Abstract
Bispecific antibodies (Bs-Abs) containing an anti-CD3 and an anti-TAA specificity can recruit T cells to the tumor for cancer immunotherapy. To be effective, efficient activation at the tumor site is a prerequisite. This can be achieved by triggering both the T-cell receptor and the co-stimulatory molecule CD28. We engineered two recombinant cross-interacting Bs-Abs (CriBs-Abs) by incorporating a peptide tag and its cognate single-chain variable fragment (scFv), respectively, into a pair of (tumor × CD3) and (tumor × CD28) binding Bs-Abs. A 30-fold lower concentration of the activating CriBs-Ab as compared to non interacting Bs-Ab was sufficient for strong T-cell activation in the presence of tumor cells. One thousand-fold higher concentrations of both CriBs-Abs were required for marginal T-cell activation (70-fold below maximal response) in the absence of tumor cells. An optimized stoichiometry (1 : 1000) of activating versus co-stimulating CriBs-Ab thus allowed low doses of activating CriBs-Ab to induce tumor-cell dependent T-cell activation when used in combination with high concentrations of the pre-targeted co-stimulating CriBs-Ab in vitro. This indicates a large window of operation in which only tumor cell dependent T-cell activation is induced and systemic tumor cell independent T-cell activation is avoided, while ensuring optimal activation with a low concentration of the activating CriBs-Ab, which has the highest potential to induce toxic effects in vivo.
Keywords: T lymphocytes, Co-stimulation, Bispecific antibodies, Tumor immunotherapy, Recombinant antibodies, CD28
Introduction
Bispecific antibodies (Bs-Abs) are artificial antibodies with dual specificity. They can be used in tumor immunotherapy, for example, when one specificity is targeting a tumor associated antigen (TAA) and the other an activating receptor on an immune effector cell. Such Bs-Abs have been used in attempts to redirect the immune system against various types of cancer. Bs-Abs designed to redirect the activity of T cells typically recognize both a TAA and the T-cell-receptor (TCR)/CD3 complex. However, stimulation via the TCR alone (signal 1) may lead to inappropriate T-cell activation and eventually result in anergy or apoptosis [47]. Appropriate T-cell activation requires concomitant triggering of co-stimulatory receptors, such as CD28 (signal 2) [9]. Therefore, in early clinical trials using Bs-Abs providing only signal 1, T-cell pre-activation was used [8, 39, 41]. This approach is patient specific and thus labor intensive. An alternative strategy administers additional IL-2 i.v. [12, 22, 33], but this proved to be toxic in humans. When complementing the activating Bs-Abs (giving signal 1) with a co-stimulating anti-CD28 monoclonal antibody (moAb) (giving signal 2), T-cell activation in vitro improved significantly [6, 25, 26]. Animal studies, however, frequently failed to demonstrate that the co-stimulating moAb provided an additional benefit [7, 15], although some reported an improved therapeutic outcome [10]. Clinical trials as well revealed only a limited response [37]. To improve T-cell retargeting and activation, the activating Bs-Ab was complemented with a second co-stimulating Bs-Ab targeting a TAA and the CD28-receptor. Comparison of the two strategies in vitro showed that the presence of the second Bs-Ab markedly improved T-cell activation potential [6, 38]. This improvement was also visible in animal studies. In different tumor models, therapy with a combination of two Bs-Abs led to complete tumor eradication in mice [21, 30, 44, 45]. However, in clinical studies again only a limited therapeutic response was obtained [28]. Assembling both signal 1 and 2 on a single entity using an anti-(tumor × CD3 × CD28) trispecific antibody (Ts-Ab) [27] showed a high T-cell activation capacity which was tumor-cell independent, and thus unsuited for therapy.
One explanation for the discrepancy in efficacy of Bs-Abs between animal and clinical studies could be the generally low tumor targeting efficiency of antibodies in humans as compared to the results in mice [20]. Increasing the concentration of the CD3 binding Bs-Ab is limited due to an increased chance of toxicity induced by aggregating CD3 binding antibodies. So improvement of Bs-Ab therapy should include better targeting molecules that have tumor restricted activity at low concentrations of the CD3 binding antibody. Several groups have demonstrated that a higher accumulation of BsAb at the tumor site is achieved by increasing the functional affinity (avidity) of the Bs-Ab for the tumor by incorporating a bivalent tumor specificity [1, 31, 40]. In an alternative strategy an effector molecule is administered some time after a pre-targeting agent, which typically recognizes both the tumor cell and the effector molecule. Such pre-targeting strategies are currently being evaluated in radio-immunotherapy (RIT), and have been reported to markedly improve tumor localization while sparing normal tissue [5, 19]. Targeting by an “affinity enhancement system” combines both strategies: a bivalent radiolabeled effector molecule cross-links the cell-bound pre-targeting Bs-Ab, thus resulting in the formation of a more stable complex due to an increase in the avidity [2, 4, 29].
Here we evaluate a model in which two separate Bs-Abs target the tumor cell and either the CD3- or the CD28-receptor on a T-cell. A mutual cross-interaction was incorporated in both Bs-Abs, thus ideally inducing the assembly of a more stable complex at the tumor cell through an enhanced avidity. Moreover, the presence of the cross-interaction is expected to lead to co-ligation of the CD3- and CD28-receptors on recruited T cells, ensuring correct T-cell activation. We analyzed the tumor-cell dependent and independent T-cell activating characteristics of the cross-interacting Bs-Abs (CriBs-Abs) in a pre-targeting strategy in comparison to the corresponding non-CriBs-Abs.
Materials and methods
Animals
Female C57BL/6 (H-2b) mice were purchased from the Broekman Instituut Eindhoven, The Netherlands. Spleen cell preparations were made from 9–14-week-old mice.
Cell lines
HEK293T, a human embryonic kidney cell line transfected with SV40 largeT-Ag (SV40TtsA1609) [17], was used for transient eukaryotic expression. The influenza A/H3 HA-specific and H-2b -restricted CD4+ murine T-cell clone T-HA was developed and maintained as previously described [16]. Briefly, T-HA cells were cultured in vitro and restimulated biweekly with 10 ng/ml bromelain-cleaved hemagglutinin (BHA) and 5×107 syngenic spleen cells from C57BL/6 mice (3,000 rad gamma irradiated). Recombinant murine IL-2 (30 IU/ml) was added on day 3 after restimulation, and the cells were expanded by renewal of culture medium and rIL-2 every 2 days. Culture medium consisted of RPMI 1640 buffered with 12.5 mM HEPES and supplemented with 10% fetal calf serum (FCS), 2 mM GlutaMAX-1 (Life Technologies), 100 U/ml penicillin, 100 μg/ml streptomycin, 1 mM sodium pyruvate, and 5×10−5 M 2-mercaptoethanol. MO4I4 cells are C3H mouse-derived MO4 fibrosarcoma cells transfected with the human placental alkaline phosphatase (hPLAP) gene [24, 48].
Isolation of the hamster anti-murine CD28 single-chain variable fragment (scFv)
mRNA was isolated from the 37.51 hybridoma using the Fast Track 2.0 kit (Invitrogen, San Diego, CA, USA). cDNA was prepared using Ready to go You-prime First-strand Beads (Amersham Biosciences, Uppsala, Sweden). The VH-gene was isolated using the RPAS purification module (Amersham Biosciences). The VL-gene was isolated by a nested PCR using a degenerated forward primer based on the N-terminal amino acid sequence of the light chain of the mAb. (EFVLTQPKSVSE, determined using Edman degradation), and the RPAS backward primers in the CL and VL domain. The scFv was assembled by introducing a [(G)4S]3 linker in between the VH and VL gene, using a splice-overlap PCR reaction with primers designed on the basis of the VH and VL gene sequences. The scFv was cloned into the eukaryotic expression vector pCAGGS [42]. DNA amplification was performed with Vent-DNA polymerase (New England Biolabs, Beverly, MA, USA). The sequences of all cloned PCR fragments were verified. Specific binding of the 37.51 scFv to the CD28-receptor and to the CD28-receptor positive cells was demonstrated with ELISA and FACS, respectively (data not shown).
Plasmids and gene assembly
Restriction enzymes, DNA modifying enzymes and DNA polymerase were used as recommended by the manufacturers. DNA was amplified with Vent-DNA polymerase (New England Biolabs, Beverly, MA, USA). E6, 2c11 and 37.51 denote the gene fragments of a mouse anti-human placental alkaline phosphatase (anti-hPLAP), a hamster anti-murine T-cell receptor associated CD3ε chain (anti-CD3) monoclonal antibody (moAb), and a hamster anti-murine CD28-co-stimulatory receptor (anti-CD28) mAb, respectively. Expression plasmids were constructed in pCDNA3.1zeo- (Invitrogen) and pCAGGS. The cloning of the light chain (E6L) of the parental murine anti-hPLAP mAb (IgG2b/κ) in pSV51E6L and of the heavy chain E6Fd fragment in pCAGGS has been described [14, 46]. The 2c11 scFv gene and the 37.51 scFv gene, both in the VH-VL orientation (with VH and VL connected through a (G4S)3 linker), were fused to the C-terminus of the E6Fd gene, via a (G4S)3 and a G(PQ)5PGP linker, respectively. The genes encoding the mutual reactive couple, P-peptide (PSTAIRE) and an anti-P scFv (4D21) [18], were fused to the C-terminus of the E6L gene, via an EGVP or a DVDGGSRGDGPSPG linker, respectively. Genes were assembled by introduction of suitable restriction sites using modifying PCR primers. All PCR-derived fragments were sequence verified after cloning.
Production and purification of antibody fragments
For transient expression, HEK293T cells were transfected by Ca3(PO4)2 precipitation [43]. Cells were seeded at 4×106 cells/175 cm2 20 h before transfection. DNA of each expression plasmid (14 μg) was added to the cells, and 24 h later the cells were covered with supplemented Dulbecco’s modified Eagle’s medium (DMEM) containing 10% FCS or 5 mg/L bovine insulin, 5 mg/L transferrin and 5 μg/L selenium. Medium was harvested every 48 h after transfection. For stable expression lines, SP2/0-Ag14 cells were electroporated, cultured in selective medium, subcloned and screened for production. Purification of the BsAb was performed as described [51], generating >98% pure protein free of aggregates and endotoxin. Proteins were fractionated by 10% SDS-PAGE and visualized using Coomassie Brilliant Blue (CBB). Protein concentration was measured with the Micro BCA Protein Assay Reagent Kit (Pierce Chemical Company).
Determination of antibody KD values
For determination of the KD values for the anti-CD3 and anti-CD28 specificities, 2×105 T-HA cells were incubated with various dilutions of purified CriBs-Abs in 100 μl of PBS, 1% BSA, 0.02% sodium-azide (PBS/BSA) for 1 h at 0°C. Cells were then washed with PBS/BSA and incubated for 1 h in 100 μl PBS/BSA containing 10 μg/ml FITC-conjugated goat anti-mouse (Fab’)2 (Organon Teknika, Durham, NC, USA). After a final wash, cells were analyzed by FACS. The inverse of the determined fluorescence was plotted as a function of the inverse of antibody concentration to determine KD by the Lineweaver-Burk method [35]. KD values were determined by the following equation: 1/(F−Fback)=1/Fmax + (KD/Fmax)(1/[Ab]), where F=fluorescence units, Fback=background fluorescence and Fmax was calculated from the plot. KD for the anti-TAA specificities was determined by ELISA: soluble TAA (hPLAP) was coated and subsequently incubated with a serial dilution of the Bs-Ab. The bound Bs-Ab was revealed with an anti-His tag Ab conjugated to HRP.
Flow cytometry
2×105 T-HA, MO4I4 or MO4 cells were incubated with 5 μg/ml Abs in 100 μl of PBS, 1% BSA, 0.02% PBS/BSA for 1 h at 0°C. Cells were then washed with PBS/BSA and incubated for 1 h in 100 μl PBS/BSA containing 10 μg/ml FITC-conjugated goat anti-mouse (Fab’)2 (Organon Teknika), or anti-hamster FITC-coupled antiserum (Sera-Lab). After a final wash, cells were analyzed by flow cytometry (FACSCalibur; Becton Dickinson, Sunnyvale, CA,USA).
Proliferation and cytokine assays
The T-HA cells, cultured with murine recombinant IL-2 (mIL-2) produced in our laboratory (VIB Protein Expression & Purification Core, Ghent, Belgium) were harvested and washed three times to remove mIL-2. MO4I4 tumor cells were pre-treated with mitomycine C at 37°C in the dark for 12 h. After removal of mitomycine C (by washing three times), 1×104 treated MO4I4 cells were co-cultured with 1×104 T-HA cells in a round-bottom well in the presence of the indicated antibody derivatives. All experiments were performed in triplicate. Proliferation was measured after 72 h by radioactive thymidine uptake. After adding 0.5 μCi/well [3H]thymidine ([3H]TdR, 1 mCi/ml, Amersham Biosciences), cells were incubated for 24 h, and then harvested on glass fiber filters. Incorporated [3H]TdR was measured on a Topcount scintillation counter (Packard Instrument, Meriden, CT, USA). For IL-2 and IFN-γ assays, 50 μl of supernatant was collected after 18 h. Secreted IL-2 and IFN-γ were determined by ELISA.
ELISA
The plates were coated overnight at room temperature with 50 μl of the desired protein (e.g. anti-IL2 or anti-IFNγ mAb) at 10 μg/ml in carbonate/bicarbonate buffer pH 9.6. The wells were then blocked with 300 μl of 1% BSA in carbonate/bicarbonate buffer for 1 h at 37°C. After 1 h incubation (37°C) with 50 μl supernatant or with 50 μl of a dilution of a pure antibody fragment in 0.1% BSA in carbonate/bicarbonate buffer, bound IL-2 or IFN-γ was detected by incubation with biotinylated anti-IL2 or anti-IFNγ, followed by incubation with streptavidin conjugated to HRP. Known concentrations of recombinant murine IL-2 and IFN-γ (VIB Protein Expression & Purification Core) were used as standard. In between each step the wells were washed three times with PBS + 0.1% Tween 20. When alkaline phosphatase was used, wells were washed with 10% DEA before adding 50 μl p-nitrophenyl phosphate (PNPP). When horseradish peroxidase (HRP) was used, 50 μl of the TMB substrate reagent set (Pharmingen, San Diego, CA, USA) were added. After 30 min incubation, A405 was measured in a microplate reader.
Surface plasmon resonance
Affinity analysis was performed using a BIAcore 2000 (BIAcore, Uppsala Sweden). One of the purified CriBs-Ab couple was immobilized on a CM5 chip in 50 mM acetate buffer pH 4. The other CriBs-Ab was used as an analyte in concentrations of 200, 100 and 50 nM, at a flow rate of 10 μl/min. The antibodies were eluted in 50 mM glycine-HCl pH 2.0. The koff, kon, KD and KA values were calculated from the sensorgram using BIAeval 3.0 software.
Results
Construction and characterization of bispecific and CriBs-Abs
The highly efficient heterodimerization of L and Fd chains in mammalian cells constitutes a platform suitable for generating fully functional, disulfide-stabilized Bs-Ab and Ts-Ab recombinant antibody derivatives of intermediate molecular size (75–100 kDa) [46]. Because the heterodimerizing Fab fragment already contributes a binding moiety, C-terminal fusion of a scFv to the L and/or Fd chains can generate bispecific Fab-scFv or trispecific Fab-(scFv)2 heterodimers.
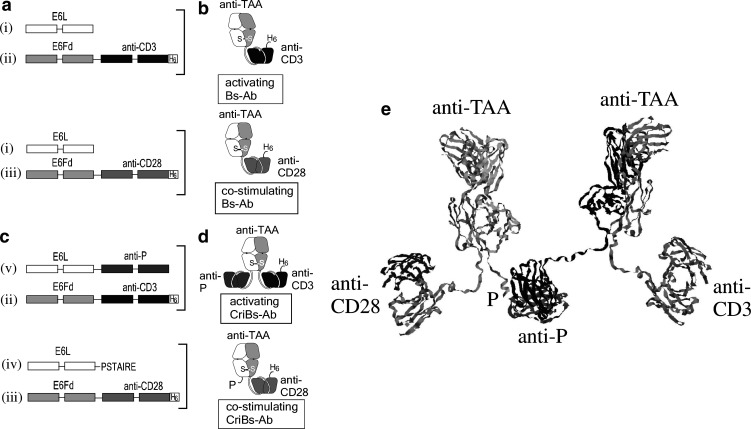
In this study we used the E6 Fab fragment that targets the hPLAP tumor marker, present on human testis and ovarian carcinomas [11, 32]. For construction of the activating BsAb anti-(TAA × CD3), the E6Fd chain was fused at its C-terminus to the gene encoding an anti-murine CD3ε scFv ([13] (Fig. 1a). This fusion gene was then co-expressed with the E6L chain for production of the anti-(TAA × CD3) Bs-Ab (Fig. 1b). Co-expression of the E6L chain with a fusion of the gene encoding an anti-murine CD28 scFv (isolated from the 37.51 hybridoma) to the C-terminus of the E6Fd-chain (Fig. 1a), led to the production of the co-stimulating Bs-Ab anti-(TAA × CD28) (Fig. 1b). In order to construct CriBs-Abs, one Bs-Ab (TAA × CD28) was modified to contain a peptide tag P, while the other Bs-Ab (TAA × CD3) was converted to a trispecific antibody by inserting a scFv specific to the P-peptide. The CriBs-Abs were thus made by additionally extending the E6L-chain with the PSTAIRE peptide (P), and with the 4D21 scFv [18] that recognizes this peptide (anti-P) (Fig. 1c). Co-expression of both extended E6L and E6Fd chains led to the production of an activating CriBs-Ab, anti-(TAA × CD3×P), and a co-stimulating CriBs-Ab, anti-(TAA × CD28)-P (Fig. 1d). For transient expression, HEK293T cells were used and yields of 1–5 mg/L were obtained. Stable cell lines of transfected SP2/0 myeloma cells could be obtained by electroporation. Expression levels of primary isolates varied between 5 mg/L and 200 mg/L, and could be improved by increasing the antibiotic used for selection. The three-dimensional model of the CriBs-Abs indicated no steric hindrance of the tumor and T cell binding sites after P/anti-P interaction (Fig. 1e). Bs-Abs and CriBs-Abs were purified from cell culture supernatants as described [51], generating >95% pure protein (Fig. 2b), free of aggregates (Fig. 2a) and endotoxins.
Fig. 1.
Construction of Bs-Abs and CriBs-Abs. a Overview of the gene constructs for expression of the activating and co-stimulating Bs-Ab: the E6 Fab light chain gene (E6L) (i) and the E6 Fab heavy chain gene (E6H) extended with the gene encoding an anti-murine CD3ε scFv (ii) or an anti-murine CD28 scFv (iii). b Co-expression of Fab light chain genes and Fab heavy chain scFv fusion genes leads to the production of an activating and a co-stimulating Bs-Ab. c Overview of the gene constructs for expression of the activating and co-stimulating CriBs-Abs: the E6 Fab light chain gene (E6L) extended with the DNA encoding PSTAIRE peptide (P) (iv) or the anti-P scFv (v) and the E6 Fab heavy chain gene (E6H) extended with the gene encoding an anti-murine CD3ε scFv (ii) or an anti-murine CD28 scFv (iii). d Co-expression of Fab light chain scFv fusion genes and Fab heavy chain scFv fusion genes, leads to the production of an activating and a co-stimulating CriBs-Ab. e Molecular model (ribbon representation) of the CriBs-Ab complex
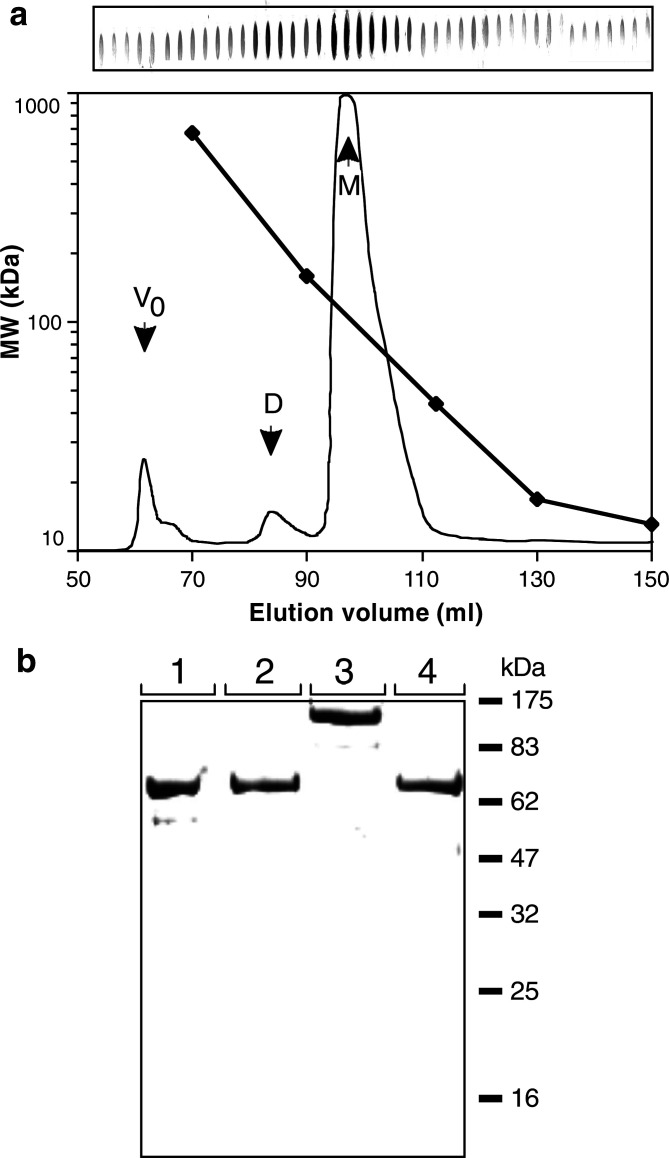
Fig. 2.
Purification of the Bs-Abs and the CriBs-Abs. a Purification of the activating Bs-Ab: chromatogram with integrated molecular weight calibration curve of the polishing step with size exclusion chromatography. A slot blot of the different fractions was developed with a goat anti-mouse κ light chain antibody, followed by an alkaline phosphatase conjugated anti-goat antibody. Purification of the other Bs-Abs and CriBs-Abs gave similar chromatograms (data not shown). M monomers; D dimers; V0 void. b SDS-page (CBB stained) of the purified Bs-Abs and CriBs-Abs. Lanes: 1 activating Bs-Ab; 2 costimulating Bs-Ab; 3 activating CriBs-Ab; 4 costimulating CriBs-Ab
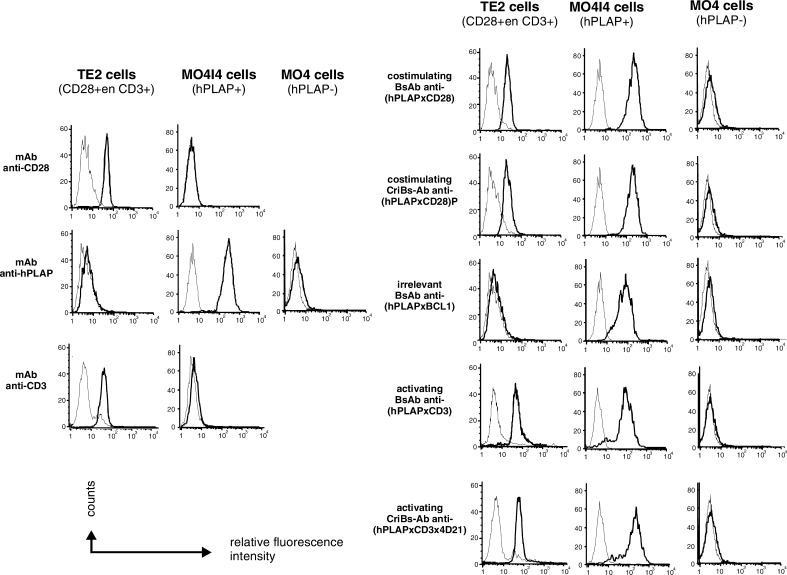
The functionality of all the binding sites of the Bs-Abs as well as the CriBs-Abs was verified in a flow-cytometry-based assay (Fig. 3). All antibody derivatives with an anti-hPLAP specificity, as well as the parental anti-hPLAP mAb, were found to bind specifically onto hPLAP+ MO4I4 tumor cells but not the parental hPLAP-negative MO4 tumor cells. The parental anti-CD3 and anti-CD28 mAbs could not recognize the tumor cells. CD3+ and CD28+ T-HA cells were recognized by the anti-CD3 and anti-CD28-containing antibody derivatives, by the parental anti-CD3 and anti-CD28 mAbs, but not by the anti-hPLAP mAb. The specificity of the T-cell binding was shown by using an irrelevant Bs-Ab (anti-(hPLAP × BCL1)), which could not recognize T-HA cells.
Fig. 3.
Binding of the Bs-Abs and the CriBs-Abs on tumor cells and T cells, assessed by flow cytometric analysis. Cells were incubated with the indicated Bs-Ab or CriBs-Ab and the cell-bound Abs were detected with fluorescein-conjugated anti-mouse Ig (Fab’)2 antibody (thick lines). As a control, cells were incubated with the parental mAbs and the cell-bound mAbs were detected with fluorescein-conjugated anti-mouse Ig (Fab’)2 antibody (anti-hPLAP) or with anti-hamster FITC-conjugated serum (anti-CD3 and anti-CD28). Background fluorescence was measured by incubating the cells with the detection antibodies (thin lines). Cells with bound mAbs, Bs-Abs or CriBs-Abs showed a shift in immunofluorescence intensity
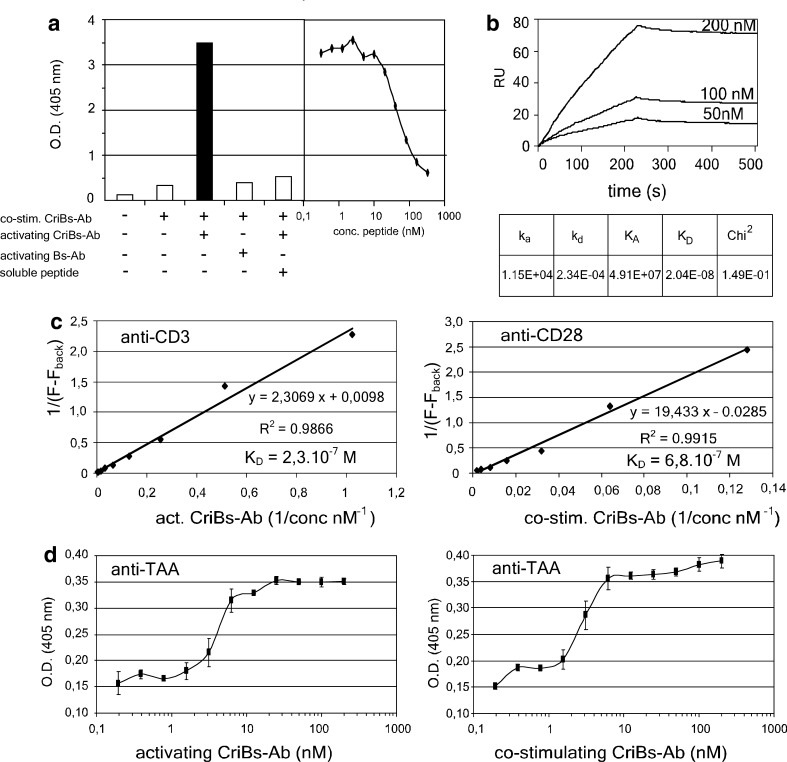
Cross-interaction of the CriBs-Abs was verified by ELISA by coating with the purified P-containing CriBs-Ab and binding of the anti-P CriBs-Ab, followed by addition of soluble hPLAP and detection via its alkaline phosphatase activity. In order to keep the OD of the first coating low, the concentration of the P-containing CriBs-Ab used for coating was optimized to 25 nM (data not shown). Binding of the anti-P CriBs-Ab onto the coated P-containing CriBs-Ab was then detected by the elevated trapping of hPLAP activity (Fig. 4a). As a first control, the activating anti-P CriBs-Ab was substituted with the corresponding Bs-Ab lacking the anti-P moiety. This control revealed no binding on the coated P-containing CriBs-Ab. An additional control was performed with increasing concentrations of soluble blocking P-peptide, which resulted in inhibition of the cross-interaction between the two CriBs-Abs. Both controls confirm the functional P/anti-P interaction. The binding characteristics of the cross-interaction were further examined using surface plasmon resonance (Fig. 4b). The KD of binding was found to be 2.10−8 M, with a kon of 115×104 M−1 s−1 and a koff of 234×10−4 s−1. These values predict a slow formation of the complex, but also a slow dissociation once the complex is formed.
Fig. 4.
Characterization of the CriBs-Abs. a Demonstration of the cross-interaction in an ELISA experiment. An ELISA plate was coated with 25 nM of the P-containing CriBs-Ab and incubated with 50 nM anti-P-containing CriBs-Ab. Mutual binding of the two antibodies was then detected by the elevated trapping of hPLAP activity. As a control, the anti-P CriBs-Ab was substituted with the corresponding Bs-Ab lacking the anti-P moiety. An additional control was performed by adding a dilution series of soluble blocking P-peptide to the anti-P containing CriBs-Ab. b Biologic interaction analysis of the cross-interaction using SPR on a BIAcore 2000: purified co-stimulating CriBs-Ab was coupled to a CM5 chip and increasing concentrations of purified activating CriBs-Ab were used as an analyte. c Determination of the anti-CD3 and the anti-CD28 affinities in the CriBs-Abs by a flow-cytometry-based assay. T-HA cells were incubated with various dilutions of purified activating (anti-CD3) or co-stimulating (anti-CD28) CriBs-Ab (1–500 nM), followed by incubation with 10 μg/ml FITC-conjugated goat anti-mouse Fab. KD is determined by the Lineweaver-Burk method. d The anti-hPLAP affinities of both CriBs-Abs were compared in ELISA. A plate was coated with 10 μg/ml hPLAP and subsequently incubated with a serial dilution (starting at 200 nM) of the activating CriBs-Ab or the co-stimulating CriBs-Ab. The bound CriBs-Abs were detected with an anti-His tag Ab, conjugated to HRP. All experiment were performed in triplicate
The KD values of both the anti-CD3 and the anti-CD28 specificities were estimated using a flow-cytometry-based T-cell binding assay [3], and calculated by Lineweaver-Burk kinetic analysis [35]. The KD of the anti-CD3 and the anti-CD28 specificities were found to be 23×10−7 M and 68×10−7 M, respectively (Fig. 4c). A comparable affinity for the TAA (hPLAP) was found for both CriBs-Abs using ELISA (Fig. 4d). Here, soluble hPLAP was coated and incubated with a serial dilution of either CriBs-Ab, starting from equimolar concentrations (200 nM). The bound CriBs-Abs were detected with an anti-His tag mAb. The estimated KD (3–5×10−9 M) was in line with affinity measurements previously performed on the E6 Fab fragment by itself and when it is incorporated in a Ts-Ab format (32×10−9 M) [46]. In conclusion, tumor binding of the CriBs-Abs was found to be 50–100 fold stronger than binding to the T cells, and ten fold stronger than the cross-interacting affinity of the CriBs-Abs.
Induction of T-cell activation in the absence of tumor cells
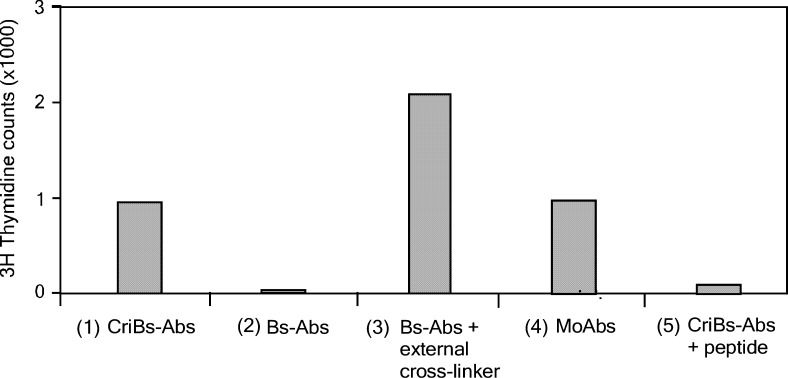
Having established the cross-interactive binding of the CriBs-Abs, we verified whether such an interaction results in a complex capable of stimulating T cells. Formation of a complex in which both the anti-CD3 and the anti-CD28 specificities are present is expected to result in tumor cell independent T cell activation, similarly to a Ts-Ab containing both the anti-CD28 and anti-CD3 specificities [27]. As shown in Fig. 5, co-incubation of cultured T-cells with both the activating and the co-stimulating CriBs-Abs resulted in T-cell proliferation, while the corresponding Bs-Abs lacking the cross-interaction could not induce T-cell proliferation. A comparable response was obtained with the parental, bivalent binding anti-CD3 and anti-CD28 mAbs and when the corresponding Bs-Abs were externally cross-linked via an anti-κ light chain polyclonal Ab (Fig. 5). A somewhat lower proliferation was noticed with the CriBs-Abs and the mAbs, as compared to the Bs-Abs in combination with the polyclonal external cross-linker. This difference might be explained by the fact that the polyclonal Ab promotes aggregate formation, resulting in the cross-linking of several TCR/CD3 complexes and CD28-receptors. However, the internal cross-interaction in the CriBs-Ab pair induces the formation of heterodimers consisting of one TCR/CD3 complex and one CD28-receptor. When using soluble mAbs, homodimers consisting of two TCR/CD3 complexes or two CD28-receptors are formed. As a control, the P/anti-P cross-interaction of the CriBs-Abs was blocked by adding an excess of soluble P-peptide. This resulted in loss of the T-cell activating potential of the CriBs-Abs.
Fig. 5.
Induction of T-cell activation in the absence of tumor cells. T cells were co-cultured with (1) 80 nM of soluble activating and co-stimulating CriBs-Abs, (2) 80 nM of soluble activating and co-stimulating Bs-Abs, (3) 80 nM of soluble activating and co-stimulating Bs-Abs externally cross-linked via an anti-mouse kappa light chain polyclonal Ab, (4) 80 nM soluble anti-CD3 and anti-CD28 mAbs, or (5) 80 nM of soluble activating and co-stimulating CriBs-Abs with 400 nM of blocking peptide. The data are representative of three independent experiments
These experiments confirm the functional cross-interaction of the CriBs-Ab pair that results in the formation of an activating complex at the surface of the T cell. However, in order to obtain this tumor-cell-independent activation, high concentrations of both CriBs-Abs were needed, and activation remained low as compared to full T-cell activation, which gave 60-fold higher proliferation rates (data not shown).
Induction of T-cell activation in the presence of tumor cells
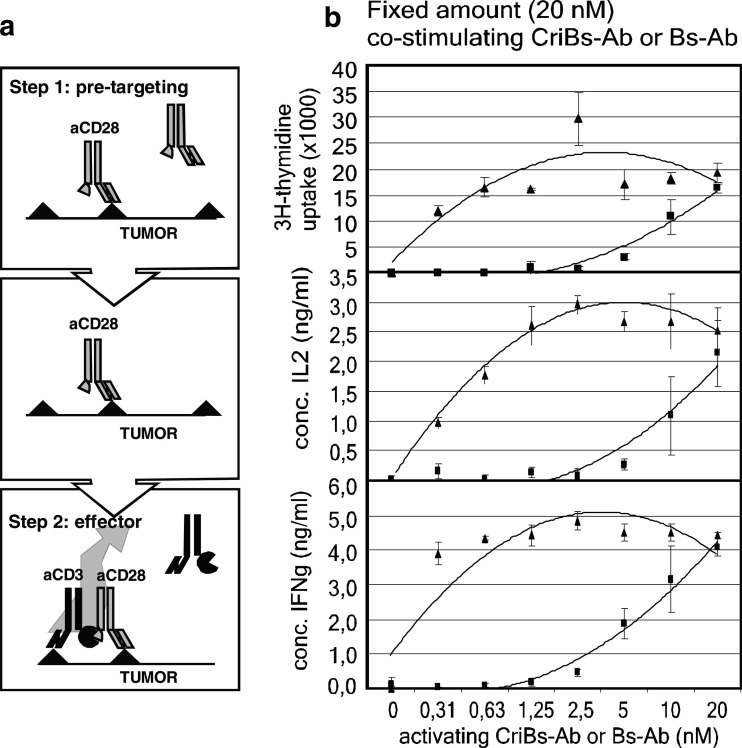
We next evaluated whether the cross-interacting functionality inherent to the CriBs-Abs results in enhanced tumor-cell dependent T-cell activation, compared to the homologous Bs-Abs lacking the cross-interaction. An in vitro proliferation assay mimicking an in vivo pre-targeting strategy was used (Fig. 6a). In a first step, mitomycin treated tumor cells were incubated with the co-stimulating CriBs-Ab or Bs-Ab. In a second step, after removal of unbound antibody, the activating CriBs-Ab or Bs-Ab was added. The tumor cells prepared in this way were then incubated with resting T cells. T-cell activation was measured by cell proliferation, and IL-2 and IFN-γ production. A fixed amount of co-stimulating antibody (20 nM) was combined with increasing concentrations of activating antibody (0–20 nM). When using the CriBs-Abs, the proliferative response and IL-2 and IFN-γ production rapidly reached a plateau value at concentrations of 0.63–1.25 nM of activating CriBs-Ab (Fig. 6b). When using the Bs-Ab, the plateau value for the three parameters was reached only at the highest concentration (20 nM) of activating Bs-Ab (Fig. 6b). In terms of proliferation and IL-2 induction, the activating potential of the activating CriBs-Ab was at least 30-fold higher than that of its non-cross-interactive counterpart (0.3 vs 10 nM). Comparison of IFN-γ production suggests an even greater improvement in activation potential, as the amount of secreted IFN-γ at 0.3 nM of activating CriBs-Ab was still markedly higher than at 10 nM of activating Bs-Ab. As a control, one of the CriBs-Abs was exchanged with its non-cross-interacting equivalent, generating the same level of response as that obtained with the nonCriBs-Ab pair (data not shown), thus confirming that the built-in cross-interaction is necessary and sufficient for the enhanced activation potential of the CriBs-Abs. The results show a marked increase (>30-fold) of tumor-cell dependent T cell activating capacity of CriBs-Abs as compared to Bs-Abs lacking the cross-interaction, thus allowing the use of a lower concentration of CD3 binding antibody.
Fig. 6.
Induction of T-cell activation in the presence of tumor cells. a The pre-targeting strategy. b Mitomycin treated tumor cells were pre-incubated for 1 h with a fixed amount (20 nM) of co-stimulating Bs-Ab (open square) or CriBs-Ab (open traingle) followed by a 1 h incubation with a serial dilution (0–20 nM) of activating Bs-Ab (open square) or CriBs-Ab (open traingle). Pre-incubated tumor cells were then co-incubated with T cells. T-cell activation was measured by proliferation, IL-2 production and IFN−γ production. Each condition was tested in triplicate
Influence of the ratio and concentration range of the CriBs-Abs on specific and non-specific T cell activation
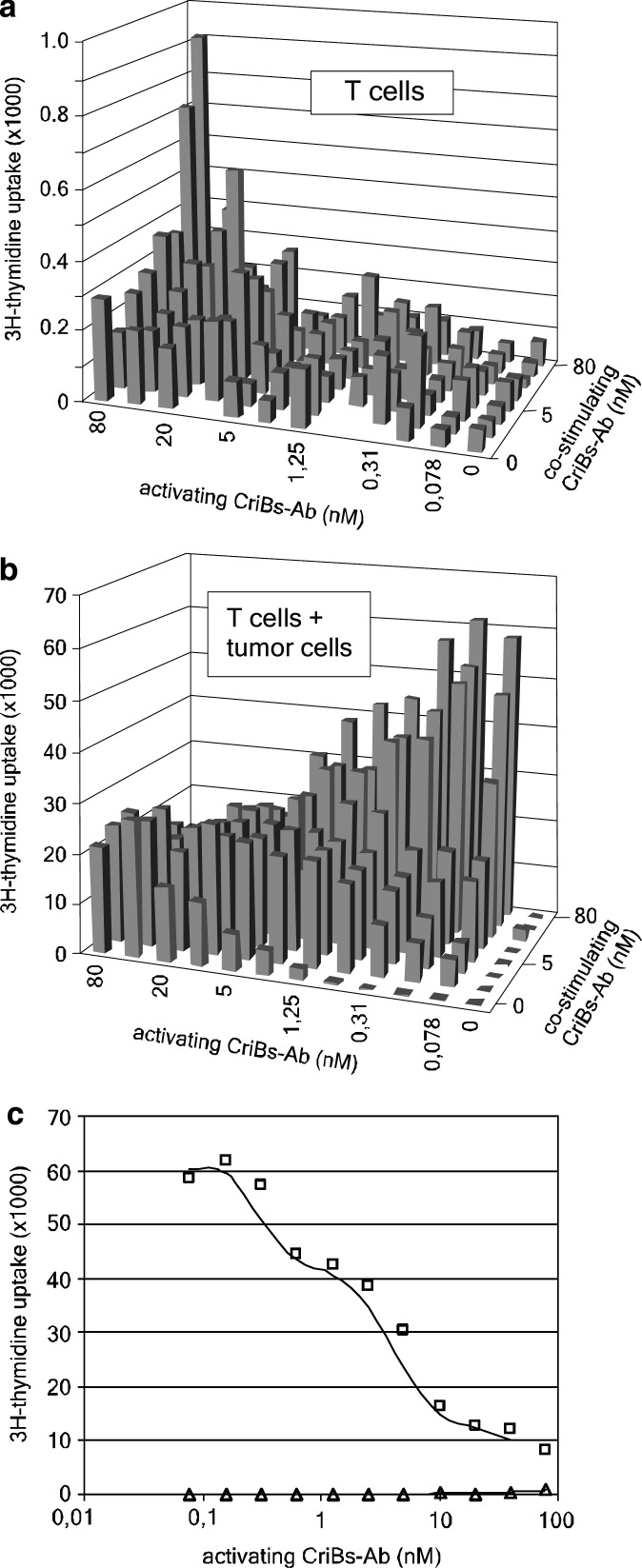
We determined the ratio of activating versus co-stimulating CriBs-Ab at which maximal tumor-cell dependent and minimal tumor-cell independent T-cell activation is obtained. T cells were co-incubated with a criss-cross serial dilution of both CriBs-Abs. In the absence of tumor cells, high concentrations of both CriBs-Abs (>20 nM) were required to reach marginal T-cell proliferation (Fig. 7a). When diluting the activating CriBs-Ab at a constant high concentration of the co-stimulating CriBs-Ab (80 nM), a decreasing proliferative response was observed. In the presence of tumor cells, a much stronger proliferative response was obtained, with the highest response at high concentrations of co-stimulating CriBs-Ab (80 nM) in combination with the lowest concentration (<0.08 nM) of activating CriBs-Ab (Fig. 7b).
Fig. 7.
Determination of the optimal ratio of activating versus co-stimulating CriBs-Ab. In a proliferation assay, T cells were co-incubated with a criss-cross serial dilution of both CriBs-Abs (0–80 nM) in a pretargeting setting performed in a 96 well plate, in the absence (a) or presence (b) of mitomycin treated tumor cells. A separate graph was made from the same data displayed in a and b, at a constant concentration of 80 nM co-stimulating CriBs-Ab and a serial dilution (0–80 nM) of the activating CriBs-Ab, in the absence (open traingle) or presence (open square) of mitomycine treated tumor cells (c)
As a result, two opposite dose-response curves with or without tumor cells were observed, as illustrated in a cross-section of the criss-cross dilution experiment at 80 nM of co-stimulating CriBs-Abs (Fig. 7c). Decreasing the concentration of the activating CriBs-Ab resulted in an increasing proliferative response in the presence of tumor cells. This implies that in the presence of sufficient pre-targeting reagent (the co-stimulating CriBs-Ab), a large window of operation is available, in which T-cell activation is dependent on the presence of tumor cells: at concentrations of activating CriBs-Ab below 10 nM no T-cell activation is seen in the absence of tumor cells, while in the presence of tumor cells T cell activation is maximal at 0.08 nM (the lowest concentration tested). Furthermore, in the same experiment the highest level of T-cell activation observed without tumor cells was 60-fold lower than in the situation where tumor cells were present to aggregate the antibodies.
Affinity enhancement effect of CriBs-Abs
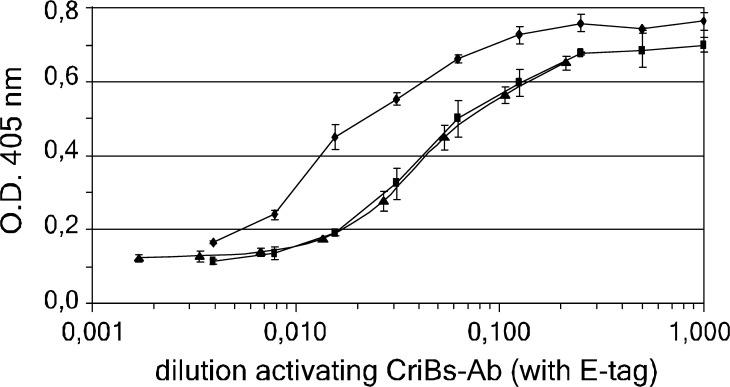
Efficient tumor targeting of Bs-Abs is important in obtaining an efficient tumor cell-dependent T-cell activation, especially in humans. Increasing the functional affinity of the Bs-Abs can improve tumor targeting. In the pre-targeting strategy followed in the previous experiment, the activating CriBs-Ab was able to recognize two epitopes present on the tumor cells: the hPLAP TAA, present on the tumor cell surface, and the P-tag, present on the pre-targeted co-stimulating CriBs-Ab. To verify whether this dual interaction results in an affinity enhancement, an ELISA experiment was performed. Soluble TAA (hPLAP) was coated and incubated with equimolar amounts of either the co-stimulating CriBs-Ab or the co-stimulating Bs-Ab, and then an activating CriBs-Ab containing a C-terminal E-tag was added. Detection with an anti-E-tag mAb revealed a twofold to threefold increased avidity of the activating CriBs-Ab for the TAA that was pre-incubated with the co-stimulating CriBs-Ab compared to the TAA that was pre-incubated with the co-stimulating Bs-Ab (Fig. 8). An excess of soluble blocking P-peptide was added to CriBs-Ab containing anti-P, resulting in inhibition of the affinity enhancement effect. This control confirms the dependence of the affinity enhancement on the presence of the P/anti-P interaction. The experiment was repeated using different concentrations of the coated TAA and gave comparable results (data not shown). In accordance with the ‘affinity enhancement system’ [2, 4, 29], cross-linking two monovalent binding molecules is expected to result in improved in vivo tumor targeting.
Fig. 8.
The affinity enhancement effect caused by the cross-interaction. An ELISA plate was coated with the soluble TAA (hPLAP) and incubated with 40 nM of the co-stimulating CriBs-Ab (filled circle), the co-stimulating CriBs-Ab with an excess of blocking P-peptide (filled traingle) or the co-stimulating Bs-Ab (filled square). The subsequent incubation step was performed with a serial dilution (1/2) of cell culture supernatant containing an E-tagged activating CriBs-Ab. Bound E-tagged CriBs-Ab was revealed with an anti-E-tag mAb conjugated to HRP. Each condition was tested in triplicate
Discussion
When redirecting T cells to a tumor using Bs-Abs, effective activation of the recruited T cells at the tumor site is essential for the development of a tumoricidal immune response. Therefore, the biological requirements for T-cell priming and expansion have to be met. Co-stimulation via receptors such as CD28 is crucial in this process [9], as stimulation via the TCR alone favors anergy or apoptosis [47]. It has been shown that in cis presentation of both signals is beneficial in promoting T-cell activation. Moreover, anti-CD3 and anti-CD28 mAb presented to the T cell in cis by immobilization on the same bead resulted in a Th1-type cytokine profile, a response considered beneficial for tumor rejection [34].
We here evaluate a pre-targeting strategy in which signal 1 (anti-TCR) and 2 (anti-CD28) are given by two separate CriBs-Abs. By using a pre-targeting strategy in which the co-stimulating CriBs-Ab is added before the activating CriBs-Ab, a more optimal T-cell signal containing both the anti-CD3 and anti-CD28 specificity can be reconstituted at the tumor site, while avoiding the simultaneous presence of both signals systemically. Such a pair of CriBs-Abs was engineered by incorporating a peptide (P) and a scFv specific for this (anti-P) into, respectively, an anti-(TAA × CD28) and an anti-(TAA × CD3) Bs-Ab. Both CriBs-Abs were shown to mutually interact with an affinity of 20 nM. Comparing the CriBs-Abs with their non-cross-interacting counterparts in the absence of tumor cells revealed a T-cell proliferative response similar in strength to the response induced by the Bs-Abs cross-linked via an external cross-linker (polyclonal serum). In the absence of the cross-linking, a monovalent anti-CD3 and anti-CD28 specificity on two separate Bs-Abs was not sufficient to induce T-cell activation at any concentration. This confirms the functionality of the cross-interaction and the fact that T-cell triggering can result from the heterodimerization of the TCR with the CD28 co-stimulatory receptor.
In the presence of tumor cells and using sufficiently high Ab-concentrations, both the CriBs-Abs and their non-cross-interacting counterparts induced similar strong proliferative responses, caused by a concentration of Ab at the cell–cell interaction synapse through recognition of the TAA on the tumor cells. However, lowering the concentrations of the activating antibody led to a rapid loss of activity using the non-CriBs-Abs, while stimulation could be maintained at a plateau value using more than 30-fold lower concentrations of the CriBs-Abs. Interestingly, at these low concentrations of activating CriBs-Ab, high levels of the co-stimulating CriBs-Ab were required, yielding at its extreme a theoretically optimized stochiometry of 1 : 1000 anti-CD3:anti-CD28. The presence of high concentrations of co-stimulating antibody is expected to induce tumor cell driven clustering of co-stimulatory receptors. Recruitment to this cluster of minute amounts of T-cell receptors by the cross-interacting activating CriBs-Ab is apparently sufficient to induce optimal T-cell activation. Possibly, this association promotes TCR/CD3 signaling through induced proximity of associated signaling molecules and/or by a CD28 mediated partitioning of TCR/CD3 complexes into lipid rafts, which are themselves enriched in signaling molecules [23, 50]. In fact, this effect of optimized stoichiometry mimicks the real-life situation where a T-cell becomes activated by a few TCR ligands in the presence of an abundance of co-stimulatory ligands. This effect is impossible to achieve with an anti-(TAA × CD3×CD28) trispecific single molecule, in which the anti-CD3 and anti-CD28 specificities are present in a fixed 1 : 1 stoichiometry. We can thus conclude that the pre-targeting strategy with the CriBs-Abs as described holds an important advantage over former strategies as the concentration of the most toxic component, the activating CriBs-Ab, can be reduced while preserving the full potential of the reagents. No toxicity has so far been reported for a co-stimulatory Bs-Ab (targeting the CD28-receptor), while the toxicity of the activating anti-CD3 is dose limiting.
Pre-targeting of the co-stimulatory CriBs-Ab to the tumor cells creates two binding sites on the cells for the activating CriBs-Ab. ELISA revealed a threefold increase in the avidity of the activating CriBs-Ab for the TAA preloaded with the co-stimulatory CriBs-Ab. Although potentially important for increasing the tumor targeting of the activating CriBs-Ab when administered to the organism, this marginal increase in functional affinity seems insufficient to explain the efficient T-cell activation at low concentrations of activating CriBs-Ab.
When considering an application for tumor therapy, systemic T-cell activation, leading to a severe and potentially life-threatening inflammatory response, needs to be avoided. Systemic toxicity mediated by Bs-Abs may be caused by several mechanisms. When combining the anti-CD3 and anti-CD28 specificities on a single molecule, tumor-independent T-cell activation was observed [27]. Toxicity due to Fc-receptor mediated aggregation of an anti-(TAA × CD3) Bs-Ab has also been reported [36]. Even the use of high doses of a monovalent anti-(TAA × CD3) Bs-Ab lacking an Fc-tail has been reported to cause systemic response and accompanying toxicity in humans [49]. This emphasizes the need for a system that on the one hand uses low doses of activating Bs-Ab and on the other hand assembles co-stimulating and activating functions in a tumor-driven manner, thus warranting a tumor-restricted but potent T-cell activation. The T-cell activating characteristics of the co-stimulating/activating CriBs-Ab pair used in a pre-targeting strategy clearly approaches these requirements. In the presence of tumor cells, a minimal concentration of activating CriBs-Ab (<0.08 nM) induces maximal T-cell activation, provided enough co-stimulating CriBs-Ab is tumor cell-bound. In contrast, in the absence of tumor cells a high concentration (>10 nM) of both co-stimulating and activating CriBs-Ab is required for T-cell activation. Thus, in a therapeutic setting, T-cell activation may be restricted to the tumor site using this two-step approach. First, high doses of the co-stimulating CriBs-Ab can be administered. Based on the high affinity of the CriBs-Ab for the tumor TAA (KD=3–5×10−9 M), and the 100-fold lower affinity for the CD28 (or CD3) receptor on the T cell (KD=23 to 68×10−7 M), the fractional occupancy ([Ab]/(KD + [Ab])) of antibody binding to the tumor will be higher than the fractional occupancy of antibody binding T cells. Once the non-tumor-bound co-stimulating CriBs-Ab is sufficiently (<10 nM) cleared from the circulation, low non-toxic doses (<10 nM) of the activating CriBs-Ab can be administered. This would minimize the possibility of systemic T-cell responses but yet enable T-cell activation at low reagent concentrations when cross-interacting the activating and co-stimulatory signals specifically at the tumor site.
Although current results are limited to in vitro data, the experiments indicate that we designed a promising and novel approach to ensure optimal and tumor-dependent T-cell activation at much lower concentrations of activating Bs-Abs, by crosslinking the CD28 and the CD3 T-cell receptors into a single complex.
Acknowledgements
An Willems is a research associate with the Fonds Wetenschappelijk Onderzoek (FWO)—Vlaanderen. We thank T. Van Belle and D. Ginneberghe (Ghent University, Belgium) for the use of T-HA cells. Dr. M. Hall (University of Birmingham, U.K.) and Dr. M. De Broe (University of Antwerp, Belgium) are acknowledged for donating HEK293T cells and MO4I4 cells, respectively.
Abbreviations
- Ab
Antibody(-ies)
- Bs-Ab
Bispecific Ab
- Ts-Ab
Trispecific Ab
- CriBs-Ab
Cross-interacting bispecific Ab
- hPLAP
Human placental alkaline phosphatase
- scFv
Single-chain variable fragment
- TAA
Tumor associated antigen
References
- 1.Adams GP, Schier R, McCall AM, Crawford RS, Wolf EJ, Weiner LM, Marks JD. Prolonged in vivo tumour retention of a human diabody targeting the extracellular domain of human HER2/neu. Br J Cancer. 1998;77(9):1405–1412. doi: 10.1038/bjc.1998.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barbet J, Kraeber-Bodere F, Vuillez JP, Gautherot E, Rouvier E, Chatal JF. Pretargeting with the affinity enhancement system for radioimmunotherapy. Cancer Biother Radiopharm. 1999;14(3):153–166. doi: 10.1089/cbr.1999.14.153. [DOI] [PubMed] [Google Scholar]
- 3.Benedict CA., MacKrell AJ, Anderson WF. Determination of the binding affinity of an anti-CD34 single-chain antibody using a novel, flow cytometry based assay. J Immunol Methods. 1997;201(2):223–231. doi: 10.1016/S0022-1759(96)00227-X. [DOI] [PubMed] [Google Scholar]
- 4.Boerman OC, van Eerd J, Oyen WJ, Corstens FH. A 3-step pretargeting strategy to image infection. J Nucl Med. 2001;42(9):1405–1411. [PubMed] [Google Scholar]
- 5.Boerman OC, Van Schaijk FG, Oyen WJ, Corstens FH. Pretargeted radioimmunotherapy of cancer: progress step by step. J Nucl Med. 2003;44(3):400–411. [PubMed] [Google Scholar]
- 6.Bohlen H, Hopff T, Manzke O, Engert A, Kube D, Wickramanayake PD, Diehl V, Tesch H. Lysis of malignant B cells from patients with B-chronic lymphocytic leukemia by autologous T cells activated with CD3×CD19 bispecific antibodies in combination with bivalent CD28 antibodies. Blood. 1993;82(6):1803–1812. [PubMed] [Google Scholar]
- 7.Bohlen H, Manzke O, Titzer S, Lorenzen J, Kube D, Engert A, Abken H, Wolf J, Diehl V, Tesch H. Prevention of Epstein-Barr virus-induced human B-cell lymphoma in severe combined immunodeficient mice treated with CD3xCD19 bispecific antibodies, CD28 monospecific antibodies, and autologous T cells. Cancer Res. 1997;57(9):1704–1709. [PubMed] [Google Scholar]
- 8.Bolhuis RL, Lamers CH, Goey SH, Eggermont AM, Trimbos JB, Stoter G, Lanzavecchia A, di Re E, Miotti S, Raspagliesi F, et al. Adoptive immunotherapy of ovarian carcinoma with bs-MAb-targeted lymphocytes: a multicenter study. Int J Cancer Suppl. 1992;7:78–81. [PubMed] [Google Scholar]
- 9.Chambers CA. The expanding world of co-stimulation: the two-signal model revisited. Trends Immunol. 2001;22(4):217–223. doi: 10.1016/S1471-4906(01)01868-3. [DOI] [PubMed] [Google Scholar]
- 10.Cochlovius B, Kipriyanov SM, Stassar MJ, Schuhmacher J, Benner A, Moldenhauer G, Little M. Cure of Burkitt’s lymphoma in severe combined immunodeficiency mice by T cells, tetravalent CD3×CD19 tandem diabody, and CD28 costimulation. Cancer Res. 2000;60(16):336–341. [PubMed] [Google Scholar]
- 11.De Broe ME, Pollet DE. Multicenter evaluation of human placental alkaline phosphatase as a possible tumor-associated antigen in serum. Clin Chem. 1988;34(10):1995–1999. [PubMed] [Google Scholar]
- 12.De Gast GC, Van Houten AA, Haagen IA, Klein S, De Weger RA, Van Dijk A, Phillips J, Clark M, Bast BJ. Clinical experience with CD3 × CD19 bispecific antibodies in patients with B cell malignancies. J Hematother. 1995;4(5):433–437. doi: 10.1089/scd.1.1995.4.433. [DOI] [PubMed] [Google Scholar]
- 13.De Jonge J, Brissinck J, Heirman C, Demanet C, Leo O, Moser M, Thielemans K. Production and characterization of bispecific single-chain antibody fragments. Mol.Immunol. 1995;32(17–18):1405–1412. doi: 10.1016/0161-5890(95)00089-5. [DOI] [PubMed] [Google Scholar]
- 14.De Sutter K, Feys V, Vande Voorde A, Fiers W. Production of functionally active murine and murine::human chimeric F(ab’)2 fragments in COS-1 cells. Gene. 1992;113(2):223–230. doi: 10.1016/0378-1119(92)90399-A. [DOI] [PubMed] [Google Scholar]
- 15.Demanet C, Brissinck J, De Jonge J, Thielemans K. Bispecific antibody-mediated immunotherapy of the BCL1 lymphoma: increased efficacy with multiple injections and CD28-induced costimulation. Blood. 1996;87(10):4390–4398. [PubMed] [Google Scholar]
- 16.Dooms H, Desmedt M, Vancaeneghem S, Rottiers P, Goossens V, Fiers W, Grooten J. Quiescence-inducing and antiapoptotic activities of IL-15 enhance secondary CD4+ T cell responsiveness to antigen. J Immunol. 1998;161(5):2141–2150. [PubMed] [Google Scholar]
- 17.DuBridge RB, Tang P, Hsia HC, Leong PM, Miller JH, Calos MP. Analysis of mutation in human cells by using an Epstein-Barr virus shuttle system. Mol Cell Biol. 1987;7(1):379–387. doi: 10.1128/mcb.7.1.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eeckhout D, Fiers E, Sienaert R, Snoeck V, Depicker A, De Jaeger G. Isolation and characterization of recombinant antibody fragments against CDC2a from Arabidopsis thaliana. Eur J Biochem. 2000;267(23):6775–6783. doi: 10.1046/j.1432-1033.2000.01770.x. [DOI] [PubMed] [Google Scholar]
- 19.Goldenberg DM, Chang CH, Sharkey RM, Rossi EA, Karacay H, McBride W, Hansen HJ, Chatal JF, Barbet J. Radioimmunotherapy: is avidin-biotin pretargeting the preferred choice among pretargeting methods? Eur J Nucl Med Mol Imaging. 2003;30(5):773–776. doi: 10.1007/s00259-002-1089-6. [DOI] [PubMed] [Google Scholar]
- 20.Govindan SV, Goldenberg DM, Hansen HJ, Griffiths GL. Advances in the use of monoclonal antibodies in cancer radiotherapy. Pharm Sci Technol Today. 2000;3(3):90–98. doi: 10.1016/S1461-5347(00)00241-8. [DOI] [PubMed] [Google Scholar]
- 21.Grosse-Hovest L, Brandl M, Dohlsten M, Kalland T, Wilmanns W, Jung G. Tumor-growth inhibition with bispecific antibody fragments in a syngeneic mouse melanoma model: the role of targeted T-cell co-stimulation via CD28. Int J Cancer. 1999;80(1):138–144. doi: 10.1002/(SICI)1097-0215(19990105)80:1<138::AID-IJC25>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 22.Haagen IA. Performance of CD3xCD19 bispecific monoclonal antibodies in B cell malignancy. Leuk Lymphoma. 1995;19(5–6):381–393. doi: 10.3109/10428199509112195. [DOI] [PubMed] [Google Scholar]
- 23.Harder T. Raft membrane domains and immunoreceptor functions. Adv Immunol. 2001;77:45–92. doi: 10.1016/s0065-2776(01)77014-9. [DOI] [PubMed] [Google Scholar]
- 24.Hendrix PG, Dauwe SE, VanDe Voorde A, Nouwen EJ, Hoylaerts MF, De Broe ME. Radiolocalisation and imaging of stably HPLAP-transfected MO4 tumours with monoclonal antibodies and fragments. Br J Cancer. 1991;64(6):1060–1068. doi: 10.1038/bjc.1991.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hombach A, Mathas S, Jensen M, Tillmann T, Menges M, Diehl V, Kruis W, Pohl C. Activation of resting T cells against the CA 72–4 tumor antigen with an anti-CD3/CA 72–4 bispecific antibody in combination with a costimulatory anti-CD28 antibody. Anticancer Res. 1997;17(3C):2025–2032. [PubMed] [Google Scholar]
- 26.Hombach A., Tillmann T, Jensen M, Heuser C, Sircar R, Diehl V, Kruis W, Pohl C. Specific activation of resting T cells against CA19–9+ tumor cells by an anti-CD3/CA19–9 bispecific antibody in combination with a costimulatory anti-CD28 antibody. J Immunother. 1997;20(5):325–333. doi: 10.1097/00002371-199709000-00001. [DOI] [PubMed] [Google Scholar]
- 27.Jung G, Freimann U, Von Marschall Z, Reisfeld RA, Wilmanns W. Target cell-induced T cell activation with bi- and trispecific antibody fragments. Eur J Immunol. 1991;21(10):2431–2435. doi: 10.1002/eji.1830211020. [DOI] [PubMed] [Google Scholar]
- 28.Jung G, Brandl M, Eisner W, Fraunberger P, Reifenberger G, Schlegel U, Wiestler OD, Reulen HJ, Wilmanns W. Local immunotherapy of glioma patients with a combination of 2 bispecific antibody fragments and resting autologous lymphocytes: evidence for in situ t-cell activation and therapeutic efficacy. Int J Cancer. 2001;91(2):225–230. doi: 10.1002/1097-0215(200002)9999:9999<::AID-IJC1038>3.3.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 29.Karacay H, Sharkey RM, McBride WJ, Griffiths GL, Qu Z, Chang K, Hansen HJ, Goldenberg DM. Pretargeting for cancer radioimmunotherapy with bispecific antibodies: role of the bispecific antibody’s valency for the tumor target antigen. Bioconjug Chem. 2002;13(5):1054–1070. doi: 10.1021/bc0200172. [DOI] [PubMed] [Google Scholar]
- 30.Katayose Y, Kudo T, Suzuki M, Shinoda M, Saijyo S, Sakurai N, Saeki H, Fukuhara K, Imai K, Matsuno S. MUC1-specific targeting immunotherapy with bispecific antibodies: inhibition of xenografted human bile duct carcinoma growth. Cancer Res. 1996;56(18):4205–4212. [PubMed] [Google Scholar]
- 31.Kipriyanov SM, Moldenhauer G, Schuhmacher J, Cochlovius B, Vonder Lieth CW, Matys ER, Little M. Bispecific tandem diabody for tumor therapy with improved antigen binding and pharmacokinetics. J Mol Biol. 1999;293(1):41–56. doi: 10.1006/jmbi.1999.3156. [DOI] [PubMed] [Google Scholar]
- 32.Koshida K, Stigbrand T, Munck-Wikland E, Hisazumi H, Wahren B. Analysis of serum placental alkaline phosphatase activity in testicular cancer and cigarette smokers. Urol Res. 1990;18(3):169–173. doi: 10.1007/BF00295842. [DOI] [PubMed] [Google Scholar]
- 33.Kroesen BJ, Buter J, Sleijfer DT, Janssen RA, van der Graaf WT, The TH, de Leij L, Mulder NH. Phase I study of intravenously applied bispecific antibody in renal cell cancer patients receiving subcutaneous interleukin 2. Br J Cancer. 1994;70(4):652–661. doi: 10.1038/bjc.1994.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levine BL, Bernstein WB, Connors M, Craighead N, Lindsten T, Thompson CB, June CH. Effects of CD28 costimulation on long-term proliferation of CD4+ T cells in the absence of exogenous feeder cells. J Immunol. 1997;159(12):5921–5930. [PubMed] [Google Scholar]
- 35.Lineweaver H, Burk D. The determination of enzyme dissociation constants. J Am Chem Soc. 1934;56:658. doi: 10.1021/ja01318a036. [DOI] [Google Scholar]
- 36.Link BK, Kostelny SA, Cole MS, Fusselman WP, Tso JY, Weiner GJ. Anti-CD3-based bispecific antibody designed for therapy of human B-cell malignancy can induce T-cell activation by antigen-dependent and antigen-independent mechanisms. Int J Cancer. 1998;77(2):251–256. doi: 10.1002/(SICI)1097-0215(19980717)77:2<251::AID-IJC14>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 37.Manzke O, Tesch H, Borchmann P, Wolf J, Lackner K, Gossmann A, Diehl V, Bohlen H. Locoregional treatment of low-grade B-cell lymphoma with CD3xCD19 bispecific antibodies and CD28 costimulation. I. Clinical phase I evaluation. Int J Cancer. 2001;91(4):508–515. doi: 10.1002/1097-0215(200002)9999:9999<::AID-IJC1068>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 38.Mazzoni A, Mezzanzanica D, Jung G, Wolf H, Colnaghi MI, Canevari S. CD3-CD28 costimulation as a means to avoiding T cell preactivation in bispecific monoclonal antibody-based treatment of ovarian carcinoma. Cancer Res. 1996;56(23):5443–5449. [PubMed] [Google Scholar]
- 39.Miotti S, Negri DR, Valota O, Calabrese M, Bolhuis RL, Gratama JW, Colnaghi MI, Canevari S. Level of anti-mouse-antibody response induced by bi-specific monoclonal antibody OC/TR in ovarian-carcinoma patients is associated with longer survival. Int J Cancer. 1999;84(1):62–68. doi: 10.1002/(SICI)1097-0215(19990219)84:1<62::AID-IJC12>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 40.Nielsen UB, Adams GP, Weiner LM, Marks JD. Targeting of bivalent anti-ErbB2 diabody antibody fragments to tumor cells is independent of the intrinsic antibody affinity. Cancer Res. 2000;60(22):6434–6440. [PubMed] [Google Scholar]
- 41.Nitta T, Sato K, Yagita H, Okumura K, Ishii S. Preliminary trial of specific targeting therapy against malignant glioma. Lancet. 1990;335(8686):368–371. doi: 10.1016/0140-6736(90)90205-J. [DOI] [PubMed] [Google Scholar]
- 42.Niwa H, Yamamura K, Miyazaki J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene. 1991;108(2):193–199. doi: 10.1016/0378-1119(91)90434-D. [DOI] [PubMed] [Google Scholar]
- 43.O’Mahoney JV, Adams TE. Optimization of experimental s influencing reporter gene expression in hepatoma cells following calcium phosphate transfection. DNA Cell Biol. 1994;13(12):1227–1232. doi: 10.1089/dna.1994.13.1227. [DOI] [PubMed] [Google Scholar]
- 44.Renner C, Pfreundschuh M. Tumor therapy by immune recruitment with bispecific antibodies. Immunol Rev. 1995;145:179–209. doi: 10.1111/j.1600-065x.1995.tb00082.x. [DOI] [PubMed] [Google Scholar]
- 45.Renner C, Bauer S, Sahin U, Jung W, van Lier R, Jacobs G, Held G, Pfreundschuh M. Cure of disseminated xenografted human Hodgkin’s tumors by bispecific monoclonal antibodies and human T cells: the role of human T-cell subsets in a preclinical model. Blood. 1996;87(7):2930–2937. [PubMed] [Google Scholar]
- 46.Schoonjans R, Willems A, Schoonooghe S, Fiers W, Grooten J, Mertens N. Fab chains as an efficient heterodimerization scaffold for the production of recombinant bispecific and trispecific antibody derivatives. J Immunol. 2000;165(12):7050–7057. doi: 10.4049/jimmunol.165.12.7050. [DOI] [PubMed] [Google Scholar]
- 47.Schwartz RH. T cell clonal anergy. Curr Opin Immunol. 1997;9(3):351–357. doi: 10.1016/S0952-7915(97)80081-7. [DOI] [PubMed] [Google Scholar]
- 48.Smans KA, Hoylaerts MF, Narisawa S, Millan JL, De Broe ME. Bispecific antibody-mediated lysis of placental and germ cell alkaline phosphatase targeted solid tumors in immunocompetent mice. Cancer Res. 1995;55(19):4383–4390. [PubMed] [Google Scholar]
- 49.Tibben JG, Boerman OC, Massuger LF, Schijf CP, Claessens RA, Corstens FH. Pharmacokinetics, biodistribution and biological effects of intravenously administered bispecific monoclonal antibody OC/TR F(ab’)2 in ovarian carcinoma patients. Int J Cancer. 1996;66(4):477–483. doi: 10.1002/(SICI)1097-0215(19960516)66:4<477::AID-IJC11>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 50.Viola A, Lanzavecchia A. T-cell activation and the dynamic world of rafts. Apmis. 1999;107(7):615–623. doi: 10.1111/j.1699-0463.1999.tb01450.x. [DOI] [PubMed] [Google Scholar]
- 51.Willems A., Leoen J, Schoonooghe S, Grooten J, Mertens N. Optimizing expression and purification from cell culture medium of trispecific recombinant antibody derivatives. J Chromatogr B. 2003;786(1–2):161–176. doi: 10.1016/S1570-0232(02)00813-9. [DOI] [PubMed] [Google Scholar]