Abstract
In this study, a human melanoma vaccine induced antibody responses in mice that varied significantly from animal to animal. BALB/c mice were immunized to a xenogenic human polyvalent melanoma vaccine that has been used in phase II clinical trials in over 600 patients. Mice were bled biweekly for up to 6 weeks to measure antibody responses. IgG antibody responses to the melanoma vaccine components were detectable within 2 weeks but were much stronger at 4 and 6 weeks. When the pooled sera were further analyzed by Western blot, a complex pattern of antigens was detected. When individual sera from identically immunized mice were assayed by Western blot, a consistent, reproducible pattern of antigen recognition was not seen. Rather, we found significantly different antibody responses among the mice. Both the intensity of antibody responses and the pattern of antigens recognized varied from animal to animal. Although there appeared to be immunodominant antigens that produced antibody responses in most mice, no single antigen induced antibody responses in all mice. These results demonstrate that polyvalent vaccines induce heterogeneous antibody responses in mice treated identically. Analysis of the response of selected melanoma patients immunized to the same vaccine revealed similar antibody responses to the antigens in the melanoma vaccine. Heterogeneity may hamper interpretation of vaccine immunogenicity and relevant tumor antigens in humans.
Keywords: Heterogeneous antibody response, Murine model, Polyvalent cancer vaccines
Introduction
There is a strong interest in developing vaccines for melanoma, as current therapies are of limited effectiveness and can be associated with serious adverse effects. This appears feasible, as vaccines can markedly augment resistance to this type of cancer in animals, and some have led to improved clinical outcome in randomized trials in humans [1–4, 6, 8–12, 14–17].
The strategies used to produce melanoma vaccines may be shifting toward construction of polyvalent vaccines that contain multiple melanoma-associated antigens, as such vaccines are more likely to contain clinically relevant antigens [1, 4, 15]. Polyvalent vaccines may be more likely to stimulate tumor-protective immune responses and are more likely to circumvent the antigenic heterogeneity of tumors [1, 9]. Polyvalent vaccines do have limitations including the dilution of relevant antigen concentrations by irrelevant components and the potential for inducing autoimmune responses [5]. Furthermore, the stimulation of immune responses against multiple targets on tumor cells is more likely to kill these cells than responses directed against only a single antigen target. Additionally, little is known about the characteristics of the immune responses induced by cancer vaccines that contain multiple antigens and in particular whether the responses are invariably directed to a limited number of immunodominant antigens.
To address this question, we analyzed the antibody responses to individual antigens in mice immunized to a polyvalent melanoma vaccine that contains multiple antigens [1, 2, 8–12]. Qualitative measurements of IgG responses were performed by Western blot analysis. The results have important implications for the design, construction, and analysis of potency of polyvalent cancer vaccines as well as the interpretation of immune responses in patients treated with these vaccines.
Materials and methods
Mice
All animal studies were approved by the Institutional Animal Care and Use Committee and conformed to the principles of laboratory animal care. Female BALB/c mice (5–6 weeks old, 15 gm) were obtained from Taconic Labs (Germantown, NY, USA) and maintained in the Berg animal facility of the NYU Medical Center. Mice were allowed to recover from transit and acclimated for 1 week prior to use. Mice were housed in cages with a maximum of five per cage and individuals identified by ear punch patterns.
Melanoma patient sera
All sera were collected from melanoma patients participating in clinical trials of the melanoma vaccine over several years at the NYU Medical Center. All clinical trials were approved by the NYU Institutional Review Board, and patients provided informed consent. Sera were stored at −70°C until analyzed.
Polyvalent vaccine
The model polyvalent vaccine used for these studies was a partially purified, melanoma vaccine prepared from the material shed into culture medium by three lines of human melanoma cells adapted to long-term growth in serum-free medium as previously described [1]. The vaccine contains multiple proteins ranging in size from <25 to >300 kDa that can stimulate antibody responses in humans [1].
Immunization
Mice were immunized weekly ×4 by subcutaneous injection into the abdominal region, switching sides weekly to minimize local reactions. The vaccine was admixed with alum as an adjuvant by mixing equal volumes of vaccine and a 1:4 dilution of Alhydrogel 2% (EM Sargeant Pulp and Chemical Company, Clifton, NJ, USA) in sterile saline to provide 20 μg of vaccine and 0.5 mg of alum in a final volume of 0.2 ml per vaccine dose. In some experiments, the same dose of vaccine was administered without alum in a final volume of 0.2 ml of saline. Mice were weighed prior to each immunization to determine overall health. Blood was collected from the retroorbital sinus plexus prior to immunization and at 2, 4, and 6 weeks after immunization had begun. All sera were stored at −80°C until analyzed.
Assay of antibody responses
Western blot analysis was used to detect antibody responses to individual antigens in the vaccine. Briefly, vaccine antigens were separated on 8% SDS-PAGE mini slab gels loaded with 60 μg of vaccine protein/gel, and blotted onto Immobulin-P membranes (Millipore, Bedford, MA, USA) pre-soaked in methanol. The profile of blotted proteins was determined by staining the membrane with fast green [13]. The blots were blocked with 5% milk in PBS, cut into individual strips (approximately 2 mm wide), and incubated at 4°C overnight with mouse serum diluted 1:100 in 5% milk. The strips were washed 6× with 0.05% Tween 20/PBS, then incubated with biotinylated goat antimouse IgG or biotinylated goat antihuman IgG (ICN/Cappel, Costa Mesa, CA, USA) diluted 1:500 in 5% milk/PBS for 1 h at room temperature on a shaker. The strips were washed extensively with 0.05% Tween 20/PBS and then incubated with 1:1,000 diluted avidin/peroxidase conjugate (ExtrAvidin; Sigma, St Louis, MO, USA) for 1 h at room temperature with shaking. Strips were washed extensively then developed with luminol substrate (Western Lightning, Perkin Elmer Life Sciences, Boston, MA, USA). Images were captured with a Kodak 440 Image Station (Eastman Kodak, Rochester, NY, USA). Software provided with the image station was used to measure and compare pixel densities of individual bands in each lane. A mixture of pre-stained molecular weight standard proteins or broad range protein standards (GibcoBRL, Invitrogen, Carlsbad, CA, USA) was run in separate lanes on every gel and blotted. The molecular weight strip was cut, scanned directly into the computer, then aligned with the images from luminol staining. A standard curve of molecular weights vs migration was generated by the software and used to identify molecular weights of antigen bands.
Results
All mice in these experiments were healthy and active throughout the course of immunization and gained weight over the immunization period (data not shown). In addition there were no signs of local toxicity at site of vaccine or vaccine plus alum injections.
Antibody responses to melanoma vaccine immunization
The ability of a polyvalent human melanoma vaccine to induce antibody responses was initially evaluated by immunizing a group of five syngeneic, BALB/c mice weekly ×4 to the vaccine alone or adjuvanted to alum. Blood was collected from each mouse at baseline and 2, 4, and 6 weeks after the first immunization. In this initial study, blood from each mouse was pooled and the resultant sera used to measure immune responses. IgG antibodies responses against the vaccine were measured by Western blot assay as shown in Fig. 1. No antibodies were detected in the preimmune bleed of any group of mice as shown for the pooled sera from both the vaccine-alone group and the vaccine-plus-alum group seen in lanes 2 and 6, respectively. In all of these experiments, no preexisting antibodies to any vaccine component (data not shown) were ever detected. An IgG antibody response against vaccine was evident 2 weeks after the first immunization and became stronger after 4 and 6 weeks. Immunization to vaccine alone induced antibody to a single antigen band at 110 kDa. The response was stronger at 4 and 6 weeks, as seen in the darker bands and measured quantitatively by the pixel densities of the bands (data not shown). When mice were immunized to vaccine with alum the initial response at 2 weeks (Fig. 1, lane 7) was similar with a single antigen band at 110 kDa. However at 4 and 6 weeks, multiple antigen bands were evident (lanes 8 and 9) and the intensities were much greater. The antibodies were directed to several antigens, with the strongest responses being directed to antigens of 110, 102, 95, 80, and 55 kDa, and weaker responses to antigens of 250 and 50 kDa.
Fig. 1.
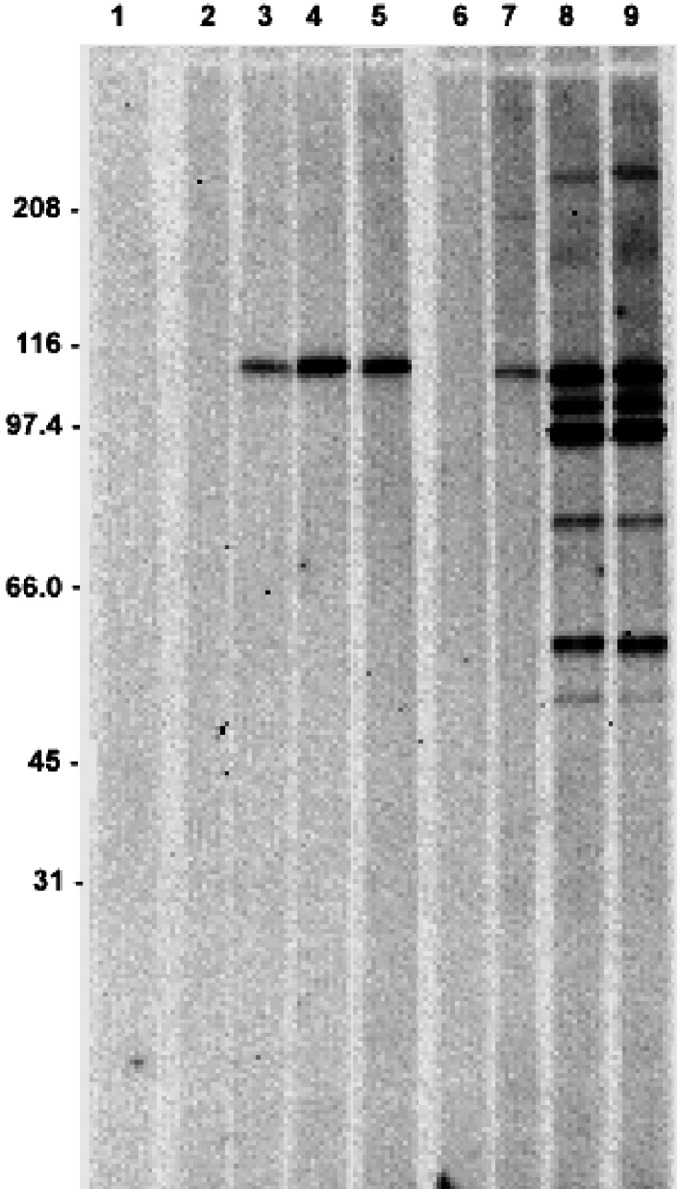
Western blot analysis of vaccine-induced IgG antibody responses at different time points. Five mice were immunized to a polyvalent melanoma vaccine (lanes 2–5) or vaccine admixed with alum (lanes 6–9) weekly ×4. Pooled sera were tested for antibodies against antigens in the vaccine at baseline (lanes 2 and 6) and 2 (lanes 3 and 7), 4 (lanes 4 and 8), and 6 weeks (lanes 5 and 9) after the first immunization. Lane 1 represents a no-serum negative control.
Heterogeneity in the antibody response of individual mice to vaccine immunization
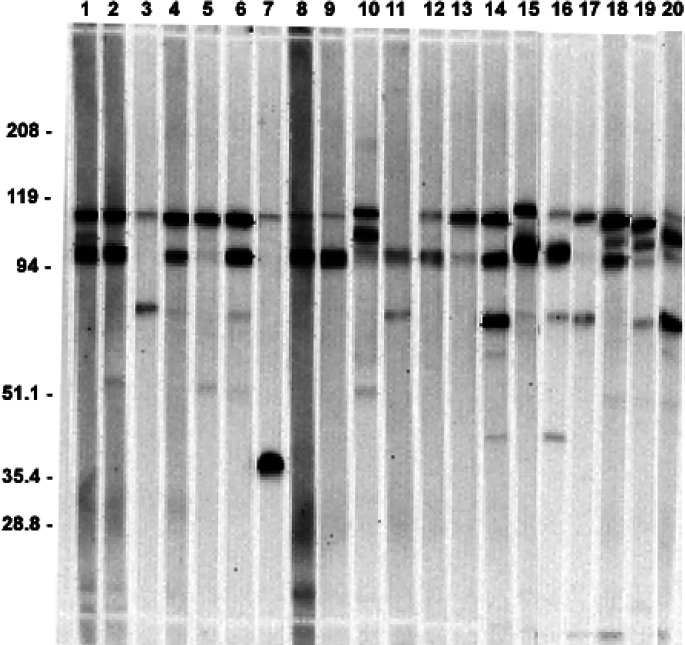
The reproducibility of antibody responses to individual antigens among different mice was evaluated by immunizing identically, and at the same time, a group of 20 mice to the melanoma vaccine adjuvanted to alum. The presence and quality of the antibody responses in individual mice was measured by Western blot of sera collected 6 weeks after immunization was begun. As shown in Fig. 2, all mice developed a detectable antibody response to at least one antigen. In almost all cases, the antibodies were directed to one or more of the antigens initially shown for pooled sera in Fig. 1. There was considerable heterogeneity in the spectrum and in the specificity of the antibody responses among the mice. Some mice developed strong responses to several antigens, while others developed a weak response to only a single antigen. More strikingly, some mice developed a strong antibody responses to one antigen but not to another (strong response of mouse #5 to the 110-kDa antigen but lack of response to the 95-kDa antigen), while the reverse was true for another mouse (see response of mouse #11 to the 95-kDa antigen but lack of response to the 110-kDa antigen). The most immunogenic antigens were those with molecular weights of 110 and 95 kDa. These antigens stimulated strong antibody responses in almost all mice. Of the 20 mice, 19 showed a response to the 110-kDa band and over half of these (11 of the 19) had a very strong response characterized by a dense staining band, while the other mice had weak to moderate responses to this antigen. A total of 15 mice developed antibodies to the 95-kDa band with eight very strong responses. The 80-kDa antigen stimulated strong antibody responses in only two mice and weaker responses in another four mice. One mouse (#7) developed a very strong antibody response to a 38-kDa antigen that no other mice recognized.
Fig. 2.
Western blot assay of sera from 20 mice immunized to vaccine + alum. Bands represent mouse IgG antibodies against human melanoma vaccine using sera collected at week 6.
Vaccine antigens recognized by antibodies from melanoma patients
The IgG response of pooled mouse sera was compared with that of four selected melanoma patients. The patients were selected on the basis of previous analysis showing antibody responses to an antigen with molecular weight of 110 kDa expressed on melanoma cells [9]. All sera were from patients immunized six or seven times to the vaccine used in this study. The data (shown in Fig. 3) indicate a very similar antigen recognition pattern seen in the pooled sera of immunized mice. The pattern of proteins in the vaccine is shown in lane 7 of Fig. 3. The vaccine contains many components that are not immunogenic in either mice or melanoma patients. In fact, visualization of the protein profile by fast green stain does not reveal a detectable protein band corresponding to the dominant 110-kDa antigen.
Fig. 3.
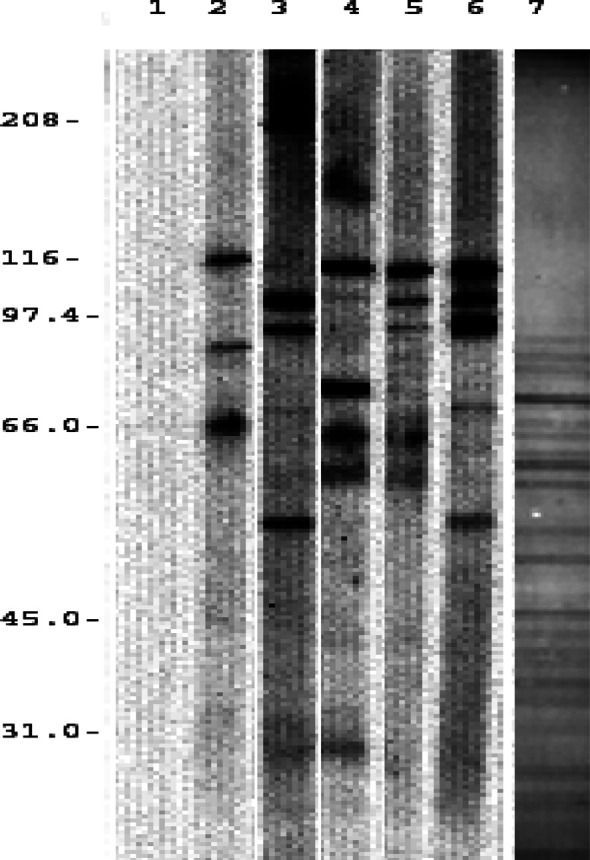
Comparison of antigens recognized by melanoma patients and mice. The Western blot analyses measured IgG responses to vaccine antigens. Lanes 2–5 show IgG antibodies against vaccine from patients immunized seven times with vaccine. Pooled sera from mice immunized to the same vaccine plus alum at week 6 are shown in lane 6. Lane 1 is a PBS control. Lane 7 shows the pattern of proteins on the blot membrane detected by fast green protein stain.
Discussion
The results of this study indicate that there is a striking heterogeneity in the antibody responses of syngeneic mice immunized identically to a mixture of antigens present in a polyvalent human melanoma vaccine. The pattern of antigens recognized by melanoma patients immunized to the vaccine was similar.
Groups of syngeneic mice were immunized identically to a vaccine containing a broad range of antigens. All mice developed an antibody response to at least one of the antigens in the vaccine, but the pattern of antigens recognized by antibodies in different mice was distinctly variable. Some mice developed strong antibody reactions to multiple antigens in the vaccine, others developed strong responses to one or two antigens, while still others developed relatively weak reactions to only some antigens. This heterogeneous response was not due to differences in the immunogenicity of the antigens, as antigens that failed to stimulate an immune response in one mouse could do so in another, while the reverse was true for other antigens. Nor was the heterogeneity due to differences in the immune reactivity of the mice, as some mice that failed to develop an antibody response to a strong immunogen (such as the 110-kDa antigen) did develop strong responses to other antigens (such as the 102-kDa antigen), which in general is a weaker immunogen as evidenced by stimulation of antibody responses in few mice. Nor could the heterogeneity in response be ascribed to genetic variations in the animals, as all mice were syngeneic. Finally, the heterogeneity could not result from variations in the vaccine or immunization procedure because all mice were immunized concurrently to the vaccine. The antibody heterogeneity was seen in mice immunized to vaccine adjuvanted with alum, which was needed to boost overall antibody responses. While the role of alum in the heterogeniety cannot be determined, it seems unlikely to be the major factor. We have seen an identical pattern of antibody responses in mice immunized to the same vaccine encapsulated in liposomes. In any case, the results are relevant to human cancer vaccines, which often require adjuvants to boost immunogenicity.
The vaccine components that were immunogenic in mice also induced immune responses in melanoma patients. The patients were selected from those that had previously tested positive for an antibody response to the 110-kDa antigen [9]. The patients showed a remarkable similarity to mice in developing antibodies to the same vaccine antigens. Even among the four patients analyzed, the individual pattern of antigens recognized varied considerably, mirroring that seen in individual mice. This heterogeneity in antibody response resembles that previously observed in melanoma patients immunized to the same vaccine [9], although in that case variation due to differences in the HLA phenotype of the patients or to subtle difference in the immunization procedure could not be excluded.
An explanation for the heterogeneity in response may be derived from the clonal selection mechanism of immune responses. The antibody specificity of the response in an individual mouse may be the result of the random encounter of B or T cells with a particular antigen during the initial immunization. Once activated these clones may then be preferentially further stimulated and selected over other antibody responses by subsequent immunizations.
Whatever the mechanism(s) responsible for the heterogeneity in antibody responses, the fact that animals immunized identically to a cocktail of antigens react variably and unpredictably to the different antigens in the mixture has several implications. At a mechanistic level, it indicates that antibody response to antigens is not simply a function of the immunogenic potency of the molecule. Strongly immunogenic antigens that induce antibody in most animals fail to do so in some others, while antigens that are weakly immunogenic can, nonetheless, stimulate strong responses in some animals that fail to generate antibodies against stronger immunogens. At a practical level, this further supports construction of polyvalent vaccines from multiple antigens, so as to increase the proportion of patients in whom immune responses can be induced.
These results point to the difficulty in interpreting immune responses in patients given polyvalent vaccines. It also complicates the creation of potency assays that measure the biological activity of vaccines [7], and which are required by the FDA before widespread use of a vaccine can be approved.
Acknowledgement
This research was supported in part by grant CA096804 (to DJ) from the National Institutes of Health.
References
- 1.Bystryn J-C, Shapiro RL, Oratz R. Cancer vaccines: clinical applications: partially purified tumor antigen vaccines. In: DeVita V, Hellman S, Rosenberg SA, Lippincott JB, editors. Biologic therapy of cancer. 2. Philadelphia: 1995. pp. 668–679. [Google Scholar]
- 2.Bystryn J-C, Oratz R, Shapiro RL, Harris MN, Roses DF, Zeleniuch-Jacquotte A, Chen DL, Rivas MC. Double-blind, placebo-controlled, trial of a shed, polyvalent, melanoma vaccine in stage III melanoma. Proc Am Soc Clin Oncol. 1999;434:1673. [Google Scholar]
- 3.DiFronzo LA, Gupta RK, Essner R, Foshag LJ, O‘Day SJ, Wanek LA, Stern SL, Morton DL. Enhanced humoral immune response correlates with improved disease-free and overall survival in American joint committee on cancer stage II melanoma patients receiving adjuvant polyvalent vaccine. J Clin Oncol. 2002;20:3242–3248. doi: 10.1200/JCO.2002.01.065. [DOI] [PubMed] [Google Scholar]
- 4.Finn OJ. Cancer vaccines: between the idea and the reality. Nat Rev Immunol. 2003;8:630–641. doi: 10.1038/nri1150. [DOI] [PubMed] [Google Scholar]
- 5.Gross DA, Graff-Dubois S, Opolon P, Cornet S, Alves P, Bennaceur-Griscelli A, Faure O, Guillaume P, Firat H, Chouaib S, Lemonnier FA, Davoust J, Miconnet I, Vonderheide RH, Kosmatopoulos K. High vaccination efficiency of low-affinity epitopes in antitumor immunotherapy. J Clin Invest. 2004;113:425–433. doi: 10.1172/JCI19418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hsueh EC, Essner R, Foshag LJ, Ye W, Morton DL. Active immunotherapy by reinduction with a polyvalent allogeneic cell vaccine correlates with improved survival in recurrent metastatic melanoma. Ann Surg Oncol. 2002;5:486–492. doi: 10.1245/aso.2002.9.5.486. [DOI] [PubMed] [Google Scholar]
- 7.Keilholz U, Weber J, Finke JH, Gabrilovich DI, Kast WM, Disis ML, Kirkwood JM, Scheibenbogen C, Schlom J, Maino VC, Lyerly HK, Lee PP, Storkus W, Marincola F, Worobec A, Atkins MB. Immunologic monitoring of cancer vaccine therapy: results of a workshop sponsored by the society for biological therapy. J Immunother. 2002;15:97–138. doi: 10.1097/00002371-200203000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Livingston P. The unfulfilled promise of melanoma vaccines. Clin Cancer Res. 2001;7:1837–1838. [PubMed] [Google Scholar]
- 9.Miller K, Abeles G, Oratz R, Zeleniuch-Jacquotte A, Cui J, Roses DF, Harris M, Bystryn J-C. Improved survival of melanoma patients with an antibody response to immunization to a polyvalent melanoma vaccine. Cancer. 1995;75(2):495–502. doi: 10.1002/1097-0142(19950115)75:2<495::aid-cncr2820750212>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 10.Reynolds SR, Oratz R, Shapiro RL, Fotino M, Hao P, Vukmanovic S, Bystryn J-C. Stimulation of CD8+ T cell responses to MAGE-3 and MELAN A/MART-1 by immunization to a polyvalent melanoma vaccine. Int J Cancer. 1997;72:1–5. doi: 10.1002/(SICI)1097-0215(19970917)72:6<972::AID-IJC9>3.3.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 11.Reynolds SR, Celis E, Sette A, Oratz R, Shapiro RL, Johnston D, Fotino M, Bystryn J-C. HLA-independent heterogeneity of CD8+ T cell responses to MAGE-3, Melan A/MART-1, gp100, tyrosinase, MC1R and TRP-2 in vaccine-treated melanoma patients. J Immunol. 1998;161:6970–6976. [PubMed] [Google Scholar]
- 12.Shapiro RL, Johnston D, Oratz R, Harris MN, Roses DF, Zeleniuch-Jacquotte A, Beck A, Bystryn J-C. Effect of GM-CSF liposomes on the immunogenicity of a polyvalent melanoma vaccine. Proc Am Soc Clin Oncol. 1999;440:1695. [Google Scholar]
- 13.Shen HD, Choo KB, Lin WL, Lin RY, Han SH. An improved scheme for the identification of antigens recognized by specific antibodies in two-dimensional gel electrophoresis and immunoblotting. Electrophoresis. 1990;11:878–882. doi: 10.1002/elps.1150111018. [DOI] [PubMed] [Google Scholar]
- 14.Slingluff CL, Jr, Petroni GR, Yamshchikov GV, Barnd DL, Eastham S, Galavotti H, Patterson JW, Deacon DH, Hibbits S, Teates D, Neese PY, Grosh WW, Chianese-Bullock KA, Woodson EMH, Wiernasz CJ, Merrill P, Gibson J, Ross M, Engelhard VH. Clinical and immunological results of a randomized phase II trial of vaccination using four melanoma peptides either administered in granulocyte–macrophage colony-stimulating factor in adjuvant or pulsed on dendritic cells. J Clin Oncol. 2003;21:4016–4026. doi: 10.1200/JCO.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Sosman JA, Weeraratna AT, Sondak When will melanoma vaccines be proven effective? J Clin Oncol. 2004;22:1–3. doi: 10.1200/JCO.2004.11.950. [DOI] [PubMed] [Google Scholar]
- 16.Tjoa BA, Elgamal A-AA, Murphy GP. Vaccine therapy for prostate cancer. Urol Clin North Am. 1999;26(2):365–374. doi: 10.1016/s0094-0143(05)70076-8. [DOI] [PubMed] [Google Scholar]
- 17.Zhang S, Zhang HS, Reuter VE, Slovin SF, Scher HI, Livingston PO. Expression of potential target antigens for immunotherapy on primary and metastatic prostate cancers. Clin Can Res. 1998;4:295–302. [PubMed] [Google Scholar]