Abstract
Previous studies by others using transplantable murine tumor models have demonstrated that the administration of antibodies that block CTLA-4 interaction with B7 can provoke the elimination of established tumors, and that the tumor suppression is mediated by T-cells and/or cells expressing NK1.1. Studies from our lab have established in a human/severe combined immunodeficient (SCID) mouse chimeric model that autologous peripheral blood leukocytes (PBL) can suppress the growth of tumor xenografts in a PBL dose-dependent fashion, and that this suppression is dependent upon the patient’s T and NK cells. Using this human/mouse chimeric model, we sought to determine whether an antibody blockade of CTLA-4 would enhance the anti-tumor response of a patient’s PBL. It was first important to determine whether the tumor suppression observed in the SCID model was dependent upon CD28/B7 co-stimulation. Blockade of B7 with a human CTLA-4-Ig fusion protein completely abrogated the lymphocyte-mediated tumor suppression, confirming in this model that tumor suppression is dependent upon a CD28/B7 co-stimulation. Using two different CTLA-4 specific monoclonal antibodies, we observed that CTLA-4 blockade significantly enhanced the human lymphocyte-mediated tumor suppression in mice co-engrafted with PBL and tumor cells. This enhancement was observed in both an allogeneic setting (in which the PBL were allogeneic with respect to the tumor) and an autologous setting (in which the PBL and tumor were from the same patient). These results sustain the notion that human anti-tumor immune response can be augmented (in vivo) by blocking the interaction between CTLA-4 and B7.
Keywords: Human CTLA-4, Lymphocytes, SCID Mice, Xenografts
Introduction
Tumors (both in mice and humans) express antigens that when processed and presented to T cells by antigen presenting cells may provoke a CD4+ and/or CD8+ effector T-cell mediated anti-tumor response [1–3]. However, whether prompted naturally or by active immunization with the tumor-associated antigens, these responses are often unable to control tumor progression and metastasis. While many different reasons have been suggested for the failure of host T cells to eradicate tumors (including several possible tumor escape mechanisms [4] and the development of tolerance to tumor antigens [5]), it remains unclear why tumors are able to progress in the face of the host’s anti-tumor immune response. Most likely, tumor control depends upon both the magnitude of the initial immune response, and the ability to sustain the response for a prolonged period. This hypothesis is well-supported by studies in which the degree of tumor growth suppression was found to be directly related to the level of the anti-tumor response generated [6]. A logical extension of this hypothesis is that if one were able to enhance and prolong the anti-tumor response, it should be possible to better control tumor growth.
Several important discoveries relating to T-cell activation and regulation have provided valuable insights with respect to the development of novel approaches that are designed to enhance and prolong T-cell immune responses. Recognition that T cells require a second co-stimulatory signal for activation (in addition to the one transduced by the antigen receptor), represents a most important insight that has laid the foundation for manipulation of both the strength and duration of lymphocyte responses [7, 8]. The major co-stimulatory molecule on both CD4+ and CD8+ T cells was determined to be CD28, and its engagement by either B7-1 or B7-2 on antigen presenting cells provides the requisite second T cell activation signal [8, 9]. The discovery that CTLA-4, a close homolog of CD28, is up-regulated on activated T cells, and binds B7-1 and B7-2 with considerably greater avidity than CD28 led to the prediction that CTLA-4 may also play a role in T cell activation [10–13]. The use of function-blocking CTLA-4 specific antibodies in studies conducted both in vitro and in vivo suggests that ligation of CTLA-4 on T cells results in the transduction of an inhibitory signal [14, 15]. The inhibitory role of CTLA-4 was confirmed by the discovery that knockout mice lacking CTLA-4 develop uncontrolled lymphocyte activation and proliferation resulting in massive enlargement of lymph nodes and spleen [16, 17].
The precise molecular mechanisms by which T cells integrate TCR stimulation with the co-stimulatory signal of CD28 and the inhibitory signals of CTLA-4 remain unclear. However, since CD28 is expressed constitutively on naïve T cells, it may be used to initiate immune responses, whereas CTLA-4 is only expressed following T cell activation and thus, may function to terminate the response [14, 18, 19]. This led investigators to speculate that by blocking the CTLA-4 mediated termination signal (with function blocking antibodies), it would be possible to prolong anti-tumor immune responses, resulting in enhanced suppression (or eradication) of tumors. Initial experiments with two different murine transplantable tumors established that the antibody blockade of CTLA-4 enhanced the weak immune response elicited by these tumors to a level sufficient for tumor rejection [20]. In subsequent experiments, it was established that the CTLA-4 blockade, when used in conjunction with other therapies (such as vaccination with irradiated tumor cells transfected with GM-CSF), results in a substantial synergistic enhancement of the anti-tumor response [21].
Much of the published data supporting the ability of CTLA-4 blockade to enhance anti-tumor responses have been generated using murine tumor models. To determine whether a CTLA-4 blockade would have a similar effect upon human lymphocytes, and their response to human tumors, a human/SCID mouse chimeric model has been designed and tested. In this “SCID-Winn” model, human peripheral blood leukocytes (PBL) are mixed with a suspension of a patient’s tumor cells and following injection into SCID mice, the anti-tumor immune response of the engrafted human PBL is monitored in vivo. Previous studies using the SCID-Winn model have established that: (1) PBL (both allogeneic and autologous with respect to the tumor) suppress tumor growth in a dose-dependent fashion, (2) tumor suppression is mediated by T cells and NK cells, and (3) tumor suppression requires the presence of both human IL-12 and IFN-γ (secreted in situ by the engrafted human leukocytes) [22–24]. In this report, we establish that the human lymphocyte-mediated tumor suppression observed in the SCID-Winn model, is dependent upon the co-stimulation provided by B7 interaction with CD28. Furthermore, we provide direct evidence that CTLA-4 blockade enhances human lymphocyte-mediated tumor suppression in vivo.
Materials and methods
Animals, reagents and cell lines
CB.17 scid/scid mice were obtained from our breeding colony. All mice were maintained in microisolation cages (Lab Products, Inc., Maywood, NJ, USA) under pathogen-free conditions in the animal maintenance facilities of Roswell Park Cancer Institute. The human lung carcinoma cell line RPCI-2E9 is a human squamous cell carcinoma cell line that was originally established in our laboratory from a resected patient tumor [24]. The human lung carcinoma cell line RPCI-9530 is an adenocarcinoma cell line that was established in a similar manner [23]. Cells were maintained in DMEM/Nutrient mixture F-12 media (Life Technologies, Inc., Grand Island, NY, USA), supplemented with 10% heat inactivated FCS. The cells were incubated at 37°C in 5% CO2. They were allowed to grow to 80% confluence and were harvested with 0.25% trypsin/1 mM EDTA in HBSS.
Human PBL’s were obtained under an IRB approved protocol by leukophoresis of peripheral blood donated by a healthy donor or patient #9530, from whom the cell line RPCI-9530 was established. RBC’s were lysed with freshly prepared 0.83% NH4Cl solution, and the HuPBL’s were washed twice with DMEM (as previously described) [24].
Antibodies and Fusion Proteins
Human CTLA-4*Ig, a fusion protein and a human fusion protein L-6, an Ig control, were gifts from Bristol-Myers Squibb (Princeton, NJ, USA) and were generated as reported previously [11]. The human CTLA-4*Ig is a soluble genetic fusion molecule comprising the extracellular domain of CTLA-4 joined to an Ig Cγ1 domain. The binding properties and the functional effects of this molecule on T and B lymphocyte responses have been previously reported [11]. 10A8, an IgG1 murine anti-human CTLA-4 monoclonal antibody, was obtained from Bristol Myers Squibb and was originally produced by P. Linsley [25]. The 10A8 antibody binds strongly to CTLA-4 transfected COS cells or immobilized CLTA-4 and inhibits the binding of CTLA-4 to B7+ CHO cells [25]. 2C3 is an IgG1 anti-hapten antibody developed in our laboratory and used as an isotype control antibody. P2 is an IgG2 humanized monoclonal antibody directed against human CTLA-4. P2 and control IgG2 were gifts from Pfizer Global Research and Development (Groton, CT, USA). The P2 antibody was shown to inhibit the binding of soluble B7.1 and B7.2 to CTLA-4 immobilized on plastic plates as is described next.
Competition ELISA
In order to confirm the ability of the humanized anti-human monocolonal antibody to bind to CTLA-4, a competition ELISA was performed. Briefly, a microtiter 96-well plate was coated with human CTLA-4-Ig fusion protein at 1 mg/ml. An amount of 250 μl of quench solution (500 ml BBS +2.5 ml normal rabbit serum +5 gm BSA) was added to each well for 1 h at room temperature. P2 was added at varying concentrations to each well, with 0.5 μg/ml of B7.1 (CD80) or B7.2 (CD86). After 1 h., the wells were washed three times and rabbit anti-human IgHRP was added. After 1 h., the wells were washed again. O-phenylenediamine (OPD) was added for 15 min., the reaction was stopped by adding 6N HCl to each well and the plates were analyzed on an ELISA reader at a single wavelength of 490. Complete blockade of anti-CTLA-4 binding to CTLA-4 with either B7.1 or B7.2 was seen at 0.1 μg/ml or less of P2 antibody (data not shown).
Dendritic cell-mixed lymphocyte reaction
The in vitro effects of CTLA4*Ig and anti-hCTLA-4 mAb on human lymphocyte proliferation were assessed through the use of a dendritic cell mixed lymphocyte reaction (DC*MLR). Human PBL were stimulated with allogenic dendritic cells, in the presence of either hCTLA-4*Ig or anti- hCTLA-4 mAb or their respective controls. Dendritic cells were cultured using the following technique: HuPBL were cultured for 1 h. at 37°C after which the non-adherent cells were removed and the adherent cells were cultured at 37°C overnight. The non-adherent cells were collected and replated in 100 ng IL-4 and 50 ng of GM-CSF for 7 days. The dendritic cells were then matured by the addition of monocyte condition medium, as previously described [26]. HuPBL’s were obtained by leukophoresis of a second healthy donor and cultured in RPMI 1,640 plus 10% heat-inactivated FCS at a concentration of 5×106 cells/ml. The dendritic cells from the first leukophoresis were irradiated with 5,000 rad and then introduced into the new HuPBL culture at a ratio of 10:1. For the CTLA-4*Ig experiments, either L-6 or CTLA-4*Ig was added at a dose of 50 μg/ml. In a parallel series of experiments, either 10A8, (a murine anti-human CTLA4 mAb) or 2C3, (an isotype control), was added at a dose of 1 μg per 1×104 HuPBL. Additional groups of HuPBL without irradiated dendritic cells, and with or without anti-hCTLA4 mAb, were also cultured in a similar manner. The cells were then incubated at 37°C in 5%CO2. In the anti-CTLA4 mAb experiments, 100 μl of culture media was drawn on days 0, 2, 4 and 7 for determination of human IFN-γ concentration.
The concentration of human IFN-γ in the culture media or sera of the engrafted mice was determined by an ELISA developed in our laboratory as described previously [24]. Briefly, 96-well microtiter plates were coated with a mouse antihuman IFN-γ monoclonal antibody (M-700A; Endogen, Cambridge, MA, USA) overnight at 4°C. Standards and unknowns were added to the wells and incubated with biotin-labeled antihuman-IFN-γ monoclonal antibody M-701 (Endogen). Bound antibody was detected by the addition of avidin-horseradish peroxidase conjugate and the substrate o-phenylenediamine (Sigma Chemical Co., St. Louis, MO, USA).
After 7 days, cell proliferation was measured using a colorimetric MTT assay, as previously described [27]. Briefly, 100 μl suspension from each group was added to each of the eight wells in a column of a 96-well Elisa plate. Stock MTT solution (5 mg/ml in PBS) was added at 10 μl of MTT to 100 μl medium to each well. The plates were incubated at 37°C for 4 h. Acid isopropanol (200 μl of 0.04N HCl in isopropanol) was added to all wells and mixed thoroughly to dissolve the dark blue crystals. The plates were then read on an ELISA reader at a single wavelength of 540 nm.
HuPBL and human tumor xenograft implantation in SCID mice
Freshly harvested tumor cells, (either RPCI-2E9 or RPCI-9530) were washed twice in DMEM and mixed with HuPBL’s at the appropriate effector to target ratios. In the allogenic experiments, RPCI-2E9 cells were co-engrafted with HuPBL obtained from leukophoresis of a healthy donor. For the autologous experiments, RPCI-9530 cells were co-engrafted with HuPBL obtained from the same donor from whom the RPCI-9530 cell line was originally established.
To measure tumor suppression in response to CTLA-4 stimulation, hCTLA-4*Ig was added to the HuPBL and tumor cells at a dose of 10 μg per 1×106 HuPBL. L-6 was added at an equivalent dose to control groups. After resuspension in 1 ml of sterile DMEM, the cell/antibody suspension was inoculated subcutaneously (0.2 ml/mouse) into the ventral caudal midline area of SCID mice. When investigating the effects of anti-human CTLA-4 antibody treatment in vivo, either anti-hCTLA-4 mAb 10A8 or P2 was added in a similar fashion, at a dose of 5 μg per 1×104 HuPBL. IgG2 control antibody was added at an equivalent dose to control animals. Tumor growth was monitored once a week until the animals were moribund (i.e.when tumor lengths became 15 mm in diameter) or after 90 days. Subcutaneous tumors were measured using a caliper. The longest dimension (a) and perpendicular width (b) were determined, and tumor volume (v) was calculated according to the formula v=ab2/2. In some experiments, mice treated with anti-hCTLA-4 mAb were bled for determination of serum levels of human IFN-γ on day 5 after implantation. The concentration of human IFN-γ in the sera of SCID mice was determined by ELISA as described above.
Statistical ansalysis
The statistical difference between each group was determined by the unpaired Student’s t-test. A ‘p’ value less than 0.05 was interpreted as statistically significant.
Results
Suppression of lymphocyte activation in vitro by B7 blockade with hCTLA-4 fusion protein and enhancement of lymphocyte activation in vitro by CTLA-4 blockade with anti-CTLA-4 antibody
Before using the B7 and CTLA-4 blocking reagents in vivo, we sought to confirm that these reagents had function-blocking activity against human lymphocytes in vitro. The first reagent, human CTLA-4-Ig fusion protein, was expected to prevent the costimulatory second signal that is necessary for T cell activation by binding to B7 on the surface of antigen presenting cells. The second reagent tested for its function-blocking activity was the monoclonal antibody specific for human CTLA-4. Since this antibody is reported to bind the CTLA-4 molecule at or near the recognition site for B7, it would be expected to have a function-blocking activity and would, thus, prevent the T cell inhibitory signal that results from the interaction between B7 and CTLA-4.
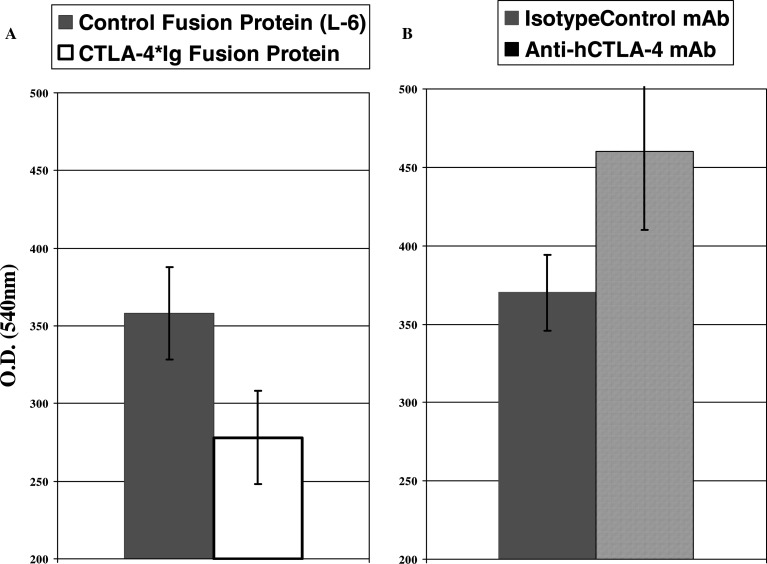
The in vitro model of T cell activation used to test these two reagents was a mixed one-way lymphocyte reaction (MLR) in which human lymphocytes are stimulated to proliferate by allogeneic dendritic cells (DC). Because allogeneic DC express both B7.1 and B7.2 on their surface, they are very efficient activators of the responding T cells. Therefore, the MLR represents a reliable method for stimulation of T cells that reflects activation events which occur in vivo. In these experiments, irradiated mature stimulator DC were incubated with responder lymphocytes at a stimulator to effector ratio of 10:1 and lymphocyte proliferation was monitored after 7 days in culture. The effect of each of the blocking reagents on lymphocyte proliferation was determined by comparing the proliferation of cells in cultures incubated with the inhibitory reagent to cultures incubated with control reagents. It was established that the proliferation of cells in culture with the human CTLA-4-Ig fusion protein was partially inhibited compared to cells incubated with a control fusion protein L-6 (Fig. 1a). In contrast, the lymphocytes in the cultures incubated with anti-CTLA-4 monoclonal antibody exhibited an enhanced proliferation when compared to cells in the DC/lymphocyte cultures incubated with an isotype control monoclonal antibody (Fig. 1b). These results, i.e. partial suppression of T-cell proliferation with CTLA-4-Ig fusion protein, and enhancement of proliferation with anti-CTLA-4 antibody, are consistent with what would be expected if each reagent were functionally active.
Fig. 1.
Changes in lymphocyte proliferation of allo-stimulated HuPBL in the presence of either B7 or CTLA-4 blocking agents. The in vitro effects of CTLA4*Ig and anti-hCTLA-4 mAb on lymphocyte proliferation were assessed through the use of a dendritic cell mixed lymphocyte reaction (DC*MLR). Human PBL were cultured with or without irradiated mature allogenic dendritic cells (stimulator to effector ratio of 10 to 1), in the presence of either hCTLA-4*Ig or anti- hCTLA-4 mAb or isotype controls. Lymphocyte proliferation was monitored after 7 days in culture by colorimetric MTT assay (see “Materials and Method”). The proliferation of cells in culture with the human CTLA-4-Ig fusion protein was inhibited compared to cells incubated with a control fusion protein L-6 (a). In contrast, the lymphocytes in the cultures incubated with anti-CTLA-4 monoclonal antibody 10Ag exhibited an enhanced proliferation when compared to cells in the DC/lymphocyte cultures incubated with an isotype control monoclonal antibody (b). This experiment was also performed using the P2 antibody. Error bars represent the standard deviation from the mean
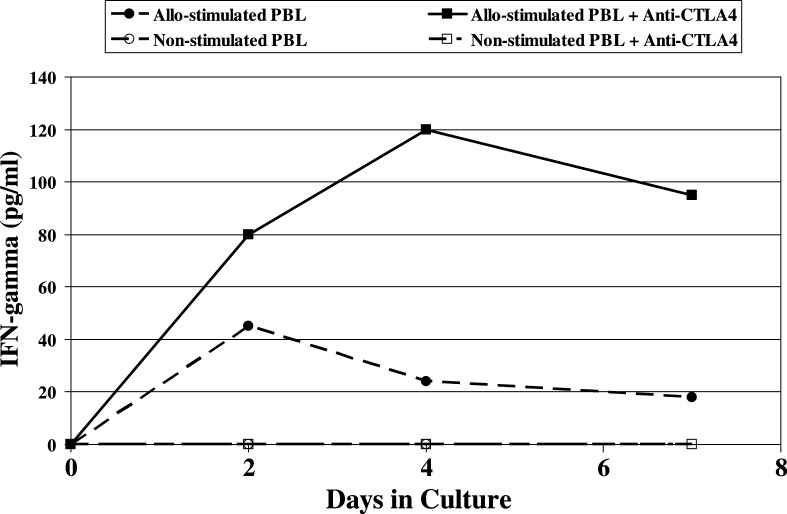
In addition to proliferation, T cells produce IFN-γ following activation, and the production of IFN-γ is often used as a quantifiable indicator of T-cell activation. Human IFN-γ was detected in the supernatant fluid of the PBL/allogeneic DC cocultures confirming that the PBL were, in fact, activated by allostimulation (Fig. 2). The amount and duration of IFN-γ production by the allostimulated PBL was significantly enhanced by the antibody blockade of CTLA-4 (P=0.01). Minimal IFN-γ was detected in cultures of PBL without allogeneic DC (with or without the addition of anti-CTLA antibody) (Fig.2). These results further support the notion that the anti-CTLA-4 antibody has function blocking activity which results in an enhanced T-scell activation.
Fig. 2.
Levels of hIFN-γ production by allo-stimulated HuPBL with or without CTLA-4 blockade. Human PBL were cultured with or without irradiated mature allogenic dendritic cells (stimulator to effector ratio of 10:1), in the presence of the P2 anti-hCTLA-4 mAb or isotype controls. Hundred Microlitres of culture media was drawn on days 0, 2, 4 and 7. IFN-γ was detected in the supernatant fluid of the cocultures of PBL and allogeneic DC confirming that the PBL were activated by allostimulation. The amount and duration of IFN-γ production by the allostimulated PBL was significantly enhanced by the antibody blockade of CTLA-4 (P=0.01). Minimal IFN-γ was detected in cultures of PBL without allogeneic DC (with or without the addition of anti-CTLA-4 antibody)
Human T-Cell mediated suppression of human tumor xenografts in SCID mice is dependent upon costimulation by B7 ligation of CD28
Previous studies have established that human PBL when coengrafted with tumor cells into the subcutis of SCID mice are able to suppress tumor growth in a PBL dose dependent fashion [23, 24]. Tumor suppression in this human/SCID mouse chimeric model (i.e. SCID/Winn model) was shown to require human T and NK cells. Moreover, this tumor suppression was abrogated in mice treated with function blocking antibodies specific for either human IL-12 or human IFN-γ [23].
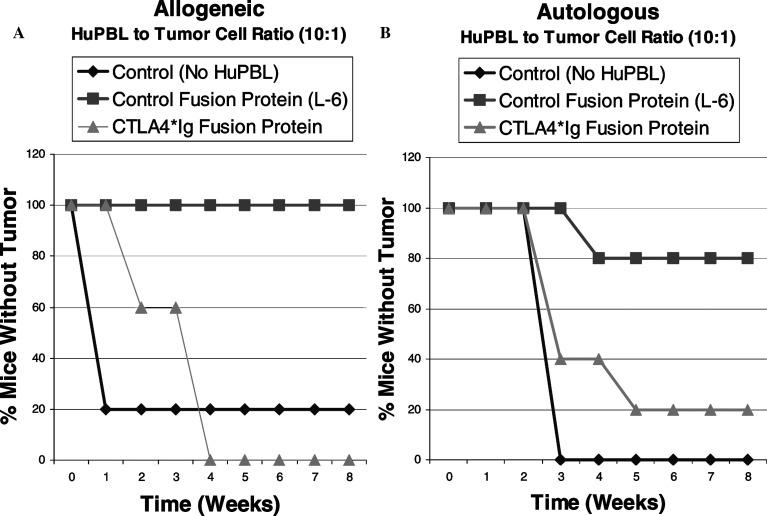
The SCID/Winn model was used here to investigate the ability of anti-CTLA-4 to enhance the human lymphocyte-mediated anti-tumor response in an in vivo setting. The suitability of the SCID/Winn model to address the effects of CTLA-4 blockade would depend upon whether the T-cell mediated anti-tumor response observed in the model were dependent upon a CD28/B7 costimulation. If the anti-tumor response were dependent upon this costimulation, one would expect to be able to inhibit this response by a blockade of B7 with the human CTLA-4-Ig fusion protein. As illustrated in Figure 3, T-Cell mediated tumor suppression was unaffected by the control fusion protein but it was reversed when human CTLA-4-Ig fusion protein was added to the PBL/tumor xenograft (Fig. 3). This reversal of tumor inhibition with the B7 blockade was observed both in an allogeneic setting (In which the PBL were obtained from a healthy volunteer and co-engrafted with a human tumor lung tumor cell line (Fig.3A)], and in an autologous setting [In which the tumor cell line and the PBL were derived from the same patient (Fig.3B)]. In both groups, a significant suppression of in vivo tumor growth was observed at a PBL to tumor cell ratio of 10:1 and this PBL-mediated tumor suppression was abrogated at a dose of 10 μg of CTLA-4-Ig fusion protein to 1×106 lymphocytes.
Fig. 3.
Effect of B7 blockade with hCTLA-4*Ig fusion protein on T-lymphocyte dependent tumor suppression. Freshly harvested tumor cells were mixed with either allogeneic (a) or autologous (b) HuPBL’s at effector to target ratio of 10:1, with or without hCTLA-4*Ig fusion protein, or a control fusion protein (L-6) at a dose of 10 μg per 1×106 HuPBL. Tumors were then inoculated subcutaneously into the ventral caudal midline area of SCID mice and tumor growth was monitored. Control mice were inoculated with tumor cells only. Groups consisted of five mice, and data are presented as tumor-free survival. The T-cell mediated tumor suppression, seen in both the allogeneic and autologous model, was reversed when B7 blockade was achieved by adding human CTLA-4-Ig fusion protein to the xenograft. This demonstrates that the lymphocyte mediated tumor suppression observed in the human SCID mouse chimeric model is dependent upon the B7/CD28 costimulation of T cellss
We conclude from these studies that the human lymphocyte-mediated tumor suppression observed in the human SCID mouse chimeric model is dependent upon the B7-CD28 costimulation of T cells, and that this model is an appropriate one for evaluating the effects of CTLA-4 blockade on the human lymphocyte mediated anti-tumor responses in vivo.
Antibody blockade of CTLA-4 enhances human lymphocyte mediated tumor suppression in the human/SCID mouse chimeric model
To assess the ability of anti-CTLA-4 to enhance the anti-tumor response of human PBL in the SCID/Winn model, a sub-optimal ratio of PBL:tumor cells was used. In preliminary experiments, it was determined for both allogeneic and autologous PBL that engraftment of mice with a 10:1 ratio of PBL to tumor cells resulted in a near complete inhibition of tumor growth in vivo (data not shown). At a 2.5:1 effector to target ratio no tumor suppression was seen with the allogeneic cells and at a 5:1 ratio of autologous PBL to tumor cells, no tumor suppression was observed (data not shown).
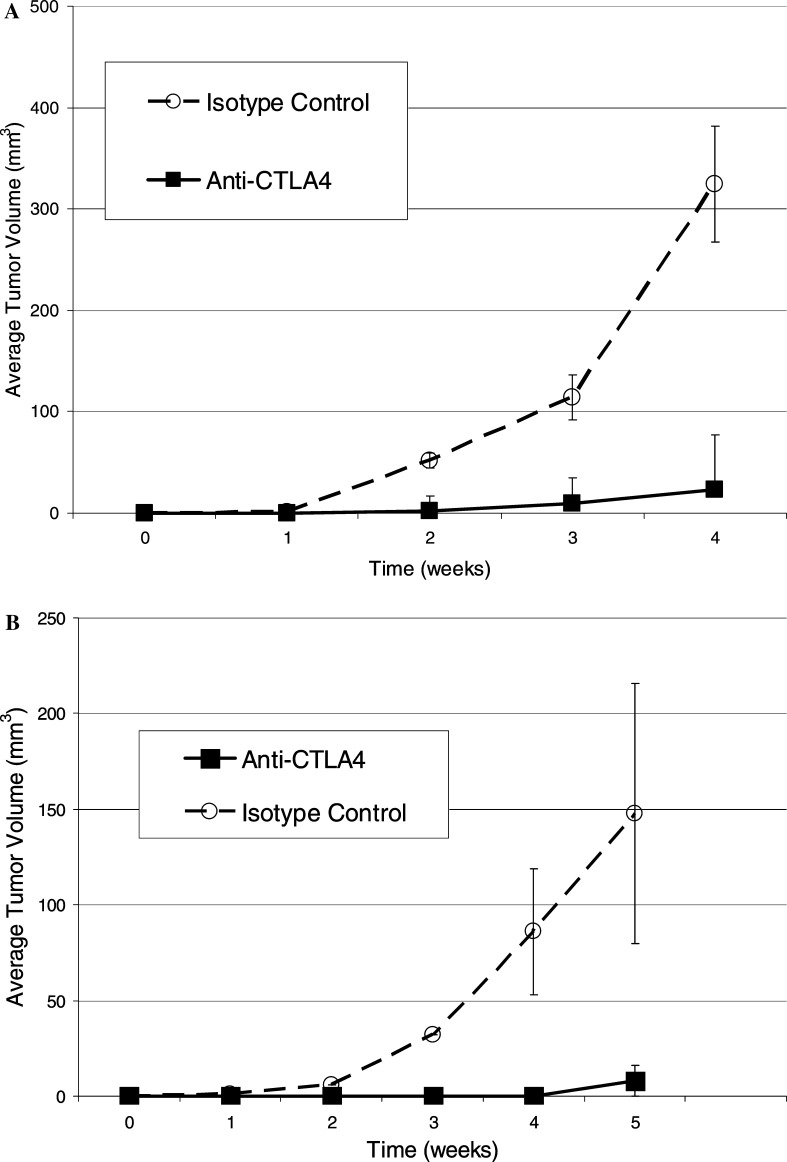
Using mice engrafted with a sub-optimal ratio of PBL to tumor, the effects of anti-CTLA-4 treatment were evaluated in vivo. Representative results of four separate experiments are presented in Fig 4a (allogeneic, at an E:T ratio of 2.5 to 1) and Fig. 4b (autologous, at an E:T ratio of 5:1) are representative of two separate experiments. These data show that human PBL at this sub-optimal dose, were unable to suppress tumor growth. An enhancement of tumor suppression was observed following anti-CTLA-4 treatment in both the allogeneic (P=0.04) and autologous settings (P=0.002) as compared to mice treated with an isotype control antibody.
Fig. 4.
Effect of CTLA-4 blockade on allogeneic and autologous SCID/Winn assay. a RPCI-2E9 cells were washed twice in DMEM and mixed with HuPBL’s obatined from a leukophoresis of a healthy donor, at an effector to target (E:T) ratio of 2.5 to 1. Either anti-hCTLA-4 mAb P2 or IgG2 isotype control antibody was added at a dose of 5 μg per 1×104 HuPBL prior to subcutaneous inoculation (0.2 ml/mouse) into the ventral caudal midline area of SCID mice. Tumor growth was carefully monitored with subcutaneous tumors measured weekly using a caliper. Each group consisted of five mice, (Bars, SE). Addition of anti-CTLA-4 mAb resulted in significant enhancement of tumor suppression (P=0.04). Similar effect was observed in four other experiments with L2 or 10A8 anti-CTLA-4 antibody. b RPCI-9350 cells were washed twice in DMEM and mixed with HuPBL obtained from leukophoresis of donor from whom the cell line 9530 was originally established, at an effector to target (E:T) ratio of 5:1. Either anti-hCTLA-4 mAb P2 or IgG2 isotype control antibody was added at a dose of 5 μg per 1×106 HuPBL prior to subcutaneous inoculation (0.2 ml/mouse) into the ventral caudal midline area of SCID mice. Tumor growth was monitored with subcutaneous tumors measured weekly using a caliper. Each group consisted of five mice (bars, SE). Addition of anti-CTLA-4 mAb resulted in significant enhancement of tumor suppression (P=0.002). This experiment was done twice with similar results
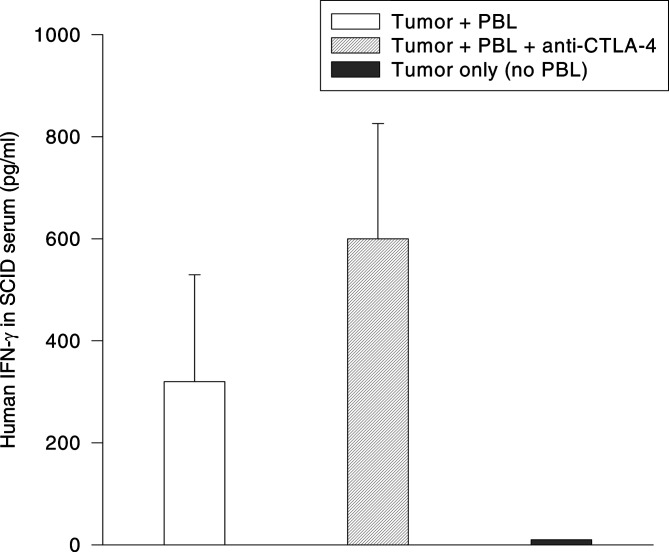
It was previously established that tumor suppression observed in the SCID/Winn model correlates with, and is dependent upon, the production of IFN-γ by human T cells in the tumor xenograft [23]. In view of these findings, it was anticipated that an increase in the serum level of human IFN-γ may occur concomitantly with the anti-CTLA-4 induced enhancement of the T-cell mediated tumor suppression. To test this hypothesis, mice were co-engrafted with PBL and tumor at a ratio of 2.5:1 and treated with either anti-CTLA-4 or an isotype control antibody. The mice were then bled 5 days after implantation and the serum levels of human IFN-γ quantified by ELISA. As shown in Fig. 5, human IFN-γ was detected in the sera of mice engrafted with human PBL+tumor cells, but not in the sera of mice engrafted with tumor only. Anti-CTLA-4 treatment resulted in an increase in the day 5 serum concentration of human IFN-γ compared to mice that did not receive anti-CTLA-4 therapy (Fig. 5). While this increase did not quite reach the level of statistical significance, these data are consistent with the increase in IFN-γ observed when human lymphocytes+DC’s were co-cultured with anti-CTLA-4 in vitro (Fig. 2).
Fig. 5.
Effect of anti-CTLA-4 treatment on the production of IFN-γ by human PBL co-engrafted with tumor cells into SCID mice. SCID mice were co-engrafted s.c. with 1.0×106 RPCI-9530 tumor cells and 2.5×106 allogeneic human PBL on day 0. One group of mice received P2 anti-CTLA-4 antibody along with the tumor/PBL inoculum. A control group of mice was engrafted with tumor only in the absence of PBL. Individual mice were bled on day 5 post-engraftment, and the quantity of human IFN-γ present in the sera was determined by ELISA. Data are plotted as mean serum IFN-γ concentration on day 5 post-engraftment (N=5 mice per group). Statistical significance among the three groups was calculated using the unpaired Student’s t-test (P=0.076 for Tumor+PBL vs. Tumor+PBL+anti-CTLA-4; P < 0.01 for Tumor+PBL vs. Tumor only; P < 0.01 for Tumor+PBL+anti-CTLA-4 vs. Tumor only).
Collectively, these studies indicate that human PBL-mediated tumor suppression is dependent upon CD28/B7 co-stimulation and that the suppression can be enhanced by the blockade of CTLA-4 in vivo.
Discussion
The function of B7-CTLA4 interaction, and the mechanisms by which CTLA-4 blockade may augment immunity, remain somewhat controversial [28–30]. The majority of the preclinical data supporting the use of CTLA-4 blockade to enhance anti-tumor responses have been generated using murine tumor models. We sought to examine whether CTLA-4 blockade would have a similar effect upon human lymphocytes, and their response to human tumors. To accomplish this, we utilized a human/SCID mouse chimeric model (“SCID-Winn model‘’) where human peripheral blood leukocytes (PBL) are mixed with a suspension of a tumor cells and following injection into SCID mice, the anti-tumor immune response of the engrafted human PBL is monitored in vivo. In this report, we establish that the human lymphocyte-mediated tumor suppression observed in the SCID-Winn model, is dependent upon the co-stimulation provided by B7 interaction with CD28. Furthermore, we provide direct evidence that CTLA-4 blockade enhances human lymphocyte-mediated tumor suppression in vivo. This enhancement was observed both in a setting in which the responding cells were allogeneic with respect to the tumor, and in a setting where the responding cells and tumor cells were derived from the same patient, i.e. autologous.
The results presented here support the ongoing evaluation of anti-CTLA-4 antibodies for tumor immunotherapy. However, these results also suggest that CTLA-4 blockade would be best utilized under inflammatory conditions, where the attenuating effects of CTLA-4 blockade can augment the proliferation of highly-reactive T-cells. In vitro, the addition of CTLA-4 antibodies to HuPBL in the absence of co-stimulation failed to induce IFN-gamma production, and the anti-tumor response seen in the SCID-Winn assay is clearly dependent upon B7:CD28 co-stimulation.
Initial experiments with CTLA-4 blockade demonstrated that anti-CTLA-4 as a single agent resulted in the rejection of transplantable murine tumors [20]. Phase I
clinical trials have also generated optimism. Several prostate cancer patients treated with anti-CTLA-4 antibody showed significant decreases in serum levels of prostate-specific antigen (PSA), indicating a reduction in tumor burden [18]. Histological examination of tumor biopsy tissue from these patients after therapy revealed the presence of inflammatory infiltrates within the tumor microenvironment as well as extensive tumor necrosis.
However, other murine studies have shown that CTLA-4 blockade was ineffective as a single agent when used against transplantable tumors that are inherently poorly immunogenic, such as B16 melanoma and SM1 mammary carcinoma [21, 31]. This calls into question whether anti-CTLA-4 antibodies would be effective as a single agent therapy for human cancer. Instead, CTLA-4 blockade may be better utilized as an adjuvant to active immunization. Several models demonstrated that the enhancement of antigen presentation by GM-CSF could be enhanced with CTLA-4 blockade, and that this strategy is effective as a vaccine therapy [21, 31, 32]. CTLA-4 blockade may also be used in combination with low-dose chemotherapy, where the cell death induced by chemotherapy may prime an immune response that can be augmented by anti-CTLA-4 [33]. In clinical trials, CTLA-4 blocking antibody infused into advanced cancer patients stimulated extensive tumor necrosis with lymphocyte and granulocyte infiltrates in metastatic melanoma patients and the reduction or stabilization of CA-125 levels in two of two metastatic ovarian carcinoma patients previously vaccinated with irradiated, autologous granulocyte-macrophage colony stimulating factor-secreting tumor cells [34, 35].
In conclusion, the human lymphocyte-mediated tumor suppression observed in a human-SCID chimeric model is dependent upon the co-stimulation provided by B7:CD28 co-stimulation, and CTLA-4 blockade enhances this human lymphocyte-mediated immune response in vivo. This data, as well as the early results of phase I clinical trials demonstrate the promise of blockade of CTLA-4-mediated inhibition as a potential strategy for the enhancement of T-cell responses in active immunization.
Acknowledgments
The authors thank James P. Allison for reading the manuscript and providing critical comments and helpful suggestions. We greatly appreciate the help from Mr. Robert Parsons and the DLAR staff at RPCI for their help and support with the SCID mouse colony. We are grateful to Dr. Ron Gladue from Pfizer, Inc. for the gift of the P2 anti-CTLA-4 antibody and the isotype control antibody and to Dr. Robert Peach from Bristol-Myers Squibb who supplied us with the CTLA-4 Ig fusion protein, the L6 fusion protein control and the anti-CTLA-4 antibody 10A8. We thank Ms. Sandra Yokota and Ms. Jenni Loyall for their excellent technical support and Cheryl Zuber for helping with the preparation and typing of this manuscript.This work was supported in part by U.S. Public Health Service Grants CA79879 and CA96528 (to R.B.B.).
Abbreviations
- CTLA-4
Cytotoxic T Lymphocyte associated Antigen 4
- SCID
Severe combined immunodeficient
- MLR
Mixed lymphocyte reaction
- PBL
Peripheral blood leukocytes
- DC
Dendritic cells
- NK
Natural killer cells
References
- 1.Boon T, van der Bruggen P. Human tumor antigens recognized by T lymphocytes. J Exp Med. 1996;183:725–729. doi: 10.1084/jem.183.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosenberg SA. A new era for cancer immunotherapy based on the genes that encode cancer antigens. Immunity. 1999;10:281–287. doi: 10.1016/s1074-7613(00)80028-x. [DOI] [PubMed] [Google Scholar]
- 3.Cohen PA, Peng L, Plautz GE, et al. CD4+ T cells in adoptive immunotherapy and the indirect mechanism of tumor rejection. Critical Reviews in Immunology. 2000;20:17–56. [PubMed] [Google Scholar]
- 4.Marincola FM, Jaffee EM, Hicklin DJ, Ferrone S. Escape of human solid tumors from T-cell recognition: molecular mechanisms and functional significance. Adv Immunol. 2000;74:181–273. doi: 10.1016/s0065-2776(08)60911-6. [DOI] [PubMed] [Google Scholar]
- 5.Pardoll D. Does the immune system see tumors as foreign or self. Annu Rev Immunol. 2003;21:807–839. doi: 10.1146/annurev.immunol.21.120601.141135. [DOI] [PubMed] [Google Scholar]
- 6.Perez-Diaz A, Spiess PJ, Restifo NP, et al. Intensity of the vaccine-elicited immune response determines tumor clearance. J Immunol. 2002;168:338–347. doi: 10.4049/jimmunol.168.1.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chambers CA, Allison JP. Co-stimulation in T cell responses. Curr Opin Immunol. 1997;9:396–404. doi: 10.1016/s0952-7915(97)80087-8. [DOI] [PubMed] [Google Scholar]
- 8.Mueller DL, Jenkins MK, Schwartz RH. Clonal expansion versus functional clonal inactivation: A costimulatory signalling pathway determines the outcome of T cell antigen receptor occupancy. Annu Rev Immunol. 1989;7:445–480. doi: 10.1146/annurev.iy.07.040189.002305. [DOI] [PubMed] [Google Scholar]
- 9.Sharpe AH, Freeman GJ. The B7-CD28 superfamily. Nat Immunol. 2002;2:116–126. doi: 10.1038/nri727. [DOI] [PubMed] [Google Scholar]
- 10.Brunet JF, Denizot F, Luciani MF, et al. A new member of the immunoglobulin superfamily- CTLA-4. Nature. 1987;328:267–270. doi: 10.1038/328267a0. [DOI] [PubMed] [Google Scholar]
- 11.Linsley PS, Brady W, Urnes M, et al. CTLA-4 is a second receptor for the B-cell activation antigen B7. J Exp Med. 1991;174:561–569. doi: 10.1084/jem.174.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Linsley PS, Greene JL, Brady W, et al. Human B7-1 (CD80) and B7-2 (CD86) bind with similar avidities but distinct kinetics to CD28 and CTLA-4 receptors. Immunity. 1994;1:793–801. doi: 10.1016/s1074-7613(94)80021-9. [DOI] [PubMed] [Google Scholar]
- 13.van der Merwe PA, Bodian DL, Daenke S, et al. CD80 (B7-1) binds both CD28 and CTLA-4 with a low affinity and very fast kinetics. J Exp Med. 1997;185:393–403. doi: 10.1084/jem.185.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walunas TL, Lenschow DJ, Bakker CY, et al. CLTA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1:405–413. [PubMed] [Google Scholar]
- 15.Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T-cells to stimulation. J Exp Med. 1995;182:459–465. doi: 10.1084/jem.182.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Waterhouse P, Penninger JM, Timms E, et al. CTLA-4 deficiency causes lymphoproliferative disorder with early lethality. Science. 1995;1995(270):985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 17.Tivol EA, Borriello F, Schweitzer AN, et al. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role for CLTA-4. Immunity. 1995;3:541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 18.Egen JG, Kuhns MS, Allison JP. CLTA-4: new insights into its biological function and use in tumor immunotherapy. Nat Immunol. 2002;3:611–618. doi: 10.1038/ni0702-611. [DOI] [PubMed] [Google Scholar]
- 19.Lee KH, Holdorf AD, Dustin ML, et al. T cell receptor signaling precedes immunological synapse formation. Science. 2002;295:1539–1542. doi: 10.1126/science.1067710. [DOI] [PubMed] [Google Scholar]
- 20.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 21.van Elsas A, Hurwitz AA, Allison JP. Combination immunotherapy of B16 melanoma using anti-CTLA-4 and GM-CSF producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J Exp Med. 1999;190:355–366. doi: 10.1084/jem.190.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bankert RB, Egilmez NK, Hess SD. Human/SCID chimeric models for preclinical evaluation of anti-cancer therapies: Applications, limitations and future directions. Trends Immunol. 2001;22:368–393. doi: 10.1016/s1471-4906(01)01943-3. [DOI] [PubMed] [Google Scholar]
- 23.Egilmez NK, Hess SD, Chen FA, et al. CD4+ effector T-cells mediate an indirect IL-12 and IFN-gamma-dependent suppression of autologous lung tumor xenografts in SCID mice. Cancer Res. 2002;62:2611–2617. [PubMed] [Google Scholar]
- 24.Iwanuma Y, Chen FA, Egilmez NK, et al. Antitumor immune response of human peripheral blood lymphocytes co-engrafted with tumor nito severe immunodeficient mice. Cancer Research. 1997;57:2937–2942. [PubMed] [Google Scholar]
- 25.Linsley PS, Greene JL, Tan P, et al. Co-expression and functional cooperation of CTLA-4 and CD28 on activated T-lymphocytes. J Exp Med. 1992;176:1595–1604. doi: 10.1084/jem.176.6.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bender A, Sapp M, Schuler G, et al. Improved methods for the generation of dendritic cells from non-proliferating progenitors in human blood. J Immunol Methods. 1996;196:121–135. doi: 10.1016/0022-1759(96)00079-8. [DOI] [PubMed] [Google Scholar]
- 27.Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods. 1993;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 28.Liu Y. Is CTLA-4 a negative regulator for T-cell activation. Immunol Today. 1997;18:569–72. doi: 10.1016/s0167-5699(97)01170-5. [DOI] [PubMed] [Google Scholar]
- 29.Thompson CB, Allison JP. The emerging role of CTLA-4 as an immune attenuator. Immunity. 1997;7:445–450. doi: 10.1016/s1074-7613(00)80366-0. [DOI] [PubMed] [Google Scholar]
- 30.Yang Y. Enhanced induction of antitumor T-cell responses by cytotoxic T lymphocyte associated molecule-4 blockade: The effect is manfiested only at the restricted tumor-bearing stages. Cancer Res. 1997;57:4036–4041. [PubMed] [Google Scholar]
- 31.Hurwitz AA, Yu TF, Leach DR, Allison JP (1998) CLTA-4 blockade synergizes with tumor-derived GM-CSF for treatment of an experimental mammary carcinoma. Proc Natl Acad Sci USA (10067–10071) [DOI] [PMC free article] [PubMed]
- 32.Hurwitz AA, Foster BA, Kwon ED, et al. Combination immunotherapy of primary prostate cancer ina transgenic mouse model using CTLA-4 blockade. Cancer Res. 2000;60:2444–2448. [PubMed] [Google Scholar]
- 33.Mokyr MB, Kalinchenko T, Gorelik L, Bluestone JA. Realization of the therapeutic potential of CTLA-4 blockade in low-dose chemotherapy-treated tumor-bearing mice. Cancer Res. 1998;58:5301–5304. [PubMed] [Google Scholar]
- 34.Phan GQ, Yang JC, Sherry RM, et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci USA. 2003;100:8372–8377. doi: 10.1073/pnas.1533209100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hodi FS, Mihm MC, Soiffer RJ, et al. Biologic activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proc Natl Acad Sci USA. 2003;100:4712–4717. doi: 10.1073/pnas.0830997100. [DOI] [PMC free article] [PubMed] [Google Scholar]