Abstract
Standard allogeneic stem cell transplantation (alloSCT) has provided a cure for chronic myeloid leukaemia (CML) over the last 25 years, but is only an option for a minority of patients. It was hoped that the introduction of imatinib mesylate (IM), a specific tyrosine kinase inhibitor that targets the Bcr-Abl oncogene product, would provide long-term remission or even cure for those patients without a donor, but studies have shown that IM does not eliminate leukaemic stem cells in CML patients. To overcome this problem of molecular persistence, research is underway to combine reduced intensity stem cell transplant or non-donor-dependent immunotherapies with IM with the aim of increasing cure rate, reducing toxicity and improving quality of life. The alternative approach is to combine IM or second-generation agents with other novel drugs that interrupt key signalling pathways activated by Bcr-Abl. This article will focus on the latest immunotherapy and molecularly targeted therapeutic options in CML and how they may be combined to improve the outcome for CML patients in the future.
Keywords: Allogeneic stem cell transplantation, Chronic myeloid leukaemia, Cytotoxic T lymphocytes, Dendritic cells, Imatinib, Immunotherapy
Introduction
Chronic myeloid leukaemia (CML) is a clonal disease of stem cell origin which is characterised by the presence of the Philadelphia chromosome (Ph+), which is designated t(9;22)(q34;q11) [78], and its fusion gene product, Bcr-Abl, a constitutively active tyrosine kinase. Bcr-Abl activates a range of intracellular signalling pathways and alters the behaviour of CML stem cells in a number of ways. It increases stem cell turnover of Bcr-Abl+ cells and thereby confers a proliferative advantage over normal haemopoietic stem cells, allowing the malignant clone to suppress and replace normal haemopoiesis [12, 33]. In addition, it has transforming activity both in vitro and in vivo [25], alters cellular adhesion [9] and triggers multiple pathways which block apoptosis [6].
Chronic myeloid leukaemia has three stages: the chronic phase (CP), accelerated phase (AP) and blast crisis (BC). CP is characterised by a leucocytosis due to increased granulopoiesis with hepatosplenomegaly as a result of leukaemic infiltration and can last from several months to several years. In AP there is an increase in the number of immature cells in the bone marrow (BM) or blood, and it may be associated with additional cytogenetic abnormalities (i.e. clonal evolution). BC may be of myeloid or lymphoid lineage and behaves like an acute leukaemia. It carries a very poor prognosis.
Numerous research studies have shown that CML is a disease that is likely to be susceptible to immune attack (as reviewed in [16]). The use of allogeneic stem cell transplantation (alloSCT) and donor lymphocyte infusion (DLI) in the treatment of CML offers not only the possibility of cure, but also demonstrates the effectiveness of immunotherapy in malignant disease. As well as being a paradigm for the use of immunotherapy, with the introduction of the Abl-specific tyrosine kinase inhibitor imatinib mesylate (IM; Glivec, Gleevec, formerly known as STI571), CML has become a model disease for the use of molecularly targeted therapies in malignant disease.
Immunotherapy based on availability of a suitable donor
Despite the advances in understanding the molecular biology of CML and the development of new therapies, in particular IM, only a minority of patients are cured. The first evidence that cancer cells were susceptible to immune attack was discovered in the early 1960s with the animal models used for haemopoietic SCT [63]. It was more than 20 years until this was reproduced in human SCT [37, 56], with the observation that patients who survived alloSCT had a lower relapse rate compared with autologous, syngeneic [38], or T-cell–depleted SCT [41, 54]. The power of the graft-versus-leukaemia (GvL) effect was first demonstrated in CML using steady state unprimed DLI to induce remission following relapse post-alloSCT and in the setting of minimal residual disease (MRD) [59]. The GvL effect relies on major and minor HLA mismatches between donors and recipients for disease eradication [86], although donor T lymphocytes may also recognise and destroy as-yet-unidentified leukaemia-specific antigens [5]. Donor T-cell responses to minor histocompatibility antigen differences may contribute up to 35% of the total GvL response in leukaemic patients [58].
Donor lymphocyte infusion is given in incremental doses with the aim of harnessing the GvL effect. A response to DLI can be expected between 3 and 12 months postinfusion. The success rate of DLI for relapse of CML posttransplant is at least 70% for patients with haematologic relapse and as high as 90% in patients with molecular relapse only [60]. The GvL effect is greatest in those patients who have posttransplant graft-versus-host disease (GvHD), with GvL and GvHD being driven by minor genetic mismatches between donor and recipient. However, alloSCT/DLI is only an option for a minority of patients (~30%) who are young (<50–55 years of age) and have a matched donor. The procedure is associated with significant morbidity and mortality (20–40%), most commonly due to opportunistic infections and GvHD [81, 84]. For those patients who do receive an alloSCT, this type of immunotherapy offers a leukaemia-free survival rate of up to 70% [24].
With the success of alloSCT/DLI in curing CML by exploiting the GvL effect, interest in the late 1990s focused on reduced intensity ‘mini’ transplant regimens (reduced intensity stem cell transplant, RISCT). These less intense transplant conditioning regimens allow transplantation of older patients (up to age 70) and reduce regimen related toxicities as compared with standard alloSCT [21, 85]. A recent study [71] showed that of 24 patients with CML transplanted in first CP, 21 remained alive and disease free after a median of 42 months of follow-up. The GvL effects induced by donor immunocompetent lymphocytes eradicated all host haemopoietic cells, as evidenced by molecular testing.
Molecularly targeted therapy
The limited availability of alloSCT as an option for many patients and its associated morbidity and mortality has led to a continued search for specific drug therapies for CML. One such therapy is IM, which targets abl, kit and PDGFR. This compound was designed by Ciba-Geigy (now Novartis), and its structure was based on that of the protein kinase ATP-binding site; IM competes with ATP to block abl-coded kinase activity [13].
In vitro studies showed that IM inhibited proliferation of Bcr-Abl+ cells both in CML cell lines and CML patient samples [27, 30]. Further in vivo work showed that IM eradicated Bcr-Abl+ tumours in nude mice [61]. The effectiveness of IM in these studies led to its rapid introduction into clinical trials [32].
The introduction of IM has radically changed the treatment options for newly diagnosed patients with CML. A recent prospective, multicentre, phase III, randomised study (the IRIS trial) [20, 69] compared IM with interferon-α plus cytosine arabinoside (IFN-α+Ara-C) (the most successful nontransplant option for treatment of CML prior to the introduction of IM) in newly diagnosed CML patients in CP. The rate of major cytogenetic response (MCR; 0–35% of cells in metaphase Ph+) at 18 months was 87.1% in the IM group compared with 34.7% in the IFN-α+Ara-C group (p<0.001). The rates of complete cytogenetic response (CCR) were 76.2% and 14.7%, respectively (p<0.001). At 24 months, only 7.5% of patients remained on IFN-α+Ara-C, so analysis focused on the IM arm, in which 86% and 79% remained in MCR and CCR, respectively. In addition, at 18 months, progression-free survival (PFS) in the IM arm was 96.7% compared with 91.5% in the control arm, and at 24 months, PFS in the IM arm was 90% with an overall survival of 96%. IM was also better tolerated than IFN-α+Ara-C [49]. Therefore, these studies demonstrated the superiority of IM to IFN-α+Ara-C in terms of disease response and tolerability, but did not show an absolute survival advantage for IM. During the course of this study, molecular responses were assessed at regular intervals by RT-PCR in peripheral blood (PB) for bcr-abl transcripts [35, 55]. Patients responding to IM had a three to four log reduction in transcript levels compared with their starting value. Therefore, other than alloSCT, IM induces a much higher rate of CCR than any other licensed therapy.
However, an emerging problem in CP CML is molecular persistence. With IM, approximately 75% of patients in CP achieve a CCR, but remain RT-PCR positive [35, 57]. It is hypothesised that this MRD is due to the quiescent stem cell population, as in vitro, quiescent CML stem cells [51, 52] are completely insensitive to IM at concentrations up to ten times higher than those achievable in vivo [44], while proliferating cells remain exquisitely sensitive. In addition, an in vivo study to assess presence of Bcr-Abl+ cells by fluorescence in situ hybridisation (FISH) and levels of Bcr-Abl mRNA by RT-PCR demonstrated that samples from different CML patients collected at different time points displayed persistence of Bcr-Abl+ progenitors despite continued IM therapy [10]. This is further indication that IM does not eliminate malignant primitive progenitors in CML patients.
Although more common in AP and BC, another problem seen in CP CML is resistance to IM. This has resulted in IM being less successful in advanced disease [31, 82, 89]. Some patients in AP do respond well, and although many patients treated in BC achieve some haematological improvement, these benefits are not maintained, with the majority of patients in the advanced stages of CML developing resistance to IM. Resistance can be primary, i.e. no response to IM after initial therapy, or secondary, i.e. loss of response. The most common mechanisms of IM resistance are mutations of the Bcr-Abl kinase catalytic domain that interfere with IM binding (P-loop) [83] and over-expression of the Bcr-Abl gene [43].
Alternative immunotherapeutic approaches
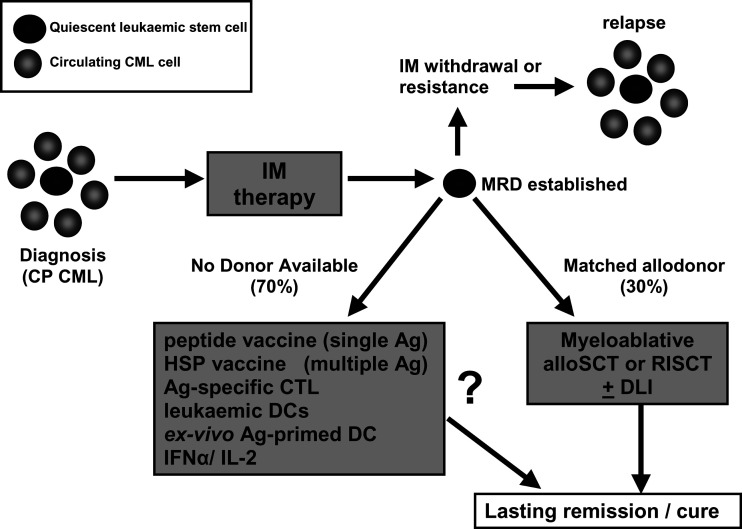
As alloSCT is only available to a minority of CML patients, and IM alone is unlikely to be curative, a number of different approaches have been investigated to harness the patient immune response to combat MRD and induce long-lasting remission (Fig. 1). In CML immunotherapy, the priority is to capitalise on the MRD state established after IM therapy, and push the immune response to eliminate the ‘silent minority’ of quiescent leukaemic stem cells.
Fig. 1.
Immunotherapeutic approaches for the treatment of CML post-IM. Diagnosis of CP CML is by detection of Ph+ or Bcr-Abl+ cells in PB and BM. It is now clear that there is also a population of quiescent leukaemic stem cells resident in PB and BM. IM therapy induces CCR and establishes MRD in the majority of patients. However IM-resistant quiescent cells remain and if therapy is withdrawn or resistance occurs, these cells eventually proliferate and induce relapse. AlloSCT or RISCT offer the best chance for lasting remission or cure, with or without supplementary graded DLI. In patients without a suitable donor, several immunotherapy approaches are being investigated. These include vaccination with Bcr-Abl peptides, HSP complexes, or adoptive therapy with expanded tumour-specific CTLs. Leukaemic APCs naturally expressing tumour antigens, or DCs primed with additional tumour antigens are also being studied as vaccine agents. Interleukin 2 (IL-2) and IFN-α can be used singly or as an adjunct to IM therapy as immunostimulatory agents, improving overall response through a variety of mechanisms.
The adaptive immune response is a complex interaction between antigen-presenting cells (APCs), effector cells such as cytotoxic T lymphocytes (CTLs) and costimulatory factors. Experimental approaches to autologous CML immunotherapy use vaccines, leukaemic and nonleukaemic APCs and CTLs to direct the immune system against defined or undefined CML antigens.
The ideal vaccine candidate would be an antigen expressed only on tumour cells but common to all patients and tumour types. It should be highly immunogenic, able to induce both humoral and cellular immune responses and should be essential for tumour cell survival, and thus not susceptible to mutation or deletion.
CML-specific antigens
Chronic myeloid leukaemia is particularly attractive as a model for immunotherapy. Bcr-Abl, or the reciprocal fusion Abl-Bcr [8], would appear to be ideal candidate targets for immunotherapy, as they express novel epitopes (fusion point peptides) that are recognised by the immune response (Table 1). T cells specific for the Bcr-Abl b3a2 fusion point have been observed in CML patients, but restricted to those with HLA-A3, A11 or B8 haplotypes [23]. Phase I and II vaccination studies are underway in CML patients using Bcr-Abl b3a2 peptides plus adjuvant, and show interesting preliminary results. Vaccination of patients was well tolerated with no significant side effects, and resulted in peptide-specific T-cell proliferation [76]. Peptide-specific IFN-γ–producing CD8+ cells were also observed, but only in HLA-A3 or A11 patients [18]. In another recent phase I study in IM-treated CML patients with stable disease for at least 6 months, three to six vaccination cycles resulted in reduced Ph+ levels in nine of the nine patients, and five of the nine went on to CCR. Of the patients in CCR, three of the five were also negative by Q-RT-PCR. Patients receiving IFN-α therapy also responded positively to Bcr-Abl peptide vaccination [11]. This indicates that b3a2 Bcr-Abl vaccination is a strong candidate for immunotherapy of CML in combination with IM or IFN-α therapy. Further trials are currently underway using Bcr-Abl peptide vaccination in CML patients, examining dose escalation in b3a2-positive patients responding to IM, regardless of HLA type (R.E. Clark, personal communication).
Table 1.
Current approaches for immunotherapy of CML. Ags antigens, APC antigen-presenting cell, CRCL chaperone-rich cell lysate, CTL cytotoxic T lymphocyte, DC dendritic cell
| Target | Method of delivery | Advantages | Disadvantages | Stage of development |
|---|---|---|---|---|
| CML-specific Ags (b3a2 Bcr-Abl fusion peptides) | Vaccine or DC loading | Easy to monitor response; ubiquitous CML Ag | Certain HLA types may have better response than others | Phase II |
| Tumour-associated Ags (Pr3/myeloblastin, WT1, survivin, etc.) | Vaccine or Ag-specific CTL isolation | Easy to monitor response; Ags expressed at high levels in CML cells | Single Ag targeting can lead to tumour escape mechanisms; Ags also expressed on other tissues | Preclinical and phase I studies |
| Tumour-derived material HSP complexes (HSP70, Grp94, CRCL) | Vaccine | All tumour Ags available; specific mechanism for uptake | Complex preparation for individual patients; difficult to monitor specific responses | Phase II |
| Leukaemic APC | DC derived from Ph+ CD34+ cells or monocytes | All tumour Ags available; supplies costimulatory factors to improve immune response | Ph+ DC less efficient APC than normal DC; difficult to monitor specific responses | Pilot and preclinical studies |
| Immunostimulators (IFN-α, IL-2) | Subcutaneous | Induce a variety of immune responses which can attack CML | Requires low tumour cell burden; can be associated with toxicity | IFN-α current therapy; IL-2 phase II as single-agent pilot studies combined with IM |
Shared tumour antigens
A number of proteins are present at greatly increased levels in CML and other malignancies, but are absent or expressed at low levels in normal cell lineages. These proteins may be involved in maintenance of the leukaemic phenotype and are therefore useful targets for directed immunotherapy. Proteinase-3 (Pr3) or myeloblastin is highly expressed in myeloid haematological malignancies. Pr3-specific CTLs have been identified in CML patients and are implicated in clearance of malignant cells [67]. A multimeric peptide derived from Pr3 (Pr1 nonamer) has been used in vaccination trials, and resulted in both immunological and clinical responses in CML patients. Good clinical response was correlated with the induction of Pr1-specific CTLs with a central memory (CCR7+) phenotype, indicative of a self-renewing population [95]. However, there is good evidence that IM therapy down-regulates Pr3 expression on CML tumour cells, which could potentially affect CTL recognition and clearance [14]. Another candidate molecule is Wilms’ tumour 1 (WT-1) protein, present in both haematological and solid tumour malignancies. Studies have identified WT-1–specific CTLs in CML patients [7]. Animal and in vitro models have demonstrated that these CTLs deplete leukaemic, but not normal CD34+ stem cells [39, 40], suggesting they may be effective in eradicating the quiescent stem cells present in MRD.
Other potential targets for immunotherapy include survivin, an antiapoptotic molecule preferentially up-regulated in CML cells by the Bcr-Abl/MAPK signalling pathway [17, 96], and telomerase, an enzyme involved in disease progression and potentially linked to IM resistance [3]. Other leukaemia-associated molecules have been identified by serological screening, including RHAMM, CML66 and CML28 antigens [3, 47, 48]. However, a potential problem with targeting a single protein or epitope for immunotherapy is the possibility of immune escape. Under pressure from the immune response, the malignant clone may down-regulate, mutate or delete the target epitope, evading specific CTL attack [75]. By targeting a range of different defined and undefined tumour antigens, the possibility of immune evasion is concomitantly lessened.
Heat shock protein vaccines
Heat shock proteins (HSPs) are ubiquitous protective intracellular molecules induced by cellular stress, which act as chaperones for peptides. HSPs isolated from tumour cells carry an array of tumour-specific peptides capable of inducing specific immune responses (Table 1). Phase II clinical trials are currently underway using patient-specific HSP immunization (AG858 and Oncophage trials, http://www.antigenics.com). In phase I trials, vaccination with AG858 (composed of HSP70 complexes) produced no adverse effects and resulted in maintenance of stable disease in 14/14 patients, and measurable reduction in Ph+ cells and Bcr-Abl expression in 10 of the 14 patients. Increased IFN-γ expression in T cells was seen in eight of the ten responders [62]. Recent preclinical studies have shown that chaperone-rich cell lysates (CRCLs) from tumour cells have a greater ability to stimulate mixed leukocyte reactions and proinflammatory IL-12 expression than separate individual HSP fractions [45], which may simplify preparation of vaccine material.
Cell-based immunotherapy
Dendritic cells (DCs) are now recognised as central to the specific induction of immune responses to cancer, and much research has gone into harnessing such responses for cancer immunotherapy [91]. DCs for use in immunotherapy may be obtained by a number of different methods (Fig. 2). Prior to the introduction of IM, the majority of DCs obtained by these methods would have been Ph+, and there is evidence that circulating Ph+ DCs have altered function [34, 65]. After IM therapy, immune function has been shown to improve via eradication of Ph+ DCs [94]. Antigen-presenting activity of recovering Ph− DCs is also increased [66, 80]. However, new evidence is emerging which suggests that IM administration can inhibit the development and function of DCs from CD34+ progenitors [1, 87], and can inhibit mitogen-mediated T-cell proliferation [29]. In both cases, this seems to be modulated through down-regulation of NFκB levels, and implies that long-term treatment with IM may interfere with some aspects of the immune response.
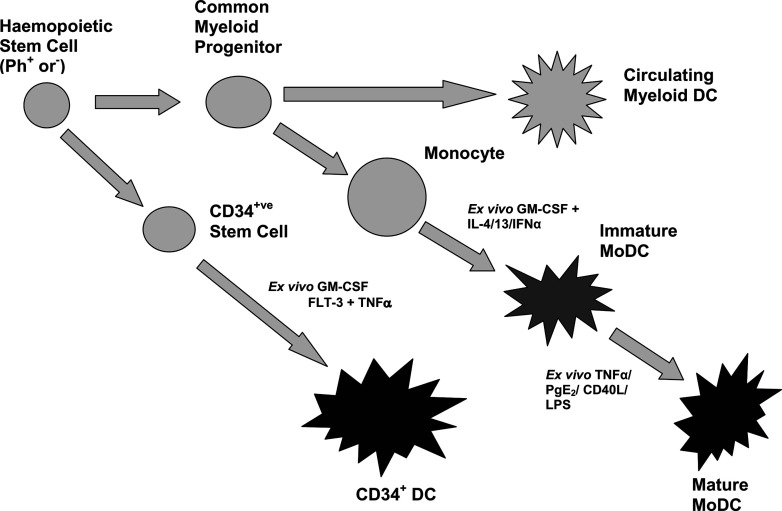
Fig. 2.
DC types available for clinical use. DCs may be generated from Ph+ or Ph− precursors. Circulating DCs, CD34+ cells and monocytes are collected by apheresis and enriched using magnetic beads coated with monoclonal antibodies specific for surface markers. DCs may be generated from CD34+ cells by ex vivo culture with cytokine cocktails such as GM-CSF, FlT-3 and TNF-α. Monocytes can be converted to fully mature DCs via a two-stage process. Monocytes are cultured with cytokine cocktails such as GM-CSF and IL-4/IL-13/IFN-α to produce immature monocyte-derived DCs (MoDCs), antigen primed and then cultured with TNF-α/prostaglandin E2/CD40L cocktails to generate fully mature DCs.
Circulating DCs are present in the PB of CML patients, but only at low levels (<1%), making their collection for immunotherapy impractical. Furthermore, preliminary results from their use in clinical studies have been disappointing [88]. Large numbers of DCs can be generated ex vivo from patient CD34+ stem cells cultured with GM-CSF and TNF-α [19], or monocyte precursors cultured with GM-CSF plus IL-4, IL-13 or IFN-α [36, 68, 79]. The latter method has been favoured in CML. Although potentially compromised functionally, DCs generated from Ph+ precursors have been shown to induce CML-specific T-cell responses that have immunotherapeutic potential [22]. Autologous Bcr-Abl+ DCs have been administered to patients in a number of phase I trials with minimal side effects. However, clinical response has generally been poor, with few remissions reported [74]. The immune responses induced by this vaccination approach rely on presentation of leukaemic antigens inherently expressed by the leukaemic DC. An alternative approach is to generate more specifically targeted responses (and hopefully more effective responses) by loading (or ‘priming’) the Ph+ or Ph− DCs with tumour-specific antigens.
DCs generated from monocyte precursors go through an initial immature phase, characterised by an increased ability to uptake and process exogenous tumour antigens which may be presented to the DCs as autologous tumour lysate, purified peptides (e.g. b3a2) or in conjunction with HSP. An alternate approach to DC priming is to load DCs by transfection with tumour antigen mRNA, such as survivin [96]. DC priming for CML therapy is still relatively uncommon in comparison with other malignancies [77]. Priming is usually followed by a second maturation step (Fig. 2). There is evidence that immature DCs do not migrate correctly, and can induce tolerogenic responses [28], so it is crucial that DCs achieve a fully mature state if they are to be used as an anticancer therapy.
Immature DCs are converted to a mature phenotype using microbial products, cytokines or CD40 ligand [4]. Mature DCs secrete inflammatory cytokines (IL-2, IL-12) [46], express costimulatory markers (CD80/CD83/CD86) and chemokine receptors involved in migration to lymph nodes (CCR7) and are highly effective at converting naïve T cells into tumour antigen–specific CTLs. Takahashi et al. [88] used b3a2 fusion peptide-primed DCs in a pilot clinical trial, and observed peptide-specific immune responses in three of the three patients vaccinated with mature DCs but only one of the three patients given immature DCs. No clinical improvement was observed in either group. However, a recent study using a murine Bcr-Abl+ leukaemia model combined CRCL-loaded DCs and IM to improve tumour-specific immune responses and survival, and demonstrated that IM could successfully be used in conjunction with immunotherapy [97].
A further adaptation of these approaches has been reported by Osman et al. [72, 73]: rather than using patient-derived DCs, donor DCs were primed with peptides or tumour cells to induce donor CTLs ex vivo. These donor DCs or ex vivo generated tumour-specific CTLs could be used as a supplement to alloSCT, with the aim of increasing the GvL effect without exaggerating GvHD [72, 73].
Immunostimulators
IFN-α has been a standard therapy for CP CML for over 10 years. The introduction of IM has largely replaced its role as a first-line agent, but its pluripotent effects may still play a key part in eradication of MRD. It has numerous antitumour effects, including increased tumour cell apoptosis, activation and up-regulation of IL-2 and IFN-γ production by T cells, and increased NK cell cytotoxicity [26]. IFN-α also induces rapid differentiation of monocytes into activated DCs [36], which may be a key mechanism in CML tumour eradication. The T-cell growth factor IL-2 can induce tumour-specific CTLs and memory T-cell proliferation and expression of IFN-γ, which consequently maintains antitumour activity. Low-dose IL-2 has been used as a therapy for advanced CML [42, 92], and IL-2 and IFN-α in combination have been used to prevent relapse in high-risk CML patients after alloSCT [93]. Both agents have been used singly or together, and combined with IM significantly reduced Bcr-Abl levels in stable disease, but were only effective in patients with low tumour burdens [15]. This suggests their role should be to boost and maintain immune responses induced by cellular immunotherapy of CML, once IM has established MRD.
The future
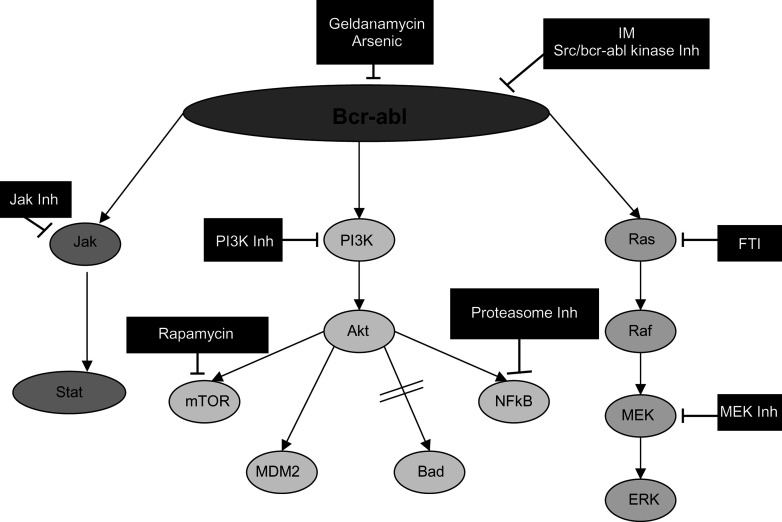
Due to the success of IM, other candidate agents for targeted therapy in CML are rapidly emerging (Fig. 3). It is anticipated that to enhance disease eradication and prevent emergence of resistant clones, rationally designed combinations of drugs to interrupt key survival pathways in the most appropriate cell population will be required. To date, a wide range of conventional and novel agents have been combined with IM in vitro (e.g. hydroxyurea, Ara-C, IFN-α, arsenic trioxide and FTI [90]). Although many of these studies have been informative, few have addressed cytotoxicity at the stem cell level [53]. Evidence of additive or synergistic effects of combinations in vitro with cell lines and primary CML patient material formed the basis for phase I/II trials of IM with IFN-α [50, 70] or Ara-C [64]. However, evidence of improved response rates and survival will be reliant upon the results from large randomised trials such as the German CML study IV (http://www.kompetenznetz-leukaemie.de) or the SPIRIT trial about to launch in the UK (http://www.spirit-cml.org). Although some signal transduction inhibitors have been shown to potentiate the leukaemia-specific cytotoxicity of conventional agents, others induce cell cycle arrest, thus antagonising the action of agents that require proliferative activity to elicit cytotoxicity. Therefore the choice of agents to combine and the scheduling of multiple drugs will be critical to improve the treatment of CML.
Fig. 3.
Signal transduction pathways affected by Bcr-Abl and sites of inhibition. Bcr-Abl can affect many downstream signalling pathways including Jak/Stat, PI3K/Akt and Ras/Raf/MEK/ERK. Sites of inhibition and their inhibitors are indicated in the figure. Inh inhibitor, FTI farnesyl transferase inhibitor.
Another therapeutic approach is to combine molecularly targeted therapy, e.g. IM, with RISCT. This gives the possibility of inducing tolerance in the recipient to donor T cells in a relatively nontoxic fashion, thereby creating an engraftment pattern which would be sensitive to further manipulation with effector T cells given in the form of graded DLI. A number of studies looking at this are currently underway [2].
In our opinion, a number of strategies need to be pursued to eradicate quiescent CML stem cells. Firstly, further investigation is required to elucidate the most effective drug combinations for eradicating disease and preventing resistance. Secondly, for patients with a matched donor, clinical trials combining IM with RISCT/DLI to achieve a molecular ‘cure’ need to continue and be expanded. Thirdly, much research is still necessary to determine the optimum immunotherapeutic approach in the absence of a matched donor, and, critically, all those in the immunotherapy field need to be encouraged to continue their research and not consider immunotherapy redundant in the era of IM.
In conclusion, a truly effective, long-lasting immunotherapy for all CML patients is still some way off. Future developments in CML therapy seem likely to combine different approaches to integrate and reinforce the patient immune response against remaining quiescent CML stem cells in conjunction with combinations of drugs which reverse quiescence and target the genetic and molecular abnormalities present in CML cells.
References
- 1.Appel S, Boehmler AM, Grunebach F, Muller MR, Rupf A, Weck MM, Hartmann U, Reichardt VL, Kanz L, Brummendorf TH, Brossart P. Imatinib mesylate affects the development and function of dendritic cells generated from CD34+ peripheral blood progenitor cells. Blood. 2004;103:538–544. doi: 10.1182/blood-2003-03-0975. [DOI] [PubMed] [Google Scholar]
- 2.Avery S, Nadal E, Davis J, Apperley J, Goldman J, Marin D. Infusion of peripheral blood stem cells collected at diagnosis can improve the level of cytogenetic response in CML patients on imatinib whose treatment is limited by cytopaenia. Blood. 2003;102:318b. [Google Scholar]
- 3.Bakalova R, Ohba H, Zhelev Z, Ishikawa M, Shinohara Y, Baba Y. Cross-talk between Bcr-Abl tyrosine kinase, protein kinase C and telomerase-a potential reason for resistance to Glivec in chronic myelogenous leukaemia. Biochem Pharmacol. 2003;66:1879–1884. doi: 10.1016/j.bcp.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 4.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 5.Barrett AJ, van Rhee F. Graft-versus-leukaemia. Baillieres Clin Haematol. 1997;10:337–355. doi: 10.1016/s0950-3536(97)80011-x. [DOI] [PubMed] [Google Scholar]
- 6.Bedi A, Zehnbauer BA, Barber JP, Sharkis SJ, Jones RJ. Inhibition of apoptosis by BCR-ABL in chronic myeloid leukaemia. Blood. 1994;83:2038–2044. [PubMed] [Google Scholar]
- 7.Bellantuono I, Gao L, Parry S, Marley S, Dazzi F, Apperley J, Goldman JM, Stauss HJ. Two distinct HLA-A0201-presented epitopes of the Wilms tumor antigen 1 can function as targets for leukemia-reactive CTL. Blood. 2002;100:3835–3837. doi: 10.1182/blood.V100.10.3835. [DOI] [PubMed] [Google Scholar]
- 8.Berke Z, Andersen MH, Pedersen M, Fugger L, Zeuthen J, Haurum JS. Peptides spanning the junctional region of both the abl/bcr and the bcr/abl fusion proteins bind common HLA class I molecules. Leukemia. 2000;14:419–426. doi: 10.1038/sj.leu.2401703. [DOI] [PubMed] [Google Scholar]
- 9.Bhatia R, Wayner EA, McGlave P, Verfaillie CM. Interferon-alpha restores normal adhesion of chronic myelogenous leukaemia hematopoietic progenitors to bone marrow strom by correcting impaired beta 1 integrin receptor function. J Clin Invest. 1994;94:384–391. doi: 10.1172/JCI117333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhatia R, Holtz M, Niu N, Gray R, Snyder DS, Sawyers CL, Arber DA, Slovak ML, Forman SJ. Persistence of malignant hematopoietic progenitors in chronic myelogenous leukemia patients in complete cytogenetic remission following imatinib mesylate treatment. Blood. 2003;101:4701–4707. doi: 10.1182/blood-2002-09-2780. [DOI] [PubMed] [Google Scholar]
- 11.Bocchia M, Gentili S, Abruzzese E, Fanelli A, Iuliano F, Tabilio A, Amabile M, Forconi F, Pirrotta MT, Gozzetti A, Ippoliti M, Raspadori I, Lauria F. Imatinib plus CMLVAX100 (p210 derived multipeptide vaccine): induction of complete molecular responses in patients with chronic myeloid leukaemia (CML) showing persistent residual disease during treatment with imatinib mesylate. Blood. 2003;102:30a. [Google Scholar]
- 12.Brummendorf TH, Holyoake TL, Rufer N, Barnett MJ, Schulzer M, Eaves CJ, Eaves AC, Lansdorp PM. Prognostic implications of differences in telomere length between normal and malignant cells from patients with chronic myeloid leukemia measured by flow cytometry. Blood. 2000;95:1883–1890. [PubMed] [Google Scholar]
- 13.Buchdunger E, Zimmermann J, Mett H, Meyer T, Muller M, Druker BJ, Lydon NB. Inhibition of the Abl protein-tyrosine kinase in vitro and in vivo by a 2-phenylaminopyrimidine derivative. Cancer Res. 1996;56:100–104. [PubMed] [Google Scholar]
- 14.Burchert A, Wolfl S, Schmidt M, Brendel C, Denecke B, Cai D, Odyvanova L, Lahaye T, Muller MC, Berg T, Gschaidmeier H, Wittig B, Hehlmann R, Hochhaus A, Neubauer A. Interferon-alpha, but not the ABL-kinase inhibitor imatinib (STI571), induces expression of myeloblastin and a specific T-cell response in chronic myeloid leukemia. Blood. 2003;101:259–264. doi: 10.1182/blood-2002-02-0659. [DOI] [PubMed] [Google Scholar]
- 15.Burton CH, Avery S, Ranger A, Nadal E, Nathan I, Olavarria E, Apperley J, Goldman J. Use of RT-PCR to monitor the possible benefit of adding interferon-alpha to imatinib for patients with CML in chronic phase. Blood. 2003;102:908a. [Google Scholar]
- 16.Campbell JD, Cook G, Holyoake TL. Evolution of bone marrow transplantation—the original immunotherapy. Trends Immunol. 2001;22:88–92. doi: 10.1016/S1471-4906(00)01857-3. [DOI] [PubMed] [Google Scholar]
- 17.Carter BZ, Schober WD, McQueen T, Andreeff M. Regulation of survivin expression through bcr-abl/MAPK cascade: survivin as a therapeutic target in STI571 resistant CML cells. Blood. 2003;102:651a. doi: 10.1182/blood-2003-03-0960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cathcart K, Pinilla-Ibarz J, Korontsvit T, Schwartz J, Zakhaleva V, Papadopoulos EB, Scheinberg DA. A multivalent bcr-abl fusion peptide vaccination trial in patients with chronic myeloid leukemia. Blood. 2004;103:1037–1042. doi: 10.1182/blood-2003-03-0954. [DOI] [PubMed] [Google Scholar]
- 19.Caux C, Massacrier C, Vanbervliet B, Dubois B, Durand I, Cella M, Lanzavecchia A, Banchereau J. CD34+ hematopoietic progenitors from human cord blood differentiate along two independent dendritic cell pathways in response to granulocyte-macrophage colony-stimulating factor plus tumor necrosis factor alpha: II. Functional analysis. Blood. 1997;90:1458–1470. [PubMed] [Google Scholar]
- 20.Cervantes F. Durability of responses to imatinib in newly diagnosed chronic phase chronic myeloid leukaemia: 24 month update from the IRIS study. Blood. 2003;102:181a. [Google Scholar]
- 21.Champlin R, Khouri I, Shimoni A, Gajewski J, Kornblau S, Molldrem J, Ueno N, Giralt S, Anderlini P. Harnessing graft-versus-malignancy: non-myeloablative preparative regimens for allogeneic haematopoietic transplantation, an evolving strategy for adoptive immunotherapy. Br J Haematol. 2000;111:18–29. doi: 10.1046/j.1365-2141.2000.02196.x. [DOI] [PubMed] [Google Scholar]
- 22.Choudhury A, Gajewski JL, Liang JC, Popat U, Claxton DF, Kliche KO, Andreeff M, Champlin RE. Use of leukemic dendritic cells for the generation of antileukemic cellular cytotoxicity against Philadelphia chromosome-positive chronic myelogenous leukemia. Blood. 1997;89:1133–1142. [PubMed] [Google Scholar]
- 23.Clark RE, Dodi IA, Hill SC, Lill JR, Aubert G, Macintyre AR, Rojas J, Bourdon A, Bonner PL, Wang L, Christmas SE, Travers PJ, Creaser CS, Rees RC, Madrigal JA. Direct evidence that leukemic cells present HLA-associated immunogenic peptides derived from the BCR-ABL b3a2 fusion protein. Blood. 2001;98:2887–2893. doi: 10.1182/blood.V98.10.2887. [DOI] [PubMed] [Google Scholar]
- 24.Clift RA, Anasetti C. Allografting for chronic myeloid leukaemia. Baillieres Clin Haematol. 1997;10:319–336. doi: 10.1016/s0950-3536(97)80010-8. [DOI] [PubMed] [Google Scholar]
- 25.Daley GQ, Van Etten RA, Baltimore D. Induction of chronic myelogenous leukemia in mice by the P210bcr/abl gene of the Philadelphia chromosome. Science. 1990;247:824–830. doi: 10.1126/science.2406902. [DOI] [PubMed] [Google Scholar]
- 26.de Castro FA, Palma PV, Morais FR, Simoes BP, Carvalho PV, Ismael SJ, Lima CP, Voltarelli JC. Immunological effects of interferon-alpha on chronic myelogenous leukemia. Leuk Lymphoma. 2003;44:2061–2067. doi: 10.1080/1042819031000110973. [DOI] [PubMed] [Google Scholar]
- 27.Deininger MW, Goldman JM, Lydon N, Melo JV. The tyrosine kinase inhibitor CGP57148B selectively inhibits the growth of BCR-ABL-positive cells. Blood. 1997;90:3691–3698. [PubMed] [Google Scholar]
- 28.Dhodapkar MV, Steinman RM, Krasovsky J, Munz C, Bhardwaj N. Antigen-specific inhibition of effector T cell function in humans after injection of immature dendritic cells. J Exp Med. 2001;193:233–238. doi: 10.1084/jem.193.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dietz AB, Souan L, Knutson GJ, Bulur PA, Litzow MR, Vuk-Pavlovic S. Imatinib mesylate inhibits T cell proliferation in vitro and delayed-type hypersensitivity in vivo. Blood. 2004;104:1094–1099. doi: 10.1182/blood-2003-12-4266. [DOI] [PubMed] [Google Scholar]
- 30.Druker BJ, Tamura S, Buchdunger E, Ohno S, Segal GM, Fanning S, Zimmermann J, Lydon NB. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells. Nat Med. 1996;2:561–566. doi: 10.1038/nm0596-561. [DOI] [PubMed] [Google Scholar]
- 31.Druker BJ, Sawyers CL, Kantarjian H, Resta DJ, Reese SF, Ford JM, Capdeville R, Talpaz M. Activity of a specific inhibitor of the BCR-ABL tyrosine kinase in the blast crisis of chronic myeloid leukemia and acute lymphoblastic leukemia with the Philadelphia chromosome. N Engl J Med. 2001;344:1038–1042. doi: 10.1056/NEJM200104053441402. [DOI] [PubMed] [Google Scholar]
- 32.Drummond MW, Holyoake TL. Tyrosine kinase inhibitors in the treatment of chronic myeloid leukaemia: so far so good. Blood Rev. 2001;15:85–95. doi: 10.1054/blre.2001.0152. [DOI] [PubMed] [Google Scholar]
- 33.Eaves AC, Cashman JD, Gaboury LA, Eaves CJ. Clinical significance of long-term cultures of myeloid blood cells. Crit Rev Oncol Hematol. 1987;7:125–138. doi: 10.1016/s1040-8428(87)80022-7. [DOI] [PubMed] [Google Scholar]
- 34.Eisendle K, Lang A, Eibl B, Nachbaur D, Glassl H, Fiegl M, Thaler J, Gastl G. Phenotypic and functional deficiencies of leukaemic dendritic cells from patients with chronic myeloid leukaemia. Br J Haematol. 2003;120:63–73. doi: 10.1046/j.1365-2141.2003.03979.x. [DOI] [PubMed] [Google Scholar]
- 35.Feng L, Drummond MW, Byrne J, Shepherd PC, Apperley JF, O’Brien SG, Craddock C, Clark RE, Cervantes F, Lennard AL, Holyoake TL, Szydlo RM, Goldman JM, Kaeda JS. Molecular monitoring of complete cytogenic responders following treatment with imatinib (STI571, Glivec) for CML: a report from the UKSTI571 study group. Blood. 2002;100:1425. doi: 10.1182/blood-2002-05-1389. [DOI] [PubMed] [Google Scholar]
- 36.Gabriele L, Borghi P, Rozera C, Sestili P, Andreotti M, Guarini A, Montefusco E, Foa R, Belardelli F. IFN-alpha promotes the rapid differentiation of monocytes from patients with chronic myeloid leukemia into activated dendritic cells tuned to undergo full maturation after LPS treatment. Blood. 2004;103:980–987. doi: 10.1182/blood-2003-03-0981. [DOI] [PubMed] [Google Scholar]
- 37.Gale RP, Horowitz MM, Bortin MM. IBMTR analysis of bone marrow transplants in acute leukaemia. Advisory Committee of the International Bone Marrow Transplant Registry (IBMTR) Bone Marrow Transplant. 1989;4(suppl 3):83–84. [PubMed] [Google Scholar]
- 38.Gale RP, Horowitz MM, Ash RC, Champlin RE, Goldman JM, Rimm AA, Ringden O, Stone JA, Bortin MM. Identical-twin bone marrow transplants for leukemia. Ann Intern Med. 1994;120:646–652. doi: 10.7326/0003-4819-120-8-199404150-00004. [DOI] [PubMed] [Google Scholar]
- 39.Gao L, Bellantuono I, Elsasser A, Marley SB, Gordon MY, Goldman JM, Stauss HJ. Selective elimination of leukemic CD34(+) progenitor cells by cytotoxic T lymphocytes specific for WT1. Blood. 2000;95:2198–2203. [PubMed] [Google Scholar]
- 40.Gao L, Xue SA, Hasserjian R, Cotter F, Kaeda J, Goldman JM, Dazzi F, Stauss HJ. Human cytotoxic T lymphocytes specific for Wilms’ tumor antigen-1 inhibit engraftment of leukemia-initiating stem cells in non-obese diabetic-severe combined immunodeficient recipients. Transplantation. 2003;75:1429–1436. doi: 10.1097/01.TP.0000061516.57346.E8. [DOI] [PubMed] [Google Scholar]
- 41.Goldman JM, Gale RP, Horowitz MM, Biggs JC, Champlin RE, Gluckman E, Hoffmann RG, Jacobsen SJ, Marmont AM, McGlave PB, et al. Bone marrow transplantation for chronic myelogenous leukemia in chronic phase. Increased risk for relapse associated with T-cell depletion. Ann Intern Med. 1988;108:806–814. doi: 10.7326/0003-4819-108-6-806. [DOI] [PubMed] [Google Scholar]
- 42.Goodman M, Spector N, Rodrigues G, Cassileth PA. Interleukin-2 therapy for advanced chronic myeloid leukemia. Leukemia. 1998;12:1682–1684. doi: 10.1038/sj.leu.2401200. [DOI] [PubMed] [Google Scholar]
- 43.Gorre ME, Mohammed M, Ellwood K, Hsu N, Paquette R, Rao PN, Sawyers CL. Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science. 2001;293:876–880. doi: 10.1126/science.1062538. [DOI] [PubMed] [Google Scholar]
- 44.Graham SM, Jorgensen HG, Allan E, Pearson C, Alcorn MJ, Richmond L, Holyoake TL. Primitive, quiescent, Philadelphia-positive stem cells from patients with chronic myeloid leukemia are insensitive to STI571 in vitro. Blood. 2002;99:319–325. doi: 10.1182/blood.V99.1.319. [DOI] [PubMed] [Google Scholar]
- 45.Graner MW, Zeng Y, Feng H, Katsanis E. Tumor-derived chaperone-rich cell lysates are effective therapeutic vaccines against a variety of cancers. Cancer Immunol Immunother. 2003;52:226–234. doi: 10.1007/s00262-002-0359-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Granucci F, Vizzardelli C, Pavelka N, Feau S, Persico M, Virzi E, Rescigno M, Moro G, Ricciardi-Castagnoli P. Inducible IL-2 production by dendritic cells revealed by global gene expression analysis. Nat Immunol. 2001;2:882–888. doi: 10.1038/ni0901-882. [DOI] [PubMed] [Google Scholar]
- 47.Greiner J, Ringhoffer M, Taniguchi M, Schmitt A, Kirchner D, Krahn G, Heilmann V, Gschwend J, Bergmann L, Dohner H, Schmitt M. Receptor for hyaluronan acid-mediated motility (RHAMM) is a new immunogenic leukemia-associated antigen in acute and chronic myeloid leukemia. Exp Hematol. 2002;30:1029–1035. doi: 10.1016/S0301-472X(02)00874-3. [DOI] [PubMed] [Google Scholar]
- 48.Greiner J, Ringhoffer M, Taniguchi M, Hauser T, Schmitt A, Dohner H, Schmitt M. Characterization of several leukemia-associated antigens inducing humoral immune responses in acute and chronic myeloid leukemia. Int J Cancer. 2003;106:224–231. doi: 10.1002/ijc.11200. [DOI] [PubMed] [Google Scholar]
- 49.Hahn EA, Glendenning GA, Sorensen MV, Hudgens SA, Druker BJ, Guilhot F, Larson RA, O’Brien SG, Dobrez DG, Hensley ML, Cella D. Quality of life in patients with newly diagnosed chronic phase chronic myeloid leukemia on imatinib versus interferon alfa plus low-dose cytarabine: results from the IRIS Study. J Clin Oncol. 2003;21:2138–2146. doi: 10.1200/JCO.2003.12.154. [DOI] [PubMed] [Google Scholar]
- 50.Hochhaus A, Fischer T, Brummendorf TH, Schoch C, Muller MC, Merx K, Berger U, Gschaidmeier H, Hehlmann R. Imatinib (Glivec) and pegylated interferon a2a (Pegasys) phase I/II combination study in chronic phase myelogenous leukaemia (CML) Blood. 2002;100:164a. [Google Scholar]
- 51.Holyoake T, Jiang X, Eaves C, Eaves A. Isolation of a highly quiescent subpopulation of primitive leukemic cells in chronic myeloid leukemia. Blood. 1999;94:2056–2064. [PubMed] [Google Scholar]
- 52.Holyoake TL, Jiang X, Jorgensen HG, Graham S, Alcorn MJ, Laird C, Eaves AC, Eaves CJ. Primitive quiescent leukemic cells from patients with chronic myeloid leukemia spontaneously initiate factor-independent growth in vitro in association with up-regulation of expression of interleukin-3. Blood. 2001;97:720–728. doi: 10.1182/blood.V97.3.720. [DOI] [PubMed] [Google Scholar]
- 53.Holyoake TL, Allan EK, Graham SJ, Godden JL, Mountford JC, Gilmour D, Richmond L, Jorgensen HG. Combination therapies including imatinib do not eradicate quiescent chronic myeloid leukaemia stem cells in vitro. Blood. 2003;102:71a. [Google Scholar]
- 54.Horowitz MM, Gale RP, Sondel PM, Goldman JM, Kersey J, Kolb HJ, Rimm AA, Ringden O, Rozman C, Speck B, et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990;75:555–562. [PubMed] [Google Scholar]
- 55.Hughes T, Branford S. Molecular monitoring of chronic myeloid leukemia. Semin Hematol. 2003;40:62–68. doi: 10.1053/shem.2003.50044. [DOI] [PubMed] [Google Scholar]
- 56.Hughes TP, Economou K, Mackinnon S, Vlitos M, Arthur CK, Guo AP, Rassool F, Apperley JF, Hows J, Goldman JM. Slow evolution of chronic myeloid leukaemia relapsing after BMT with T-cell depleted donor marrow. Br J Haematol. 1989;73:462–467. doi: 10.1111/j.1365-2141.1989.tb00281.x. [DOI] [PubMed] [Google Scholar]
- 57.Hughes TP, Kaeda J, Branford S, Rudzki Z, Hochhaus A, Hensley ML, Gathmann I, Bolton AE, van Hoomissen IC, Goldman JM, Radich JP. Frequency of major molecular responses to imatinib or interferon alfa plus cytarabine in newly diagnosed chronic myeloid leukemia. N Engl J Med. 2003;349:1423–1432. doi: 10.1056/NEJMoa030513. [DOI] [PubMed] [Google Scholar]
- 58.Kloosterboer FM, van Luxemburg-Heijs SA, van Soest RA, Barbui AM, van Egmond HM, Strijbosch MP, Kester MG, Marijt WA, Goulmy E, Willemze R, Falkenburg JH. Direct cloning of leukemia-reactive T cells from patients treated with donor lymphocyte infusion shows a relative dominance of hematopoiesis-restricted minor histocompatibility antigen HA-1 and HA-2 specific T cells. Leukemia. 2004;18:798–808. doi: 10.1038/sj.leu.2403297. [DOI] [PubMed] [Google Scholar]
- 59.Kolb HJ, Mittermuller J, Clemm C, Holler E, Ledderose G, Brehm G, Heim M, Wilmanns W. Donor leukocyte transfusions for treatment of recurrent chronic myelogenous leukemia in marrow transplant patients. Blood. 1990;76:2462–2465. [PubMed] [Google Scholar]
- 60.Kolb HJ, Schattenberg A, Goldman JM, Hertenstein B, Jacobsen N, Arcese W, Ljungman P, Ferrant A, Verdonck L, Niederwieser D, et al. Graft-versus-leukemia effect of donor lymphocyte transfusions in marrow grafted patients. European group for blood and marrow transplantation working party chronic leukemia. Blood. 1995;86:2041–2050. [PubMed] [Google Scholar]
- 61.le Coutre P, Mologni L, Cleris L, Marchesi E, Buchdunger E, Giardini R, Formelli F, Gambacorti-Passerini C. In vivo eradication of human BCR/ABL-positive leukemia cells with an ABL kinase inhibitor. J Natl Cancer Inst. 1999;91:163–168. doi: 10.1093/jnci/91.2.163. [DOI] [PubMed] [Google Scholar]
- 62.Li Z, Qiao Y, Laska E, Liu B, Glynn L, Kulko J, Bona RD, Gaffney J, Hegde UP, Moyo V, Srivastava PK. Autologous heat shock protein 70-peptide complex as a vaccine for chronic myeloid leukaemia iun chronic phase: an updated phase I study. Blood. 2003;102:911a. [Google Scholar]
- 63.Mathe G, Amiel JL, Schwarzenberg L, Cattan A, Schneider M. Adoptive immunotherapy of acute leukemia: experimental and clinical results. Cancer Res. 1965;25:1525–1531. [PubMed] [Google Scholar]
- 64.Mauro MJ, O’Dwyer M, Stone RM, Walker T, Druker B. Preliminary evaluation of the combination of imatinib mesylate (Gleevec) with low-dose Ara-C as initial therapy for newly diagnosed chronic phase CML. J Natl Cancer Inst. 2002;101:165a. [Google Scholar]
- 65.Mohty M, Jarrossay D, Lafage-Pochitaloff M, Zandotti C, Briere F, de Lamballeri XN, Isnardon D, Sainty D, Olive D, Gaugler B. Circulating blood dendritic cells from myeloid leukemia patients display quantitative and cytogenetic abnormalities as well as functional impairment. Blood. 2001;98:3750–3756. doi: 10.1182/blood.V98.13.3750. [DOI] [PubMed] [Google Scholar]
- 66.Mohty M, Jourdan E, Ben Mami N, Vey N, Damaj G, Blaise D, Isnardon D, Olive D, Gaugler B. Imatinib and plasmacytoid dendritic cell function in chronic myeloid leukemia patients. Blood. 2004;103:4666–4668. doi: 10.1182/blood-2003-09-3220. [DOI] [PubMed] [Google Scholar]
- 67.Molldrem JJ, Lee PP, Wang C, Felio K, Kantarjian HM, Champlin RE, Davis MM. Evidence that specific T lymphocytes may participate in the elimination of chronic myelogenous leukemia. Nat Med. 2000;6:1018–1023. doi: 10.1038/79526. [DOI] [PubMed] [Google Scholar]
- 68.Morse MA, Lyerly HK, Li Y. The role of IL-13 in the generation of dendritic cells in vitro. J Immunother. 1999;22:506–513. doi: 10.1097/00002371-199911000-00005. [DOI] [PubMed] [Google Scholar]
- 69.O’Brien SG, Guilhot F, Larson RA, Gathmann I, Baccarani M, Cervantes F, Cornelissen JJ, Fischer T, Hochhaus A, Hughes T, Lechner K, Nielsen JL, Rousselot P, Reiffers J, Saglio G, Shepherd J, Simonsson B, Gratwohl A, Goldman JM, Kantarjian H, Taylor K, Verhoef G, Bolton AE, Capdeville R, Druker BJ. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348:994–1004. doi: 10.1056/NEJMoa022457. [DOI] [PubMed] [Google Scholar]
- 70.O’Dwyer ME, Mauro MJ, Kuyl KM, Paquette R, Sawyers CL, Druker B. Preliminary evaluation of the combination of imatinib mesylate (Gleevec) in combination with low dose interferon-alpha for the treatment of chronic phase CML. Blood. 2001;100:846a. [Google Scholar]
- 71.Or R, Shapira MY, Resnick I, Amar A, Ackerstein A, Samuel S, Aker M, Naparstek E, Nagler A, Slavin S. Nonmyeloablative allogeneic stem cell transplantation for the treatment of chronic myeloid leukemia in first chronic phase. Blood. 2003;101:441–445. doi: 10.1182/blood-2002-02-0535. [DOI] [PubMed] [Google Scholar]
- 72.Osman Y, Takahashi M, Zheng Z, Koike T, Toba K, Liu A, Furukawa T, Aoki S, Aizawa Y. Generation of bcr-abl specific cytotoxic T-lymphocytes by using dendritic cells pulsed with bcr-abl (b3a2) peptide: its applicability for donor leukocyte transfusions in marrow grafted CML patients. Leukemia. 1999;13:166–174. doi: 10.1038/sj/leu/2401311. [DOI] [PubMed] [Google Scholar]
- 73.Osman Y, Takahashi M, Zheng Z, Toba K, Liu A, Furukawa T, Aizawa Y, Shibata A, Koike T. Activation of autologous or HLA-identical sibling cytotoxic T lymphocytes by blood derived dendritic cells pulsed with tumor cell extracts. Oncol Rep. 1999;6:1057–1063. doi: 10.3892/or.6.5.1057. [DOI] [PubMed] [Google Scholar]
- 74.Ossenkoppele GJ, Stam AG, Westers TM, de Gruijl TD, Janssen JJ, van de Loosdrecht AA, Scheper RJ. Vaccination of chronic myeloid leukemia patients with autologous in vitro cultured leukemic dendritic cells. Leukemia. 2003;17:1424–1426. doi: 10.1038/sj.leu.2402979. [DOI] [PubMed] [Google Scholar]
- 75.Pawelec G. Tumour escape: antitumour effectors too much of a good thing? Cancer Immunol Immunother. 2004;53:262–274. doi: 10.1007/s00262-003-0469-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pinilla-Ibarz J, Cathcart K, Korontsvit T, Soignet S, Bocchia M, Caggiano J, Lai L, Jimenez J, Kolitz J, Scheinberg DA. Vaccination of patients with chronic myelogenous leukemia with bcr-abl oncogene breakpoint fusion peptides generates specific immune responses. Blood. 2000;95:1781–1787. [PubMed] [Google Scholar]
- 77.Ridgway D. The first 1000 dendritic cell vaccinees. Cancer Invest. 2003;21:873–886. doi: 10.1081/CNV-120025091. [DOI] [PubMed] [Google Scholar]
- 78.Rowley JD. Letter: a new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and Giemsa staining. Nature. 1973;243:290–293. doi: 10.1038/243290a0. [DOI] [PubMed] [Google Scholar]
- 79.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 1994;179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sato N, Narita M, Takahashi M, Yagisawa K, Liu A, Abe T, Nikkuni K, Furukawa T, Toba K, Aizawa Y. The effects of STI571 on antigen presentation of dendritic cells generated from patients with chronic myelogenous leukemia. Hematol Oncol. 2003;21:67–75. doi: 10.1002/hon.705. [DOI] [PubMed] [Google Scholar]
- 81.Sawyers CL. Chronic myeloid leukemia. N Engl J Med. 1999;340:1330–1340. doi: 10.1056/NEJM199904293401706. [DOI] [PubMed] [Google Scholar]
- 82.Sawyers CL, Hochhaus A, Feldman E, Goldman JM, Miller CB, Ottmann OG, Schiffer CA, Talpaz M, Guilhot F, Deininger MW, Fischer T, O’Brien SG, Stone RM, Gambacorti-Passerini CB, Russell NH, Reiffers JJ, Shea TC, Chapuis B, Coutre S, Tura S, Morra E, Larson RA, Saven A, Peschel C, Gratwohl A, Mandelli F, Ben-Am M, Gathmann I, Capdeville R, Paquette RL, Druker BJ. Imatinib induces hematologic and cytogenetic responses in patients with chronic myelogenous leukemia in myeloid blast crisis: results of a phase II study. Blood. 2002;99:3530–3539. doi: 10.1182/blood.V99.10.3530. [DOI] [PubMed] [Google Scholar]
- 83.Shah NP, Nicoll JM, Nagar B, Gorre ME, Paquette RL, Kuriyan J, Sawyers CL. Multiple BCR-ABL kinase domain mutations confer polyclonal resistance to the tyrosine kinase inhibitor imatinib (STI571) in chronic phase and blast crisis chronic myeloid leukemia. Cancer Cell. 2002;2:117–125. doi: 10.1016/S1535-6108(02)00096-X. [DOI] [PubMed] [Google Scholar]
- 84.Silver RT, Woolf SH, Hehlmann R, Appelbaum FR, Anderson J, Bennett C, Goldman JM, Guilhot F, Kantarjian HM, Lichtin AE, Talpaz M, Tura S. An evidence-based analysis of the effect of busulfan, hydroxyurea, interferon, and allogeneic bone marrow transplantation in treating the chronic phase of chronic myeloid leukemia: developed for the American society of hematology. Blood. 1999;94:1517–1536. [PubMed] [Google Scholar]
- 85.Slavin S, Nagler A, Naparstek E, Kapelushnik Y, Aker M, Cividalli G, Varadi G, Kirschbaum M, Ackerstein A, Samuel S, Amar A, Brautbar C, Ben-Tal O, Eldor A, Or R. Nonmyeloablative stem cell transplantation and cell therapy as an alternative to conventional bone marrow transplantation with lethal cytoreduction for the treatment of malignant and nonmalignant hematologic diseases. Blood. 1998;91:756–763. [PubMed] [Google Scholar]
- 86.Spierings E, Wieles B, Goulmy E. Minor histocompatibility antigens-big in tumour therapy. Trends Immunol. 2004;25:56–60. doi: 10.1016/j.it.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 87.Taieb J, Maruyama K, Borg C, Terme M, Zitvogel L. Imatinib mesylate impairs Flt3L-mediated dendritic cell expansion and antitumor effects in vivo. Blood. 2004;103:1966–1967. doi: 10.1182/blood-2003-10-3475. [DOI] [PubMed] [Google Scholar]
- 88.Takahashi T, Tanaka Y, Nieda M, Azuma T, Chiba S, Juji T, Shibata Y, Hirai H. Dendritic cell vaccination for patients with chronic myelogenous leukemia. Leuk Res. 2003;27:795–802. doi: 10.1016/S0145-2126(03)00011-0. [DOI] [PubMed] [Google Scholar]
- 89.Talpaz M, Silver RT, Druker BJ, Goldman JM, Gambacorti-Passerini C, Guilhot F, Schiffer CA, Fischer T, Deininger MW, Lennard AL, Hochhaus A, Ottmann OG, Gratwohl A, Baccarani M, Stone R, Tura S, Mahon FX, Fernandes-Reese S, Gathmann I, Capdeville R, Kantarjian HM, Sawyers CL. Imatinib induces durable hematologic and cytogenetic responses in patients with accelerated phase chronic myeloid leukemia: results of a phase 2 study. Blood. 2002;99:1928–1937. doi: 10.1182/blood.V99.6.1928. [DOI] [PubMed] [Google Scholar]
- 90.Topaly J, Zeller WJ, Fruehauf S. Combination therapy with imatinib mesylate (STI571): synopsis of in vitro studies. Br J Haematol. 2002;119:3–14. doi: 10.1046/j.1365-2141.2002.03806.x. [DOI] [PubMed] [Google Scholar]
- 91.Turtle CJ, Hart DN. Dendritic cells in tumor immunology and immunotherapy. Curr Drug Targets. 2004;5:17–39. doi: 10.2174/1389450043490640. [DOI] [PubMed] [Google Scholar]
- 92.Vey N, Blaise D, Lafage M, Olive D, Viens P, Baume D, Camerlo J, Stoppa AM, Gabus R, Brandely M, Hercend T, Maraninchi D. Treatment of chronic myelogenous leukemia with interleukin-2: a phase II study in 21 patients. J Immunother. 1999;22:175–181. doi: 10.1097/00002371-199903000-00009. [DOI] [PubMed] [Google Scholar]
- 93.Vivancos P, Granena A, Jr, Sarra J, Granena A. Treatment with interleukin-2 (IL-2) and interferon (IFN(alpha 2b)) after autologous bone marrow or peripheral blood stem cell transplantation in onco-hematological malignancies with a high risk of relapse. Bone Marrow Transplant. 1999;23:169–172. doi: 10.1038/sj.bmt.1701532. [DOI] [PubMed] [Google Scholar]
- 94.Wang L, Butt NM, Atherton MG, Clark RE. Dendritic cells become BCR-ABL negative in chronic myeloid leukaemia patients successfully treated with imatinib. Leukemia. 2004;18:1025–1027. doi: 10.1038/sj.leu.2403342. [DOI] [PubMed] [Google Scholar]
- 95.Wieder ED, Kant S, Lu S, He Z, Molldrem JJ. PR1 peptide vaccination of myeloid leukaemia patients induces PR1-specific CTL with high CCR7 expression. Blood. 2003;102:611a. [Google Scholar]
- 96.Zeis M, Siegel S, Wagner A, Schmitz M, Marget M, Kuhl-Burmeister R, Adamzik I, Kabelitz D, Dreger P, Schmitz N, Heiser A. Generation of cytotoxic responses in mice and human individuals against hematological malignancies using survivin-RNA-transfected dendritic cells. J Immunol. 2003;170:5391–5397. doi: 10.4049/jimmunol.170.11.5391. [DOI] [PubMed] [Google Scholar]
- 97.Zeng Y, Graner MW, Feng H, Li G, Katsanis E. Imatinib mesylate effectively combines with chaperone-rich cell lysate-loaded dendritic cells to treat bcr-abl+ murine leukemia. Int J Cancer. 2004;110:251–259. doi: 10.1002/ijc.20115. [DOI] [PubMed] [Google Scholar]