Abstract
As a vaccine vector, Listeria monocytogenes targets the innate immune system, resulting in a cytokine response that enhances antigen-presenting cell function as well as inducing a Th1 profile. It also enhances cell-mediated immunity by targeting antigen delivery in antigen-presenting cells to both the MHC class I pathway of exogenous presentation that activates CD8 T cells and the MHC class II pathway that processes antigen endogenously and presents it to CD4 T cells. In this review, we describe the development of vaccine constructs that target the human papillomavirus 16 (HPV-16) E7 antigen, and we characterize their effects on tumor regression as well as various immune parameters both innate and adaptive. In particular, we describe the effect on tumor angiogenesis, induction of antitumor suppressor factors like CD4+CD25+ T cells and regulatory cytokines TGF-β and IL-10, homing and infiltration of antigen-specific CD8+ T cells to the tumor, and also effects of the vaccines on antigen-presenting cells, especially focusing on dendritic cell maturation and ability to influence tumor regression. We believe that the identification of several immune parameters that correlate with antitumor efficacy, and of some that have a negative correlation, may have wider application for other cancer immunotherapeutic approaches.
Keywords: Listeria monocytogenes, Vaccine vector, Immune correlates, Human papillomavirus, Cancer vaccines, Mouse models
Introduction
Cervical carcinoma is a gynecological disease that has been identified as the second leading cause of cancer among women worldwide. Human papillomaviruses (HPVs) are responsible for more than 95% of the nearly 450,000 cervical cancers that occur each year throughout the world. HPV is a double-stranded DNA tumor virus that belongs to the papovavirus family and inhabits the squamous epithelium of the mucocutaneous surface. These viruses can be classified into different risk classes, with the high-risk HPV type 16 being the most prevalent in cervical carcinoma cases.
In many developing countries with poor screening techniques, HPV affects millions of men and women annually in the form of genital warts and diseases of the cervix and anogenital region, although in the United States, the cancer rate is relatively low at 13,500 cases per year [2]. Recently, clinical trials to prevent HPV infection, such as those of immunization with human papillomavirus-like particles (VLPs) have been extremely successful [21]. Despite these exciting results for treatment of cervical precancers, the duration of immunity in these trials is unknown, and reversing advanced, invasive cervical lesions with immunotherapeutics is a much more difficult challenge.
One of the most successful methods of anticancer immunotherapy involves using immunodominant peptides from tumor antigens to generate high levels of circulating T cells against cancer antigens [20]. Recent controlled trials have shown some success in inducing precursor regression with vaccines targeting viral oncoproteins. Two of the HPV gene products—early transforming proteins E6 and E7—are almost ideal candidates for vaccine approaches against HPV neoplasia because they are the only open reading frames from the HPV genome that are constitutively expressed in HPV-transformed tissues and are thought to be necessary to maintain the transformed state of these cells [22]. In addition, there is a wealth of evidence that these two proteins are immunogenic in humans, with the production of both humoral and cell-mediated responses [27]. CD8+ cytotoxic T cells are essential to antitumor therapy, as shown by studies demonstrating the presence of CD8+ cells in the lymphocyte infiltration of cervical tumors, which can be cultured and demonstrate CTL activity against certain tumor antigens. Therefore, the development of a vaccine that results in a strong adaptive immune response in individuals with inadequate protective immunity to HPV E6 or E7, would be essential for any approach to successfully treat HPV-associated cancer.
Intracellular bacteria are able to generate a strong cell-mediated immune response within the host. Our lab and those of others have demonstrated the ability of Listeria monocytogenes to be used as a vaccine vector in inducing directed immune responses to added antigens. L. monocytogenes is especially attractive, as compared to other intracellular bacteria such as Salmonella or BCG, as a vaccine vector due to its unusual life cycle. As facultative intracellular bacteria, listeriae are taken up primarily by antigen-presenting cells (APCs) such as macrophages and dendritic cells (DCs) and enter their phagosomes (Fig. 1). The majority of bacteria are killed and digested in the lysosomal compartment in vivo, thus targeting the antigens to the MHC class II pathway for antigen processing and cell surface presentation. At the same time, some of the bacteria will escape into the cytosol through the actions of the listeriolysin O (LLO) protein [19]. Antigens from the Listeria that multiply in the cytosol are presented in an MHC class I–restricted manner to T cells, allowing the expansion of CD8+ T cells, which are necessary for clearance of virally infected host cells as well as being important for direct killing of tumor cells [14]. The MHC class II antigen presentation of bacteria-derived antigens in the lysozome induce a CD4+ T-cell response, which is also necessary for a robust cell-mediated immune response.
Fig. 1.
Antigen processing and targeting of passenger antigens by L. monocytogenes compared to other facultative intracellular bacteria. Facultative intracellular bacteria such as Salmonella, BCG, and L. monocytogenes are taken up into APCs by phagocytosis (a). Listeria alone is capable of lysing the phagosome’s membrane by secreted virulence factors and is released into the cytoplasm (b). Any secreted protein can be cleaved by the proteosome, and the resulting peptides are transported into the endoplasmic reticulum where they are loaded onto MHC class I molecules. The class I MHC molecules are sent to the cell membrane where they present the peptides to CD8+ T cells. In contrast (a), Salmonella, BCG, and L. monocytogenes that fail to lyse the phagosomal-lysosomal membrane are degraded, and their proteins are broken down into peptides that can be loaded onto MHC class II molecules and presented to CD4+ T cells
HPV 16-E7 expressing L. monocytogenes vaccine constructs
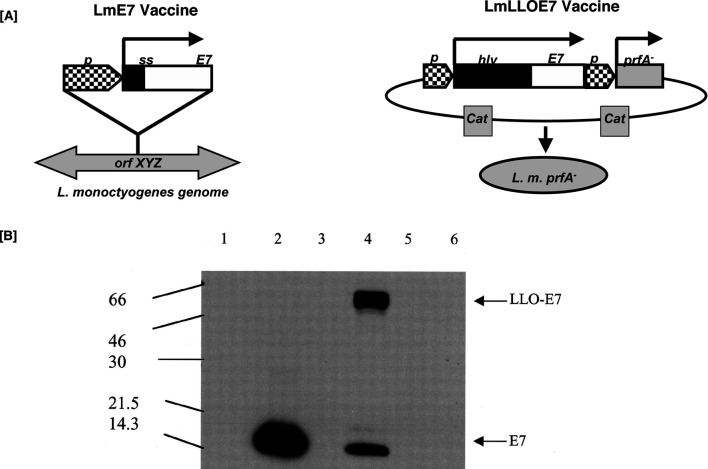
We have designed two recombinant forms of L. monocytogenes vectors that express human papillomavirus (HPV) protein E7 [3]. The two vectors differ in the forms of the HPV E7 that are expressed. As shown in Fig. 2, the Lm-E7 vector is transformed with a single copy of the gene that is integrated into the Listeria genome. The Lm-LLO-E7 vector has the E7 protein expressed as multiple copies from a plasmid and the E7 protein is fused with the nonfunctional first 420 amino acids of the listeriolysin protein. Lm-E7 secretes E7 that migrates at approximately 14 kDa, and Lm-LLO-E7 expresses and secretes a 67-kDa LLO-E7 fusion protein as well as the 14-kDa E7 protein as verified by anti-E7 Western blot (Fig. 2). The Lm-E7 construct is modeled after the Lm-Gag recombinant that has previously been demonstrated to induce effective antiviral immunity to the HIV Gag antigen [10–12]. Lm-LLO-E7 is modeled after the Lm-LLO-NP strain that has shown remarkable effectiveness as an immunotherapeutic targeting the artificial tumor Ag, NP [15– 17].
Fig. 2.
Lm-E7 and Lm-LLO-E7 vaccine constructs. a Lm-E7 was generated by introducing a gene cassette into the orfZ domain of the L. monocytogenes genome. Lm-LLO-E7 was generated by transforming the prfA− strain XFL-7 with the plasmid pGG-55. b Lm-E7 and Lm-LLO-E7 secrete E7. E7 expression was analyzed by Western blot from Lm-Gag (lane 1), Lm-E7 (lane 2), Lm-LLO-NP (lane 3), Lm-LLO-E7 (lane 4), XFL-7 (lane 5), and wild-type Listeria 10403S (lane 6). Reproduced with permission of the authors and publishers [3]
The model that we utilize is the TC-1 tumor model, which has several distinct advantages. It is a C57Bl/6–derived tumor that has been immortalized with HPV-16 E6 and E7 and transformed with oncogenic ras [7]. This tumor expresses E6 and E7 protein constitutively at low levels, grows rapidly in syngeneic mice, and has been used extensively to test HPV immunotherapeutics. In our system, Lm-E7 and Lm-LLO-E7 were compared for their abilities to impact on TC-1 growth. Subcutaneous tumors were established on the left flank of C57BL/6 mice. Seven days later, tumors had reached a palpable size of 4–5 mm in diameter, and the mice were treated on days 7 and 14 with 0.1 LD50 Lm-E7, Lm-LLO-E7, or, as controls, Lm-Gag and Lm-LLO-NP. Tumors were measured every 3 days, and mice were sacrificed at day 60 or when tumors reached 2 cm in diameter. While Lm-E7 had no effect on tumor growth, Lm-LLO-E7 induced complete regression of 75% of established TC-1 tumors (Fig. 3).
Fig. 3.
Lm-LLO-E7 induces complete regression of established TC-1 tumors. C57BL/6 mice (eight per group) received 2×105 TC-1 cells by s.c. injection on the left flank. Mice were treated on days 7 and 14 following tumor challenge with 0.1 LD50 Lm-LLO-E7, Lm-E7, Lm-LLO-NP, or Lm-Gag, or were left untreated. The average tumor diameter was measured with calipers and is shown for each mouse. Mice were sacrificed when tumor diameter reached approximately 2.0 cm. Tumor measurements for each time point are shown only for surviving mice. Reproduced with permission of the authors and publishers [3]
Role for innate immunity in immunotherapy with Listeria
One issue that arises from these results is the necessity to understand the immune parameters that correlate with this obvious difference in the in vivo ability of these two contructs to cause effective tumor regression. For instance we can see that in Fig. 3, the slowing of TC-1 growth in Lm-E7–treated mice compared with naive controls is clearly due to innate immune mechanisms, since the isogenic control, Lm-Gag, slows tumor growth to the same extent.
Listeria induces an inflammatory cytokine cascade early after infection
Listeria induces a potent cellular immune response and a Th1-type cytokine profile by infecting professional APCs in the liver and spleen, both of which are thought to be useful for tumor immunotherapy. As described in Fig. 4, phagocytosis of Listeria by innate immune cells such as macrophages results in the secretion of cytokines IL-12, IL-1, and TNF-α and also activates the macrophage, which in turn results in the destruction of the bacteria by an oxidative burst. IL-1 maintains this activated state of the macrophage and also neutrophils. IL-12 and TNF-α act on NK cells, which are stimulated to produce IFN-γ. This IFN-γ in turn results in the expansion of a Th0 T-cell population, which is further influenced by IL-12 and TNF to differentiate into a Th1 phenotype, which also secrete IFN-γ, TNF-α, and IL-2. This Th1-type cytokine profile is known to be most effective for tumor immunity, and the cytokine cascade initiated by macrophages eventually activates both the innate and adaptive arms of the cellular immune response.
Fig. 4.
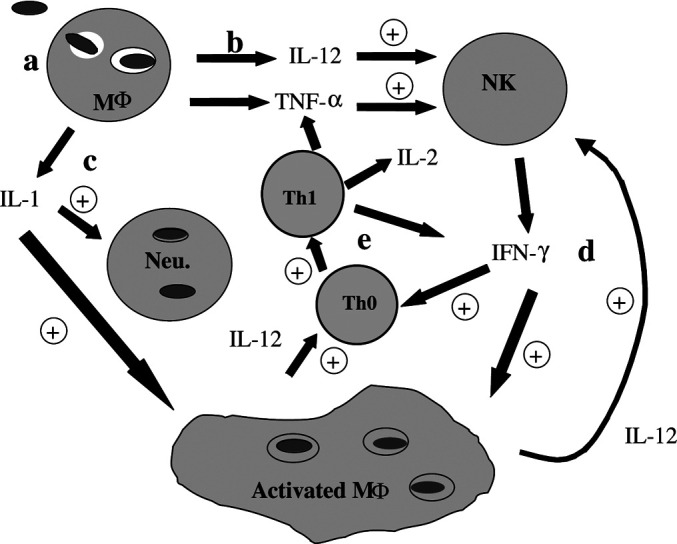
L. monocytogenes induces a cytokine cascade early in infection. a Bacteria are taken up by phagocytic cells including macrophages (Mϕ) and neutrophils. Cell wall components activate macrophages to produce b IL-12 and TNF-α that activate NK cells, and c IL-1 that activates neutrophils. Activated NK cells produce d IFN-γ that acts on macrophages to up-regulate antigen-processing machinery and increase the further production of IL-12, and e it drives the maturation of Th0 cells to Th1 cells. Reproduced with permission of the authors and publishers, from Gunn et al. [4]
The effect of Listeria on tumor angiogenesis
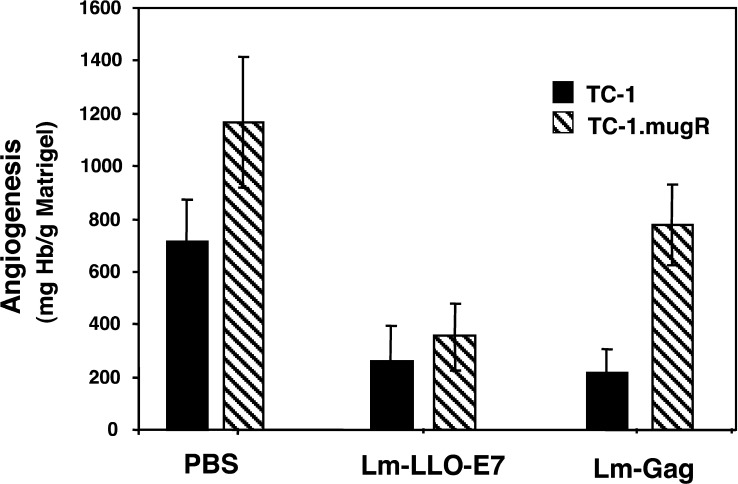
Many human tumors lose responsiveness to IFN-γ, providing a possible mechanism for the tumor to avoid immune recognition and destruction. In fact, tumor responsiveness to IFN-γ has been shown to be required for the effectiveness of IL-12 antiangiogenesis therapy [9, 24]. To determine whether the control of tumor growth through Listeria-induced innate immune mechanisms is by an angiostatic mechanism, we developed a tumor model where TC-1 cells were transfected with a dominant-negative IFN-γRα chain thus rendering them unresponsive to IFN-γ. The clone, TC-1.mugR, displayed equivalent levels of the E7 tumor antigen and increased surface levels of IFN-γRα, and yet remained unresponsive to IFN-γ. In vivo Matrigel assays were used to measure the ability of Lm-LLO-E7 and Lm-Gag to inhibit tumor angiogenesis stimulated by TC-1 and TC-1.mugR. Using hemoglobin content as an index of Matrigel vascularization, tumor cells were mixed with Matrigel matrix and subcutaneously implanted into C57BL/6 mice. Mice were immunized on day 7 with Lm-LLO-E7, Lm-Gag, or PBS. As shown in Fig. 5, a significantly reduced level of vascularization was found in TC-1 tumors of mice treated with either Lm-LLO-E7 or Lm-Gag compared to the PBS-treated group (statistical differences between groups were determined by Student’s t test). Lm-LLO-E7 inhibited angiogenesis in both TC-1 and TC-1.mugR tumors, and therefore, this effect was not dependent on sensitivity to IFN-γ. However, the ability of Lm-Gag to inhibit tumor angiogenesis was dependent on sensitivity to IFN-γ, as Lm-Gag was unable to inhibit angiogenesis in TC-1.mugR (Fig. 5). We demonstrate that suppression of tumor angiogenesis by a nonspecific L. monocytogenes recombinant (Lm-Gag) requires tumor sensitivity to IFN-γ, consistent with previous studies investigating IL-12 antitumor therapy.
Fig. 5.
Listeria inhibits angiogenesis in TC-1 and TC-1.mugR tumors. Naive C57BL/6 mice were subcutaneously injected with Matrigel containing 1×105 TC-1 or 1×105 TC-1.mugR tumor cells. On day 7, mice were injected intraperitoneally with PBS, Lm-LLO-E7, or Lm-Gag. Seven days after treatment, Matrigel plugs were harvested and angiogenesis was quantified by determining the hemoglobin content of individual pellets. Data shown represent the mean ± SEM. Adapted with permission of the authors, from Dominiecki et al. (2005) Cancer Immunol Immunother 54:477--488
Adaptive immune parameters that are influenced by Listeria vaccine vectors
As discussed earlier in Fig. 3, only Lm-LLO-E7 was capable of complete regression of TC-1 tumors, whereas Lm-E7 did not show very significant levels of tumor regression. Are there differences between the two vaccines at the effector level of adaptive immunity that are responsible for differences in tumor efficacy?
To further analyze the abilities of the two recombinants to induce E7-specific CD8+ T cells, mice were immunized and boosted with Lm-E7 or Lm-LLOE7, and their splenocytes were stained with H-2Db tetramers loaded with the E7 peptide. Both E7 vaccine strains induce activated CD8+ T-cell responses in the spleen of similar magnitude. Lm-LLO-E7 induces approximately 8.2% E7-specific tetramer positive CD8+ T cells in the spleen which is comparable to the 12.8% induced by the Lm-E7 vaccine [3]. However, cytotoxic T-lymphocyte (CTL) induction in the spleen is a necessary but not a sufficient condition for effective immunotherapy. Using in vivo depletion of T cells or cytokines following immunotherapeutic vaccination of implanted TC-1 with either Lm-E7 or Lm-LLO-E7, we found that both CD8+ and CD4+ T cells, in addition to IFN-γ, were crucial to the full efficacy of Lm-LLO-E7. Conversely, depletion of CD8+ T cells or IFN-γ had no effect on the incapacity of Lm-E7 to influence the growth of TC-1 in C57BL/6 mice whereas depletion of CD4+ cells in Lm-E7 mice showed improved anti–TC-1 response. Similar results were also obtained when TC-1–bearing mice were treated with an anti–TGF-β mAb (2G7) before and after administration of Lm-E7 or Lm-LLO-E7. While treatment with 2G7 had no apparent effect on mice that received Lm-LLO-E7, a significant number of 2G7-treated Lm-E7–vaccinated mice remained tumor free [3]. Taken together, these data suggested that Lm-E7 and Lm-LLO-E7 might induce qualitatively different CD4+ T-cell subsets that could impact on the antitumor efficacy of Lm-E7–induced CD8+ T cells.
Role of regulatory T cells in preventing tumor regression by Listeria vaccine vector
It has become increasingly clear that even while vaccine and other immunotherapeutics strategies are capable of raising high levels of antitumor T cells, tumors often grow despite lymphocytic infiltration. There are a variety of active mechanisms that may limit the effectiveness of immune stimulation and prevent cancer regression. These mechanisms may be due to the impact of lymphocyte-related factors or tumor-related factors [5, 6]. Recent studies have shown that large numbers of CD4+CD25+ cells capable of suppressing effector T-cell activity can invade the peripheral blood and tumor microenvironment of human non–small cell lung cancers, ovarian cancers, and other invasive cancers [8, 28]. Studies show that when these cells are depleted using mAb, transplanted tumors in mice can be rejected by the host immune system, suggesting that CD4+CD25+ cells may mitigate the immune response to cancers and that removal of this subpopulation in vivo could evoke effective antitumor immunity [13, 26].
To determine whether a CD4+CD25+ suppressor T-cell population was responsible for the different tumor-regressing capabilities of the Lm-E7 versus LmLLO-E7 vaccines, using flow cytometry, we examined the prevalence of CD4+CD25+ T cells in different organs from tumor-bearing mice that were challenged with either vaccine. There were significantly higher numbers of CD4+CD25+ T cells in the spleen and the tumor of the Lm-E7 vaccinated mice when compared to Lm-LLO-E7 vaccinated mice. In the spleen, Lm-E7 vaccinated mice showed an average of 13.5% CD4+CD25+ T cells compared to 9% in Lm-LLO-E7 vaccinated mice. Even more strikingly we found 29% CD4+CD25+ T cells in the tumors of Lm-E7 mice compared to those of Lm-LLO-E7 vaccinated mice (13%). Both these differences were statistically significant; however, we could find no significant differences in the tumor-draining lymph nodes, peripheral blood, or liver. These results suggest that increased numbers of CD4+CD25+ T lymphocytes are seen infiltrating the tumor and spleens of Lm-E7 vaccinated tumor-bearing mice compared to Lm-LLO-E7 tumor bearing mice.
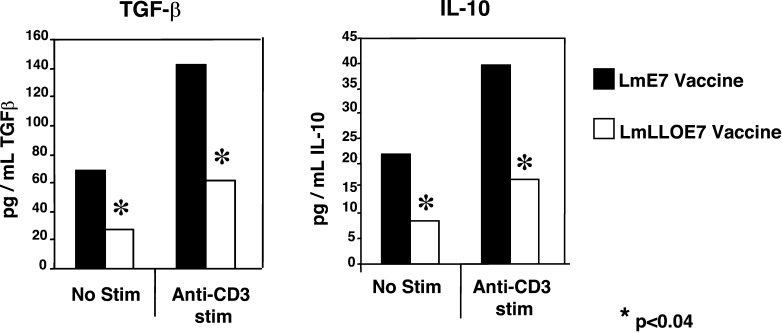
We further characterized the mechanism of action of these CD4+CD25+ suppressor T cells and correlated the data from the TGF-β depletion experiments described above, by looking at whether these cells could impact on tumor efficacy via suppressor cytokines like TGF-β or IL-10. We stimulated T cells isolated from the tumor and spleen of vaccinated as well as control mice via the T-cell receptor (TCR) and collected the supernatants and tested for the presence of suppressor cytokines (Fig. 6). We found that while there was no significant difference in the amount of these cytokines produced in the spleen of the different groups, there was a significant increase in both TGF-β and IL-10 in the tumor-infiltrating lymphocytes produced by the Lm-E7 vaccinated group versus the Lm-LLO-E7 vaccinated group.
Fig. 6.
CD4+ tumor-infiltrating T cells produce TGF-β and IL-10. Mice were injected with TC-1 tumors on day 0, followed by 0.1 LD50 of either Lm-E7 or Lm-LLO-E7 on days 7 and 14 or left unvaccinated. Splenocytes and tumor cells were incubated with or without anti-CD3/APC stimulation for 3 days, and supernatants were collected and analyzed. a TGF-β was detected using a highly sensitive MLEC assay that detects all three forms of TGF-β. Supernatants were added to mink lung epithelial cells (MLEC) expressing PAI-1 with firefly luciferase. TGF-β activates PAI-1, and the amount of TGF-β present is quantitated by the amount of luciferase activity. b IL-10 was detected using the commercially available ELISA kit from Pharmingen (San Diego, CA, USA) according to protocols provided by the manufacturer. Adapted with permission of the authors S.F. Hussain and Y. Paterson (2004) J Immunother 27:339--346
Role of tumor-infiltrating cytotoxic T lymphocytes in antitumor effectiveness of the Listeria vaccines
The difference in tumor regression in the two vaccine groups is so striking that the question still remains whether the induction of suppressor T cells is the only explanation as to why Lm-E7–induced CTLs do not impact on tumor growth, unlike their Lm-LLO-E7 counterparts. In fact CTL induction in the spleen is a necessary but not a sufficient condition for effective immunotherapy, and it is unclear whether these cells are found in the tumor. In other words, how well does infiltration of CD8+ T cells into tumors correlate with antitumor effectiveness?
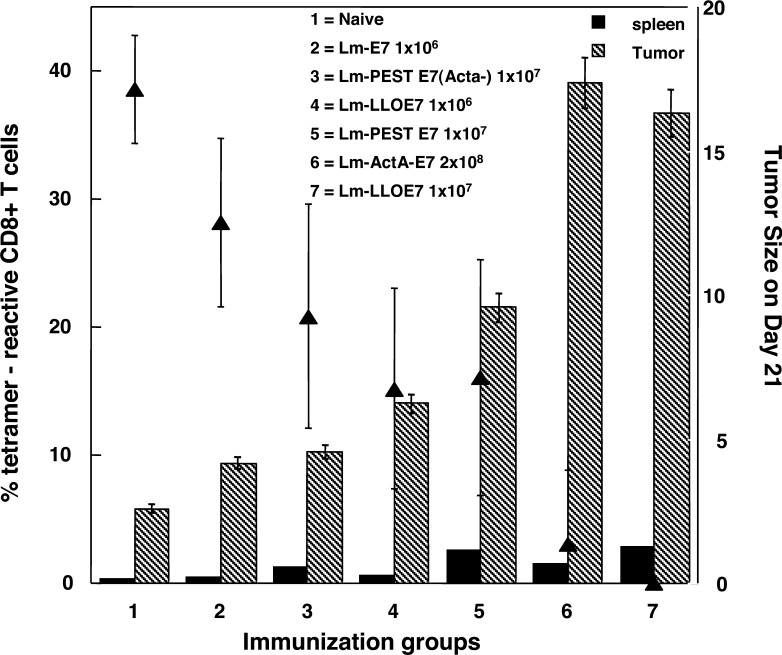
We have compared CD8+ T-cell induction in the spleen with infiltration into the tumor in mice immunized using six different vaccine strategies (Fig. 7). TC-1 cells (2×105 cells in 500 μl of Matrigel) were injected s.c., and 0.1 LD50 or 0.01 LD50 of each Listeria vaccine construct was injected i.p. on days 7 and 14. We used a range of recombinant Listeria expressing different molecular forms of E7 and both optimal and suboptimal vaccine strategies for these constructs in order to provide a range of antitumor efficacy as measured by average tumor size within the vaccine group on days 21 or 28. Spleens and Matrigel plugs were removed on day 21, and underwent collagenase/DNAse digestion. Three-color FACS was performed on the resultant single cell populations for either intracellular IFN-γ or E7-specific tetramer and CD8 and CD62L, and then we quantitated either CD8+ E7 tetramer+ T cells or CD8+ IFN-γ+ T cells. (Figure 7 shows the data for tetramer staining only; similar results were obtained for IFN-γ.) We compared the tumor efficacy, as measured by the ability of the vaccine to regress the tumor, with CD8+ T-cell induction in the spleen and with the number of tumor-infiltrating lymphocytes. In fact the correlation is marked for the tumor-infiltrating lymphocytes (TILs) identified on day 21, with the size of the tumors as determined on days 21 or 28 (R=0.9184, for E7 tetramer+ CD8+ T cells on day 28; and R=0.94386, for IFN-γ+CD8+ T cells on day 28). In contrast, the correlation with the induction of T cells in the spleen is poor (R=0.7 and R=0.3, respectively).
Fig. 7.
Frequency of CD8+ T cells in the spleen and tumors of C57BL/6 mice treated with different Listeria constructs. The dose of vaccine injected i.p into each mouse is the value for LD50 as indicated next to the construct name. The solid triangles represent the average tumor diameter at day 21, following tumor implantation s.c at day 0 and vaccine doses at days 7 and 14. The hatched bars represent the percentage of E7 tetramer+ CD8+ T cells at day 21 in the tumor and the solid bars represent those in the spleen. All numbers are an average from eight mice in vaccine group. Numbers 1–7 indicate the different vaccines used in this experiment. Lm-E7 and Lm-LLO-E7 are described in [3]; Lm-ActA-E7 is described in [23]; Lm-PEST-E7 is described in Sewell et al. (2004) Cancer Res 64:8821--8825. Lm-PEST-E7 (ActA-) is similar to Lm-Pest-E7 except that the host Listeria strain has the ActA gene deleted
We have shown that depletion of CD8+ T cells in mice that have been vaccinated with Lm-LLO-E7 almost completely abrogates tumor regression. This is consistent with the above correlation of increased numbers of E7-specific CD8+ T cells infiltrating the tumor in the most effective tumor-regressing vaccine, thus suggesting a major role for this effector T-cell population in the tumor. One of the mechanisms by which T cells infiltrate the tumor is through the action of chemokines. Chemokine receptors on the T cells also allow the cells to home to sites of inflammation where chemokines are being produced. Some of the possible differences between the Lm-E7 and Lm-LLO-E7 vectors are that the two vectors induce different chemokine profiles, chemokine receptor profiles, or even Th1 cytokine profiles. We are currently utilizing RNAse protection assays to examine cytokine and chemokine production in Lm-LLO-E7–induced CD8+ T cells as compared to the “conventional” CD8+ responses induced by Lm-E7.
Role of antigen-presenting cells in listerial antitumor efficacy
To successfully establish the efficacy of these listerial vaccines, it is critical to understand the reason Lm-LLO-E7 and Lm-E7 induce such qualitatively and quantitatively different populations of CD4+ and CD8+ T cells. One obvious explanation is the effect the individual vaccines may have on APCs. DCs are professional APCs that initiate adaptive immune responses by priming both CD4+ and CD8+ T cells. Mature DCs can be induced to express a variety of costimulatory molecules which are essential for the activation of naive T cells [1, 25]. We therefore hypothesized that the difference in the CD4+ T-cell population induced by the two vaccines may be due to differences in the ability of each of these vectors to render immature DCs effective APCs [18].
Differences in surface expression of costimulatory molecules on DCs
To analyze the expression of costimulatory molecules on DCs infected with two Listeria cancer vaccines, bone marrow–derived DCs were left uninfected or infected with Lm-E7 or Lm-LLO-E7 at a multiplicity of infection (MOI) of 1,000. After 24 h of infection, surface molecules on DCs were measured by flow cytometry. As described in Table 1, Lm-LLO-E7 strongly enhanced CD86 expression (86%) compared to Lm-E7 (40%). Both recombinants slightly increased CD80 expression level at 24 h culture. MHC class II expression was also enhanced in the Lm-LLO-E7 group, with 93% of DCs MHC class II+, whereas similar levels (43–55%) were expressed on the uninfected and Lm-E7 infected groups. Lm-LLO-E7 also up-regulated B7-H1high (63%) compared to uninfected DCs (34%), while Lm-E7 down-regulated the levels of B7-H1high (11% and 1%). Similarly, B7-DChigh was up-regulated by Lm-LLO-E7 (85%) compared to uninfected DCs (67%) and Lm-E7 levels (63%). Lm-LLO-E7 also strongly increased the expression level of the CD40 molecule (46%) compared to Lm-E7 (26%) and uninfected DCs (19%). Taken together, our data demonstrated that Lm-LLO-E7 induces more efficient DC maturation than Lm-E7.
Table 1.
MHC II, B7, and CD40 expression on DCs after Lm vaccine infection. Bone marrow–derived DCs (106/ml) were cultured with 109 CFU/ml of Lm-E7, Lm-LLO-E7, or left untreated in RPMI 1640 medium without antibiotics in a 24-well plate at 37°C. After 1 h incubation, 50 mg/ml of gentamicin was added to kill the remaining extracellular bacteria. Cell culture continued at 37°C for 24 h. Cells were stained with either PE-labeled mAbs specific for mouse CD11c, or FITC-labeled mAb specific for mouse CD80, CD86, B7H1, B7DC, or CD40 MHC class II molecules. Isotype-matched mouse IgG was used as a negative control. Then 7AAD (10 ml) was added to all samples 10 min before cells were analyzed on a FACS flow cytometer. The values represent the percentage of CD11c+ cells
| DC marker (%) | Uninfected (%) | Lm-E7 (%) | Lm-LLO-E7 (%) |
|---|---|---|---|
| MHC II | 55 | 43 | 93 |
| CD80 | 83 | 94 | 93 |
| CD86 | 45 | 40 | 86 |
| B7H1 | 34 | 11 | 63 |
| B7DC | 67 | 63 | 85 |
| CD40 | 19 | 26 | 46 |
Effect of the vaccines on antigen presentation in vitro and in vivo
We then demonstrated that these Lm-LLO-E7–matured DCs are also functionally superior to Lm-E7–induced DCs at stimulating an effector T-cell response both in vitro and in vivo. Firstly, Lm-LLO-E7–infected DCs and Lm-E7–infected DCs were analyzed for their ability to stimulate proliferation of T cells in vitro. Using the proliferation marker CFSE, Figure 8 shows that 4.3% of the T cells exposed to Lm-LLO-E7 DCs underwent cell division or proliferation as compared to the Lm-E7 group, where all the T cells remained undivided, indicating that Lm-LLO-E7–infected DCs alone are capable of stimulating a naive CD8+ T-cell proliferative response in vitro (Fig. 9).
Fig. 8.
Dendritic cells matured with Lm-LLO-E7 stimulate naive T-cell proliferation. Bone marrow DCs from C57BL/6 mice were left uninfected or pulsed with Lm-E7 (1:1,000) or Lm-LLO-E7 (1:1,000) for 4 h. CD3+ T cells were isolated from splenocytes from naive C57BL/6 mice and labeled with CFSE. CFSE-labeled CD3+ T cells (75,000) were incubated in a 96-well plate at 37°C for 3 days with either soluble anti-CD3 antibody (5 μg/ml) and APC (anti-CD3 antibody), 150,000 uninfected DCs (DC alone), 150,000 DCs pulsed with Lm-E7 (Lm-E7 DC), or 150,000 DCs pulsed with Lm-LLO-E7 (Lm-LLO-E7 DC). Cells were then washed and labeled with anti-CD8 antibody and examined by FACS. a Cells from each group were gated on CD8+ T cells and analyzed for dilution of CFSE stain. b Each cell division as determined by CFSE dilution from the histogram analysis in (a) was gated, and the percentage of cells in each population was tabulated. Reproduced with permission of the authors and publishers [18].
Fig. 9.
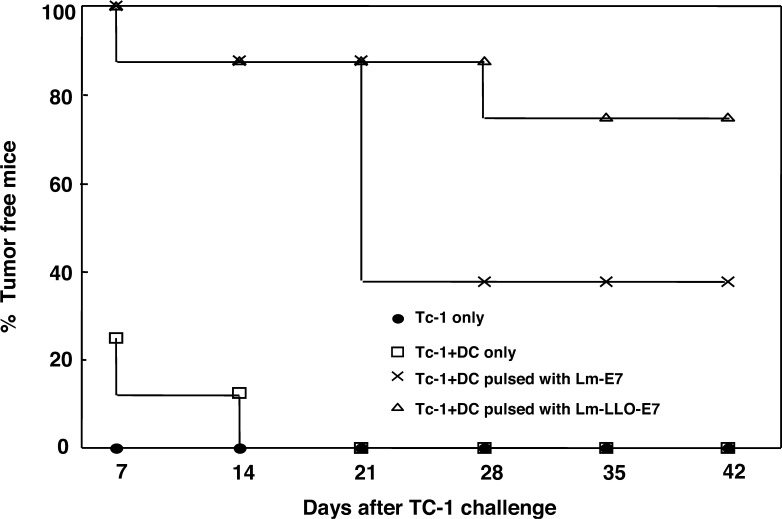
Lm-LLO-E7–pulsed DCs give better protection than Lm-E7–pulsed DCs against TC-1 challenge. Lm-LLO-E7 enhances in vivo antitumor response. Bone marrow–derived DCs (106/ml) were pulsed with 109 CFU/ml of Lm-E7 or Lm-LLO-E7 in RPMI 1640 medium without antibiotics. After 1 h incubation, 50 μg/ml of gentamicin was added. After 30 min, cells were washed and cultured in antibiotic-free medium for 4 h. After washing twice with PBS, 1×105 pulsed DCs, unpulsed DCs, or PBS alone was mixed with 2×105 TC-1 cells and injected s.c. in the left flank of C57BL/6 mice. Reproduced with permission of the authors and publishers [18].
To compare the in vivo antitumor efficacy of Lm-E7– and Lm-LLO-E7–infected DCs, we examined the impact on mice injected s.c. with TC-1 tumor cells together with DCs either unpulsed or pulsed with Lm-LLO-E7 or Lm-E7. In mice receiving Lm-LLO-E7–pulsed DCs, 75% of mice remained tumor free 42 days after TC-1 challenge. Whereas, only 37.5% of mice receiving Lm-E7–pulsed DCs remained tumor free, confirming that Lm-LLO-E7–pulsed DCs also have better in vivo antitumor efficacy than Lm-E7–pulsed DCs.
Conclusions
A critical issue in mounting an effective immune response to antigens expressed by HPV-induced tumors is the fact that they are poorly immunogenic. Our model system for cervical cancer demonstrates that the powerful immune response induced by infection with L. monocytogenes secreting the HPV-16 E7 tumor antigen is able to completely eradicate established tumors. In this review, we try to shed light on the primary immune mechanisms that are engendered by this vaccine system. These include characterizing the effects on innate as well as adaptive immune parameters. We demonstrate that effective Listeria-induced innate immunity helps slow tumor growth through angiostatic mechanisms. On a cellular level, instead of potentially inducing tumor immunity, Lm-E7–induced CD4+ T cells are suppressive in nature, which correlates with impaired tumor regression by this vaccine. This shows that recombinant bacterial vectors have the potential to induce suppressive immunity and reinforces the importance of analyzing cellular immune responses to vaccines.
In contrast, we show that antigen-specific CD8+ T cells play a critical role in tumor regression. Our results demonstrate that both Lm-LLO-E7 and Lm-E7 vaccines induce similar numbers of E7 tetramer+ CD8+ T cells in the spleen, however, there are increased numbers of antigen-specific CD8+ T cells present in the tumors of Lm-LLO-E7 vaccinated mice as compared to those induced by other vaccine constructs. This suggests that the ability of the CD8+ T cells to home to and infiltrate the tumor differs between the Listeria vaccine models, and it is a focus in our lab to determine if this difference is due to qualitative differences in their chemokine receptor and adhesion receptor expression or any other markers that could affect penetration of the tumor. We also analyzed the possible reasons for the two vaccines inducing such different immune responses. Indeed, vaccine efficacy correlates closely with DC maturation status and function as determined by in vitro costimulatory molecule expression levels and in vivo tumor regression analysis.
Identification of such tumor homing markers, understanding antigen presentation of these vaccines, recognizing regulatory molecules that are immune suppressive, and correlating these immunological parameters with tumor regression efficacy of the vaccines, are factors that are critical in the development and improvement of cancer immune therapies. We believe that our findings may provide more general lessons that are required for effective antitumor immunotherapy.
Acknowledgements
This work was supported by NIH Grant CA 69632 and ACS Grant TURSG LIB-01-168-01. We wish to thank past and current members of the Paterson laboratory, including Gregory Beatty, Mary Dominiecki, George Gunn, Zhen-Kun Pan, John Pappas, Duane Sewell, and Vafa Shahabi, for permission to describe their as-yet-unpublished results.
Footnotes
This article is a symposium paper from the second international conference “Strategies for Immune Therapy,” 29 February–3 March 2004, Würzburg, Germany; summarized by G. Pawelec and C. Gouttefangeas.
References
- 1.Carreno BM, Collins M. The B7 family of ligands and its receptors: new pathways for costimulation and inhibition of immune responses. Annu Rev Immunol. 2002;20:29–53. doi: 10.1146/annurev.immunol.20.091101.091806. [DOI] [PubMed] [Google Scholar]
- 2.Crum CP, Rivera MN. Vaccines for cervical cancer. Cancer J. 2003;9:368–376. doi: 10.1097/00130404-200309000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Gunn GR, Zubair A, Peters C, Pan ZK, Wu TC, Paterson Y. Two Listeria monocytogenes vaccine vectors that express different molecular forms of human papilloma virus-16 (HPV-16) E7 induce qualitatively different T cell immunity that correlates with their ability to induce regression of established tumors immortalized by HPV-16. J Immunol. 2001;167:6471–9. doi: 10.4049/jimmunol.167.11.6471. [DOI] [PubMed] [Google Scholar]
- 4.Gunn GR, Zubair A, Paterson Y (2002) Harnessing bacteria for cancer immunotherapy. In: Goebel W, Dietrich G (eds) Vaccines: delivery systems, chap 14. Horizon Scientific Press, Wymondham
- 5.Kiessling R, Wasserman K, Horiguchi S, Kono K, Sjoberg J, Pisa P, Petersson M. Tumor-induced immune dysfunction. Cancer Immunol Immunother. 1999;48:353–362. doi: 10.1007/s002620050586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levey DL, Srivastava PK. Alterations in T cells of cancer-bearers: whence specificity? Immunol Today. 1996;17:365–368. doi: 10.1016/0167-5699(96)10013-X. [DOI] [PubMed] [Google Scholar]
- 7.Lin KY, Guarnieri FG, Staveley-O’Carroll KF, Levitsky HI, August JT, Pardoll DM, Wu TC. Treatment of established tumors with a novel vaccine that enhances major histocompatibility class II presentation of tumor antigen. Cancer Res. 1996;56:21–26. [PubMed] [Google Scholar]
- 8.Liyanage UK, Moore TT, Joo HG, Tanaka Y, Herrmann V, Doherty G, Drebin JA, Strasberg SM, Eberlein TJ, Goedegebuure PS, Linehan DC. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol. 2002;169:2756–2761. doi: 10.4049/jimmunol.169.5.2756. [DOI] [PubMed] [Google Scholar]
- 9.Majewski S, Marczak M, Szmurlo A, Jablonska S, Bollag W. Interleukin-12 inhibits angiogenesis induced by human tumor cell lines in vivo. J Invest Dermatol. 1996;106:1114–8. doi: 10.1111/1523-1747.ep12340161. [DOI] [PubMed] [Google Scholar]
- 10.Mata M, Paterson Y. Th1 T cell responses to HIV-1 Gag protein delivered by a Listeria monocytogenes vaccine are similar to those induced by endogenous listerial antigens. J Immunol. 1999;163:1449–1456. [PubMed] [Google Scholar]
- 11.Mata M, Travers PJ, Liu Q, Frankel FR, Paterson Y. The MHC class I-restricted immune response to HIV-gag in BALB/c mice selects a single epitope that does not have a predictable MHC-binding motif and binds to Kd through interactions between a glutamine at P3 and pocket D. J Immunol. 1998;161:2985–2993. [PubMed] [Google Scholar]
- 12.Mata M, Yao ZJ, Zubair A, Syres K, Paterson Y. Evaluation of a recombinant Listeria monocytogenes expressing an HIV protein that protects mice against viral challenge. Vaccine. 2001;19:1435–1445. doi: 10.1016/S0264-410X(00)00379-0. [DOI] [PubMed] [Google Scholar]
- 13.Onizuka S, Tawara I, Shimizu J, Sakaguchi S, Fujita T, Nakayama E. Tumor rejection by in vivo administration of anti-CD25 (interleukin-2 receptor alpha) monoclonal antibody. Cancer Res. 1999;59:3128–3133. [PubMed] [Google Scholar]
- 14.Pamer EG, Sijts AJ, Villanueva MS, Busch DH, Vijh S. MHC class I antigen processing of Listeria monocytogenes proteins: implications for dominant and subdominant CTL responses. Immunol Rev. 1997;158:129–136. doi: 10.1111/j.1600-065x.1997.tb00999.x. [DOI] [PubMed] [Google Scholar]
- 15.Pan ZK, Ikonomidis G, Lazenby A, Pardoll D, Paterson Y. A recombinant Listeria monocytogenes vaccine expressing a model tumour antigen protects mice against lethal tumour cell challenge and causes regression of established tumours. Nat Med. 1995;1:471–477. doi: 10.1038/nm0595-471. [DOI] [PubMed] [Google Scholar]
- 16.Pan ZK, Ikonomidis G, Pardoll D, Paterson Y. Regression of established tumors in mice mediated by the oral administration of a recombinant Listeria monocytogenes vaccine. Cancer Res. 1995;55:4776–4779. [PubMed] [Google Scholar]
- 17.Pan ZK, Weiskirch LM, Paterson Y. Regression of established B16F10 melanoma with a recombinant Listeria monocytogenes vaccine. Cancer Res. 1999;59:5264–5269. [PubMed] [Google Scholar]
- 18.Peng X, Hussain SF, Paterson Y. The ability of two Listeria monocytogenes vaccines targeting human papillomavirus-16 E7 to induce an antitumor response correlates with myeloid dendritic cell function. J Immunol. 2004;172:6030–6038. doi: 10.4049/jimmunol.172.10.6030. [DOI] [PubMed] [Google Scholar]
- 19.Portnoy DA, Chakraborty T, Goebel W, Cossart P. Molecular determinants of Listeria monocytogenes pathogenesis. Infect Immun. 1992;60:1263–7. doi: 10.1128/iai.60.4.1263-1267.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosenberg SA, Yang JC, Schwartzentruber DJ, Hwu P, Marincola FM, Topalian SL, Restifo NP, Dudley ME, Schwarz SL, Spiess PJ, Wunderlich JR, Parkhurst MR, Kawakami Y, Seipp CA, Einhorn JH, White DE. Immunologic and therapeutic evaluation of a synthetic peptide vaccine for the treatment of patients with metastatic melanoma. Nat Med. 1998;4:321–327. doi: 10.1038/nm0398-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schiller JT, Lowy DR. Papillomavirus-like particle based vaccines: cervical cancer and beyond. Expert Opin Biol Ther. 2001;1:571–581. doi: 10.1517/14712598.1.4.571. [DOI] [PubMed] [Google Scholar]
- 22.Seedorf K, Oltersdorf T, Krammer G, Rowekamp W. Identification of early proteins of the human papilloma viruses type 16 (HPV 16) and type 18 (HPV 18) in cervical carcinoma cells. EMBO J. 1987;6:139–144. doi: 10.1002/j.1460-2075.1987.tb04731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sewell DA, Douven D, Pan ZK, Rodriguez A, Paterson Y. Regression of HPV-positive tumors treated with a new Listeria monocytogenes vaccine. Arch Otolaryngol Head Neck Surg. 2004;130:92–97. doi: 10.1001/archotol.130.1.92. [DOI] [PubMed] [Google Scholar]
- 24.Sgadari C, Angiolillo AL, Tosato G. Inhibition of angiogenesis by interleukin-12 is mediated by the interferon-inducible protein 10. Blood. 1996;87:3877–3882. [PubMed] [Google Scholar]
- 25.Sharpe AH, Freeman GJ. The B7-CD28 superfamily. Nat Rev Immunol. 2002;2:116–26. doi: 10.1038/nri727. [DOI] [PubMed] [Google Scholar]
- 26.Shimizu J, Yamazaki S, Sakaguchi S. Induction of tumor immunity by removing CD25+CD4+ T cells: a common basis between tumor immunity and autoimmunity. J Immunol. 1999;163:5211–5218. [PubMed] [Google Scholar]
- 27.Tindle RW, Frazer IH. Immune response to human papillomaviruses and the prospects for human papillomavirus-specific immunisation. Curr Top Microbiol Immunol. 1994;186:217–253. doi: 10.1007/978-3-642-78487-3_12. [DOI] [PubMed] [Google Scholar]
- 28.Woo EY, Chu CS, Goletz TJ, Schlienger K, Yeh H, Coukos G, Rubin SC, Kaiser LR, June CH. Regulatory CD4(+) CD25(+) T cells in tumors from patients with early-stage non-small cell lung cancer and late-stage ovarian cancer. Cancer Res. 2001;61:4766–4772. [PubMed] [Google Scholar]