Abstract
Adoptive immunotherapy with antitumor effector cells is an attractive therapeutic approach in metastatic renal cell carcinoma (RCC). The aim of the work was to enhance in vitro activation of lymphocytes with optimal cytotoxic activity against tumor cells. We evaluated a procedure based on the use of dendritic cells (DCs) loaded with irradiated tumor cells (DC-Tu) to stimulate lymphocytes. Experimental conditions were established with cells from healthy donors and melanoma cell lines. Procedures were then applied to cells from RCC patients. A total of 30 tumor biopsies, 14 proximal lymph nodes, and 17 peripheral blood samples from 30 patients were used. When lymphocytes were stimulated in vitro with DC-Tu, they responded to tumor cells with an increased cytolytic activity for all the assays with donor cells (n=18). For RCC patients, DC-Tu stimulation improved the final cytotoxic activity in only half of the assays (16/31). When significantly enhanced (>10%, n=8), responder cells resulted in a final 43% cytotoxicity against autologous RCC cells. Mechanism of lysis was at least in part class I mediated. Effector cells have no lytic activity against normal renal cells. Percentage of cells with regulatory T-cell phenotype was not found to be enhanced in the DC-Tu stimulated lymphocytes. Individual differences were observed in the characteristics of DCs generated from RCC patients in contrast to that observed in donors and could explain why lymphocyte stimulation was not improved by DC-Tu in half of the RCC assays. T-cell spreading was suitable for a therapeutic use (>109 cells) irrespective of the procedure (with or without DC-Tu stimulation) or the tissular origin of lymphocytes from patients. Data show that precursors of selective antitumor effector cells are present in patients with RCC and can be amplified in vitro either with or without DC-Tu stimulation. One of these populations could be chosen for an adoptive transfer immunotherapy.
Keywords: Immunity, Tumor antigen, Antigen-presenting cell
Introduction
Prognosis of renal cell carcinoma (RCC) is poor. Thirty percent of patients have metastases at diagnosis and response of metastatic RCC to conventional treatment is dramatically low [16, 25]. The finding that RCC is a immunogenic tumor offered some hope of treatment [3, 6]. Indeed, although patients with advanced-stage RCC have a defective immune response [24], immunotherapy with IL-2 and interferon α administration gave durable responses for about 5–10% of the patients with an overall rate of response from 15% to 39% depending on the series [13, 16, 27]. T lymphocytes, NK, and NKT cells are the final effector cells of a successful antitumor immune response. For these reasons, the adoptive transfer of effector cells is a particularly attractive therapeutic approach [8]. It should allow treatment of patients with metastatic disease. Tumor-infiltrating lymphocytes (TIL) can be expanded from RCC biopsies [1, 5]. However pioneering clinical trials with TIL and IL-2 have not given more than a minimal increase in the survival rate in metastatic RCC patients [9, 10]. The reason for this lies in part in the methods used for ex vivo lymphocyte expansion. We addressed the question of how to enhance in vitro activation of lymphocytes with optimal cytotoxic activity against tumor cells.
Induction and expansion of cytotoxic T lymphocytes (CTLs) requires optimal antigen presentation and T-cell costimulation. The capacity of dendritic cells (DCs) to induce antitumor response has been demonstrated in a series of in vitro [18, 26] and in vivo experiments [22, 21, 28, 7]. We evaluated a procedure based on the use of DCs loaded with irradiated or apoptotic tumor cells (DC-Tu) in a controlled cytokinic environment. Experimental conditions were first established with cells from healthy donors and melanoma cell lines. The procedure was then applied to cells from RCC patients. The use of autologous cells as a source of tumor antigen is a promising way to circumvent the need for identifying tumor-specific antigens. The aim of the work was to characterize the resulting effector cells. Their antitumor cytotoxic activity was analysed with the objective of cell therapy use.
Materials and methods
Healthy donors
Peripheral blood (PB) samples were collected from eight different HLA-A2 healthy donors. Lymphocytes and DCs were prepared as described below. The allogenic M74 and SK23 human melanoma cell lines expressing the HLA-A2 restricted MelanA-Mart127–35 epitope were used for the processing of tumor antigens by DCs from healthy donors.
Patients
Thirty patients, 62±11 years of age, with histologically confirmed renal cell carcinoma (RCC) were enrolled in this study. They were derived from patients undergoing surgery in the Department of Urology of the Rennes University Hospital Center in France, from 1998 to 2002. Tumors were classified according to the 1997 TNM staging [15] and evaluated with the Fuhrman grading system [12]. Forty-six percent of patients were TNM T3, 31% were T2, and 23% T1. Twenty-seven percent were grade IV, 65% grade III, and 8% grade II. Nine patients were metastatic at time of surgery. Location of metastasis was lung (n=4), mediastinal lymph node (n=2), bone (n=2), and one was peritoneal metastasis. Tumor biopsies, proximal lymph nodes, and PB were directly obtained in the operating room each time it was possible: 29 tumor biopsies, 1 bone metastasis (R 56), 14 lymph node and 17 PB samples were finally used in the study. Resected RCC tumors and lymph nodes were immediately dissected under sterile conditions. Tumor biopsies measured less than 1 cm3. Normal, necrotic tissue and blood was dissected away from tumor tissue. The study was approved by the regional ethics committee (CCPPRB de Rennes).
Isolation of cells from peripheral blood
Peripheral blood mononuclear cells (PBMC) were isolated from blood of either HLA-A2 healthy donors or RCC patients by density gradient separation (UniSep, Novamed, Valbiotech). Cells were resuspended in X-VIVO 10 culture medium in 25-cm2 culture flasks and incubated at 37°C for 2 h after which the nonadherent cells were removed with several gentle washings. Nonadherent cells were used for PB lymphocyte cultures. DCs were generated as described by Sallusto and Lanzavecchia [29]: adherent cells were incubated at 37°C in X-VIVO 10 medium supplemented with 1,000-U/ml hrGM-CSF (Leucomax, Schering-Plough) and 400-U/ml hrIL-4 (Biosource Int.) and 10% fetal calf serum. DCs were collected by vigorous rinsing after 7 days in culture.
Growth of tumor-infiltrating lymphocytes (TIL), lymph node lymphocytes (LNL), and peripheral blood lymphocytes (PBL)
Tumor and lymph node were minced into small pieces and treated as described previously [5]. Briefly, each 1–2 mm3 fragment was explanted immediately or after freezing into a 12-well tissue culture plate with lymphocyte culture medium X-VIVO 15 supplemented with 2% l-glutamine, 2% pyruvate and 1% nonessential amino acids (Bioproducts, Gagny, France), 50-μg/ml streptomycin, 50-IU/ml penicillin (ICN Biomedicals, Aurora, Ohio, USA) and 150-IU/ml hrIL-2 (Proleukin, Chiron, Suresnes, France). PB lymphocytes were nonadherent cells collected after density gradient separation and cultured in the same medium. Cultures were done at 37°C in 5% CO2 and observed microscopically daily. For TIL and LNL (RCC patients), lymphocytes were stringently separated from tumor cells either by filtration through a 40–70-μm cell strainer (Falcon, Becton Dickinson, New Jersey, USA) or by difference in adherence. Assays consisted in the characterization of the lymphocytes isolated from tumor, lymph nodes, and PB, when cultured without any stimulation and of the same submitted to stimulation with DC-Tu: 18 assays with PB lymphocytes from donors (3 donors), 31 with lymphocytes from patients (21 patients). Duration of culture was 13–35 days depending on the expanding index of the lymphocytes and complete disappearance of tumor cells in the culture. Comparisons between the two procedures (with or without DC-Tu stimulation) were done at the same time.
Tumor cells
Small fragments of tumor biopsies were seeded into a 25-cm2 culture flask in a minimum volume of RPMI 1640 (Eurobio, Les Ullis, France) containing 10% FCS (Gibco, Life Technology, Germany), 2% l-glutamine, 50-μg/ml streptomycin, and 50-IU/ml penicillin in a 37° humidified 5% CO2 incubator to obtain the primary cell culture line. The M74 and SK23 melanoma cell lines and the NK-cell sensitive K562 erythroleukemia cell line were maintained in RPMI culture medium identically supplemented.
Tumor loading of DC
In assays with HLA-A2 donors, DC were incubated overnight with tumor cells (DC-Tu) in a 1:100 DC to tumor cell ratio. Tumor cells were either 75 Gy irradiated M74 and SK23 cells (DC-Tu irr, n=9) or apoptotic bodies of the same (DC-Tu but, n=9). Controls were unstimulated lymphocytes (n=7) or lymphocytes stimulated with unloaded DC (n=7). The preparation of apoptotic bodies was adapted from the published method [4]. Briefly, cells were treated for 3 days with 5-mM butyrate. Floating dead cells were removed every day and pooled at 4°C. On the 3rd day, apoptotic cells were incubated for 1 h at 37°C to remove adherent cells and supernatant was centrifuged at 800 rpm for 15 min. Apoptotic bodies were characterized as 39±5% annexine V-FITC positive/propidium iodine negative cells (Kit 2375, Immunotech) by FACScan analysis.
In RCC assays, DCs were incubated overnight with 75 Gy irradiated autologous tumor cells in 1:100 DC to tumor cell ratio. Tumor cells were from a 0–1 passage primary culture at the time of DC loading.
Lymphocyte stimulation with DC-Tu
After 7 days in culture in the presence of IL-2, lymphocytes were stimulated with DCs. In healthy donors assays, stimulation was done in a DC to lymphocyte ratio of 1:10 or 1:100, with DC-Tu irr, DC-Tu but, or unloaded DC (DC). Coculture was maintained in 150-IU/ml rhIL-2 X-VIVO 15 medium. The recovery of viable cells, phenotype, and cytotoxic activity against melanoma cell line (M74 or SK23 depending on the corresponding DC loading) or K562 was measured 7 days after stimulation. Controls were done with lymphocytes cultured alone. The T CD8 cell clone M77-84 specific for MelanA-Mart127–35 epitope (a gift of F. Jotereau, Nantes) was used as a positive control (80% specific lysis).
For assays with RCC samples (from 21 patients), lymphocytes from tumor (n=5), lymph node (n=9), or PB (n=17) were separately stimulated with autologous DC-Tu (in a 1:100 DC to lymphocyte ratio). Phenotype of responding cells and cytolytic activity against autologous tumor cells was measured 7 days after stimulation. Data were compared using the χ-square and the nonparametric Mann and Whitney statistical tests.
Cell surface phenotype
The cell surface phenotype was analyzed by two- or three-color immunofluorescence analysis. Cells (3–5×105) were suspended in phosphate-buffered saline (PBS), pH 7.4, supplemented with 0.5% bovine serum albumin (BSA), and incubated for 30 min at 4°C in the dark, in the presence of the appropriate dilution of FITC-, PE-, or PC5-conjugated MAbs. Anti-CD45, anti-CD3, anti-CD4, anti-CD8, anti-CD56, anti-HLA-DR, anti-CD25, anti-CD152 (CTLA-4) and isotype controls (Immunotech, Marseille, France) were used for characterization of lymphocyte phenotype. Anti-CD11c, anti-CD123, anti-HLA-DR, lineage cocktail of MAbs (anti-CD3, anti-CD14, anti-CD16, anti-CD19, anti-CD20, anti-CD56) (Becton Dickinson France, Le pont de Claix, France), anti-CD14, anti-CD83, anti-CD40 (Immunotech) and anti-CD80, anti-CD86 (Serotec, Oxford, UK), were used for characterization of the DCs phenotype. Isotype-matched murine immunoglobulins conjugated to the same fluorochromes were used as negative controls. After lysis of erythrocytes (Lyse & Fix IO Test Coulter, Marseille, France), cells were then washed twice in PBS-BSA and kept in 0.3% formol until cytofluorometric analysis. Data were acquired on the FACScan flow cytometer (Becton Dickinson) using Cell Quest software, from 1,000 to 10,000 events for each sample.
Cytotoxicity assays
Target cells (K562 and tumor cells) were labeled with 3.7×107 Bq of 51Cr sodium chromate/106 cells (Na2 51CrO4, specific activity 200 mCi/mg, Amersham Life Sciences, Buckinghamshire, UK). After 1 h at 37°C, cells were washed three times and resuspended in RPMI 1640 medium at a concentration of 5×104 cells/ml. Cells were added at 50:1 effector to target cell ratio (E:T) in the wells of 96-well U-bottom plates (100 μl/well) for 4 h. Assays were performed in triplicate. Chromium release was assessed in culture supernatants, using a COBRA gamma counter (Packard Instument, Rungis, France). Specific activity was calculated using the formula (mean experimental cpm − mean spontaneous cpm)/(mean maximum cpm − mean spontaneous cpm)x100. Spontaneous release was less than 30% of maximal release in all the assays. Specific activity >10% was considered as a significant lytic activity. Cold target inhibition experiments were performed by addition of a 2- to 100-fold excess of unlabeled K562 to tumor autologous target cells. To determine whether tumor lysis was class I restricted, we performed blocking assays in which target cells were preincubated 45 min at 37°C with W6.32 antibody (mouse antihuman HLA-ABC, MLA81XZ, Serotec, Oxford, UK; in complete absence of FCS) before use in the 51Cr release assay. To evaluate the selectivity of antitumor reactivity of effectors, cytotoxicity assays were performed in parallel against autologous normal cells. Normal renal cells could be derived from a healthy area of the resected kidney for two patients (R175, R191) and five DC-Tu–stimulated lymphocyte populations could be tested on the autologous normal renal cells or monocytes.
Final large scale expansion of lymphocytes
For seven assays with RCC patients, lymphocytes were collected after 7 days in culture after DC stimulation or not. They were then incubated with feeder cells in 96-well U-bottom plates as described by Jotereau [20]. Feeder cells consisted of PBMC from healthy donors (EFS, Rennes), and EBV-transformed B cell line (LAZ 388) at a rate of 4×104 50 Gy irradiated PBMC, 2×104 75 Gy irradiated LAZ and 1,000 to 10,000 lymphocytes per well. At the beginning of the expansion, 15 μg of PHA-L (Sigma, France) and 150-IU/ml IL-2 were added to supplemented lymphocyte medium. After 7 days, lymphocytes were transferred to a 500-ml capacity life-cell tissue culture flask (Baxter/Fenwall, Deerfield, Illinois, USA) and cultured in the presence of 300–1,000-IU/ml IL-2. Medium was added every day to adapt the concentration to 1×106 cells/ml.
Results
Growth, phenotype, and cytotoxic activity of lymphocytes from HLA-A2 healthy donors after DC stimulation
We first examined T-cell activation by loaded DC from healthy donors in the above described culture conditions. DC prepared from the PBMC of healthy donors expressed high levels of CD11c and class II molecules (HLA-DR), and did not express lineage-specific markers (lin) of T (CD3), B (CD19), macrophages (CD14), or NK (CD56) cells. These cells were immature DCs characterized as CD11c+/DR+/lin−, DR+/CD86+, and DR+/CD40+, but expressing low levels of DR+/CD83+ and of DR+/CD80+ (Table 1).
Table 1.
. Characteristics of DCs generated from PBMC. Data are from assays with healthy donors (n=8) and RCC patients (n=10). Number of DCs obtained from 10-ml PB and percentage of cells expressing different DC markers as indicated; nd no data
| Healthy controls (n=8) | RCC patients (n=10) | |
|---|---|---|
| Number of DCs | nd | 1.7±1.6 |
| Phenotype | ||
| CD11c+DR+lin− (%) | 93±6 | 64±27 |
| CD80+ (%) | 3±2 | 15±26 |
| CD86+ (%) | 63±22 | 77±20 |
| CD83+ (%) | 1±1 | 3±3 |
| DR−lin− (%) | 3±3 | 10±10 |
In our results, PBL from donors did not expand when cultured alone. However, expansion was obvious after stimulation with autologous DCs. The expansion index was 15±4 (n=10) and 17±4 (n=12), 7 days after stimulation with respectively DC-Tu in 1:10 or 1:100 DC to lymphocyte ratios. Loading the DCs with treated tumor cells enhanced the lymphocyte growth already observed with unloaded DC (p<0.02 for DC-Tu but).
The majority of cells for which growth was stimulated by DCs and DC-Tu were T lymphocytes (97±3% were CD3+CD56− T cells), significantly enhanced (p<0.01) if compared with unstimulated controls (67±4%), but without any change in TCD8/TCD4 ratio (1.4±0.5). No expansion of NK cells was observed in these populations: 1±1% CD3−CD56+ cells in all of the DC-stimulated lymphocyte populations.
Lymphocytes stimulated with DCs loaded with tumor cells (irradiated or apoptotic bodies) provided a significant lytic activity (respectively 18±7% and 14±4%, p<0.01) against tumor cell lines (M74 and SK23). Controls and lymphocytes stimulated with unloaded DCs exhibited no lytic activity. Lysis of the NK-sensitive K562 cells was enhanced only in DC-Tu-but–stimulated lymphocytes (23±18%, p<0.04). This data led us to choose irradiation of tumor cells for the assays with RCC cells.
Expansion of cytotoxic effector from tumor, lymph node, or peripheral blood lymphocytes from RCC patients
In a first step of the work with samples from patients, lymphocytes were cultured using 150-IU/ml IL-2. For TIL and LNL, stringent separation of lymphocytes from tumor cells was done (without any stimulation with DCs). Tumor cell cultures could be established from 29/30 RCC biopsies.
First, depending on the patient, and also the tissue origin, lymphocytes exhibited naturally occurring morphological differences. TIL usually have morphological features characteristic of activated T cells. However, PBL from patients resembled quiescent lymphocytes (as observed in all naïve healthy donors), but in some cases, activated T forms were evident.
Cytolytic activity of lymphocytes derived from PB was usually lower than for lymphocytes derived from proximal lymph nodes or tumors (Table 2). Lymphocytes could be isolated from 24/30 tumor or metastasis biopsies (TIL). They were submitted to a complete analysis when they were obtained in a sufficient count. This was when lymphocytes could be precociously separated from tumor cells. For 10/17 patients, PBL exhibited no lytic activity (<10%), whereas this was observed in only 4/24 TIL and in 3/14 LNL. By contrast, 8/24 TIL and 7/14 LNL exhibited more than 30% cytolytic activity (data not shown). These results are in agreement with a natural selective homing of lymphocytes in the tumor.
Table 2.
Characteristics of TIL, LNL, or PBL from RCC patients. Phenotype and cytolytic activity of lymphocytes simply cultured in presence of 150-IU/ml IL-2. Analyses were done at day 13–35 of culture. The percentage of positive cells for lymphocyte markers was measured in a 99% CD45+ population for TIL (n=24), LNL (n=14), and PBL (n=17). Cytolytic activity was measured against autologous (auto) tumor and K562 target cells. Data are mean ± SD
| Phenotype (%) | Cytolytic activity (%) | |||||
|---|---|---|---|---|---|---|
| CD3+ | T CD4+ | T CD8+ | CD3-CD56+ | auto | K562 | |
| TIL (n=24) | 71±24 | 49±27 | 23±18 | 17±17 | 27±20 | 54±33 |
| LNL (n=14) | 72±15 | 54±14 | 20±14 | 13±8 | 37±31 | 59±33 |
| PBL (n=17) | 76±17 | 48±20 | 30±23 | 18±16 | 11±13 | 35±23 |
Large individual differences were observed between patients for TIL, LNL, and PBL (Table 3). Correlation could not be found between the percentages of CD3+, CD8+, or CD56+ cells, on the one hand, and the level of autologous or NK-cytolytic activity or with the presence or absence of tumor cells in the cultures at time of seeding, on the other. The only significant link was that a high cytolytic activity in LNL was found to be related to advanced clinical stage (p<0.01).
Table 3.
. Individual characteristics of DC-Tu–stimulated lymphocytes from RCC patients. Phenotype and cytolytic activity of TIL, LNL, or PBL simply cultured with IL-2(-) or DC-Tu stimulated(+). The percentage of positive cells for lymphocyte markers was measured in a 99% CD45+ population. Cytotoxicactivity was measured against K562 target and autologous (auto) tumor cell; nd no data
| Phenotype (%) | Cytolytic activity (%) | |||||
|---|---|---|---|---|---|---|
| Patient | ± DC-Tu stim | T CD4+ | T CD8+ | CD3-CD56+ | auto | K562 |
| PBL R56a | - | 16 | 35 | 46 | 13 | 65 |
| + | 57 | 37 | 3 | 100 | 100 | |
| PBL R62a | - | 66 | 12 | 9 | 40 | 69 |
| + | 92 | 6 | 0 | 13 | 22 | |
| PBL R70a | - | 61 | 25 | 9 | 8 | 18 |
| + | 43 | 31 | 10 | 3 | 9 | |
| PBL R77a | - | 63 | 20 | 15 | 0 | 27 |
| + | 32 | 20 | nd | 7 | 77 | |
| PBL R78a | - | 45 | 25 | 16 | 0 | 60 |
| + | 70 | 11 | 7 | 0 | 100 | |
| PBL R79a | - | 87 | 7 | 1 | 2 | 5 |
| + | 79 | 16 | 1 | 7 | 3 | |
| PBL R103a | - | 51 | 15 | 31 | 0 | 54 |
| + | 78 | 11 | 11 | 0 | 42 | |
| PBL R104b | - | 55 | 40 | 8 | 11 | nd |
| + | 57 | 33 | 13 | 2 | nd | |
| PBL R116a | - | nd | nd | nd | 19 | 6 |
| + | 66 | 38 | 0 | 26 | 2 | |
| PBL R118a | - | 30 | 7 | 58 | 44 | 57 |
| + | 44 | 11 | 40 | 4 | 20 | |
| PBL R119a | - | 43 | 29 | 11 | 7 | 20 |
| + | 32 | 59 | 4 | 29 | 36 | |
| PBL R123a | - | 71 | 11 | 11 | 18 | 13 |
| + | 32 | 6 | 1 | 11 | 4 | |
| PBL R159a | - | 26 | 85 | 0 | 4 | 15 |
| + | 29 | 68 | 1 | 5 | 15 | |
| PBL R162b | - | 19 | 73 | 9 | 0 | 52 |
| + | 31 | 65 | 2 | 10 | 45 | |
| PBL R191a | - | 44 | 29 | 24 | 4 | nd |
| + | 39 | 46 | 15 | 0 | nd | |
| LNL R62a | - | 74 | 12 | 11 | 73 | 97 |
| + | 80 | 7 | 8 | 43 | 74 | |
| LNL R70a | - | 36 | 55 | 8 | 2 | 19 |
| + | 44 | 43 | 8 | 0 | 78 | |
| LNL R77a | - | 31 | 10 | 30 | 7 | 100 |
| + | 43 | 11 | 29 | 0 | 71 | |
| LNL R78a | - | 44 | 11 | 11 | 1 | 8 |
| + | 53 | 21 | 24 | 0 | 52 | |
| LNL R79a | - | 87 | 13 | 0 | 3 | 0 |
| + | 87 | 13 | 0 | 1 | 0 | |
| LNL R110b | - | 40 | 53 | 5 | 0 | 17 |
| + | 67 | 14 | 8 | 66 | 50 | |
| LNL R127a | - | 71 | 26 | 5 | 44 | 49 |
| + | 71 | 29 | 10 | 60 | 61 | |
| LNL R131a | - | 50 | 40 | 9 | 18 | 72 |
| + | 60 | 38 | 4 | 22 | 70 | |
| LNL R175a | - | 79 | 12 | 9 | 11 | nd |
| + | 83 | 14 | 4 | 12 | nd | |
| TIL R56a | - | 82 | 13 | 4 | 88 | 100 |
| + | 82 | 16 | 1 | 63 | 58 | |
| TIL R60a | - | 53 | 67 | 4 | 15 | 20 |
| + | 64 | 47 | 2 | 27 | 17 | |
| TIL R62a | - | 65 | 4 | 6 | 7 | 32 |
| + | 72 | 7 | 3 | 10 | 42 | |
| TIL R175a | - | 86 | 16 | 3 | 14 | nd |
| + | 87 | 17 | 2 | 28 | nd | |
| TIL R191a | - | 34 | 28 | 35 | 15 | 60 |
| + | 38 | 65 | 3 | 3 | 13 | |
aLymphocytes were expanded immediately after being taken
bLymphocytes were expanded immediately after freezing
Autologous tumor lysis was often elevated concomitantly to high NK activity. Nevertheless, when autologous tumor lysis was evaluated in the presence of unlabeled K562 cells, final cytolytic activity was not found reduced excepted for PBL R118 presenting a high percentage of NK cells (Table 3).
For 9/10 of the populations we studied (6 PBL, 2 LNL, 2 TIL), less than 3% of the cells were found with the regulatory T-cell phenotype CD4+CD25+CTLA−4+. No relation was found between this phenotype and the cytolytic activity against autologous tumor cells.
Generation of DCs from RCC patients
DCs prepared from PBL of RCC patients expressed high levels of CD11c and HLA-DR, but large individual differences could be recorded for the lineage-specific markers cocktail (lin), CD123 marker (17±22%), and expression of B7.1 (Table 1). As previously observed for healthy donors, B7-2 molecules (CD86), but not CD83 marker, were expressed on the DC. The final number of DCs generated from 10-ml PB was also heterogeneous depending on the patient (Table 1). The percentage of immature myeloid cells (DR-lin-) could reach 22% in one patient (R127).
Ability of autologous DC-Tu irr to induce cytotoxic effectors
Because of the difficulty in collecting a sufficient number of TIL for complete investigations, only five TIL populations could be stimulated with DC-Tu. By contrast, 9/14 LNL and 15/17 PBL were at a count suitable to be evaluated in DC-Tu stimulation assays.
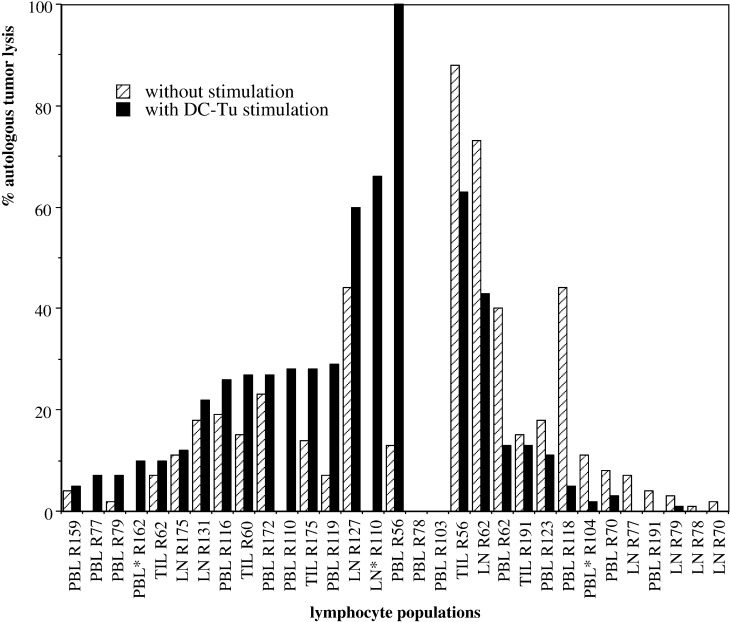
Lymphocytes from tumor, LN, and PB were assayed for their cytolytic activity 7–10 days after stimulation with autologous DC-Tu irr and compared with nonstimulated controls (evaluated after the same number of days in culture). For 16/31 assays, DC-Tu-irr stimulation enhanced effector cells able to lyse autologous tumor cells (Fig. 1). This enhancement was more than 10% for eight assays: two TIL, two LNL, and four PBL. It concerned populations characterized by a rather low cytolytic activity if not stimulated (12±14%). Increases were significant and resulted in a 43% mean final lysis. By contrast, in 13 other assays a decrease in autologous tumor lysis was observed after DC-Tu-irr stimulation. This decrease was more than 10% for 4/13 patients (Fig. 1). These patients were remarkable: the cytolytic activity of lymphocytes without DC stimulation was naturally greater than 40% (61±20%). For 11 assays, cytolytic activity remained <10% whether with or without DC-Tu-irr stimulation (Fig. 1). No correlation was found between any of the individual DC parameters and the final cytolytic activity of the lymphocytes stimulated with these cells (DC-Tu irr). Nor were any correlations found between the tumor stage or grade, the survival of patients, and the final effect of DC-Tu stimulation. We could simply observe that the lymphocytes from the metastatic RCC (7/9 metastatic RCCs were evaluated for DC-Tu stimulation) always presented a significant cytolytic activity either without (3/7) or after DC-Tu stimulation (4/7). Nul cytotoxic activity was only observed with nonmetastatic RCC patients.
Fig. 1.
Cytolytic activity of lymphocytes from RCC patients after stimulation with autologous-tumor-loaded DCs. Lymphocytes were stimulated or not with DC-Tu irr. Autologous tumor cell lysis (%) was measured 7 to 10 days after stimulation. Data are the individual values of 31 lymphocyte populations derived from 21 RCC patients. Lymphocytes were expanded immediately after taking or after freezing (*). Patients with cytolytic activity increased by DC-Tu stimulation are ranged on the left part, and with decreased activity on the right part of the figure
The percentage of T cells with a regulatory T-cell phenotype (CD4+CD25+CTLA-4+) was not enhanced after DC-Tu-irr stimulation: 4/5 populations were below 4%.
Lytic activity of effector cells
To assess the mechanism of the lysis mediated by cytolytic effectors, we performed experiments measuring the lysis of autologous target cells in presence of anti–class I MAb W6.32 (n=25). Lymphocytes were either stimulated by DC-Tu or simply cultured in the presence of IL-2 alone. In 5/25 assays (3 after stimulation, 2 without stimulation), cytolytic activity against autologous tumor was reduced from 27±13% to 9±6% specific lysis by W6.32, arguing for at least in part a MHC–class I mediated mechanism of lysis. By contrast, cytolytic activity was enhanced in 17 assays (7 after stimulation,10 without stimulation) from 16±13% to 30±13% specific lysis by pretreating targets with W6.32. In three assays, lysis was not changed.
Cold target inhibition experiments were also performed. In the presence of K562 cells (X2-20), autologous cytolytic activity was not reduced (17 assays with nonstimulated lymphocytes). In the presence of K562 (X100) autologous and NK cytolytic activity was decreased, but significant specific lysis remained in 2/5 assays.
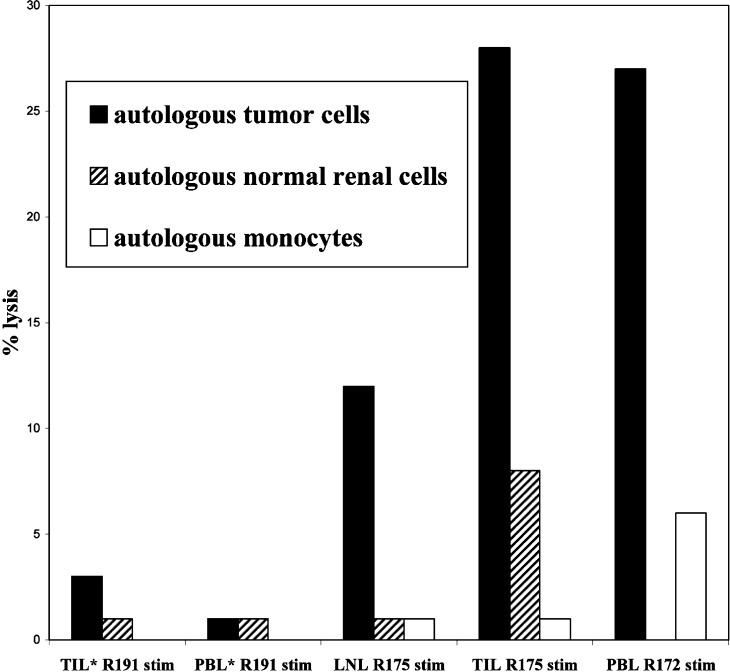
In addition, for 6/7 assays with DC-Tu-stimulated lymphocytes, lysis of normal renal cells or monocytes was nul or by far lower (1/7) than lysis of carcinoma cells (Fig. 2).
Fig. 2.
Cytolytic activity of lymphocytes against autologous tumor cells and normal cells. DC-Tu stimulated lymphocytes were evaluated for cytolytic activity against autologous normal renal cells (maintained in culture for patients R191 and R175) and monocytes. Lymphocytes were expanded immediately after taking or after freezing (*)
Final expansion of lymphocytes suitable for therapy
Since a great number of effector cells should be required for a therapeutic use, lymphocytes were submitted to an expansion process. After a culture step on feeder cells, 50×106 lymphocytes were transferred to 500-ml capacity Life-Cell tissue culture bags and expanded with 1,000-IU IL-2. Lymphocytes were from tumors, lymph nodes, or PB, either stimulated with DC-Tu or not. They could be expanded to a count suitable for therapy (>109) in less than 30 days. Similar results were obtained for seven RCC patients.
Discussion
Adoptive immunotherapy with immune effector T cells is an attractive approach to treating RCC since it allows treatment of patients with advanced metastatic disease. However, the functional quality of the cell therapy product needs to be improved for better clinical responses than those observed in pioneering clinical trials [2, 10, 17, 23].
Before testing samples from RCC patients, we first examined T-cell activation by DCs from healthy donors. We observed that PBMC from healthy donors treated with IL-4 and GM-CSF generated DCs expressing immature DC-membrane markers, B7.2 costimulation, and CD40 activation molecules. Using DCs loaded with either irradiated cells or apoptotic bodies of tumor cells, we repeateadly observed the induction of T-cell differentiation as assessed by proliferation and cytolytic responses. Enhancement of cytolytic activity against tumor cells was induced only when DCs were loaded with tumor material. NK-like activity was also induced since K562 lysis was enhanced in spite of low percentages of CD3−CD56+ cells (below 2%).
In a first step with samples from patients, lymphocytes were cultured using IL-2 with stringent separation of lymphocytes from tumor cells, without any stimulation with DCs. Cytolytic activity of lymphocytes against autologous tumor was variable but could reach more than 50%. It was in part suppressed by cold target inhibition experiment with K562 cells: the majority of these lymphocytes provided a NK-like activity. This activity was not correlated with the number of NK cells in the bulk. Regulatory T cells were below 3% of the cells. Taken together, these observations show that antitumor effector cells are present and can be expanded with a simple IL-2 culture procedure. Cytolytic activity of lymphocytes derived from tumor-invaded tissues was higher than that of tumor-cell-free lymph nodes. This is in agreement with a natural homing of lymphocytes on the tumor. However, separation of lymphocytes from tumor cells at an early step of the culture was needed to expand a lymphocyte population with significant cytotoxic activity.
When lymphocytes from RCC patients were stimulated in vitro with autologous DC-Tu, results were different from what was observed with cells from healthy donors. Our hypothesis is that it was not simply because of differences in minor antigens between healthy donors cells and melanoma cell lines since preliminary data indicate that MelanA-Mart1–specific T cells were significantly enhanced by DC-Tu stimulation in the M74-healthy donors model (Alban Gervais, personal communication of our group).
Stimulation of lymphocytes with DC-Tu resulted in two different effects. In half of the patients, stimulation enhanced the final cytotoxicity against autologous tumor. Cytolytic activity against normal cells was nul or very low. When significant (>10%), this enhancement reached a final 43% antitumor lytic activity. This was observed with T-cell populations characterized by a rather low cytolytic activity if not stimulated (12±14%). On the contrary, for other assays, the DC-Tu stimulation led to a decreased ability of lymphocytes to lyse autologous tumor. This was observed with lymphocytes characterized by a rather high cytotoxic activity when nonstimulated (61±20%). Our hypothesis is that DC-Tu stimulation has a better chance of favoring generation of antitumor cytolytic effectors when lymphocytes were naïve T cells. Clear improvements were observed with PBL.
We observed that irrespective of the procedure (with or without stimulation with DCs) or the tissue origin of lymphocytes from patients, T-cell expansion enabled us to collect a count suitable for therapeutic use (>109 in less than 30 days).
To explain the significant reduction in cytolytic activity observed with 4/31 of the assays, several mechanisms could be put forward. First, further stimulation after previous repeated in vivo stimulation of memory effector cells could induce their ultimate differentiation and final apoptosis [31]. Another potential mechanism that might influence the course of the coculture comes from DC characteristics. Though we could not observe any correlation between DC markers and modulation of the final lymphocyte functionality, it is important to recall that the nature of DCs can determine the outcome of induced immune response, ranging from immunity to tolerance. In healthy donors, DC phenotype was the same and similar to that described by others [18]. When loaded with tumor antigens, they activated effector T cells in vitro. By contrast in RCC patients, we found that phenotype and final yield of DCs from 10-ml PB was heterogeneous. Their characteristics were similar to those reported by others [1, 26]. The hypothesis of induction of regulatory T cells [19] was investigated, but these cells were not found to be enhanced after DC-Tu stimulation. Previous reports have shown that DCs from RCC tumors were defective [30], and others described the capacity of RCC cells to influence in vitro differentiation of DC [24].
Taken together, our data confirm that precursors of antitumor effector cells are present in patients with RCC and can be activated in vitro. DC-Tu stimulation was particularly efficient when precursors were not activated by a simple IL-2 expansion. The mechanism of lysis was not fully determined: it was variable from one patient to another. A part of the lysis was class I mediated for some populations either DC-Tu stimulated or not. However, the use of W6.32 MAb to block the class I molecules, enhanced the cytolytic activity of the cells for several lymphocyte populations. In spite of the fact that all cytotoxic activities were measured in absence of complement, ADCC mechanism could not be excluded from an explanation of this enhancement. Another explanation is that the binding of MAb to class I molecules could switch a negative signal and favor the lysis of target cells by a class I independant mechanism. Inhibitory and stimulatory NK-like receptors are known to be expressed on CD8 αβ and CD8 γδ T cells and can modulate antigen-specific T-cell response [14]. Preliminary results indicate that CD8+CD94+ Τ cells were present in our unstimulated or DC-Tu–stimulated lymphocyte populations.
Our conclusion is that DC-Tu stimulation is an additional method for T-cell expansion. Presently, the two procedures (with and without DC-Tu stimulation) need to be used in parallel for each patient. One of these populations should be chosen (taking into account the cytolytic activity 7 days after DC-Tu stimulation) for final large-scale expansion for an adoptive transfer cell therapy.
Acknowledgements
We are grateful to Marc Grégoire (INSERM U419, Nantes) for helpful discussion of the data.
Footnotes
This work was supported by grants from the Comité Grand Ouest de La Ligue Contre le Cancer and from the Faculté de Médecine de Rennes.
References
- 1.Almand J Immunol. 2001;166:678. doi: 10.4049/jimmunol.166.1.678. [DOI] [PubMed] [Google Scholar]
- 2.Belldegrun J Urol. 1993;150:1384. doi: 10.1016/s0022-5347(17)35785-3. [DOI] [PubMed] [Google Scholar]
- 3.Bernhard Int J Cancer. 1994;59:837. doi: 10.1002/ijc.2910590621. [DOI] [PubMed] [Google Scholar]
- 4.Boisteau Apoptosis. 1997;1997:403. doi: 10.1023/a:1026461825570. [DOI] [PubMed] [Google Scholar]
- 5.Bouet-Toussaint Eur Cytokin Network. 2000;11:217. [PubMed] [Google Scholar]
- 6.Caignard Int J Cancer. 1996;66:564. doi: 10.1002/(SICI)1097-0215(19960516)66:4<564::AID-IJC23>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 7.Delneste Immunity. 2002;17:353. [Google Scholar]
- 8.Dudley J Immunother. 2002;25:243. doi: 10.1097/00002371-200205000-00007. [DOI] [Google Scholar]
- 9.Figlin J Urol. 1997;158:740. doi: 10.1097/00005392-199709000-00012. [DOI] [PubMed] [Google Scholar]
- 10.Figlin J Clin Oncol. 1999;17:2521. doi: 10.1200/JCO.1999.17.8.2521. [DOI] [PubMed] [Google Scholar]
- 11.Finke J Immunother. 1992;11:1. doi: 10.1097/00002371-199201000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Fuhrman Am J Surg Pathol. 1982;6:655. doi: 10.1097/00000478-198210000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Goey Annals of Oncology. 1996;7:887. doi: 10.1093/oxfordjournals.annonc.a010790. [DOI] [PubMed] [Google Scholar]
- 14.Groh Nature Immunology. 2001;2:255. doi: 10.1038/85321. [DOI] [PubMed] [Google Scholar]
- 15.Guinan Cancer. 1997;80:992. doi: 10.1002/(SICI)1097-0142(19970901)80:5<992::AID-CNCR26>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 16.Hänninen J Urol. 1996;155:19. [Google Scholar]
- 17.Hayakawa Urol Int. 1994;53:117. doi: 10.1159/000282651. [DOI] [PubMed] [Google Scholar]
- 18.Hoffmann Cancer Res. 2000;60:3542. [PubMed] [Google Scholar]
- 19.Jonuleit Trends in immunology. 2001;22:394. doi: 10.1016/S1471-4906(01)01952-4. [DOI] [PubMed] [Google Scholar]
- 20.Jotereau J Immunother. 1991;10:405. doi: 10.1097/00002371-199112000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Kugler Nat Med. 2000;6:332. doi: 10.1038/73193. [DOI] [PubMed] [Google Scholar]
- 22.Liau J Neurosurg. 1999;90:1115. doi: 10.3171/jns.1999.90.6.1115. [DOI] [PubMed] [Google Scholar]
- 23.Mathiot Eur J Cancer. 1995;31A:1552. doi: 10.1016/0959-8049(95)00314-9. [DOI] [PubMed] [Google Scholar]
- 24.Menetrier-Caux Cancer Res. 2001;61:3096. [PubMed] [Google Scholar]
- 25.Motzer J Clin Oncol. 1999;17:2530. doi: 10.1200/JCO.1999.17.8.2530. [DOI] [PubMed] [Google Scholar]
- 26.Mulders Clin Cancer Res. 1999;5:445. [PubMed] [Google Scholar]
- 27.Negrier N Engl J Med. 1998;338:1272. doi: 10.1056/NEJM199804303381805. [DOI] [PubMed] [Google Scholar]
- 28.Nestlé Nat Med. 1998;4:328. doi: 10.1038/nm0398-328. [DOI] [PubMed] [Google Scholar]
- 29.Sallusto J Exp Med. 1994;179:1109. [Google Scholar]
- 30.Thurnher Int J Cancer. 1996;68:1. doi: 10.1002/(SICI)1097-0215(19960927)68:1<1::AID-IJC1>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 31.Weng Proc Natl Acad Sci USA. 1995;92:11091. [Google Scholar]