Abstract
Prostate-specific antigen (PSA) is a valuable marker antigen for prostate cancer. Lately considerable interest has been generated in the prospect of developing a vaccine for prostate cancer with PSA-derived peptide epitopes to induce cytotoxic T-cell (CTL) response. We report here that T cells capable of exhibiting PSA epitope-specific effector function—in their native state, i.e, without having to be further stimulated, in vitro—are detectable in more than half of the prostate cancer patients we studied. Ex vivo cultured autologous dendritic cells (DC) were used to present four HLA-A2–binding PSA peptide epitopes to freshly isolated peripheral blood lymphocytes (PBL) from patients and healthy volunteers. Ten out of 14 patients' PBL recognized at least one of the four peptides and 6 out of 10 patients' PBL recognized more than one peptide antigen as measured by IFN-γ secretion upon stimulation of the PBL with the peptide antigen. Intracytoplasmic cytokine analysis for IFN-γ in purified CD8+ cells after stimulation with peptide antigens was tested in 6 patients and this technique demonstrated a similar response. Freshly isolated and purified CD8+ cells when tested, also recognized the epitopes, as measured by IFN assay, when presented by transporter associated with antigen-processing (TAP) deficient T2 cells in an MHC-I restricted fashion. PBL from 9 normal donors when tested in identical fashion did not show any IFN-γ production in recognition to the peptide antigens. Interestingly, neither of these CD8+ T cells having IFN-γ–producing ability did show any cytolytic activity in their native state against peptide loaded target cells or tumor cells when tested in cytotoxicity assay. In long term cocultures stimulation of purified CD8+ T cells with matured DC pulsed with PSA peptides generated a PSA-specific CTL response in 4 of 6 patients studied and in 2 of 9 normal donors. While our observations of CTL generation are consistent with the prior reports that have demonstrated that specific CD8+ CTL could be generated which recognize PSA-derived epitopes by in vitro stimulation by one means or another, this observation that IFN-γ–producing CD8+ T cells are present in patients which are antigen experienced, and do not require in vitro stimulation, is novel and has major implications for prostate cancer vaccine preparation.
Keywords: PSA, CD8+ T cells, IFN-γ, CTL activity
Introduction
Cytotoxic T lymphocytes (CTL) are considered to be one of the most imporrtant effector cells in antitumor immunity. It is now clear that many cancer patients harbor T cells that can be stimulated to recognize autologous tumor cells on the basis of defined specificity [5, 16, 17, 26, 32]. Although a large body of information on T-cell-mediated antitumor response has been generated in the melanoma model, the evidence of cellular (and serologic) antitumor immune response has been amply demonstrated in a large variety of tumor types including prostate cancer [1, 5, 8, 9, 10, 14,16, 17, 26, 32, 41]. Studies in this area have revealed that tumor-specific T cells can be generated from tumor involved or uninvolved in draining lymph nodes, from tumor tissues, and from circulating PBL after in vitro stimulation. Very little information is available on whether T cells that can respond to stimulation by a given tumor-associated epitope have previously encountered this antigen in vivo and, if they have, how these epitopes are presented and what events follow such presentation. Several promising prostate associated antigens and peptide epitopes that can stimulate T cells from prostate cancer patients have been identified. Some of these antigens and peptide epitopes have provided a basis for vaccines in clinical trials [23, 24, 25, 33,34,39]. The majority of T cells that bear receptors for a given tumor-associated antigen are antigen inexperienced, as they seldom exhibit memory-type response upon exposure to antigen, or these cells await appropriate antigen presentation or their activation is prevented by surrounding supressive factors [29, 38]. In a recent report, McNeel et al. [21] have documented spontaneous recognition of certain class II determined epitopes by CD4+ T cells in prostate cancer patients. PSA-specific T helper-1 (TH1)–type responses were detected in patients with metastatic disease but not in patients with early stage disease. Similarly, Ponniah et al. have shown PSA-specific TH1 responses in 5 out of 14 patients with chronic prostatitis [28]. In an earlier report, McNeel et al. have shown IgG-type PSA-specific antibody mediated immunity in metastatic prostate cancer patients indicating the involvement of TH-type T cells [20]. Here we provide evidence of prostate epitope specific CD8+ T cells in freshly isolated PBL from prostate cancer patients, and demonstrate a reasonably effective method to generate peptide specific CTL in in vitro coculture. These findings add further support to a role for T-cell-mediated immunity in prostate cancer and the potential clinical relevance of vaccines designed to increase the cellular immune response to prostate associated antigens.
Materials and methods
Patient samples
Normal controls were adult males with no history of prostate cancer. All patients had biopsy-proven prostatic adenocarcinoma. Both groups of subjects gave informed consent in accordance with Institutional Review Board guidelines. Peripheral blood samples were drawn by routine phlebotomy and peripheral blood mononuclear cells (PBMC) were isolated by Ficoll-Hypaque gradient centrifugation [22].
Peptides
We used four HLA-A2–binding PSA peptides (9): PSA 16–25 VLVASRGRAV referred to as PSA-1, PSA 141–150 FLTPKKLQCV referred to as PSA-2, PSA 146–154 KLQCVDLHV referred to as PSA-3 and PSA 151–159 DLHVISNDV referred to as PSA-4. Sequences are described in NCBI database No. AJ459783 and are Homo sapiens mRNA for prostate-specific antigen hKLK3 gene (human kallikrein gene). As HLA-A2–binding unrelated peptide we used influenza nucleoprotein-derived peptide, GILGFVFTL referred to as Flu peptide. Another melanoma-associated but self-antigen, Mart-1–derived HLA-A2–binding peptide AAGIGILTV, was used as another control peptide. Melanoma-associated antigen MAGE-1–derived HLA-A1–binding peptide EADPTGHSY was used as negative control for HLA-A2–binding peptides. Peptides were synthesized and purified by HPLC in University core facility.
Cell lines
The T2 cell line was a gift of Dr. Peter Creswell, Yale University [3]. This cell line is intracellular transport of antigen-processing-deficient (TAP-deficient) and HLA-A2 positive. Peptide molecules that have a binding motif for HLA-A2 can promptly be loaded onto empty A2 molecules on these cells. HLA-A2 positive and PSA positive prostate tumor cell line LN CaP [10, 15] was obtained from ATCC. LN CaP cell line was established from a needle aspiration biopsy of the supraclavicular lymph node of a 50-year-old Caucasian male with confirmed diagnosis of metastatic carcinoma by Professor G. P. Murphy's group in 1977. Details of the cell line have been described by Horoszewicz et al. [15]. All the viable LN CaP cells were found to express HLA-A2. Although culture supernatant collected form LN CaP did not show any detectable amount of PSA, immunocytochemical analysis revealed that these cells are PSA positive. A melanoma tumor cell line CS-M was established in our laboratory from a HLA-A2 positive metastatic melanoma patient. These cells do express high levels of HLA-A2 melanoma-associated antigen Mart-1 but do not express PSA (unpublished). These CS-M cells were used as control tumor in the assays. Cells were cultured in Iscove's Modified Dulbecco's Medium (IMDM) as described below.
DC culture for antigen presentation in short-term T-cell stimulation assays
DC used in this study were peripheral blood mononuclear cell–derived myeloid cells cultured in vitro as described [35]. Briefly, plastic adherent cells (mostly monocytes/macrophages) were isolated from a Ficoll-Hypaque gradient–derived mononuclear cell population. The adherent cells were then cultured in Iscove's Modified Dulbecco's Medium supplemented with 10% FBS, l-arginine (0.55 mM), l-asparagine (0.24 mM), and l-glutamine (1.5 nM), henceforth described as complete medium (CM). Interleukin-4, 1,000 U/ml (R&D Systems, Minneapolis, MN) and Granulocyte macrophage-CSF, 1,000 U/ml (Immunex, Seattle, WA) were added to the culture from day 1 and the cultures were continued for 7 days. Phenotypic analysis of the cultured DC revealed that they are CD1a+, CD80+, CD86+, CD19-, MHC-II+. About 10% of the cells were CD14+ and less than 0.5% were CD3+.
DC maturation for in vitro CTL generation
DC after 7 days of regular cultures were further matured after stimulation with LPS (100 ng/ml) and IFN-γ (250 U/ml) for 24 h. These matured DC were used in CTL generation in long-term cultures.
Isolation of CD8+ cells from PBL
CD8+ cells were purified from total PBL by using antibody-coated Dynal magnetic beads (Oslo, Norway) according to the manufacturer's instructions. Purity of the isolated cells was 90–95%.
Phenotypic analysis
The immunofluorescence procedure for phenotypic analysis by cytofluorography has been described [22].
Peptide binding to HLA-A2 molecules
Peptide-binding studies to the HLA molecules and stability of the MHC peptide complexes were measured according to the methods described by Nijman et al. [30] and Terasawa et al. [40]. Briefly, T2-A2 cells were washed with Ca++ mG++ free phosphate buffered saline (PBS) and 1×106 cells were incubated overnight in serum-free IMDM in 24-well plates with different concentrations (10–50 μg/ml 1) of peptides at 37°C in 5% C02. Cells were then washed two times in PBS and were incubated in ice with 10 μg of HLA-A2–specific monoclonal antibody (One Lambda) for 1 hr. The cells were then washed two times and incubated with 1:100 dilution of FITC conjugated antimouse IgG (Becton Dickinsion). The cells were immediately analyzed in FACScan with appropriate isotype control and data were gathered from 10,000 live cells.
Microcytotoxicity assay
The 51Cr release microcytotoxicity assay has been described [22]. Freshly prepared or cryopreserved target cells were labeled with 51Cr with good efficiency. The spontaneous release of the radioactivity from the 51Cr-labeled targets was usually <12%. All the experiments in cytotoxicity assay were carried out in presence of 50-fold excess of cold K-562 cells to overcome the nonspecific killing activity. Cold target inhibition assay was done using 51Cr-labeled and peptide-pulsed T2 cells competing with unlabeled and appropriate peptide-pulsed T2 cells.
Cytokine synthesis assay
Freshly isolated or cryopreserved PBL or CD8+ cells were stimulated with autologous dendritic cells pulsed with PSA peptide epitopes or T2 (TAP-deficient HLA-A2 cells; 3) cells pulsed with peptide epitopes (at peptide concentration of 25 μg/ml) in U-bottom 96-well cluster plates (Costar, Cambridge, MA) in 0.2 ml of medium for 24 hr. Supernatants were assayed for the presence of IFN-γ by using ELISA kits (Immunotech, Miami, FL) using manufacturer's protocol.
Intracellular analysis for cytokine production
The method for intracellular analysis of cytokine production by cells has been described [27]. Briefly, the effector cells were stimulated with peptide-pulsed DC for a total period of 6 hr at 37°C (responder to stimulator ratio = 10:1). Two hours after initiation of the culture nonstimulatory doses of PMA (0.5 ng/ml) and Ionomycin (4 ng/ml, Sigma, St Louis, MO) were added for an additional 4 hr at 37°C. Brefeldin A (1 μg/ml, Sigma) was added 2 hr before harvesting the cells. Cells were washed and stained with FITC-conjugated anti-CD8 antibody (Pharmigen, San Diego, CA) for 30 min on ice. Cells were then fixed for 25 min with 4% paraformaldehyde (Sigma) and permeabilized with PBS/BSA/Saponin for 10 min. Cells were then stained with human anti–IFN-γ antibody conjugated with alo pico cyanin (APC; Pharmigen, San Diego, CA), and analyzed on FACS using Cell Quest Software. Data were analyzed after acquiring 10,000 viable cells in each point.
Results
Patient demographics
The median age of 14 patients with prostate cancer who were HLA-A2+ was 72 (range 50–81). Ten patients had clinically localized (T1c or T2a) disease which had been treated with either radical prostatectomy or radiation therapy and leuprolide. Two patients had locally recurrent disease and were being treated with leuprolide. Two patients had bone metastases which had progressed following an orchiectomy. Serum PSA level was elevated at the time of diagnosis in all patients, and was elevated at the time of blood sampling in 2/14 patients. Nine HLA-A2 normal males with no history of prostate disease served as controls.
Peptide binding to HLA-A2 molecule
All the PSA-derived peptides we have used in this study were tested for binding to HLA-A2 molecules according to the method described in "Materials and methods." Figure 1 shows that these peptides bind to the HLA-A2+ cells. The binding of the PSA peptides is comparable to that of the HLA-A2–determined melanoma antigen–derived Mart-127–35 peptide. We then tested the stability of the four PSA peptides–HLA-A2 complexes. Peptides were added at a concentration of 50 μg/ml. Free unbound peptides were washed away and incubated with brefeldin-A (1μg /ml, Sigma) to block the delivery of new class I molecules to the cell surface, and the stability of the complex was analyzed at different times, at 2, 4, 6, and 8 h. No significant difference was observed between the PSA peptides (data not shown).
Fig. 1.
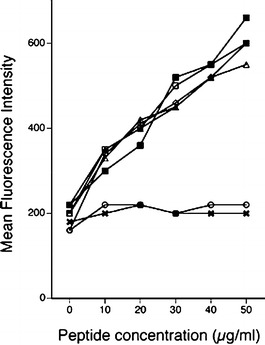
Binding of PSA-derived peptides to HLA-A2 on T2 A2 cells. Peptides were analyzed for binding to T2 A2 cells as described. This describes the binding of peptides to A2 when different concentrations (10–50 μg/ml) of peptides were used. X T2 cells without any peptide, open circle T2 + MAGE-1, open square T2 + PSA-1, solid square T2 + PSA-2, open triangle T2 + PSA-3, solid triangle T2 + PSA-4, open diamond T2 + MART-1
Expression of surface molecules by cultured DC
Figure 2 describes the expression of various surface markers typically expressed by DC. Fig. 2A describes several markers on 7-day cultured DC in GM-CSF- and IL-4-containing medium. Seven-day cultured DC were further stimulated with LPS and IFN to mature them. Figure 2B shows significant increase in all the necessary molecules on matured DC required for T-cell stimulation.
Fig. 2A, B.
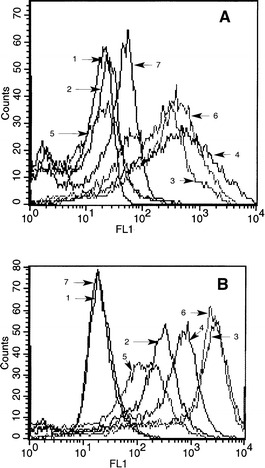
Phenotypic analysis of dendritic cells. A Different surface markers on DC after 7 days in culture in presence of GM-CSF and IL-4, immature DC. B Surface markers on DC further matured with LPS + IFN-γ for additional 24 hr, matured DC. 1 Isotypecontrol, 2 CD80, 3 CD86, 4 MHC-I, 5 CD83, 6 CD40, and 7 CD14. MFI of various markers on immature DC as in A are Isotype = 8.3, CD80 = 17.0, CD86 = 289.1, MHC-I = 139.0, CD83 = 9.2, CD40 = 232.5, and CD14 = 21.7. MFI of various markers on matured DC in 2B are Isotype = 14.2, CD80 = 148.7, CD86 = 1118, MHC-1 = 356.9, CD83 = 71.2, CD40 = 1137.6, and CD14 = 15.9.
Functional analysis of PBL upon ex vivo presentation of PSA peptide epitopes
IFNγ secretion by PBL as measured by ELISA
We tested 14 patients and 9 normal donors who are HLA-A2 positive for T-cell response to PSA-derived HLA-A2.1–restricted peptide epitopes, in vitro, without rounds of stimulation and restimulation. Using four HLA-A2.1–determined PSA-derived peptides as epitopes and employing a simple in vitro cytokine synthesis assay as a readout of "memory type response," we tried to determine if we could identify PSA epitope-specific T cells following short-term (24 hr) culture. Table 1 shows that upon stimulation by the peptide-loaded autologous DC, freshly isolated PBL from 10/14 patients synthesized IFN-γ in response to at least one of the peptides. (Cf. results for normal donors in Table 2.) We considered a two-fold increase of IFN-γsecretion by T cells over DC control or a value of 25 pg/ml (if the DC control was zero) as a positive response to a particular peptide. The experiments were repeated at least twice in all the cases, and in some cases it was repeated three times with essentially similar results.
Table 1.
Patients. Interferon-γ production by PBL from prostate cancer patients after stimulation with peptide-pulsed autologous dendritic cells. DC from plastic adherent macrophage and monocyte lineage of cells from PBMC were cultured with GM CSF(1,000 U/ml) and IL-4 (1,900 U/ml) for 5 to 7 days. DC were pulsed with any of the four peptides; PSA-1 (VLVASRGRAV), PSA-2 (FLTPKKLQCV), PSA-3 (KLQCVDLHV), PSA-4 (DLHVISNDV), or Flu (GILGFVFTL). Respective autologous PBL was added to peptide (25 µg/ml) pulsed DC at DC/PBL = 1:10 (104 DC/105 PBL) in 96-well U-bottom tissue culture plates in 200 μl of culture medium. Plates were incubated at 37°C for 24 h. Culture supernatant was collected and assayed for the presence of IFN-γ with ELISA kits. Significant differences were found between columns of DC only and DC+PSA-2, t(df)=2.44, p<.05; and between columns of DC+PSA-2 and DC+PSA-4, t(df)=2.20, p<.05
| PBL from patient | IFN-γ pg/ml after stimulation with DC pulsed with peptides | |||||
|---|---|---|---|---|---|---|
| DC only | DC+PSA-1 | DC+PSA-2 | DC+PSA-3 | DC+PSA-4 | Flu peptide | |
| PD | 0 | 0 | 75 | 100 | 0 | 120 |
| JM | 50 | 80 | 100 | 0 | 0 | 0 |
| NC | 0 | 0 | 0 | 0 | 0 | NT |
| AB | 0 | 0 | 0 | 0 | 0 | NT |
| RS | 0 | 100 | 0 | 0 | 0 | 80 |
| PH | 0 | 0 | 120 | 0 | 0 | 60 |
| JK | 25 | 0 | 200 | 0 | 0 | 100 |
| GD | 30 | 85 | 800 | 95 | 90 | 120 |
| HW | 0 | 0 | 0 | 0 | 0 | 0 |
| FB | 0 | 0 | 0 | 0 | 0 | 50 |
| FG | 30 | 40 | 350 | 75 | 40 | NT |
| RO | 40 | 75 | 200 | 100 | 175 | 50 |
| RV | 0 | 0 | 100 | 50 | 50 | NT |
| RT | 0 | 100 | 100 | 50 | 50 | 60 |
Table 2.
Normal donors
| PBL from donor | IFN-γ pg/ml after stimulation with DC pulsed with peptides | |||||
|---|---|---|---|---|---|---|
| DC only | DC+PSA-1 | DC+PSA-2 | DC+PSA-3 | DC+PSA-4 | Flu peptide | |
| RLS | 0 | 0 | 0 | 0 | 0 | 80 |
| RZ | 10 | 10 | 0 | 0 | 10 | 120 |
| CM | 15 | 10 | 10 | 10 | 10 | 120 |
| PC | 0 | 0 | 0 | 0 | 0 | 50 |
| JS | 10 | 10 | 10 | 10 | 10 | 60 |
| RK | 0 | 15 | 10 | 15 | 15 | 0 |
| DD | 15 | 20 | 10 | 25 | 10 | 100 |
| JDS | 25 | 25 | 20 | 20 | 30 | 180 |
| TS | 10 | 0 | 0 | 0 | 0 | 100 |
IFN-γ by CD8+ cells as detected by intracellular cytoplasm staining
The nature of the T cells responding to the short-term primary stimulation was examined in intracytoplasmic cytokine and phenotypic analysis by flowcytometry. Figure 3 shows a representative experiment that demonstrates significant increase in the number of intracytoplasmic IFN-γ positive CD8+ cells upon stimulation. As shown, when stimulated with PSA peptides, the increase in the IFN-γ–producing CD8+ cells varied from 0.38%, 0.93%, 0.64%, or 0.83% from the baseline of (DC control) 0.20%. This increase is significant considering the enormous diversity of antigen-specific T-cell receptor( TCR)–bearing cells in circulation. No significant increase was observed when Mart-1 A2 peptide was used (Fig. 3G). Interestingly these CD8+ cells showed no spontaneous cytolytic effect when tested in a 51Cr-release assay against peptide-pulsed T2 cells, peptide-pulsed DC, or against tumor cell line LN CaP (data not shown).
Fig. 3A–G.
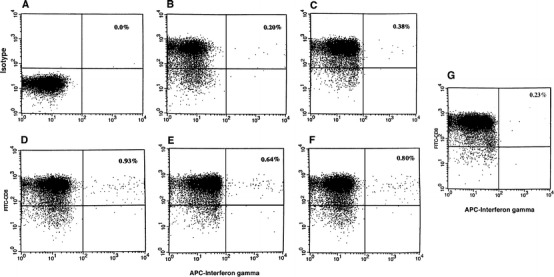
Intracellular cytokine analysis. CD8+ cells from PBL were isolated by positive selection using Dynal magnetic beads. Cells were stimulated with PSA-peptide-pulsed autologous DC for 6 h. Cells were stained with anti-CD8 antibody conjugated with FITC (Y axis) and then stained for intracellular staining with antihuman IFN-γ conjugated with APC (X axis) according to the method described. A Isotype matched control. B Cells stimulated with DC only. C Cells stimulated with PSA-1 peptide-pulsed DC. D Cells stimulated with PSA-2 pulsed DC. E Cells stimulated with PSA-3-pulsed DC. F Cells stimulated with PSA-4-pulsed DC. G Cells stimulated with Mart-1 peptide-pulsed DC
IFN-γ secretion by CD8+ T cells stimulated by T2 cells and peptide as measured by ELISA
We then examined whether these CD8+ T cells are capable of recognizing the antigens when presented by cells other than dendritic cells. We used TAP-deficient T2 cells to present the antigens to purified CD8+ cells. We pulsed T2 cells with PSA-2 (since the majority of the patients recognized this peptide) or PSA-4 and used these to stimulate CD8+ cells from patients. This was also performed in the presence and absence of antibody to HLA-A1 and HLA-A2 (1 μg/ml). A representative experiment with the patient PD in Fig. 4 shows that these T cells do respond to the antigen when presented with T2 cells and this recognition is HLA-A2 restricted. This nonresponsiveness to PSA-4 by the patient as shown earlier in Table 1 can be seen in this assay also.
Fig. 4.
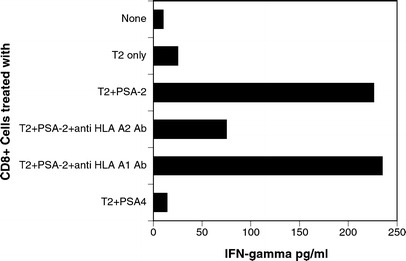
Response to PSA peptides presented by T2 cells. Purified CD8+ cells from a patient (PD) who was responding against PSA-2 were stimulated with T2 cells pulsed with PSA-2 in presence and absence of anti-HLA-A2 antibody (1 μg/ml). PSA-4 peptide was used as control for the experiment
In vitro generation of CTL response
As mentioned earlier, the IFN-γ–responsive T cells did not show any cytolytic activity. As such, standard long-term cocultures were set up to address whether by repetitive stimulation with epitope-pulsed DC, one could generate a PSA-peptide-specific CTL response against these peptides. Autologous DC were matured with LPS (100 ng/ml) and IFN-γ (250 U/ml) (Fig. 2B). Matured DC were then pulsed with one of the four peptides (100 μg/ml) and β-2 microglobulin (2.5 μg/ml, Sigma) [11]. Purified CD8+ T cells were then added to DC and were cultured for 7 days (ratio of DC to T cells = l:10). On day 8, T cells were restimulated with the same peptide-antigen-pulsed matured DC. Cultures were then continued for another 7 days in presence of IL-2 (50 U/ml). Responding cells were tested for their cytolytic activity against peptide-pulsed T2 cells and the prostate tumor cell line LNCaP (HLA-A2+, PSA+) as target cells (in the presence of a 50-fold excess of cold K-562 cells). Figure 5 shows a representative experiment with CD8+ cells from a patient (PD) whose fresh PBL responded against PSA-2 and PSA-3 and not against PSA-1 or PSA-4 in the initial screening (Table 1). As shown, specific CTL activity against all four peptides could be generated by long-term in vitro culture (without having cross-reactivity against any other peptides). When PSA-1, PSA-2, and PSA-4 peptides were used individually to generate specific CTL response, a significant level of CTL reactivity was observed against that peptide which was used in generation and also against the prostate cancer cell line LN CaP but no killing activity was observed against the melanoma tumor CS-M (Figs. 5A, 5B, 5D). Interestingly when PSA-3 peptide was used in CTL-generation assay, it only generated PSA-3 CTL and not against.tumor target LN CaP (Fig. 5C). Of the six patients whose T cells responded to multiple peptides in IFN assay in Table 1, we could generate specific CTL in four cases. Out of nine normal donors, we were able to generate CTL activity in two cases against peptides PSA-1, PSA-2, and PSA-4, but not against PSA-3 (data not shown). For further confirmation of the specificity of the peptide-specific CTLs generated in culture, a cold target inhibition experiment was done using 51Cr-labeled and peptide-pulsed T2 cells as hot targets, and unlabeled and appropriate peptide-pulsed T2 cells as cold targets. As it can be observed in Table 3, increasing doses of peptide-pulsed cold targets eliminate the lysis of respective peptide-pulsed hot target cells. This experiment further confirms the specificity of the CTLs generated in the cocultures.
Fig. 5A–D.
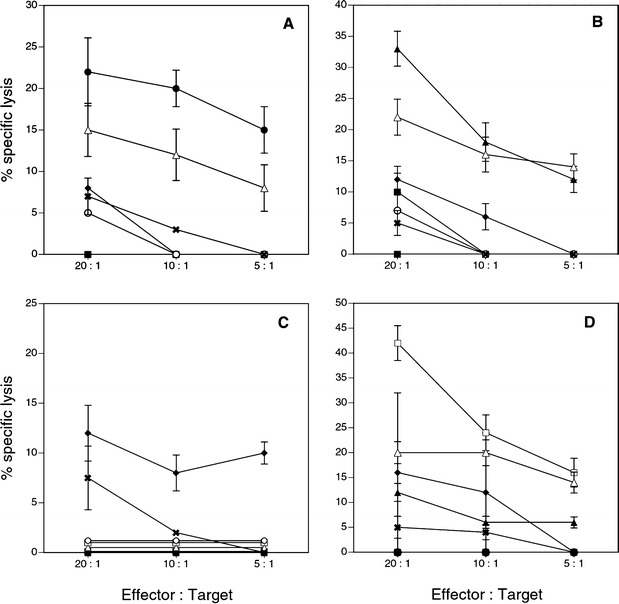
Cytotoxicity assay with in vitro expanded peptide-specific CTL. CD8+ cells were stimulated in vitro with matured autologous DC pulsed with one of the PSA-derived peptides. Cytotoxicity was measured by 51Cr release from labeled different target cells. Target cells were T2 cells alone, T2 cells pulsed with one of the four PSA peptides, T2 cells pulsed with Flu peptide, HLA-A2 positive and PSA positive prostate cancer cell line LN CaP, and HLA-A2 positive and PSA negative melanoma cell line CS-M. A CTL activity with responding CD8+ cells stimulated with DC pulsed with PSA-1. B CTL activity with responding CD8+ cells stimulated with DC pulsed with PSA-2. C CTL activity with responding CD8+ cells stimulated with DC pulsed with PSA-3. D CTL activity with responding CD8+ cells stimulated with DC pulsed with PSA-4. Solid square % specific lysis of the target T2 cells alone. Solid circle % specific lysis of the target T2 cells pulsed with peptide PSA-1. Solid triangle % specific lysis of the target T2 cells pulsed with peptide PSA-2. Solid diamond % specific of the target T2 cells pulsed with peptide PSA-3. Open square % specific lysis of the target T2cells pulsed with peptide PSA-4. Open circle % specific lysis of the target T2 cells pulsed with Flu peptide. Open triangle % specific lysis of the target LN CaP tumor cells. X % specific lysis of HLA A2 positive melanoma tumor target CS-M cells
Table 3.
Cold target inhibition assay with CTL. Inhibition of cytolysis of labeled targets (hot) by unlabeled targets (cold). In addition to 50-fold excess of cold K-562 cells, specificity of CTL generated in vitro was further confirmed in cold target inhibition assay using unlabeled peptide (against which CTL was generated) pulsed T2 cells as the cold target: such as 51Cr labeled PSA-1-pulsed T2 cells as hot target competing with unlabeled PSA-1-pulsed T2 cells and so on
| CTL generated in coculture | Hot target to cold target ratio | ||||
|---|---|---|---|---|---|
| 1:0 | 10:1 | 5:1 | 1:1 | 1:5 | |
| Against PSA-1 | 20 | 20 | 8 | 2 | 0 |
| Against PSA-2 | 38 | 28 | 11 | 1 | 0 |
| Against PSA-3 | NT | NT | NT | NT | NT |
| Against PSA-4 | 33 | 20 | 10 | 4 | 0 |
Discussion
Considerable interest has been generated lately regarding the prospect of developing vaccine for prostate cancer with prostate-associated antigen-derived CTL-determined peptide epitopes. While the clinical results of the early cancer vaccine trials with peptide alone or loaded onto DC [12, 22, 23, 24, 32, 33, 34], in general, have been somewhat modest, the field clearly remains open to further innovation. The great majority of work that has demonstrated T-cell response to tumor-associated epitopes has usually employed the traditional technique of several rounds of in vitro stimulation and restimulation of the host's T cells by a variety of mechanisms to fully activate them prior to assay for immune response [1, 5, 7, 9, 16, 32, 41]. While this approach has demonstrated that specific tumor epitope reactive T cells can be generated from many cancer patients, it does not clearly demonstrate whether these hosts harbored antigen-experienced T cells. Lately, ELISPOT assays and tetramer analyses coupled with simultaneous evaluations of T cell activation markers have confirmed, however, that indeed some cancer patients do harbor antigen-experienced T cells [2, 19, 31, 36]. Nonetheless, the clear evidence for PSA-reactive circulating T cells that do not require repetitive stimulation ex vivo is not available.
For the first time, we demonstrate that, irrespective of the clinical status, IFN-producing CD8+ T cells are detectable in 10 out of 14 patients. Our study reveals a number of interesting features of reactivity against PSA-derived epitopes in prostate cancer patients. First, CD8+ T cells in their native state, from a sizeable number of patients (10/14), exhibited a IFN-γ response to at least one epitope, and 6/14 responded to multiple epitopes. It is however noteworthy that these effectors did not show cytotoxic activity against peptide-loaded T2 cells, peptide-loaded DC, or prostate tumor cell line LNCaP in their native state (data not shown). Yet cytotoxic function against a broad repertoire of PSA epitopes could be generated by long-term stimulation by peptide-loaded matured DC (Fig. 5). Thus a major disparity between IFN response and cytotoxic response seems to exist with these effector cells in their native state. Presently, it is not clear if the disparity is a reflection of a different threshold for activation of IFN response vs CTL response or if this represents a form of "dysfunctional" CTL response such as that shown by others [19, 29, 38]. Second, certain PSA epitopes seem to be more immunogenic. Of the four PSA epitopes studied, PSA-2 was recognized more often by fresh T cells in the IFN assay. Immunogenecity of these peptides could not be attributed to their binding affinity to HLA-A2 molecules. There was no significant difference in binding of these peptides to HLA-A2 molecules (Fig. 1), and stability of the complex was observed (data not shown). Nonetheless CD8+ cells could be cytolytically activated in vitro against the less dominant epitopes also when such epitopes were presented in the correct context by matured DC. This affirms the role of DC (APC) in induction of cytolytic T cell response as demonstrated by Guerder et al. [13]. Third, the demonstration of IFN response by freshly isolated T cells suggests that this might be a form of memory response. Fourth, although Alexander et al. [1] have shown that CTL generated in in vitro culture did not recognize internally expressed epitope, our study in contrast clearly shows that CTL generated in vitro with peptide-loaded DC are fully reactive against the prostate cancer cell line (Fig. 5). Unfortunately we could not test CTL activity against autologous tumor cells.
In the prostate cancer model it has been shown that prostatic-antigen-specific T cells can be generated in in vitro stimulation/restimulation techniques [1, 8, 9, 10]. In a recent observation, McNeel et al. have shown the existence of naturally occuring PSA-specific Th cells in patients [21]. Very little information is available on whether T cells that can respond to stimulation by a given tumor-associated epitope having encountered antigen, in vivo, and if so how these epitopes are presented and what is the outcome of such presentation. The T cells that bear receptors for a given TAA are either antigen inexperienced—as they seldom exhibit a memory response upon exposure to antigen—are anergic, or are not presented with antigens appropriately; or their activation is prevented by the suppressive factors present in the microenvironment [29, 37, 38]. In this study we failed to detect reactivity against some of the peptides in initial analysis with some of the patient's PBL (Table 1), yet we could generate peptide-specific CTL against those peptides by presenting such peptides with matured DC (Fig. 5). Nonreactivity of T cells to certain peptides can not be due to differential binding of the peptides to HLAA2 molecules (Fig. 1). Further, the study of this system using PSA antigen which is not present during early stages of development may provide insight into the generation of immune responses to self-antigens. It may also provide a model to study the control of CTL function in CD8+ cells which have already acquired antigen specificity.
It should be pointed out that in generation of PSA-peptide-specific CTL in long-term coculture our system has four critical elements. We used only CD8+ cells in the reaction. This removes CD4+ T-reg cells [4, 7], if any, from the priming reaction. Second, we used fairly high concentrations of peptide (100 μg/ml) to activate all low-to-intermediate-affinity T cells. Third, we matured DC with LPS and IFN-γ to obtain immunogenic DC matured in an inflammatory setting (see Fig. 2B). Fourth, the responses are amplified with one additional stimulation. However, in the issue of inducibility of PSA-epitope-specific CTLS, there is a novel aspect in our observations. It is that prostate cancer patients do harbor PSA-epitope-experienced CD8+ T cells and that the cytolytic dysfunctional state can be corrected by a matured DC-based stimulation technique in vitro. A more comprehensive examination of these issues would provide a better understanding of the unresponsive state in prostate cancer patients and help in the generation of more effective approaches to immunotherapy.
Acknowledgements
We thank B. Mukherji for many valuable suggestions and critical review of the manuscript. We also thank Robert B. Clark for critical review of the manuscript.
Abbreviations
- PSA
prostate-specific antigen
- DC
dendritic cells
- APC
antigen-presenting cells
- CTL
cytotoxic T lymphocytes
- PBL
peripheral blood lymphocytes
- PBMC
peripheral blood mononuclear cells
Footnotes
The work was supported by a grant from General Clinical Research Center, University of Connecticut Health Center, Farmington, CT 06030.
References
- 1.Alexander Urology. 1998;51:150. doi: 10.1016/S0090-4295(97)00480-9. [DOI] [Google Scholar]
- 2.Altman Science. 1996;274:94. [PubMed] [Google Scholar]
- 3.Anderson J Immunol. 1993;151:3407. [PubMed] [Google Scholar]
- 4.Baecher-Allan J Immunol. 2001;167:1245. doi: 10.4049/jimmunol.167.3.1245. [DOI] [PubMed] [Google Scholar]
- 5.Boon J Exp Med. 1996;183:725. doi: 10.1084/jem.183.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chakraborty Clin Immunol. 2000;94:88. doi: 10.1006/clim.1999.4826. [DOI] [PubMed] [Google Scholar]
- 7.Chakraborty J Immunol. 1999;162:5576. [PubMed] [Google Scholar]
- 8.Corman Clin Exp Immunol. 1998;114:166. doi: 10.1046/j.1365-2249.1998.00678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Correale J Natl Cancer Inst. 1997;89:293. doi: 10.1093/jnci/89.4.293. [DOI] [PubMed] [Google Scholar]
- 10.Correale J Immunol. 1998;161:3186. [PubMed] [Google Scholar]
- 11.Cox Science. 1990;247:715. doi: 10.1126/science.2137259. [DOI] [PubMed] [Google Scholar]
- 12.Dhodapkar J Exp Med. 2001;193:233. doi: 10.1084/jem.193.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guerder J Immunol. 1995;155:5167. [PubMed] [Google Scholar]
- 14.Heiser J Immunol. 2000;164:5508. doi: 10.4049/jimmunol.164.10.5508. [DOI] [PubMed] [Google Scholar]
- 15.Horoszewicz Can Res. 1983;43:1809. [PubMed] [Google Scholar]
- 16.Houghton A. Cancer antigens: immune recognition of self and altered self. J Exp Med. 1994;180:1–4. doi: 10.1084/jem.180.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Israeli Cancer Res. 1993;33:227. [PubMed] [Google Scholar]
- 18.Kappler Cell. 1987;49:273. [Google Scholar]
- 19.Lee PP, Yee C, Savage PA, Davis MM. Characterization of circulating T cells specific for tumor associated antigens in melanoma patients. Nature Med. 1999;5:677–685. doi: 10.1038/9525. [DOI] [PubMed] [Google Scholar]
- 20.McNeel J Urology. 2000;164:1825. [PubMed] [Google Scholar]
- 21.McNeel Prostate. 2001;47:222. doi: 10.1002/pros.1066. [DOI] [PubMed] [Google Scholar]
- 22.Mukherji J Exp Med. 1989;169:1961. doi: 10.1084/jem.169.6.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murphy Prostate. 1996;29:371. doi: 10.1002/(SICI)1097-0045(199612)29:6<371::AID-PROS5>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 24.Murphy Prostate. 1999;39:54. doi: 10.1002/(SICI)1097-0045(19990401)39:1<54::AID-PROS9>3.0.CO;2-U. [DOI] [Google Scholar]
- 25.Murphy Prostate. 2000;43:59. [Google Scholar]
- 26.Pardoll Clin Immunol. 2000;95:S44. doi: 10.1006/clim.1999.4819. [DOI] [PubMed] [Google Scholar]
- 27.Perez-Diez Cancer Res. 1998;58:5305. [PubMed] [Google Scholar]
- 28.Ponniah Prostate. 2000;44:49. doi: 10.1002/1097-0045(20000615)44:1<49::AID-PROS7>3.3.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 29.Nanda NK, Sercarz EE. Induction of anti-self immunity to cure cancer. Cell. 1995;82:13–17. doi: 10.1016/0092-8674(95)90047-0. [DOI] [PubMed] [Google Scholar]
- 30.Nijman Eur J Immunol. 1993;23:1215. doi: 10.1002/eji.1830230603. [DOI] [PubMed] [Google Scholar]
- 31.Romero J Exp Med. 1998;188:1641. doi: 10.1084/jem.188.9.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosenberg Immunol Today. 1997;18:175. doi: 10.1016/s0167-5699(97)84664-6. [DOI] [PubMed] [Google Scholar]
- 33.Salgaller Expert Opin Investig Drugs. 2000;9:1217. doi: 10.1517/13543784.9.6.1217. [DOI] [PubMed] [Google Scholar]
- 34.Salgaller Crit Rev Immunol. 1998;18:109. doi: 10.1615/critrevimmunol.v18.i1-2.120. [DOI] [PubMed] [Google Scholar]
- 35.Sallustro J Exp Med. 1994;179:1109. [Google Scholar]
- 36.Schmittel J Immunol Methods. 1997;210:167. doi: 10.1016/S0022-1759(97)00184-1. [DOI] [PubMed] [Google Scholar]
- 37.Schwartz Cold Spring Harbor Symp Quant Biol. 1989;56:605. doi: 10.1101/sqb.1989.054.01.072. [DOI] [PubMed] [Google Scholar]
- 38.Sercarz Ann Rev Immunol. 1993;11:729. doi: 10.1146/annurev.immunol.11.1.729. [DOI] [PubMed] [Google Scholar]
- 39.Simmons Prostate. 1999;39:291. doi: 10.1002/(SICI)1097-0045(19990601)39:4<291::AID-PROS10>3.3.CO;2-0. [DOI] [Google Scholar]
- 40.Terasawa Clin Can Res. 2002;8:41. [Google Scholar]
- 41.Xue Prostate. 1997;30:73. [Google Scholar]


