Abstract
Human skin tumours often regress spontaneously due to immune rejection. Murine skin tumours model this behaviour; some regress and others progress in syngeneic immunocompetent hosts. Previous studies have shown that progressor but not regressor skin tumours inhibit dendritic cell (DC) migration from the tumour to draining lymph nodes, and transforming growth factor-β1 (TGF-β1) has been identified as a responsible factor. To determine whether increased production of TGF-β1 in the absence of other differences inhibits DC migration from the tumour and enables it to evade immune destruction, a murine regressor squamous cell carcinoma clone was transfected with the gene for TGF-β1. This enhanced growth in vitro and in vivo, causing it to become a progressor. TGF-β1 transfection reduced the number of infiltrating DCs by about 25%. Quantitation of CD11c+ E-cadherin+ (epidermally derived) DCs in lymph nodes determined that TGF-β1 reduced the number of DCs that migrated from the tumour to undetectable levels. This was supported by showing that TGF-β1 reduced DC migration from cultured tumour explants by greater than tenfold. TGF-β1 transfection also reduced the number of infiltrating CD4 and CD8 T cells. Thus, TGF-β1 production by skin tumours is sufficient to immobilise DCs within the tumour, preventing their migration to lymph nodes. This reduces the number of T cells that infiltrate the tumour, preventing regression. Thus, TGF-β1 is a key regulator of whether skin tumours regress or progress.
Keywords: Dendritic cells, Transforming growth factor-β1, Skin tumours, Tumour escape
Introduction
Human skin tumours frequently undergo partial or complete spontaneous regression, during which the tumour is destroyed by the immune system so that it disappears in the absence of therapeutic intervention. Th1-like cell mediated immunity characterised by a T cell infiltrate producing γ-interferon and lymphotoxin appears to be responsible for spontaneous regression of human skin tumours [1]. Ultraviolet radiation (UVR)-induced murine skin tumour clones model this behaviour as they can be divided into regressors which are destroyed by the immune system, and progressors which evade immune destruction when transplanted into syngeneic immunocompetent mice. Spontaneous regression of murine tumours also results from immune destruction [2]. It is, however, unclear how progressor tumours evade the immune system.
Several tumour evasion mechanisms that prevent activation of protective immunity, enabling tumours to grow progressively in immunocompetent hosts have been proposed [3]. By comparing spontaneously regressing with progressing skin tumours, we recently showed that progressor but not regressor skin tumours inhibit dendritic cell (DC) migration from the tumour to draining lymph nodes [4]. Transforming growth factor-β1 (TGF-β1) was identified as one factor produced by the progressor tumours that immobilise DC within the tumours [5].
Dendritic cells are potent antigen presenting cells that are important for the induction of immunity to tumours. The number of tumour infiltrating DC can be of prognostic value [6]. They take up antigen and migrate to local lymph nodes where they present the antigen to T cells, inducing immunity [7]. Studies in animal models and humans have shown that DC are able to induce immunity to skin cancers [2, 8, 9]. Tumours are immunologically destroyed when DC take up antigen and migrate to lymph nodes, but escape immune destruction if DC are subverted so that they do not migrate to draining lymph nodes, or macrophages become the major cell that takes up antigen [10, 4]. Several studies have shown that DC in tumour-bearing animals and cancer patients are defective [11, 12].
TGF-β1 knockout mice lack Langerhans cells (LC), which are epidermal DC [13], and in vitro development of DC precursors into LC requires TGF-β1 [14]. As the skin expresses high levels of TGF-β1 [15], epidermal TGF-β1 is likely to have a profound influence on DC and therefore skin immunity.
This suggests that skin tumours protect themselves from immune destruction by producing TGF-β1 that immobilises DC within the tumour, preventing their migration to lymph nodes and therefore T cell activation. TGF-β1 inhibition of cytotoxic T cells is known to be an important mechanism by which tumours evade immune destruction [16]. However, it is not clear whether overexpression of TGF-β1 alone, in the absence of other differences, is capable of immobilising DC within the tumour, thus reducing T cell activation and inhibiting spontaneous regression. To directly study this, we transfected a murine UV-induced regressor skin tumour clone with the gene for TGF-β1 so that it produced higher levels of this cytokine, and show here that this converted the regressor into a progressor tumour and substantially reduced DC migration from the tumour to draining lymph nodes.
Materials and methods
Transfection, cell culture and TGF-β1 determination
The original cell line was a progressor squamous cell carcinoma (SCC) called UV 13.1 (kind gift from Dr M. Kripke, University of Texas). It was cultured from a skin tumour which arose on a chronically UV irradiated C3H/HeN mouse. Immediately prior to transfection, to ensure the clonal nature of the cells studied, it was subcloned and a regressor tumour cell clone was selected for transfection. A simian TGF-β1 DNA construct mutated to produce only bioactive and no latent forms of TGF-β1 (kindly provided by Ignacio Anegon, Nantes, France) [17] was cloned into the pIRES2-EGFP vector (Clontech, Palo Alto, CA, USA) [18]. The SCC cell clone was transfected with the TGF-β1-pIRES-EGFP or the vector alone using lipofectamine (Gibco BRL, Grand Island, NY, USA). Single cell clones were obtained using the limited dilution method and expanded. Two TGF-β1 transfected clones, called TGF (A) and TGF (B), an empty vector transfected clone (VCo), and the original untransfected clone (UCo) were studied. These TGF-β1 transfected clones were used because they produced different levels of TGF-β1 (see below) and therefore resulted from transfection of different cells.
Cells were routinely cultured in Dulbecco’s modified medium (DMEM) containing 10 mM HEPES (Gibco BRL) and 10% foetal calf serum (FCS) (Gibco BRL Life Technologies, Auckland, New Zealand) at 37°C in a humidified atmosphere containing 5% CO2 in air and trypsinised to detach from the flasks.
A volume of 5×106 cells/ml/well were cultured in 24-well plates (1 ml per well) for 24 h in DMEM containing 10 mM HEPES and 5% mouse serum. Supernatant was collected and treated with 1 M HCl for 1 h at 4°C and then neutralised with 1 M NaOH to activate any latent TGF-β1. TGF-β1 was detected by ELISA with the OptEIA Set human TGF-β1 kit (Pharmingen, San Diego, USA).
In vitro proliferation assay
Tumour cells (5×104 in 200 μl per well) were cultured at 37°C/5% CO2 in 96 well flat-bottomed plates with 1 μCi [methyl 3H]-thymidine (Amersham Pharmacia, Amersham, UK). rhTGF-β1 (R&D Systems, Minneapolis, MN, USA) was included in some experiments. After 24 h cells were harvested for liquid scintillation counting.
Animals
Inbred C3H/HeN mice were obtained from the University of Sydney (Camperdown, Australia) and female ARC(s)-nude athymic mice from the Animal Resources Centre (ARC, Perth, Australia). All mice were aged between 8 weeks and 12 weeks at the commencement of each experiment. Standard rations and water were supplied ad libitum. All experiments were conducted with approval from the University of Sydney Animal Ethics Committee.
In vivo growth of tumours
Tumour cell clones propagated in culture were resuspended at 2×106 viable cells/50 μl phosphate buffered saline (PBS) and injected s.c. into both flanks of female mice. Tumour growth was monitored twice per week up to day 35 in C3H/HeN mice and day 14 in athymic mice by measuring two perpendicular diameters of each tumour with vernier callipers (Mitutoyo, Tokyo, Japan). The mean of the two measured diameters was used as the tumour diameter. Tumours were excised from the mice before they reached a diameter of 8 mm to avoid ulceration. The TGF (B) cell clone was only followed up to day 24 due to several large tumours that had to be removed at that time point for ethical reasons. In one experiment, mice were injected i.p. with 100 μl PBS containing either 2.5 μg chicken anti-hTGF-β1 antibody (type IgY, R&D Systems) or 2.5 μg chicken control IgY (R&D Systems) immediately after tumour inoculation.
Histopathology and immunohistochemistry of tumour tissue
Mice were sacrificed on day 11 after inoculation with tumour cells. Tumours were removed, snap-frozen and stored in liquid nitrogen until required. For immunohistochemistry 6 μm cryostat sections were dried overnight at 4°C. For CD3, CD4, CD8 and CD205 staining they were fixed for 10 min with acetone at 4°C, for CD11c staining they were fixed for 10 min with 0.2% paraformaldehyde in PBS at room temperature. Sections were blocked with 1% (w/v) bovine serum albumin (BSA; Sigma) and 5% (v/v) goat serum (Gibco BRL) in Tris buffered saline (pH 7.3) for 20 min prior to incubation with the primary antibody for 1 h at room temperature. Anti-CD3 (rat IgG2b, KT3), anti-CD4 (rat IgG2b, GK1.5), anti-CD8α (rat IgG2b, YTS-169), anti-CD11c (hamster IgG, N418) and anti-CD205 (rat IgG2a, NLDC-145) monoclonal antibodies (all American Type Culture Collection, Rockville, MD, USA) were used as hybridoma culture supernatants. Rat IgG2b, rat IgG2a and hamster IgG (Pharmingen) were used as isotype controls. Subsequent incubation with biotinylated goat anti-rat or hamster antibody (Caltag, Burlingame, CA, USA) for 1 h at room temperature was followed by streptavidine-alkaline phosphatase (Amersham, Buckinghamshire, UK) and new Fuchsin based substrate as previously described [19]. The stained sections were blinded and the number of stained cells in randomly selected fields were counted by light microscopy until the total area evaluated approximated 0.5–1 mm2.
For histopathological assessment, the tumours from C3H/HeN mice were fixed in formalin and embedded in paraffin. Sections were stained with hematoxylin and eosin and examined in a blinded fashion by a histopathologist specialising in skin tumours.
In vitro migration of DC from tumours
Tumours that had been grown in C3H/HeN mice for 11 days were removed and cultured to induce DC migration from the tissue as previously described [20]. Each tumour was divided into two halves, one of which was freshly snap-frozen. The other half was cultured for 24 h prior to snap freezing. Twenty-four hours were found optimal to detect DC migration from these tumours in preliminary experiments. Frozen sections were stained and counted for DC as described above in a blinded manner by a single investigator (FW). For each tumour specimen, DC were counted in three replicate sections until the total area counted was 0.5–1 mm2 so that 100–300 DC were counted per specimen, in order to obtain an accurate assessment of DC density. The difference in CD11c positive cells between fresh and cultured tumour tissue was a measure of the number of DC migrated out of the tumour explants [5].
In vivo migration of DC from tumours to lymph nodes
Tumour draining (inguinal) and non-draining cervical lymph nodes were removed from C3H/HeN mice at day 11 of tumour growth and mechanically disrupted into cell suspensions. Total cell counts were determined using a hemocytometer, with dead cells being identified by trypan blue (0.4%) dye exclusion. DC recently migrated from the skin tumour and normal epidermis to the lymph nodes were identified by double labelling for E-cadherin and CD11c and flow cytometry as we have described previously [21]. From each sample 50,000 events were routinely acquired and analysed using a FACScalibur flow cytometer and CellQuest software (Becton Dickinson, Franklin Lakes, NJ, USA) to determine the percentage of CD11c+ E-cadherin+ cells. Isotype controls were subtracted. The percentage of CD11c+ E-cadherin+ cells was multiplied by the total number of cells to determine the number of CD11c+ E-cadherin+ cells in each lymph node.
Statistical analysis
Results are presented as means + SEM. For all experiments statistical analysis was performed using Fisher’s PLSD analysis of variance (ANOVA), except for in vivo tumour growth that was analysed by repeated measures ANOVA. The empty vector transfected cell clone (VCo) was chosen as control for statistical comparison in all experiments. P<0.05 was regarded as significant.
Results
Characterization of TGF-β1-transfected cell clones
The empty vector transfected control (VCo) secreted 11 pg/ml; the original untransfected control (UCo) 13 pg/ml; and the two TGF-β1 transfected clones, called TGF (A) and TGF (B) secreted 114 and 64 pg/ml, respectively. Thus transfection increased TGF-β1 production about 5 to 10-fold. The two TGF-β1 transfected clones had fibroblastoid spindle cell morphology in contrast to the empty vector transfected and untransfected clones that had round epitheloid cell morphology in culture. Thus TGF-β1 caused an epithelial mesenchymal transition. There was no obvious difference in morphology of the clones producing different levels of TGF-β1. There were no obvious histopathologic differences in tumour grade, mitotic rate, number of apoptotic cells, necrosis and stromal reaction between the tumours grown in immunocompetent mice. All tumours were identified as SCC, grade 2.
TGF-β1 augments in vitro proliferation of the SCC clone
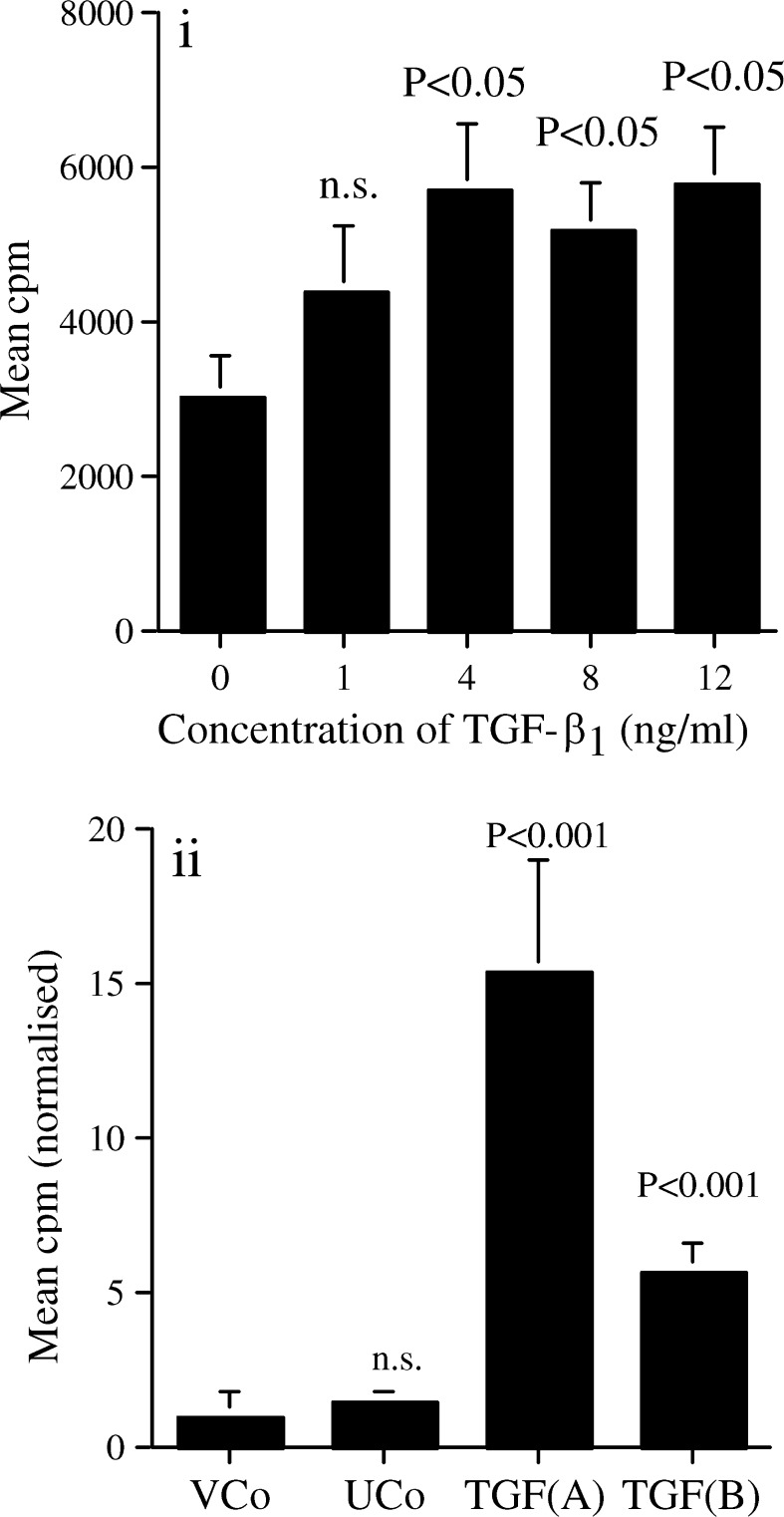
TGF-β1 significantly enhanced [3H]-thymidine uptake of the untransfected clone (Fig. 1a). Additionally, both TGF-β1 transfected cell clones had significantly enhanced proliferation rates compared to the vector transfected control (Fig. 1b) with TGF (A), which produced the highest amount of TGF-β1, having the highest cell growth rate in vitro.
Fig. 1.
TGF-β1 augments growth of the SCC clone. a Proliferation of the untransfected control SCC clone was assessed after 24 h of culture with different TGF-β1 concentrations by [3H]-thymidine uptake. Results shown are representative of three experiments (n=6 in each experiment). b In vitro proliferation of untransfected (UCo), vector transfected (VCo) and the two TGF-β1 transfected cell clones during 24 h culture assessed by [3H]-thymidine uptake. Six independent experiments normalised to the VCo to enable them to be pooled (n=6 in each experiment). Mean + SE shown. Statistical analysis by ANOVA compared to the cell clone without added TGF-β1, or to the VCo control
TGF-β1 enhances growth of tumours in athymic and syngeneic mice
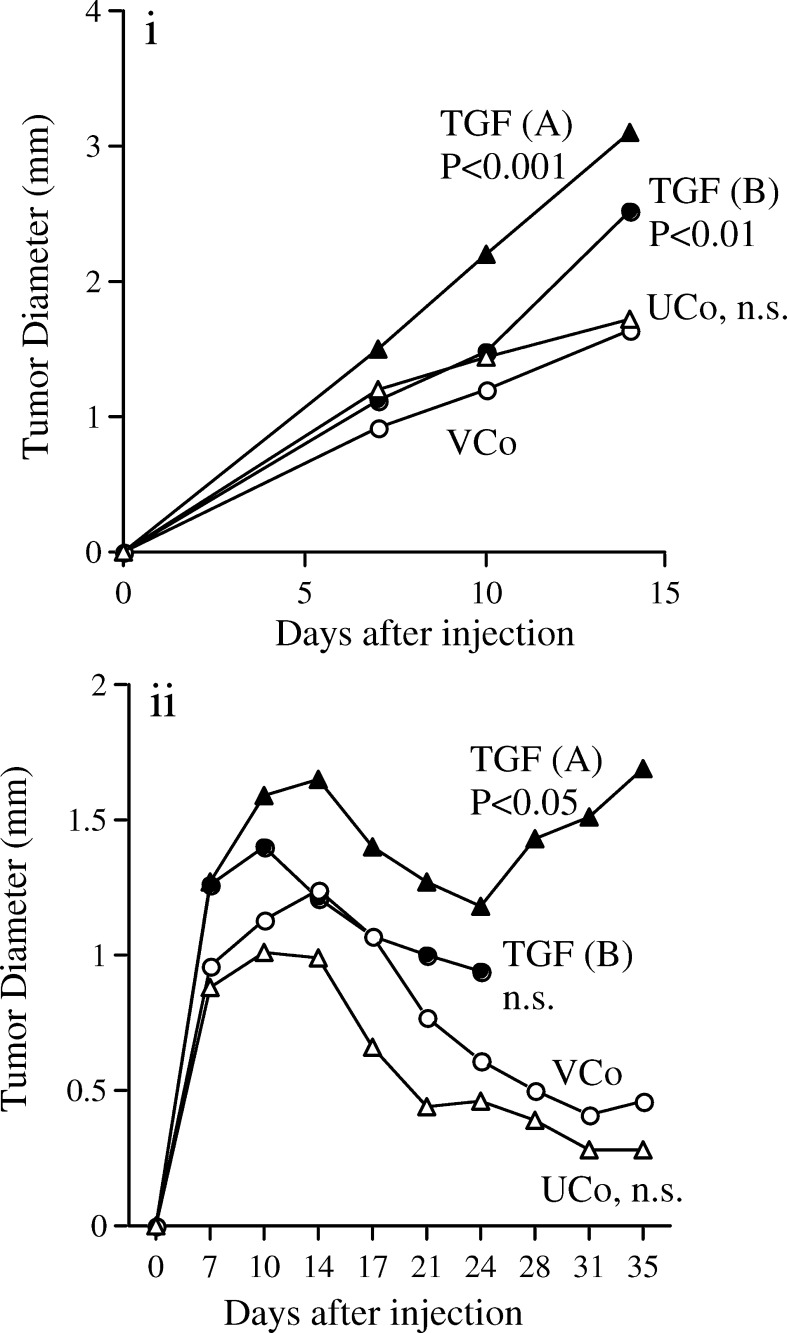
In athymic mice, all injected cell clones showed continuous and progressive growth without regression. The TGF-β1 transfected clones had significantly enhanced in vivo growth compared to the vector transfected control (Fig. 2a). There was no statistical difference between the untransfected and the vector transfected controls. These experiments could not be continued beyond day 14 as many tumours became so large that the mice had to be euthenased for ethical reasons; tumours continued to grow in those mice, which could be kept beyond day 14.
Fig. 2.
TGF-β1 enhances growth of tumours in athymic and syngeneic mice. Diameters of tumours growing in athymic mice (a) and in C3H/HeN mice (b). UCo untransfected control, VCo vector transfected control, TGF (A) and TGF (B) two clones transfected with TGF-β1. Results are a pool of three independent experiments; n=24 (UCo, TGF (A)) or 36 (VCo, TGF (B)) in athymic mice and n=36 for all cell clones in syngeneic C3H/HeN mice. Statistical analysis by repeated measures ANOVA compared to VCo. For TGF (B) the tumours grew so rapidly from day 24 that the mice needed to be culled for ethical reasons and hence there is no data past this time point
Upon transplantation into syngeneic C3H/HeN mice, tumours grew progressively up to day 10–14 followed by a phase of partial or complete regression (Fig. 2b). As regression was not observed in athymic mice, this was likely due to the immune system becoming effectual at about day 10–14. During the tumour progression phase (days 0–14), the TGF-β1 expressing cell clones had increased in vivo growth; however, only TGF (A) reached statistical significance. In the tumour regression phase (after day 14), TGF (A) overcame regression and again grew progressively, leading to significantly enhanced regrowth. TGF (B) behaved similarly with many tumours growing so rapidly that the mice needed to be culled at day 24 for ethical reasons. Therefore complete data could not be obtained past day 24 resulting in no significant effect up to this time point. Tumours on mice that were not culled were 24% larger by the end of the experiment on day 35 than they were on day 24.
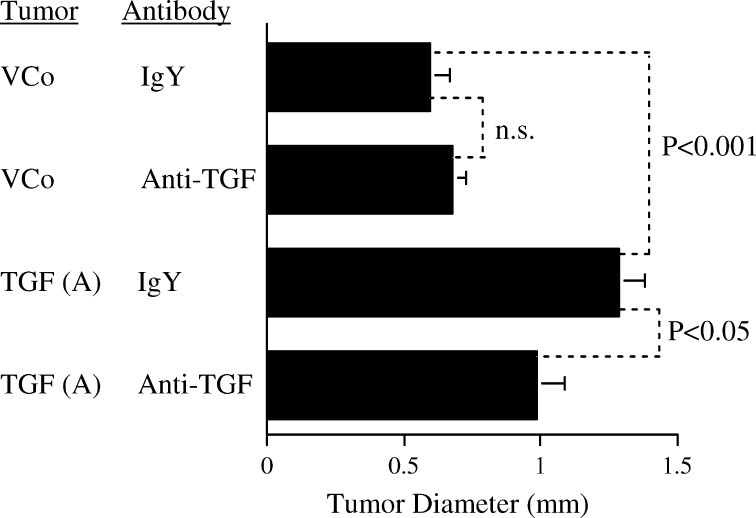
To confirm that the change in growth of the transfected cell clones resulted from increased TGF-β1 production, antibody neutralisation studies were performed in vivo (Fig. 3). Measurement of tumour diameters showed significantly enhanced growth of TGF (A) compared to the vector transfected cell clone in mice receiving control IgY. This growth stimulation was significantly abrogated by i.p. injection of anti-TGF-β1 antibody in the TGF (A) group. Anti-TGF-β1 antibody did not significantly alter growth of the vector only transfected control cell clone.
Fig. 3.
TGF-β1 neutralisation reverses the effect of TGF-β1 transfection on growth in syngeneic mice. Mice received i.p. injections of neutralizing anti-TGF-β1 or control IgY antibody immediately after transfer of vector control (VCo) or TGF (A) tumours. Ten tumours per group. Diameter was assessed after 3 days of growth, mean + SEM shown. Statistical analysis by ANOVA
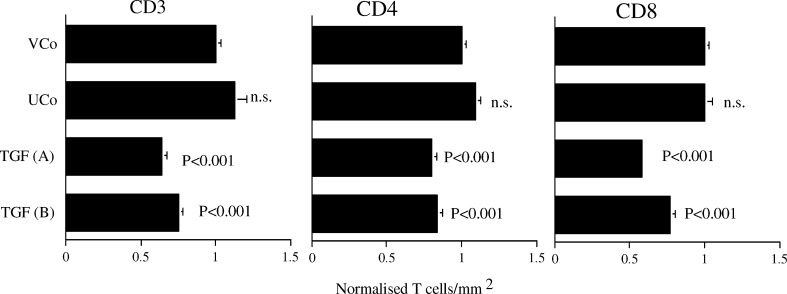
TGF-β1 decreases the number of infiltrating CD3+, CD4+ and CD8+ T cells in tumours growing in syngeneic mice
Tumours grown in C3H/HeN mice were removed on day 11 after injection, which was at the peak of growth and just prior to the commencement of regression. This time was chosen to investigate the cellular events that lead to regression, and avoided confounding events associated with the mediation of regression at later time points. TGF (A) and TGF (B) were both infiltrated with significantly decreased numbers of CD3, CD4 and CD8 positive T cells (Fig. 4). The average numbers of cells in frozen sections of vector transfected control tumours in two independent experiments were 1,114 and 1,248 CD3 positive cells, 686 and 697 CD4 positive cells and 571 and 529 CD8 positive cells per mm2. There was no difference between the untransfected and vector transfected controls.
Fig. 4.
TGF-β1 decreases the number of infiltrating CD3+, CD4+ and CD8+ T cells in tumours growing in syngeneic mice. The number of positive cells in frozen sections normalised to the vector control (VCo; n=23) to enable two independent experiments to be pooled (means + SEM). UCo (n=6), TGF(A) (n=11) and TGF (B) (n=21). Statistical analysis by ANOVA compared to VCo
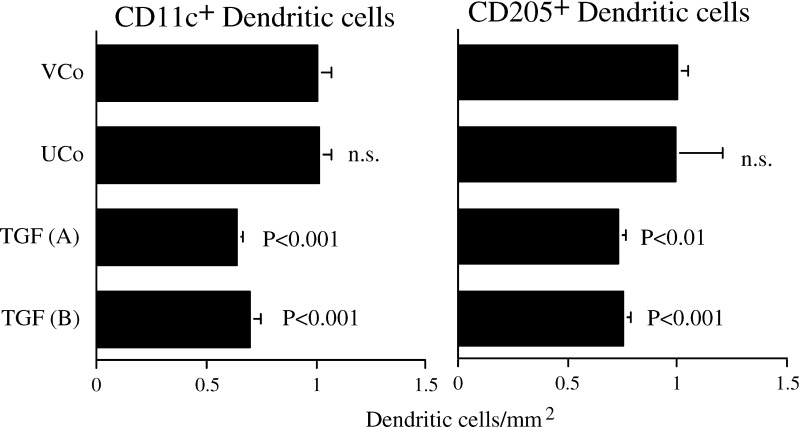
TGF-β1 decreases the number of infiltrating CD11c+ and CD205+ DCs in tumours growing in syngeneic mice
Both TGF-β1-transfected tumours were infiltrated with significantly decreased DC identified with anti-CD11c or CD205 (Fig. 5). There was no statistically significant difference between the untransfected and vector-transfected tumours for either DC marker. The number of CD11b+ macrophages in the tumours was very low (<10 cells/mm2, compared to 100–300 CD11c positive cells/mm2). Thus, any low level CD11c expression on macrophages did not substantially contribute to the number of CD11c+ cells.
Fig. 5.
TGF-β1 decreases the number of infiltrating CD11c+ and CD205+ dendritic cells in tumours growing in syngeneic mice. The number of positive cells in frozen sections normalised to the vector control (VCo; n=22) to enable two independent experiments to be pooled (means + SEM). Remainder of legend as for Fig. 4
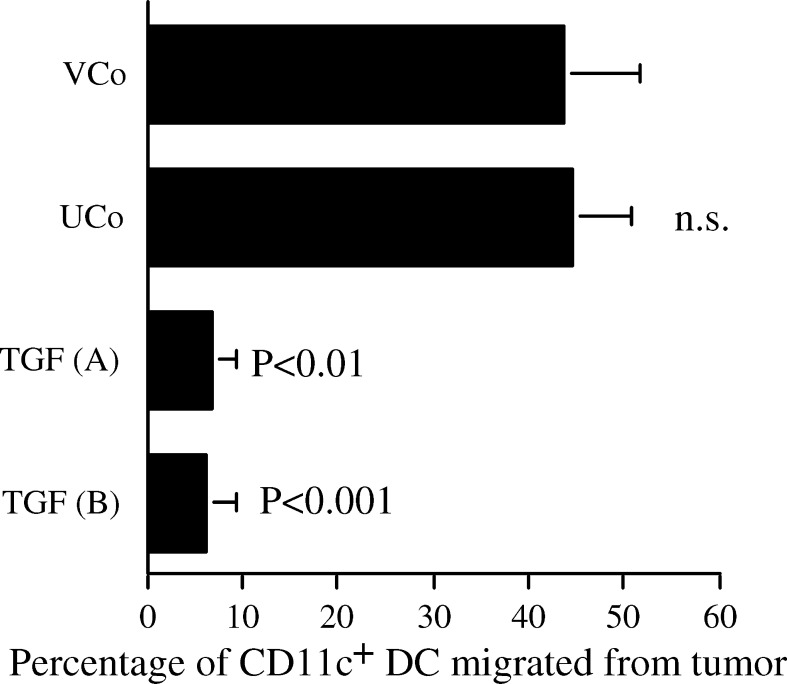
TGF-β1 inhibits migration of dendritic cells out of the tumour
About 45% of DC were stimulated to migrate from the vector-transfected and untransfected control tumours by culture, with no significant differences between these groups. TGF-β1 transfection significantly reduced migration of CD11c+ DC from the tumours to 6.5% and 6.0% (TGF (A) and (B), respectively) (Fig. 6). It is unclear why the two TGF-β1 transfected tumours inhibited DC migration to similar extents despite producing different amounts of TGF-β1, but it is possible that the maximum inhibitory concentration was being produced by both tumours. Similar results were observed when DC were identified by staining for CD205 (results not presented).
Fig. 6.
TGF-β1 inhibits migration of dendritic cells out of the tumour. CD11c+ cells in frozen sections were stained by immunohistochemistry and counted per mm2 of tumour. For each tumour the difference in CD11c+ cells in halves, which were freshly frozen or cultured for 24 h was calculated as dendritic cells which migrated from the tumour. To express DC migration independently of the reduced absolute DC density in TGF-β1 transfected tumours (Fig. 5), the number of DC retained in each cultured half was expressed as a percentage of DC in the respective freshly frozen half. Mean + SEM of two independent experiments. VCo (n=15), UCo (n=4), TGF (A) (n=5) and TGF (B) (n=16). Statistical analysis by ANOVA compared to VCo
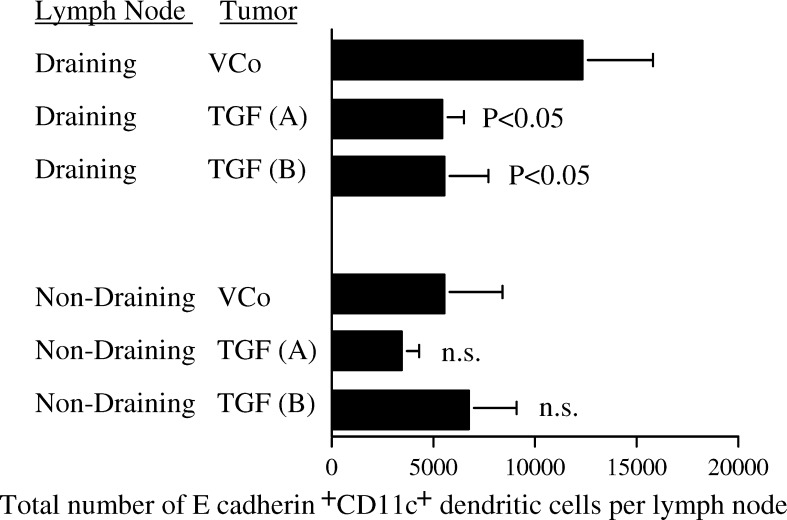
TGF-β1 reduces the number of tumour-derived dendritic cells in draining but not non-draining lymph nodes
CD11+DC infiltrating these tumours were E-cadherin+, and therefore those recently migrated from the SCC and surrounding epidermis to the lymph nodes were detected by double labelling with CD11c and E-cadherin and flow cytometry (Fig. 7). The number of total cells in both draining and non-draining lymph nodes was not altered by transfection with TGF-β1, nor was the number of single positive CD11c+ cells. E-cadherin+ CD11c+ cells only made up about 0.15% of all CD11c+ DC indicating that only a small fraction of the lymph node DC had recently arrived from the tumour or epidermis.
Fig. 7.
TGF-β1 reduces the number of tumour-derived dendritic cells in draining but not non-draining lymph nodes. Tumour and epidermal-derived dendritic cells were identified as E-cadherin and CD11c double positive cells by flow cytometry in tumour draining inguinal and non-tumour draining cervical lymph nodes. Results from two independent experiments pooled. VCo (n=10), TGF (A) (n=10) and TGF (B) (n=10). The untransfected control is not shown for clarity but was not statistically different to the VCo. Statistical analysis by ANOVA compared to VCo
Lymph nodes draining each of the two TGF-β1-transfected tumour clones contained about 5,000 E-cadherin+ CD11c+ cells, which was about half the number found in lymph nodes draining vector-transfected control tumours (Fig. 7). This was a statistically significant difference. In contrast, TGF-β1 transfection did not significantly alter the number of E-cadherin+ CD11c+ cells in non-draining lymph nodes. Thus, TGF-β1 transfection of the SCC reduced the number of DC that migrated from the tumour to draining lymph nodes.
Discussion
Increasing TGF-β1 production by gene insertion into a regressor tumour converted it into a progressor tumour that escaped immune destruction. The immunological changes included reduced infiltration by CD4 and CD8 T cells as well as DC. Additionally, the TGF-β1 inhibited those DC infiltrating the tumour from migrating out of the tumour to draining lymph nodes. These are characteristics of UV-induced progressor tumours [4] and suggest that production of TGF-β1 by the tumour cells is a key factor in enabling tumours to escape immune-mediated regression.
TGF-β1 suppresses growth and differentiation of normal keratinocytes [22, 23]; however, it stimulates the growth of tumour cells [24], giving them a growth advantage over non-transformed cells [25]. Consistent with these previous studies, growth of the cutaneous SCC used in these experiments was stimulated by TGF-β1.
Production of TGF-β1 caused a second peak in growth of the tumours, overcoming regression in immunocompetent mice. This was probably due to TGF-β1 produced by the tumours reducing both CD4 and CD8 T cell infiltration into the tumour. TGF-β1 is commonly reported to inhibit T lymphocyte activation [26]. In support of this, a previous study showed that TGF-β1 transfection of a regressor tumour inhibited the activation of cytotoxic T cells (CTL), and inhibited regression in partially immunosuppressed mice [27].
E-cadherin anchors DC to keratinocytes in the epidermis [28], where they are called LC. It is downregulated when DC are activated, enabling them to migrate out of the epidermis [29]. Only CD11c+ DC that have migrated recently from the epidermis or epidermally derived tumours to lymph nodes display E-cadherin, and although downregulated, levels of E-cadherin are readily detectable by flow cytometry [21, 30, 31]. Thus E-cadherin+ DC in lymph nodes originate from the SCC and surrounding epidermis. Only about 0.15% of total lymph node DC retained sufficient levels of E-cadherin to be detected in our study, suggesting that the DC detected as E-cadherin+ reached the lymph node within a relatively short time period before E-cadherin levels decreased to become undetectable.
Non-tumour-draining lymph nodes in mice bearing vector or TGF-β1 transfected tumours contained about 5,000 CD11c+ E-cadherin+ DC. These DC most likely recently arrived from the epidermis. As there was no detectable effect of TGF-β1 from transfected tumours on DC in non-draining lymph nodes, the effect of TGF-β1 was limited to the tumour. Insufficient amounts of TGF-β1 were produced by the tumours to have systemic effects on DC. In support of this we could not detect elevated levels of TGF-β1 in the serum of mice bearing these tumours (results not presented). Lymph nodes draining the control tumours contained about twice as many CD11c+ E-cadherin+ DC as non-draining lymph nodes, suggesting that about 5,000 of these came from the tumour, and about 5,000 from the epidermis. In contrast, lymph nodes draining TGF-β1 tranfected tumours contained about 5,000 CD11c+ E-cadherin+ DC that could all be accounted for as DC from the epidermis. Therefore, it appears that TGF-β1 substantially prevented DC migration from the tumour to draining lymph nodes. If any DC migrated from the TGF-β1 transfected tumours to lymph nodes, they were too few to be detectable.
Dendritic cell migration from explant cultures is a well-characterised procedure for studying DC migration from tissues [20]. Our studies here showed that TGF-β1 production by tumours reduced migration of DC from cultured tumours by about tenfold, consistent with our experiments showing no detectable CD11c+ E-cadherin+ DC had migrated from the TGF-β1 producing tumours to draining lymph nodes.
By transfecting the gene for TGF-β1 into a regressor SCC, we have shown that production of this cytokine is sufficient to convert it into a progressor in the absence of other differences. TGF-β1 has a biphasic role in skin carcinogenesis, reducing the formation of benign lesions, but enhancing malignant conversion [32]. TGF-β1 may have an important role late in tumourogenesis, after the cells lose growth inhibition in response to this cytokine. Its ability to suppress immunity to the developed tumour may enable the tumour to escape immune destruction. Our studies show that TGF-β1 immobilises DC within the tumour, reducing their migration to lymph nodes by over tenfold. This is likely to be one of the mechanisms by which TGF-β1 inhibits the activation of cell-mediated immunity, and therefore, prevents spontaneous regression.
Acknowledgments
This work was supported by the National Health and Medical Research Council of Australia, the Melanoma and Skin Cancer Research Institute, University of Sydney, the Fondation René Touraine and the University of Innsbruck, Austria. The Centre for Immunology and the Melanoma and Skin Cancer Research Institute are supported by New South Wales Health Research and Development Infrastructure grants.
Abbreviations
- DC
Dendritic cell
- LC
Langerhans cell
- TGF (A) and (B)
Transforming growth factor-β1 transfected clones
- TGF-β1
Transforming growth factor-β1
- UVR
Ultraviolet radiation
- UCo
Untransfected clone
- VCo
Empty vector transfected clone
References
- 1.Halliday GM, Barnetson RSC. Spontaneous regression. In: Chu AC, Edelson RL, editors. Malignant tumors of the skin. London: Arnold; 1999. pp. 411–424. [Google Scholar]
- 2.Cavanagh LL, Halliday GM. Dendritic epidermal T cells in ultraviolet-irradiated skin enhance skin tumor growth by inhibiting CD4(+) T-cell-mediated immunity. Cancer Res. 1996;56:2607–2615. [PubMed] [Google Scholar]
- 3.Byrne SN, Halliday GM. High levels of fas ligand and MHC class II in the absence of CD80 or CD86 expression and a decreased CD4(+) T cell infiltration, enables murine skin tumours to progress. Cancer Immunol Immunother. 2003;52:396–402. doi: 10.1007/s00262-003-0380-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lucas AD, Halliday GM. Progressor but not regressor skin tumours inhibit Langerhans’ cell migration from epidermis to local lymph nodes. Immunology. 1999;97:130–137. doi: 10.1046/j.1365-2567.1999.00751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Halliday GM, Le S. Transforming growth factor-beta produced by progressor tumors inhibits, while IL-10 produced by regressor tumors enhances, Langerhans cell migration from skin. Int Immunol. 2001;13:1147–1154. doi: 10.1093/intimm/13.9.1147. [DOI] [PubMed] [Google Scholar]
- 6.Iwamoto M, Shinohara H, Miyamoto A, Okuzawa M, Mabuchi H, Nohara T, Gon G, Toyoda M, Tanigawa N. Prognostic value of tumor-infiltrating dendritic cells expressing CD83 in human breast carcinomas. Int J Cancer. 2003;104:92–97. doi: 10.1002/ijc.10915. [DOI] [PubMed] [Google Scholar]
- 7.Byrne SN, Halliday GM. Dendritic cells: making progress with tumour regression? Immunol Cell Biol. 2002;80:520–530. doi: 10.1046/j.1440-1711.2002.01122.x. [DOI] [PubMed] [Google Scholar]
- 8.Nestle FO, Alijagic S, Gilliet M, Sun YS, Grabbe S, Dummer R, Burg G, Schadendorf D. Vaccination of Melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nat Med. 1998;4:328–332. doi: 10.1038/nm0398-328. [DOI] [PubMed] [Google Scholar]
- 9.Hersey P, Menzies SW, Halliday GM, Nguyen T, Farrelly ML, DeSilva C, Lett M. Phase I/II study of treatment with dendritic cell vaccines in patients with disseminated melanoma. Cancer Immunol Immunother. 2004;53:125–134. doi: 10.1007/s00262-003-0429-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Byrne SN, Halliday GM. Phagocytosis by dendritic cells rather than MHC II high macrophages is associated with skin tumour regression. Int J Cancer. 2003;106:736–744. doi: 10.1002/ijc.11274. [DOI] [PubMed] [Google Scholar]
- 11.Gabrilovich DI, Ciernik IF, Carbone DP. Dendritic cells in antitumor immune responses 1 defective antigen presentation in tumor-bearing hosts. Cell Immunol. 1996;170:101–110. doi: 10.1006/cimm.1996.0139. [DOI] [PubMed] [Google Scholar]
- 12.Chaux P, Favre N, Martin M, Martin F. Tumor-infiltrating dendritic cells are defective in their antigen-presenting function and inducible B7 expression in rats. Int J Cancer. 1997;72:619–624. doi: 10.1002/(SICI)1097-0215(19970807)72:4<619::AID-IJC12>3.3.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 13.Borkowski TA, Letterio JJ, Farr AG, Udey MC. A role for endogenous transforming growth factor beta 1 in Langerhans cell biology: the skin of transforming growth factor beta 1 null mice is devoid of epidermal Langerhans cells. J Exp Med. 1996;184:2417–2422. doi: 10.1084/jem.184.6.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caux C, Massacrier C, Dubois B, Valladeau J, Dezutter-Dambuyant C, Durand I, Schmitt D, Saeland S. Respective involvement of TGF-beta and IL-4 in the development of Langerhans cells and non-Langerhans dendritic cells from CD34(+) progenitors. J Leukoc Biol. 1999;66:781–791. doi: 10.1002/jlb.66.5.781. [DOI] [PubMed] [Google Scholar]
- 15.Gruschwitz M, Muller PU, Sepp N, Hofer E, Fontana A, Wick G. Transcription and expression of transforming growth factor type beta in the skin of progressive systemic sclerosis: a mediator of fibrisis? J Invest Dermatol. 1990;94:197–203. doi: 10.1111/1523-1747.ep12874503. [DOI] [PubMed] [Google Scholar]
- 16.Beck C, Schreiber H, Rowley DA. Role of TGF-beta in immune-evasion of cancer (Review) Microsc Res Tech. 2001;52:387–395. doi: 10.1002/1097-0029(20010215)52:4<387::AID-JEMT1023>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 17.Josien R, Douillard P, Guillot C, Muschen M, Anegon I, Chetritt J, Menoret S, Vignes C, Soulillou JP, Cuturi MC. A critical role for transforming growth factor-beta in donor transfusion-induced allograft tolerance. J Clin Invest. 1998;102:1920–1926. doi: 10.1172/JCI4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fairlie WD, Russell PK, Wu WM, Moore AG, Zhang HP, Brown PK, Bauskin AR, Breit SN. Epitope mapping of the transforming growth factor-beta superfamily protein, macrophage inhibitory cytokine-1 (MIC-1): identification of at least five distinct epitope specificities. Biochemistry. 2001;40:65–73. doi: 10.1021/bi001064p. [DOI] [PubMed] [Google Scholar]
- 19.Patel A, Halliday GM, Cooke BE, Barnetson RS. Evidence that regression in keratoacanthoma is immunologically mediated: a comparison with squamous cell carcinoma. Br J Dermatol. 1994;131:789–798. doi: 10.1111/j.1365-2133.1994.tb08580.x. [DOI] [PubMed] [Google Scholar]
- 20.Larsen C, Steinman R, Witmer-Pack M, Hankins D, Morris P, Austyn J. Migration and maturation of Langerhans cells in skin transplants and explants. J Exp Med. 1990;172:1483–1493. doi: 10.1084/jem.172.5.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Byrne SN, Halliday GM, Johnston LJ, King NJC. Interleukin−1β but not tumor necrosis factor is involved in West Nile virus-induced Langerhans cell migration from the skin in C57BL/6 mice. J Invest Dermatol. 2001;117:702–709. doi: 10.1046/j.0022-202x.2001.01454.x. [DOI] [PubMed] [Google Scholar]
- 22.Dahler AL, Cavanagh LL, Saunders NA. Suppression of keratinocyte growth and differentiation by transforming growth factor beta 1 involves multiple signaling pathways. J Invest Dermatol. 2001;116:266–274. doi: 10.1046/j.1523-1747.2001.01243.x. [DOI] [PubMed] [Google Scholar]
- 23.Dissanayake NS, Mason RS. Modulation of skin cell functions by transforming growth factor-beta-1 and Acth After Ultraviolet Irradiation. J Endocrinol. 1998;159:153–163. doi: 10.1677/joe.0.1590153. [DOI] [PubMed] [Google Scholar]
- 24.Nugent MA, Lane EA, Keski-Oja J, Moses HL, Newman MJ. Growth stimulation, altered regulation of epidermal growth factor receptors, and autocrine transformation of spontaneously transformed normal rat kidney cells by transforming growth factor beta. Cancer Res. 1989;49:3884–3890. [PubMed] [Google Scholar]
- 25.Reiss M. Transforming growth factor-beta and cancer—a love–hate relationship. Oncol Res. 1997;9:447–457. [PubMed] [Google Scholar]
- 26.Ebert EC. Inhibitory effects of transforming growth factor-beta (TGF-beta) on certain functions of intraepithelial lymphocytes. Clin Exp Immunol. 1999;115:415–420. doi: 10.1046/j.1365-2249.1999.00824.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Torre-Amione G, Beauchamp RD, Koeppen H, Park BH, Schreiber H, Moses HL, Rowley DA. A highly immunogenic tumor transfected with a murine transforming growth factor type beta 1 cDNA escapes immune surveillance. Proc Natl Acad Sci USA. 1990;87:1486–1490. doi: 10.1073/pnas.87.4.1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang A, Amagai M, Granger LG, Stanley JR, Udey MC. Adhesion of epidermal Langerhans cells to keratinocytes mediated by E-cadherin. Nature. 1993;361:82–85. doi: 10.1038/361082a0. [DOI] [PubMed] [Google Scholar]
- 29.Schwarzenberger K, Udey MC. Contact allergens and epidermal proinflammatory cytokines modulate Langerhans cell E-cadherin expression in situ. J Invest Dermatol. 1996;106:553–558. doi: 10.1111/1523-1747.ep12344019. [DOI] [PubMed] [Google Scholar]
- 30.Borkowski TA, Vandyke BJ, Schwarzenberger K, Mcfarland VW, Farr AG, Udey MC. Expression of E-cadherin by murine dendritic cells: E-cadherin as a dendritic cell differentiation antigen characteristic of epidermal Langerhans cells and related cells. Eur J Immunol. 1994;24:2767–2774. doi: 10.1002/eji.1830241129. [DOI] [PubMed] [Google Scholar]
- 31.Johnston LJ, Halliday GM, King NJC. Phenotypic changes in Langerhans Cells after infection with arboviruses—a role in the immune response to epidermally acquired viral infection. J Virol. 1996;70:4761–4766. doi: 10.1128/jvi.70.7.4761-4766.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cui W, Fowlis DJ, Bryson S, Duffie E, Ireland H, Balmain A, Akhurst RJ. TGFbeta1 inhibits the formation of benign skin tumors, but enhances progression to invasive spindle carcinomas in transgenic mice. Cell. 1996;86:531–542. doi: 10.1016/s0092-8674(00)80127-0. [DOI] [PubMed] [Google Scholar]