Abstract
In this phase I/II study, we evaluated the feasibility, safety and efficacy of allogeneic dendritic cells (DCs) with or without cyclophosphamide in the treatment of patients with metastatic renal cell carcinoma (RCC). Immunomagnetic beads were used to isolate CD14+ monocytes from healthy donor leukapheresis products, and CD83+ antigen-pulsed monocyte-derived DCs (moDCs) loaded with tumor lysate and keyhole limpet hemocyanin (KLH) were generated. Twelve patients were treated with allogeneic moDCs alone, while ten patients also received cyclophosphamide on days 4 and 3 prior to vaccination. Of the 22 patients enrolled, 20 received full treatment consisting of at least three vaccinations at monthly intervals. Two mixed responses with substantial tumor regression were observed. In 3 patients, disease stabilization occurred, in 13 patients disease progressed and 4 patients were lost to follow-up. Overall, immune responses against KLH and tumor lysate were weak or absent; however, the strongest increases in antigen-independent and KLH-specific responses were observed in the 2 patients with mixed responses. In addition, 1 of them showed a substantial increase in oncofetal antigen (OFA)-specific IFN-γ production. Importantly, the 2 mixed responders and 1 patient with stable disease belonged to the cyclophosphamide group. Median overall survival in the cyclophosphamide group was 23.2 and 20.3 months in the group that received allogeneic moDCs alone. Allogeneic immunotherapy with moDCs is feasible and well tolerated. However, the immunogenicity of allogeneic moDCs is clearly less pronounced than that of autologous moDC immunotherapy. Cyclophosphamide may have the capacity to augment DC-induced antitumor immunity.
Keywords: Dendritic cells, Allogeneic immunotherapy, Renal cell carcinoma, Tumor vaccine, Cyclophosphamide
Introduction
Renal cell carcinoma (RCC) is resistant to chemotherapy, hormonal treatments and radiation [1]. Due to spontaneous regressions, which were observed in isolated cases, and clinical responses occurring after immunotherapeutic interventions, RCC is considered an immunogenic tumor. However, most therapies in the past had only limited success [2]. This has resulted in the implementation of new modalities based on the immune system’s unique ability to retrieve, specifically recognize and kill microbial invaders as well as internal enemies such as malignant body cells [3–6].
Dendritic cells (DCs), the most potent antigen-presenting cells (APC), play a central role in the induction of T- and B-cell immune responses [7]. Immature DCs can be cultured in sufficient numbers from abundant circulating monocytes using GM-CSF and IL-4 (monocyte-derived DCs, moDCs) [8–10]. Inflammatory stimuli induce a final maturation step, which is characterized by a re-programming that enhances the immunostimulatory potential of the DCs [11, 12]. In clinical studies, DCs were found to be safe and to induce promising immune and clinical responses [13].
The role of allogeneic response in tumor immunity has recently been reviewed [14]. Accordingly, allogeneic MHC molecules on the injected DCs should activate patient T cells with alloreactivity, which occur at high precursor frequencies. Among the responding cells, T cells may be present with specificity, for tumor peptides presented on self-MHC molecules. In addition, the strong CD4+T-cell response against allogeneic MHC will provide T cell help for the self-restricted responses to tumor peptides. Moreover, innate immune responses against allogeneic MHC should generate an immunostimulatory milieu, which favors antitumor immune responses. Finally, under such immunostimulatory conditions, endogenous patient DCs may acquire antigens from the injected allogeneic DCs and cross-present them to self-restricted T cells [15].
Such considerations prompted us to treat metastatic RCC patients with CD83+ allogeneic moDCs loaded with tumor lysate and KLH. In an attempt to further enhance vaccination efficacy, a cohort of patients received treatment with low-dose cyclophosphamide prior to each vaccination. Low-dose cyclophosphamide has been described to down-regulate suppressor T-cell activity, which may be a prerequisite for the success of tumor immunotherapy [16, 17].
Patients and methods
Patient eligibility, study design and evaluation criteria
All patients included in this single-center study had RCC with clear-cell histology and bi-dimensionally measurable metastatic lesions. All patients had their primary tumor removed. Patients with solitary brain metastasis, malignancies other than RCC within the last 5 years, treatment with immunosuppressive drugs, other immunotherapies or chemotherapies within 4 weeks prior to the start of treatment, pregnancy or lactation, presence of acute or chronic infections and HIV or viral hepatitis or a Karnofsky index <60 were excluded from the study. Prior to inclusion, every patient underwent a physical examination, evaluation of the clinical history and determination of hematological and biochemical parameters. Furthermore, computed tomography (CT) of brain, chest and abdomen and a bone scan were performed. The study protocol was reviewed and approved by the Austrian Advisory Board for Pharmaceutical Products. All patients were informed about the investigative character of the study and gave written informed consent. Clinical response evaluation was based on CT scans performed prior to inclusion and 1 month after the third vaccination. A complete response was defined as the absence of all evidence of disease for at least 1 month. A partial response was defined as a decrease of at least 30% in tumor mass lasting for at least 1 month with no progression of any lesion or appearance of new lesions. Complete or partial response of the original lesions with the appearance of at least one new lesion was referred to as mixed response. All patients were evaluated for response. Survival-time measurements started from the first vaccination.
Tumor cell culture and preparation of lysate
Autologous tumor tissue was the preferred source of tumor antigen. Alternatively, a permanent RCC cell line (A-498) was used in cases where no autologous tissue was available. The A-498 cell line is HLA-A2+ and expresses tumor antigens such as Her-2/neu and MUC-1 [18, 19]. The expression of Her-2/neu and MUC-1 on the A-498 cell line used in the present study was confirmed in our laboratory (data not shown). After mechanical disintegration, tissue was digested with 1 mg/ml of type 1 collagenase (EC 3.4.24.3; Sigma, St. Louis, MO, USA) and 40 U/ml of type 1 desoxyribonuclease (DNase; EC 3.1.21.1; Sigma, St. Louis, MO, USA), washed extensively and the resulting cell suspension was seeded in 75 cm2 culture flasks (Costar,Corning Inc., NY, USA) at a cell density of 3–5×104 cells per ml of RPMI 1640 supplemented with 10% fetal bovine serum (FBS; Hyclone, Logan, UT, USA). On day 2 or 3, non-adherent cells were removed and fresh medium was added. Subconfluent cells were adapted to FBS-free medium for the last 48 h and then harvested. Random samples were tested for the expression of G250, which is considered to be one of the best markers for RCC [20, 21]. G250 expression could be detected in all samples tested.
Cell lysates were generated by repeated freeze-thaw cycles (liquid nitrogen, 37°C water bath), irradiated with 100 Gy and stored at −80°C. Protein concentrations of the lysates were determined using the Bio-Rad Protein Assay (Bio-Rad Laboratories, Munich, Germany).
Culture of moDCs
Healthy volunteers (n=7) negative in HIV, hepatitis and neopterin testing were subjected to standard leukapheresis at the local Institute for Blood Transfusion. Informed consent was signed by all volunteers. Leukapheresis was performed with the Cobe Spectra cell separator. Using the MNC program at a continuous whole-blood inlet flow rate ranging from 50 to 70 ml/min, 3–5 l of whole blood were processed. Anticoagulant Citrate Dextrose Solution A (ACD-A, Baxter, Austria) at a 1:12 ratio was used for anticoagulation.
The CD14+ monocytes were separated from leukapheresis products by positive selection using the CD14 Reagent, the CliniMACS Tubing Set 600 (for up to 20×109 cells) and the CliniMACS Instrument. All steps were carried out according to the manufacturer’s instructions (Miltenyi Biotec, Bergisch Gladbach, Germany).
The CD14+ cells (50×106 in 50 ml) were cultured in 162 cm2 cell culture flasks in AIM-V (GIBCO, Paisley, Scotland, UK) containing 1% heat-inactivated human AB plasma (local Institute of Blood Transfusion), 10 mM HEPES (Biowhittaker, Verviers, Belgium) and 50 μM 2-mercaptoethanol as well as a combination of recombinant human GM-CSF (1,000 U/ml; Leucomax, Novartis) and recombinant human IL-4 (1,000 U/ml; Strathmann Biotech, Hannover, Germany). After 2 days of culture, 50 ml of fresh medium containing supplements were added. Day-5 moDCs were harvested and frozen in liquid nitrogen using a standard protocol (50% AIM-V, 40% human AB plasma, 10% DMSO). Two days before vaccination, moDCs were thawed, counted and 18×106 cells were replated in 6-well plates at 1.8×106 cells per well in 6 ml of fresh medium containing GM-CSF (1,000 U/ml) and IL-4 (250 U/ml) as well as 1% heat-inactivated human AB plasma.
All cells were pulsed with RCC lysate (10 μg/ml) and half of the cells also with KLH (10 μg/ml; Calbiochem-Novabiochem, San Diego, CA, USA). Maturation was induced on day 5 by stimulation with a “cocktail” consisting of 1,000 U/ml of recombinant human TNF-γ, IL-1ß (5 ng/ml), IL-6 (10 ng/ml)(all from R&D Systems, Minneapolis, MN, USA) and 1 μM prostaglandin E2 (Prostin E2, Pharmacia & Upjohn, Vienna, Austria) [22]. After 48 h, moDCs were harvested, washed, counted in the presence of trypan blue and resuspended in lactated Ringer’s solution containing 1% heat-inactivated human AB plasma. On average, 12×106 cells of 18×106 plated cells were recovered after antigen loading and maturation (66.7%).
An aliquot of the cells was removed for phenotypic analysis and sterility testing. Sterility tests included the incubation of the test sample in both solid and fluid media (soybean–casein digest, fluid thiolglycolate and Sabouraud-4% fluid) to detect the growth of contaminating bacteria (anaerobic and aerobic, gram-negative and gram-positive) and fungi (yeast and mold), respectively.
Patient treatment
A treatment cycle consisted of 3 vaccinations in monthly intervals. Different donors were used for each immunization to avoid dominant alloreactive T-cell responses. Administration was i.d. and i.v. (ratio 3:7). Thereafter, a CT re-evaluation was performed, and in cases of disease progression, three additional vaccinations were offered to the patient.
In 18 of the 22 patients, a standard delayed-type hypersensitivity (DTH) testing for recall antigens (tetanus, diphtheria, Streptococcus, tuberculin, Candida albicans, Trichophyton mentagrophytes and Proteus mirabilis; Multitest Immignost, Biosyn, Fellbach, Germany) was performed prior to treatment and considered positive when at least one antigen induced induration and redness of more than 2 mm in mean diameter.
The first ten patients entering the study received pretreatment with 300 mg/m2 of cyclophosphamide on day 3 and 4 prior to each vaccination. Cyclophosphamide was infused over a period of 2 h. Additionally, 3,000-ml fluid during therapy, as well as sodium 2-mercaptoethane sulfonate (mesna) at a dose of 300 mg/m2 before chemotherapy as well as 4 and 8 h after chemotherapy were administered to prevent hemorrhagic cystitis.
For i.v. application, moDCs were resuspended in 10 ml of lactated Ringer’s solution containing 1% autologous serum and administered through a peripheral i.v. catheter. In the case of i.d. administration, moDCs were resuspended in 0.5 ml of lactated Ringer’s solution containing 1% autologous serum.
Antigen-specific proliferation and cytokine production
PBMCs were harvested before and after treatment. PBMCs (106/ml) were stimulated in triplicates in flat-bottomed 96-well plates with tumor lysate (10 μg/ml), KLH (10 μg/ml) or OFA [23] (1 μg/ml). Cultures were pulsed during the last 16 h with 1 μCi [3H] thymidine (1 μCi/well=37 kBq/well, ICN Biomedicals, Eschwege, Germany) per well. Cells were harvested on glass fiber filters using a Skatron cell harvester (Skatron Instruments, Lier, Norway) and analyzed in a liquid scintillation counter.
Results
Patient characteristics and immune status
Between May 2000 and October 2001, 22 patients (8 women and 14 men) with a mean age of 55.2 years at the first vaccination were enrolled in this phase I/II study. All patients gave written informed consent before study entry. To assess the cellular immune status, skin testing for DTH against recall antigens was performed before the start of treatment. Only 6 of 18 patients tested showed at least one positive reaction, indicating that the immune system was compromised in the majority of the patients. Performance state was >60% according to Karnofsky. Pathological examination of the primary tumors was performed to select RCC tumors of the clear-cell type. Patient characteristics are summarized in Table 1.
Table 1.
Patient characteristics at initial diagnosis
| Patient no. | Age (years) | Gender | Prior therapy | Localization of metastases |
|---|---|---|---|---|
| 1 | 57 | Male | IL-2/IFN-α | Lung |
| 2 | 54 | Male | None | Lung, lymph node |
| 3 | 60 | Male | None | Lung |
| 4 | 44 | Male | None | Lymph node |
| 5 | 46 | Female | None | Lung |
| 6 | 40 | Female | IL-2/IFN-α | Lung lymph node |
| 7 | 51 | Male | None | Lung |
| 8 | 65 | Female | None | Liver, bone |
| 9 | 48 | Male | None | Lung, liver |
| 10 | 54 | Female | None | Liver, lymph node, local recurrence |
| 11 | 58 | Male | IL-2/IFN-α | Lung, lymph node, liver |
| 12 | 75 | Female | None | Lung, lymph node |
| 13 | 67 | Male | None | Lung |
| 14 | 52 | Male | None | Liver, bone, local recurrence |
| 15 | 64 | Male | None | Lung |
| 16 | 51 | Male | None | Lung, lymph node, local recurrence |
| 17 | 47 | Male | None | Lung, lymph node, soft tissue |
| 18 | 54 | Female | None | Bone |
| 19 | 56 | Female | None | Lung |
| 20 | 56 | Male | None | Lung |
| 21 | 44 | Male | IL-2/IFN-α | Lung |
| 22 | 72 | Female | None | Lung |
Vaccine generation and patient treatment
The mean number of PBMCs obtained by leukapheresis was 87×108, ranging from 60 to 140×108. Monocytes were isolated immunomagnetically using anti-CD14 beads. The mean number of CD14+ cells was 10×108, ranging from 7 to 16×108 resulting in a mean number of 4.6×108 (2–9×108) immature DCs. On average, 66.7% of the plated immature DCs were recovered after antigen loading and maturation.
Tumor cell cultures were successful in all 16 patients in whom autologous tissue was available. In the remaining six patients lysate of the A-498 cell line [18, 19] had to be used. In some patients (n=5), cultures were tested for the expression of the G250 antigen, which is considered to be the best marker of RCC [20, 21]. All cultures were found to contain G250+ cells (data not shown). Tumor cell cultures, immature DC bulk cultures and the final vaccine consisting of tumor lysate-pulsed, CD83+ moDCs were subjected to sterility testing. All tests remained negative.
Twenty patients received the three planned vaccinations, whereas two patients progressed early and died before completion of therapy (Table 2). The mean number of vaccinations was 3.68, ranging from three to six with an average moDC dose administered per patient of 8.26×106 moDCs (2.5–10×106). The percentage of CD83+ cells ranged from 64.9 to 99, with a mean of 86.6 (SD=8.4).
Table 2.
Patient treatment and clinical outcome
| Patient no. | Cy | Tumor lysate | No. of vaccinations | Average moDC dose (×106) | Survival (months) | Outcome |
|---|---|---|---|---|---|---|
| 1 | + | A-498 | 3 | 2.5 | – | LFU |
| 2 | + | Autologous | 3 | 10 | 5.7 | MR |
| 3 | + | Autologous | 3 | 8 | 24.9+ | SD |
| 4 | + | Autologous | 6 | 4.3 | 27.1+ | PD |
| 5 | + | Autologous | 3 | 10 | – | LFU |
| 6 | + | A-498 | 6 | 3.2 | 29.1 | PD |
| 7 | + | Autologous | 3 | 5.7 | – | LFU |
| 8 | + | Autologous | 6 | 5 | 27.9 | MR |
| 9 | + | Autologous | 2 | 3.5 | 2.8 | PD |
| 10 | + | Autologous | 3 | 10 | 3.8 | PD |
| 11 | − | A-498 | 3 | 9.5 | 22.9+ | SD |
| 12 | − | Autologous | 3 | 10 | 18.9+ | SD |
| 13 | − | Autologous | 3 | 10 | 19.1+ | PD |
| 14 | − | A-498 | 3 | 10 | 5.2 | PD |
| 15 | − | Autologous | 3 | 10 | 20.3 | PD |
| 16 | − | autologous | 3 | 10 | 20.1 | PD |
| 17 | − | Autologous | 6 | 10 | 10.7 | PD |
| 18 | − | Autologous | 3 | 10 | 5.1 | PD |
| 19 | − | A-498 | 6 | 10 | 21.6+ | PD |
| 20 | − | Autologous | 3 | 10 | 20.9+ | PD |
| 21 | − | A-498 | 6 | 10 | – | LFU |
| 22 | − | Autologous | 1 | 10 | 1.1 | PD |
Cy cyclophosphamide, LFU lost to follow-up
Toxicity
Treatment was well tolerated with no or moderate flu-like symptoms. Body temperature was increased in ten patients (>37.5°C) but did not exceed 39°C. Side effects from cyclophosphamide treatment were tolerable and did not require therapy, with the exception of one patient from the cyclophosphamide group who developed severe thrombocytopenia (WHO grade IV) after the second vaccination. Subsequent examinations revealed antibodies against glycoprotein IIb/IIIa but excluded heparin-induced thrombopenia and made drug-induced, platelet-directed toxicity unlikely. Finally, analysis of hematopoietic chimerism remained negative. The patient was treated with corticosteroids and rituximab and could be discharged after 14 days with stable thrombocyte counts. The cause of thrombopenia could not be identified. In principle, thrombopenia could also have been induced as part of paraneoplasia [24–26]. In conclusion, it cannot be definitively excluded that moDC-based immunization had exacerbated pre-existing, immune-mediated platelet destruction.
Clinical outcome
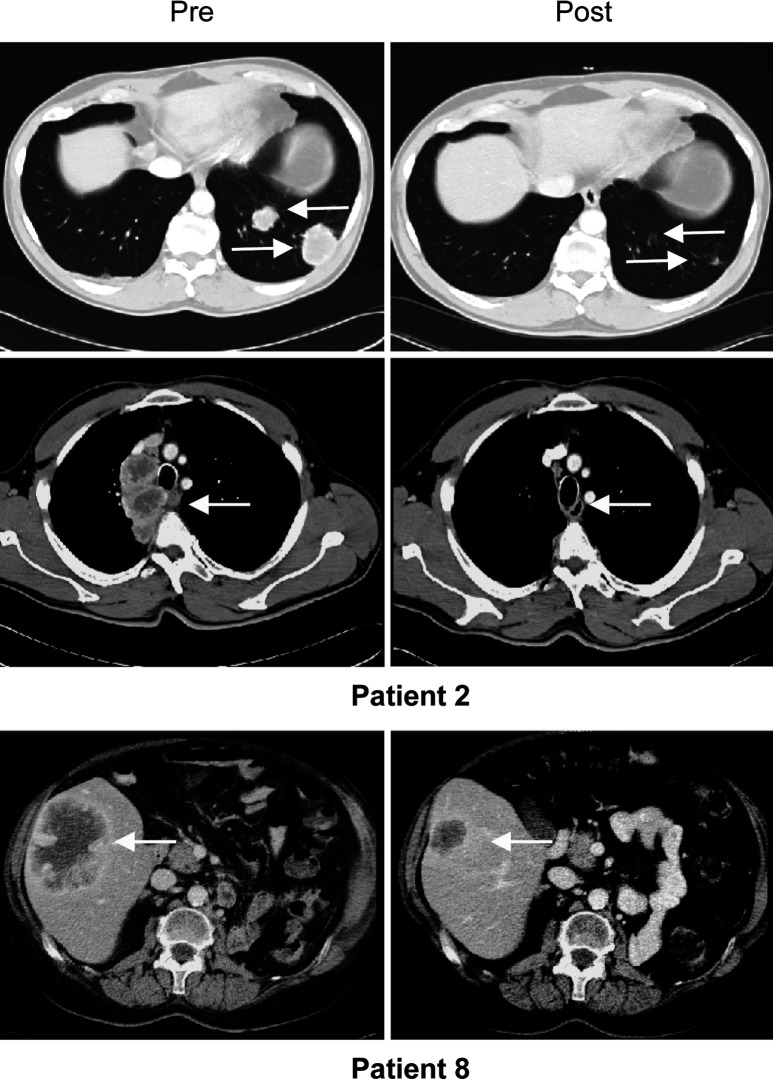
Of the 20 patients who had received full treatment (=at least three vaccinations), mixed responses were observed in two patients (nos. 2 and 8). One of them (patient 2) showed a complete response of his lung metastases and partial regression of mediastinal lesions 1 month after the third vaccination (Fig. 1). However, the patient concomitantly developed two brain lesions and died 2 months later. The other (patient 8) showed regression of a large liver metastasis (Fig. 1; duration 3.9 months). This patient developed a new bone metastasis in the right pelvis and died 2 years later. At the time of evaluation, three patients had been stable for 19, 23 and 25 months, respectively (mean: 22.3 months). One patient with multiple lung metastases remained stable for 16 months but then developed a bone metastasis. In 12 patients, disease continuously progressed. Of the 13 patients with progressive disease, four are currently alive with a mean follow-up of 22.2 months, whereas nine died after a mean time of 11 months; four patients were lost to follow-up (three patients from the cyclophosphamide group). It is worth noting that the two mixed responses (patients 2 and 8) as well as one stabilization (patient 3) occurred in the cyclophosphamide group. Median overall survival was 23.2 months in the cyclophosphamide group and 20.3 months in the group that received allogeneic moDCs alone.
Fig. 1.
Computed tomography scans of two patients with mixed responses. Arrows point at responding lesions.
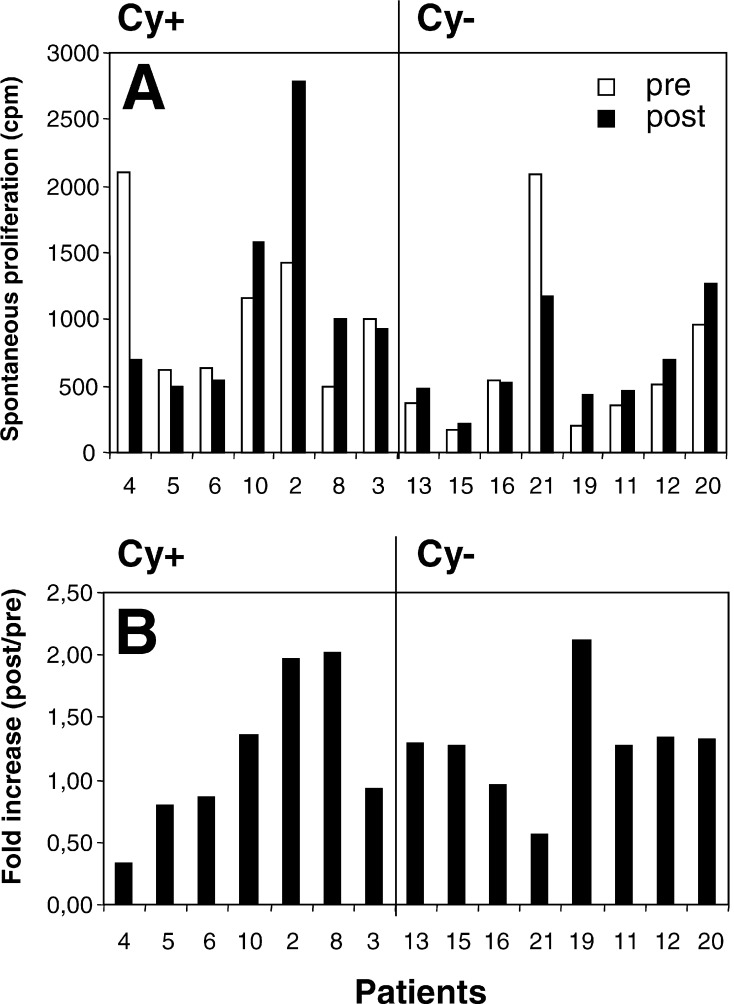
Antigen-independent proliferation
A recurrent observation in the immunomonitoring experiments was the vaccination-induced increase of PBMC proliferation in the absence of exogenous antigen. When we compared antigen-independent proliferation before the first and after the third vaccination, nine of 15 evaluated patients showed an increase in proliferation (Fig. 2a, b). Three patients (2, 8 and 19) were outstanding with an approximately twofold increase in antigen-independent proliferation (Fig. 2b). Figure 2a indicates that the increase in patient 19 occurred at a low level (from 206 to 436 cpm). In contrast, patients 2 and 8 displayed increases from 1,416 to 2,791 cpm and from 499 to 1,007 cpm, respectively. This is intriguing since patients 2 and 8 had tumor regression (Fig. 1 and Table 2).
Fig. 2.
Increase in antigen-independent (spontaneous) proliferation. Data are presented as cpm before (pre) and after (post) treatment (a) or as fold increase (b).
Antigen-specific responses: KLH
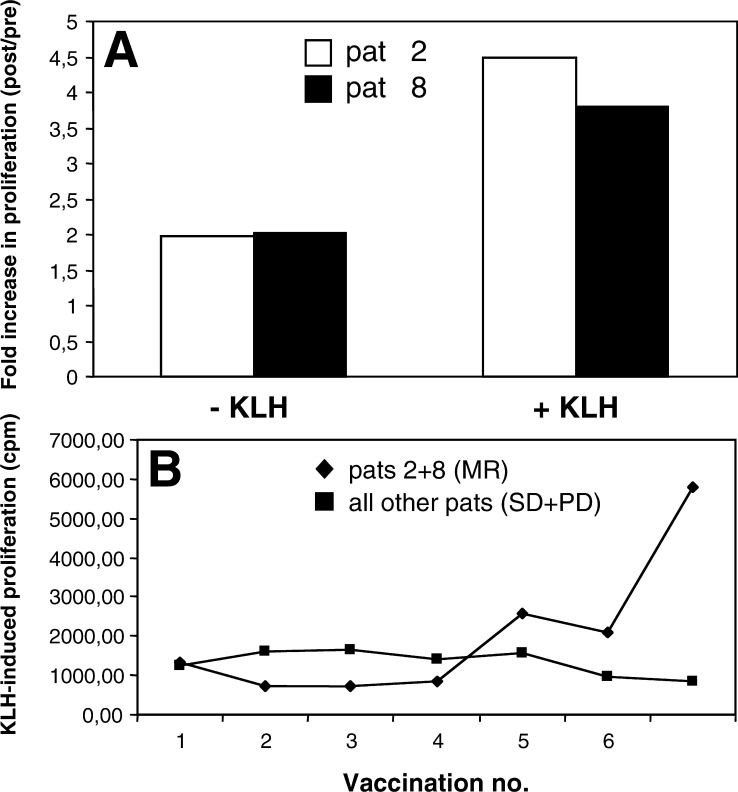
Antigen-induced proliferation was assessed before the first treatment and 1 month after the last. Monitoring of KLH-specific responses could be performed in six patients of the cyclophosphamide group and in eight patients of the group not treated with cyclophosphamide. Overall, KLH-specific responses were weak. As mentioned above, after treatment the two patients with tumor regression (patients 2 and 8) showed a twofold increase in proliferation even in the absence of antigen (Fig. 2). In the presence of KLH, a 4.5-fold increase (patient 2) and a 3.8-fold increase (patient 8) was measurable (Fig. 3a). In patient 2, an increase in IFN-γ production was also noted (0 pg/ml before and 333 pg/ml after treatment).
Fig. 3.
Increase in KLH-specific immunity. a Fold increase in the two patients with tumor regression (patients 2 and 8). b Development of KLH-specific immunity as a function of the number of vaccinations.
The increase in proliferation observed in patients 2 and 8 appeared to be due to KLH immunity developing after the fourth vaccination (Fig. 3b). The remaining patients failed to elicit significant KLH immunity. Thus, the two patients with tumor regression had the strongest proliferative responses of all patients tested.
Antigen-specific responses: tumor lysate and OFA
We failed to detect significant proliferative or cytokine responses against tumor lysate. Several patients had pre-existing immunity against OFA, which was, however, not enhanced by allogeneic DC vaccination in the majority of the patients (data not shown). Patient 2 again showed an unusual response with an increase in OFA-induced IFN-γ production from 191 pg/ml (before treatment) to 579 pg/ml (after treatment).
Discussion
Dendritic cells naturally induce immune responses against various antigens and may therefore be useful to stimulate immune responses against tumor-associated antigens. The safety and immunogenicity of cultured autologous DCs has been tested and confirmed in over 30 clinical trials [27]. In prior studies from our department, autologous moDCs induced antigen-specific immune responses in all patients tested and clinical responses in some patients [23, 28, 29]. We found that mature, autologous moDCs pulsed with KLH induced vigorous proliferative responses after a single vaccination [23]. Increases ranged from 6- to 904-fold. In contrast, in the present study mature, allogeneic moDCs pulsed with KLH failed to induce comparable responses after one or more vaccinations. Our decision to use allogeneic moDCs was also based on the assumption that antigen transfer occurs between the injected allogeneic moDCs and endogenous DCs, which subsequently elicit antigen-specific responses [30, 31]. Recent work has indeed confirmed that antigen transfer between migrating and resident DCs in lymph nodes is important for the induction of immune responses [15]. However, it was also shown that the injected DCs and endogenous DCs had to express the same MHC class II allotype to induce optimal T-cell activation. Thus, the limited antigen transfer in our allogeneic setting may be responsible for the failure to induce strong immune responses.
The lack of strong immune responses after allogeneic moDC vaccination is in accordance with relatively poor clinical responses. Whereas two complete responses and one partial response as well as seven stabilizations were observed in the autologous setting (27 patients) [23], only two mixed responses and three stabilizations were noticed in the present allogeneic setting (20 patients). Nevertheless, it was interesting to note that the two mixed responders (patients 2 and 8) showed enhanced proliferative responses in both antigen-independent (Fig. 2) and KLH-dependent fashion (Fig. 3a, b) whereas non-responders failed to mount comparable responses (Fig. 3b).
Cyclophosphamide has been used in the treatment of human tumors for more than 40 years. By itself, cyclophosphamide is not cytotoxic but is rapidly converted to highly active alkylating metabolites by liver enzymes. Cytotoxic chemotherapy with cyclophoposphamide can also be immunomodulatory and down-regulate the activity of suppressor cells [16, 17, 32]. Intriguingly, the two mixed responses and one stabilization occurred in the group that received pretreatment with cyclophosphamide (Table 2). In a mouse model, cyclophosphamide was able to augment T-cell responses against sheep red blood cells [33]. However, in this model the enhancing effect of cyclophosphamide could only be observed under conditions of suboptimal antigen dose. One possible interpretation of the present data is that under the suboptimal conditions of the allogeneic setting used in the present study, cyclophosphamide was able to augment immune and clinical responses, at least in some patients (Figs. 1, 2 and 3).
Acknowledgements
We thank Joseph H. Coggin Jr and Adel Barsoum for providing recombinant oncofetal antigen. This work was supported by a grant of the kompetenzzentrum medizin tirol (KMT) to Martin Thurnher.
References
- 1.Motzer RJ, Bander NH, Nanus DM. Renal-cell carcinoma. N Engl J Med. 1996;335:865. doi: 10.1056/NEJM199609193351207. [DOI] [PubMed] [Google Scholar]
- 2.Mulders P, Figlin R, deKernion JB, Wiltrout R, Linehan M, Parkinson D, deWolf W, Belldegrun A. Renal cell carcinoma: recent progress and future directions. Cancer Res. 1997;57:5189. [PubMed] [Google Scholar]
- 3.Pawelec G, Rees RC. Cancer vaccination progress. Trends Mol Med. 2002;8:545. doi: 10.1016/S1471-4914(02)02430-9. [DOI] [PubMed] [Google Scholar]
- 4.Ward S, Casey D, Labarthe MC, Whelan M, Dalgleish A, Pandha H, Todryk S. Immunotherapeutic potential of whole tumour cells. Cancer Immunol Immunother. 2002;51:351. doi: 10.1007/s00262-002-0286-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whelan M, Whelan J, Russell N, Dalgleish A. Cancer immunotherapy: an embarrassment of riches. Drug Discov Today. 2003;8:253. doi: 10.1016/S1359-6446(03)02633-3. [DOI] [PubMed] [Google Scholar]
- 6.Thurnher M, Rieser C, Höltl L, Papesh C, Ramoner R, Bartsch G. Dendritic cell-based immunotherapy of renal cell carcinoma. Urol Int. 1998;61:67. doi: 10.1159/000030291. [DOI] [PubMed] [Google Scholar]
- 7.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 8.Sallusto F, Cella M, Danieli C, Lanzavecchia A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J Exp Med. 1995;182:389. doi: 10.1084/jem.182.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 1994;179:1109. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Romani N, Gruner S, Brang D, Kampgen E, Lenz A, Trockenbacher B, Konwalinka G, Fritsch PO, Steinman RM, Schuler G. Proliferating dendritic cell progenitors in human blood. J Exp Med. 1994;180:83. doi: 10.1084/jem.180.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cella M, Engering A, Pinet V, Pieters J, Lanzavecchia A. Inflammatory stimuli induce accumulation of MHC class II complexes on dendritic cells. Nature. 1997;388:782. doi: 10.1038/42030. [DOI] [PubMed] [Google Scholar]
- 12.Rieser C, Böck G, Klocker H, Bartsch G, Thurnher M. Prostaglandin E2 and tumor necrosis factor alpha cooperate to activate human dendritic cells: synergistic activation of interleukin 12 production. J Exp Med. 1997;186:1603. doi: 10.1084/jem.186.9.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brossart P, Wirths S, Brugger W, Kanz L. Dendritic cells in cancer vaccines. Exp Hematol. 2001;29:1247. doi: 10.1016/S0301-472X(01)00730-5. [DOI] [PubMed] [Google Scholar]
- 14.Fabre JW. The allogeneic response and tumor immunity. Nat Med. 2001;7:649. doi: 10.1038/89008. [DOI] [PubMed] [Google Scholar]
- 15.Kleindienst P, Brocker T. Endogenous dendritic cells are required for amplification of T cell responses induced by dendritic cell vaccines in vivo. J Immunol. 2003;170:2817. doi: 10.4049/jimmunol.170.6.2817. [DOI] [PubMed] [Google Scholar]
- 16.North RJ. Cyclophosphamide-facilitated adoptive immunotherapy of an established tumor depends on elimination of tumor-induced suppressor T cells. J Exp Med. 1982;155:1063. doi: 10.1084/jem.155.4.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berd D, Maguire HC, Jr, Mastrangelo MJ. Potentiation of human cell-mediated and humoral immunity by low-dose cyclophosphamide. Cancer Res. 1984;44:5439. [PubMed] [Google Scholar]
- 18.Giard DJ, Aaronson SA, Todaro GJ, Arnstein P, Kersey JH, Dosik H, Parks WP. In vitro cultivation of human tumors: establishment of cell lines derived from a series of solid tumors. J Natl Cancer Inst. 1973;51:1417. doi: 10.1093/jnci/51.5.1417. [DOI] [PubMed] [Google Scholar]
- 19.Brossart P, Stuhler G, Flad T, Stevanovic S, Rammensee HG, Kanz L, Brugger W. Her-2/neu-derived peptides are tumor-associated antigens expressed by human renal cell and colon carcinoma lines and are recognized by in vitro induced specific cytotoxic T lymphocytes. Cancer Res. 1998;58:732. [PubMed] [Google Scholar]
- 20.Mulders P, Bleumer I, Oosterwijk E. Tumor antigens and markers in renal cell carcinoma. Urol Clin North Am. 2003;30:455. doi: 10.1016/s0094-0143(03)00024-7. [DOI] [PubMed] [Google Scholar]
- 21.Oosterwijk E, Bander NH, Divgi CR, Welt S, Wakka JC, Finn RD, Carswell EA, Larson SM, Warnaar SO, Fleuren GJ, et al. Antibody localization in human renal cell carcinoma: a phase I study of monoclonal antibody G250. J Clin Oncol. 1993;11:738. doi: 10.1200/JCO.1993.11.4.738. [DOI] [PubMed] [Google Scholar]
- 22.Jonuleit H, Kuhn U, Muller G, Steinbrink K, Paragnik L, Schmitt E, Knop J, Enk AH. Pro-inflammatory cytokines and prostaglandins induce maturation of potent immunostimulatory dendritic cells under fetal calf serum-free conditions. Eur J Immunol. 1997;27:3135. doi: 10.1002/eji.1830271209. [DOI] [PubMed] [Google Scholar]
- 23.Höltl L, Zelle-Rieser C, Gander H, Papesh C, Ramoner R, Bartsch G, Rogatsch H, Barsoum AL, Coggin JH, Jr, Thurnher M. Immunotherapy of metastatic renal cell carcinoma with tumor lysate-pulsed autologous dendritic cells. Clin Cancer Res. 2002;8:3369. [PubMed] [Google Scholar]
- 24.Schwartz KA, Slichter S J, Harker LA. Immune-mediated platelet destruction and thrombocytopenia in patients with solid tumours. Br J Haematol. 1982;51:17. doi: 10.1111/j.1365-2141.1982.tb07285.x. [DOI] [PubMed] [Google Scholar]
- 25.Tarraza HM, Carroll R, De Cain M, Jones M. Recurrent ovarian carcinoma: presentation as idiopathic thrombocytopenic purpura and a splenic mass. Eur J Gynaecol Oncol. 1991;12:439. [PubMed] [Google Scholar]
- 26.Kamra D, Boselli J, Sloane BB, Gladstone DE. Renal cell carcinoma induced Coombs negative autoimmune hemolytic anemia and severe thrombocytopenia responsive to nephrectomy. J Urol. 2002;167:1395. doi: 10.1097/00005392-200203000-00049. [DOI] [PubMed] [Google Scholar]
- 27.Schuler G, Schuler-Thurner B, Steinman RM. The use of dendritic cells in cancer immunotherapy. Curr Opin Immunol. 2003;15:138. doi: 10.1016/S0952-7915(03)00015-3. [DOI] [PubMed] [Google Scholar]
- 28.Höltl L, Rieser C, Papesh C, Ramoner R, Bartsch G, Thurnher M. CD83+ blood dendritic cells as a vaccine for immunotherapy of metastatic renal-cell cancer. Lancet. 1998;352:1358. doi: 10.1016/s0140-6736(05)60748-9. [DOI] [PubMed] [Google Scholar]
- 29.Höltl L, Rieser C, Papesh C, Ramoner R, Herold M, Klocker H, Radmayr C, Stenzl A, Bartsch G, Thurnher M. Cellular and humoral immune responses in patients with metastatic renal cell carcinoma after vaccination with antigen pulsed dendritic cells. J Urol. 1999;161:777. doi: 10.1097/00005392-199903000-00009. [DOI] [PubMed] [Google Scholar]
- 30.Knight SC, Iqball S, Roberts MS, Macatonia S, Bedford PA. Transfer of antigen between dendritic cells in the stimulation of primary T cell proliferation. Eur J Immunol. 1998;28:1636. doi: 10.1002/(SICI)1521-4141(199805)28:05<1636::AID-IMMU1636>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 31.Turley SJ, Inaba K, Garrett WS, Ebersold M, Unternaehrer J, Steinman RM, Mellman I. Transport of peptide-MHC class II complexes in developing dendritic cells. Science. 2000;288:522. doi: 10.1126/science.288.5465.522. [DOI] [PubMed] [Google Scholar]
- 32.Livingston PO, Cunningham-Rundles S, Marfleet G, Gnecco C, Wong GY, Schiffman G, Enker WE, Hoffman MK. Inhibition of suppressor-cell activity by cyclophosphamide in patients with malignant melanoma. J Biol Response Mod. 1987;6:392. [PubMed] [Google Scholar]
- 33.Askenase PW, Hayden BJ, Gershon RK. Augmentation of delayed-type hypersensitivity by doses of cyclophosphamide which do not affect antibody responses. J Exp Med. 1975;141:697. doi: 10.1084/jem.141.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]