Abstract
We have previously reported the identification of a unique thymocyte-specific surface molecule, JL1, which was detected using the monoclonal antibody (mAb), anti-JL1. Interestingly, JL1 was shown to be expressed in most leukemias, irrespective of their immunophenotype, and subpopulations of normal bone marrow (BM) mononuclear cells (MNCs). Here we investigated the potential usefulness of the anti-JL1 mAb as a therapeutic tool for leukemia. We demonstrated that the proliferation of cultured human leukemia cells was dramatically inhibited in vitro by anti-JL1 mAb conjugated with the polypeptide toxin, gelonin, but not by gelonin alone. We then systematically investigated the reactivity of the anti-JL1 mAb against normal human tissues to evaluate possible side effects along with various hematopoietic and nonhematopoietic tumor cell lines. All of 33 types of normal tissues except thymus and subpopulation of BM MNCs were clearly devoid of JL1 expression. Among tumor cell lines, all the nonhematopoietic cell lines tested were negative for JL1 expression, while some hematopoietic cell lines contained JL1 antigen. Collectively, the results showed the cytotoxic effects of anti-JL1-based immunotoxin against JL1-positive leukemic cells, sparing most normal tissues other than thymocytes and some BM MNCs. Therefore, we strongly suggest that gelonin-conjugated anti-JL1 mAb immunotoxin could be developed as a potential immunotherapeutic agent in the treatment of various types of JL1-positive acute leukemias.
Keywords: JL1, Leukemia, Immunotoxin, Gelonin
Introduction
Numerous applications of monoclonal antibodies (mAbs) have been proposed in cancer detection and therapy for leukemia (for review, see references 5, 15, 22 and 30). Recent innovative antibody engineering techniques and a better understanding of a growing list of tumor antigens have led to a rapid increase in research and clinical trials of antibody-based tumor-targeted therapies [12, 21]. Despite the major technological advances in the improvement of affinities to their antigens, and the reduced immunogenicity and enhanced delivery of potent cytotoxic agents, the effective therapeutic use of mAbs has been largely limited by the lack of suitable tumor-specific targets. These tumor-specific antigens should allow the selective accumulation of antibodies in leukemic cells for cytotoxic activity with the least unwanted toxicity to normal cells. In past trials of mAbs for the treatment of acute leukemia, mAbs reacting with glycolipids or cell surface antigens, such as CD5, CD10, CD25, CD33, CD45 and CDw52, have been investigated as potential therapeutic agents [2, 4, 6, 8, 12, 16, 18].
The concept of targeting cells selectively via cell surface antigens can be accomplished by using mAbs armed with toxins, that is immunotoxins (ITs) [1, 14, 17, 29]. Toxic agents widely used in the preparation of ITs are the ribosome-inactivating proteins (RIPs) which inhibit protein synthesis via catalytic cleavage of an N-glycosidic bond in the 28S rRNA [3]. Gelonin toxin from the seeds of Gelonium multiflorum is a 30-kDa single-chain polypeptide classified as a type I RIP. Gelonin has several advantages over other types of RIPs, such as ricin or abrin which are composed of an A chain and a lectin-like B chain [25]. Free gelonin, which lacks the B chain that reacts with cell-surface carbohydrates, is much less toxic to normal mammalian cells in vitro and in vivo, and appears to be less immunogenic than the larger heterodimers [24].
The JL1 molecule is a 120–130-kDa surface molecule selectively expressed on subpopulation of human thymocytes [19]. Despite restricted distribution of the JL1 antigen in normal tissues and cells, anti-JL1 mAb recognizes various types of leukemias of myeloid and B-cell origin and T-cell lineage [20]. The anti-JL1 mAb binds to human leukemia Molt-4 cells with an affinity constant of 1.7×109 l/mol and 5100–9600 binding sites per cell [7]. Furthermore, confocal microscopic analysis has shown that JL1 antigen/anti-JL1 antibody complexes are readily internalized into the intracellular compartment upon the engagement of JL1 with anti-JL1 mAb [26]. These findings, together with recent advances in the enhancement of antibody-mediated cytotoxicity, prompted us to evaluate the possibility that anti-JL1 mAb might be used as an immunotherapeutic agent for leukemia.
In the study reported here, we evaluated the immunotherapeutic potential of gelonin-conjugated anti-JL1 mAb ITs in terms of: (1) the cytotoxic effect of the IT against JL1-positive leukemic cell lines and (2) the level of its cross-reactivity with normal tissues. We demonstrated that gelonin-conjugated anti-JL1 mAb possessed a selective cytotoxic activity against leukemic cells in vitro. We also suggest that since JL1 is not expressed on normal cells other than thymocytes and some lineage-committed hematopoietic cells, the possibility for the IT to attack normal tissues would be negligible. These results imply that gelonin-conjugated anti-JL1 mAb could be developed as a potential immunotherapeutic agent.
Materials and methods
Tissues and cells
Normal human tissue specimens were collected shortly after surgery or autopsy at Chungbuk University Hospital, immediately snap-frozen in liquid nitrogen, and stored at −70°C. Cryostat sections (5 μm) of Tissue-Tek-embedded tissues were prepared and frozen at −20°C until staining. All paraffin-embedded tissues were selected from the archives of the Department of Pathology, Chungbuk University Hospital. The cell lines used for this study were obtained from the American Type Culture Collection (Rockville, Md.) or the German Collection of Microorganisms and Cell Cultures (Braunschweig, Germany), and placed in culture in our laboratory. Cells were maintained in Dulbecco's modified Eagle's medium (DMEM) or RPMI-1640 (Gibco, Grand Island, N.Y.) with 10% fetal bovine serum (Hyclone, Logan, Utah), 50 U/ml penicillin and 50 μg/ml streptomycin (Gibco).
Tables 1, 2, 3 and 4 list the normal tissues, primary cells and hematopoietic and nonhematopoietic cell lines studied, respectively.
Table 1.
Tissue distribution of JL1 antigen determined by immunohistochemistry (F frozen tissue, P formalin-fixed paraffin-embedded tissue)
| Tissue tested | No. positive/no. tested (%) | Tissue tested | No. positive/no. tested (%) | ||
|---|---|---|---|---|---|
| Lymph node | F, P | 0/8 (0) | Prostate | F, P | 0/1 (0) |
| Tonsil | F, P | 0/3 (0) | Ovary | F, P | 0/8 (0) |
| Thymus | F, P | 6/6 (100) | Salpinx | F | 0/3 (0) |
| Spleen | F, P | 0/4 (0) | Uterus | F, P | 0/4 (0) |
| Cerebrum | F, P | 0/4 (0) | Endometrium | F | 0/3 (0) |
| Cerebellum | F | 0/4 (0) | Kidney | F, P | 0/9 (0) |
| Skeletal muscle | P | 0/1 (0) | Salivary gland | P | 0/1 (0) |
| Spinal cord | F | 0/1 (0) | Urinary bladder | F | 0/1 (0) |
| Esophagus | F | 0/3 (0) | Tendon | F | 0/1 (0) |
| Stomach | F, P | 0/10 (0) | Breast | F | 0/2 (0) |
| Ileum | F, P | 0/2 (0) | Skin | F | 0/4 (0) |
| Colon | F, P | 0/3 (0) | Cartilage | F | 0/1 (0) |
| Liver | F, P | 0/7 (0) | Vein | F | 0/1 (0) |
| Exocrine pancreas | F, P | 0/4 (0) | Lung | F, P | 0/8 (0) |
| Appendix | F | 0/4 (0) | Thyroid | F, P | 0/2 (0) |
| Epididymis | F | 0/2 (0) | Adrenal gland | F, P | 0/5 (0) |
| Testis | F, P | 0/5 (0) | Endocrine pancreas | F, P | 0/4 (0) |
| Seminal vesicle | P | 0/1 (0) | Placenta | F | 0/1 (0) |
Table 2.
JL1 expression profiles in primary cells (++ positivity of >90% by flow cytometry, − complete negativity)
| Primary cells | Intensity of expression |
|---|---|
| Nucleated red blood cells | − |
| Lymphocytes | − |
| Monocytes | − |
| Granulocytes | − |
| Platelets | − |
| Thymocytes | ++ |
| Splenocytes | − |
| AC133+CD34+ bone marrow stem cells | − |
Table 3.
JL1 expression profiles in hematopoietic cell lines (++ positivity of >90% by flow cytometry, + positivity of <90%, − complete negativity)
| Cell line | Origin | Intensity of expression |
|---|---|---|
| Jurkat | T cell origin | ++ |
| Molt-4 | T-ALL | ++ |
| CEM-CCRF | T-ALL | ++ |
| Reh | ALL (B-ALL) | ++ |
| H9 | Cutaneous T-cell lymphoma | − |
| HUT-78 | Cutaneous T-cell lymphoma | − |
| K562 | Erythroleukemia | − |
| HEL92.1.7 | Erythroleukemia | + |
| IM-9 | EBV-transformed B cell | ++ |
| 1A2 | Lymphoblastoid EBV+ | − |
| Raji | EBV-transformed B cell | ++ |
| BJAB | North American Burkitt's lymphoma | − |
| Ramos | Burkitt's lymphoma | ++ |
| KG1 | AML | − |
| HL-60 | Promyelocytic leukemia | ++ |
| HPB-ALL | Leukemia | ++ |
| RPMI-8226 | Myeloma | − |
| U937 | Histiocytic | − |
| THP-1 | Acute monocytic leukemia | − |
| MutuIIIp122 | EBV-transformed B cell (latency III) | − |
| HDLM-2 | Hodgkin's lymphoma | − |
| L428 | Hodgkin's lymphoma | − |
| KMH2 | Hodgkin's lymphoma | + |
| LCL | EBV-transformed B cell | + |
Table 4.
JL1 expression profiles in nonhematopoietic cell lines (− complete negativity)
| Cell line | Origin | Intensity of expression |
|---|---|---|
| RD-ES | Ewing's sarcoma | − |
| SK-N-MC | Neuroepithelioma | − |
| SK-N-SH | Neuroblastoma | − |
| NT-2 | Teratocarcinoma | − |
| HNT | Neuron cell derived from NT2 | − |
| IMR-32 | Neuroblastoma | − |
| WERI-Rb | Retinoblastoma | − |
| Y79 | Retinoblastoma | − |
| H4 | Neuroglioma | − |
| A172 | Glioblastoma | − |
| HEPG2 | Adenocarcinoma, liver | − |
| A375 | Malignant melanoma | − |
| SCC | Squamous cell carcinoma | − |
| HELA | Cervical carcinoma | − |
| D407 | Transformed retinal pigment epithelium | − |
| FLF | Fetal lung fibroblast | − |
| A253 | Epidermal carcinoma | − |
Antibodies and conjugation
The murine mAb, anti-JL1, was produced as previously described [19]. The ascites form of anti-JL1 mAb was produced after injection of hybridoma cells into pristane-pretreated Balb/c mice. The mAb was purified using protein A chromatography [10]. Fluorescein isothiocyanate (FITC) conjugates were prepared using a Fluorescence-EX protein labeling kit or fluorescence 5-thiosemicarbazide (Molecular Probes, Eugene, Ore.), and biotinylation of anti-JL1 mAb was also performed with biotin hydrazine (Pierce, Rockford, Ill.) according to the manufacturer's protocols [11, 13].
Immunohistochemical analysis
Immunostaining was carried out using an avidin-biotin complex immunoperoxidase method. Tissues on slides were fixed with cold acetone before immunostaining. Paraffin sections were deparaffinized in xylene and hydrated through ethanol to phosphate buffer (0.1 M, pH 7.3). Sections were sequentially blocked with 3% H2O2 for 5 min and in 10% (vol/vol) normal goat serum for 20 min. The sections were incubated with mouse anti-JL1 mAb in a humid chamber for 2 h at room temperature. After rinsing with phosphate-buffered saline (PBS), the sections were incubated for 30 min at room temperature with affinity-purified biotinylated goat anti-mouse IgG, and then in a 1:1000 dilution of streptavidin conjugated with horseradish peroxidase (HRP) (DiNonA, Seoul, Korea). The chromogen was developed for 10 min with liquid 3,3′-diaminobenzidine (DAB) (DiNonA) in the presence of 0.3% H2O2. Counterstaining was not performed since the staining patterns of the serial sections with hematoxylin-eosin were available.
Biotinylated tyramine-based amplified immunohistochemistry
Biotinylated tyramine-based immunohistochemical staining was carried out following the procedure described above for conventional immunohistochemical as far as the HRP-conjugated streptavidin incubation step. Biotinylated tyramine solution (DiNonA) was then applied for 10 min at room temperature. After three consecutive 5-min washes in PBS, HRP-conjugated streptavidin was applied again for 20 min at room temperature. Following three additional 5-min washes with PBS, the chromogen was developed in liquid DAB and the slides were further processed according to the conventional immunohistochemical staining method described above.
Immunofluorescence staining and flow cytometric analysis
One-, two- and three-color flow cytometric analysis was done for anti-JL1 mAb screening. After incubation of 106 cells with the relevant fluorochrome-conjugated and/or biotinylated mAbs in PBS containing 1% bovine serum albumin and 0.1% sodium azide for 30 min at 4°C, the cells were washed with PBS and stained with fluorochrome-conjugated streptavidin (Caltag Laboratories, South San Francisco, Calif.). Flow cytometric analysis was performed with a FACScalibur system (Becton Dickinson Immunocytometry Systems, San Jose, Calif.).
Preparation of JL1 mAb-conjugated gelonin IT
A large amount of gelonin was purchased from Pierce. Conjugation of JL1 mAb with gelonin was performed as described previously [17]. The conjugation steps are described briefly below.
Activation of mAb with N-succinimidyl-3-(2-pyridyldithio)propionate (SPDP)
A threefold molar excess of sulfo-LC-SPDP, prepared in dry dimethyl formamide, was added to 10 mg purified JL1 mAb in conjugation buffer (0.1 M sodium phosphate, 0.15 M NaCl, pH 7.5) and incubated for 30 min at room temperature. Excess SPDP was removed on a Sephadex G-25 column equilibrated with conjugation buffer containing 0.5 mM ethylene diaminetetraacetic acid (EDTA).
Thiolation of gelonin with 2-iminothiolane
2-Iminothiolane in degassed nitrogen-bubbled deionized water was added to 10 mg gelonin in 50 mM TEA/HCl, 10 mM EDTA, pH 8.0, and incubated for 90 min at 4°C under nitrogen. Excess 2-iminothiolane was removed by gel filtration on a Sephadex G-25 column equilibrated with conjugation buffer with 10 mM EDTA. The presence of EDTA in this buffer helps to prevent oxidation of the sulfhydryl groups and resultant disulfide formation.
Conjugation of SPDP-activated JL1 mAb with thiolated gelonin
SPDP-modified JL1 mAb in conjugation buffer with 0.5 mM EDTA was mixed with a fivefold molar excess of 2-iminothiolane-modified gelonin. The mixture was incubated for 20 h at 4°C under a nitrogen blanket. To block unreacted sulfhydryl groups, iodoacetamide was added to a final concentration of 2 mM and the mixture incubated for 1 h at room temperature.
Purification
The unconjugated gelonin was removed by passage of the conjugate solution over a column of immunobilized protein A (Pierce). Purified IT, JL1-gelonin complex was eluted with ImmunoPure elution buffer (Pierce) and dialyzed against PBS. To check for contamination of the free gelonin, the dialysate was subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis under non-reducing conditions and Coomassie blue staining. No band was detectable near 30 kDa corresponding to free gelonin whereas the lane loaded with free gelonin showed a band at that position.
Colony-forming assays and statistical analysis
Clonogenic assays for progenitor cells capable of forming granulocytic and monocytic (CFU-GM), monocytic (CFU-M) and erythroid (CFU-E) colonies were performed as described previously using a methyl cellulose assay system [9, 27, 28]. Mononuclear cells (MNC) obtained by density-gradient separation were incubated in liquid culture for 4 h with free gelonin, 10 nM JL1-gelonin IT, or 100 nM JL1-gelonin IT at a cell density of 1.0×106/ml, washed twice in α-minimal essential medium, and then plated at 2.0×105/ml in a semisolid culture medium from Stem Cell Technologies (Vancouver, BC, Canada). Briefly, the growth medium consisted of 1.0% methyl cellulose in Iscove's MDM, 30% fetal bovine serum, 1% bovine serum albumin, 10−4 M 2-mercaptoethanol, 2 mM l-glutamine, and recombinant cytokines including 50 ng/ml stem cell factor, 10 ng/ml granulocyte-macrophage colony-stimulating factor, 10 ng/ml IL-3, and 3 U/ml erythropoietin. All cultures were incubated at 37°C and 95% humidity in an atmosphere containing 5% CO2, and the colonies of different types were scored after 14 days of culture using an inverted microscope. Data are presented as the means±SD from four independent experiments. The Mann-Whitney U-test was applied to verify the significance of differences between groups. P-values less than 0.05 were considered statistically significant. The processing and statistical analysis of the data were performed using SPSS software version 6.1.2 (SPSS, Chicago, Ill.).
Results
Cytotoxicity of JL1 mAb-conjugated gelonin is specific for cells that express JL1 antigen
We have previously demonstrated that JL1 molecules are rapidly internalized into the intracellular compartment within 4 h of incubation upon engagement of anti-JL1 mAb, indicating that JL1 antigen may be an excellent target to deliver cytotoxins to JL1-positive leukemic cells if anti-JL1 mAb is conjugated with an appropriate potent toxin [26]. Therefore, we investigated whether anti-JL1-based IT could kill tumor cells effectively. Due to the several known advantages of gelonin over other types of toxins [24, 25], we conjugated gelonin with anti-JL1 mAb and the ITs were produced as described in Materials and methods. The binding specificities of anti-JL1 IT were tested against JL1-positive Molt-4 cells using a cellular ELISA and flow cytometric analysis. The conjugates retained the same specificity for JL1 antigen as unconjugated JL1 mAb (data not shown).
Gelonin-conjugated anti-JL1 mAb was tested for its ability to inhibit the proliferation of JL1-positive and/or JL1-negative cell lines. The growth-inhibitory effects were determined by trypan blue exclusion, and dose-response curves were generated by estimating the inhibitory effects of JL1-gelonin IT on Molt-4 cells (JL1-positive) and U937 cells (JL1-negative) in culture (Fig. 1). In the presence of JL1-gelonin IT in the concentration range 10–0.01 nM, Molt-4 cell proliferation was inhibited in a dose-dependent manner, with a half reduction in cell number after 96 h incubation with 1 nM IT. The growth rate of Molt-4 cells exposed to unconjugated JL1 mAb concomitantly with free gelonin was not reduced.
Fig. 1a–c.
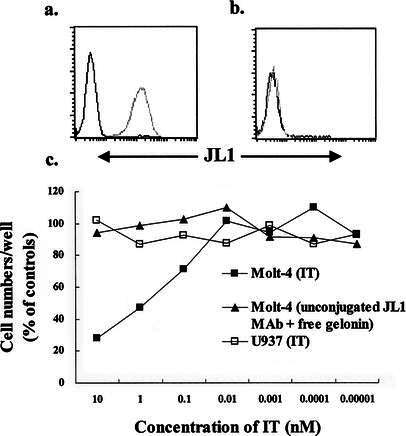
JL1 expression on the surface of Molt-4 cells (a) and U937 cells (b). c Dose response profiles of JL1-gelonin IT in Molt-4 (JL1-positive) cells and U937 (JL1-negative) cells. Starting at a density of 105/ml, cells were incubated for 96 h at 37°C with JL1-gelonin IT and unconjugated JL1 mAb with free gelonin. The treatments are given in parentheses. Trypan blue was added and live cells were counted under a microscope
We also examined the cytotoxic effect of JL1-gelonin IT by determining cell viability at various cell densities at high concentrations of IT. At 17 nM IT, no live Molt-4 cells were observed below a density of 12.5×103 cells/ml (Fig. 2). However, JL1-gelonin IT did not inhibit the proliferation of JL1-negative U937 cells and showed no evidence of cytotoxicity even at the high concentration of 17 nM. These results suggest that the inhibition of cell proliferation was due to the specific binding of mAb to cell surface antigen and the cytotoxic activity of the IT, but not due to nonspecific toxicity of the antibody or the toxin itself. The specific targeting of the IT, anti-JL1-gelonin, to JL1-positive leukemic cell lines was confirmed by a competition assay. In this experiment, the proliferation capacity of Molt-4 cells was restored by preincubation with free JL1 mAb (Fig. 3).
Fig. 2.
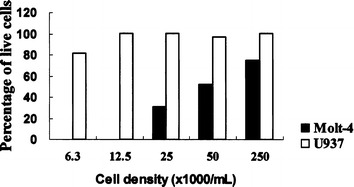
In vitro cytotoxicity of 17 nM JL1-gelonin IT in Molt-4 cells (JL1-positive) and U937 cells (JL1-negative). Cell viability was determined by trypan blue exclusion. The ordinate indicates the percentage of live cells after incubation for 72 h at 37°C in the presence of 17 nM JL1-gelonin IT
Fig. 3a, b.
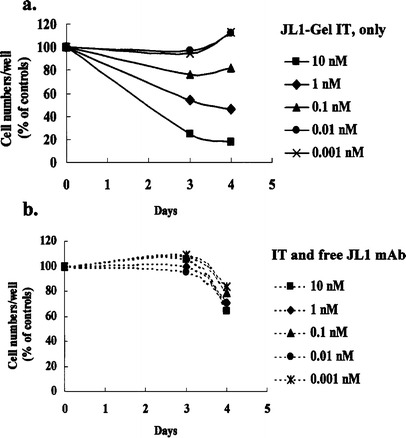
Competition assay with free JL1 mAb. Starting at a density of 105/ml, Molt-4 cells were incubated for 72 or 96 h at 37°C in the presence of only JL1-Gel IT (a) or JL1-Gel IT with free JL1 mAb (1.2 μM) (b). Trypan blue was added and live cells were counted under a microscope
No effect of JL1-gelonin IT on clonogenic activities of early myeloid cells in normal bone marrow (BM) MNCs
To examine the effect of JL1-Gel IT on early myeloid precursors, clonogenic assays for CFU-GM, CFU-M, and CFU-E were performed after normal BM MNCs were incubated with free gelonin, 10 nM JL1-Gel IT, or 100 nM JL1-Gel IT, respectively. Figure 4 indicates that there was no effect of JL1-Gel IT on the clonogenic activity of early myeloid cells in normal BM (P>0.05). These findings suggest that JL1-Gel IT has little, if any, nonspecific toxic effect on normal hematopoiesis of the myeloid lineage.
Fig. 4.
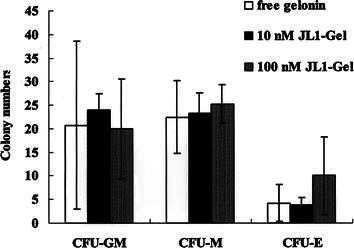
No effect of JL1-Gel IT on the clonogenic activity of early myeloid cells in normal BM MNCs. The results are shown as the number of CFU-GM, CFU-M, and CFU-E per 2.0×105 BM MNCs plated from the corresponding samples. All values are presented as the means±SD from four independent experiments
Restricted distribution of the JL1 antigen on normal cells and tissues
To use anti-JL1 mAb in leukemia patients, it is mandatory to evaluate the expression of JL1 in all types of normal cells and tissues. The reactivity of the anti-JL1 mAb was examined in 35 normal tissues derived from different organ sites by conventional or tyramine-based signal-amplified immunohistochemical analysis. Among these tissues, all except thymus showed completely negative reactivity to anti-JL1 mAb (Table 1). As shown for thymus, anti-JL1 mAb worked equally well on cryoconserved and formalin-fixed paraffin-embedded tissue. Since the catalyzed signal-amplification system using biotinylated tyramine improved the detection limit by more than 200-fold, there was no possibility of false-negative results due to lesser staining in the paraffin-embedded tissue of skeletal muscle, seminal vesicle and salivary gland.
In accordance with the immunohistochemistry results, JL1 antigen expression was not detectable in a large panel of primary cells (Table 2) as well as nonhematopoietic cell lines (Table 4). Most remarkably, JL1 antigen was not detected even in normal lymphoid tissues such as tonsil, lymph node, spleen, or mucosa-associated lymphoid tissue. In contrast, the majority of leukemic and EBV-transformed cell lines were JL1-positive (Table 3). This unique pattern of JL1 expression makes anti-JL1-based IT a selective bullet against leukemic cells, minimizing unwanted side effects on normal cells.
Discussion
The concept of targeting cytotoxic agents specifically against tumor cells has found concrete application in the construction of ITs. The recent development of tumor-targeted cytotoxic agents shows their potential for the treatment of hematologic malignancies, as suggested by preclinical studies and clinical trials with a variety of ITs [1, 17]. We have previously demonstrated that JL1 antigen is expressed in most acute leukemias irrespective of immunological subtype [20]. In order to establish the usefulness of anti-JL1 mAb-based ITs for tumor-targeted immunotherapy, two criteria need to be satisfied: selective binding of the mAb to leukemic cells with low reactivity with normal cells and tissues, and a sufficient amount of mAb delivered into tumor cells for cytotoxic activity. In this context, we first reevaluated the expression patterns of the JL1 molecule in a series of normal tissues and cell types. As shown in Tables 1, 2, 3 and 4, surprisingly, the expression of the JL1 molecule was narrowly restricted to thymocytes and some BM MNCs among the normal tissues tested. Recently, we have dissected the detailed expression of JL1 during normal hematopoiesis and have shown that JL1 is not expressed in noncommitted pluripotent stem cells and is restricted to the specific stage of lymphoid and myelomonocytic precursors [23]. This unique pattern of distribution of JL1 antigen and the specificity of antigen-antibody binding suggest that anti-JL1 mAb could be the most promising candidate for therapeutic trials in acute leukemia.
A recent study by Suh et al. [26] has shown that JL1 antigen on the surface of leukemic cells is readily internalized into the intracellular compartment after binding to anti-JL1 mAb. The endocytosis of the JL1 antigen-antibody complex was visualized by immunofluorescence confocal microscopy. This effective endocytic capacity of JL1 antigen-antibody complex suggests the potential of JL1 mAb as a carrier of immunoconjugates. This property of the JL1 molecule and anti-JL1 mAb is a valuable feature for anti-JL1 mAb-based tumor-targeted therapies for leukemia. To produce ITs, we conjugated anti-JL1 mAb with gelonin and found that gelonin-conjugated anti-JL1 mAb IT effectively destroyed JL1-positive Molt-4 cells in vitro via specific binding. We suggest that JL1 antigen is sufficiently tumor-specific to be of relevance to immunotherapy.
Considering that the affinity constant of anti-JL1 mAb is 1.7×109 l/mol and there are approximately 5400 binding sites (range 5100–9600) per Molt-4 cell, we can calculate the amount of IT required for the complete killing of leukemic cells, based on the assumption that leukemic cells have the same level of JL1 expression as Molt-4 cells. At a concentration of 0.01 nM of JL1-gelonin IT and a Molt-4 cell density of 105/ml, the mean ratio of JL1-gelonin ITs to JL1 molecules is 11.1 (range 6.25–11.8), at which growth inhibition started to be detected. The ratio of about 1000 was required to inhibit Molt-4 cell proliferation by 50%. Furthermore, for complete killing of leukemic cells, the ratio reached about 20,000 (at 17 nM IT for 1.25×104 Molt-4 cells/ml) and this is equivalent to 14.5 μg IT per human body to eradicate 6×107 leukemic cells. Since free gelonin is toxic to cells at a concentration of 200 nM and 1010 leukemic cells are assumed to be present even in patients in complete remission, it seems that we could not use JL1-gelonin IT for the initial reduction of a large tumor burden. However, this IT may be useful for eradicating any remaining leukemic cells in a patient's BM after high-dose chemotherapy or for removing contaminating leukemic cells from BM cells for transplantation in an immunochemopurging autologous bone marrow transplantation protocol.
With recent advances in antibody engineering techniques and the development of new immunoconjugates, clinical trials of antibody-based therapy for cancer are reemerging. However, the effective therapeutic use of mAbs for acute leukemias has been extremely limited mainly by the absence of appropriate tumor-specific targets. The JL1 molecule expressed on leukemic cells is unique in that its expression was almost undetectable in all types of normal cells other than lymphoid precursors. However, it remains to be determined whether the anti-JL1 mAb-based ITs also have effects against leukemic cells in vivo. Since the effective use of mAbs for cancer immunotherapy requires escape from the human immune response, we have developed a humanized anti-JL1 mAb, and the capacity of this form to specifically bind JL1 antigen is under investigation.
We conclude that JL1-gelonin IT is active in vitro against acute leukemia, and that this IT merits further evaluation for use in the treatment of leukemia or for ex vivo purging of cells for BM transplantation.
Acknowledgements
This work was supported in part by a research grant (no. M1010400012401J00000551) from the National Research Laboratory Program of the Korea Ministry of Science and Technology and the 01' DiNonA R&D Project, Seoul, Korea. We are grateful to S.K. Chung (Keimyung University Hospital, Taegu) for helpful technical support.
Footnotes
Y.K.S. and Y.L.C. contributed equally to this work.
References
- 1.Amlot Blood. 1993;82:2624. [PubMed] [Google Scholar]
- 2.Appelbaum FR (1999) Antibody-targeted therapy for myeloid leukemia. Semin Hematol 36 [Suppl 6]: 2 [PubMed]
- 3.Barbieri Biochim Biophys Acta. 1993;1154:237. doi: 10.1016/0304-4157(93)90002-6. [DOI] [PubMed] [Google Scholar]
- 4.Bernstein Leukemia. 2000;14:474. doi: 10.1038/sj.leu.2401663. [DOI] [PubMed] [Google Scholar]
- 5.Caron Curr Opin Oncol. 1994;6:14. doi: 10.1097/00001622-199401000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Caron Clin Cancer Res. 1998;4:1421. [PubMed] [Google Scholar]
- 7.Chung Nucl Med Biol. 1997;24:433. doi: 10.1016/s0969-8051(97)00026-7. [DOI] [PubMed] [Google Scholar]
- 8.Dillman J Clin Oncol. 1994;12:1497. doi: 10.1200/JCO.1994.12.7.1497. [DOI] [PubMed] [Google Scholar]
- 9.Eaves CJ (1995) Assays of hematopoietic progenitor cells. In: Beutler E, Lichtman MA, Coller BS, Kipps TJ (eds) Williams hematology, 5th edn. McGraw-Hill, New York. p L22
- 10.Gagnon P (1996) Protein A affinity purification: In: Purification tools for monoclonal antibodies, 1st edn. Validated Biosystems, Tucson, p 158
- 11.Hardy RR (1986) Purification and coupling of fluorescent proteins for use in flow cytometry. In: Weir DM, Herzenberg LA, Blackwell CC, Herzenberg LA (eds) Handbook of experimental immunology, 1st edn. Blackwell Scientific, Oxford, p 31
- 12.Harris WJ, Cunningham C (1995) Clinical studies with murine and chimeric monoclonal antibodies. In: Antibody therapeutics, 1st edn. R.G. Landes, Austin, p 39
- 13.Hermanson GT (1996) Tags and probes. In: Bioconjugate techniques, 1st edn. Academic Press, San Diego, p 297
- 14.Hertler J Clin Oncol. 1989;7:1932. doi: 10.1200/JCO.1989.7.12.1932. [DOI] [PubMed] [Google Scholar]
- 15.Maloney Curr Opin Hematol. 1999;6:222. doi: 10.1097/00062752-199907000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Matthews Blood. 1999;94:1237. [PubMed] [Google Scholar]
- 17.McGraw Cancer Immunol Immunother. 1994;39:367. doi: 10.1007/BF01534423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Osterborg J Clin Oncol. 1997;15:1567. doi: 10.1200/JCO.1997.15.4.1567. [DOI] [PubMed] [Google Scholar]
- 19.Park J Exp Med. 1993;178:1447. doi: 10.1084/jem.178.4.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park Leukemia. 1998;12:1583. doi: 10.1038/sj.leu.2401161. [DOI] [PubMed] [Google Scholar]
- 21.Sahin Curr Opin Immunol. 1997;9:709. doi: 10.1016/s0952-7915(97)80053-2. [DOI] [PubMed] [Google Scholar]
- 22.Scott Curr Opin Immunol. 1997;9:717. doi: 10.1016/s0952-7915(97)80054-4. [DOI] [PubMed] [Google Scholar]
- 23.Shin Am J Pathol. 2001;158:1473. doi: 10.1016/s0002-9440(10)64098-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sivam Cancer Res. 1987;47:3169. [PubMed] [Google Scholar]
- 25.Stirpe J Biol Chem. 1980;255:6947. [PubMed] [Google Scholar]
- 26.Suh J Controlled Release. 2001;72:171. doi: 10.1016/S0168-3659(01)00273-5. [DOI] [PubMed] [Google Scholar]
- 27.Sutherland Gee. 1991;AP:a. [Google Scholar]
- 28.Testa Molineux. 1993;G:a. [Google Scholar]
- 29.Vitetta ES (1990) Immunotoxins: new therapeutic reagents for autoimmunity, cancer, and AIDS. J Clin Immunol 10 [6 Suppl]:15S [DOI] [PubMed]
- 30.Vitetta Cancer Res. 1994;54:5302. [PubMed] [Google Scholar]


