Abstract
The effect of a cationic liposome non-coding plasmid DNA complex on the growth of an intracerebral glioblastoma in an immunocompetent syngeneic mouse strain was evaluated. Previous studies of extraneural tumors in mice have demonstrated that such complexes containing plasmid DNA are capable of stimulating a potent Th-1 cytokine immune-mediated response with a dramatic inhibition of tumor growth. A DOTIM-cholesterol cationic liposome complexed to non-coding plasmid DNA (EV-CLDC) was administered intravenously (i.v.) at weekly intervals to 6-week-old male mice of the B6D2F1 strain at either 3, 10 or 17 days post-inoculation (DPI) of 4C8 glioblastoma cells. Tumor growth was monitored by volumetric image analysis obtained from sequential weekly magnetic resonance imaging studies of the brain. Experiments were terminated between 30 to 38 DPI. Terminal tumor volumes calculated from histological sections directly correlated with tumor volumes from corresponding MR images. The EV-CLDC administered at 3 DPI resulted in a statistically significant (P<0.0001) sustained inhibition of tumor growth compared with tumors in mice administered only individual components of the EV-CLDC. The EV-CLDC similarly inhibited growth of longer established glioblastomas. Histopathologic evaluation of terminal tumors did not find any hemorrhage, edema or necrosis in either the EV-CLDC-treated or control tumors. The results indicate that an i.v.-administered EV-CLDC can significantly inhibit the growth of a brain tumor in immunocompetent syngeneic mice.
Keywords: Brain tumor, Cationic liposome-DNA complex, EV-CLDC, Glioblastoma, Mice, B6D2F1 strain, MR imaging, 4C8 cells
Introduction
Cationic liposome-DNA complexes (CLDCs) have shown promise as a delivery vehicle for effective gene expression in systemic gene therapy of some solid cancers in mice. The major advantages of CLDCs over viral-dependent delivery systems are their ease of preparation, safety, low toxicity and absence of high neutralizing antibody levels. Systemic administration of CLDCs encoding for either tumor suppressor genes, anti-angiogenic factors or cytokines has demonstrated potent antitumor activity in a number of different types of extraneural tumors in vivo [6, 8, 16, 18, 21]. An unexpected finding was that noncoding (empty vector) CLDC (EV-CLDC) complexes alone could induce growth inhibition of tumors in extraneural sites in immunocompetent animals [9, 12, 16, 25]. Subsequent experiments in mice have revealed that plasmid DNA (pDNA) complexed to cationic liposomes activates both systemic innate and adaptive anti-tumor immune responses [14, 15, 22, 26]. This activation leads to sustained and potent tumor-specific immunity against many solid primary or metastatic tumors in extraneural locations [2, 8, 9, 16, 20, 22, 26]. Unmethylated CpG motifs within bacterial or pDNA appear critical for induction of the specific immune antitumor response to EV-CLDC [14, 15, 26]. Oligodeoxynucleotides (ODN) containing immunostimulatory CpG motifs (CpG-ODN) have induced regression of subcutaneous (s.c.) neuroblastomas in syngeneic immunocompetent mice [4]. CpG-ODNs have also enhanced anti-tumor responses generated by vaccine-based strategies ,e.g., in s.c. neuroblastomas [21].
Because of the dramatic inhibition or rejection of solid extraneural tumors evoked by EV-CLDC, it is tempting to assume that similar responses might occur with tumors in the CNS. However, there is little data to support any direct correlation in therapeutic efficiency of immunostimulatory-based strategies between extraneural and neural tumors. Long-term survival of rats with an intracerebral glioma has been achieved by direct intratumoral inoculation of ODN-CPG motifs alone [5]. However, it is not clear whether systemic delivery of EV-CLDC would be as effective against intracerebral tumors as with extraneural tumors. In CNS tumors intrinsic differences resulting from the blood brain barrier, e.g., tumor vascular permeability and the so-called immunologically privileged status of the brain, might confound a similar response. Thus, this study was designed to assess any effect of a systemically delivered EV-CLDC on the growth of an intracerebral orthotopic glioblastoma in immunocompetent mice.
Materials and methods
Cell line
The 4C8 cell tumor line, derived from a spontaneously arising glioma in a transgenic MP/c-neu mouse, was provided by Dr. C.A. Dyer (Children’s Hospital of Philadelphia, Pa.) and maintained in culture essentially as previously described [10]. Briefly, cells were maintained initially in medium consisting of DMEM supplemented with glucose, sodium bicarbonate and 10% heat-inactivated newborn calf serum (NCS). Two passages prior to inoculation, cells were maintained in medium supplemented with 5% heat-inactivated normal mouse serum rather than NCS to avoid potential antigenic components in calf serum. Cells were cultured to confluence, harvested using EDTA (Sigma-Aldrich Co. St Louis, Mo.) and resuspended at a final cell density of 1×106 cells/5 µl in CM supplemented with 5% normal mouse serum (NMS). The cells were tested to be free from mycoplasma contamination by an established protocol [11].
Preparation of cationic lipid DNA complexes
The cationic liposomes used to prepare the CLDC were comprised of DOTIM [octadecenoylloxy (ethyl-2-heptadecenyl-3-hydroxyethyl) imidazolinium chloride; Valentis Corp., Burlingame, Calif.] and cholesterol (Avanti Polar Lipids, Alabaster, Ala.) in equimolar ratios as reported previously [8, 17]. Briefly, the lipids were dried down in round-bottomed tubes under vacuum, then rehydrated with 5% dextrose in water with periodic shaking. The liposomes were then filtered through sequentially smaller filters to a final diameter of 0.2 m. The expression vector PCR3.1 (InVitrogen, San Diego, Calif.), without a gene insert, was used as a source of pDNA. The plasmid was purified from E. coli using a Qiagen endotoxin free plasmid purification kit (EndoFree Mega, Qiagen Inc. Calif.). Purified preparations were quantified by spectrophotometry and agarose gel analysis. Plasmids were predominantly supercoiled and free of detectable RNA and chromosomal DNA. Endotoxin content was less than 0.01 EU/µg pDNA (LAL assay; Biowhittaker, Walkersville, Md.). Plasmid DNA for injection was resuspended in distilled water prior to use. For in vivo injection EV-CLDC were prepared immediately prior to injection by gently mixing cationic lipids with pDNA at a ratio of 32 nmol total lipid to 1.0 µg pDNA, to a final concentration of 100 µg pDNA per ml in a sterile solution of 5% dextrose in water. All CLDCs were maintained at room temperature and were injected i.v. within 30–60 min of preparation. DOTIM-cholesterol and pDNA alone were prepared at the same concentrations as for EV-CLDC.
Mice
Specific pathogen-free adult B6D2F1 male mice 4 to 6 weeks of age purchased from Jackson Laboratories (Bar Harbor, Me.) were quarantined for a week after their arrival and acclimatized before initiation of experiments. Mice were housed in laminar flow units at the UC Davis Medical Science campus. All mouse experiments were conducted according to protocols approved by the University of California Davis Animal Care and Use Committee. All EV-CLDC and control solutions were slowly injected as 200-μl boluses via the lateral tail vein of the mice. Mice were maintained lightly anesthetized using gaseous isofluorane anesthesia during the i.v. injection procedures.
Intracerebral tumor model
For induction of the intracerebral glioblastomas, mice were deeply anesthetized with pentobarbital intraperitoneally (i.p.) at a dose of 65 mg/kg body weight. Mice were secured in a stereotactic head frame (David Kopf Instruments, Tujunga, Calif.), and 1×106 4C8 cells in a 5-μl volume were stereotactically injected into the left cerebral cortex at the level of the bregma, 2.0 mm from midline, at a depth of 2.0 mm, essentially as described [24].
In the first experiment, there were three control groups of mice and one experimental group. Control groups received a total of four i.v. injections of either (1) 5% dextrose in water (D5W; n=10), (2) 20 ug pDNA in D5W (n=5) or (3) 640 nmol DOTIM-cholesterol in D5W (n=4). Mice in the experimental group received four injections of EV-CLDC (n=10). The first injections were administered i.v. beginning on 3 DPI and then subsequent injections were given at 7-day intervals. Mice were killed at 30 DPI.
In the second experiment, the control group received D5W (n=4), while the experimental group of mice received EV-CLDC (n=5). Mice in all groups received a total of four injections that were started at 3 DPI, and then subsequent injections were given at 7-day intervals. Mice were killed at 38 DPI.
In the third experiment, mice received a total of three or four injections at 7-day intervals, but the initiation of treatment was delayed as indicated below. The control group received four injections of D5W (n=4) at 7-day intervals beginning at 10 DPI. Mice in the second group received four injections of EV-CLDC (n=6) at 7-day intervals starting at 10 DPI. Treatment of the last group with EV-CLDC (n=5) was started at 17 DPI, and two additional injections were administered 7 days later. All surviving mice were killed at 35 DPI.
Magnetic resonance (MR) imaging
All mouse brain MR images were acquired under general anesthesia (50 mg/kg pentobarbital i.p.) using a Bruker Biospec DBX scanner (Bruker Medical, Billeria, Mass.) interfaced to an Oxford 7.0 Tesla/183 clear-bore magnet (Oxford Instruments, Oxford, UK). The scanner was equipped with Bruker G0–60 (60-mm bore) microgradients capable of 95 gauss/cm maximum gradient strength and a Bruker 35-mm diameter 10-rung birdcage send-receive RF coil. Image data were obtained and reconstructed using the scanner’s software (Paravision software, v 2.0., Bruker Medical). Selective sinc pulses of 2-ms duration were used for excitation and refocusing. Both gradient echo and spin echo sequences were used with a 1.0-mm slice thickness and a 1.2-mm interslice distance. Specific acquisition parameters were as follows: gradient echo; matrix dimensions (256, 256); recovery time (TR) 232 ms; echo time (TE) 7 ms; pulse angle 30°; spin echo; matrix dimensions (128, 128); TR 3,000 ms, TE 60 ms.
Tumor volumetric quantitation from MR images and HE sections
Tumors were localized as well demarcated areas of decreased signal intensity on both gradient and spin echo sequence images. Tumors were first detected on MR images at approximately 18–20 DPI. Sequential MR images of brain with a 1.2-mm interslice distance were acquired and tumor area for each slice was calculated using NIH Image 1.62 software (NIH, Besthesda, Md.). Distance was calibrated using internal standardized scales present on selected MR images.
Sequential hematoxylin-eosin (HE)-stained sections routinely processed from serial 1-mm-thick brain slices were digitally scanned using a Kodak Professional RF3 3570 Scanner (Eastman Kodak Co., Rochester, N.Y.) and Adobe Photoshop v.6.01 software (Adobe Systems Inc., San Jose, Calif.). The tumor area for each section was calculated using the NIH Image 1.62 software. Distance was calibrated from a 1-cm micrometer using scanned images, and the final tumor volume (mm3) was calculated using the formula, volume (mm3) = sum of tumor areas (mm2) × 1.0.
Pathology studies
After mice were killed with an overdose of pentobarbital given i.p., their brains were removed immediately and immersion-fixed in 10% buffered formalin. Using a 1-mm mouse brain tissue block slicer (Zivic Laboratories Inc., Zelienople, Pa.), transverse serial 1-mm thick sections of the fixed brain were cut, embedded in sequence in paraffin by routine processing techniques, then sectioned at 5 µm. These sequential serial sections were stained with HE, and the tumors evaluated microscopically for presence or absence of intratumoral necrosis or hemorrhage or of peritumoral edema.
Statistical analysis
To satisfy normality and ANOVA model assumptions, logarithmic transformation of data from all experiments was done. In experiment 1, the Tukey-Kramer method for multiple comparisons was used to analyze tumor volume data from MR images. In experiment 2, data from the two groups were analyzed using a Tukey’s means comparison in a three-factor mixed effect ANOVA model. Standard regression analysis was done to determine the relationship between MRI and HE tumor volumes. In experiment 3, data were analyzed using Tukey’s means comparison.
Results
EV-CLDC, but not the individual components, inhibits intracerebral tumor growth
In the first experiment, the effect of EV-CLDC and of each of the individual components was evaluated on the growth of the mouse glioblastoma for 30 DPI. Tumor growth was measured by sequential MR imaging and volumetric analysis. The mean tumor volumes from control groups administered either D5W, cationic liposome or DNA were compared with those tumors given EV-CLDC. In a pilot study (data not shown), earliest tumor growth could be first detected by MR imaging at 18–20 DPI; therefore, subsequent MR imaging studies were done starting at 23 DPI and repeated at 30 DPI. At 23 DPI (Fig. 1) in the EV-CLDC group (n=10) mean tumor volume was 9.06 mm3 (SD =2.75) and was significantly smaller than the control groups. Mean tumor volumes were 35.27 cm3 (SD =19.46) for D5W treated mice (n=10); 34.88 cm3 (SD =14.65) for the cationic liposome (DOTIM-cholesterol) (n=4) and 23.31 cm3 (SD =4.5) for the DNA group (n=5). At 30 DPI, mean tumor volume in the EV-CLDC treated group was 12.12 cm3 (SD =4.29); the D5W group was 99.32 cm3; the cationic liposome group was 90.96 cm3 (SD =28.77), and the DNA group was 77.64 cm3 (SD =21.16). At 23 DPI, the EV-CLDC group had significantly smaller volumes by MR imaging than either the DNA treated mice (P=0.0047), the D5W group (P<0.0001) or the cationic liposome alone group (P<0.0001). Further, the DNA group did not have significantly different volumes than the D5W group (P=0.9056) or liposome group (P=0.9317), and the two latter groups did not differ significantly from one another (P=1.0; Fig. 1). At 30 DPI, tumor volumes for the EV-CLDC group were significantly smaller than in each of the other groups (P<0.0001), but the other groups did not differ significantly from each other, e.g., DNA vs. D5W (P=0.998), DNA vs. liposome alone (P=0.996) or D5W vs. liposome alone (P=1.0) (Fig. 1). The DNA and D5W groups had significantly larger volumes at 30 DPI compared with 23 DPI (P=0.0021 and P<0.0001), respectively. The cationic liposome group was marginally but not significantly larger on 30 DPI vs. 23 DPI (P=0.0506). The EV-CLDC group, however, did not have significantly different volumes between these two time points (P=0.8827). In summary, there were statistically significant differences between the tumor volumes in the EV-CLDC-treated group and in each of those groups where tumors were treated with only individual components of the EV-CLDC. The differences in tumor volumes were consistently maintained throughout the duration of the experiment and at each of the sequential time points.
Fig. 1.
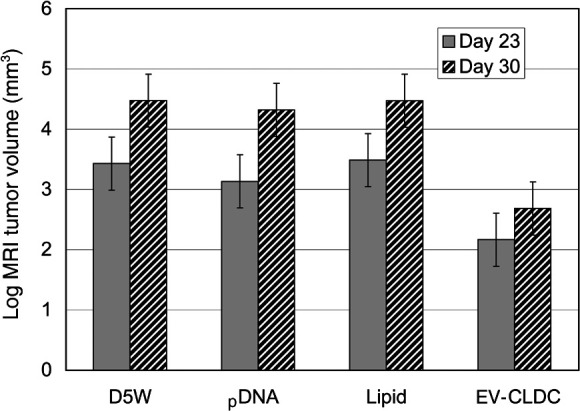
EV-CLDC but not the individual components inhibited intracranial glioblastoma growth in mice as determined by serial volumetric quantification from MR images. Note the statistically significant inhibition of tumor growth (P<0.0001) by DOTIM-cholesterol complexed with pDNA (EV-CLDC) as measured by tumor volume obtained from MR images at both 23 and 30 DPI compared to volumes of tumors similarly treated with either control (D5W), pDNA or cationic liposome (lipid) alone. Logarithmic transformation of data was analyzed by the Tukey-Kramer method for multiple comparisons
The tumor-inhibiting effect of EV-CLDC was reproducible and consistent
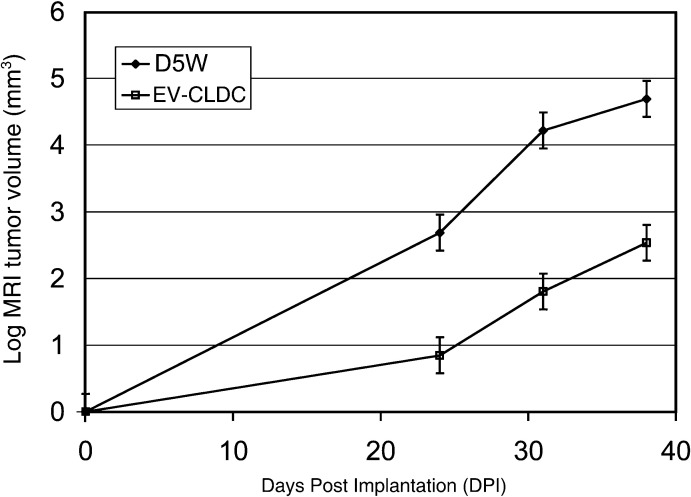
The second experiment was designed to test the reproducibility of the EV-CLDC effect on the mouse glioblastoma. Serial MR imaging was done at 24, 31 and terminally at 38 DPI in mice from both D5W (n=4) control and EV-CLDC (n=5) treated groups. All mice received a total of four injections, which were given at weekly intervals beginning at 3 DPI (Fig. 2). At 38 DPI, terminal tumor volumes of all mice were calculated from both MR imaging and HE-stained brain sections, and the resulting mean tumor volumes were compared. As determined by sequential MR imaging, mice in the D5W group had average tumor volumes of 14.5 mm3 (SD =6.37) at 24 DPI, whereas the EV-CLDC group had an average volume of 2.22 mm3 (SD =2.56). At 31 DPI, the average volumes were 68.6 mm3 (SD =18.04) and 6.27 mm3 (SD =4.65) for the respective groups. At 38 DPI the one surviving mouse in the D5 W group had a tumor volume of 100 mm3, while the EV-CLDC group had an average of volume 15.14 mm3 (SD =11.19).
Fig. 2.
Comparison of tumor volumes between EV-CLDC- and control (D5W)-treated tumors obtained from sequential MR images at 24, 31 and 38 DPI. There was a statistically difference (P=0.002) between tumor volumes at each of the time points for each group. With treatments starting at 3 DPI, there was a consistent suppression of growth in the EV-CLDC group. Log data were analyzed using a Tukey’s mean comparison in a three-factor mixed effect ANOVA model
As was found in experiment one, there was a significant and constant difference in tumor volumes between the D5W and EV-CLDC groups averaged over each of the time points (P=0.0001), with those from the EV-CLDC group significantly smaller (P=0.0027). There were also significant and constant differences (P=0.0002) between tumor volumes of each group. In summary the EV-CLDC growth inhibition was statistically significant in duplicated experiments.
Tumor volumes calculated from MR imaging closely correlated with those from HE sections
Terminal tumor volumes calculated from MR imaging of all mice surviving to either 30 DPI (first experiment) or 38 DI (second experiment) were compared with those calculated from HE sections of the same tumors (Fig. 3). These tumors were from both EV-CLDC treated and untreated (D5W) control mice. Using regression analysis there was a statistically significant and consistent relationship between the MRI (n=8) and HE (n=8) volumes (P=0.0007) from mice in both experiments. The correlation between the MRI- and HE-derived tumor volumes was significant with an r 2 of 93.6%.
Fig. 3.
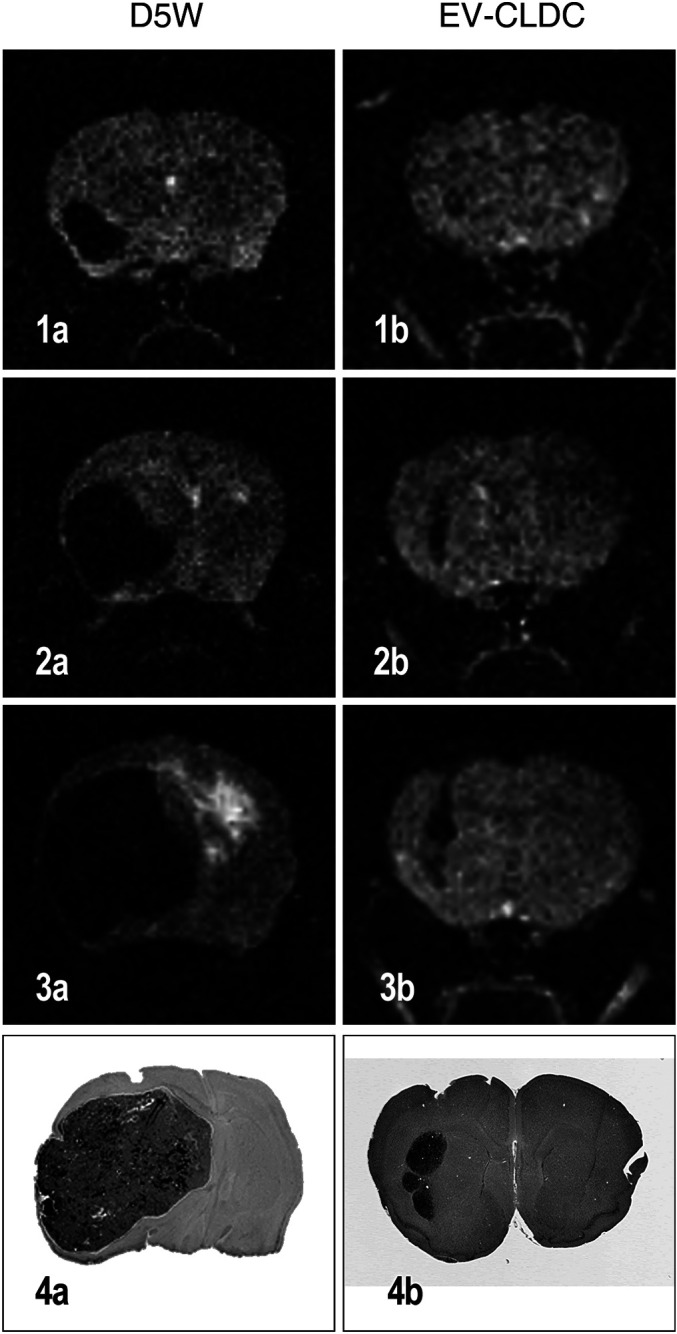
Comparison of serial MR images of both a control (a) D5W- and EV-CLDC-treated (b) mouse with a glioblastoma at 24 (1a,b), 31 (2a,b) and at 38 DPI (3a,b) illustrating the differences in tumor size at each time point. Also note the difference in terminal tumor size on histologic sections from the same mouse in each group killed at 38 DPI (4a, b). Regression analysis demonstrated a consistent and statistically significant constant relationship between comparison of tumor volumes obtained from the terminal MR images and HE sections (P=0.007); r 2 was 93.6%
In summary, assessment of tumor size by MR imaging was found to be closely correlated with that determined by histologic morphometry. Thus, MR imaging allowed temporal and precise evaluation of the tumor response to EV-CLDC administration.
EV-CLDC treatment is also consistently effective in inhibiting growth of longer established tumors
Because administration of EV-CLDCs dramatically suppressed the growth of immature tumors, the effect of this treatment regime on established intracranial tumors was then assessed. Thus, in experiment 3, EV-CLDC treatment was withheld until either 10 DPI for group 2 (n=6) or 17 DPI for group 3 (n=5), and subsequent injections were given at 7-day intervals. The termination of all surviving mice was on 35 DPI (Fig. 4). The control group (group 1) was treated with D5W starting at 10 DPI. Tumor volumes calculated from HE sections at 35 DPI in group 1 were an average of 104.94 mm3 (SD =52.7), in group 2 of 12.51 mm3 (SD =14.0) and in group 3 19.24 mm3 (SD =16.02). Terminal HE volumes in group 1 were significantly greater when compared with groups 2 and 3 (P=0.0045 and P=0.0304, respectively). However, there was no significant difference between groups 2 and 3 (P=0.5779; Fig. 4). In summary, these data demonstrated that repeated EV-CLDC administration effectively suppressed the growth of longer established intracerebral tumors.
Fig. 4.
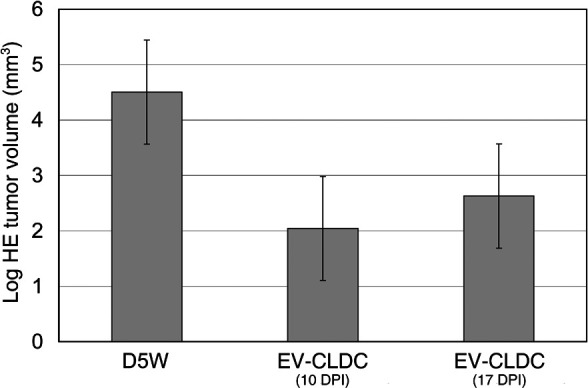
Comparison of terminal tumor volumes from HE sections at 38 DPI between the control group and groups initially treated with EV-CLDC at either 10 or 17 DPI. The statistically significant difference between tumor volumes of the control group (D5W) and those at either 10 or 17 DPI of P=0.004 and P=0.030, respectively, indicates that treatment is also effective in inhibiting the growth of longer established tumors. The logarithmic transformation of volumetric data was analyzed by a Tukey’s means comparison
Pathology studies
Histologically, the tumors in both groups were sharply demarcated, but no intratumoral necrosis, hemorrhage or peritumoral edema was seen (Fig. 3). The main difference in overall pathologic evaluation between the groups was only in the size of the tumors.
Discussion
This study has demonstrated that repeated systemic administration of an EV-CLDC containing non-coding pDNA elicited a sustained growth inhibition of an intracerebral glioblastoma in syngeneic immunocompetent mice. This growth inhibition, monitored by serial MR imaging, occurred in both early and longer established glioblastomas. The inhibition was statistically significant compared with the effect of administration of the individual components of the EV-CLDC, namely pDNA, cationic liposome or the D5W vehicle. Although a similar effect has been documented with lung metastases, intraperitoneal mesothelioma and ovarian carcinoma in mice [8, 9, 12, 20, 22, 26], this study is the first demonstration in which systemically administered EV-CLDC resulted in growth inhibition of an intracerebral syngeneic tumor. No toxicity was observed even after repeated weekly administration of either EV-CLDC, cationic liposome or pDNA alone.
Because of the consistently significant correlation found between tumor volumes calculated from terminal MR images and the corresponding HE sections, accurate volumetric analysis of in vivo tumor growth was obtained by sequential MR imaging. Importantly, there was no histologic evidence in any tumors of either intratumoral necrosis or hemorrhage or of peritumoral edema, each of which potentially could have been confounding factors in modifying the tumor volume as calculated from the MR images. Although the histologic tumor volume was consistently smaller than that calculated from MR images in all groups, this difference was attributed to shrinkage artefact expected from routine fixation and paraffin embedding. Thus, it could be demonstrated that repeated weekly injections of EV-CLDC resulted in sustained inhibition of tumor growth for up to 38 DPI. Further, it was demonstrated that the initial inhibitory effect was sustained throughout the experimental period and not from intermittent remissions or exacerbations of tumor growth. Serial MR imaging also demonstrated that a similar degree of growth inhibition by EV-CLDC occurred in longer established brain tumors. Our findings indicate the usefulness of this technique for serial in vivo monitoring of responses to EV-CLDC and potentially for evaluation of other experimental therapeutic treatments of either solid primary or metastatic brain tumors.
The pathogenic mechanism(s) by which this growth inhibition was mediated depend upon anti-angiogenic as well as cell-mediated inflammatory cell responses. Immunophenotyping of extraneural tumors in mice treated with different EV-CLDC formulations has revealed a spectrum of inflammatory cell infiltrates including NK cells, macrophages, and T and B lymphocytes [8, 9, 20, 26]. These cellular infiltrates indicate that both innate and acquired immunity contributes to the anti-tumor effect of EV-CLDC. Based on several studies, a potential scenario for the effect is that immunostimulatory CpG motifs in pDNA activate NK cells and stimulate antigen-presenting cells within the CNS and peripheral lymphoid tissue, leading to the production of inflammatory cytokines, e.g., TNF–α, IL-12 and IFN-γ [2, 4, 9, 20]. Unmethylated CpG motifs in the pDNA are apparently critically dependent upon assembly within a cationic liposome for activation of an immune response [12, 16, 20, 23, 26]. A comparable effect cannot be induced with eukaryotic DNA, methylated bacterial DNA or Freund’s complete or incomplete adjuvant [20, 25]. However, the differences in the degree and cellular composition of the inflammatory response noted between various cationic lipid formulations may be reflected in the extent of anti-tumor activity [2].
The increased levels of inflammatory cytokines promote lytic activity of NK cells, increased expression of co-stimulatory molecules on APC and upregulated expression of class I and class II MHC molecules. Ultimately, the rapid rise in serum levels of Th-1 cytokines would lead to development of a tumor-specific cell-mediated immune response [9, 14, 15, 16, 20, 26]. However, only CLDC formulations that can concomitantly stimulate NK cells and Thy-1 cytokine production (TNF-a, IL-12, IL-6 and IFN-γ) are capable of simulating tumor-specific cytotoxic T lymphocytes and significantly inhibiting tumor growth in extraneural tumors [12, 22, 25, 26].
Despite the dramatic regression of various extraneural tumors in mice given an EV-CLDC systemically, there have been no comparable studies on response of primary or metastatic tumors in the CNS. However, ODN-containing immunostimulatory CpG motifs, injected near well-established s.c. neuroblastomas in mice, have led to a dramatic reduction in tumor size [4]. Also, there has been successful treatment of implanted intracranial gliomas established in rats following one intratumoral injection with ODN containing immunostimulatory CpG motifs [5]. It is encouraging that our results confirm EV-CLDC given systemically to mice with intracerebral glioblastomas is dramatically effective despite possible perturbation of the anti-tumoral immune response engendered by the blood brain barrier. It would be of interest next to compare the degree of growth inhibition with systemic EV-CLDC vs. that from direct intratumoral injection of ODN-CpG formulations [5]. Since ODN-CpGs are rapidly eliminated by absorption to serum proteins and nuclease degradation, systemic EV-CLDC would offer advantages of ease of administration and effectiveness against metastatic CNS tumors [1]. Further, immunophenotyping studies are planned to determine whether the inflammatory cell profile in these EV-CLDC treated tumors is similar to that described in tumors in extraneural sites [8, 9, 20, 22, 26].
A second mechanism may involve release of inflammatory cytokines, e.g., IL-12 and IL-18, which have potent and synergistic anti-angiogenic effects and tumor regression in extraneural sites in mice [3, 7, 13, 19]. The effects of these cytokines are largely mediated by IFN-γ secreted from T and NK cells [7]. It is most likely that these cytokines may play some direct or synergistic role contributing to the growth inhibition by EV-CLDC though their relative roles await further study.
In summary, dramatic growth inhibition of an intracerebral glioblastoma in mice has been achieved with a systemically administered EV-CLDC and serially monitored by MR imaging. The success of this immunostimulatory regimen suggests that such EV-CLDC formulations need to be more closely evaluated for their potential efficacy against other primary or metastatic CNS tumors.
Acknowledgements
We would like to thank Dr. J.L. Grandy, Ms. I. Espiritu and Mr. J.S. deRopp for their excellent administrative and technical help and the Paul C. and Borghild T. Petersen Foundation for their financial support.
References
- 1.Barry Hum Gene Ther. 1999;10:2461. doi: 10.1089/10430349950016816. [DOI] [PubMed] [Google Scholar]
- 2.Bramson Cancer Gene Ther. 2000;7:353. doi: 10.1038/sj.cgt.7700143. [DOI] [PubMed] [Google Scholar]
- 3.Cao FASEB. 1999;13:2195. doi: 10.1096/fasebj.13.15.2195. [DOI] [PubMed] [Google Scholar]
- 4.Carpentier Cancer Res. 1999;59:5429. [PubMed] [Google Scholar]
- 5.Carpentier Clin Cancer Res. 2000;6:2469. [PubMed] [Google Scholar]
- 6.Chen Cancer Res. 1999;59:3308. [PubMed] [Google Scholar]
- 7.Coughlin J Clin Invest. 1998;101:1441. doi: 10.1172/JCI1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dow Hum Gene Ther. 1999;10:2961. doi: 10.1089/10430349950016375. [DOI] [PubMed] [Google Scholar]
- 9.Dow J Immunol. 1999;163:1552. [PubMed] [Google Scholar]
- 10.Dyer J Neuropath Exp Neurol. 1995;54:852. doi: 10.1097/00005072-199511000-00012. [DOI] [PubMed] [Google Scholar]
- 11.Fitch FW, Gajewski TS, Yokoyama WM (1997) Diagnosis and treatment of mycoplasma-contaminated cultures. In: Colligan JE, Kruisbeek AM, Margulies DH, Shevach EM, Strober W (eds) Current protocols in immunology, vol. 4. John Wiley and Sons, New York, pp A.3E.1–A.3E.4 [DOI] [PubMed]
- 12.Hofland Biochem Biophys Res Comm. 1995;207:492. doi: 10.1006/bbrc.1995.1215. [DOI] [PubMed] [Google Scholar]
- 13.Jean Neurosurgery. 1998;42:850. doi: 10.1097/00006123-199804000-00097. [DOI] [PubMed] [Google Scholar]
- 14.Krieg Nature. 1995;374:546. doi: 10.1038/374546a0. [DOI] [PubMed] [Google Scholar]
- 15.Krieg Annu Rev Immunol. 2002;20:709. doi: 10.1146/annurev.immunol.20.100301.064842. [DOI] [PubMed] [Google Scholar]
- 16.Lanuti Cancer Res. 2000;60:2955. [PubMed] [Google Scholar]
- 17.Liu J Biol Chem. 1995;27:24864. doi: 10.1074/jbc.270.42.24864. [DOI] [PubMed] [Google Scholar]
- 18.Liu J Biol Chem. 1999;274:13338. doi: 10.1074/jbc.274.19.13338. [DOI] [PubMed] [Google Scholar]
- 19.Park J Immunol. 2001;167:1644. doi: 10.4049/jimmunol.167.3.1644. [DOI] [PubMed] [Google Scholar]
- 20.Rudginsky Mol Ther. 2001;4:347. doi: 10.1006/mthe.2001.0463. [DOI] [PubMed] [Google Scholar]
- 21.Sandler Cancer Res. 2003;63:394. [Google Scholar]
- 22.Siders Mol Ther. 2002;6:519. doi: 10.1006/mthe.2002.0697. [DOI] [PubMed] [Google Scholar]
- 23.Tan Hum Gene Ther. 1999;10:2153. doi: 10.1089/10430349950017149. [DOI] [PubMed] [Google Scholar]
- 24.Weiner J Neuropath Exp Neurol. 1999;58:54. [Google Scholar]
- 25.Whitmore Gene Ther. 1999;6:1867. doi: 10.1038/sj.gt.3301026. [DOI] [PubMed] [Google Scholar]
- 26.Whitmore Cancer Immunol Immunother. 2001;50:503. doi: 10.1007/s002620100227. [DOI] [PMC free article] [PubMed] [Google Scholar]