Abstract
Dendritic cells (DC) are activated by pathogens, cytokines and activated T cells. We investigated the impact of a transient initial DC stimulation on the kinetics of maturation using a combination of double-stranded RNA and TNFα and subsequent restimulation by T cell-derived stimuli. Transient stimulation of DC was sufficient to start an irreversible program of phenotypic maturation which proceeded in the absence of the initial stimulus. Transiently stimulated DC secreted lower amounts of IL-12 during the 48-h period of the first stimulation than cells activated for 48 h. Although both DC preparations expressed the same level of maturation-associated markers at 48 h, DC stimulated for shorter periods preserved higher sensitivity to boosting upon subsequent stimulation by T cell-derived signals. We showed that DC initially stimulated for shorter periods were more potent stimulators of T lymphocytes and they induced a more polarized Th1 response. These results indicate that short exposure of DC to maturation stimuli enables an efficient defensive immune response induction by differentially regulating phenotypic maturation and cytokine production of DC.
Keywords: Dendritic cell, Maturation, Kinetics, Immune response, Immunotherapy
Introduction
Dendritic cells (DC) are considered to be the most efficient "professional" antigen-presenting cells (APC) that have the ability to prime naive T cells and initiate primary immune responses [4]. They differentiate from hematopoietic stem cells and exist in two distinct states commonly termed immature and mature. Immature DC reside in peripheral tissues, sample the environment through the uptake of surrounding antigens, continuously migrate into lymph nodes and recent reports suggest their role in the maintenance of peripheral tolerance against self-antigens [10, 14]. To efficiently prime naive T lymphocytes, DC have to be activated (matured). DC maturation is triggered by products derived from microbial and viral pathogens, such as LPS, CpG DNA, peptidoglycan and double-stranded RNA. Additional DC stimulation is achieved and modulated by proinflammatory cytokines such as TNFα, IL-1β and prostaglandin E2 or by a variety of noninflammatory and pathogen-unrelated factors such as histamine, heparin and ATP [23]. These auxiliary stimuli influence the nature of the maturational process and provide DC with different capacities for T cell effector subset priming [18].
Maturation is a complex process associated with a decreased antigen-capturing capacity and the orchestrated secretion of various cytokines, particularly IL-12, the prototypical Th1-polarizing cytokine [34], and IL-10 that exerts an inhibitory effect on DC maturation [32]. The upregulation of MHC class I and class II and of costimulatory molecules synergistically enhances the antigen-presenting capacities of DC and their T cell-stimulatory potential. Maturing DC also acquire augmented migratory functions through a switch in the expression of chemokine receptors [28], and this culminates in the arrival of activated DC in the T-cell zone of lymph nodes to interact with T cells. DC maturation thus has to be viewed as a process that coordinately regulates DC antigen capturing, processing and presentation, expression of costimulatory molecules and cytokine secretion in time and space.
In vivo migration studies have shown that the majority of DC reach the lymph nodes within 4 to 8 h after an initial maturation signal and their influx continues up to 48 h [36]. Thus their exposure to a maturation-inducing pathogen, in the case of localized infection, is likely to be only transient and the T cell-stimulatory properties of maturing DC are further enhanced by subsequent interactions with activated antigen-specific T cells. As recently reported, prolonged in vitro stimulation leads to the development of DC that have already exhausted their IL-12 production capacity and become refractory to further stimulation [11, 19]. Analogous results have been obtained in an in vivo model based on the systemic administration of soluble Toxoplasma gondii antigens [26]. These exhausted DC preferentially prime Th2 and nonpolarized T cells [20]. Based on these results, Lanzavecchia and Sallusto have proposed an elegant model in which recently activated DC secreting IL-12 preferentially drive Th1 polarization, whereas DC surviving in the T-cell areas or DC entering secondary lymphoid organs later (>24 h) after maturation exhaust their IL-12-producing capacity and participate in the downregulation of the immune response and in the formation of the central memory T-cell pool [22].
Intriguingly, the T cell-stimulatory capacity of DC depends directly on the surface levels of costimulatory molecules that reach their highest levels later in the maturation process. Thus recently activated DC, secreting the Th1-polarizing cytokine IL-12, and leaving the tissues providing maturation signals, do not yet express the maximal levels of these costimulatory molecules and cannot act as the most efficient APC. Moreover, before encountering antigen-specific T cells, maturing DC are likely to undergo a number of non-cognate interactions, and additional activating signals provided by activated T cells may be available only later, when exhausted DC can no longer be restimulated.
To gain an insight into these discrepancies, we investigated the impact of a transient initial stimulation of monocyte-derived DC on the kinetics of their phenotypic and functional maturation. It is important to consider that DC are exposed sequentially to several maturation stimuli, i.e. first to pathogen and inflammatory cytokines while in peripheral tissues and then to T cells once they have reached the lymph nodes. We followed this two-step activation model by using the combination of synthetic double-stranded RNA [polyriboinosinic polyribocytidylic acid, poly(I:C)] and TNFα as the strong first maturation signals [31], mimicking the presence of pathogen in the inflamed tissue, and subsequent restimulation by two molecules representing activated T cell-derived stimuli: CD40L and IFNγ [29]. We investigated the impact of a transient initial stimulation of monocyte-derived DC on the kinetics of their phenotypic and functional maturation, on their potential to be restimulated by activated T cell-derived stimuli and on their T-cell priming capacities. Our results indicate that short exposure of DC to maturation stimuli enabled an efficient defensive immune response to be mounted by differentially regulating phenotypic maturation and cytokine production of DC. They also have direct implications for the protocols used for the production of DC for immunotherapy trials.
Materials and methods
Media and cell lines
Complete culture medium (CM) was used for culture of lymphocytes and DC and consisted of X-Vivo 15 (BioWhittaker, Walkersville, Md.) plus 2 mM l-glutamine (Gibco BLR, Paisley, UK) and 1% penicillin/streptomycin (Gibco). Mouse fibroblasts transfected with a human CD40L (3T6) (kindly provided by Prof. R. Bataille, INSERM, U463, Nantes, France) were cultured in RPMI-1640 (BioWhittaker) supplemented with 10% heat-inactivated fetal calf serum (Eurobio, Les Ulis, France), 2 mM l-glutamine, and 1% penicillin/streptomycin at 37°C in an atmosphere containing 5% CO2.
Generation of immature DC
Immature monocyte-derived DC were generated from the leukapheresis products of five healthy donors obtained from the Etablissement Français du Sang in Nantes after obtaining informed consent. Peripheral blood mononuclear cells (PBMC) were separated from leukapheresis harvests by Ficoll-Paque (Amersham, Uppsala, Sweden) gradient centrifugation and cultured in gas-permeable hydrophobic bags (Baxter) at 5×106 cells/ml in CM with 500 U/ml of GM-CSF (Leucomax, Novartis, Rueil-Malronion, France) and 15 ng/ml of IL-4 (AbCys, Paris, France). Fresh cytokines were added on day 4 of culture. On day 7, immature DC were separated from the cell suspension by elutriation (counterflow centrifugation) (Beckman Coulter, Villepinte, France), yielding a DC population >90% pure as demonstrated by FACS analysis.
DC maturation
Day-7 immature DC were seeded in 24-well plates (Falcon, Le Pont de Claix, France) at 1×106 DC/ml in CM with GM-CSF and IL-4. Concomitant treatment with TNFα at 20 ng/ml (AbCys) and poly(I:C) 50 μg/ml (Sigma, St Quentin Fallavier, France) was used to induce DC maturation as previously described [30]. At indicated time-points, maturation stimuli were removed by centrifugation and DC were replated in CM with GM-CSF and IL-4. For the second stimulation, DC were cultured for 48 h on a layer of murine fibroblasts transfected with human CD40L in the presence of 1000 U/ml of IFNγ (AbCys).
Flow cytometric analysis of cell-surface phenotype and intracellular staining
For the phenotypic analysis, FITC- or PE-conjugated monoclonal antibodies (mAbs) against the following molecules were used: CD36, CD40, CD80, HLA-DR, DC-LAMP (Immunotech, Villepinte, France), CD14, CD83, CD86, HLA-ABC (BD Pharmingen, Le pont de Claix, France), and CCR7 (R&D Systems, Abingdon, UK). Cells were stained for 30 min at 4°C and washed twice in phosphate-buffered saline (PBS) plus 0.1% bovine serum albumin (BSA). For the intracellular detection of DC-LAMP, cells were first fixed in 4% paraformaldehyde for 10 min at room temperature and washed in PBS plus 0.1% BSA. Cells were then permeabilized with 0.1% saponin and incubated with PE-DC LAMP for 30 min. Stained DC were analyzed on a FACScalibur system (Becton Dickinson) using Cell Quest Pro software. The DC population was gated according to its forward-scatter and side-scatter properties and dead cells were excluded on the basis of propidium iodide (Sigma) or TO-PRO3 iodide (Molecular Probes, Montlucon, France) staining. Appropriate negative controls were included and at least 5000 viable DC were counted in each experiment.
Cytokine detection
At each time-point, supernatants were collected and stored at 80°C until analysis using commercially available ELISA kits (Pharmingen) to measure production by DC of IL-12 p70 (the bioactive heterodimer of IL-12) and IL-10 following the manufacturer's procedure. For the detection of IL-12 p35 and p40 mRNA, RT-PCR was performed as follows. DC (106/ml) were stimulated with the combination TNFα and poly(I:C). Unstimulated DC were used as negative control. Total RNA was isolated at various time-points using Trizol (Gibco) extraction. Complementary DNA was synthesized from the total RNA using a first-strand cDNA synthesis kit for RT-PCR (Roche Molecular Biochemical, Mannheim, Germany). DNA amplification was carried out on an MJ Research PTC-200 thermal cycler (Waterton, Mass.). The oligonucleotide primers (Genosys-Sigma, Cambridge, UK) used for PCR were 5′-CCTTCACCACTCCCAAAACCT/5′-TGAAATTCAGGGCCTGCATC for IL-12 p35 and 5′-GGATGCCCCTGGAGAAATGG/5′-CTCCCAGCTGACCTCCACCT for IL-12 p40 resulting in products of 407 bp and 655 bp, respectively. A total of 40 PCR cycles were conducted under the following conditions: 30 s at 94°C, 30 s at 63°C and 1 min at 72°C followed by final extension for 5 min at 72°C. Amplification of β-actin was performed as a control for cDNA integrity. Ethidium bromide-stained PCR products were analyzed on a 1.2% agarose gel.
Th1/Th2 polarization
Allogeneic naive T cells were cocultured with DC at a ratio of 10:1. After 7 days, T cells were further expanded for 1 week with IL-2 (20 U/ml) (AbCys). On day 14, T cells were stimulated with 15 ng/ml of phorbol 12-myristate acetate (Sigma) and 1 μg/ml of calcium ionomycin (Sigma) for 6 h and the production of IL-4 and IFNγ was detected by intracellular staining using PE-IL-4 and FITC-IFNγ (Becton Dickinson). Brefeldin A (10 μg/ml) (Sigma) was added for the last 4 h.
Mixed leukocyte reaction (MLR)
Graded numbers of DC matured for the indicated times were cultured in triplicate in U-bottomed 96-well plates (Falcon) with 1×105 allogeneic lymphocytes in a final volume of 200 μl. Proliferation of allogeneic T cells was determined after 4 days by uptake of 3H-thymidine (1 μCi/well) for the last 18 h.
Antigen-presentation assay
Immature DC (1×106) were cultured in 1 ml CM in 24-well plates (Falcon) and pulsed overnight with 24 μg/ml of keyhole limpet hemocyanin (KLH) (Calbiochem, Meudon, France). Pulsed DC were matured and used as stimulators for autologous T lymphocytes (1×105) at the DC/T ratios of 1:10, 1:20, 1:40 and 1:80 in U-bottomed 96-well plates in a final volume of 200 μl/well. Proliferation of autologous lymphocytes was measured in triplicate after 7 days of culture by incorporation of 3H-thymidine (1 μCi/well) for the last 18 h of assay.
Statistical analysis
Student's t-test was used to evaluate the differences between the two groups. P values <0.05 were considered statistically significant.
Results
Kinetics of DC maturation induced by a 48-h treatment with TNFα plus poly(I:C)
The generation of DC in hydrophobic bags prevented the maturation of cells during the differentiation period that may have resulted from mechanical activation and enabled a homogeneous cell population with a typical immature phenotype to be obtained. Treatment of immature DC for 48 h with TNFα plus poly(I:C) led to the complete maturation of all cells, reflected by appropriate changes in the phenotypic profile (Fig. 1). We first determined the kinetics of DC maturation during a 72-h continuous exposure to TNFα plus poly(I:C) (Fig. 2). All cells in culture responded homogeneously to the activation stimuli used and we thus present the mean fluorescence intensity values (MFI) of the markers studied. Two types of profiles were observed. The first group of intracellular (DC-LAMP) and cell surface (CD80, CD83, CD86, HLA-DR) molecules followed activation kinetics characterized by a gradual increase of expression during culture, reaching a plateau between 24 and 48 h, depending on the donor. In contrast, CD40 and HLA-ABC followed a slightly different expression profile with rapid upregulation after 12 h of culture, reaching maximal expression from 16 to 20 h after the start of exposure to TNFα plus poly(I:C). A similar, albeit inverted, profile was seen for CD36 and CD14 (not shown) downregulation, as we observed its rapid decrease within 12 h. Of particular note was the unique expression profile of CCR7, which was rapidly upregulated between 2 and 4 h after the start of stimulation, and then decreased slightly or remained stable.
Fig. 1.
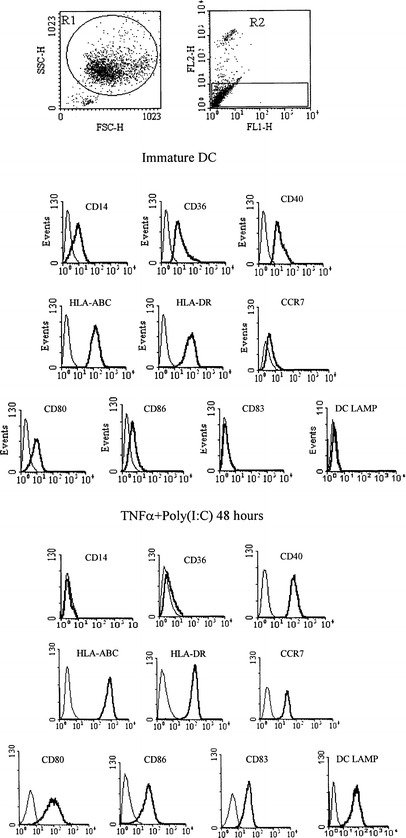
Phenotype of immature DC and of DC activated for 48 h by continuous TNFα plus poly(I:C) treatment. Day-7 immature DC were elutriated and cultured for 48 h in the presence (lower panel) or absence (upper panel) of TNFα plus poly(I:C). DC were gated according to their morphological proprieties (R1) and only viable cells (propidium iodide negative) were included in the final analysis. The thick lines represent the specific expression of the investigated molecule, and the thin lines represent the isotype control staining. The profiles are representative of 20 independent experiments
Fig. 2.
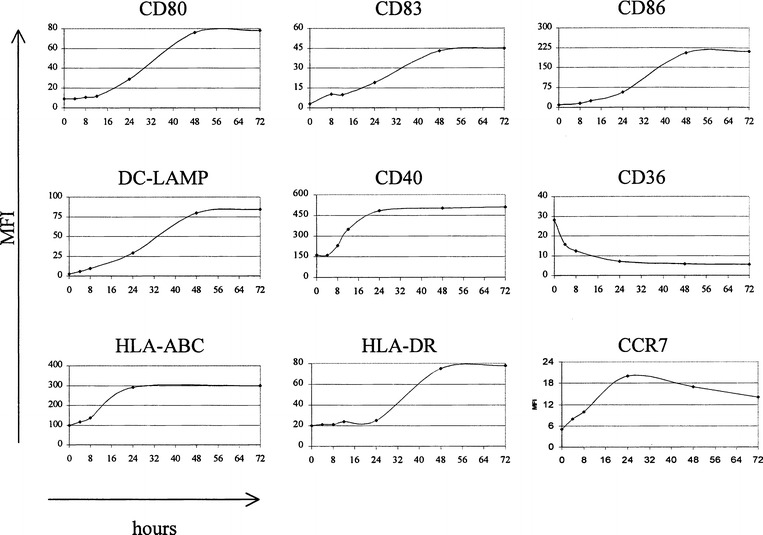
Kinetics of changes in the expression pattern of different surface or intracellular (DC-LAMP) molecules during 72 h continuous treatment with TNFα plus poly(I:C). Labeled cells were analyzed at the indicated time-points by flow cytometry, and the values represent mean fluorescence intensity (MFI). The level of nonspecific fluorescence, obtained with an isotype-matched irrelevant mAb were set at MFI 4. Representative results of one out of six independent experiments with different DC preparations are shown
Simultaneously, we studied the kinetics of p70 IL-12 and IL-10 production by stimulated DC (Fig. 3). IL-10 was detected in culture supernatants as early as 1 h after the start of stimulation and it continued to be produced throughout the whole culture period, i.e. until at least 48 h. Secretion of the biologically active heterodimer p70 IL-12 was detectable only after 12 h and virtually all IL-12 was secreted within 12–24 h after the start of stimulation. These findings correlated with the results obtained for the expression of p35 and p40 subunits of p70 IL-12 at the mRNA levels. We detected p35 mRNA in the sample taken 4 h after the start of TNFα plus poly(I:C) exposure and p40 mRNA, the regulatory subunit permitting formation of the biologically active heterodimer, was detectable in cells treated for longer than 12 h. Messenger RNA for p35 and p40 subunits could no longer be detected in DC treated for 48 h.
Fig. 3.
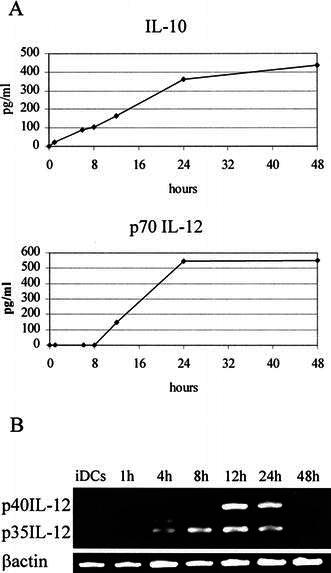
A Kinetics of IL-10 and p70 IL-12 production by DC during 48 h continuous exposure to TNFα plus poly(I:C). Concentrations of cytokines present in the culture supernatants were determined at different time-points by ELISA. B Analysis of p35 and p40 IL-12 mRNA expression by RT-PCR. Representative results of one out of four experiments with different DC preparations are shown
Phenotypic DC maturation is irreversible, proceeds in the absence of maturation stimuli and transiently activated DC can be further reboosted by stimulation with CD40L plus IFNγ
It is conceivable that, in the case of localized infection, the exposure of immature DC to activating stimuli is limited and ceases after the DC leave the inflamed tissue. We thus analyzed the 48-h phenotype of DC matured for restricted periods to evaluate the length of the initial stimulation necessary for the initiation of irreversible maturation. For this purpose, immature DC were stimulated for the indicated times and then washed twice before being replated in CM without TNFα plus poly(I:C). For each treatment, DC phenotype and cytokine production were evaluated at the time of removal of the maturation stimuli and 48 h after the start of the assay. All cells activated with TNFα plus poly(I:C) showed a uniform phenotype at 48 h, whatever the period of initial DC activation (Fig. 4). Indeed, only 15 min of treatment (the shortest period tested) sufficed to induce the same level of phenotypic maturation as a prolonged 48-h exposure.
Fig. 4.
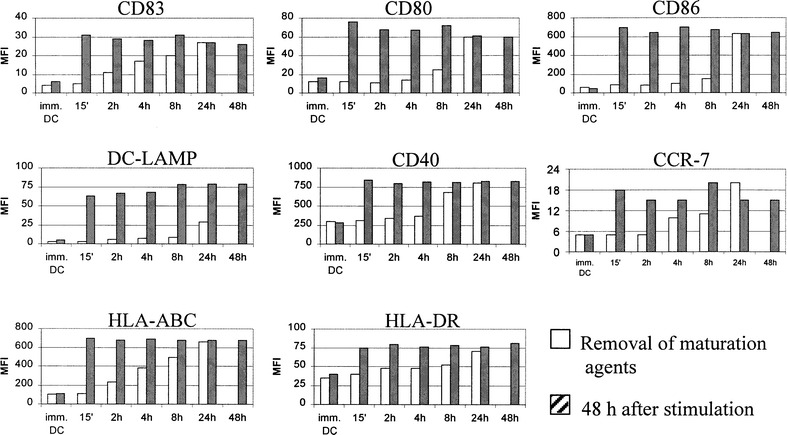
Phenotypic DC maturation by exposure to TNFα plus poly(I:C) is an irreversible phenomenon proceeding independently of further presence of maturation stimuli. Immature DC were stimulated for the indicated times with TNFα plus poly(I:C). The expression of maturation-associated phenotypic markers was determined by FACS at the moment of removal of maturation agents and 48 h after the initial stimulation. Representative results of one out of three independent experiments are shown
In the next set of experiments, we investigated the potential of mature DC to react to a second signal. DC initially exposed to TNFα plus poly(I:C) for different times were harvested after 48 h of culture and restimulated by the combination of two physiologically relevant T cell-derived signals, CD40L plus IFNγ for a further 48 h. As shown in Fig. 5, DC exposed to TNFα plus poly(I:C) for 2 to 8 h preserved their capacity to further upregulate the expression of their costimulatory and maturation-associated markers. In contrast, DC stimulated for 24 or 48 h became refractory to the second stimulation as no or only moderate increases in the investigated molecules were observed.
Fig. 5.
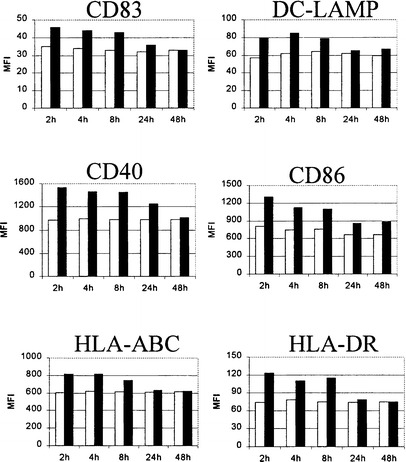
The effect of the restimulation by CD40L plus IFNγ on the phenotypic properties of DC initially matured by TNFα plus poly(I:C). DC were first exposed to TNFα plus poly(I:C) for the indicated times. Maturational agents were then removed and all DC preparations were restimulated with a human CD40L-transfected cell line plus IFNγ (1000 U/ml) 48 h after initiation of the culture. The expression of maturation-associated phenotypic markers on either CD40L plus IFNγ-restimulated (■) or non-restimulated (□) DC was analyzed by FACS 48 h after the second stimulation. The results presented are representative of one out of five experiments conducted with DC generated from different donors
Kinetics of p70 IL-12 production by maturing DC depends on the time of the initial exposure to TNFα plus poly(I:C)
Consistent with the uniform mature phenotype, all studied 48-h DC preparations produced comparable levels of IL-10 (data not shown). Interestingly, this was not the case for the second cytokine tested, p70 IL-12. DC cultured with TNFα plus poly(I:C) for prolonged periods (>8 h) produced high levels of IL-12, while shorter exposures were sufficient to initiate only low levels of p70 IL-12 secretion during the first 48 h of stimulation (Fig. 6A).
Fig. 6.
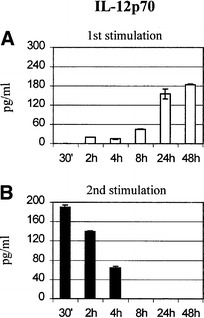
Concentrations of p70 IL-12 in 48-h culture supernatants of DC initially stimulated for different times. DC were first exposed to TNFα plus poly(I:C) for the indicated times. Maturation agents were then removed and all DC preparations were restimulated with a human CD40L-transfected cell line plus IFNγ (1000 U/ml) 48 h after initiation of the culture. The concentration of p70 IL-12 in the culture supernatants was determined for each condition by ELISA (A) 48 h after initial stimulation with TNFα plus poly(I:C) (□) and (B) 48 h after restimulation in the presence of CD40L-transfected fibroblasts and 1000 U/ml IFNγ (■). Representative results of one out of four independent experiments are shown
Conversely, CD40L plus IFNγ restimulation of transiently activated DC elicited the secretion of p70 IL-12 while restimulated DC initially exposed to TNFα plus poly(I:C) for more than 8 h showed no IL-12 production (Fig. 6B), although they still produced low levels of IL-10 (not shown). The levels of cytokines secreted by DC generated from different donors varied but their kinetics remained unchanged.
Thus the ability of mature DC to respond 48 h after first activation to a second stimulation decreased with the time of exposure to the initial maturation agents. In our model, this was true for the kinetics of phenotypic changes and for p70 IL-12 production.
T cell-stimulatory and polarizing capacities of DC stimulated with TNFα plus poly(I:C)
We next investigated the impact of DC maturation kinetics as presented above on their T cell-stimulatory and polarizing capacity. First, DC were activated for different times and used directly as stimulators in a standard 4-day MLR (Fig. 7A). DC stimulated for 4 or 8 h reproducibly induced slightly higher proliferation of allogeneic T lymphocytes than DC treated for 12, 24 or 48 h. However, DC treated for 2 h (the preparation demonstrated to retain the highest capacity for being reboosted by CD40L plus IFNγ restimulation) did not promote T-cell proliferation above the levels observed for immature DC. The same, with an even more pronounced tendency, was observed in an autologous setting, using KLH-pulsed DC matured for different times. Again, DC exposed to TNFα plus poly(I:C) for 4 or 8 h consistently induced higher proliferation of autologous naive T cells while DC activated for 2 h were repeatedly unable to trigger their expansion (Fig. 7B). Alternatively, immature DC were exposed to TNFα plus poly(I:C) for the indicated times, TNFα plus poly(I:C) was then removed, and all DC preparations were used as stimulators of allogeneic or autologous lymphocytes 48 h after the initial activation. Under these conditions, DC initially treated for 2 h stimulated T cells to a level comparable to that stimulated by DC matured for 4 and 8 h and significantly better (P<0.01) than DC initially exposed to TNFα plus poly(I:C) for 24 and 48 h (Fig. 7C, D).
Fig. 7A–D.
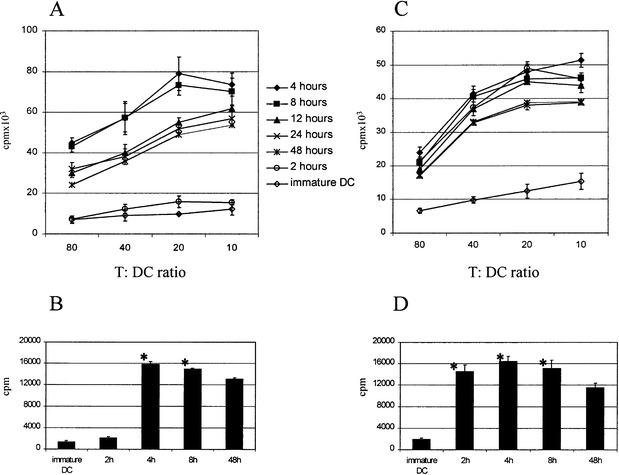
T cell-stimulatory characteristics of DC as a function of time of initial exposure to TNFα plus poly(I:C). A Allogeneic mixed leukocyte reaction. Graded numbers of DC matured for different times by TNFα plus poly(I:C) were used to stimulate allogeneic T lymphocytes. Proliferation was measured after 18 h of 3H-thymidine incorporation on day 4. B Stimulation of autologous lymphocytes by different KLH-pulsed DC preparations. Immature DC were pulsed overnight with KLH and exposed to TNFα plus poly(I:C) for the indicated times and used immediately as stimulators of autologous T lymphocytes. Proliferation of autologous lymphocytes was determined after 7 days by means of 3H-thymidine incorporation for the last 18 h. C, D Alternatively, immature DC were exposed to TNFα plus poly(I:C) for the indicated times. TNFα plus poly(I:C) was then removed, and all DC preparations were used as stimulators of allogeneic (C) or autologous (D) lymphocytes 48 h after the initial activation. The values are means±SD of triplicate determinations. Similar results were obtained in six separate experiments. Asterisks indicate significantly higher activated DC-induced proliferation of autologous T lymphocytes than proliferation induced by DC exposed to TNFα plus poly(I:C) for 48 h (P<0.01)
Simultaneously, we investigated the impact of different maturation times on the type of Th response generated. Expanded CD4 T cells were evaluated for IL-4 and IFNγ production by intracellular staining. As shown in Fig. 8, all preparations of TNFα plus poly(I:C)-matured DC preferentially induced a Th1 response. The proportion of IL-4-producing cells never exceeded 1.5%. The Th1-polarizing effect was especially pronounced for 48-h DC preparations initially exposed to TNFα plus poly(I:C) for 4 and 8 h, whereas for 2, 24 and 48-h exposures, a higher fraction of cells remained nonpolarized.
Fig. 8.
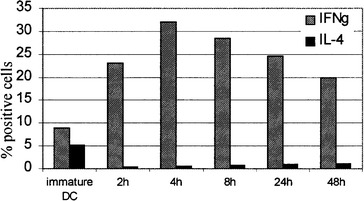
Th-polarizing capacity of DC initially matured for different times. 48-h preparations of DC activated for the indicated times were used to stimulate allogeneic T lymphocytes at a ratio of 1:10. After 14 days, the responder T cells were restimulated with PMA/calcium ionomycin for 6 h and the production of IL-4 and IFNγ was measured by intracellular labeling. Results, show the percentage of CD4 T cells (gated by means of CD4-PerCP staining) secreting IFNγ and IL-4 in three independent experiments
Discussion
The recognition of the presence of pathogen by immature DC triggers the sequence of events commonly termed maturation, resulting in DC capable of priming the primary immune response. DC maturation coordinately regulates the phenotypic and functional changes and ensures the transition between immature DC sampling their microenvironment for the presence of pathogens to the mature DC present in secondary lymphatic organs and effectively stimulating naive T cells. Activated T cells reciprocally activate DC via CD40L-CD40 [27] and tumor necrosis factor-related activation-induced cytokine (TRANCE) receptor activator of nuclear factor kappa B (RANK) signaling leading to increased expression of costimulatory molecules, secretion of cytokines and enhanced T-cell responses [3].
In the setting of localized infection, the exposure of DC to invading pathogen and simultaneously produced inflammatory cytokines is likely to be only transient, as the concentration gradients decline after DC exit the site of infection and migrate towards the T cell area of the proximal lymph nodes via afferent lymphatics. In this study, we investigated the impact of a transient initial stimulation of monocyte-derived DC on the kinetics of their phenotypic and functional maturation, on their potential to be restimulated by activated T cell-derived stimuli and on their T cell-priming capacities. The combination TNFα plus poly(I:C) used for the first stimulation matured the entire DC population after a continuous 48-h exposure, with a gradual increase in the expression of investigated maturation-associated phenotypic markers, reaching a maximum between 24 and 48 h after stimulation, depending on the donor. In accordance with previous reports [20], we detected a rather stable production of IL-10 throughout the whole culture period. Immature DC secreted low levels of IL-10 and a majority of the maturation stimuli described further promoted its production during maturation. IL-10 neutralization has been reported to be sufficient to induce spontaneous DC maturation [8]. Thus this continuous IL-10 production by monocyte-derived DC possibly represents a mechanism preventing an exaggerated or dysregulated immune response. A different secretion profile was observed for p70 IL-12, as its production was restricted to a narrow time-window between 12 and 24 h after maturation.
We showed that a strong maturation stimulus starts the complete irreversible phenotypic maturation program which proceeds independently of its further presence. However, we noted important differences for the production of p70 IL-12, a major Th1-priming cytokine, as its secretion was dependent on the long-term presence of poly(I:C). The production of high quantities of p70 IL-12 in 48-h culture supernatants required exposure to poly(I:C) for at least 8 h and was highest for the cells treated for 48 h. In the next series of experiments, phenotypically uniform 48-h DC populations initially exposed to TNFα plus poly(I:C) for different times were restimulated by a combination of CD40L and IFNγ, T cell-derived stimuli known to synergize in their activating effect on DC maturation. This second stimulation efficiently reboosted those DC that were treated with TNFα plus poly(I:C) for 8 h or less. We observed a further increase in the expression of maturation-associated phenotypic markers that was more pronounced at the level of costimulatory molecules and maturation-specific markers than for the molecules associated with antigen presentation, i.e. MHC class I and class II whose levels increased moderately. Moreover, restimulation of DC previously exposed to TNFα plus poly(I:C) for less than 8 h resulted in the production of high quantities of p70 IL-12 as opposed to virtually no detectable p70 IL-12 in DC initially activated for more than 12 h.
The dissociation between the uniform course of maturation-related phenotypic marker expression and strikingly different cytokine production profiles of DC stimulated by TNFα plus poly(I:C) for various times is likely to have been caused by the different requirements for initiation of the intracellular signaling pathways. Poly(I:C) is a molecule promoting p70 IL-12 secretion in our model, as TNFα stimulation does not lead to its production [21, 30]. Recently, the action of poly(I:C) has been shown to be mediated by TLR-3, a member of the Toll-like receptor family [2]. Interestingly, DC originating from knockout mice lacking MyD88, an adapter protein implicated in the upstream events of TLR signaling, have been shown to mature phenotypically upon activation while their capacity to produce cytokines is severely reduced. Thus, MyD88-dependent and MyD88-independent pathways could represent the evolutionary mechanism ensuring the spatial dissociation of phenotypic and functional DC maturation. As similar observations have been reported for LPS [16], known to act through TLR-4 [7], it would be interesting to compare the characteristics of LPS-stimulated DC to the response of DC stimulated by poly(I:C). In future, the dissection of the signal transduction pathways engaged by individual receptors under different signaling conditions will help to reveal both common and stimulus-specific maturation programs.
The capacity of DC to produce IL-12 in response to a second activation signal has recently been reported to decline with the time of activation [11, 19]. Following the initial LPS stimulation, this phenomenon of "exhaustion" leads to higher Th2 priming [20]. In all these models DC were treated continuously with the initial maturation stimuli and restimulated the second time immediately after their removal. We extended these observations and showed that DC activated for short periods, i.e. less than 8 h, preserve their capacity to produce p70 IL-12 upon restimulation even after they complete the program of phenotypic maturation.
We further showed that DC initially stimulated for shorter periods, i.e. less than 8 h were significantly more potent stimulators of both allogeneic and autologous T lymphocytes and they induced a more polarized Th1 response. However, in contrast to the results reported by Langenkamp et al. [20], we did not observe any Th2 priming by DC stimulated for 24 or 48 h. This discrepancy could be explained by the TNFα plus poly(I:C) activation model used in our study, as poly(I:C) induces the development of extremely potent Th1-inducing DC in contrast to the LPS stimulation used by Langenkamp et al. These findings confirm that immune responses to different types of pathogens are associated with different types of effector responses directed by polarized Th1 or Th2 cell subsets [9, 25].
It is important to note that DC stimulated for 2 h and immediately used as stimulators repeatedly failed to activate T lymphocytes. It is conceivable that, although the maturation program has already been initiated, the expression of costimulatory and antigen presentation-associated molecules has not yet reached the threshold needed for T-cell priming. Notably, the switch in the expression of CCR7, a molecule indicating the capacity of DC to migrate to the lymph nodes, occurred between 2 and 4 h after DC stimulation. It is tempting to speculate that the synchronized CCR7 expression prevents the interaction between suboptimally activated DC and naive T lymphocytes. Several recent reports challenge the current paradigm that consider immature DC as a cell type responsible for the induction of immune tolerance and suggest that costimulation is required to anergize T cells [1, 24]. In the light of these reports indicating the role of DC activation in the induction of peripheral T-cell tolerance, we are currently investigating whether T cells primed by DC stimulated for less than 2 h are actively anergized or whether they simply ignore the presented antigens because of the low levels of costimulatory molecules at the moment of initial contact.
The stimulatory and polarizing properties of mature DC are determined by the initial maturation stimuli. Pathogen-derived stimuli stimulating IL-12 production are known to induce a potent Th1 response while DC modulated by histamine [6] and prostaglandin E2 [17] prime Th2 lymphocytes. Th1 vs Th2 priming is also influenced by the relative DC:T cell ratio [33] and the quantity of antigen [13]. Our results further show that the kinetics of maturation and the length of initial exposure of DC to a pathogen in an inflamed tissue are important variables determining the final outcome of immune response.
Altogether, our results permit us to envisage the following scenario. Upon encounter between DC and a pathogen invading peripheral tissue, phenotypic DC maturation is triggered by ligation of the corresponding TLRs and further modulated by concomitantly secreted proinflammatory cytokines. Following the pathogen-related maturation signal, DC rapidly upregulate CCR7 and home to the lymph nodes in large numbers as early as 4 h after infection [35, 36]. In vivo, maturing DC most probably have to undergo a number of noncognate interactions before meeting the antigen-specific naive T lymphocyte. During this period, phenotypic maturation continues and the expression of costimulatory and antigen-presentation molecules gradually increases. The interaction with a T cell specific for a presented MHC-restricted peptide elicits T lymphocyte activation accompanied by the upregulation of CD40L, TRANCE [15], and production of IFNγ. These signals then reciprocally activate DC, prolong their survival, trigger the production of p70 IL-12 and enhance their T cell-activating properties. Thus, short initial exposure to maturation agents permits the restriction of cytokine production to the context of the DC:T-cell cognate interaction, and preserves the capacity of DC to enhance their costimulatory properties upon this encounter. It would be of interest to investigate the characteristics of DC during systemic infections where the sustained exposure of DC to pathogens and cytokines could lead to the exhaustion of their capacity to be reboosted. The inefficient restimulation of exhausted DC could contribute to the impaired immune response described in septic patients [5, 12].
Finally, our results are important for the use of DC in immunotherapy. In vitro protocols for DC generation have enabled the extensive study of DC physiology, and these procedures are also used to obtain large amounts of DC for cell-based cancer immunotherapies. A thorough knowledge of DC maturation kinetics and the nature of primed T cells is beneficial for forthcoming immunotherapy trials, as it is important to use DC irreversibly committed to terminal maturation, yet capable of migrating to secondary lymphoid organs, being boosted by activated T lymphocytes and secreting cytokines determining the desired polarization of the immune response.
Acknowledgements
We thank Isabelle Barbieux and Marie-Aude Robin for excellent technical assistance and Pierre-François Cartron for his help with the molecular biology experiments. This work was supported by grants from La ligue Départementale (Loire Atlantique et Vendée) Contre le Cancer, l'Association Nationale de Recherche Contre le Cancer, INSERM and the Ministere de la Recherche et de la Technologie. R.S. is a recipient of a postdoctoral fellowship from La ligue Nationale Contre le Cancer.
Footnotes
R.S. and G.B. contributed equally to this work.
References
- 1.Albert Nat Immunol. 2001;2:1010. doi: 10.1038/ni722. [DOI] [PubMed] [Google Scholar]
- 2.Alexopoulou Nature. 2001;413:732. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 3.Bachmann J Exp Med. 1999;189:1025. doi: 10.1084/jem.189.7.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banchereau Annu Rev Immunol. 2000;18:767. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 5.Bone Ann Intern Med. 1996;125:680. doi: 10.7326/0003-4819-125-8-199610150-00009. [DOI] [PubMed] [Google Scholar]
- 6.Caron J Immunol. 2001;167:3682. doi: 10.4049/jimmunol.167.7.3682. [DOI] [PubMed] [Google Scholar]
- 7.Chow J Biol Chem. 1999;274:10689. doi: 10.1074/jbc.274.16.10689. [DOI] [PubMed] [Google Scholar]
- 8.Corinti J Immunol. 2001;166:4312. doi: 10.4049/jimmunol.166.7.4312. [DOI] [PubMed] [Google Scholar]
- 9.de J Immunol. 2002;168:1704. doi: 10.4049/jimmunol.168.4.1704. [DOI] [PubMed] [Google Scholar]
- 10.Dhodapkar J Exp Med. 2001;193:233. doi: 10.1084/jem.193.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ebner J Immunol. 2001;166:633. doi: 10.4049/jimmunol.166.1.633. [DOI] [PubMed] [Google Scholar]
- 12.Ertel Blood. 1995;85:1341. [PubMed] [Google Scholar]
- 13.Hosken J Exp Med. 1995;182:1579. doi: 10.1084/jem.182.5.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jonuleit J Exp Med. 2000;192:1213. doi: 10.1084/jem.192.9.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Josien J Exp Med. 2000;191:495. doi: 10.1084/jem.191.3.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaisho J Immunol. 2001;166:5688. doi: 10.4049/jimmunol.166.9.5688. [DOI] [PubMed] [Google Scholar]
- 17.Kalinski Adv Exp Med Biol. 1997;417:363. doi: 10.1007/978-1-4757-9966-8_59. [DOI] [PubMed] [Google Scholar]
- 18.Kalinski Immunol Today. 1999;20:561. doi: 10.1016/s0167-5699(99)01547-9. [DOI] [PubMed] [Google Scholar]
- 19.Kalinski J Immunol. 1999;162:3231. [PubMed] [Google Scholar]
- 20.Langenkamp Nat Immunol. 2000;1:311. doi: 10.1038/79758. [DOI] [PubMed] [Google Scholar]
- 21.Lanzavecchia Curr Opin Immunol. 2001;13:291. doi: 10.1016/s0952-7915(00)00218-1. [DOI] [PubMed] [Google Scholar]
- 22.Lanzavecchia Cell. 2001;106:263. [Google Scholar]
- 23.Mellman Cell. 2001;106:255. doi: 10.1016/s0092-8674(01)00449-4. [DOI] [PubMed] [Google Scholar]
- 24.Menges J Exp Med. 2002;195:15. doi: 10.1084/jem.20011341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pulendran J Immunol. 2001;167:5067. doi: 10.4049/jimmunol.167.9.5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reis Immunity. 1999;11:637. [Google Scholar]
- 27.Ridge Nature. 1998;393:474. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- 28.Sallusto Eur J Immunol. 1998;28:2760. doi: 10.1002/(SICI)1521-4141(199809)28:09<2760::AID-IMMU2760>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 29.Snijders Int Immunol. 1998;10:1593. doi: 10.1093/intimm/10.11.1593. [DOI] [PubMed] [Google Scholar]
- 30.Spisek Cancer Immunol Immunother. 2001;50:417. doi: 10.1007/s002620100215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spisek Cancer Res. 2002;62:2861. [PubMed] [Google Scholar]
- 32.Steinbrink J Immunol. 1997;159:4772. [PubMed] [Google Scholar]
- 33.Tanaka J Exp Med. 2000;192:405. doi: 10.1084/jem.192.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trinchieri Immunol Res. 1998;17:269. doi: 10.1007/BF02786451. [DOI] [PubMed] [Google Scholar]
- 35.van J Invest Dermatol. 1994;103:217. doi: 10.1111/1523-1747.ep12393088. [DOI] [PubMed] [Google Scholar]
- 36.Xia J Exp Med. 1995;181:1275. doi: 10.1084/jem.181.4.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]


