Abstract
The ability of heat shock proteins (HSPs) to increase the potency of protein- and DNA-based vaccines has been previously reported. We have constructed several plasmid-based vectors encoding chimeric proteins containing prostate-specific antigen (PSA) fused to Mycobacterium tuberculosis hsp70, M. bovis hsp65, Escherichia coli DnaK (hsp70), or human hsp70. Immunizing mice with these plasmids induced CD8+ cytotoxic T lymphocytes (CTLs) specific to human PSA and protected mice from a subsequent subcutaneous challenge with PSA-expressing tumors. We did not observe a significant difference either in the levels of PSA-specific CTLs or in protection against tumor challenge in mice immunized with plasmids expressing PSA-HSP chimeric proteins, as compared to mice receiving a conventional PSA-expressing DNA plasmid. Our data indicate that using HSPs as fusion partners for tumor-specific antigens does not always result in the enhancement of antigen-specific CTL responses when applied in the form of DNA vaccines.
Keywords: CTL, DNA vaccine, Heat shock protein, Prostate-specific antigen
Introduction
Immunization with naked DNA plasmids encoding protein antigens has been applied in active specific cancer immunotherapy with the intention of eliciting immune responses against tumor-associated antigens [22, 25, 28]. Our studies focus on the development of DNA vaccines targeting prostate-specific antigen (PSA). PSA is a 30–33-kDa secreted serine protease, which is almost exclusively produced by epithelial cells that line prostatic acini and ducts [18, 26]. The PSA level in serum is a widely used tumor marker [1, 17]. Human PSA gene (or KLK3) is a member of the human kallikrein gene family. PSA-related genes were identified in nonhuman primates, but no homologue was found in other mammalian species, including mice [10]. Human PSA is immunogenic both in mice and in nonhuman primates [8, 27]. Immunization with a naked DNA plasmid encoding PSA generates both humoral and cellular immune responses against PSA in mouse models [11] and, more importantly, in Rhesus macaques [12]. Since PSA is a secreted protein and is not present on the cell surface, induction of PSA-specific CD8+ cytotoxic T lymphocytes (CTLs), rather then antibodies, is believed to be a critical requirement for successful targeting of PSA-expressing cells.
Our objective in this study was to increase the potency of a PSA DNA vaccine to induce CTL responses against PSA. We were encouraged by the finding that linkage of Mycobacterium tuberculosis hsp70 to the human papillomavirus E7 antigen increased the potency of E7 DNA vaccine dramatically, and also provided protection in mice challenged with E7-expressing tumors [3]. It was shown that a DNA vaccine containing the E7-hsp70 fusion gene increased the frequency of E7-specific CD8+ T cells by at least 30-fold relative to a vaccine containing the wild-type E7 gene. The potent induction of CTLs was also demonstrated when the E7-hsp70 fusion gene was used in the form of self-replicating RNA [4] or DNA [9] vectors.
The exact mechanism of enhancement of CD8+ T-cell responses by heat shock proteins (HSPs) in the form of chimeric DNA vaccines is still under investigation. The mode of action may be different when HSPs are applied for protein-based versus DNA-based vaccines [3, 20]. To elucidate further the potency of HSPs of different origins and their ability to modulate the antigen-specific CTL responses when used in the context of DNA vaccines, we constructed a panel of PSA-HSP hybrid DNA constructs with M. tuberculosis hsp70, M. bovis hsp65, Escherichia coli DnaK (hsp70), and human hsp70. These HSPs were previously shown to increase antigen-specific CTL responses when administered in the form of antigen-linked chimeric proteins [2, 5, 21, 24].
We found that immunizing with the PSA and the different PSA-HSP DNA plasmids induced CD8+ CTLs against human PSA, and protected mice from the subcutaneous challenge with PSA-expressing tumors. No significant difference between the PSA and the different PSA-HSP plasmids in the ability to induce PSA-specific CTLs or mediate tumor protection was observed. Therefore, we conclude that HSPs are not universally efficient in enhancing DNA-based tumor vaccines.
Materials and methods
Plasmid DNA vectors and cloning of PSA-HSP constructs
A gene coding for the full-length human PSA protein [14] was kindly provided by Dr Tim Ratliff (Washington University, St Louis, MO, USA). The pVax1 and pcDNA3 vectors were obtained from Invitrogen (Carlsbad, CA, USA). The following HSP genes were kindly provided: the pSJ70 plasmid containing cDNA for human inducible hsp70 by Dr Satish Jindal (Neo Genesis, MA, USA); the hsp65-pcDNA3 plasmid with M. bovis hsp65 cDNA and p70ex plasmid with M. tuberculosis hsp70 cDNA by Dr Ruurd van der Zee (Utrecht University, The Netherlands). The cDNA for E. Coli DnaK was amplified from E. Coli genomic DNA by PCR with the forward (5′-ATGGGTAAAATAATTGGTATCGA-3′) and backward (5′-TTATTTTTTGTCTTTGACTTCTTCAAATT-3′) primers, and cloned into a pcDNA3 plasmid resulting in the DnaK-pcDNA3 vector. The pVax1-PSA vector containing cDNA for human PSA, under the control of the human CMV promotor, was previously constructed in our laboratory.
The plasmid DNA vectors encoding for the PSA-HSP chimeric proteins were constructed with the pVax1 plasmid as a backbone. The PSA cDNA sequence without a stop codon was PCR amplified, and inserted upstream of the HSP cDNA sequences into pSJ70, hsp65-pcDNA3, p70ex and DnaK-pcDNA3. The resulting cDNAs, encoding the fusion proteins, were cloned into the pVax1 plasmid under the control of the CMV promotor. The final plasmids, pVax-PSA-hsp70Hum, pVax-PSA-hsp65Myc, pVax-PSA-hp70Myc, and pVax-PSA-DnaK encoding the PSA-HSP fusion proteins, were sequenced to confirm the integrity of the PSA-reading frame in the fusion constructs. For mouse immunizations, the plasmids were purified with EndoFree Plasmid Mega or Giga kits (Qiagen, Valencia, CA, USA) and dissolved in sterile PBS.
Tumor cell lines
The murine MC57 (H-2b) fibrosarcoma cell line, the EL4 (H-2b) thymoma cell line and the B16F10 (H-2b) melanoma cell line were from ATCC (Manassas, VA, USA). Cells were cultured in DMEM medium (BioWhittaker, East Rutherford, NJ, USA), supplemented with 10% FCS (BioWhittaker), 2-mM L-glutamine, and 25 μg/ml gentamicin.
EL4/PSA and B16/PSA are clonally derived cell lines, stably transfected with pcDNA3-PSA and pTracer-CMV2-PSA, respectively, expressing human PSA. EL4/PSA and B16/PSA cells were grown in the presence of selective antibiotics G418 (500 μg/ml; Invitrogen) and Zeocin (20 μg/ml; Invitrogen), respectively. The conditioned medium from the PSA-transfected cell lines was routinely screened for presence of PSA with the Prostatus PSA Free/Total method (Wallac Oy, Turku, Finland) at the Department of Clinical Chemistry (Karolinska Hospital, Stockholm). All cell lines were routinely checked for the presence of mycoplasma with the Mycoplasma PCR ELISA kit (Roche, Indianapolis, IN, USA).
Detection of PSA and PSA-HSP proteins in vitro
The presence of PSA and each of the PSA-HSP chimeric proteins in cell lysates and culture supernatant was detected by Western blotting after transient transfection of MC57 cells with the corresponding plasmid DNA. For the cell transfections, the Lipofectamine 2000 reagent (Invitrogen) was used according to the manufacturer’s instructions. Twenty-four hours after transfection, MC57 cells were trypsinized, divided in half, and cultured for 10 h in fresh medium containing 0.5% FCS with or without addition of GolgiPlug reagent (a protein transport inhibitor containing brefeldin A; Pharmingen, San Diego, CA, USA). Afterward, the culture supernatant and the cells were collected separately. The cell lysate samples for Western blotting were prepared by dissolving the cell pellets (0.5×106 cells) in 400 μl of 1XNuPage LDS reducing sample buffer (Invitrogen), followed by denaturation for 10 min at 95°C. Any remaining cells were removed from the culture supernatant by centrifugation at 12,000 g for 10 min. For protein precipitation, 500-μl aliquots of culture supernatant were incubated for 1 h at −20°C with four volumes of pure acetone, followed by centrifugation at 12,000 g for 10 min. The culture supernatant samples were prepared by dissolving the precipitated protein pellets in 100 μl of the 1XNuPage LDS reducing sample buffer (Invitrogen), followed by denaturation for 10 min at 95°C. The 30 μl of the proteins from the cell lysate and the culture supernatant samples were separated in NuPage 4–12% Bis-Tris gels (Invitrogen) and transferred to an Immobilon-P PVDF membrane (Millipore, Bedford, MA, USA). The PSA-HSP proteins were detected either by rabbit polyclonal anti-human PSA (DAKO, Carpinteria, CA, USA), or mouse monoclonal anti-human hsp70/hsc70, M. tuberculosis hsp71, M. bovis hsp65, and E. coli DnaK (StressGen, San Diego, CA, USA) primary antibodies. The secondary, peroxidase-labeled anti-rabbit or mouse antibodies, and ECL Advance Western blotting detection kit, were obtained from Amersham Pharmacia Biotech (Uppsala, Sweden).
Mice and immunizations
C57Bl/6(H-2b) mice at 8–12 weeks of age were purchased from Karolinska Institute’s animal breeding facility. For intramuscular (i.m.) immunizations, mice were anesthetized with 4% Isofluran (Baxter Medical AB, Kista, Sweden). Plasmid DNA (50 μl) in PBS was injected bilaterally into each anterior tibial muscle. Each mouse received three immunizations with 70-μg plasmid, every 10 days, if not stated differently. The Ethical Committee for Animal Research in Stockholm approved all animal experiments.
Splenocyte isolation and in vitro restimulation
Two weeks after the last immunization, mice were euthanized and spleens were removed. The splenocyte suspensions were prepared by passage through a 40-μm cell strainer (Becton Dickinson, San Jose, CA, USA), and then the red blood cells were lysed in ACK buffer (0.15-M NH4Cl, 10-mM KHCO3, 0.1-mM EDTA, pH 7.2–7.4). Splenocytes were cultured in DMEM medium supplemented with 5% FCS, 10-mM HEPES, 2-mM L-glutamine, 1% non-essential amino acids, 25 μg/ml gentamicin, 5×10−5-M 2-mercaptoethanol, and 20 IU/ml human recombinant IL-2 (Proleukin; Chiron, Emeryville, CA, USA). For in vitro restimulation, 2×106 cells/ml of splenocytes were cultured for 5 days with irradiated (5,000 rad) EL4/PSA cells at a ratio of 10:1.
Intracellular cytokine staining for IFN-γ
The frequency of PSA-specific CD8+ T cells in bulk splenocyte cultures was determined after 5 days of in vitro restimulation. The splenocytes were incubated with confluent B16/PSA or B16 cells at 2×106 splenocytes per well in a 24-well plate for 6 h. To inhibit secretion of IFN- γ, GolgiPlug (Pharmingen) reagent was added to the wells during the last 3 h of incubation. The cells were then stained for surface CD8 and intracellular IFN-γ expression. Cells were fixed and permeabilized with the CytoFix/CytoPerm Plus kit (Pharmingen) according to the manufacturer’s instructions. The following antibodies were used for flow cytometry: rat IgG2a-FITC antimouse CD8a and rat IgG1-PE antimouse IFN-γ (Pharmingen). A rat IgG antibody (Sigma, St Louis, MO, USA) was added during all staining steps to block nonspecific binding. The splenocyte samples were analyzed on a FACSCalibur (Becton Dickinson), and 5×104–1×105 events were collected. Acquisition and analysis of samples was performed using the CELLQuest software (Becton Dickinson). The proper compensation during data collection was set using splenocytes stimulated with PMA/ionomycin (Sigma).
Cytotoxicity assay
The cytolytic activity of bulk splenocyte cultures against EL4/PSA and EL4 targets was determined after 5 days of in vitro restimulation. The EL4 and EL4/PSA cells were labeled with 1–2 μCi/μl of Na51CrO4 (Amersham Pharmacia Biotech) at 37°C for 1 h. Serial dilutions of splenocytes were incubated in triplicate in 200 μl of medium with 1×104 target cells. After 5 h, 100 μl of supernatant was collected and the amount of 51Cr released into the medium was measured by an automatic γ-counter (Wallac, Upplands Vasby, Sweden). The percentage of specific lysis was calculated by the formula [(E − S) / (T − S)]×100%. E represents the experimental release, S is the spontaneous release, and T is the total release in the presence of 5% Triton X-100. The spontaneous release never exceeded 10% of the total release.
In vivo tumor protection experiment
Five mice in a group received three i.m. immunizations with 70-μg plasmid, every 10 days. One week after the last immunization each mouse was challenged subcutaneously in the right flank with 5×104 EL4/PSA cells. Tumor growth was examined by palpation and inspection twice a week, and mice with apparent tumor growth were euthanized.
Statistical analysis
All data are expressed as mean ± SD derived from at least four independent experiments. A comparison of the data from intracellular cytokine stainings and cytotoxicity assays between different immunization groups was made using a two-tailed Student’s t-test with the significance level of p≤0.05. Statistical analysis of the tumor challenge data was conducted by Cox’s regression (SAS 8.2 software), with the level of significance defined as p<0.05.
Results
Expression and extracellular secretion of PSA-HSP fusion proteins in vitro
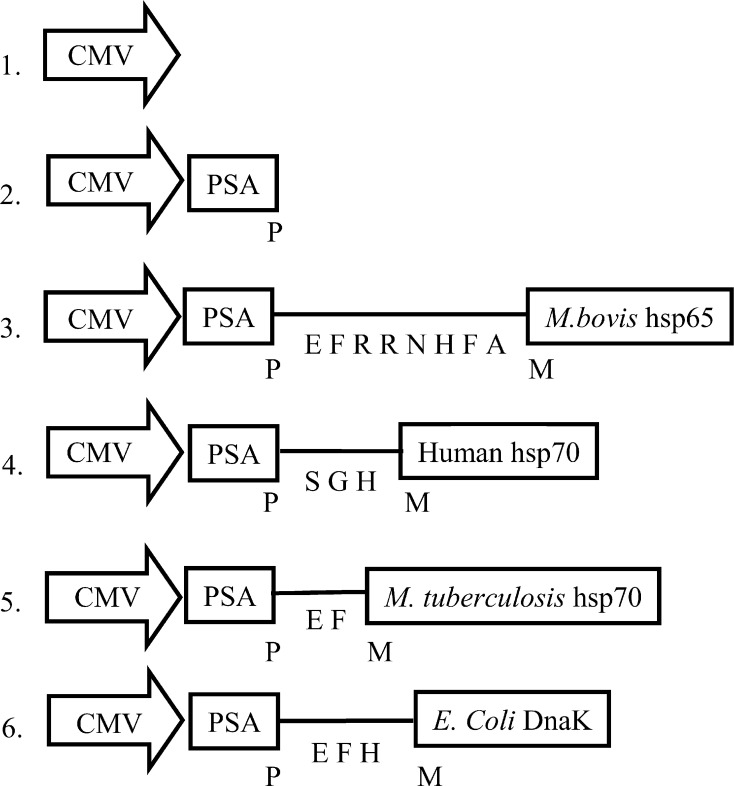
The PSA-HSP constructs contain human PSA cDNA fused at the C-terminus with cDNA for the different HSPs (Fig. 1). The additional amino acid sequences between PSA and HSP proteins are derived from the cloning procedures and were confirmed by DNA sequencing.
Fig. 1.
Schematic representation of the PSA-HSP plasmid vectors used for immunizations. The chimeric PSA-HSP protein junction sequences are shown in letters. The plasmid vectors are numbered for convenient referencing: 1 pVax, 2 pVax-PSA, 3 pVax-PSA-hsp65Myc, 4 pVax-PSA-hsp70Hum, 5 pVax-PSA-hsp70Myc, and 6 pVax-PSA-DnaK
Targeting the E7-Hsp70 proteins for secretion was suggested to provide more efficient “cross priming” of E7-hsp70 fusion proteins to APCs, especially after intramuscular immunizations with E7-HSP70 DNA vaccines [7, 23]. Therefore, we analyzed secretion of the PSA-HSP fusion proteins after transient transfection of mouse MC57 cells in vitro. Since it can not be excluded that proteins accumulate in the culture supernatant due to the release from dying cells, the secretion of the PSA-HSP proteins was analyzed by blocking the secretion pathways with GolgiPlug reagent (a protein transport inhibitor containing brefeldin A). Addition of GolgiPlug reagent didn’t block the expression of the PSA and the different PSA-HSP fusion proteins, since the proteins were detected in the cell lysate samples both in presence or absence of the GolgiPlug reagent (Fig. 2a). The PSA-HSP proteins migrated with a net molecular weight in the range of 100–120 kDa, corresponding to their predicted sizes. Presence of the HSP proteins in the fusions was confirmed by Western blot analysis with antibodies against the different HSPs (data not shown). The PSA and the PSA-hsp70Human fusion protein were actively secreted, since they were absent in the culture supernatant in the presence of GolgiPlug reagent (Fig. 2b, c). In contrast, all other PSA-HSP proteins were also present in the culture supernatant containing GolgiPlug reagent, which possibly rules out their active secretion and might be explained by the release from dying cells caused by increased toxicity of these proteins for the cells.
Fig. 2a–c.
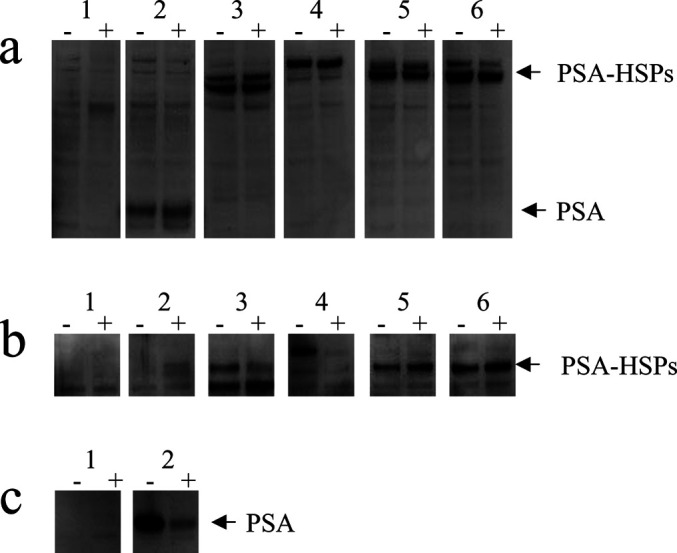
Western blot analysis of PSA-HSP chimeric protein expression. MC57 cells were transfected with the different PSA-HSP–expressing constructs and cultured for 10 h in the presence (“+”) or absence (“−”) of GolgiPlug reagent. PSA or PSA-HSP fusion proteins were detected with antibodies against human PSA, in total cell lysates (a) and culture supernatant (b–c). The loaded samples are numbered in accordance with Fig. 1
Immunizations with PSA and PSA-HSP DNA vaccines generated similar levels of PSA-specific CTLs
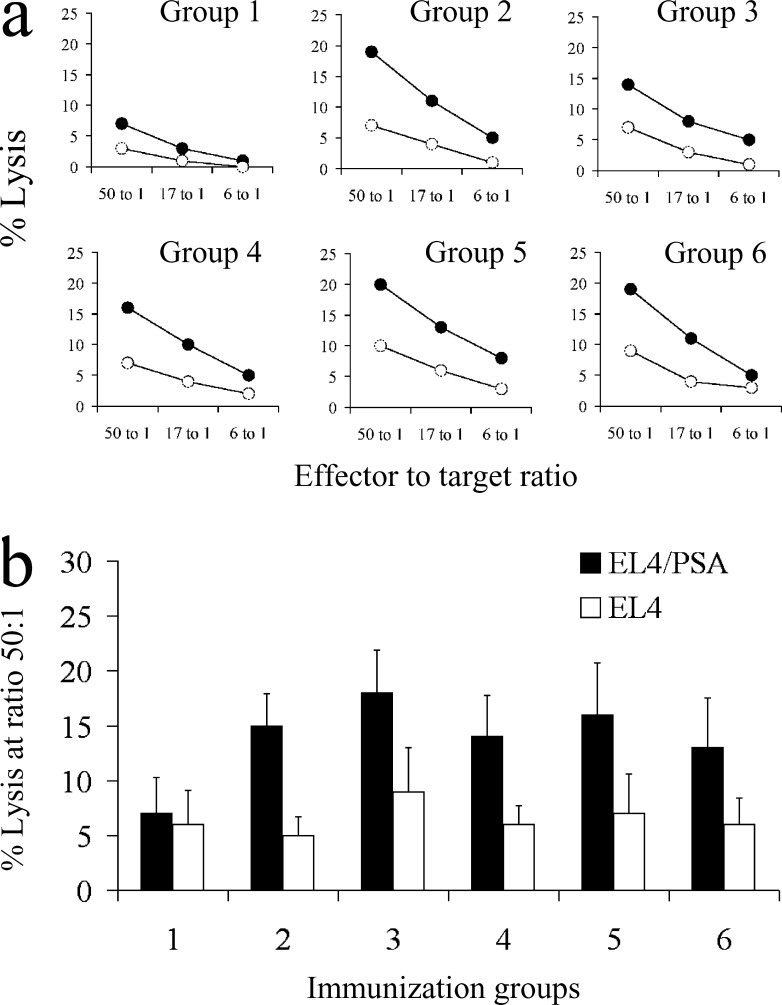
We further determined if immunizing with the PSA-HSP plasmids leads to the generation of PSA-specific CTLs in mice. In each group, four C57Bl/6 mice were immunized i.m. three times every 10 days. Two weeks after the last immunization, splenocytes from each mouse were analyzed. As immunodominant H-2b restricted peptides have not been identified for PSA, EL4/PSA cells stably transfected to express PSA were used for restimulation of the PSA specific precursor CTLs. After 5 days of in vitro restimulation, PSA-specific lytic activity was detected in a cytotoxicity assay with EL4 and EL4/PSA as target cells (Fig. 3a). Restimulation of the splenocytes with EL4 cells never resulted in lytic activity against EL4 or EL4/PSA target cells (data not shown). As shown in Fig. 3b, immunizations with each of the PSA-HSP DNA vaccines resulted in PSA-specific lytic activity. However, no significant difference in levels of PSA-specific lytic activity between the mice immunized with pVax-PSA or the different PSA-HSP plasmids was found.
Fig. 3a, b.
Detection of PSA-specific lytic activity in mice immunized with PSA-HSP plasmids. a Splenocytes were restimulated for 5 days with EL4-PSA cells and then tested against EL4 (open circles) or EL4-PSA targets (solid circles) in a 5-h cytotoxicity assay. A representative experiment is shown for one mouse from each immunization group. b Comparison of PSA-specific lytic activity in mice immunized with the different PSA-HSP plasmids and then assayed against EL4 or EL4-PSA targets in a cytotoxicity assay. The mean value from four mice analyzed individually and standard deviation are shown. The immunization groups are numbered in accordance with Fig. 1
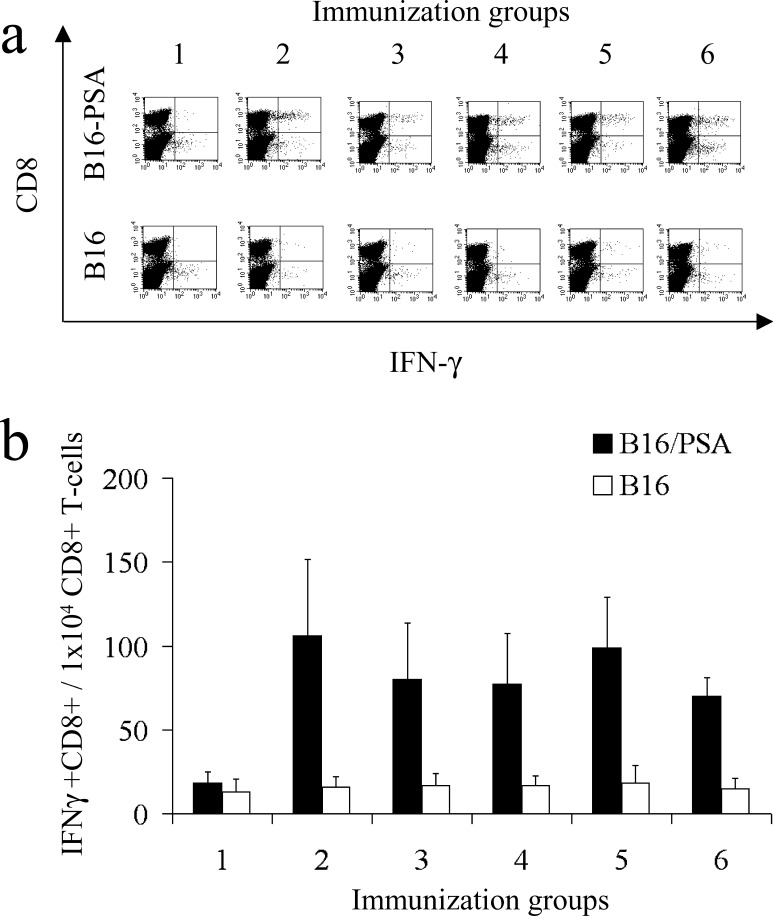
To analyze the PSA-specific CTLs quantitatively we also performed intracellular cytokine staining experiments to detect CD8+ T cells producing IFN-γ in response to PSA. After 5 days of in vitro restimulation with EL4/PSA cells, splenocytes from immunized mice were cocultured with B16 or B16/PSA cells. Representative panels of intracellular cytokine–staining experiments for each immunization group are shown in Fig. 4a. We detected PSA-specific effector CTLs after immunization with all of the PSA-HSP DNA vaccines (Fig. 4b). In agreement with the data from the cytotoxicity assay, no significant differences in levels of PSA-specific effector CTLs, between the groups immunized with pVax-PSA and the different PSA-HSP plasmids were found.
Fig. 4a, b.
Quantification of PSA-specific CD8+ T cells in mice immunized with PSA-HSP plasmids. After 5 days of in vitro restimulation with EL4-PSA cells, splenocytes were incubated with B16 or B16-PSA tumor cells, stained with antibodies against CD8 and IFN-γ, and analyzed by flow cytometry. a A representative experiment is shown for one mouse from each immunization group. b Quantitative comparison of PSA-specific CD8+ T cells in mice immunized with the different PSA-HSP plasmids. The mean value from four mice analyzed individually and standard deviation are shown. Immunization groups are numbered in accordance with Fig. 1
Mice vaccinated with PSA and PSA-HSP DNA vaccines generated similar in vivo tumor protection
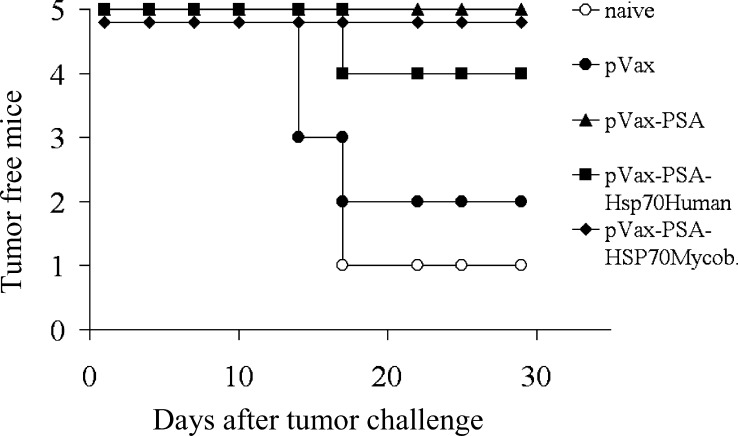
To determine whether the in vitro anti-PSA immune CTL response elicited by our DNA vaccines reflects their immunogenicity in vivo, we performed a tumor protection experiment using EL4/PSA cells for subcutaneous tumor challenge (Fig. 5). Statistical analysis revealed that mice immunized with pVax-PSA, pVax-PSA-hsp70Mycob (non-secreted PSA-HSP), or pVax-PSA-hsp70Human (secreted PSA-HSP) were significantly better protected (p<0.05) against tumor challenge then naïve mice, while pVax empty vector had no statistically significant effect compared with the naïve group. In between the three protected groups there was no statistical difference. The experiment was performed twice with reproducible results, confirming the in vitro data on the generation of PSA-specific CTLs.
Fig. 5.
In vivo tumor protection experiments in mice vaccinated with PSA and PSA-HSP DNA vaccines. Mice were immunized with the different PSA-HSP plasmids and challenged with 5×104 EL4/PSA tumor cells. Mice immunized with pVax-PSA, pVax-PSA-hsp70Human, or pVax-PSA-hsp70Mycob were significantly better protected (p<0.05) against tumor challenge then naïve mice
Discussion
In this study we showed that immunization with the PSA and the different PSA-HSP DNA vaccines generated PSA-specific CTLs in mice, as judged both by cytotoxicity and intracellular cytokine–staining (ICCS) assays. The detected levels of PSA-specific CTLs were similar to those observed by Chen et al. [3] for the E7 antigen. In contrast to the unmodified E7 DNA vaccine that was not able to generate any E7-specific CTLs, we found that our conventional PSA DNA vaccine (pVax-PSA plasmid) was able to prime PSA-specific CTLs that comprised up to 2% of the CD8+ T-cell pool. This suggests that human PSA protein in our murine model has high intrinsic immunogenicity. Further modification of human PSA by fusion with the different HSP proteins did not increase levels of PSA-specific CTLs in our system. Thus our data suggest that the mere linkage of antigen to HSPs in DNA vaccines does not always result in an increase in immunogenicity.
The mechanism of HSP’s action was mainly studied by immunizations with reconstituted HSP-peptide complexes [6, 20] and recombinant chimeric antigen-HSP proteins [21]. The adjuvant effect of HSPs has been attributed to the unique chaperone property of HSPs when extracellular antigen-HSP complexes are taken up and processed by antigen-presenting cells (APCs) via the MHC class I–restricted pathway (also known as “cross priming”) [3, 9, 20, 21].
The application of adjuvant activity of the HSP proteins for DNA vaccines was originally provided by Chen et al. for the E7 antigen [3]. It was shown that the DNA vaccine containing a fusion gene consisting of Mycobacterium hsp70 and HPV-16 E7 increased the frequency of E7-specific CD8+ T cells by at least 30-fold relative to a vaccine containing the wild-type E7 gene alone. In further studies the same effect was observed with the human hsp70 [7]. The following E7-Hsp70 fusion DNA vaccines were designed to encode a secreted form of the E7-Hsp70 fusion proteins, which could enable more efficient “cross priming” of E7-hsp70 fusion proteins to APCs [7, 23]. Since data was not provided on secretion of these fusion proteins, nor immune response compared with a fusion gene lacking a secretion signal, the contribution of “cross priming” to the observed immune response is still unclear.
We based the design of our PSA-HSP plasmids on these findings and fused the different HSP genes to the C-terminus of the PSA gene. Since PSA is a secreted protein with a N-terminus–located secretion signal [15], such a design would suggest an efficient secretion of the PSA-HSP fusion proteins. In transfection experiments in vitro, by blocking protein transport with GolgiPlug reagent, we were able to demonstrate that only PSA-hsp70 human fusion protein was efficiently secreted, while other fusion proteins were not secreted and appeared in culture supernatant presumably after cell death (Fig. 2). Although the constructs showed differences in secretion properties, no significant difference in PSA-specific immune response was observed comparing the fusion genes in DNA vaccinations (Figs. 3 and 4). This suggests that “cross priming” provided by secreted proteins contributes only marginally to the induction of PSA-specific immune response in our model system.
Differences in intrinsic immunogenicity of HPV-16 E7 and Trypanosoma cruzi KMP11 [19] versus PSA protein might explain the contradictions between our results and former studies concerning the applicability of HSPs in DNA vaccination[7, 19, 23]. Both unmodified E7 and KMP11 applied in DNA vaccination induce only low levels of specific CTL responses [16, 19]. Furthermore, for E7 it was shown in previous studies that various modifications including the addition of protein import and export signals [16], or increasing the steady state level of E7 by codon optimization [13], dramatically enhances the E7-specific immune response. Thus fusion of E7 with HSPs in DNA vaccines provides only one additional modification resulting in an increase of E7 immunogenicity. However, a general applicability of this strategy to other antigens is questionable, particularly for antigens with high intrinsic immunogenicity.
In summary, our data indicate that the fusion of a tumor antigen to HSPs in DNA vaccines does not always result in the enhancement of the antigen-specific CTL responses, but needs to be evaluated for each tumor antigen separately.
Acknowledgements
We thank Mr W David Culp Jr and Ms Ashley Miller for the critical assistance with preparation of the manuscript. This study was supported by the Cancer Society in Stockholm, the Swedish Cancer Society, Karolinska Institute Funds, Swedish Research Council, Wallenberg’s Foundation, and Swedish Society for Medical Research.
References
- 1.Armbruster Clin Chem. 1993;39:181. [PubMed] [Google Scholar]
- 2.Barrios Clin Exp Immunol. 1994;98:229. doi: 10.1111/j.1365-2249.1994.tb06130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen Cancer Res. 2000;60:1035. [Google Scholar]
- 4.Cheng J Immunol. 2001;166:6218. doi: 10.4049/jimmunol.166.10.6218. [DOI] [PubMed] [Google Scholar]
- 5.Chu Clin Exp Immunol. 2000;121:216. doi: 10.1046/j.1365-2249.2000.01293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ciupitu J Exp Med. 1998;187:685. doi: 10.1084/jem.187.5.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hauser Methods. 2003;31:225. doi: 10.1016/S1046-2023(03)00136-1. [DOI] [PubMed] [Google Scholar]
- 8.Hodge Int J Cancer. 1995;63:231. doi: 10.1002/ijc.2910630215. [DOI] [PubMed] [Google Scholar]
- 9.Hsu Gene Ther. 2001;8:376. doi: 10.1038/sj.gt.3301408. [DOI] [PubMed] [Google Scholar]
- 10.Karr Cancer Res. 1995;55:2455. [PubMed] [Google Scholar]
- 11.Kim Oncogene. 1998;17:3125. doi: 10.1038/sj.onc.1201736. [DOI] [PubMed] [Google Scholar]
- 12.Kim Oncogene. 2001;20:4497. doi: 10.1038/sj.onc.1204542. [DOI] [PubMed] [Google Scholar]
- 13.Liu Virology. 2002;301:43. doi: 10.1006/viro.2002.1584. [DOI] [PubMed] [Google Scholar]
- 14.Lundwall FEBS Lett. 1987;214:317. doi: 10.1016/0014-5793(87)80078-9. [DOI] [PubMed] [Google Scholar]
- 15.McCormack Urology. 1995;45:729. doi: 10.1016/S0090-4295(99)80076-4. [DOI] [PubMed] [Google Scholar]
- 16.Michel Virology. 2002;294:47. doi: 10.1006/viro.2001.1321. [DOI] [PubMed] [Google Scholar]
- 17.Oesterling J Urol. 1991;145:907. doi: 10.1016/s0022-5347(17)38491-4. [DOI] [PubMed] [Google Scholar]
- 18.Papsidero J Natl Cancer Inst. 1981;66:37. [PubMed] [Google Scholar]
- 19.Planelles Infect Immun. 2001;69:6558. doi: 10.1128/IAI.69.10.6558-6563.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suto Science. 1995;269:1585. doi: 10.1126/science.7545313. [DOI] [PubMed] [Google Scholar]
- 21.Suzue Proc Natl Acad Sci U S A. 1997;94:13146. doi: 10.1073/pnas.94.24.13146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang Nature. 1992;356:152. doi: 10.1038/356152a0. [DOI] [PubMed] [Google Scholar]
- 23.Trimble C, Lin CT, Hung CF, et al. Comparison of the CD8+ T cell responses and antitumor effects generated by DNA vaccine administered through gene gun, biojector, and syringe. Vaccine. 2003;21(25–26):4036–4042. doi: 10.1016/s0264-410x(03)00275-5. [DOI] [PubMed] [Google Scholar]
- 24.Udono Int Immunol. 2001;13:1233. doi: 10.1093/intimm/13.10.1233. [DOI] [PubMed] [Google Scholar]
- 25.Ulmer Science. 1993;259:1745. doi: 10.1126/science.8456302. [DOI] [PubMed] [Google Scholar]
- 26.Wang Invest Urol. 1997;17:159. [Google Scholar]
- 27.Wei Cancer Immunol Immunother. 1996;42:362. doi: 10.1007/s002620050295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wolff Science. 1990;247:1465. [Google Scholar]