Abstract
Introduction: An extended phase I trial was conducted in a total of 26 patients with ovarian cancer. The objectives were to assess the safety and tolerability of idiotypic vaccination using the murine monoclonal antibody HMFG1 (anti-MUC1), and to develop robust assays to monitor humoral immune responses generated against either the antibody or MUC1. Material and methods: All patients had undergone standard debulking surgery (where appropriate) and at least one regimen of platinum-based chemotherapy. Eligibility criteria included: (a) residual disease at the end of chemotherapy, (b) relapsed disease, and (c) pathologically confirmed second complete remission following salvage chemotherapy. Patients received a priming dose of 25 mg of HMFG1 either intravenously or intraperitoneally, followed by up to six intradermal doses of HMFG1 in 10% Alhydrogel at intervals of 1 month. The three dose levels were 0.5 mg, 1 mg and 5 mg. We devised modifications of published protocols for the measurement of anti-idiotypic and anti-MUC1 antibody responses and also extended the use of the IAsys resonant mirror biosensor to measure the kinetics of the idiotypic network response in these patients. Results: There were no serious adverse events at any dose level. The trial confirmed that all doses could be administered safely with minimal toxicity. No clinical responses were seen in patients with evaluable disease. ELISA for anti-idiotypic antibodies (Ab2) showed significant levels in patients who completed the protocol. There were no significant differences in the levels of Ab2 generated by the different doses of antibody. These results were confirmed by biosensor assays for Ab2, which also showed affinity maturation of the Ab2 response as patients progressed through the vaccination protocol. Biosensor assays also demonstrated no difference in the affinity of Ab2 generated by different booster doses of HMFG1. ELISA for anti-MUC1 antibodies showed less consistent results, with very small but statistically significant rises in anti-MUC1 signals seen in 38% of patients who completed the vaccination regimen. Discussion: The clinical endpoints of safety and tolerability were met. The assays developed for this project have shown reproducibility and may provide surrogate endpoints to assess vaccination for future trials. The use of similar biosensors may be of particular relevance for monitoring of humoral immune responses in other vaccine trials. The low levels of anti-MUC1 antibodies generated may correspond with the lack of clinical efficacy in the few patients with evaluable disease.
Keywords: Intradermal, Monoclonal antibody, MUC1
Introduction
The overall survival for epithelial ovarian cancer remains around 40% at 5 years [1]. This is regardless of the high proportion of complete remissions that can be obtained by a combination of optimal primary surgery followed by platinum-taxane chemotherapy [2]. It is clear that residual microscopic disease is not being eradicated by standard management, and no additional conventional therapy has been shown to improve survival [3].
MUC1 is a cell-surface glycoprotein which is both aberrantly glycosylated and overexpressed in many carcinomas including those of the breast, pancreas and ovary [4]. The protein backbone is composed of multiple tandem repeats (up to 100) of a peptide of 20 amino acids. Both the carbohydrate side chains and various epitopes within the peptide are recognised to some extent by the immune system. The molecule has been a target for immunotherapy both with monoclonal antibodies directed against peptide epitopes and with a vaccine based on the carbohydrate side chains [5].
Idiotypic network interactions have formed the basis for an enormous body of work describing humoral immune responses. The original hypothesis [6] predicted that an antibody (Ab1) generated against a given antigen also itself elicited a humoral immune response leading to antibodies directed against its idiotope. This second level of antibodies (Ab2), less specifically termed “anti-idiotypic,” themselves elicit a further level of anti-anti-idiotypic antibodies (Ab3) which should include species whose antigen-binding sites target the same antigen as Ab1. Where murine antibodies are administered, for example as cancer therapy, these antibodies are seen as foreign antigens, eliciting the generation of human antimouse antibodies (HAMA) in the recipient, mostly directed against the murine Fc region, but some of which are anti-idiotypic.
A phase I/II study of intraperitoneal yttrium 90–labelled HMFG1 (murine IgG1, anti-MUC1 [7]) suggested improved survival for patients in first complete remission compared with historical controls [8]. The low-level of radioactivity (1,110 MBq) delivered by this targeting strategy is unlikely to account for any antitumour effect. Humoral immune responses have been documented in these patients [9], and this prompted the question as to whether the HMFG1 was functioning as an idiotypic cancer vaccine.
Monoclonal antibodies (MAbs) have been used in vaccine strategies for other tumours (melanoma and lymphoma) with objective responses [10, 11]. The use of radiolabelled MAbs for radioimmunoscintigraphy in ovarian cancer patients has been associated with both Ab2 formation and improvements in survival [12, 13], leading to therapeutic trials of unlabelled MAbs [14, 15]. We therefore conducted a phase I trial to assess the safety and tolerability of repeated intradermal vaccination with HMFG1.
We also sought to develop assays to assess the immune response to unlabelled HMFG1 for possible use as surrogate markers of successful vaccination. We have previously reported in brief our observations using the resonant mirror biosensor in this group of patients [16].
The IAsys resonant mirror biosensor measures antigen-antibody binding in a nonisotopic, nonenzymatic system, where interaction between fixed antigen and antibody in the sample causes changes in the evanescent wave associated with a dielectric resonant layer applied to the reaction surface [17, 18]. Resonance changes are detected using polarised laser light which is reflected back to a detector/transducer. Software connected to the IAsys device records changes in the resonant angle in arcseconds on the y-axis of a continuous display, with time on the x-axis. The FASTfit software provided with the IAsys biosensor analyses the data using nonlinear curve-fitting equations. This system enables the kinetics of antigen-antibody interactions to be studied in real time. Changes in both quantity and affinity can be measured. Reference standards are required to measure absolute levels, but to demonstrate maturation of the Ab2 response, as in idiotypic vaccination protocols, relative changes compared with pretreatment levels may be sufficient.
We restricted our measurements to (1) dissociation rate constant, k off, which is inversely proportional to affinity, and (2) initial slope of the binding curve as a measure of quantity of Ab2 or HAMA, in accordance with previous studies by ourselves and other investigators [19–22].
Materials and methods
Patient eligibility
All patients had histological proof of epithelial ovarian cancer. All had undergone standard debulking surgery (where appropriate) and at least one regimen of platinum-based chemotherapy. Patients were eligible if they had either:
residual disease at the end of chemotherapy,
relapsed disease, or
pathologically confirmed second complete remission following salvage chemotherapy.
Performance status of greater than 50% Karnowsky score and life expectancy >3 months were eligibility criteria for all disease states.
All patients received written information and gave written informed consent prior to entry. The trial was approved by the ethics committees of the Hammersmith and Royal Marsden hospitals. It was conducted in accordance with the Declaration of Helsinki and according to the guidelines in the document “Good Clinical Practice for Trials on Medicinal Products in the European Community” (Directive 91/507/EEC).
Patients were ineligible if they were currently receiving chemotherapy or radiotherapy, or if they had received any prior immunotherapy.
Regimen
All patients received an initial dose of 25 mg of unlabelled, unconjugated HMFG1 (Hybridoma Development Unit, Imperial Cancer Research Fund, UK), the same dose of antibody used in the radioimmunotherapy trial [23]. Patients undergoing planned laparoscopy (as an entry requirement for our concurrent phase III trial of adjuvant intraperitoneal radioimmunotherapy) were offered entry into the vaccine trial with i.p. priming if they were found to have residual disease, excluding them from the radioimmunotherapy trial. Patients who presented with obvious macroscopic disease or who would not normally undergo laparoscopy were offered entry into the trial with intravenous priming. Sufficient numbers of patients thus received intravenous (n=10) or intraperitoneal (n=16) priming to provide some indication of any obvious immunological differences due to route of administration.
Patients then received up to six intradermal booster injections of HMFG1 admixed with 10% (by volume) Alhydrogel (aluminium hydroxide) vaccine adjuvant (Superfos Biosector, Uppsala, Sweden). Boosters were given at intervals of 4 weeks. There were three dose levels: 0.5 mg, 1 mg and 5 mg per vaccination.
Blood was taken before each dose of MAb for routine biochemistry and haematology, CA125 and for assays of immune responses. Final samples were taken 1 month after the final booster dose.
Patients were assessed clinically at each visit, with toxicity recorded according to the common toxicity criteria. Radiologic imaging was performed as clinically indicated and not as part of the trial protocol. CA125 results were obtained with and without non-Ab2 human antimouse antibodies (HAMA) correction using Immunoglobulin Inhibiting Reagent (Bioreclamation, East Meadow, NY, USA) as previously described [24].
ELISA for anti-idiotypic antibodies (Ab2)
The assay was designed to measure Ab2 with as little as possible interference from HAMA. It is less prone to interference by anti-Fc HAMA than some published protocols that we have used [25, 26].
HMFG1 and control MAb (H17E2, murine IgG1, anti-PLAP [27]) were covalently bound to carbohydrate-binding 96-well microtitre plates (Labcoat, Costar) according to the manufacturer’s instructions. These plates are coated with a hydrazide which forms covalent bonds with periodate-activated carbohydrates or glycosylated molecules. This, in the case of antibodies, should lead to a regular, site-specific orientation of the immunoglobulin molecules, with the Fc region bound to the plate and the antigen-binding site free to bind Ab2. HAMA cross-reactivity was further minimised by diluting all samples 1:100 in buffer containing 100 μg/ml AUA1 (murine IgG1, anti-CEA [28]).
Diluted serum samples were then assayed in triplicate on prepared ELISA plates, each plate divided so that both anti-HMFG1 and anti-Fc (control MAb) assays were performed on the same plate for each serum sample. A standard ELISA protocol was followed and Ab2 were detected using horseradish peroxidase–conjugated goat antihuman antibody (Sigma, St Louis, MO, USA) as second layer and o-phenylenediamine as chromogenic substrate.
Isotype-matched control antibody (H17E2) provides an assay of anti-isotype binding as a background signal for each sample. The protocol therefore additionally measures the success with which non-Ab2 HAMA has been excluded from the final anti-idiotypic signal.
ELISA for anti-MUC1 antibodies
This ELISA was designed to detect circulating, uncomplexed anti-MUC1 antibodies. Advanced ovarian cancer patients may show spontaneous anti-MUC1 antibodies, with 20% possessing circulating immune complexes of MUC1–anti-MUC1 [29]. All patients had pretreatment serum assayed and therefore any changes in signal should be attributable either to the development of additional anti-MUC1 antibodies as a consequence of vaccination (i.e. anti-anti-idiotypic antibodies, Ab3) or as a result of tumour progression.
Ninety-six-well high-binding ELISA plates (MaxiSorp, Nunc) were coated with 50 μl of recombinant glutathione-s-transferase (GST) / MUC1 fusion protein (generously provided by Dr D. Snary, Applied Development Laboratory, Imperial Cancer Research Fund) at a concentration of 25 μg/ml in PBS, 15-mM glutathione. Serum was assayed at 1:100 dilutions. Serial dilutions of humanised HMFG1 [30] were used as a positive control and to generate a calibration curve. Second-layer antibody and substrate were as above.
Interassay comparison was made by repeating assays of identical serum samples on separate equipment and in separate laboratories. Interassay correlation was good, with a correlation coefficient, r 2, of 0.915 (data not shown).
Biosensor studies on anti-idiotypic antibodies
Briefly, HMFG1 (50 μg/ml in 200 μl of 10-mM sodium acetate, pH 4.5–5) was bound to the activated carboxymethyl dextran coating of IAsys cuvettes (Affinity Sensors, Cambridge, UK) using 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide and N-hydroxysuccinimide in the EDC-coupling kit supplied by the manufacturer (Affinity Sensors, Cambridge, UK). Extraneous binding sites were blocked using 1-M ethanolamine, pH 8.5. Phosphate-buffered saline containing 0.05% Tween 20 was used as wash buffer throughout, and the reaction surface was regenerated between experiments using Gentle Elution Buffer (Pierce and Warriner, UK).
Serum samples were diluted 1:4 in either wash buffer (for studies on total HAMA) or wash buffer containing isotype-matched MAb (AUA1, 150 μg/ml) for Ab2 studies. Wash buffer (190 μl) was added to the cuvette and the machine readout was followed until a flat baseline was achieved. Ten microlitres of sample was then added to the cuvette, with mixing ensured by a small propeller that sits in the chamber of the cuvette. The association curve was followed typically for 5 min. The cuvette was then aspirated and washed twice rapidly, and the dissociation phase of the curve followed. The cuvette reaction surface was regenerated with 200 μl of Gentle Elution Buffer for 2 min. Three rapid washes were then performed and the readout allowed to return to flat baseline before proceeding to the next sample.
Protocols for antigen immobilisation using the EDC-coupling kit and a review of the use of FASTfit software can be found elsewhere [31].
CA125 measurements and HAMA correction using Immunoglobulin Inhibiting Reagent
Patients who develop HAMA after MAb therapy may show spurious elevations in tumour markers without evidence of tumour recurrence. This is due to nonspecific cross-linking of the Fc regions of murine “catcher” and “tracer” antibodies used in immunoassays [32]. Immunoglobulin Inhibiting Reagent (IIR) was studied as a means of eradicating such interference, as described previously [24].
Immunoglobulin Inhibiting Reagent (Bioreclamation, NY, USA) is a purified (>96%) preparation of immunoglobulins (IgG and IgM) from multiple species, but principally murine IgG (subtypes IgG2a, IgG2b and IgG3) derived from BALB/c mice. The objective is to absorb specifically heterophilic antibodies in order to neutralise their interference in immunoassays. The manufacturers quote a binding affinity of >109 M−1 for heterophile HAMA (Dr M Gatz, Bioreclamation, personal communication). IIR was supplied as 40 mg of lyophilised immunoglobulin in individual Eppendorf tubes. Serum (500 μl) was added to each tube, mixed by repeated inversion and allowed to stand for 1 h at room temperature. Paired samples were then analysed by the Tumour Marker Reference Laboratory, Charing Cross Hospital, using the standard in-house assay (Cobas Core CA125 II Enzyme Immunoassay, Roche).
Results
Clinical results
A total of 26 patients were entered between March 1996 and August 1997. The characteristics of these patients are shown in Table 1. Thirteen patients completed all planned vaccinations, the remainder discontinuing trial treatment due to clinical disease progression.
Table 1.
Patient characteristics
| Cohort 1 | Cohort 2 | Cohort 3 | |
|---|---|---|---|
| Booster HMFG1 dose (mg) | 0.5 | 1 | 5 |
| Number of patients | 8 | 6 | 12 |
| Mean age | 58 | 48 | 49 |
| Priming route (numbers i.v.:i.p.) | 4:4 | 3:3 | 4:8 |
| Number completing the regimen | 4 | 1 | 8 |
| Disease characteristics | |||
| Pathological 2nd complete remission | 1 | 0 | 3 |
| Microscopic disease | 0 | 1 | 2 |
| Residual disease <0.5 cm | 4 | 2 | 3 |
| Residual disease >0.5 cm | 3 | 3 | 4 |
Toxicity was minimal: variable discomfort at the intradermal injection sites and a self-limiting, pruritic, papular rash in two patients, similar to that reported in patients receiving intraperitoneal HMFG1 radioimmunoconjugate [33].
No clinical responses were seen in patients with measurable disease, although one patient found to have macroscopic disease at laparoscopy (not confirmed histologically) remains well without clinical progression 5 years after completing vaccination. Four patients were treated in pathologically confirmed second complete remission: three have relapsed (one greater than 2 years since completing the vaccination regimen) and one is lost to follow-up.
Anti-idiotypic antibodies (Ab2) measured by ELISA
Five patients were assayed for all time points, from pretreatment to 1 month after the sixth booster. Vaccine recipients showed only modest elevation in Ab2 1 month after the priming dose of unlabelled HMFG1, but clear sequential rises in Ab2 signal were detected as all five patients progressed through the vaccination regimen, typically with larger increments occurring after the second or third booster (data not shown).
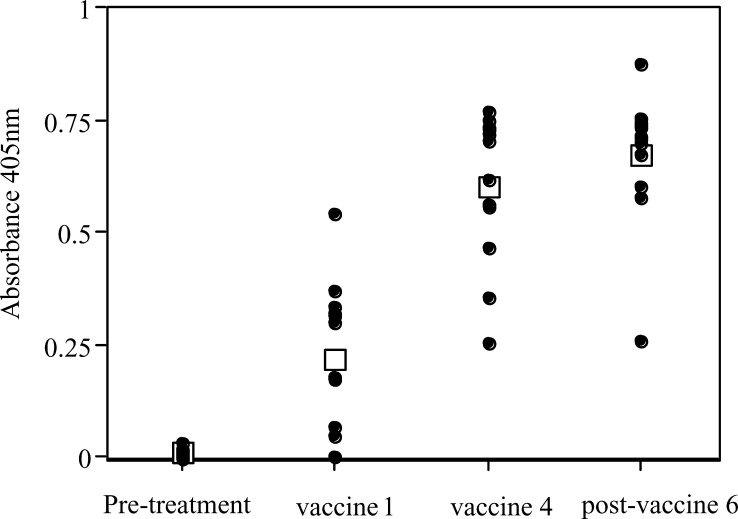
Sufficient samples from 12 patients (of the 13 who completed the vaccination protocol) were available to be assayed at the following time points: pretreatment, time of first vaccination (i.e. 1 month after priming dose of HMFG1), time of fourth vaccination and 1 month after sixth and final booster. The anti-isotypic signal obtained for each sample was subtracted from the anti-idiotypic signal to give an absorbance for Ab2. Results are shown in Fig. 1.
Fig. 1.
Anti-idiotypic antibody signals as detected by ELISA. Signals (means of triplicate samples) from patients at all dose levels are shown (there are no statistical differences between groups). Mean signals are shown as open squares. Samples were assayed immediately prior to priming dose of HMFG1, immediately before the first and fourth vaccine boosters and 1 month after the sixth and final booster
All patients generated measurable Ab2 following three vaccinations, sustained at 1 month after the final booster. This suggests that three booster doses would be sufficient in future trials. No statistically significant differences were seen in the levels of Ab2 generated by higher or lower booster doses of antibody or between patients whose priming dose was given intravenously or intraperitoneally.
Anti-MUC1/anti-anti-idiotypic antibodies (Ab3) measured by ELISA
Serum samples from 13 patients (all of whom completed the vaccination protocol) were assayed for anti-MUC1 IgG pretreatment, at the time of boosters one and four, and 1 month after the sixth and final vaccination.
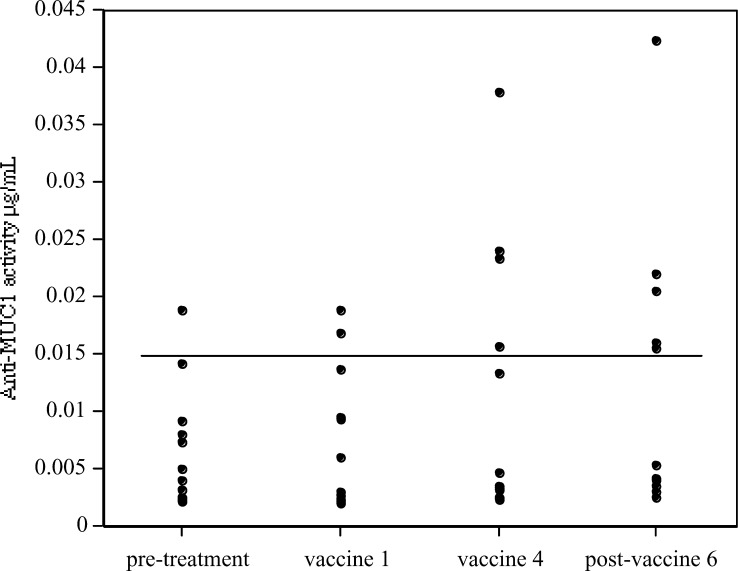
Mean pretreatment anti-MUC1 activity was equivalent to a concentration of 0.007 μg/ml of humanised HMFG1, with a figure of 0.015 μg/ml for 2 standard deviations above the mean. This was taken as the upper limit of the normal range for untreated patients.
Five patients (38%) showed increased anti-MUC1 activity above 0.015 μg/ml (Fig. 2). All the signals remain low, however, casting considerable doubt on the therapeutic value of such Ab3 induction. Paired t-testing for all 13 patients showed that Ab3 changes for the group as a whole were not statistically significant (p=0.065). Levels of anti-MUC1 did not correlate with Ab2 levels (data not shown).
Fig. 2.
Anti-MUC1 (anti-anti-idiotypic, Ab3) signals by ELISA. All points are the means of triplicate samples. Upper limit of pretreatment normal range (0.015 μg/ml) indicated by horizontal line. Five (38%) patients developed elevated anti-MUC1 activity during the vaccination protocol. Changes for the patient population as a whole were not statistically significant. One patient had pretreatment anti-MUC1 levels above the normal range, presumably reflecting the generation of antibodies in response to tumour burden
Biosensor results
We have reported previously some of our results using the resonant mirror biosensor [16]. We have assumed throughout that initial slope of the binding curve is proportional to concentration and that dissociation rate constant is inversely proportional to affinity, as discussed above. Individual patients demonstrate both a rise in quantity of Ab2 and HAMA and maturation of the affinity of each for HMFG1 as they progress through the vaccination regimen (Figs. 3 and 4).
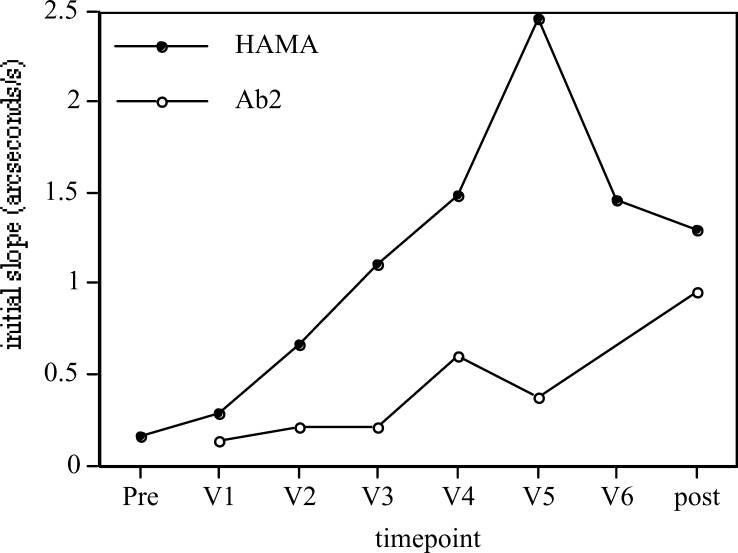
Fig. 3.
IAsys biosensor data for initial slope on one patient. Initial slope (a measure of quantity) shows tenfold increases in both HAMA and polyclonal Ab2 as the patient progresses through the vaccination regimen. Pre pretreatment, V1–V6 vaccination boosters, post 1 month post V6. The pretreatment initial slope for Ab2 was unmeasurable by FASTfit software. All points are from single-sample assays
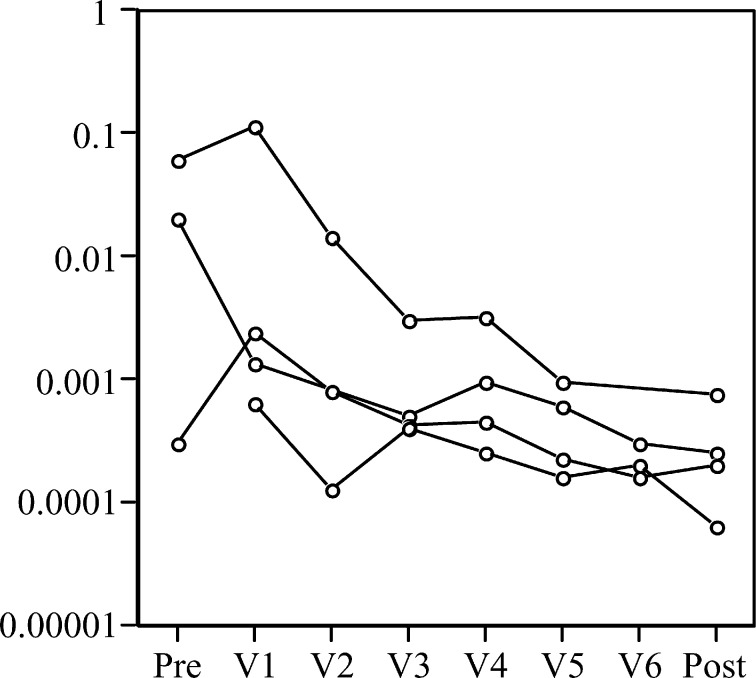
Fig. 4.
Affinity maturation of HAMA in four patients progressing through idiotypic vaccination. Dissociation rate constant (k off in s−1) shows up to 100-fold changes over the course of the vaccination regimen. The very low affinity of the small amounts of HAMA in one pretreatment sample was unmeasurable by FASTfit software
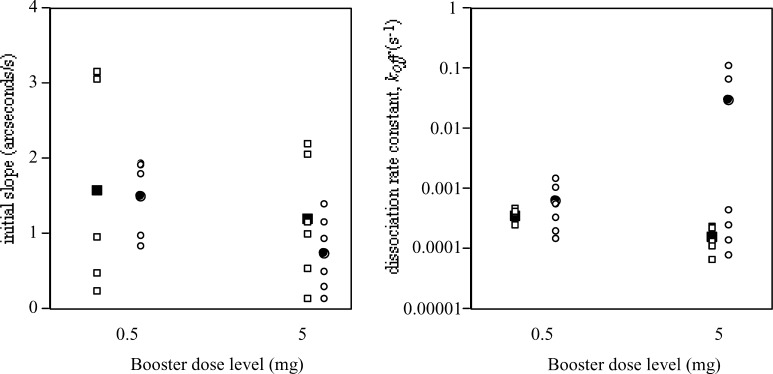
Sera from the cohorts of patients receiving 0.5-mg or 5-mg boosters were compared following the final booster to assess any differences in final Ab2/HAMA level or affinity between the two groups. The affinity and quantity of Ab2 on completion of the full protocol was not statistically different whether the booster dose was 0.5 mg or 5 mg, as previously reported [16]. Results for polyclonal HAMA showed similarly no differences in final quantity between the two groups, but a slightly higher affinity HAMA with the 5-mg dose (Fig. 5).
Fig. 5.
Biosensor results for two groups of patients 1 month after the final vaccination booster. Results for HAMA shown as squares, for Ab2 as circles. Means are shown as solid circles or squares. The graph on the left shows quantity (initial slope) and the graph on the right, dissociation rate constant (k off)
We assessed what proportion of total HAMA was polyclonal Ab2 by comparing initial slope for total HAMA with initial slope where non-Ab2 HAMA had been absorbed by incubating in buffer containing isotype-matched MAbs. Paired results for initial slope (quantity) of total HAMA and Ab2 alone were available for 10 patients: total HAMA was greater than Ab2 in six patients, and in these 50–60% of all HAMA was of Ab2 species. The initial slopes for total HAMA and Ab2 were comparable for the remaining four patients.
Unfortunately antigen supply and its fragility to regeneration rendered biosensor studies for anti-MUC1 antibodies unfeasible.
CA125 measurements with and without Immunoglobulin Inhibiting Reagent
The efficacy of IIR in correcting spurious rises in CA125 due to HAMA has been reported previously [24]. CA125 was routinely measured with and without IIR, to provide confirmatory evidence of our previous observations.
The addition of IIR to pretreatment (presumed HAMA-free) serum samples produced no significant changes in CA125 results (p=0.339, by paired t-test). Patients who completed the full vaccination protocol had statistically significant, correctable, spurious rises in CA125 results (p=0.029, by paired t-test) with a mean increase of 551 U/ml (95% confidence interval, 74 to 1,028 U/ml).
Discussion
Monoclonal antibody therapy of cancer has been investigated for over 20 years, with the emphasis drifting from passive administration of murine MAbs, to their use in targeted drug/radioisotope delivery and vaccination schedules, and back to passive administration (now of humanised and chimeric MAbs) in the mid-1990s [34]. The induction of cellular antitumour immune responses by antibody vaccination strategies has been explored by many investigators, and it is often regarded as the “holy grail” for all forms of cancer vaccination. An alternative potential mode of action is the generation of fully human antitumour antibodies, and in this regard antibody vaccination may be seen as sharing some of the goals of passive administration of humanised MAbs.
This trial has shown that repeated intradermal vaccination with HMFG1 is safe and tolerable. No clinical responses were seen, although this was not a primary endpoint.
We have used modified ELISA protocols to demonstrate that a three-dose regimen of intradermal antibody produces measurable levels of Ab2, and we have also shown (through our biosensor experiments) maturing affinity of the anti-idiotypic response as patients progress through the vaccination regimen [16]. This remains (as far as we are aware) the first time that affinity maturation has been shown in the humoral response to cancer vaccination. We have further shown the comparable efficacy of lower doses of immunogen in stimulating Ab2 production.
We have not proven that the anti-idiotypic antibodies generated in our patients included “internal image” species (Ab2β by the nomenclature of Bona et al. [35]), and formal inhibition experiments would be required to demonstrate this. The induction, however, of anti-MUC1 antibodies in some patients, albeit at low levels, is circumstantial evidence that Ab2β were generated and in turn led to the production of anti-MUC1 Ab3.
The levels of anti-MUC1 antibodies generated in this study must be considered subtherapeutic when compared with those attained with doses of exogenous humanised MAb. It is tempting, however, to speculate that high-titre, high-affinity anti-MUC1 antibodies may be achievable by vaccinating with an internal image Ab2, and this has certainly been the strategy employed by some investigators using other antibodies [10, 36, 37]. It is also unclear from our study whether additional boosters beyond six would eventually generate therapeutically active levels of anti-MUC1 antibodies.
The implicit purpose of this research—the generation of therapeutically useful levels of antitumour antibodies through idiotypic vaccination—has not been met, but the project has generated protocols that may be useful in future trials.
Monitoring by biosensor may be of particular importance to future vaccine trial strategy. The technology allows the antibody responses of individual patients to be monitored in a rapid and simple assay. Total number of vaccinations, rather than being fixed, could be tailored to the individual’s response: booster doses could be continued until the quantity or affinity of the antibody studied had reached either a plateau or a predetermined “therapeutic” level. It may then be possible to correlate objective clinical response rates with circulating Ab2 or Ab3 levels. This tool may also be employed in monitoring responses to immunogens other than monoclonal antibodies and where a humoral immune response is not the therapeutic endpoint, but rather an immunological epiphenomenon which may turn out to be a much-sought-after surrogate marker of vaccine efficacy.
Acknowledgements
The trial was funded in full by the Imperial Cancer Research Fund; Immunoglobulin Inhibiting Reagent was generously provided by Bioreclamation, East Meadow, NY, USA.
References
- 1.Pettersson FIGO. 1994;22:38. [Google Scholar]
- 2.McGuire N Engl J Med. 1996;334:1. doi: 10.1056/NEJM199601043340101. [DOI] [PubMed] [Google Scholar]
- 3.Lambert J Clin Oncol. 1993;11:440. doi: 10.1200/JCO.1993.11.3.440. [DOI] [PubMed] [Google Scholar]
- 4.Taylor-Papadimitriou Biochim Biopys Acta. 1999;1455:301. doi: 10.1016/S0925-4439(99)00055-1. [DOI] [PubMed] [Google Scholar]
- 5.Miles Br J Cancer. 1996;74:1292. doi: 10.1038/bjc.1996.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jerne Ann. 1974;Immunol:373. [PubMed] [Google Scholar]
- 7.Taylor-Papadimitriou Int J Cancer. 1981;28:17. doi: 10.1002/ijc.2910280409. [DOI] [PubMed] [Google Scholar]
- 8.Hird Br J Cancer. 1993;68:403. doi: 10.1038/bjc.1993.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Courtenay-Luck Lancet. 1988;2:894. doi: 10.1016/s0140-6736(88)92482-8. [DOI] [PubMed] [Google Scholar]
- 10.Mittelman Cancer Res. 1994;54:415. [PubMed] [Google Scholar]
- 11.Kwak N Engl J Med. 1992;327:1209. doi: 10.1056/NEJM199210223271705. [DOI] [PubMed] [Google Scholar]
- 12.Baum Hybridoma. 1993;12:583. [Google Scholar]
- 13.Baum Cancer. 1994;73:1121. doi: 10.1002/1097-0142(19940201)73:3+<1121::aid-cncr2820731353>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 14.medac. OVAREX (MAb-B43.13) (1996) A murine monoclonal antibody for the immunotherapy and immunoscintigraphy of epithelial ovarian tumours. medac Gesellschaft fur klinische
- 15.Schmolling Hybridoma. 1995;14:183. doi: 10.1089/hyb.1995.14.183. [DOI] [PubMed] [Google Scholar]
- 16.Nicholson Lancet. 1999;353:808. doi: 10.1016/S0140-6736(98)04907-1. [DOI] [PubMed] [Google Scholar]
- 17.Cush Biosens Bioelectron. 1993;8:347. [Google Scholar]
- 18.Purvis D, Pollard-Knight D, Lowe C (1997) Direct immunosensors. In: Price C, Newman D (eds) Principles and practice of immunoassay, 2nd edn. Macmillan Reference, London, p 511
- 19.Karlsson J Immunol Methods. 1993;166:75. doi: 10.1016/0022-1759(93)90330-A. [DOI] [PubMed] [Google Scholar]
- 20.Karlsson J Immunol Methods. 1995;188:163. doi: 10.1016/0022-1759(95)00203-0. [DOI] [PubMed] [Google Scholar]
- 21.George Tumor Target. 1995;1:245. [Google Scholar]
- 22.George Exp Opin Ther Patents. 1997;7:947. [Google Scholar]
- 23.Nicholson S, Bell S, McCormack M, Bomphray C, Ganesan T, Gore M, Mason P, McIndoe A, Osborne R, Rustin G, Thomas H (2000) A randomised phase III trial of adjuvant intraperitoneal radioimmunotherapy in ovarian cancer. In: Perry M (ed) ProcASCO 2000
- 24.Nicholson Int J Biol Markers. 1996;11:46. doi: 10.1177/172460089601100109. [DOI] [PubMed] [Google Scholar]
- 25.Cheung Cancer Res. 1994;54:2228. [PubMed] [Google Scholar]
- 26.Tibben Nucl Med Commun. 1995;16:853. doi: 10.1097/00006231-199510000-00009. [DOI] [PubMed] [Google Scholar]
- 27.Epenetos Br J Cancer. 1984;49:11. doi: 10.1038/bjc.1984.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Epenetos Lancet. 1982;2:1004. doi: 10.1016/S0140-6736(82)90047-2. [DOI] [PubMed] [Google Scholar]
- 29.Gourevitch Br J Cancer. 1995;72:934. doi: 10.1038/bjc.1995.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Verhoeyen Immunology. 1993;78:364. [PMC free article] [PubMed] [Google Scholar]
- 31.George AJT (2000) Use of biosensors to measure the kinetics of antibody-antigen interactions. In: George AJT, Urch C (eds) Diagnostic and therapeutic antibodies. Humana Press, Totowa, NJ, p 363 [DOI] [PubMed]
- 32.Boerman Clin Chem. 1990;36:888. [PubMed] [Google Scholar]
- 33.Maraveyas Cancer. 1994;73:1067. doi: 10.1002/1097-0142(19940201)73:3+<1067::aid-cncr2820731346>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 34.Glennie Immunol Today. 2000;21:403. doi: 10.1016/s0167-5699(00)01669-8. [DOI] [PubMed] [Google Scholar]
- 35.Bona Immunol Rev. 1984;79:25. doi: 10.1111/j.1600-065x.1984.tb00485.x. [DOI] [PubMed] [Google Scholar]
- 36.Herlyn Proc Nat Acad Sci. 1987;84:8055. doi: 10.1073/pnas.84.22.8055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reinartz Hybridoma. 1999;18:41. doi: 10.1089/hyb.1999.18.41. [DOI] [PubMed] [Google Scholar]