Abstract
The variable domain of immunoglobulin heavy chain (Ig HV) is well-characterized tumor associated antigen expressed in B-cell malignancies, which may function as a T-cell target. However, T-cell epitopes derived from shared framework regions (FRs) of each IgHV subfamily capable of inducing cytotoxic T lymphocytes (CTLs) against the B-cell malignancy, have not been identified. Using the specific PCR primers of seven IgHV gene subfamilies, we amplified the IgHV gene rearrangement for 108 cases of B-cell acute lymphoblastic leukemia (B-ALL) patients. The IgHV gene rearrangement fragments of B-ALL patients were directly sequenced then classified into seven different subfamilies. The T-cell epitopes encoded by the IgHV gene in the B-ALL patients were predicted by SYFPEITHI and BIMAS programs and compared with those from 56 representative germline IgHV sequences in the genebank. For the HLA-A*0201 locus, we found 1 or 2 top score shared epitopes from each subfamily and got 12 epitopes altogether. Results showed that ten of them were in the FRs. Using an antigen-specific T-cell expansion system, we generated the peptide-special CTLs in vitro, which were capable of killing B lymphoma cell lines that belonged to the same IgHV subfamily in a peptide-specific and HLA-restricted manner. Furthermore, we proved that the cytotoxicity of CTLs was IgHV subfamily-specific. These data indicate possible immunotherapy approaches for B-cell malignances patients based on IgHV gene subfamilies.
Keywords: T-cell epitope, Immunoglobulin, Malignancies, B-cell, Receptor, T-cell
Introduction
B-cell malignancies are clonal disorders with all tumor cells expressing the same tumor-specific immunoglobulin (Ig). The unique variable regions of Ig heavy and light chains (VH and VL) contribute to the idiotype (Id), which can serve as a target for T-cell-mediated immune response [1–3]. Previous studies had proved the effectiveness of the immune system to target the idiotype and kill lymphoma cells in vitro and in vivo [4–9]. Most early studies indicated that the cytotoxic T-cell epitopes were solely located in the complementary-determining regions (CDRs) [10–14]. However, because such vaccines have to be prepared in a personalized manner, a CDR based therapy is disadvantageous for the treatment of B-cell malignancies.
Recently, some reports have demonstrated that peptides derived from the framework regions (FRs) might also express cytotoxic T-cell epitopes. Using a bioinformatics analysis, Trojan et al. [15] predicted 272 epitopes derived from the FRs in lymphoma patients. Lou et al. [16] also demonstrated that germline IgHV peptides when presented by dendritic cells were immunogenic and could elicit a tumor-specific protective immune response in vivo. These data suggested that it was very likely that some lymphoma-shared FR epitopes could be used as the common immune targets for different patients. However, the problem on how to choose the appropriate epitopes for each patient among such a large number of predicted epitopes was still unsolved.
In 1999, Lefranc et al. [17] published 56 kinds of representative germline IgHV amino acid sequences on the website (http://imgt.cines.fr), and classified them into seven subfamilies (IgHVl–IgHV7). More than 80% of amino acid sequences in each subfamily were homologous. Malignant B-cells could also be classified into seven subfamilies according to the IgHV on the cell’s surface. In our previous experiment [18–21], we had studied the possibility of treating B-cell malignance by using the specific immune targets for each IgHV subfamily. We had constructed the IgHV1 (FR)/pcDNA3.0 and IgHV3 (FR)/pcDNA3.0 expression vectors as the DNA vaccines. Anti-lymphoma immune responses could be successfully detected in mice after immunization with these plasmids intramuscularly. Specific antibody against the same IgHV subfamily B-cell malignancies could be shown in IFA/Elisa. These results indicated the FRs-derived DNA vaccine could induce the family-specific humoral immune response.
In this paper, we examined whether some T-cell epitopes derived from framework regions (FRs) of IgHV were shared in each IgHV (IgHV1–IgHV7) gene subfamily, whether these epitopes could be used to induce the generation of peptide-special cytotoxic T lymphocytes (CTLs) in vitro, and whether these CTLs could recognize the B-cells in a same IgHV gene subfamily. These data would put forward a possibility of adopting the immunotherapy for B-cell malignances patients according to their IgHV gene subfamilies, because it is easier to prepare the specific reagent target for a group of B-cell malignance patients than for each patient respectively.
Materials and methods
Patients and healthy donors
Blood and bone-marrow samples from acute B-lymphoblastic leukemia (B-ALL) patients before treatment were collected from Hematology Center, Beijing Children’s Hospital. Peripheral blood mononuclear cells (PBMCs) were obtained from two HLA-A*0201positive healthy donors. PBMCs were isolated by Ficoll density centrifugation, and were used to produce peptide-specific CTL lines. Written consent was obtained from the participants, and the Hospital Review Board had approved the researching procedure.
Cell lines and media
HLA-A*0201-positve and IgHV1-positive lymphoma cell line NAMALWA, HLA-A*0201- negative and IgHV1-negative erythroleukemia cell line K562, as well as HLA-A*0201-positive and IgHV1 negative monocytic leukemia cell line THP-1 were used as target cells. The transporter associated with antigen-processing (TAP)- deficient cell line T2 was used to determine the binding of peptides to HLA-A*0201 molecules. Cell lines were maintained in RPMI 1640 (Hyclone, Logan, USA) supplemented with 100 U/ml penicillin G, 100 μg/ml streptomycin, 2.05 mM L-glutamine, 0.05 mM 2-mercaptoethanol (Gibco, Carlsbad, USA) and 10% heat-inactivated FCS (Gibco, Carlsbad, USA). The media used for human T-cell culture were RPMI 1640 with 0.05 mM 2- mercaptoethanol, 200 CU/ml IL-2 (PeproTech, London, UK), plus 10% human AB serum (BTD, Tianjing, CHN).
Amplification and sequencing of IgHV rearrangements
Genomic DNA was isolated from 108 cases of B-ALL patients. Polymerase chain reaction (PCR) was performed using the specific primers of seven IgHV(IgHV1–IgHV7) gene subfamilies as the sense primer and a common JH primer as the anti-sense primer [22]. All PCR products were directly sequenced utilizing the ABI sequence detection system 377–96 (Applied Biosystems, Foster City, USA). Sequences we got were translated into amino acid sequences by using the translation tool from the website (http://www.expasy.ch/tools/DNA.html). The recombination characteristics and somatic mutations of Immunoglobulin-derived nucleic sequences were analyzed through the international ImMuneGeneTics database (http://imgt.cines.fr: 8104/) and the Genebank database (http://www.nlm.nih.gov/Igblast).
Prediction of HLA epitopes in IgHV subfamily
HLA class I binding peptides were predicted by using IgHV protein sequences of 40 B-ALL cases and 56 kinds of representative germline IgHV (IgHV1–IgHV7) sequences. This allowed classification of the 40 B-ALL cases into seven subfamilies. Two independent computer prediction analysis websites were consulted to examine the binding of these peptides to HLA-A*0201 molecules: http://bimas.dcrt.nih.gov/molbio/hla_bind and http://syfpeithi. bmi-heidelberg.com/. In this study, only epitopes to HLA-A*0201 allele were predicted and examined because this HLA class I allele was expressed in approximately 30% of the patients. So, we ranked the top score peptides in each IgHV gene subfamily.
Peptides choice and synthesis
All peptides were generated by fluorenylmethoxycarbonyl synthesis (Genemed Synthesis, South San Francisco, USA). Two peptides (QLVQSGAEV and SLYLQMNSL) derived from IgHV1 and IgHV3 subfamily FRs were synthesized. And an irrelevant influenza matrix-derived peptide (FLU matrix58-66, GILGFVFTL) was used as a positive control. Peptides were dissolved in phosphate-buffered saline (Hyclone, Logan, USA) at a concentration of 1 mg/ml and stored at −70°C until they were used.
T2 binding assay
A cellular peptide-binding assay employing the TAP-deficient cell line T2 was used to evaluate the binding affinity of subfamily-shared predicted peptides to HLA-A*0201molecules [23]. T2 cells were incubated with 50 μg/ml peptide in serum-free RPMI 1640, supplemented with 5 μg/ml human β2-mG (Fluka, Buchs, SWI) for 18 h at 37°C. Next, HLA-A*0201 expression was measured by Flow Cytometry using the anti-HLA-A2 monoclonal antibody (mAb) BB7.2 followed by incubation with fluorescein isothiocyanate (FITC)–conjugated F (ab) 2 goat anti-mouse Ig (Biosource, Camarillo, USA). Fluorescence Index (FI) was calculated by the Mean Fluorescence Intensity (MFI) of HLA-A*0201 on T2 cells as determined by fluorescence activated cell sorting analysis (FACS) (Becton Dickinson, Franklin Lakes, USA), using the formula FI = [MFI (T2 cells plus peptide)/MFI (T2 cells without peptide)]-1.
Generation of peptide-specific CTLs in healthy donors
An antigen-specific T-cell expansion system in vitro was used to generate the peptide-specific CTLs as previously described [24]. Purified HLA-A*0201–positive PBMCs from healthy donors were suspended in RPMI 1640 supplemented with 10% heat-inactivated human AB serum and 0.1 mg/ml synthetic IgHV1 subfamily peptide (QLVQSGAEV) or IgHV3 subfamily peptide (SLYLQMNSL). These cells were seeded into wells of 96-well plates at a concentration of 1 × 105 cells/0.1 ml. After 7 days, half of the media were exchanged into fresh culture media, and then second stimulation was performed by addition of autologous peptide-loaded PBMCs treated with mitomycin C (35 μg/ml) as antigen presenting cells (APCs). Weekly re-stimulation was performed in the same way. Human recombinant interleukin-2 (IL-2) (PeproTech, London, UK) was added at day 8 at a final concentration of 200 U/ml, and fresh IL-2 was added every three days thereafter. After four times stimulations, the cultured cells were collected and the cytotoxicity was measured.
Identify peptides-specific CTLs by tetramer analysis
Tetramer staining was used to detect the peptide-specific CTLs according to the published method [25]. The QLVQSGAEV/HLA-A*0201 tetramer complexes were obtained from ProImmune (Oxford, UK). CTL cell lines were generated as described above. Effector CTLs were re-suspended in RPMI 1640 medium supplemented with 10% human AB serum and 5 mM NaN3 to prevent internalization of the tetramer. Cells (106) were stained with the PE-labeled tetramer at 37°C for 30 min. Staining was followed by washing and subsequent staining with FITC-labeled anti-CD8 (Becton Dickinson, Franklin Lakes, USA) at 4°C for 30 min. Cells were washed and fixed in RPMI with 1% paraformaldehyde and were analyzed within 24 h. Data was acquired using a FACS with CELL Quest software (Becton Dickinson, Franklin Lakes, USA). Flow Cytometric analysis was conducted with a minimum of 10,000 cells. The frequency of peptide-specific CTLs is presented as a fraction of peptide/HLA tetramer and CD8 double-positive lymphocytes over the total CD8+ cells. An irrelevant HBV-Sag (FLLSLGIHL/ HLA-A*0201) tetramer was a gift from the virology lab of Peking University and was used as a negative control.
Analysis of TCRβ transcripts of CTLs
The variable region rearrangements of TCRβ gene on CTLs was determined by RT-PCR, followed by fingerprinting and sequencing. A panel of 30 TCRBV-specific upstream primers in combination with one downstream TCRBC primer was used for PCR amplification [26]. While a pair of primers on the TCRβ constant region (Ca) was used as the internal control. The PCR products were first separated on 6% polyacrylamide gels, and then followed by fingerprinting on 5% denaturing polyacrylamide gels containing 7 mol/l urea as described previously [27]. In brief, samples were heated at 94°C for 5 min and chilled on ice before loading. The gels were run for approximately 4 h at 65 W, 1,600 volt and fingerprinting profiles were visualized using silver staining system. The PCR products were then purified with the Qiaquick PCR purification kit (Qiagen, Valencia, USA) and directly sequenced using the ABI sequence detection system 377–96 (Applied Biosystems, Foster City, USA). The sequences were determined by using the international ImMuneGeneTics database system (http://imgt.cines.fr:8104/) and the Genebank database system (http://www.nlm.nih.gov/blast).
Cytokine release assay and cytotoxicity assay
The cytotoxicity of the CTL lines was assessed after four times stimulations with peptide-loaded and un-loaded target cell lines using the lactate dehydrogenase (LDH) release assay kit (CytoTox Non-Radioactive Cytotoxicity Assay), (Promega, Madison USA) [28], and the supernatants were assayed for IFN-γ cytokine secretion. In brief, peptides at a final concentration of 0.1 mg/ml were loaded with target cells. Target cells were then co-cultured with effector T-cells at various effector/target (E/T) ratios (1:1,5:1,10:1) for 6 h. Spontaneous release of effector and target cells was controlled by separate incubation of the respective populations. Maximal LDH enzyme release was measured after lysis of the target cells with 0.5% Triton X-100. Cell-free supernatants were incubated in a separate 96-well plate with LDH substrate for 10 min before measuring absorbance using a microplate reader (Dynatech MR5000, Muskegon, USA) at 490 nm. The percentage of cytotoxicity was calculated according to the following formula: % Cytotoxicity = [Experimental − Effector Spontaneous − Target Spontaneous]/[Target Maximum − Target Spontaneous]×100%.
Specific lysis was assessed for each CTL line against peptide loaded and unloaded target cells. HLA restriction of lytic activity was tested against HLA-A2–negative tumor lines or by the blockade of HLA-A2 using mAb BB7.2 and its isotype control of mAb B5. Ab-blocking experiments were performed by pre-incubation with 5 mg/ml mAb with the effector cell. FLU matrix58-66 (GILGFVFTL) peptide was used as an irrelevant peptide. Data are expressed as the mean ± SEM of three independent experiments. One-way ANOVA test was used to test the significance of the specific lysis of the CTLs, and the lysis of the peptides-unloaded T2 cells was used as the control group to compare with the lysis of the different peptides-loaded T2 cells groups.
Overnight co-culture supernatants were assayed for IFN-g release with an ELISA kit (R &D Systems, Minneapolis, USA) according to instructions.
Results
Characteristics of IgHV gene rearrangements in B-ALL
Complete VHDJH rearrangements were identified in 71(66%) out of 108 B-ALL patients, which comprised 34 (47.89%)cases of monoallelic rearrangements, 30 (42.25%)biallelic rearrangements and 7 (9.86%)oligoclonal rearrangements. Among 40 B-ALL IgHV gene sequences available, (data not shown)32 cases (80%) had ≥98% of sequence homology with the most close germline IgHV gene while eight cases (20%) showed >2% of somatic mutation. The sequences were found to fall into seven subfamilies based on the classification manner of IgHV germline genes. The subfamilies distributions were as following: IgHV1 (8/40) IgHV2 (4/40) IgHV3 (11/40) IgHV4 (11/40) IgHV5 (1/40) IgHV6 (4/40) IgHV7 (1/40), similar to the reported in normal peripheral blood B cell. IgHV3 IgHV4 and IgHV1 represented 75% of rearranged IgHV subfamilies in B-ALL.
Prediction of HLA epitopes in IgHV subfamily
The HLA-binding capacities of the IgHV subfamily-shared peptides were predicted by using the BIMAS and SYFPEITHI prediction programs. We compared the major epitopes that were predicted from the malignant B-cell with those from the 56 kinds of representative IgHV germline sequences. We found that most of their epitopes are identical in IgHV1–IgHV7 subfamilies. By listing the 1 or 2 top-score epitopes that were shared in each subfamily we arrived at 12 epitopes. All the 12 epitopes had predicted binding half-lives that were more than 10 min (BIMAS) and their binding scores were higher than 21 (SYFPEITHI). The predicted peptides in each IgHV subfamily were shown in Table 1. Among these predicted peptides, ten peptides (83%) were derived from the FRs, Only two peptides were derived from the CDRs of the IgHV protein. The representative peptides (QLVQSGAEV and SLYLQMNSL) were synthesized in vitro because these two IgHV subfamilies were found in nearly half of our patients.
Table 1.
Prediction of HLA-A*0201 peptide from 40 cases B-ALL patients
| number | Sequence | subfamily | position | SYFPEITHI score | BIMAS t1/2(min) |
|---|---|---|---|---|---|
| 1 | QLVQSGAEV | IgHV1/5 | FR1(3–11) | 24 | 69.552 |
| 2 | TLVKPTQTL | IgHV2 | FR1(10–18) | 25 | 21.362 |
| 3 | SLSTSGVGV | IgHV2 | FR1/CDR1(28–36) | 25 | 68.552 |
| 4 | SLYLQMNSL | IgHV3 | FR3(78–86) | 26 | 157.227 |
| 5 | TLYLQMNSL | IgHV3 | FR3(78–86) | 24 | 157.227 |
| 6 | QLQESGPGL | IgHV4 | FR1(3–11) | 24 | 87.586 |
| 7 | QLQQWGAGL | IgHV4 | FR1(3–11) | 23 | 21.362 |
| 8 | GLLKPSETL | IgHV4 | FR1(10–18) | 26 | 38.730 |
| 9 | GLVKPSETL | IgHV4 | FR1(10–18) | 24 | 10.468 |
| 10 | QLQQSGPGL | IgHV6 | FR1(3–11) | 22 | 21.362 |
| 11 | GLVKPSQTL | IgHV6 | FR1(10–18) | 24 | 21.362 |
| 12 | VLLWFRELL | / | CDR3(106–114) | 24 | 25.196 |
Shown are the number, sequence (single-letter abbreviations for amino acids), position, subfamily, calculated score of predicted BIMAS and SYFPEITHI prediction programs. We ranked one or two top score peptides in each IgHV subfamily
FR framework region, CDR complementary-determining region, ND not determined, B-ALL acute B- lymphoblastic leukemia
Binding affinity of subfamily-shared peptides to HLA-A*0201 molecules
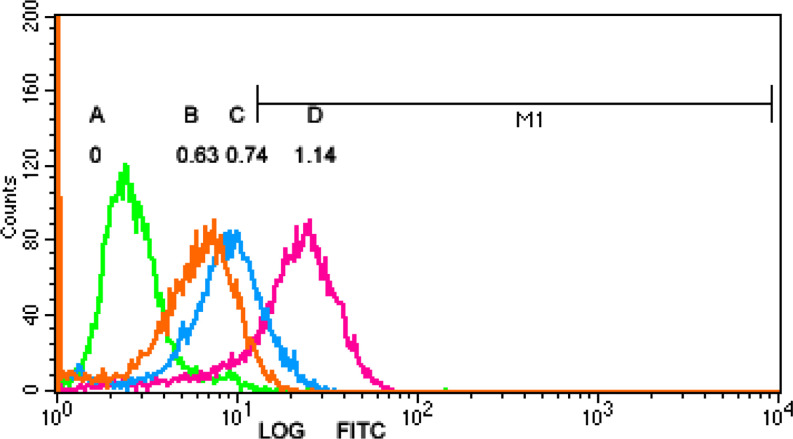
The binding affinity of IgHV1 peptide (QLVQSGAEV) and IgHV3 peptide (SLYLQMNSL) to HLA-A*0201 molecules were shown in Fig. 1. The MFI of HLA-A*0201 molecules was upregulated by 1.63±0.11 times after T2 cells incubated with IgHV1 peptide (QLVQSGAEV), the FI was 0.63. Similarly, when T2 cells were incubated with IgHV3 peptide (SLYLQMNSL), the MFI of HLA-A*0201 molecules was upregulated by 1.74±0.09 times and FI was 0.74. This result indicated that the two peptides could bind to the HLA-A*0201 molecules.
Fig. 1.
T2 Binding Assay Expression of HLA-A*0201 molecule on T2 cells without peptide and enhanced expression after addition of peptides. a no peptide. (FI=0); b IgHV1 (QLVQSGAEV) (FI=0.63); c IgHV3 (SLYLQMNSL). (FI=0.74); d a positive peptide. (FI=1.14). FI fluorescence index
Identify peptides-specific CTLs by tetramer analysis
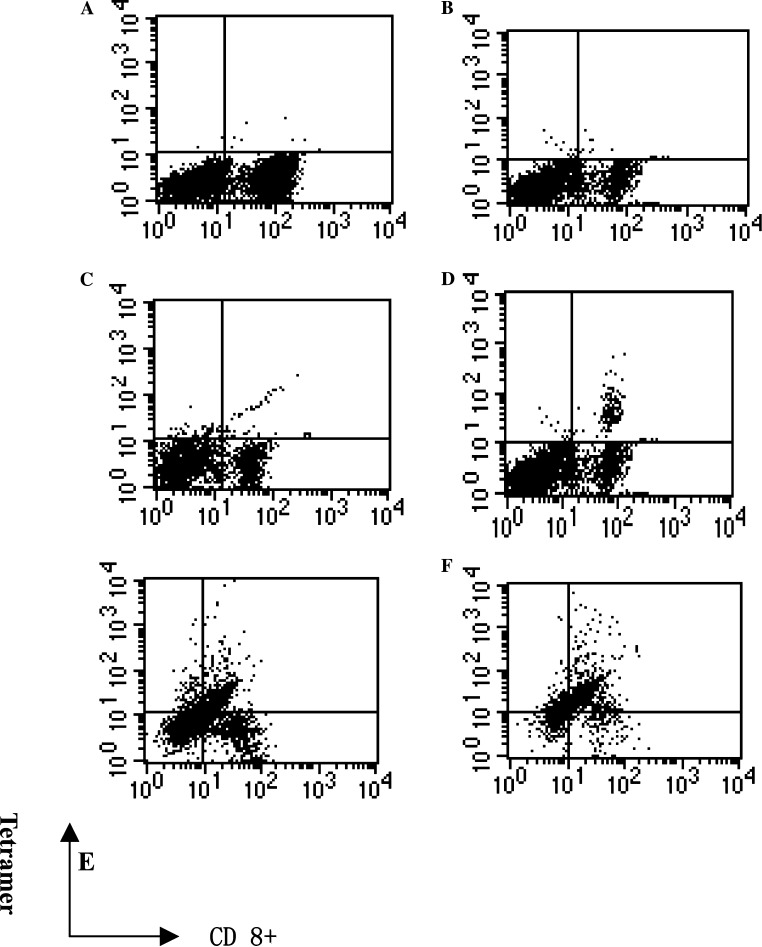
The peptide-specific CTLs were identified by tetramer analysis and were shown in Fig. 2. The frequency of peptide-specific CTLs is presented as a fraction of peptide/HLA tetramer and CD8 double-positive lymphocytes over the total CD8+ cells. The frequency of CD8 and QLVQSGAEV / HLA-A*0201 tetramer double positive cells was 0.38% before stimulation. And after the fourth stimulation, it increased to 49.38%. We observed a similar result in another donor. These figures indicated that the predicted peptides could be presented by APCs, and they were immunogenic in vitro.
Fig. 2.
Identify peptides-specific CTLs by tetramer analysis PBMCs from normal HLA-A*0201 donor were stimulated with peptides (QLVQSGAEV)-loaded autologous PBMC as the APCs every week, the frequency of CD8 and QLVQSGAEV /HLA-A*0201 tetramer double positive cells were assessed by FACS. a. CTLs stimulated by IgHV1 peptide were stained with a HCV (FLLSLGIHL/ HLA-A*0201) tetramer as a negative control; the double positive frequency was 0.02%. b CTLs stimulated by FLU peptide were stained with a QLVQSGAEV/HLA-A*0201tetramer as a negative control; the double positive frequency was 0.04%. c Before IgHV1 peptide stimulation, the frequency of CD8 and QLVQSGAEV /HLA-A*0201 tetramer double positive cells was 0.38%. d After the second stimulation, the frequency was 1.55%. e After the third stimulation, the frequency was 13.93%. f After the fourth stimulation, the frequency was 49.38%
T-Cell receptor β (TCRβ) analysis of peptide-specific CTLs
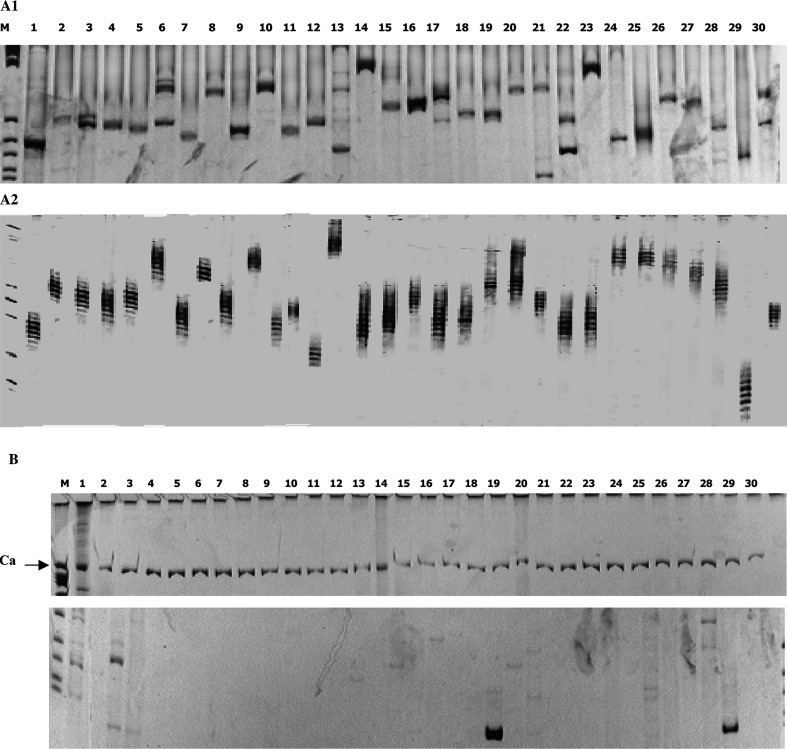
The TCRβ expressions of the peptide-special CTLs were shown in Fig. 3,a1 and a2. Before stimulation each TCR repertoire’s subfamily was expressed broadly. Each TCRβ subfamily’ amplification resulted in a number of bands that showed a Gaussian distribution in the fingerprinting profile. The samples generated unreadable sequence information due to the heterogeneous TCRβ composition of the template pool. After four times stimulations, the TCRβ repertoires were skewed in several families, and the largest clones were found to be a readable nucleotide sequences after direct sequencing. After the analysis by the IMGT/V-QUEST and IMGT/Junction Analysis database, we found that the TCRβ usage of the CTLs stimulated by the IgHV1-peptide was TRB30*05, TRBD1*01 and TRBJ2-7*01. The amino acid sequence in CDR3 region was CAWSRGDDEQYF; TCRβ usage of the CTLs stimulated by the IgHV3-peptide was TRBV7-2*04,TRBD2*02 and TRBJ2-05*01, and the amino acid sequence in the CDR3 region was CASSLSGAVETQYF. In the Gene bank database, we could not find any other identical sequences. It was highly possible that these TCRβ chains of CTLs could recognize the B-cells in the IgHV1 or IgHV3 subfamily and regulate the proliferation of B cells in vivo.
Fig. 3.
TCRβ receptor expression of CTL clone TCR BV gene usage was determined by RT-PCR, a1 Before the peptide stimulation, each TCR repertoire’s subfamily was expressed broadly. The PCR products were separated on 6% polyacrylamide gels. a2 For fingerprinting, the PCR products were separated on 5% denatured polyacrylamide gels containing 7 mol/l urea, and visualized by silver staining. Each subfamily’ amplification resulted in a number of bands that showed a Gaussian distribution. b After four stimulations, the TCR repertoires were skewed in several families. Ca (600 bp long-band) was used as an internal control
The cytotoxicity of CTLs against peptide-loaded T2 cells
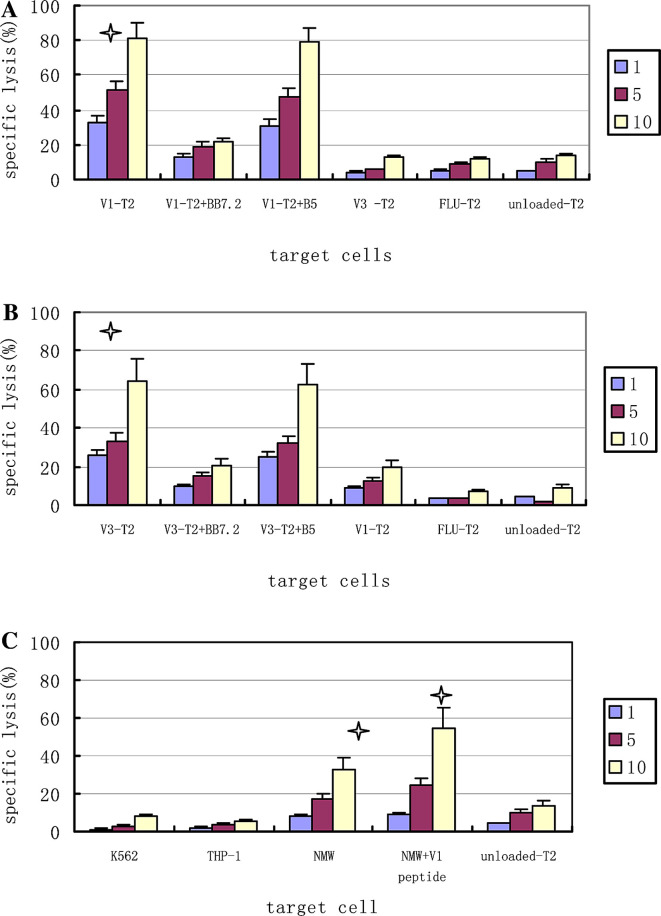
Using the peptide-loaded and peptide-unloaded T2 cells as the target cells, we analyzed the function of the peptide-specific CTLs. As indicated in Fig. 4a, after four times stimulations, the CTLs stimulated by the IgHV1 (QLVQSGAEV) peptide could kill the same peptide-loaded T2 cells. The specific lysis was 32.3±9.6%, 51.1±5.4%, and 81.5±6.5% respectively when E/T ratios were 1:1, 5:1, and 10:1. The addition of anti-HLA-A2 mAb (BB7.2) inhibited the CTL-mediated cytotoxicity. The specific lysis reduced to 13.1±1.5%, 19.3±2.8%, 21.2±7.9% (P=0.055, 0.017, 0.020 respectively) and was inhibited by 59.4%, 62.5% and 74% respectively when E/T ratios were 1:1, 5:1, and 10:1. And this supported the liklyhood that the cytotoxicity was HLA-A2 restrictive. However, the IgHV1 (QLVQSGAEV) peptide-specific CTLs could not kill the peptide-unloaded T2 cells, and the specific lysis was 4.2±2.2%, 10±2.4%, 13±4.1% respectively (P=0.045, 0.012, 0.002 respectively) when E/T ratios were 1:1, 5:1, and 10:1. Furthermore, the CTL also could not kill the T2 cell loaded with FLU peptide, though the FLU peptide consists of HLA-A*0201–high binding affinity. All these showed that CTL–mediated cytotoxicity was peptide-specific. The IgHV3 (SLYLQMNSL)-specific CTLs showed parallel data (Fig. 4b). The IgHV3 (SLYLQMNSL)-specific CTLs could not recognize the T2 cell loaded with the IgHV1peptide and the IgHV1 (QLVQSGAEV) peptide-specific CTLs could not recognize the T2 cell loaded with the IgHV3 peptide. These indicated that the lysis was subfamily-specific.
Fig. 4.
CTL cytotoxicity Cytotoxicity was determined in a standard LDH assay at E/T ratios of 1:1 and 5:1 and 10:1. a Effector T cells induced by IgHV1 (QLVQSGAEV) peptide stimulations, against T2 cells loaded with IgHV1, IgHV3, FLU peptide as target cells, preincubation with anti-HLA-A2 mAb BB7.2 and the isotype mAb B5. b Effector T cells induced by IgHV3 (SLYLQMNSL) peptide stimulations, against T2 cells loaded with IgHV1, IgHV3 or FLU peptide as targets cells and preincubation with BB7.2 and the isotype mAb B5. c Effector T cells induced by IgHV1 (QLVQSGAEV) peptide stimulations, against some tumor cell lines: NAMALWA (HLA-A*0201-positive, IgHV1-positive), THP-1 (HLA-A*0201-positive, IgHV1-negative, K562 (HLA-A*0201-nagative, IgHV1-negative), Data are expressed as the mean ± M of three independent experiments, One-way ANOVA test was used to test the significance of the specific lysis of the CTLs, and the lysis of the peptides-unloaded T2 cells as the target cells was used as the control group to compare with the lysis of the different peptides-loaded T2 cells groups as the target cells.*Significant lysis (P<0.05). V1: IgHV1 (QLVQSGAEV) peptide; V3: IgHV3 (SLYLQMNSL); FLU: irrelevant influenza matrix-derived peptide (GILGFVFTL); NAM: NAMALWA cell
The cytotoxicity of CTLs against tumor cell lines
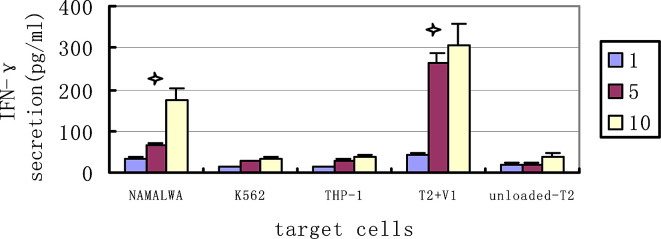
We then evaluated whether these CTL lines could also recognize HLA-matched tumor cell line expressing the IgHV1 gene endogenously. In Fig. 4c, The IgHV1 (QLVQSGAEV) peptide-specific CTLs could kill the HLA-matched and IgHV1-positive tumor cell line NAMALWA (a lymphoma cell line). The special lysis was 8.3±1.6%, 17.4±2.2%, and 32.8±2.9% respectively when E/T ratios were 1:1, 5:1, and 10:1. More interestingly, we found that killing of tumor cells increased when they were loaded with exogenous peptide, and the specific lysis increased to 9.2±1.3%, 24.6±5.8%, and 54.5±7.7% (P=0.047 at the E/T 10:1). These suggested that the mechanisms that led to increasing antigen presentation might make these tumor cells more susceptible to CTL-mediated killing. The IgHV1 (QLVQSGAEV) peptide-specific CTLs could not kill the HLA-A2–negative tumors K562. That indicated the cytotoxicity was HLA restrictive; In addition, the CTLs demonstrated no cytotoxicity against THP-1 without expressing the IgHV1 gene, regardless of its HLA-A*0201 expression status. This indicated that the cytotoxicity was peptide-specific. We also observed that CTLs stimulated by IgHV3 peptide couldnot kill the NAMALWA. This further proved that the cytotoxicity was subfamily specific. Similar data was shown by the IFN-γ secretion assay. The IFN-γ released increasingly when the CTLs had high cytotoxicity to the relevant target cells. (Fig. 5)
Fig. 5.
CTL induced IFN-γ secretion IFN-γ secretion was assayed after the IgHV1(QLVQSGAEV)peptide special-CTL lines co-culture with several tumor cell lines: NAMALWA (HLA-A*0201-positive, IgHV1-positive), THP-1 (HLA-A*0201ositive, IgHV1-negative), K562 (HLA-A*0201-nagative, IgHV1-negative), T2 (HLA-A*0201-positive). Overnight co-culture supernatants were assayed for IFN-g secretion with the ELISA method. Data are expressed as the mean ± SEM of three independent experiments. The peptide unloaded-T2 cells as the target cells were used as the control group to determine statistical significance. *Significant secretion. (P<0.05). V1: IgHV1 (QLVQSGAEV) peptide
Discussion
Trojan et al. [15] initially suggested the high possibility that some lymphomas-shared FR epitopes could be used as the common immune targets for different patients. However, how to choose the appropriate epitopes for each patient in a large number of predicted epitopes is still a problem. This study demonstrates that subfamily-specific immunotherapy for B-cell malignancies is a way to resolve this problem. Our result demonstrated that we could identify some subfamily-shared peptides in IgHV protein of the malignant B cells and these peptides could induce a subfamily-specific cell-mediated immune response in healthy donors.
Firstly, we predicted the T-cell epitopes of IgHV in the B-ALL patients by the BIMAS and SYFPEITHI prediction programs and compared them with those from 56 representative germline IgHV sequences in the genebank. We found that their major T epitopes were identical. In each IgHV subfamily, we found one or two top-score shared-epitopes and got 12 peptides from 7 subfamilies. These indicated some shared epitopes existed in the malignant B-cells subfamily.
Secondly, we proved that the subfamily-shared peptides could induce the family-specific cell-mediated immune response in healthy donors. We chose two peptides (QLVQSGAEV derived from IgHV1 and SLYLQMNSL derived from IgHV3) to synthesize in vitro for further assay, because these two subfamilies were found in nearly half of all our patients. The two peptides were clearly found to induce both peptide- and MHC- specific T cell proliferation. The CTLs stimulated by IgHV1 peptide (QLVQSGAEV) could recognize the HLA-matched B lymphoma cell line expressing the IgHV1 peptide endogenously in a peptide-specific and HLA-restricted way. From the above data, we not only proved that the subfamily-shared peptides were immunogenic in vitro, but also proved that the IgHV protein molecule was effectively processed intra-cellularly, and natural peptides, similar to the synthetic ones, could be presented by the corresponding HLA class I molecules on the malignant B-cell’s surface. Such malignant B-cells could be recognized by the CTLs stimulated by the same subfamily-shared peptide, allowing the conclusion that CTL-mediated cytotoxicity was subfamily-specific. In our paper, we had primarily proved that the shared-subfamily peptides were capable inducing special cytotoxic T-lymphocyte (CTL) expansion in vitro, and these CTLs could recognize the B-cell line in a same subfamily. Although it had been widely proved that the FRs-derived peptides could be induced by the special-CTLs in B-cell chronic lymphoblastic leukemia (B-CLL) and multiple myeloma (MM) patients in other papers [14, 15], whether these T cell responses can be elicited in patients with B-ALL is still not clear.
Although it is very convenient to treat the patients with these subfamily-shared peptides, there are still some questions that need further consideration. Firstly, these subfamily-shared epitopes were derived from the germline immunoglobulin so that the immune tolerance might be induced against these FRs in vivo, indicating that ways to increase their immunogenicity need to be further studied. Several reports have indicated that ex vivo presentation of such peptides in the context of ‘professional’ antigen-presenting cells are capable of overcoming any in vivo tolerance [16, 29]. Thus, expanding the subfamily-shared peptides-specific CTLs in vitro for adoptive immunotherapy or using the dentritic cells pulsed with high concentration of peptide to stimulate T cell in vivo are routes to overcome the tolerance and deserve further study.
In conclusion, our results demonstrated that subfamily-shared peptides could successfully lead to the generation of peptide-specific CTLs in vitro in healthy donors. These CTLs were capable of killing the malignant B-cells that belonged to the same IgHV subfamily in a peptide-specific and HLA-restricted manner. These data indicate possible immunotherapy approaches for B-cell malignances patients based on IgHV gene subfamilies.
Acknowledgements
The thorough reviewing of this manuscript by Dr. Daniel H. Conrad from the Department of Microbiology and Immunology, Virginia Commonwealth University, Medical College of Virginia, and Dr Zeng gang from the Division of Urologic Oncology, Department of Urology, David Geffen School of Medicine at UCLA are greatly appreciated. We thank Dr Dingfang-PU, Yongjin-SHI for their research suggestions and technical assistance. We also thank Jiaxia-YUAN for FCM assay, Dr Weifeng-CHEN for providing the T2 cell line. Dr Mirjam H. M. Heemskerk, (Department of Hematology, Leiden University Medical Center (LUMC), Leiden, the Netherlands) for supplying us with the primer of d TCRBV for RT-PCR. Supported by: National Natural Science Foundation of China (39970313) and grant 2002AA229011 from the ministry of Science and Technology, China.
Abbreviations
- Ig
Immunoglobulin
- IgHV
Variable domain of immunoglobulin heavy chain
- CDRs
Hypervariable complementary determining regions
- Id
Idiotype
- FRs
Framework regions
- HLA
Human-leukocyte-antigen
- CTLs
Cytotoxic T lymphocytes
- TCR
T-cell receptor
- B-ALL
B-cell acute lymphoblastic leukemia
- PBMCs
Peripheral blood mononuclear cells
- APCs
Antigen presenting cells
References
- 1.Janeway CA, Jr, Sakato N, Eisen HN. Recognition of immunoglobulin idiotypes by thymus-derived lymphocytes. Proc Natl Acad Sci USA. 1975;72(6):2357–2360. doi: 10.1073/pnas.72.6.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bogen B, Malissen B, Haas W. Idiotope-specific T cell clones that recognize syngeneic immunoglobulin fragments in the context of class II molecules. Eur J Immunol. 1986;16(11):1373–1378. doi: 10.1002/eji.1830161110. [DOI] [PubMed] [Google Scholar]
- 3.Campbell J Immunol 15. 1987;139:2825. [PubMed] [Google Scholar]
- 4.Miller N Engl J Med 4. 1982;306:517. doi: 10.1056/NEJM198203043060906. [DOI] [PubMed] [Google Scholar]
- 5.Campbell MJ, Esserman L, Byars NE, Allison AC, Levy R. Idiotype vaccination against murine B cell lymphoma. Humoral and cellular requirements for the full expression of antitumor immunity. J Immunol. 1990;145(3):1029–1036. [PubMed] [Google Scholar]
- 6.Maloney DG, Brown S, Czerwinski DK, Liles TM, Hart SM, Miller RA, Levy R. Monoclonal anti-idiotype antibody therapy of B-cell malignancies: the addition of a short course of chemotherapy does not interfere with the antitumor effect nor prevent the emergence of idiotype-negative variant cells. Blood. 1992;80(6):1502–1510. [PubMed] [Google Scholar]
- 7.Chen TT, Tao MH, Levy R. Idiotype-cytokine fusion proteins as cancer vaccines. Relative efficacy of IL-2, IL-4, and granulocyte-macrophage colony-stimulating factor. J Immunol. 1994;153(10):4775–4787. [PubMed] [Google Scholar]
- 8.Barrios Y, Cabrera R, Yanez R, Briz M, Plaza A, Fores R. Anti-idiotypic vaccination in the treatment of low-grade B-cell lymphoma. Haematologica. 2002;87(4):400–407. [PubMed] [Google Scholar]
- 9.Ruffini PA, Neelapu SS, Kwak LW, Biragyn A. Idiotypic vaccination for B-cell malignancies as a model for therapeutic cancer vaccines: from prototype protein to second generation vaccines. Haematologica. 2002;87(9):989–1001. [PubMed] [Google Scholar]
- 10.Wen YJ, Lim SH. T cells recognize the VH complementarity-determining region 3 of the idiotypic protein of B cell non-Hodgkin’s lymphoma. Eur J Immunol. 1997;27(4):1043–1047. doi: 10.1002/eji.1830270435. [DOI] [PubMed] [Google Scholar]
- 11.Fagerberg J, Yi Q, Gigliotti D, Harmenberg U, Ruden U, Persson B, Osterborg A, Mellstedt H. T-cell-epitope mapping of the idiotypic monoclonal IgG heavy and light chains in multiple myeloma. Int J Cancer. 1999;80(5):671–680. doi: 10.1002/(SICI)1097-0215(19990301)80:5<671::AID-IJC7>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 12.Wen YJ, Lim SH. Different properties of T-cell epitopes within complementarity-determining regions 1 and 2 of idiotypic VH in B-lymphoma. Scand J Immunol. 1999;50(3):296–301. doi: 10.1046/j.1365-3083.1999.00599.x. [DOI] [PubMed] [Google Scholar]
- 13.Rezvany MR, Jeddi-Tehrani M, Rabbani H, Ruden U, Hammarstrom L, Osterborg A, Wigzell H, Mellstedt H. Autologous T lymphocytes recognize the tumour-derived immunoglobulin VH-CDR3 region in patients with B-cell chronic lymphocytic leukaemia. Br J Haematol. 2000;111(1):230–238. doi: 10.1046/j.1365-2141.2000.02307.x. [DOI] [PubMed] [Google Scholar]
- 14.Hansson L, Rabbani H, Fagerberg J, Osterborg A, Mellstedt H. T-cell epitopes within the complementarity-determining and framework regions of the tumor-derived immunoglobulin heavy chain in multiple myeloma. Blood. 2003;101(12):4930–4936. doi: 10.1182/blood-2002-04-1250. [DOI] [PubMed] [Google Scholar]
- 15.Trojan A, Schultze JL, Witzens M, Vonderheide RH, Ladetto M, Donovan JW, Gribben JG. Immunoglobulin framework-derived peptides function as cytotoxic T-cell epitopes commonly expressed in B-cell malignancies. Nat Med. 2000;6(6):667–672. doi: 10.1038/76243. [DOI] [PubMed] [Google Scholar]
- 16.Lou Q, Kelleher RJ, Jr, Sette A, Loyall J, Southwood S, Bankert RB, Bernstein SH. Germ line tumor-associated immunoglobulin IgHV region peptides provoke a tumor-specific immune response without altering the response potential of normal B cells. Blood. 2004;104(3):752–759. doi: 10.1182/blood-2004-01-0105. [DOI] [PubMed] [Google Scholar]
- 17.Scaviner D, Barbie V, Ruiz M, Lefranc MP. Protein displays of the human immunoglobulin heavy, kappa and lambda variable and joining regions. Exp Clin Immunogenet. 1999;16(4):234–240. doi: 10.1159/000019115. [DOI] [PubMed] [Google Scholar]
- 18.Lin NJ, Zhu P, Zhang YP, Shi YJ, Zhang X, Bu DF, Dong YJ. The experimental study on the idiotypic nucleic Acid vaccine constructed from the genomic DNA to lymphoma. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2002;10(2):126–130. [PubMed] [Google Scholar]
- 19.Lin N, Zhu P, Zhang X, Dong Y, Ren Y, Wang Y, Li W. Specific humoral immune response against B-cell lymphoma elicited by mixed immunoglobulin fragments. Zhonghua Yi Xue Za Zhi. 2002;82(11):766–770. [PubMed] [Google Scholar]
- 20.Liu H, Zhu P, Lin NJ, Zhang Y, Bu DF, Wang YJ, Wang YQ, Yang Y. Development of IgHV family specific nucleic acid vaccine against lymphoma by construction of fusion gene of immunoglobulin heavy chain variable region and cytokine. Zhonghua Yi Xue Za Zhi. 2004;84(1):48–53. [PubMed] [Google Scholar]
- 21.Zhang Y, Zhu P, Shi Y, Liu J, Pu D, Cao X, Zhu Q, Wang Y, Ma M, Yu J. Experimental study on TCRbeta idiotypic antigenic determinants DNA vaccine to induce anti-lymphoma antibodies. Zhonghua Xue Ye Xue Za Zhi. 2002;23(2):68–72. [PubMed] [Google Scholar]
- 22.Liu Y, Zhu P, Hu YM. Characteristics of immunoglobulin heavy chain variable region genes in childhood B-cell acute lymphoblastic leukemia. Zhonghua Xue Ye Xue Za Zhi. 2004;25(1):8–12. [PubMed] [Google Scholar]
- 23.Vonderheide RH, Hahn WC, Schultze JL, Nadler LM. The telomerase catalytic subunit is a widely expressed tumor-associated antigen recognized by cytotoxic T lymphocytes. Immunity. 1999;10:673–679. doi: 10.1016/S1074-7613(00)80066-7. [DOI] [PubMed] [Google Scholar]
- 24.Valmori D, Dutoit V, Lienard D, Rimoldi D, Pittet MJ, Champagne P, Ellefsen K, Sahin U, Speiser D, Lejeune F, Cerottini JC, Romero P. Naturally occurring human lymphocyte antigen-A2 restricted CD8+ T-cell response to the cancer testis antigen NY-ESO-1 in melanoma patients. Cancer Res. 2000;60(16):4499–4506. [PubMed] [Google Scholar]
- 25.Clark RE, Dodi IA, Hill SC, Lill JR, Aubert G, Macintyre AR, Seran C. Direct evidence that leukemic cells present HLA-associated immunogenic peptides derived from the BCR-ABL b3a2 fusion protein. Blood. 2001;98(10):2887–2893. doi: 10.1182/blood.V98.10.2887. [DOI] [PubMed] [Google Scholar]
- 26.Heemskerk MH, Hoogeboom M, de Paus RA, Kester MG, van der Hoorn MA. Redirection of antileukemic reactivity of peripheral T lymphocytes using gene transfer of minor histocompatibility antigen HA-2-specific T-cell receptor complexes expressing a conserved alpha joining region. Blood. 2003;102(10):3530–3540. doi: 10.1182/blood-2003-05-1524. [DOI] [PubMed] [Google Scholar]
- 27.Yang YH, Li HF, Zhu P. The T-lymphocyte clonal proliferation in a patient with chronic eosinophilic leukemia. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2003;11(4):405–408. [PubMed] [Google Scholar]
- 28.Weidmann E, Brieger J, Jahn B, Hoelzer D, Bergmann L, Mitrou PS. Lactate dehydrogenase-release assay: a reliable, nonradioactive technique for analysis of cytotoxic lymphocyte-mediated lytic activity against blasts from acute myelocytic leukemia. Ann Hematol. 1995;70(3):153–158. doi: 10.1007/s002770050049. [DOI] [PubMed] [Google Scholar]
- 29.Harig S, Witzens M, Krackhardt AM, Trojan A, Barrett P, Broderick R, Zauls AJ, Gribben JG. Induction of cytotoxic T-cell responses against immunoglobulin V region–derived peptides modified at human leukocyte antigen–A2 binding residues. Blood. 2001;98((10):2999–3005. doi: 10.1182/blood.V98.10.2999. [DOI] [PubMed] [Google Scholar]