Abstract
Purpose: Many human tumors lose responsiveness to IFN-γ, providing a possible mechanism for the tumor to avoid immune recognition and destruction. Here we investigate the importance of tumor responsiveness to IFN-γ in the successful immunotherapy of TC1 tumors that were immortalized with human papillomavirus proteins E6 and E7. Methods: To investigate the role of IFN-γ in vivo, we constructed a variant of TC1, TC1.mugR, that is unresponsive to IFN-γ due to overexpression of a dominant negative IFN-γ receptor. Results: Using recombinant Listeria monocytogenes that express HPV-16 E7 (Lm-LLO-E7) to stimulate an antitumor response, we demonstrate that sensitivity to IFN-γ is required for therapeutic efficacy in that Lm-LLO-E7 induces regression of TC1 tumors but not TC1.mugR. In addition, we show that tumor sensitivity to IFN-γ is not required for inhibition of tumor angiogenesis by Lm-LLO-E7 or for trafficking of CD4+ and CD8+ T cells to the tumor. However, it is required for penetration of lymphocytes into the tumor mass in vivo. Conclusions: Our findings identify a role for IFN-γ in immunity to TC1 tumors and show that loss of tumor responsiveness to IFN-γ poses a challenge to antigen-based immunotherapy.
Keywords: Cytokines, Immunotherapy, Interferon-gamma, T lymphocytes, Tumor immunity
Introduction
Cervical cancer accounts for over 200,000 deaths annually and is the second leading cause of cancer-related deaths for women in the world. Human papillomavirus (HPV) is associated with more than 90% of cervical cancers [1]. In particular, HPV type 16 can be detected in approximately 50% of squamous cell carcinomas of the cervix. The malignant phenotype of HPV-16 is dependent on the expression of HPV early-region genes 6 and 7 (E6 and E7). E6 and E7 are necessary and sufficient to induce and maintain cellular transformation by disrupting the functions of the tumor suppressor proteins p53 and pRb, respectively [2]. Several lines of evidence suggest that induction of cell-mediated immunity can control both HPV infection and HPV-associated cervical cancer [3]. As a result, particular interest has developed in constructing vaccines or immunotherapies that target the HPV oncogenic proteins, E6 and/or E7, to prevent and treat HPV-associated cervical malignancies.
In animal models, the induction of anti-HPV effector T cells can prevent the growth of HPV-associated tumors and induce regression of established tumors [4–6]. We have shown that Listeria monocytogenes, a gram-positive facultative intracellular bacterium, is a potential vaccine vector for both infectious and neoplastic disease with the exquisite ability to prime both CD4+ and CD8+ T cells [6–9]. Previous studies from our laboratory [6] have shown that Lm-LLO-E7, a recombinant listerial strain expressing a fusion of the listerial protein listeriolysin O (LLO) and HPV-16 E7, can induce the regression of established TC1 tumors expressing E6 and E7. This antitumor response requires both CD4+ and CD8+ T cells and IFN-γ [6].
Several studies have demonstrated that malignant cells expressing E6 and E7 frequently lose responsiveness to IFN-γ [10, 11]. This finding may help explain the inconsistent clinical responses reported for interferon therapy of cervical cancer [12]. Because our previous study had shown that the ability of Lm-LLO-E7 to induce regression of established TC1 tumors is reduced when IFN-γ is depleted, we sought to elucidate the role of IFN-γ in the TC1 model. The aim of the present study was to determine the impact of tumor unresponsiveness to IFN-γ on the efficacy of the Lm-LLO-E7 vaccine. Using a tumor variant that is unresponsive to IFN-γ, TC1.mugR, we show that tumor sensitivity to IFN-γ is not required for the trafficking of T cells to tumors, in that TC1.mugR taken from immunized mice show an accumulation of CD4+ and CD8+ T cells at the tumor periphery, but is required for penetration of T cells into tumors and for the induction of tumor regression. These results suggest that HPV-induced tumor unresponsiveness to IFN-γ poses a potential mechanism for tumor evasion of antigen-based immunotherapy. Furthermore, the ability of IFN-γ to promote the penetration of lymphocytes into tumors may be part of the cancer immunoediting system as suggested by Dunn et al. [13].
Materials and methods
Mice and cell lines
Female C57BL/6 mice, 6–8 weeks of age, were purchased from Charles River Laboratories (Wilmington, MA, USA). TC1 is a lung epithelial cell line immortalized with HPV-16 E6 and E7 and transformed with the c-Ha-ras oncogene that is syngeneic with C57BL/6 mice [4]. EL4 is a carcinogen-induced lymphoma syngeneic with C57BL/6. CT26 is an N-nitroso-N-methylurethane–induced colon carcinoma that is syngeneic to BALB/c. Cell lines were cultured in RPMI 1640 containing 10% fetal calf serum (FCS), penicillin/streptomycin, and 2 mM L-glutamine, at 37°C with 5% CO2. EL4 cultures were supplemented with 0.05 mM β-mercaptoethanol, and TC1 cell cultures were supplemented with 1 mM sodium pyruvate, and 2 mM nonessential amino acids. “Principles of Laboratory Animal Care” (NIH publication no. 85-23, revised 1985) was followed for all animal experiments.
Transfection
The plasmid pcDNA.mugR is an expression plasmid that contains a truncated murine IFN-γRα cDNA under control of the cytomegalovirus (CMV) promoter. The truncated receptor cDNA clone was created by amplifying the murine IFN-γRα cDNA from oligonucleotides 106 to 949 encoding the IFN-γRα signal sequence, extracellular and transmembrane domains, and the first four amino acids of the intracellular domain. The truncated IFN-γRα cDNA was cloned into the EcoRI site of the pcDNA3.1 expression vector (Invitrogen, Carlsbad, CA, USA), which contains a gene for Zeocin resistance. Proper insert orientation was confirmed by sequence analysis. To create TC1 cells expressing this mutant IFN-γRα, parental cell lines were transfected with pcDNA.mugR by the calcium phosphate method. Individual Zeocin-resistant clones were screened by flow cytometry and selected based on high surface expression of the IFN-γR complex. TC1.mugR cells were maintained under similar culture conditions to those for TC1 with the addition of 250 μg/ml Zeocin (Invitrogen, Carlsbad, CA, USA).
Antigens, mAbs, and cytokines
The E7 peptide (RAHYNIVTF) is a MHC class I Db epitope from the HPV-16 E7 protein [14]. Antibodies against CD62L (clone MEL-14), CD8β.2 (clone 53-5.8), CD45 (clone 30-F11), IFN-γRα (clone GR.20), MHC class I Db (clone KH95), and murine IFN-γ (clone XMG1.2) were purchased from BD Biosciences Pharmingen (San Diego, CA, USA). Secondary FITC goat anti-mouse IgG and FITC anti-rat IgG were purchased from Sigma (St Louis, MO, USA). 7AAD viability dye was purchased from Beckman Coulter (Fullerton, CA, USA). The 28-14-8S hybridoma (anti-Db) was obtained from American Type Culture Collection (ATCC, Manassas, VA, USA). The 28-14-8S hybridoma supernatants were purified over a protein G column (Amersham Pharmacia Biotech, Piscataway, NJ, USA). The mouse anti-E7 monoclonal antibody used for Western blot analysis of E7 protein in TC1 was a gift from Dr Drew Pardoll (Johns Hopkins University School of Medicine, Baltimore, MD, USA). Recombinant murine IFN-γ was purchased from R&D Systems (Minneapolis, MN, USA).
Listeria monocytogenes strains and propagation
The construction of L. monocytogenes vaccine strain Lm-LLO-E7, which was made from the wild-type strain 10403S to stably express and secrete a fusion protein of listeriolysin O and HPV-16 E7, has been described previously [6]. Lm-Gag is a stable chromosomal recombinant of L. monocytogenes that synthesizes and secretes the HIV Gag protein [15]. Lm-E7 is a stable chromosomal recombinant of L. monocytogenes that synthesizes and secretes the HPV-16 E7 protein [6]. All strains were grown in brain heart infusion (BHI) media. Chloramphenicol (10 μg/ml) was added to Lm-LLO-E7 cultures to maintain the expression plasmid.
Matrigel angiogenesis assay
Angiogenesis assays were carried out by injecting C57BL/6 mice subcutaneously with 0.5 ml Matrigel (Collaborative Biomedical Products, Bedford, MA, USA) [16] mixed with 1×105 TC1 or TC1.mugR cells in the right flank. Seven days later, mice were treated i.p. with 0.1 LD50 of Lm-LLO-E7, Lm-Gag, or phosphate-buffered saline (PBS). After 7 days, Matrigel pellets were harvested, surrounding tissue was dissected away, and the pellets were liquefied by incubation at 4°C overnight in 300 μl PBS. To quantify angiogenesis, hemoglobin content of the liquefied pellets was assayed by the Drabkin method (Sigma Diagnostics, St Louis, MO, USA) as previously described [17].
Detection of E7 protein by Western blot
Lysates were generated by resuspension of cells in lysis buffer (50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 0.1% SDS, 1% Triton X-100, 20 μg/ml PMSF). The total protein levels of cleared lysates were assessed using the Bio-Rad DC Protein Assay (Bio-Rad Laboratories, Hercules, CA, USA). Samples (200 μg total protein) were boiled for 3 min at 95°C in reducing buffer (0.125 M Tris-HCl, pH 6.8, 4% SDS, 0.005% bromophenol blue, 20% glycerol, 0.7 M β-mercaptoethanol) before being analyzed by electrophoresis in 4–20% Tris-glycine precast gels (Invitrogen, Carlsbad, CA, USA). After electrophoresis, proteins were transferred to PVDF transfer membranes using a BioRad semidry transfer cell and stained with an anti-E7 monoclonal antibody. Probing of blots was carried out using Vectastain Mouse IgG Peroxidase Kit (Vector Laboratories, Burlingame, CA, USA). Probed blots were developed using ECL Western blotting detection reagents (Amersham Pharmacia Biotech, Piscataway, NJ, USA) and analyzed by autoradiography.
Flow cytometry and intracellular cytokine expression
To determine surface expression of murine IFN-γRα, cells were stained with primary monoclonal rat anti-mouse IFNγRα antibody and secondary goat anti-rat IgG FITC-conjugated antibody. Surface expression of MHC class I Db antigen was determined using purified 28-14-8S (anti-Db) antibody and secondary goat anti-mouse IgG FITC antibody or by direct staining using PE-anti-MHC class I Db antigen. For the assessment of IFN-γ enhancement of TC1 tumor immunogenicity, E7-specific T cells were generated from C57BL/6 mice or in some experiments, Lm-Gag was used as the immunogen as an antigen control. Mice were immunized i.p. with 0.1 LD50 of either Listeria strain, and 10 days later, single-cell suspensions of splenocytes from immunized mice were prepared. Red blood cells were removed by NH4Cl treatment. Splenocytes were cultured at 4×106/ml with 1 μM E7 peptide in RPMI 1640 containing 10% FCS, penicillin/streptomycin, 2 mM L-glutamine, 1 mM sodium pyruvate, and 0.05 mM β-mercaptoethanol. After 10 days, responder cells were harvested and washed. Responders were restimulated with live TC1, TC1 treated with murine IFN-γ for 3 days at 1,000 U/ml, or MHC class I–mismatched CT26 cells, to control for MHC specificity, at a ratio of 100:1 for 5 h in the presence of brefeldin A. For detection of cytoplasmic cytokine expression, cells were stained with PE-anti-CD8β.2 mAb and APC-anti-CD62L mAb, fixed and permeabilized with Cytofix/CytoPerm (BD Biosciences Pharmingen, San Diego, CA, USA), and stained with FITC-conjugated anti-IFN-γ mAb for 30 min on ice. The percentage of cells expressing cytoplasmic IFN-γ was determined by flow cytometry (FACSCalibur; BD Biosciences, San Diego, CA, USA).
Tumor regression studies
TC1 or TC1.mugR (1×105 or 1×106) tumor cells were implanted s.c. in C57BL/6 mice. After 2 days (when tumors were not palpable) or 7 days (when measurable tumors had grown), mice were immunized i.p. with 0.1 LD50 Lm-LLO-E7, Lm-Gag, or PBS. In some experiments, immunizations were repeated 7 days later. Tumor growth was measured every 2 days.
Tumor protection assay (Winn assay)
CD8+ T cells were prepared from C57BL/6 mice immunized i.p. with Lm-LLO-E7 or Lm-Gag, using the following procedure. Twenty mice in each group were immunized with 0.1 LD50 of either Lm-LLO-E7 (1×108 CFU) or Lm-Gag (5×105 CFU) on days 0 and 7. On day 14 the spleens were removed from each group of immunized mice and pooled. Splenocytes were harvested, red cells were lysed using Tris-buffered ammonium chloride, and adherent cells were removed on a nylon wool column. CD4+ cells were depleted using magnetic bead immunoselection (using the manufacturer’s protocol for Qiagen BioMag beads). Briefly, 1×107 cells per milliliter were incubated with 50 μg GK1.5 antibody on ice for 1 h. Subsequently, 2 ml BioMag goat anti-rat IgG beads (Qiagen) was added per spleen, incubated for 45 min on ice, and the CD4+ T cells were depleted on a magnetic separator (Polysciences). Finally, the purity of the CD8+ T cells was confirmed by FACS. No CD4+ cells could be detected in the live lymphocyte pool, 57% of which were CD8+ cells.
The tumor protection assay was performed on TC1 or TC1.mugR cells mixed with purified CD8+ T cells from each and was based on a previous method [18]. Briefly, 1×105 TC1 or 1×106 TC1.mugR cells were mixed with purified CD8+ T cells from Lm-LLO-E7– or Lm-Gag–immunized mice (ratio of one tumor cell to five CD8+ T cells) and inoculated s.c. in the right flank of C57BL/6 mice. The formation of tumors s.c. was monitored, and the mean diameter measured on days 7, 14, 21, and 28.
Histology and immunohistochemistry (IHC)
TC1 (1×105) or TC1.mugR (1×106) cells were implanted s.c. in the right flank (n=12 per group). Half of the mice in each group received the tumor cells mixed with Matrigel (Collaborative Medical Products, Bedford, MA, USA) [16]. Subsequently anti-IFN-γ antibody (1.2 mg/mouse/day) was administered on days 6, 7, 8, and 11, to six mice per group, three of which had received tumors mixed with Matrigel and three of which had received tumor cells alone. In addition on day 7, all mice were treated i.p. with 0.1 LD50 Lm-LLO-E7. Six days later, all tumors were removed and histology or IHC performed.
The tumor samples for histology were fixed in 10% neutral buffered formalin prior to standard processing through a tissue processing machine. Paraffin-embedded, 6-μm sections were cut and stained by hematoxylin and eosin (H&E).
The tumor samples for IHC were sliced to 3–4 mm and placed in zinc fixative (BD Pharmingen) for 24–48 h prior to processing through a standard tissue-processing machine. Subsequently, 6-μm sections were cut and IHC performed as per the BD Pharmingen technical booklet. For the primary antibodies, both the CD4 clone L3T4 (BD Pharmingen) and CD8 clone 53-6.7 (BD Pharmingen) were added at 1:70 dilution in 0.1 M PBS, 50 μl was added to each test section, a negative control section was incubated with an isotype control. Binding of the primary antibodies to CD4 or CD8 T cells was detected using the BD Pharmingen anti-rat Ig HRP Detection kit. Subsequently H&E and IHC slides were examined on a Nikon Microphot-FXA microscope (Nikon, Melville, NY, USA), and digital images examined using the Image-Pro Plus V3.0.1 for Windows software (Media Cybernetics, Silver Spring, MD, USA).
Statistics
Statistical significance was calculated using Student’s t test. A p value less than 0.05 was considered significant. All experiments were performed at least twice with reproducible results.
Results
Lm-LLO-E7 efficacy requires tumor sensitivity to IFN-γ
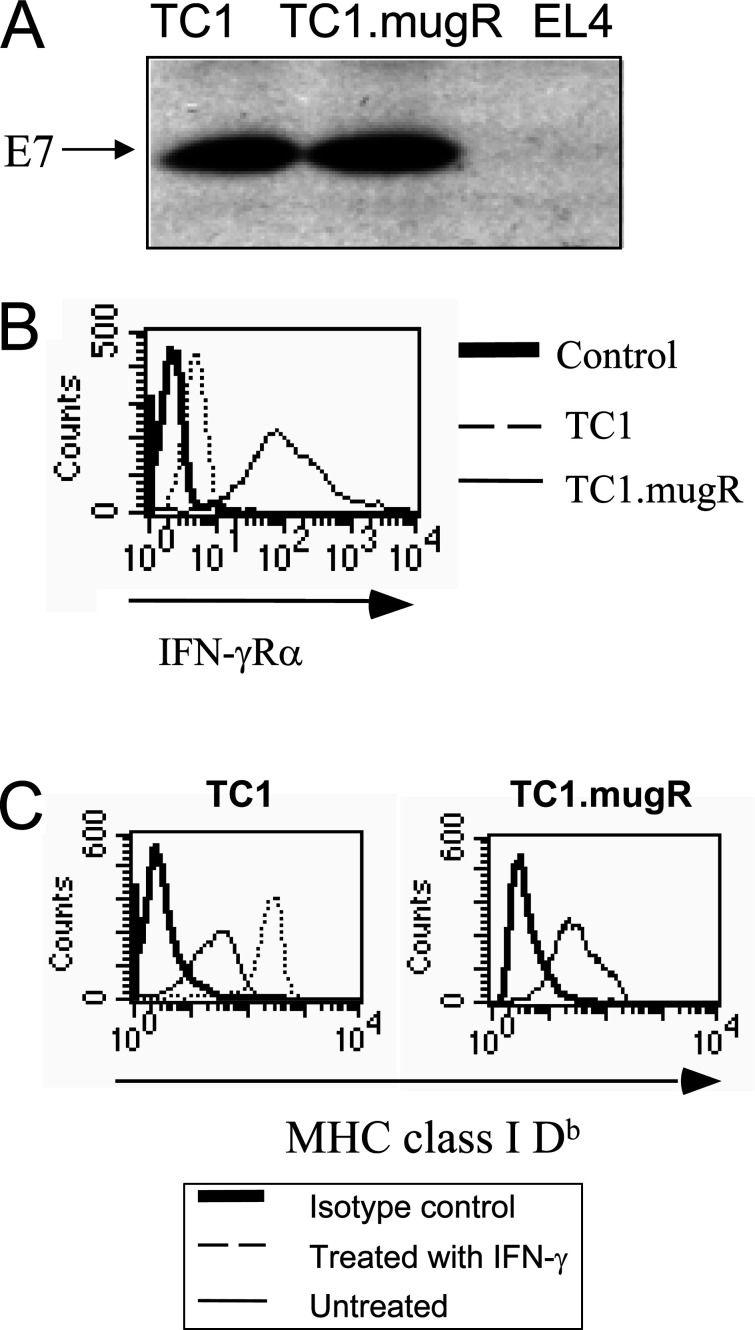
To investigate the role of tumor cell responsiveness to IFN-γ on the therapeutic efficacy of Lm-LLO-E7, TC1 cells were transfected to express high levels of a dominant negative mutant IFN-γRα that is incapable of signaling. A transfected clone called TC1.mugR that displayed equivalent levels of the E7 tumor antigen (Fig. 1A) was selected and analyzed for increased expression of IFN-γRα. As shown in Fig. 1B, flow cytometric analysis revealed that TC1.mugR displayed increased surface levels of IFN-γRα. The unresponsiveness of TC1.mugR to IFN-γ was demonstrated by analyzing surface expression of MHC class I Db. As shown in Fig. 1C, IFN-γ enhances the expression of MHC class I Db expression on the surface of TC1 but not TC1.mugR tumor cells grown in vitro. Therefore, TC1.mugR represents a variant of TC1 that is unresponsive to IFN-γ.
Fig. 1.
TC1.mugR overexpresses IFN-γRα and is unresponsive to IFN-γ. A TC1, TC1.mugR, and EL4 (negative control) tumor cells were assessed by Western blot for expression of E7 protein. B TC1 (dashed line) and TC1.mugR (thin line) were stained for expression of IFN-γRα cells with primary monoclonal rat antimouse IFNγRα antibody and secondary goat anti-rat IgG FITC-conjugated antibody. The control histogram (thick line) represents overlapping histograms of the TC1 and TC1.mugR treated just with the secondary goat anti-rat IgG FITC-conjugated antibody. C TC1 and TC1.mugR were cultured with media alone or 1,000 U/ml IFN-γ for 3 days and, subsequently, analyzed for expression of MHC class I Db expression by flow cytometry. Thick lines represent tumors stained with goat antimouse IgG FITC antibody alone. Thin lines represent tumors stained with 28-14-8S (anti-Db) antibody and secondary goat antimouse IgG FITC antibody. Dotted lines represent tumors incubated with INF-γ and stained with 28-14-8S (anti-Db) antibody and secondary goat antimouse IgG FITC antibody. The histogram for TC1.mugR treated with IFN-γ completely overlaps the one for untreated TC1.mugR
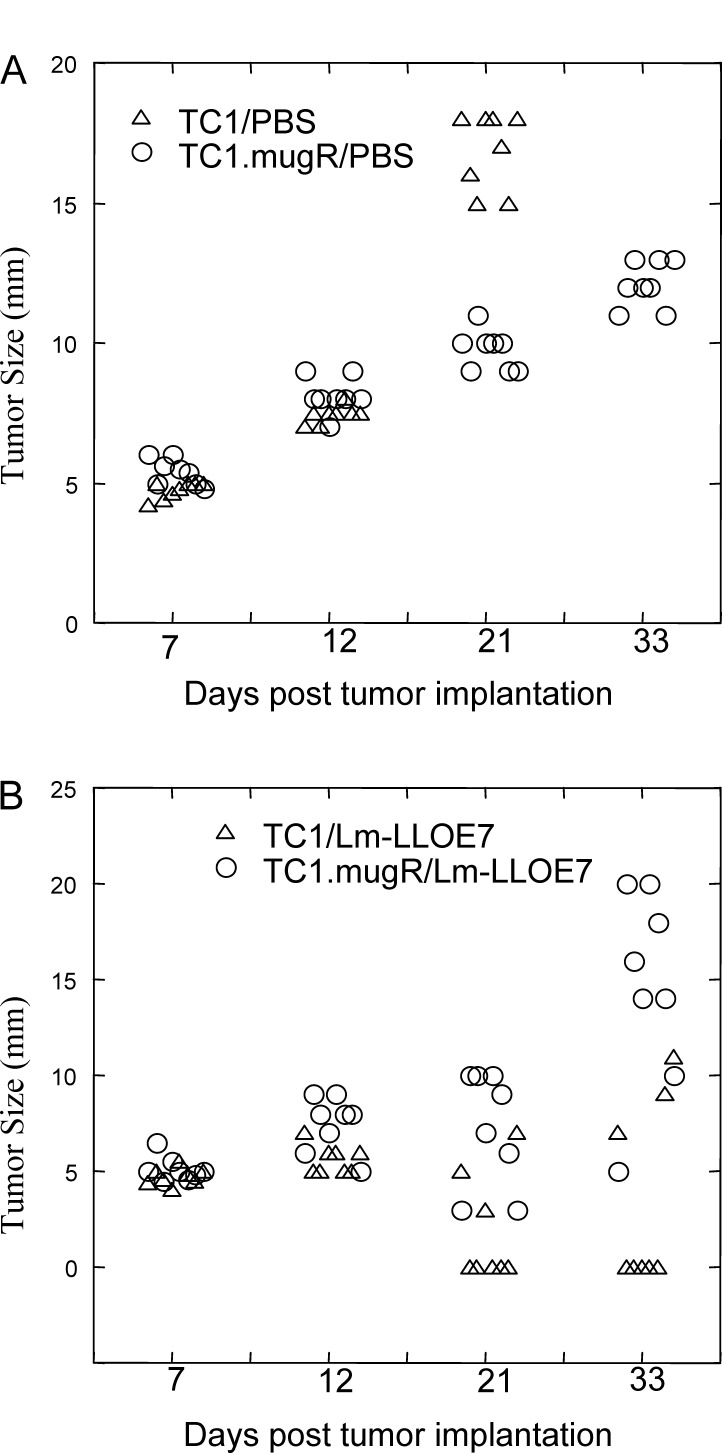
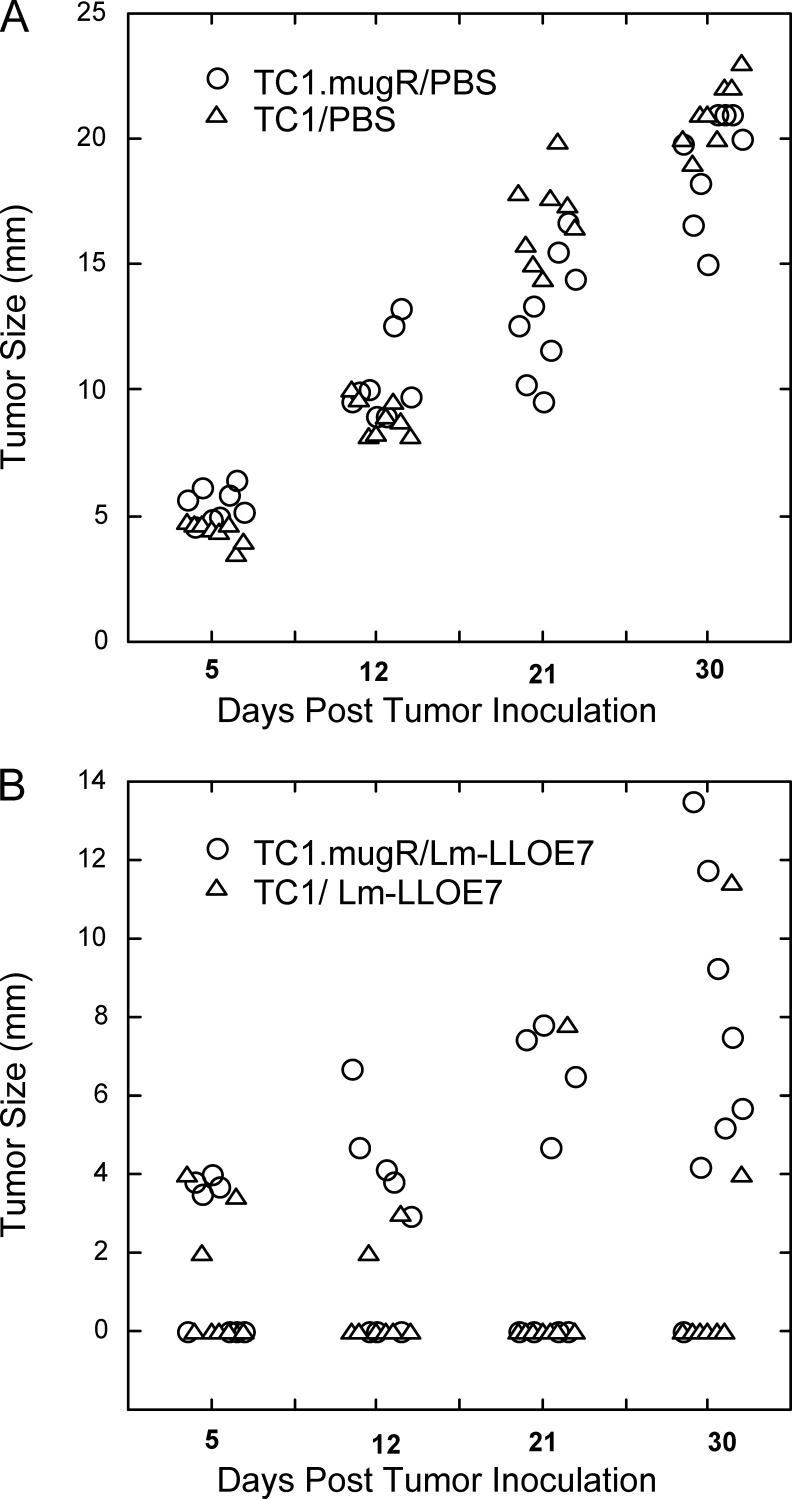
We next examined the ability of Lm-LLO-E7 to impact on TC1.mugR tumor growth. TC1 and TC1.mugR tumor cells were implanted subcutaneously in C57BL/6 mice. On days 7 and 14, mice were injected intraperitoneally with Lm-LLO-E7 or PBS. As shown in Fig. 2, Lm-LLO-E7 induced regression of TC1 tumors, with 5/8 mice tumor-free compared with 0/8 tumor-free in the mice that received PBS. But, although the expression of the mugR gene slows the growth of TC1 (Fig. 2A; p<0.001, compared with the TC1/PBS group), Lm-LLO-E7 had little impact on the growth of TC1.mugR tumors (Fig. 2B; p<0.25, on day 33 compared with the TC1.mugR/PBS group). The significantly enhanced antitumor efficacy of Lm-LLO-E7 against TC1 tumors compared with TC1.mugR tumors (p<0.001, on day 33) demonstrates the importance of TC1 responsiveness to IFN-γ for the efficacy of Lm-LLO-E7 therapy.
Fig. 2.
Inhibition of TC1 tumor growth by Lm-LLO-E7 requires TC1 responsiveness to IFN-γ. TC1 (triangles) or TC1.mugR (circles) (1×105) tumor cells were injected subcutaneously into C57BL/6 mice. On day 7 and 14 following tumor challenge when measurable tumors had grown, mice were injected i.p. with phosphate-buffered saline (PBS) (A) or Lm-LLO-E7 (B)
Immunization with Lm-LLO-E7 increases surface expression of MHC class I Db on TC1 by an IFN-γ–dependent mechanism
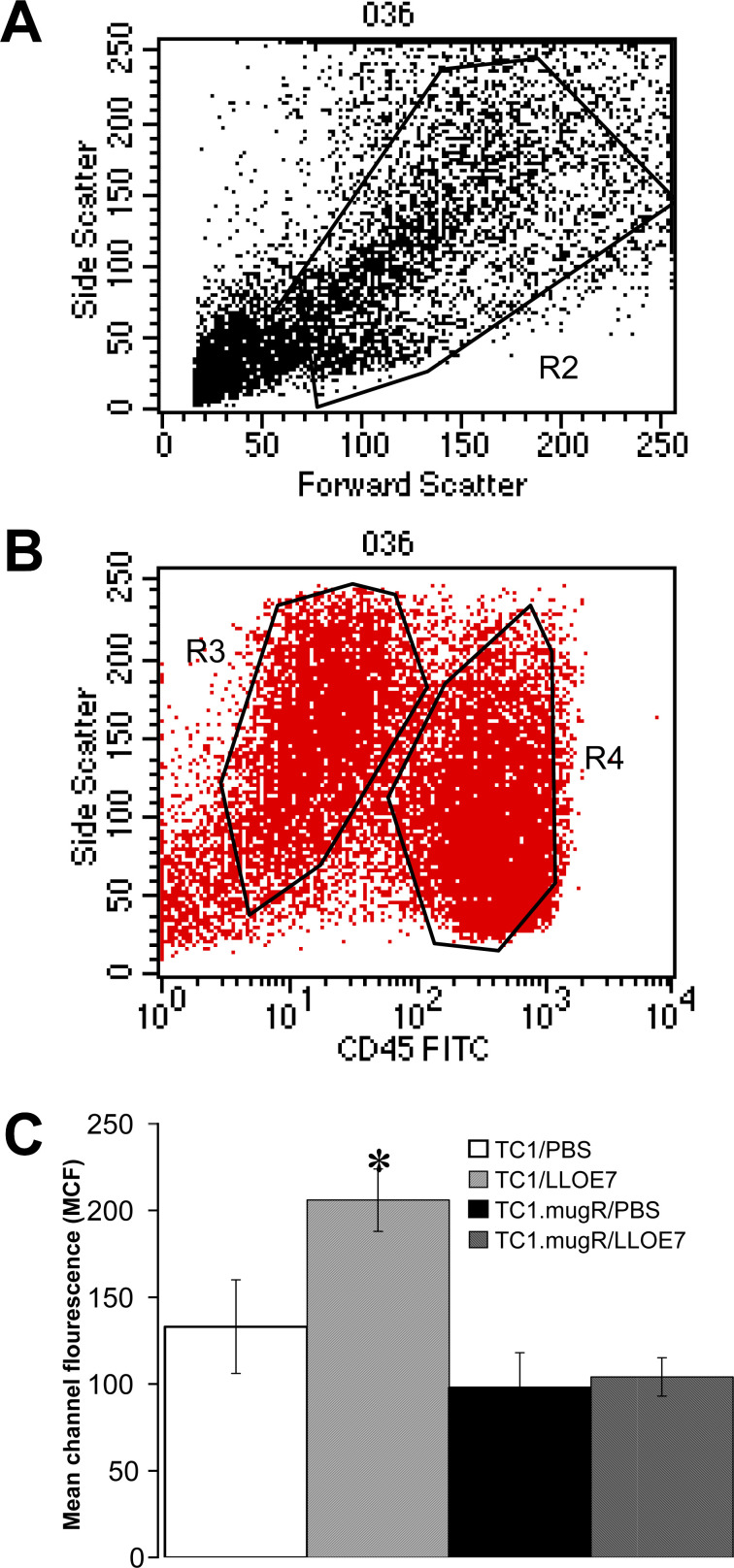
Since we had shown that MHC class I antigen is up-regulated on the surface of TC1 cells in response to IFN-γ in vitro (Fig. 1), a simple explanation for the inability of Lm-LLO-E7 to impact on TC1.mugR tumor growth could be that decreased MHC class I antigen reduces the tumor cells’ susceptibility to killing by cytotoxic T lymphocytes. We thus sought to determine if MHC class I antigen is also up-regulated on TC1 tumor cells in vivo. Lymphocytes and TC1 tumor cells were differentiated by the presence of CD45 on their surface and their side scatter profiles (Fig. 3A, B). TC1 tumor cells have low levels of CD45 and more side scatter, and lymphocytes have higher levels of CD45 and less side scatter. In Fig. 3C, we show that class I antigen is significantly up-regulated on the surface of TC1 tumor cells from mice immunized with Lm-LLO-E7 compared with TC1 from unimmunized mice (p<0.05) but not on the surface of TC1.mugR tumor cells compared with TC1.mugR from immunized mice (p<0.5). The levels of class I antigen on TC1.mugR tumor cells remained constant despite immunization. This suggests that up-regulation of MHC I in vivo on TC1 is dependent on IFN-γ induced by vaccination with Lm-LLO-E7.
Fig. 3.
Immunization with Lm-LLO-E7 increases surface expression of MHC class I Db in TC1 by an IFN-γ–dependent mechanism. C57BL/6 mice were injected subcutaneously with Matrigel containing 1×105 TC1 or TC1.mugR tumor cells. After 7 days, mice were injected i.p. with PBS or Lm-LLO-E7. After 5 days, Matrigel plugs were harvested, the surrounding tissue was digested with DNaseI and collagenase, and single-cell suspensions were made. Cells were stained for CD45 and MHC class I Db. Cells in the live lymphocyte gate (A) were further gated using CD45 and side scatter into a CD45high (gate R4) or CD45lo (gate R3) population (B). In C, percentages represent the mean channel fluorescence ± SEM for class I Db staining on CD45lo tumor cells; asterisk paired measurements that are statistically different as determined by Student’s t test.
The impact of IFN-γ on TC1 immunogenicity is minimal
The up-regulation of MHC class I on TC1 cells in response to IFN-γ, in vitro and in vivo, suggests that TC1 may become better targets for CD8+ T-cell activity in the presence of IFN-γ. However, previous work has demonstrated that tumor responsiveness to IFN-γ can either increase [19] or decrease tumor immunogenicity [20]. We thus treated TC1 cells with IFN-γ and assessed them for their ability to be recognized by E7-specific T cells. As shown in Table 1, TC1 cells are efficiently recognized by E7-specific CD8+ T cells that secrete IFN-γ upon activation. Treatment of TC1 cells with IFN-γ only modestly enhanced this recognition as assessed by an increase in the number of E7-specific CD8+ T cells secreting IFN-γ. In addition, since these measurements have been performed using TC1 treated in vitro at levels of IFN-γ that may not be reached in vivo, it is unlikely that the low level of IFN-γ enhancement of TC1 immunogenicity makes a significant contribution to the efficacy of Lm-LLO-E7.
Table 1.
IFN-γ enhances tumor recogntion in vitro. Percentage of CD8+CD62Llo cells secreting IFN-γ. E7-specific splenocytes from C57BL/6 mice were harvested and cultured in vitro with 1 μM E7 peptide. After 10 days, cells were restimulated for 7 h in the presence of brefeldin A with TC1, or TC1 treated for 3 days with 1,000 U/ml IFN-γ. The levels of intracellular IFN-γ expressed by CD8+CD62Llo cells were quantified by intracellular cytokine staining; 10,000 events were collected
| Control groupa | TC1 target cells (%) | TC1+IFN-γ (%) |
|---|---|---|
| Experiment 1 (0.24%) | 5.7 | 8.0 |
| Experiment 2 (0.44%) | 5.4 | 6.8 |
aIn experiment 1, CT26 was used instead of TC1 for antigen simulation as a control for MHC specificity. In experiment 2, the ability of splenocytes harvested from animals immunized with Lm-Gag instead of Lm-E7, to produce IFN-γ when incubated with TC1, was included as an immunogen specificity control
Lm-LLO-E7 inhibition of tumor angiogenesis is independent of tumor sensitivity to IFN-γ
Tumor responsiveness to IFN-γ has been shown to be required for the effectiveness of IL-12 antiangiogenesis therapy [21–23]. IL-12 is unable to inhibit tumor angiogenesis stimulated in a homogenous population of IFN-γ–insensitive tumor cells [24]. IL-12 production is a hallmark of early listerial responses and we have recently shown that dendritic cells infected with Lm-LLO-E7 secrete this cytokine [25]. Therefore, we investigated whether the ability of Lm-LLO-E7 to inhibit angiogenesis differed between TC1 and TC1.mugR tumors.
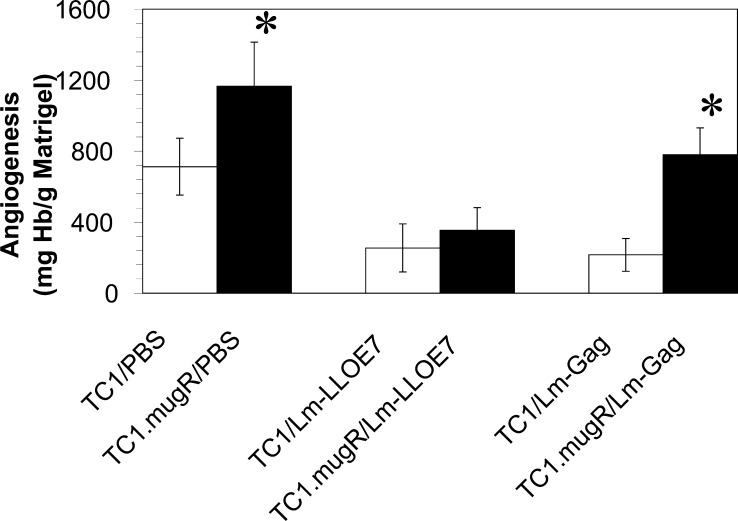
We used in vivo Matrigel assays to measure the ability of Lm-LLO-E7 and Lm-Gag to inhibit tumor angiogenesis in TC1 and TC1.mugR. Lm-Gag immunization has been shown to have a small but significant ability to slow the growth of TC1, but not TC1.mugR, in a non-antigen-specific manner ([6]; and data not shown). Using hemoglobin content as an index of Matrigel vascularization, tumor cells were mixed with Matrigel matrix and subcutaneously implanted into C57BL/6 mice. Mice were immunized on day 7 with Lm-LLO-E7, Lm-Gag, or PBS. As shown in Fig. 4, a significantly reduced level of vascularization was found in TC1 tumors of mice treated with either Lm-LLO-E7 (p<0.0005) or Lm-Gag (p<0.005) compared with the PBS-treated group. Lm-LLO-E7 inhibited angiogenesis in both TC1 (p<0.0005) and TC1.mugR tumors (p<0.001) with no significant difference between these groups (p<0.4) and therefore, this effect was not dependent on sensitivity to IFN-γ (Fig. 4). However, the ability of Lm-Gag to inhibit tumor angiogenesis was dependent on sensitivity to IFN-γ, as Lm-Gag was unable to inhibit angiogenesis in TC1.mugR but was in TC1 (p<0.05) (Fig. 4). Therefore, although tumor responsiveness to IFN-γ was required for the ability of Lm-Gag to inhibit tumor angiogenesis, Lm-LLO-E7 was equally effective in inhibiting tumor angiogenesis of either IFN-γ–sensitive or IFN-γ–insensitive tumor cells. These results suggest that Lm-LLO-E7 is inducing inhibition of angiogenesis in an antigen-dependent manner independent of an IFN-γ–mediated mechanism.
Fig. 4.
Inhibition of tumor angiogenesis by Lm-LLO-E7 does not require tumor responsiveness to IFN-γ. Naïve C57BL/6 mice were subcutaneously injected with Matrigel containing 1×105 TC1 or 1×105 TC1.mugR tumor cells. On day 7, mice were injected intraperitoneally with either PBS, Lm-LLO-E7, or Lm-Gag. Seven days after treatment, Matrigel plugs were harvested and angiogenesis was quantified by determining the hemoglobin content of individual pellets. Data shown represent the mean ± SEM; asterisks paired measurements that are statistically different as determined by Student’s t test.
Tumor sensitivity to IFN-γ is required for lymphocyte infiltration into tumors
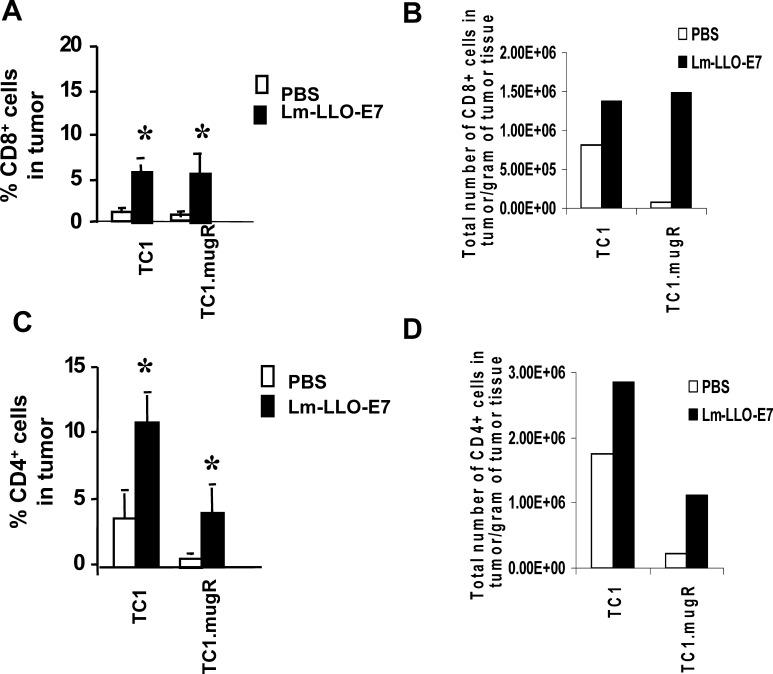
Despite the fact that Lm-LLO-E7 can inhibit tumor angiogenesis of TC1.mugR, this vaccine has little impact on the growth of TC1.mugR. This suggests that another mechanism is important in the different susceptibilities of TC1 and TC1.mugR to Lm-LLO-E7 immunization. Since IFN-γ has also been implicated in regulating the migratory capacity of T cells into the tumor mass [26, 27], we assessed the ability of T cells induced by Lm-LLO-E7 to traffic to TC1 and to TC1.mugR tumors. Using in vivo Matrigel assays and FACS as a quantitative measure of T-cell trafficking to the tumor, we found that Lm-LLO-E7 is effective in enhancing both CD8+ and CD4+ T-cell trafficking to the tumors of mice treated with Lm-LLO-E7 compared with PBS (Fig. 5A, B, respectively; p<0.03). There was no significant difference (p<0.9) between the number of CD8+ T cells in TC1 tumors compared with TC1.mugR tumors in Lm-LLO-E7–immunized mice when expressed either as the mean percentage of live cells (Fig. 5A) or as the number of CD8+ T cells per gram of tumor (Fig. 5B). However, far more CD4+ T cells were found in TC1 tumors compared with TC1.mugR tumors in mice immunized with Lm-LLO-E7 (Fig. 5B, D; p<0.003).
Fig. 5.
Lm-LLO-E7 enhances T-lymphocyte trafficking to both IFN-γ–sensitive and IFN-γ–insensitive tumors. Naïve C57BL/6 mice were injected subcutaneously with Matrigel containing 1×105 TC1 or 1×105 TC1.mugR tumor cells. On day 7, mice were injected i.p. with either PBS or Lm-LLO-E7. Seven days after treatment, Matrigel plugs were harvested and weighed. Single-cell suspensions were prepared, and the number of live cells per gram of tissue determined by trypan blue exclusion. The presence of CD8+ T cells (A, B) and CD4+ T cells (C, D) was analyzed by flow cytometry. The number of CD8+ and CD4+ T cells per gram of tissue was calculated by multiplying the cell population percentage for CD8+ or CD4+ cells by the determined number of live cells per gram of tissue. The presence of CD8+ T cells (a) and CD4+ (B) T cells were analyzed by flow cytometry. Data are mean percentage of live cells ± SEM (A, C) and number of live cells per gram of tissue (C, D); asterisks paired measurements that are statistically different as determined by Student’s t test.
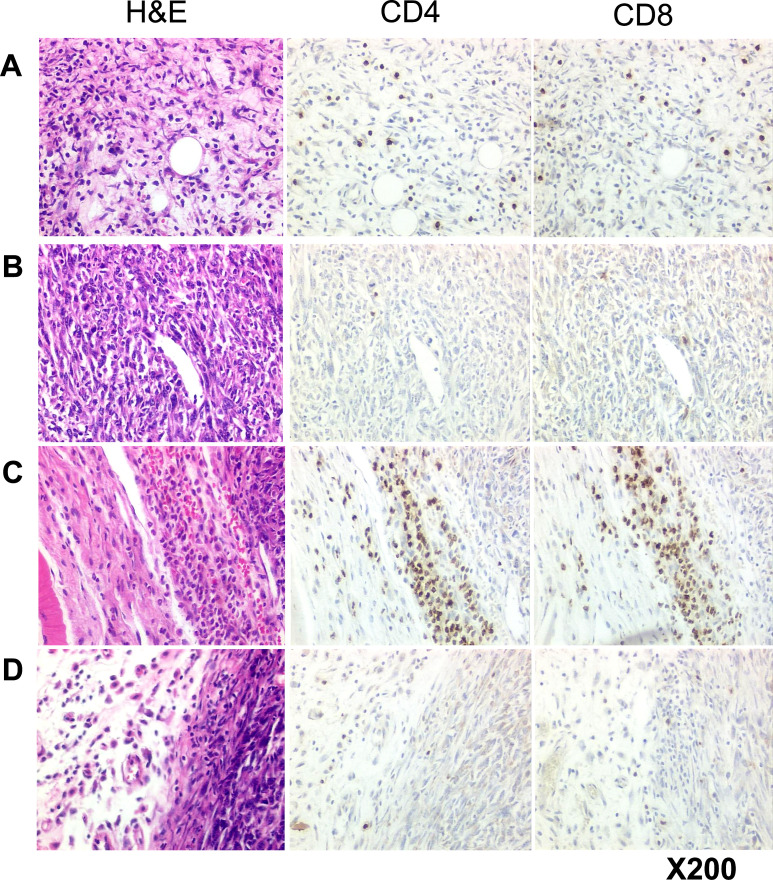
To examine the relative distribution of the CD4+ and CD8+ T cells we performed histology and IHC on TC1 and TC1.mugR tumors explanted from mice 6 days after immunization with 0.1 LD50 of Lm-LLO-E7. In addition, a subgroup of mice received anti-IFN-γ antibody to neutralize the cytokine activity (see “Materials and methods” for details). The results show that by day 13, the TC1 tumors were already undergoing substantial cell death with replacement of tumor tissue by connective tissue stroma and infiltration of the tumor with numerous CD4+ and CD8+ T cells (Fig. 6A). When the TC1 tumor margins were examined, there was evidence of a diffuse infiltrate of CD4+ and CD8+ T cells (data not shown). In contrast, TC1.mugR tumors remained unaffected by the Lm-LLO-E7 immunization and retained features of an actively growing tumor with numerous mitotic figures, little evidence of cell death, and very few infiltrating CD4+ or CD8+ T cells (Fig. 6C). When the TC1.mugR tumor margins were examined, aggregates of CD4+ and CD8+ T cells were abundantly present in the tumor connective tissue capsule (Fig. 6C). Administration of anti-IFN-γ antibody to Lm-LLO-E7–immunized mice reduced the numbers of CD4+ and CD8+ T cells in both the TC1 tumor (Fig. 6B) and the TC1 tumor margin (data not shown). In addition, the TC1 tumors displayed features of active growth and no evidence of disruption to tumor architecture resulting from immune attack. Furthermore, the TC1.mugR tumors that were explanted from mice after the combination of anti-IFN-γ antibody and Lm-LLO-E7 immunization showed no evidence of CD4+ or CD8+ T cells at the tumor margin or inside the tumor (Fig. 6D). Overall, these findings indicate a role for stromal cell sensitivity to IFN-γ in promoting the accumulation of T cells at the tumor margin and of tumor sensitivity to IFN-γ in regulating the ability of these cells to penetrate the tumor.
Fig. 6.
Responsiveness to IFN-γ is required for T-lymphocyte infiltration into the tumor mass. Naïve C57BL/6 mice (n=24) were injected subcutaneously with 1×105 TC1 or 1×106 TC1.mugR tumor cells; half of these mice received the tumor cells mixed in Matrigel and half received the tumor alone. Subsequently, on days 6, 7, 8, and 11, anti-IFN-γ antibody (1.2 mg/mouse/day) was administered to half of the mice. In addition on day 7, all mice were injected i.p. with 0.1 LD50 Lm-LLO-E7, and 6 days later all tumors were removed, and histology and IHC performed as described. Results presented are representative digital images of the tumors including A TC1 tumor, B TC1 tumor plus anti-IFN-γ, C TC1.mugR tumor margin, D TC1.mugR tumor margin plus anti-IFN-γ. No difference in lymphocytic infiltration was observed between tumor samples mixed with either Matrigel or tumor alone within any experimental group.
Lm-LLO-E7 does not induce regression of small TC1.mugR tumors
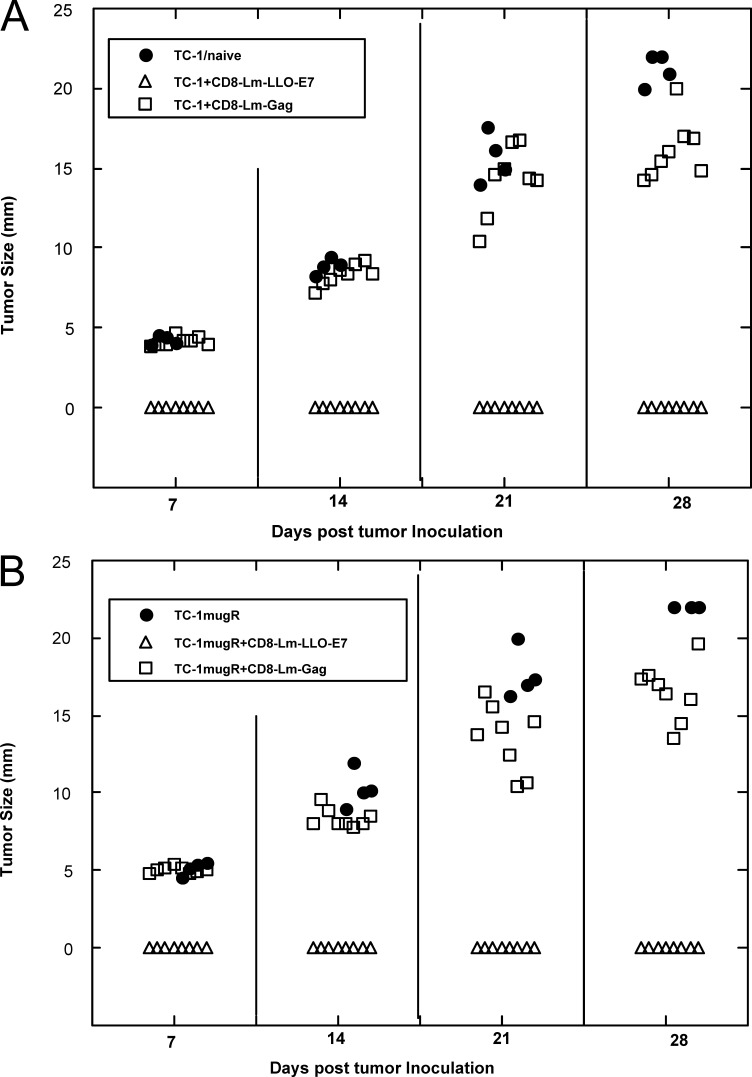
Since there appeared to be a difference between the ability of lymphocytes to penetrate the tumor mass of TC1 compared with TC1.mugR grown in vivo, we wanted to determine whether this was simply due to the tumors presenting a physical barrier to the lymphocytes. Thus, instead of treating established 5–7-mm tumors, we immunized mice 2 days after tumor implantation before the tumors had grown to a palpable size (Fig. 7). The data we obtained were very similar to those shown in Fig. 2, in that 5/8 of the mice bearing TC1 tumors became tumor-free upon immunization with Lm-LLO-E7 but only 1/8 of TC1.mugR-bearing mice became tumor-free (p< 0.01, on day 30). However, if we mixed tumor cells with CD8+ T cells isolated from mice immunized with Lm-LLO-E7 and then implanted this mixture, neither TC1 nor TC1.mugR tumors grew (Fig. 8), indicating that the failure of Lm-LLO-E7 to control tumor growth of TC1.mugR was unrelated to the ability of Lm-LLO-E7–induced CD8+ T cells to recognize the tumor. The ability of Lm-LLO-E7–specific T cells to control tumor growth was antigen specific since a similar number of Lm-Gag–specific CD8+ T cells had no impact on the growth of TC1 or TC1.mugR. Figure 8 also confirms that the up-regulation of MHC class I on TC1 cells compared with TC1.mugR cells (Figs. 1 and 3, and Table 1) does not render them more susceptible to CD8+ T-cell–mediated tumor suppression in vivo. Taken together, these data suggest that differences in the ability of the lymphocytes induced by Lm-LLO-E7 to penetrate the tumor mass of TC1 but not TC1.mugR is not dependent on the size of the tumor but may be due to differences in a chemotactic gradient induced in the tumors mediated by IFN-γ.
Fig. 7.
Lm-LLOE7 treatment does not induce regression of small TC1.mugR tumors. TC1 (triangles) or TC1.mugR (circles) (1×106) tumor cells were injected subcutaneously into C57BL/6 mice. On days 2 and 9 following tumor challenge before measurable tumors had grown, mice were injected i.p. with phosphate-buffered saline (PBS) (A) or Lm-LLO-E7 (B).
Fig. 8.
CD8+ T cells from Lm-LLO-E7–immunized mice will protect C57BL/6 mice from tumor challenge with TC1 or TC1.mugR. CD8+ T cells were purified from mice immunized with either Lm-LLO-E7 (open triangles) or Lm-Gag (open squares) as described in “Materials and methods.” These were mixed with 105 TC1 tumor cells (A) or 106 TC1.mugR tumor cells (B), at a ratio of one tumor cell to five purified CD8 T cells and inoculated s.c. in the right flank of C57BL/6 mice. Tumor growth was monitored with calipers as described.
Discussion
We have used a recombinant L. monocytogenes expressing the HPV-16 E7 protein, and two TC1 tumors that differ in their ability to be signaled by IFN-γ, to investigate the role of IFN-γ in inducing the regression of TC1. In this study, we report that the ability of Lm-LLO-E7 to induce regression of established TC1 tumors in mice is dependent on tumor sensitivity to IFN-γ. We have considered three possible mechanisms to account for these findings. First, we examined the role of MHC class I expression by the tumor. Many groups have shown that IFN-γ induces up-regulation of MHC class I antigen on tumor cells [28–30] sometimes through the restoration of the MHC class I antigen-processing machinery [31, 32]. In addition, up-regulation of MHC class I antigen has been shown to increase susceptibility of tumor cells to CTL killing [33]. However, we found that, although MHC class I expression can be up-regulated on TC1 but not TC1.mugR in vitro by rIFN-γ (Fig. 1c) and in vivo by Lm-LLO-E7 immunization (Fig. 3), this results in only a modest increase in the immunogenicity of the tumor (Table 1) that does not appear to translate into differences between the ability of CD8+ T cells to control TC1 versus TC1.mugR growth in vivo (Fig. 8). Thus increased MHC class I expression is unlikely to explain the profound differences that Lm-LLO-E7 immunization has on TC1 versus TC1.mugR tumor growth.
Previous studies have highlighted the importance of tumor sensitivity to IFN-γ for the effectiveness of various forms of therapy to inhibit tumor angiogenesis [19, 23, 33]. In contrast, it has also been reported that inhibition of tumor angiogenesis can require IFN-γR expression on nonhematopoietic cells but not tumor cells [34]. We have found that the nonspecific adjuvant effect of L. monocytogenes reported previously might involve inhibition of tumor angiogenesis. In the B16F10 melanoma, L. monocytogenes can significantly slow tumor growth [9]. This observation correlates with intratumoral expression of mRNA for many proinflammatory cytokines including IL-12 and IFN. We thus considered the possibility that Lm-LLO-E7–induced IFN-γ could slow TC1 tumor growth by an angiostatic mechanism. However, we found that, although tumor responsiveness to IFN-γ was required for the ability of the control vector, Lm-Gag, to inhibit tumor angiogenesis, Lm-LLO-E7 was equally effective in inhibiting tumor angiogenesis stimulated by either IFN-γ–sensitive or IFN-γ–insensitive tumor cells (Fig. 4). This observation suggests an alternative, IFN-γ-independent mechanism for suppression of tumor angiogenesis, mediated by the induction of E7-specific T cells.
In a final attempt to explain the mechanism by which Lm-LLO-E7 could control TC1, but not TC1.mugR, tumor growth we examined the ability of the vaccine to induce CD8+ and CD4+ T cells that trafficked to and penetrated either tumor. We found that the inability of Lm-LLO-E7 to impact on the growth of established IFN-γ–insensitive TC1.mugR tumors does not appear to be due to an inability of lymphocytes to home to the tumor, since both CD8+ and CD4+ T cells accumulate at the tumor edge of TC1.mugR (Fig. 6C). However, the ability of T cells to infiltrate the tumor tissue does require tumor sensitivity to IFN-γ Fig. 6A. Interestingly, the depletion of systemic IFN-γ at the time of immunization with Lm-LLO-E7 abrogated both the accumulation of T cells at the tumor edge of TC1.mugR (Fig. 6D) and their infiltration into TC1 (Fig. 6B). This is not likely to be due to IFN-γ influencing the immunogenicity of Lm-LLO-E7, because it has been well established that L. monocytogenes mounts robust T-cell responses in the absence of IFN-γ [35–37]. Instead, we believe that IFN-γ may act on stromal cells surrounding the tumor, stimulating the release of chemokines that encourage T-cell trafficking to TC1 and TC1.mugR. In the case of TC1, the lymphocytes do not accumulate at the tumor edge, suggesting that the penetration of lymphocytes into IFN-γ–responsive tumors is due to an active chemoattraction mediated by IFN-γ–induced chemokines secreted by tumor cells. This hypothesis is supported by our findings that even when animals were immunized at earlier time points, prior to the establishment of a tumor mass, IFN-γ–insensitive tumors did not respond to immunization with Lm-LLO-E7 (Fig. 7), but that when CD8+ T cells were mixed with the tumor they could control tumor growth (Fig. 8).
Several groups have reported that the chemokines Mig and IP-10, which are up-regulated by IFN-γ, are associated with tumor regression [38–40]. However, downstream effects of IFN-γ may also act cooperatively to induce infiltration of lymphocytes into the tumor. The mechanism by which IFN-γ facilitates the migration of CD4+ and CD8+ T cells into the tumor mass, clearly warrants further intensive study.
In summary, unresponsiveness to IFN-γ likely poses a significant barrier to the immunotherapy of many tumors. It will be important to delineate the specific mechanisms involved in stimulating lymphocyte tumor infiltration so that immunotherapeutic agents can be designed that overcome IFN-γ unresponsiveness and induce infiltration of lymphocytes into the tumor.
Acknowledgements
This work was supported by NIH grant CA69632 and ACS grant TURSG LIB-101399 (Y.P.), and NIH training grant T32CA09140 (G.L.B). We would like to thank Dr S. Farzana Hussain for critically reading this manuscript.
Abbreviations
- BHI
Brain heart infusion
- CMV
Cytomegalovirus
- CTL
Cytotoxic T lymphocyte
- Hb
Hemoglobin
- HPV
Human papillomavirus
- IFN-γ
Interferon-gamma
- IL-12
Interleukin-12
- LLO
Listeriolysin O
- Lm
Listeria monocytogenes
- MHC
Major histocompatibility class
- MME
Metalloelastase
- NK
Natural killer
- PBS
Phosphate-buffered saline
Footnotes
Mary E. Dominiecki and Gregory L. Beatty contributed equally to this work.
References
- 1.Bosch FX, Manos MM, Munoz N, Sherman M, Jansen AM, Peto J, Schiffman MH, Moreno V, Kurman R, Shah KV. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective: international biological study on cervical cancer (IBSCC) study group. J Natl Cancer Inst. 1995;87:796. doi: 10.1093/jnci/87.11.796. [DOI] [PubMed] [Google Scholar]
- 2.Mansur CP, Androphy EJ. Cellular transformation by papillomavirus oncoproteins. Biochim Biophys Acta. 1993;1155:323. doi: 10.1016/0304-419X(93)90013-3. [DOI] [PubMed] [Google Scholar]
- 3.Wu TC. Immunology of the human papilloma virus in relation to cancer. Curr Opin Immunol. 1994;6:746. doi: 10.1016/0952-7915(94)90079-5. [DOI] [PubMed] [Google Scholar]
- 4.Lin KY, Guarnieri FG, Staveley-O’Carroll KF, Levitsky HI, August JT, Pardoll DM, Wu TC. Treatment of established tumors with a novel vaccine that enhances major histocompatibility class II presentation of tumor antigen. Cancer Res. 1996;56:21. [PubMed] [Google Scholar]
- 5.Ji H, Chang EY, Lin KY, Kurman RJ, Pardoll DM, Wu TC. Antigen-specific immunotherapy for murine lung metastatic tumors expressing human papillomavirus type 16 E7 oncoprotein. Int J Cancer. 1998;78:41. doi: 10.1002/(sici)1097-0215(19980925)78:1<41::aid-ijc8>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 6.Gunn GR, Zubair A, Peters C, Pan ZK, Wu TC, Paterson Y. Two Listeria monocytogenes vaccine vectors that express different molecular forms of human papilloma virus-16 (HPV-16) E7 induce qualitatively different T cell immunity that correlates with their ability to induce regression of established tumors immortalized by HPV-16. J Immunol. 2001;167:6471. doi: 10.4049/jimmunol.167.11.6471. [DOI] [PubMed] [Google Scholar]
- 7.Pan ZK, Ikonomidis G, Pardoll D, Paterson Y. Regression of established tumors in mice mediated by the oral administration of a recombinant Listeria monocytogenes vaccine. Cancer Res. 1995;55:4776. [PubMed] [Google Scholar]
- 8.Pan ZK, Ikonomidis G, Lazenby A, Pardoll D, Paterson Y. A recombinant Listeria monocytogenes vaccine expressing a model tumour antigen protects mice against lethal tumour cell challenge and causes regression of established tumours. Nat Med. 1995;1:471. doi: 10.1038/nm0595-471. [DOI] [PubMed] [Google Scholar]
- 9.Pan ZK, Weiskirch LM, Paterson Y. Regression of established B16F10 melanoma with a recombinant Listeria monocytogenes vaccine. Cancer Res. 1999;59:5264. [PubMed] [Google Scholar]
- 10.Woodworth CD, Lichti U, Simpson S, Evans CH, DiPaolo JA. Leukoregulin and gamma-interferon inhibit human papillomavirus type 16 gene transcription in human papillomavirus-immortalized human cervical cells. Cancer Res. 1992;52:456. [PubMed] [Google Scholar]
- 11.Kim KY, Blatt L, Taylor MW. The effects of interferon on the expression of human papillomavirus oncogenes. J Gen Virol. 2000;81:695. doi: 10.1099/0022-1317-81-3-695. [DOI] [PubMed] [Google Scholar]
- 12.Tartour E, Gey A, Sastre-Garau X, Lombard Surin I, Mosseri V, Fridman WH. Prognostic value of intratumoral interferon gamma messenger RNA expression in invasive cervical carcinomas. J Natl Cancer Inst. 1998;90:287. doi: 10.1093/jnci/90.4.287. [DOI] [PubMed] [Google Scholar]
- 13.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 14.Feltkamp MC, Smits HL, Vierboom MP, Minnaar RP, de Jongh BM, Drijfhout JW, ter Schegget J, Melief CJ, Kast WM. Vaccination with cytotoxic T lymphocyte epitope-containing peptide protects against a tumor induced by human papillomavirus type 16-transformed cells. Eur J Immunol. 1993;23:2242. doi: 10.1002/eji.1830230929. [DOI] [PubMed] [Google Scholar]
- 15.Mata M, Yao ZJ, Zubair A, Syres K, Paterson Y. Evaluation of a recombinant Listeria monocytogenes expressing an HIV protein that protects mice against viral challenge. Vaccine. 2001;19:1435. doi: 10.1016/S0264-410X(00)00379-0. [DOI] [PubMed] [Google Scholar]
- 16.Kleinman HK, McGarvey ML, Liotta LA, Robey PG, Tryggvason K, Martin GR. Isolation and characterization of type IV procollagen, laminin, and heparan sulfate proteoglycan from the EHS sarcoma. Biochemistry. 1982;21:6188. doi: 10.1021/bi00267a025. [DOI] [PubMed] [Google Scholar]
- 17.Passaniti A, Taylor RM, Pili R, Guo Y, Long PV, Haney JA, Pauly RR, Grant DS, Martin GR. A simple, quantitative method for assessing angiogenesis and antiangiogenic agents using reconstituted basement membrane, heparin, and fibroblast growth factor. Lab Invest. 1992;67:519. [PubMed] [Google Scholar]
- 18.Winn HJ. In vivo methods for the assessment of antibody-mediate tumor immunity. J Natl Cancer Inst Monogr. 1972;35:13. [PubMed] [Google Scholar]
- 19.Dighe AS, Richards E, Old LJ, Schreiber RD. Enhanced in vivo growth and resistance to rejection of tumor cells expressing dominant negative IFN gamma receptors. Immunity. 1994;1:447. doi: 10.1016/1074-7613(94)90087-6. [DOI] [PubMed] [Google Scholar]
- 20.Beatty GL, Paterson Y. IFN-gamma can promote tumor evasion of the immune system in vivo by down-regulating cellular levels of an endogenous tumor antigen. J Immunol. 2000;165:5502. doi: 10.4049/jimmunol.165.10.5502. [DOI] [PubMed] [Google Scholar]
- 21.Sgadari C, Angiolillo AL, Tosato G. Inhibition of angiogenesis by interleukin-12 is mediated by the interferon-inducible protein 10. Blood. 1996;87:3877. [PubMed] [Google Scholar]
- 22.Majewski S, Marczak M, Szmurlo A, Jablonska S, Bollag W. Interleukin-12 inhibits angiogenesis induced by human tumor cell lines in vivo. J Invest Dermatol. 1996;106:1114. doi: 10.1111/1523-1747.ep12340161. [DOI] [PubMed] [Google Scholar]
- 23.Voest EE, Kenyon BM, O’Reilly MS, Truitt G, D’Amato RJ, Folkman J. Inhibition of angiogenesis in vivo by interleukin 12. J Natl Cancer Inst. 1995;87:581. doi: 10.1093/jnci/87.8.581. [DOI] [PubMed] [Google Scholar]
- 24.Coughlin CM, Salhany KE, Gee MS, LaTemple DC, Kotenko S, Ma X, Gri G, Wysocka M, Kim JE, Liu L, Liao F, Farber JM, Pestka S, Trinchieri G, Lee WM. Tumor cell responses to IFNgamma affect tumorigenicity and response to IL-12 therapy and antiangiogenesis. Immunity. 1998;9:25. doi: 10.1016/S1074-7613(00)80585-3. [DOI] [PubMed] [Google Scholar]
- 25.Peng X, Hussain SF, Paterson Y. The ability of two Listeria monocytogenes vaccines targeting human papillomavirus-16 E7 to induce an antitumor response correlates with myeloid dendritic cell function. J Immunol. 2004;172:6030. doi: 10.4049/jimmunol.172.10.6030. [DOI] [PubMed] [Google Scholar]
- 26.Ogawa M, Tsutsui T, Zou JP, Mu J, Wijesuriya R, Yu WG, Herrmann S, Kubo T, Fujiwara H, Hamaoka T. Enhanced induction of very late antigen 4/lymphocyte function-associated antigen 1-dependent T-cell migration to tumor sites following administration of interleukin 12. Cancer Res. 1997;57:2216. [PubMed] [Google Scholar]
- 27.Ogawa M, Yu WG, Umehara K, Iwasaki M, Wijesuriya R, Tsujimura T, Kubo T, Fujiwara H, Hamaoka T. Multiple roles of interferon-gamma in the mediation of interleukin 12-induced tumor regression. Cancer Res. 1998;58:2426. [PubMed] [Google Scholar]
- 28.Min W, Pober JS, Johnson DR. Kinetically coordinated induction of TAP1 and HLA class I by IFN-gamma: the rapid induction of TAP1 by IFN-gamma is mediated by Stat1 alpha. J Immunol. 1996;156:3174. [PubMed] [Google Scholar]
- 29.Seliger B, Wollscheid U, Momburg F, Blankenstein T, Huber C. Coordinate downregulation of multiple MHC class I antigen processing genes in chemical-induced murine tumor cell lines of distinct origin. Tissue Antigens. 2000;56:327. doi: 10.1034/j.1399-0039.2000.560404.x. [DOI] [PubMed] [Google Scholar]
- 30.Smahel M, Sima P, Ludvikova V, Marinov I, Pokorna D, Vonka V. Immunisation with modified HPV16 E7 genes against mouse oncogenic TC1 cell sublines with downregulated expression of MHC class I molecules. Vaccine. 2003;21:1125. doi: 10.1016/S0264-410X(02)00519-4. [DOI] [PubMed] [Google Scholar]
- 31.Wang Z, Margulies L, Hicklin DJ, Ferrone S. Molecular and functional phenotypes of melanoma cells with abnormalities in HLA class I antigen expression. Tissue Antigens. 1996;47:382. doi: 10.1111/j.1399-0039.1996.tb02573.x. [DOI] [PubMed] [Google Scholar]
- 32.Chatterjee-Kishore M, Kishore R, Hicklin DJ, Marincola FM, Ferrone S. Different requirements for signal transducer and activator of transcription 1alpha and interferon regulatory factor 1 in the regulation of low molecular mass polypeptide 2 and transporter associated with antigen processing 1 gene expression. J Biol Chem. 1998;273:16177. doi: 10.1074/jbc.273.26.16177. [DOI] [PubMed] [Google Scholar]
- 33.Beatty G, Paterson Y. IFN-gamma-dependent inhibition of tumor angiogenesis by tumor-infiltrating CD4+ T cells requires tumor responsiveness to IFN-gamma. J Immunol. 2001;166:2276. doi: 10.4049/jimmunol.166.4.2276. [DOI] [PubMed] [Google Scholar]
- 34.Qin Z, Blankenstein T. CD4+ T cell–mediated tumor rejection involves inhibition of angiogenesis that is dependent on IFN gamma receptor expression by nonhematopoietic cells. Immunity. 2000;12:677. doi: 10.1016/S1074-7613(00)80218-6. [DOI] [PubMed] [Google Scholar]
- 35.Harty JT, Schreiber RD, Bevan MJ. CD8 T cells can protect against an intracellular bacterium in an interferon gamma-independent fashion. Proc Natl Acad Sci U S A. 1992;89:11612. doi: 10.1073/pnas.89.23.11612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harty JT, Bevan MJ. Specific immunity to Listeria monocytogenes in the absence of IFN-gamma. Immunity. 1995;3:109. doi: 10.1016/1074-7613(95)90163-9. [DOI] [PubMed] [Google Scholar]
- 37.Badovinac VP, Harty JT. Adaptive immunity and enhanced CD8+ T cell response to Listeria monocytogenes in the absence of perforin and IFN-gamma. J Immunol. 2000;164:6444. doi: 10.4049/jimmunol.164.12.6444. [DOI] [PubMed] [Google Scholar]
- 38.Bukowski RM, Rayman P, Molto L, Tannenbaum CS, Olencki T, Peereboom D, Tubbs R, McLain D, Budd GT, Griffin T, Novick A, Hamilton TA, Finke J. Interferon-gamma and CXC chemokine induction by interleukin 12 in renal cell carcinoma. Clin Cancer Res. 1999;5:2780. [PubMed] [Google Scholar]
- 39.Kunz M, Toksoy A, Goebeler M, Engelhardt E, Brocker E, Gillitzer R. Strong expression of the lymphoattractant C-X-C chemokine Mig is associated with heavy infiltration of T cells in human malignant melanoma. J Pathol. 1999;189:552. doi: 10.1002/(SICI)1096-9896(199912)189:4<552::AID-PATH469>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 40.Tannenbaum CS, Tubbs R, Armstrong D, Finke JH, Bukowski RM, Hamilton TA. The CXC chemokines IP-10 and Mig are necessary for IL-12-mediated regression of the mouse RENCA tumor. J Immunol. 1998;161:927. [PubMed] [Google Scholar]