11 | NA | 1,077 | NA | 2,569 | NA |
| #12 | NA | 2,839 | NA | 1,349 | NA |
| #13 | NA | 5,105 | NA | 2,440 | NA |
| Mean | 6,405 | 1,774 | 4,026 | 1,100 | |
| ±SD | 3,075 | 1,198 | 2,424 | 819 | |
Paired TIL and PBMC were obtained from patients as described in Materials and methods
NA not available
aDetermined by immunocytochemistry, using the DO-7 Ab
bThe differences in the frequency of tetramer+ cells in PBMC versus TIL are significant at P<0.001 for wt p53264-272 tetramer and for wt p53149-157 tetramer
Fig. 2.
The log frequencies of CD3+CD8+tetramer+ cells in PBMC and TIL of an HLA-A2+ patient with SCCHN (#1). The frequencies of wt p53149-157 tetramer+ T cells as well as p53264-272 tetramer+ T cells are significantly higher in TIL than in PBMC (P<0.001) for both
Frequency of tetramer+ T cells in tumor-non-involved LN
In three patients with SCCHN, tumor-involved LN were available for analysis in addition to PBMC and TIL. The data in Table 3 show that no enrichment in wt p53 epitope-specific CD8+ T cells was evident in these LN. In fact, the frequency values for p53 tetramer+ T cells in the tumor-non-involved LN were comparable to those obtained with autologous PBMC (Table 2). The percentage of CD4+CD25+ in the tumor-non-involved LN was also similar to that in PBMC.
Table 3.
Reciprocal frequency values for tetramer+CD3+CD8+ T cells and percentages of CD4+CD25+ T cells in tumor-non-involved lymph nodes relative to autologous peripheral blood lymphocytes (PBMC) and tumor-infiltrating lymphocytes (TIL) in patients with SCCHN
| CD3+CD8+ | CD4+CD25+ | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Patient no. | wt p53264-272 tetramer+ | wt p53149-157 tetramer+ | |||||||
| Reciprocal frequency | Percentage of positive | ||||||||
| PBMC | TIL | Tumor-non-involved LN | PBMC | TIL | Tumor-non-involved LN | PBMC | TIL | Tumor-non-involved LN | |
| #1 | 5,270 | 1,207 | 5,021 | 3,022 | 668 | 6,042 | 9 | 65 | 9 |
| #12 | NA | 2,839 | 8,686 | NA | 1,349 | 7,166 | NA | 48 | 11 |
| #13 | NA | 5,105 | 10,035 | NA | 2,440 | 6,429 | NA | 34 | 12 |
| Mean | 3,050 | 7,914 | 1,486 | 6,546 | 49 | 11 | |||
| ±SD | 1,958 | 2,595 | 894 | 571 | 16 | 1.5 | |||
The frequency of wt p53 peptide-specific tetramer+CD8+ T cells and the percentages of CD4+CD25+ T cells were determined by flow cytometry, as described in Materials and methods
Percentages of CD4+CD25+ T lymphocytes in TIL versus PBMC
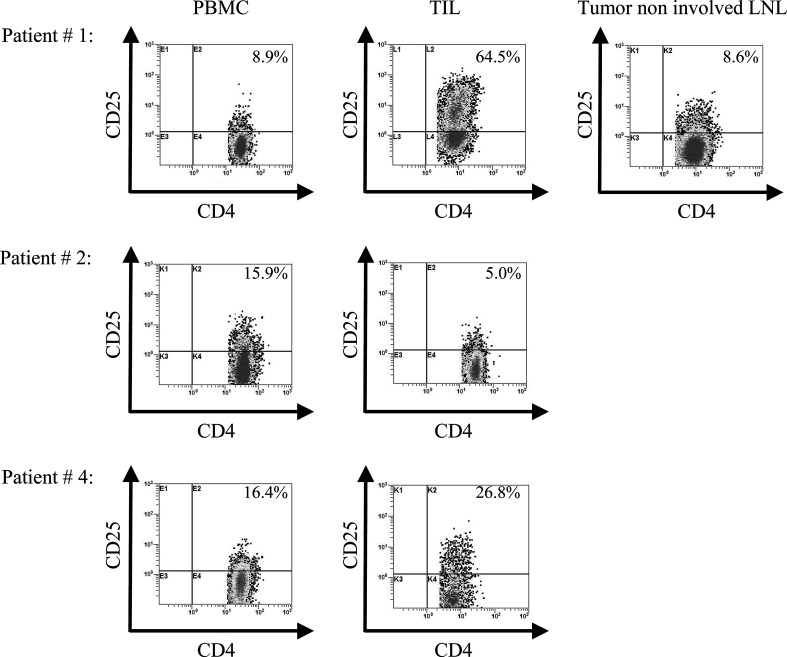
The subset of CD4+CD25+ cells, which might contain Treg, was of particular interest [2]. We first determined that the percentages of CD4+CD25+ T cells were significantly higher in TIL (P<0.003) than in autologous PBMC (Table 4). In some patients, e.g., #1 or#10, this difference was impressively greater. Figure 3 illustrates enrichment of these cells in TIL vs. PBMC in three representative patients with SCCHN. The data also demonstrate that although TIL were enriched in CD4+CD25+ T cells and tetramer+CD8+ T cells relative to PBMC, there was no correlation between the frequency of these cell subsets at the tumor site.
Table 4.
Percentages of CD4+CD25+ T cells present in paired PBMC and TIL obtained from patients with SCCHN
| Patient no. | PBMC | TIL |
|---|---|---|
| Percentage of CD4+CD25+ | ||
| #1 | 9 | 65 |
| #2 | 16 | 5 |
| #3 | 10 | 12 |
| #4 | 16 | 27 |
| #5 | NA | 19 |
| #6 | 5 | 20 |
| #7 | 8 | 25 |
| #8 | NA | 15 |
| #9 | 4 | 26 |
| #10 | 11 | 47 |
| #11 | NA | 26 |
| #12 | NA | 48 |
| #13 | NA | 34 |
| #14 | 14 | 42 |
| #15 | 15 | 51 |
| #16 | NA | 5 |
| #17 | NA | 44 |
| Mean | 11 | 30a |
| ±SD | 4.4 | 17.2 |
NA not available
aThe difference in the percentage of CD4+CD25+ Treg between PBMC and TIL is significant at P<0.003
Fig. 3.
Increased percentages of CD4+CD25+ T cells in PBMC, TIL and one tumor non-involved LN in representative patients with SCCHN. The gate was set on CD3+CD4+ cells. Patient #1 shows the highest increase in CD4+CD25+ T cells in TIL among the patients studied. The tumor-non-involved LN has the same percentage of CD4+CD25+ T cells as PBMC. Patient #2 is the only one with a decreased percent of CD4+CD25+ cells in TIL relative to PBMC
Treg among CD4+CD25+ T lymphocytes
The subset of CD4+CD25+ T cells could include activated helper T cells as well as Tregs. Tregs are thought to be responsible for downregulation of immune responses to autoantigens [24]. As wt p53 epitopes can be considered “self” as well as tumor-specific peptides, we looked for the presence of Treg in the peripheral circulation and among TIL of patients with SCCHN by multicolor flow cytometry, using antibodies to FOXp3, GITR and CTLA-4 [2, 14, 19, 27]. These markers have been used to distinguish activated CD4+ T cells from Treg [2, 27]. The data in Table 5 indicate that CD4+CD25+ TIL were highly enriched in FOXp3+, CTLA-4 as well as GITR+ T cells relative to normal or patients’ PBMC. Among TIL nearly all CD4+CD25+ T cells expressed FOXp3 surface marker and had intracytoplasmic GITR and CTLA-4+. In contrast, only a proportion of CD4+CD25+ T cells in the peripheral circulation of patients or normal donors contained Treg, as defined by these markers, while the majority were non-Treg CD4+CD25+ (i.e., activated) T cells.
Table 5.
Percentages of CD4+CD25+ and Treg populations in the peripheral blood and TIL of patients with SCCHN
| CD4+CD25+ | CD4+CD25+GITR+ | CD4+CD25+CTLA4+ | CD4+CD25+FOXp3+ | |
|---|---|---|---|---|
| Percentage (mean ± SD) | ||||
| Pt (n=5) | 7.2 ± 1.4 | 1.3 ± 0.6 | 1.5 ± 0.5 | 3.1 ± 2.0 |
| TIL #18 | 13.0 | 12.0 | 12.0 | 13.0 |
| TIL #19 | 7.6 | 5.3 | 5.6 | 7.0 |
| NC (n=4) | 10.0 ± 3.0 | 1.8 ± 1.0 | 2.0 ± 0.9 | 3.7 ± 0.9 |
Percentages of positive cells in peripheral blood mononuclear cells (PBMC) and TIL (not matched) obtained from patients with SCCHN and in PBMC of normal controls (NC) were determined by multicolor flow cytometry. Surface staining for CD4+CD25+ T cells and intracytoplasmic staining for GITR, CTLA-4 and FOXp3 were performed as described in Materials and methods.
Functional properties of TIL in SCCHN
Since TIL were shown to be enriched in CD4+CD25+FOXp3+ Treg relative to PBMC, we expected that TIL would be unresponsive to activating stimuli, and that tetramer+ tumor-specific CD8+ T cells at the tumor site would be functionally compromised. To test this hypothesis, TIL obtained from two patients (#16 and #17) with SCCHN and PBMC from patient #16 were tested for expression of TCR ζ chain in the CD3+ CD8+ T population. As shown in Table 6, both CD8+ and CD4+ TIL showed very low expression of the ζ chain, whether based on the percent values or MSFE units measurements. In contrast, CD8+ and CD4+ T cells obtained from PBMC of normal donors had uniformly high ζ chain expression (Table 6). These results suggest that the CD3+CD8+ population of effector cells among TIL is unable to signal via TCR-ζ, which is downregulated in these cells.
Table 6.
Expression of the TCR-ζ chain in SCCHN-derived T cells
| CD8+Ζ+ | CD4+Ζ+ | |
|---|---|---|
| TIL #16 | 1.3 (8,936) | 1.9 (14,540) |
| PBL #16 | 4.6 (45,205) | 4.7 (46,470) |
| TIL #17 | 1.8 (13,566) | 2.6 (21,743) |
| N PBL 1 | 92 (83,688) | 96 (85,373) |
| N PBL 2 | 98 (87,900) | 98 (76,951) |
| N PBL 3 | 97 (85,373) | 95 (90,429) |
The data are percentages of positive cells determined by flow cytometry as described in Materials and methods. The data in parentheses are the MESF units of fluorescence determined in each specimen by using the calibration beads. The cells were tested without any exogenous stimulation. Three PBL obtained from normal donors were tested in the same assays as TIL or PBL obtained from patients with SCCHN
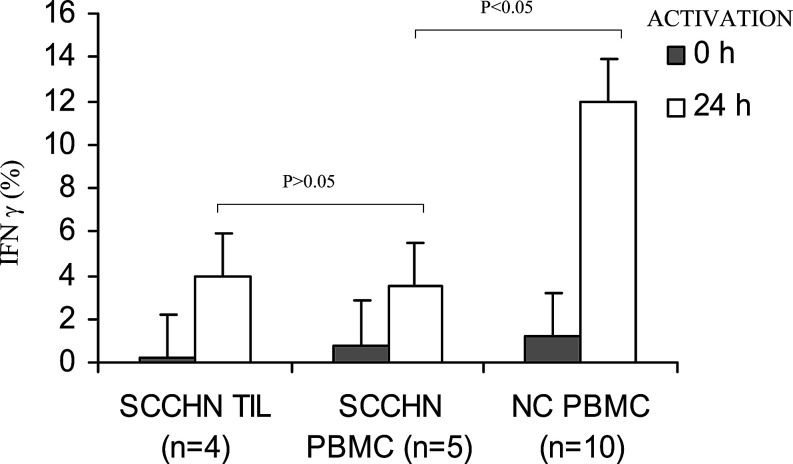
In addition, after 24 h in vitro activation of TIL with anti-CD3 Ab, IFN-γ expression was induced in only a small proportion of CD3+CD8+ TIL. Activation of PBMC obtained from five patients with SCCHN was also accompanied by IFN-γ expression in only a small percent of CD3+CD8+ T cells tested under the same conditions. In contrast, a significantly higher percentage (mean 12% ±7; P<0.05) of CD3+CD8+ T cells obtained from PBMC of normal donors expressed IFN-γ when activated with anti-CD3 mAb under the same experimental conditions. Overall, the data are consistent with decreased responsiveness of TIL and PBMC, and especially the CD8+ T-cell subset, in patients with SCCHN to the ex vivo activation, as previously reported [21, 28] (Fig. 4).
Fig. 4.
Low percentages of IFN-γ expressing CD3+CD8+ T cells in TIL or PBMC of patients with SCCHN compared to PBMC of normal donors. The cells were tested for IFN-γ expression by flow cytometry following 24 h activation with anti-CD3 Ab as described in Materials and methods. The gate was set on CD3+CD8+ T cells. The data are means ± SD
Discussion
The presence and frequency of wt p53 epitope-specific T lymphocytes was previously determined in the peripheral circulation of HLA-A2+ patients with SCCHN [8] and other malignancies [4]. However, it is not known whether these T cells are also present at tumor sites. We report here for the first time that TIL obtained from patients with HNC are significantly enriched (P<0.003) in wt p53 epitope-specific T cells, as compared to autologous PBMC. The frequency of wt p53149-157 epitope-specific T cells was higher than that of wt p53264-272 eiptope-specific lymphocytes, possibly because the p53149-157 epitope is more immunogenic than other wt p53 epitopes, it is also one of the most frequently mutated in cancer [5, 20]. These results demonstrate preferential localization to the tumor site or tumor-involved LN of wt p53 epitope-specific CD3+CD8+ effector T lymphocytes. In contrast, tumor-non-involved LN had a lower frequency of these epitope-specific CD3+CD8+ T cells, which was comparable to that found in PBMC. The observed localization of tetramer+ tumor-specific effector cells to the tumor or tumor-involved LN implies that these cells are mobilized to accumulate in situ and to exercise antitumor functions. However, numerous previous reports have indicated that T lymphocytes at tumor sites, including SCCHN, are dysfunctional and thus fail to perform expected antitumor functions [17, 21, 29, 30]. In the case of patients with SCCHN, the presence and frequency of wt p53 epitope-specific effector T cells among TIL did not correlate with tumor stage. This implies that the frequency of tetramer+CD8+ effector cells in situ has no effect on tumor progression. It appears that the tumor is successful in escaping from these tumor-specific effector T cells. Indeed, in two patients who had sufficient numbers of TIL available to test in vitro responsiveness of TIL to anti-CD3 mAb, we found that CD3+CD8+ T cells were poorly responsive as measured by IFN-γ expression. While this conclusion is based on a limited data set, our other studies support the concept of tumor escape associated with and perhaps caused by a diminished functional potential of TIL [21].
Although various mechanisms have been proposed to account for tumor escape, recent literature reports emphasize the presence at relatively high proportions of Treg in patients with cancer [2, 16, 31]. Treg were previously phenotypically defined as CD4+CD25high cells, although newer markers are currently available that appear to be more specific, including FOXp3 or GITR and CTLA-4 [2, 19, 22]. The importance of these markers is that activated CD4+CD25+ T cells can now be distinguished from true Treg. These cells have been shown to play an important role in maintaining peripheral tolerance in murine models by controlling potentially harmful autoreactive T cells [24]. Also, these cells, acting as suppressors of functions of both CD4+CD25− and CD8+ T cells, upon cell contact, have been described to be present in the peripheral circulation [23], LN [18] as well as tumors [2, 26, 32] of patients with various malignancies. It has been suggested that the presence or accumulation of Treg, which are able to produce IL-10 and inhibit T-cell responses in vivo may be responsible in part, for immune suppression was evident in patients with cancer [16, 31]. It was therefore, not surprising to observe that the percentage of CD4+CD25+ T cells was significantly, and in some cases dramatically, increased in TIL as compared to autologous PBMC, in patients with SCCHN. Further, phenotyping for Treg markers, FOXp3, GITR and CTLA-4, confirmed their enrichment in TIL. We suspect that accumulations of Treg in the tumor could be in part responsible for the observed dysfunction of the infiltrating effector cells, although we cannot rule out the possibility that the tumor-derived factors contribute as well [19]. While we did not recover sufficient numbers of T cells for classical mixing experiments for testing suppressor functions of Treg in TIL [13], we were able to show by flow cytometry that expression of the ζ chain, a signaling molecule associated with the T-cell receptor (TCR) was substantially decreased in representative CD3+CD8+ TIL isolated from SCCHN. Further, as indicated above, this TIL population was poorly responsive to anti-CD3 Ab as measured by intracellular IFN-γ expression following activation.
In conclusion, our results show that the wt p53 peptide-specific effector T cells are not only present in the circulation but also preferentially localize to tumor sites in patients with SCCHN. However, the simultaneous presence in the tumor and tumor-involved LN of an excess of Treg probably contributes in part, to poor responsiveness of these tumor-specific CD8+ T lymphocytes in situ. It is likely that the tumor or tumor-derived factors also play a role in inducing dysfunction of effector T cells in situ [19, 25, 26]. Other data from our laboratory confirm depressed functionality or spontaneous apoptosis of CD8+ T lymphocytes in patients with malignancies, including SCCHN [10, 21, 28]. These phenomena appear to be related to effects directly or indirectly mediated by the tumor, and they contribute to tumor escape from the host immune system. In addition to various other mechanisms known to facilitate tumor escape [reviewed in 29], Treg are likely to mediate suppressive effects directed at self-reactive T cells [24]. This immunosuppressive mechanism may be particularly relevant to wt p53 epitope-specific T lymphocytes, which recognize “self” peptides and, thus, are likely to be tolerized, especially at the sites of their accumulation in tumor tissues.
Acknowledgements
This work has been supported in part by the NIH grants PO1-DE12321 (TLW), P60-DE13059 and The Stout Family Fund for Head and Neck Cancer Research at the Eye and Ear Foundation of Pittsburgh.
References
- 1.Chikamatsu K, Nakano K, Storkus WJ, Appell E, Lotze MT, Whiteside TL, DeLeo AB. Generation of anti-p53 cytotoxic T lymphocytes from human peripheral blood using autologous dendritic cells. Clin Cancer Res. 1999;5:1281. [PubMed] [Google Scholar]
- 2.Curiel TJ, Coukos G, Zou L, Alvarez X, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 3.Elder EM, Whiteside TL (1992) Processing of tumors for vaccine and/or tumor infiltrating lymphocytes. In: Friedman H, Rose NR, deMacario EC, Fahey JL, Friedman H, Penn GM (Eds) Manual of clinical laboratory immunology, vol 123, 4th edn. American Society of Microbiology, Washington, p 817
- 4.Gnjatic S, Cai Z, Viguier M, Chouaib S, Guillet J-G, Choppin J. Accumulation of the p53 protein allows recognition by human CTL of a wild-type p53 epitope presented by breast carcinomas and melanomas. J Immunol. 1998;160:328. [PubMed] [Google Scholar]
- 5.Harris CC. Structure and function of the p53 tumor suppressor gene: clues and rational cancer therapeutic strategies. J Natl Cancer Inst. 1996;88:1442. doi: 10.1093/jnci/88.20.1442. [DOI] [PubMed] [Google Scholar]
- 6.Herrin V, Behrens RJ, Achtar M, Monahan B, Bernstein S, Brent-Steele T, Whiteside T, Wieckowski E, Berzofsky J, Khleif SN. Wild-type p53 peptide vaccine can generate a specific immune response in low burden ovarian adenocarcinoma. Abstr 678 Proc Am Soc Clin Oncol. 2003;22:169. [Google Scholar]
- 7.Hoffmann TK, Nakano K, Elder E, Dworacki G, Finkelstein SD, Apella E, Whiteside TL, DeLeo AB. Generation of T cells specific for the wild-type sequence p53264-272 peptide in cancer patients—implications for immunoselection of epitope-loss variants. J Immunol. 2000;165:5938. doi: 10.4049/jimmunol.165.10.5938. [DOI] [PubMed] [Google Scholar]
- 8.Hoffman TK, Donnenberg AD, Finkelstein SD, Donnenberg VS, Friebe-Hoffmann F, Myers EN, Appella E, DeLeo AB, Whiteside TL. Frequencies of tetramer+ T cells specific for the wild-type sequence p53264-272 peptide in the circulations of patients with head and neck cancer. Cancer Res. 2002;62:3521. [PubMed] [Google Scholar]
- 9.Hoffmann TK, Donnenberg V, Friebe-Hoffmann U, Meyer M, Rinaldo CR, DeLeo AB, Whiteside TL, Donnenberg AD. Competition of peptide-MHC class I tetrameric complexes with anti-CD3 provides evidence for specificity of peptide binding to the TCR complex. Cytometry. 2000;41:321. doi: 10.1002/1097-0320(20001201)41:4<321::AID-CYTO11>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 10.Hoffmann TK, Dworacki G, Meidenbauer N, Gooding W, Johnson JT, Whiteside TL. Spontaneous apoptosis of circulating T lymphocytes in patients with head and neck cancer and its clinical importance. Clin Cancer Res. 2002;8:2553. [PubMed] [Google Scholar]
- 11.Hollstein M, Sidransky D, Vogelstein B, Harris CC. p53 mutations in human cancers. Science. 1991;253:49. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- 12.Hollstein M, Shomer B, Greenblatt M, Soussi T, Hovig E, Montesano R, Harris CC. Somatic point mutations in the p53 gene of human tumors and cell lines: updated compilation. Nucleic Acids Res. 1996;24:141. doi: 10.1093/nar/24.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jonuleit H, Schmitt E, Stassen M, Tuettenberg A, Knop J, Enk AH. Identification and functional characterization of human CD4+CD25+ T cells with regulatory properties isolated from peripheral blood. J Exp Med. 2001;193:1285. doi: 10.1084/jem.193.11.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee KM, Chuang E, Griffin M, et al. Molecular basis of T cell inactivation by CTLA-4. Science. 1998;282:2263. doi: 10.1126/science.282.5397.2263. [DOI] [PubMed] [Google Scholar]
- 15.Letessier E, Sacchi M, Johnson JT, Herberman RB, Whiteside TL. The absence of lymphoid suppressor cells in tumor-involved lymph nodes of patients with head and neck cancer. Cell Immunol. 1990;130:446. doi: 10.1016/0008-8749(90)90286-Z. [DOI] [PubMed] [Google Scholar]
- 16.Liyanage UK, Moore TT, Joo HG, Tanaka Y, Herrmann V, Doherty G, Drebin JA, Strasberg SM, Eberlein TJ, Goedegebuure PS, Linehan DC. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol. 2002;169:2756. doi: 10.4049/jimmunol.169.5.2756. [DOI] [PubMed] [Google Scholar]
- 17.Marincola FM, Jaffee EM, Hicklin DJ, Ferrone S. Escape of human solid tumors from T-cell recognition: molecular mechanisms and functional significance. Adv Immunol. 2000;74:181. doi: 10.1016/s0065-2776(08)60911-6. [DOI] [PubMed] [Google Scholar]
- 18.Ng WF, Duggan PJ, Ponchel F, Matarese G, Lombardi G, Edwards AD, Isaacs JD, Lechler RI. Human CD4+CD25+ cells: a naturally occurring population of regulatory T cells. Blood. 2001;98:2736. doi: 10.1182/blood.V98.9.2736. [DOI] [PubMed] [Google Scholar]
- 19.Ramsdell F. Foxp3 and natural regulatory T cells: key to a cell lineage. Immunity. 2003;19:165. doi: 10.1016/S1074-7613(03)00207-3. [DOI] [PubMed] [Google Scholar]
- 20.Raybaud-Diogene H, Tetu B, Morency R, Fortin A, Monteil R. p53 overexpression in head and neck squamous cell carcinoma: review of the literature. Eur J Cancer B Oral Oncol. 1996;32:143. doi: 10.1016/0964-1955(95)00095-X. [DOI] [PubMed] [Google Scholar]
- 21.Reichert TE, Strauss L, Wagner EM, Gooding W, Whiteside TL. Signaling abnormalities and reduced proliferation of circulating and tumor-infiltrating lymphocytes in patients with oral carcinoma. Clin Cancer Res. 2002;8:3137. [PubMed] [Google Scholar]
- 22.Ronchetti S, Zollo O, Bruscoll S, Agostini M, Bianchini R, Nocentini G, Ayroldi E, Riccardi C. GITR, a member of the TNF receptor superfamily, is costimulatory to mouse T lymphocyte subpopulations. Eur J Immunol. 2004;34:613. doi: 10.1002/eji.200324804. [DOI] [PubMed] [Google Scholar]
- 23.Schaefer Br J Cancer. 2005;92:913. doi: 10.1038/sj.bjc.6602407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shimizu J, Yamazaki S, Sakaguchi S. Induction of tumor immunity by removing CD25+CD4+ T cells: a common basis between tumor immunity and autoimmunity. J Immunol. 1999;163:5211. [PubMed] [Google Scholar]
- 25.Soussi T. The humoral response to the tumor-suppressor gene-product p53 in human cancer: implications for the diagnosis and therapy. Immunol Today. 1996;17:354. doi: 10.1016/0167-5699(96)30019-4. [DOI] [PubMed] [Google Scholar]
- 26.Shevach EM. Fatal attraction: tumors becon regulatory T cells. Nat Med. 2004;10:900. doi: 10.1038/nm0904-900. [DOI] [PubMed] [Google Scholar]
- 27.Stephens GL, McHugh RS, Whitters MJ, Young DA, et al. Engagement of glucocorticoid-induced TNFR family-related receptor on effector T cells by its ligand mediates resistance to suppression by CD4+CD25+ T cells. J Immunol. 2004;173:5008. doi: 10.4049/jimmunol.173.8.5008. [DOI] [PubMed] [Google Scholar]
- 28.Tsukishiro T, Donnenberg AD, Whiteside TL. Rapid turnover of the CD8+CD28- T-cell subset of effector cells in the circulation of patients with head and neck cancer. Cancer Immunol Immunother. 2003;52:599. doi: 10.1007/s00262-003-0395-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Whiteside TL. Tumor-induced death of immune cells: its mechanisms and consequences. Sem Cancer Biol. 2002;12:43. doi: 10.1006/scbi.2001.0402. [DOI] [PubMed] [Google Scholar]
- 30.Whiteside TL, Rabinowich H. The role of Fas/FasL in immunosuppression induced by human tumors. Cancer Immunol Immunother. 1998;46:175. doi: 10.1007/s002620050476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wolf AM, Wolf D, Steurer M, Gasti G, Gunsilius E, Grubeck-loebenstein B. Increase in regulatory T cells in the peripheral blood of cancer patients. Clin Cancer Res. 2003;9:606. [PubMed] [Google Scholar]
- 32.Woo EY, Chu CS, Goletz TJ, Schlienger K, Yeh H, Coukos G, Rubin SC, Kaiser LR, June CH. Regulatory CD4+CD25+ T cells in tumors from patients with early-stage non-small cell lung cancer and late-stage ovarian cancer. Cancer Res. 2001;61:4766. [PubMed] [Google Scholar]