Abstract
Background. Dendritic cells (DC), the most specialized antigen-presenting cells, can be detected in the peripheral blood (PB) and divided into two subsets of populations, DC1 and DC2, endowed with different functions. The aim of this study was to evaluate the effect on DC release and on their subsets of three regimens utilized to mobilize CD34+ cells into the PB in cancer patients and in normal CD34+ cell donors. Patients and methods. The mobilizing sequences were: standard-dose epirubicin+taxol+granulocyte-colony-stimulating factor (G-CSF; 15 patients with advanced breast cancer), high-dose cyclophosphamide (CTX)+G-CSF (10 patients with breast cancer patients and 7 with non-Hodgkin's lymphoma, NHL), and G-CSF alone (5 normal donors of CD34+ cells for allogeneic transplantation). Comparative data were obtained from the steady-state PB of 20 healthy volunteers. For flow cytometric analysis, DC were gated as negative for specific lineage markers (CD3, CD11b, CD14, CD16, CD56, CD19, CD20, CD34) and positive for HLA-DR. The DC1 and DC2 subsets were defined as CD11c and CDw123 positive, respectively. Results. The percentages of DC at baseline and the time of CD34+ cell peak were: 0.48 and 0.51 for standard-dose chemotherapy (CT); 0.55 and 0.63 for breast cancer after high-dose CTX+G-CSF; 0.53 and 0.71 for NHL after high-dose CTX+G-CSF; and 0.51 and 0.54 for normal donors of CD34+ cells after G-CSF alone (all p=n.s.).Mean DC1/DC2 ratios in each study group at the time of CD34+ cell peak were 0.10, 0.12, and 0.18, respectively. Finally, in the group of healthy volunteers, the percentage of circulating DC was 0.95 and the mean DC1/DC2 ratio was 1.28. Conclusion. To our knowledge, this is the first report that demonstrates that both standard-dose or high-dose CT, when utilized together with G-CSF, do not induce DC mobilization into the PB, whereas a reversed DC1/DC2 ratio is observed. Furthermore, a lack of significant DC mobilization after G-CSF alone was also seen, in contrast to what was previously observed by others. These data should be taken in account when evaluating clinical correlations between DC number and CPC engraftment in both the transplantation setting, when monitoring the effects on the immune system of combinations of new drugs and/or cytokines, and when high numbers of DC are required for both experimental and clinical applications.
Keywords: Dendritic cells, CD34+ cell mobilization, Stem cell transplantation, Breast cancer, Non-Hodgkin's lymphoma
Introduction
Dendritic cells (DC) are a largely distributed and migratory bone-marrow-derived leukocyte subset with a peculiar efficacy in the uptake, processing, and presentation of antigens to both naive CD4+ and CD8+ cytotoxic T-lymphocytes. They are able to initiate and regulate a more effective adaptive immune response than any other previously described antigen-presenting cells (APCs) [4, 33, 36], even though their frequency has been recently been found to be altered in the peripheral blood (PB) of advanced cancer patients [17]. Among the wide DC heterogeneity, two different lineages have been identified, to date. DC 1 myeloid are lineage negative, HLA-DR+, and CD11c+, and are able to activate, through interaction between CD80 and CD28 and in the presence of IL-12, T-helper-type 1 (Th1), which produce IL-1 and IFN-γ; DC2 lymphoid, which are lineage negative, HLA-DR+, and CD123+, activate, though interaction between CD86 and CD28 in the presence of IL-10, T-helper-type 2 (Th2), which produce IL-4 and IL-10. The study of DC in vivo has been difficult because of their very low number in PB and other tissues. Another difficulty has been the failure to identify any unique surface marker that could be used to directly analyze and enumerate DC.
Additional problems derive from the fact that surface antigen expression, morphology, and function vary in association with the maturation or activation status of the DC population [16]. Despite these limitations, flow cytometric (FCM) techniques have been recently developed that allow the enumeration of DC in minimally manipulated blood samples [9, 24, 29, 32, 41].
Autologous hematopoietic progenitor cells collected from PB with leukapheresis provide a rapid and durable hematological recovery when utilized as a rescue after high-dose chemotherapy (CT) in cancer patients [15]. In the autologous transplantation setting, circulating CD34+ hematopoietic progenitor cells had become the material of choice over bone marrow (BM) for both practical reasons, i.e., the lack of a need for invasive procedures of harvesting, and for the clinical advantages, i.e., a shorter time to engraftment [19]. These cells are normally expressed on 0.01–0.1% of PB cells [34]; their mobilization from the BM into the PB can be achieved by the sole administration of cancer CT [40], but especially of myeloid growth factors (granulocyte-macrophage, GM- or granulocyte, G-colony stimulating factor, CSF) given alone [20] or in combination with both high-dose [3, 14] or standard-dose CT [42].
Conflicting results have been reported regarding the effects on the release of DC in the PB, of various CD34+ cell-mobilizing regimens [2, 13, 27]. In this work we evaluated the effects on the PB DC mobilization in cancer patients receiving both standard-dose CT or high-dose CT followed by G-CSF as CD34+ cell-mobilizing sequences and in a group of G-CSF-primed normal CD34+ cell donors.
Materials and methods
Patient population
Patients characteristics are listed in Table 1. Patients received either the standard treatment for their disease, or were enrolled in different clinical trials approved by the Institutional Review Board of the IRCCS San Matteo University Hospital, in both cases after written informed consent was obtained. Biological studies performed on patients' PB were also conducted according to institutional guidelines.
Table 1.
Patient characteristics. NHL non-Hodgkin's lymphoma
| No. of patients | Gender | Median age (years) | Stage of disease | |
|---|---|---|---|---|
| Group 1: breast cancer | 15 | M: 0, F: 15 | 59.5 (43–73) | Metastatic |
| Group 2: breast cancer/NHL | 10/7 | M: 0, F: 10 /M: 5, F: 2 | 50.1 (33–63) /52.6 (26–68) | High risk of relapse /relapsed |
| Group 3: normal CD34+ cell donors | 5 | M: 4, F: 1 | 38.6 (24–50) | - |
Age range in parentheses
We analyzed 37 subjects, shared in the following subgroups:
Group 1 consisted of 15 advanced breast cancer patients treated as a first line for metastatic disease with standard-dose CT+G-CSF.
Group 2 consisted of 10 patients with breast cancer at high risk of relapse treated following radical surgery with adjuvant CT, and 7 patients with non-Hodgkin's lymphoma (NHL) relapsed after a first-line CT. Both subgroups of patients received high-dose cyclophosphamide (CTX) followed by G-CSF for circulating progenitor cell (CPC) mobilization.
Group 3 consisted of 5 healthy donors of CD34+ cells for allogeneic CPC transplantation, treated with G-CSF alone.
As a control for the percentage of steady-state circulating DC, data were obtained from the PB of 20 healthy volunteers (laboratory personnel and blood donors).
CD34+ mobilizing regimens
To mobilize CD34+ cells, advanced breast cancer patients, after the third of six scheduled courses of standard-dose epirubicin (75 mg/m2, i.v., on day 1) and Taxol (175 mg/m2, i.v., on day 1) for six courses, received glycosylated G-CSF (Myelostim, Italfarmaco, Milan, Italy) at the dose of 300 μg/kg day−1, s.c. from the day after CT until absolute leukocyte number recovery (white blood count, WBC>5×103/μl).
On the contrary, patients with breast cancer at high risk of relapse, and NHL patients, were treated with high-dose CTX (6 g/m2) on day 1, followed by G-CSF (300 μg/kg day−1, s.c.), from the day after CTX until the day of the CD34+ cell peak. In this subgroup of patients, the target value for CPC collection was fixed: at least 20/μl CD34+ cells into the PB. Breast cancer patients also received, during the mobilization phase, s.c. recombinant erythropoietin (Globuren, Dompè Biotech, Milan, Italy) at the dose of 10,000 IU thrice weekly, while both breast and NHL patients received red blood or platelets transfusions when clinically indicated.
Finally, normal donors of CD34+ cells received G-CSF (300 µg/kg twice a day), s.c., for 4–5 days, until CD34+ count was at least 20 cells/µl.
Monitoring of CD34+ cell mobilization
In each study subjects' peripheral whole-blood samples were collected through venipuncture of an antecubital vein of the forearm. In cancer patients, the monitoring of CD34+ cells started from the beginning of the severe neutropenia (WBC <500×103/µl) until the day of CD34+ peak, whereas for normal CD34+ cell donors, it was performed throughout G-CSF administration.
The CD34+ cell count was performed on 0.5 ml of whole blood, according to the Milan Protocol [34], for all patients by our laboratory, using a Coulter XL flow cytometer (Coulter, Miami, Fla.).
Additionally, for the patients undergoing high-dose CT programs and for the CD34+ cell normal donors, the CD34+ cell count was also performed by the Immunohematology and Transfusion Service of the IRCCS San Matteo University Hospital, using the same analytical protocol and with a FACScan instrument (Becton Dickinson Immunochemistry System, BDIS, San Jose, Calif.).
Preparation of mononuclear cells
Whole-blood aliquots for DC analysis were stored in lithium heparin tubes. Blood was diluted 1:1 with Roswell Park Memorial Institute 1640 medium, added with L-glutamine, sodiumazide (NaN3) 0.05%, and fetal calf serum 2%.
Mononuclear cells were separated by stratification on Ficoll-Hypaque gradient solution density (1077 g/l) with centrifugation at 2000 rpm for 20 min. The PB mononuclear cells were collected from the interface and washed thrice with phosphate-buffered saline (PBS) at pH 7.4.
Tri-color staining for DC and flow cytometric analysis
The following mouse anti-human MoAbs directly conjugated to fluorescein isothiocyanate (FITC), R-phycoerythrin (PE), or phycoerythrin-cyanin 5.1 (PC5) were used: FITC-labeled anti-CD3 (Coulter, Miami, Fla.); CD11b; −CD16 (Immunotech, Marseille, France); −CD19; −CD20; −CD14 (Coulter, Miami, Fla.); −CD56 (Southern Biotechnology, Birmingham, Ala.); −CD34 MoAbs (Immunotech, Marseille, France); PC5-labelled anti-HLA-DR (Coulter, Miami, FL, USA); PE-labeled anti-CD11c (Immunotech, Marseille, France); and CDw123 (BD PharMingen Europe).
According to the literature, DC were detected as MHC class II expressing cells (HLA-DR+) and lacking of markers expressed by T cells (CD3, CD11b) B cells (CD19, CD20), NK (CD16, CD56), macrophages/monocytes (CD14), and hematopoietic progenitor cells (CD34−). The two DC subsets were identified as CD11c+ (DC1) and CDw123+ (DC2).
Cells labelled with PE-, FITC-, and PC5-conjugated isotype mAbs that were non-reactive to human cells were used as a control to determine the background of fluorescence.
The samples were incubated with the appropriate MoAbs for 20 min at 4°C in the dark, washed twice with the PBS solution, centrifugated for 10 min at 1400 rpm, and resuspended in 1 ml of PBS.
Fifty thousand events were analyzed for each sample with a Coulter XL flow cytometer equipped with a 480-nm argon ion laser and three fluorescence detectors with filter settings for FITC (530 nm), PE (585 nm), and PC5 (670 nm).
After instrument setting with calibrated microsphere DNA CHECK (Coulter), forward scatter (FS) and side scatter (SS) gates were set to exclude erythrocytes and debris, and markers were set to exclude background fluorescence as established using appropriate controls.
The gating strategy utilized to identify DC and subsets in PB mononuclear cells started from the identification inside the lymphocyte–monocyte light scatter of cells on the basis of lineage markers–FITC and of HLA-DR-PC-5 expression.
To identify CD11c+ (DC1) and CDw123+ (DC2) subsets among lineage-negative, HLA-DR-positive cells, the gate was set on lineage-negative cells.
Representative flow cytometric analyses are shown in Figs. 1and 2.
Fig. 1a, b.
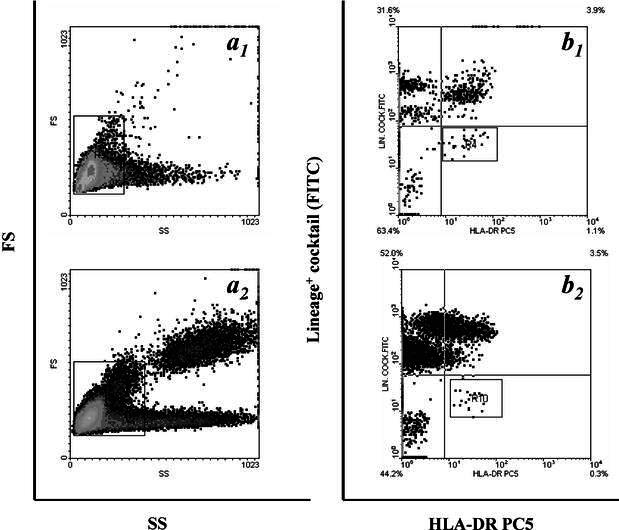
Identification of circulating dendritic cells (DC) by flow cytometry. Panel shows representative contour plot data illustrating the analytical method utilized to identify myeloid DC in peripheral blood (PB) mononuclear cells following the immunostaining described in Materials and methods from the steady state PB of a healthy volunteer (b 1) and from a breast cancer patient after high-dose CTX plus G-CSF (b 2). a 1,2 Mononuclear cells analysis regions applied to FS/SS data acquired for exclusion of granulocytes and debris. b 1,2 The same identified cell population after labelling with a cocktail FITC-conjugated MoAbs recognizing the Lin-associated antigens listed in Materials and methods and PC5-labelled anti-HLA-DR MoAb
Fig. 2a–d.
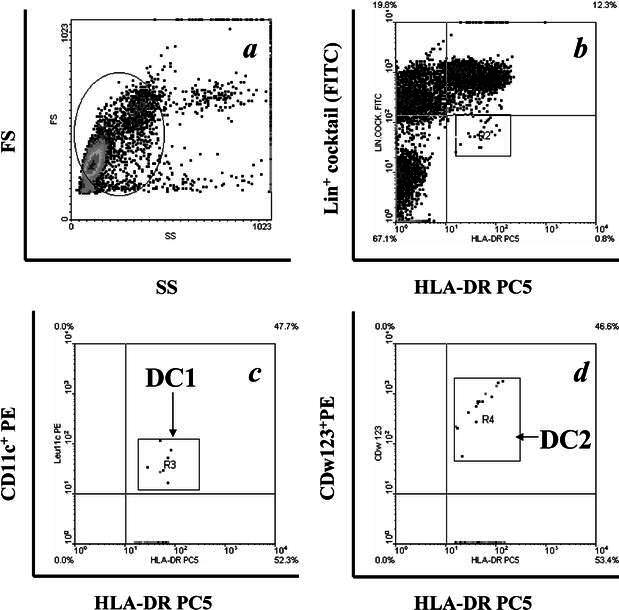
Identification of DC subsets by flow cytometry. Panel shows representative contour plot data illustrating the gating strategy utilized to identify lineage-negative/HLA DR+ cells. We started from the identification inside the lymphocyte–monocyte light scatter (a) of cells on the basis of lineage markers–FITC and of HLA-DR- PC-5 expression (b). To identify CD11c+ (DC1, c) and CDw123+ (DC2, d) subsets among lineage-negative, HLA-DR-positive cells, the gate was set on lineage-negative cells
Statistical analysis
Data are expressed as median and range. The null hypothesis was that none of the CD34+ cell-mobilizing regimens utilized did not increase the percentage of DC in PB. The alternative hypothesis was that these regimens did have an effect on this parameter. The Wilcoxon signed-rank test was used to compare DC pecentages at baseline and after CD34+ cell mobilization. The statistical package of the Excel (V98, Microsoft, Redman, Wash.) software running on a G4 Macintosh (Apple Computers, Cupertino, Calif.) was used throughout this study.
Results
Patient population
Among patients with advanced breast cancer (median age 59.5 years, age range 43–73 years) which relapsed after a median disease-free interval of 16.2 months (range 5–42 months), 7 had previously received, as adjuvant treatment, the CMF schedule (CTX 600 mg/m2+methotrexate 40 mg/m2+5-fluorouracil 600 mg/m2, all drugs being given i.v. on day 1) for six courses, whereas 8 were previously treated with the FEC schedule (CTX 600 mg/m2+epirubicin 60 mg/m2+5-fluorouracil 600 mg/m2, all drugs being given i.v., on day 1) for a total of six courses.
Breast cancer patients at high risk of relapse (median age 50.1 years, age range 33–63 years), who were candidates for a high-dose CT program, received, after induction CT with epirubicin+taxol, high-dose CTX+G-CSF as mobilizing regimen, as described above. A myeloablative phase was scheduled 1 month after mobilization.
The NHL patients (median age 52.6 years, age range 26–68 years), who relapsed after the CHOP schedule (CTX 750 mg/m2+doxorubicin 50 mg/m2+vincristine 1.4 mg/m2, all drugs being given i.v. on day 1, together with prednisone 100 mg, p.o., on days 1–5), underwent high-dose CTX+G-CSF as mobilizing regimen, in the context of a high-dose CT program for their recurrent disease, 34.1 months (range 15–55 months) after the previous CT program.
Healthy normal donors of CD34+ cells (median age 38.6 years, age range 24–50 years) for allogeneic non-myeloablative CPC transplantation received G-CSF (300 µg/kg twice a day), s.c., for 4–5 days, until CPC harvest planned when the CD34+ count was at least 20 cells/µl.
Monitoring of CD34+ mobilization
Mobilization of CD34+ cells in the breast cancer patient population belonging to group 1 happened after the third course of CT which was followed by G-CSF, with a CD34+ cell peak of 39 cells/μl (range 22–45 cells/μl) on day +9–+11 after the last day of CT.
In both breast cancer patients and NHL receiving high-dose CTX+G-CSF, the mobilization of CD34+ cells occurred at day +12 (range 9–15 days), with a median of CPCs of 52 cells/μl (range 33–67 cells/μl) for NHL and 56 cells/μl (range 35–61 cells/μl) for breast cancer patients.
In normal donors of CD34+ cells, the mobilization occurred after 4 days of G-CSF treatment, with a peak of CD34 of 61 cells/μl (range 38–72 cells/μl).
These data are summarized also in Table 2.
Table 2.
Percentage of dendritic cells (DC) in the three study groups as well as time of CD34+ cell peak and number of CD34+ cells/μl at peak
| DC at baseline | DC at day of CD34+ cell peaka | Time of CD34+ cell peak | CD34+/μL at peak | |
|---|---|---|---|---|
| Group 1: breast cancer | 0.48 (0.10–0.99) | 0.51 (0.09–1.02) | Day +9–+11 | 39 (22–45) |
| Group 2: breast cancer/NHL | 0.55 (0.09–1.57) /0.53 (0.08–1.65) | 0.63 (0.11–1.46) /0.71 (0.23–1.87) | Day +9–+15 /day +9–+15 | 52 (33–67) /56 (35–61) |
| Group 3: normal CD34+ cell donors | 0.51 (0.10–1.00) | 0.54 (0.08–0.93) | Day +4 | 61 (38–72) |
Ranges are in parentheses
aAll values non-significant
Effects of CD34+ cell mobilization on circulating DC
In breast cancer patients undergoing standard-dose CT, we detected a median percentage of DC of 0.48 (range=0.10–0.99) before starting the cytotoxic treatment and of 0.51 (range=0.09–1.02; p=n.s.) in concomitance with the CD34+ cell peak.
None of these patients presented significant increase in the percentage of circulating DC before CD34+ peak was reached.
Before starting high-dose CTX, the breast cancer patients at high risk of relapse and the relapsed NHL patients showed percentages of DC of 0.55 (range 0.09–1.57) and 0.53 (0.08–1.65), respectively (p=n.s.).
Only in the former subgroup did we record a non-significant increase in the percentage of the circulating DC in 3 patients, 2 days before CD34+ cell collection.
In concomitance with the CD34+ cell peak, breast cancer and NHL patients showed a percentage of DC of 0.63 (range 0.11–1.46) and 0.71 range (0.23–1.87), respectively (p=n.s.).
Normal donors of CD34+ cells showed a median of circulating DC of 0.51 (range 0.1–1.00) before G-CSF treatment and 0.54 (range 0.08–0.93) at the day of CD34+ cell collection (p=n.s.; Table 2).
We observed a decrease in the DC1 subset with a DC1/DC2 ratio reversed in the three study groups: 0.10 (range 0.09–0.15) in group 1; 0.12 (range 0.09–0.18) and 0.18 (range 0.15–0.22) for group-2 breast cancer and NHL patients, respectively; and 0.15 (range 0.13–0.19) for group 3.
These values before starting treatment were 1.24 (range 1.20–1.28), 1.21 (range 1.17–1.24), 1.22 (range 1.18–1.25), and 1.25 (range 1.22–1.31), respectively (Table 3).
Table 3.
DCI/DC2 ratio in the three study groups
| Baseline | Day of CD34+ cell peak | |
|---|---|---|
| Group 1: breast cancer | 1.24 (1.20–1.28) | 0.10 (0.09–0.15) |
| Group 2: breast cancer/NHL | 1.21 (1.17–1.24) /1.22 (1.18–1.25) | 0.12 (0.09–0.18) /0.18 (0.15–0.22) |
| Group 3: normal CD34+ cell donors | 1.25 (1.22–1.31) | 0.15 (0.13–0.19) |
The 20 healthy volunteers, used for internal laboratory control, showed a median percentage of DC of 0.95 (range 0.63–1.22) and a DC1/DC2 ratio of 1.25.
Discussion
In this study we utilized an FCM method to compare the effects on the population of circulating DC of different treatments able to mobilize CD34+ CPCs in cancer patients as well as in normal CD34+ donors.
The commitment of DC starts from two different BM progenitor cells. Myeloid precursors generate Langherans DC, interstitial DC and through previous differentiation in monocytes, and DC1 that express the myeloid antigens CD11c, CD13, and CD33. They require the presence of GM-CSF, IL-4, and pro-inflammatory cytokines to differentiate and maturate [38], inducing T-cell differentiation into the Th1 pathway.
Lymphoid precursors lead to plasmocytoid DC, termed DC2, interferon α/β-producing, CD11c negative but CD4 and IL-3R positive. They become mature when cultured with IL-3 and prime T cells mostly to produce a Th2 response [31, 33].
We have adapted a method for quantitation of circulating DC and the relative subpopulations DC1 and DC2, by immunostaining and FCM of lysed whole blood. The method is fast (<2 h), reproducible, requires only small volumes of blood, and can be performed using equipment available in most clinical immunology laboratories.
In the present work we were not able to demonstrate any significant increase (with respect to the steady-state DC values in the PB of healthy volunteers) of circulating DC after the mobilization of CD34+ cells with G-CSF alone (in normal donors of CD34+ cells), or with standard-dose, epirubicin/taxol-based CT followed by G-CSF, or with high-dose CTX followed by G-CSF (in cancer patients). With all the three mobilizing sequences we observed a reversal of the DC1/DC2 ratio suggesting a shift to a Th2 immune response.
Recent studies have addressed the ability of cytokines to mobilize DC into the PB. Gazitt et al. found, in a randomized study, a clear advantage for GM-CSF over G-CSF in the mobilization of DC from patients with NHL [13]. These results are in agreement with those obtained by Avigan et al. who found an increase in the mobilization of dendritic precursor cells in patients treated with GM-CSF plus G-CSF compared with G-CSF alone [2]. Arpinati et al. in an elegant work, focused on the mobilization of DC1 and DC2 subsets into the blood after treatment with G-CSF of normal donors of CD34+ cells for allogeneic transplantation. They demonstrated that G-CSF induces a selective mobilization of T-helper-2-inducing DC [1]. The same pattern has been shown by Stocchi et al. [37].
Mobilization of DC precursor cells (CD14+ cells) with G-CSF alone or with high-dose CTX plus G-CSF was studied by Morse et al. [27]. They reported no effect of CTX on the yield of DC, which was lower than that obtained with GM-CSF alone. Morse et al. [27] demonstrated that Flt3 ligand (Flt3L), a cytokine with stimulatory effects on BM progenitors [7] and that can expand in vitro different DC subsets [30], is also capable of mobilizing DC into the PB of patients with metastatic colon cancer [28], confirming preliminary experiences in which this cytokine was associated with G- or GM-CSF in normal donors [10] and in breast cancer patients [12], resulting in a similar effect of increase in the number of circulating DC.
More recently, Bolwell et al. demonstrated that standard-dose etoposide+G-CSF is able to mobilize DC1 in cancer patients, in contrast to the G-CSF alone which preferentially mobilizes DC2 better in a similar population of patients [6].
Thus, to our knowledge, this is the first report that demonstrates that both standard-dose or high-dose CT, when utilized together with G-CSF to mobilize CD34+ cells, does not simultaneously cause a mobilization of DC into the PB; furthermore, we observed also a lack of significant DC mobilization after G-CSF alone.
If the peculiar chemotherapeutic drugs we used to mobilize our patients may account for the discrepancy between our results and those reported by other authors, the lack of DC mobilization following G-CSF administration in healthy donors could be further interpreted also through the execution of functional studies on DC precursors.
At present, it is not clear if, in cancer patients undergoing CPC transplantation procedures, the mobilization of large numbers of DC could have any clinical advantages with respect to a lower DC count in PB and in CPC harvest. In particular, it should be established whether or not this parameter could have an effective role on stem-cell engrafment in the autologous setting and/or if such a role could also be hypothesized in the allogeneic setting.
Furthermore, the shift towards a Th2 response we observed in our patients could be considered as a negative immunological event, on the basis of a recent report by King et al., showing that CTL precursors undergo maturation cessation and apoptosis through a modulation of the expression of B7.1 and 2-costimulatory molecules on DC in the presence of the Th2 cytokine IL-4 [18].
Conclusion
In this preliminary phase, our results could have importance (a) when evaluating clinical correlations between DC number and CPC engraftment in both autologous and allogeneic transplantation setting, (b) when monitoring the effects on the immune system of combinations of new drugs and/or cytokines utilized as CD34+ cell mobilizing agents, particularly considering the possible functions of different DC subsets in generating further immunodepression after grafting, and (c) when high numbers of DC have to be generated for both experimental and clinical purposes [5, 8, 11, 22, 23, 25, 26, 39, 42].
Acknowledgments
Acknowledgements
The present work was supported by a M.I.U.R.(F.I.R.B.)-2001 grant and by an IRCCS San Matteo University Hospital Research Grant (to M. Danova). We thank the nursing staff of the Medical Oncology and Hematology Divisions at the IRCCS San Matteo University Hospital in Pavia for their key help in patient assistance, management, and care.
References
- 1.Arpinati Blood. 2000;95:2484. [PubMed] [Google Scholar]
- 2.Avigan Clin Cancer Res. 1999;2:2735. [PubMed] [Google Scholar]
- 3.Ballestrero J Clin Oncol. 1999;17:1296. doi: 10.1200/JCO.1999.17.4.1296. [DOI] [PubMed] [Google Scholar]
- 4.Banchereau Nature. 1998;392:245. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 5.Basak Blood. 2002;99:2869. doi: 10.1182/blood.V99.8.2869. [DOI] [PubMed] [Google Scholar]
- 6.Bolwell Blood. 2002;96:773. [Google Scholar]
- 7.Brasel Blood. 1996;88:2004. [PubMed] [Google Scholar]
- 8.Brossart Exp Hematol. 2001;29:1247. doi: 10.1016/S0301-472X(01)00730-5. [DOI] [PubMed] [Google Scholar]
- 9.Fagnoni Cytometry. 2001;45:124. doi: 10.1002/1097-0320(20011001)45:2<124::AID-CYTO1154>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 10.Fay Blood. 1999;94:1523. [Google Scholar]
- 11.Fearnley Blood. 1999;93:728. [PubMed] [Google Scholar]
- 12.Gasparetto Blood. 1999;94:367. [Google Scholar]
- 13.Gazitt J Hematother Stem Cell Res. 2001;10:177. doi: 10.1089/152581601750098471. [DOI] [PubMed] [Google Scholar]
- 14.Gianni Lancet. 1989;2:580. doi: 10.1016/s0140-6736(89)90711-3. [DOI] [PubMed] [Google Scholar]
- 15.Gratwohl Bone Marrow Transpl. 2001;27:899. doi: 10.1038/sj.bmt.1702995. [DOI] [PubMed] [Google Scholar]
- 16.Hart Blood. 1997;90:3245. [PubMed] [Google Scholar]
- 17.Hoffmann Clin Cancer Res. 2002;8:1787. [Google Scholar]
- 18.King Nat Med. 2001;7:206. doi: 10.1038/84659. [DOI] [PubMed] [Google Scholar]
- 19.Korbling Blood. 1995;85:1659. [PubMed] [Google Scholar]
- 20.Lane Transfusion. 1999;39:39. doi: 10.1046/j.1537-2995.1999.39199116893.x. [DOI] [PubMed] [Google Scholar]
- 21.Lanier Nat Med. 2001;7:1178. doi: 10.1038/nm1101-1178. [DOI] [PubMed] [Google Scholar]
- 22.Linder Eur J Clin Invest. 2002;32:207. doi: 10.1046/j.1365-2362.2002.00968.x. [DOI] [PubMed] [Google Scholar]
- 23.Luykx-de Ann Oncol. 1999;10:21. doi: 10.1023/A:1008349920664. [DOI] [Google Scholar]
- 24.Macey Cytometry. 1998;81:199. doi: 10.1002/(SICI)1097-0320(19980301)31:3<199::AID-CYTO7>3.0.CO;2-G. [DOI] [Google Scholar]
- 25.Marroquin J Immunother. 2002;25:278. doi: 10.1097/00002371-200205000-00011. [DOI] [Google Scholar]
- 26.Mayordomo Nat Med. 1995;1:1297. doi: 10.1038/nm1295-1297. [DOI] [PubMed] [Google Scholar]
- 27.Morse J Clin Oncol. 2000;18:3883. [Google Scholar]
- 28.Morse J Hematother Stem Cell Res. 1999;8:577. doi: 10.1089/152581699319731. [DOI] [PubMed] [Google Scholar]
- 29.Nuñez BMC Immunol. 2001;2:6. doi: 10.1186/1471-2172-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parajuli Exp Hematol. 2001;29:1185. doi: 10.1016/S0301-472X(01)00722-6. [DOI] [PubMed] [Google Scholar]
- 31.Robinson Eur J Immunol. 1999;29:2769. doi: 10.1002/(SICI)1521-4141(199909)29:09<2769::AID-IMMU2769>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 32.Savary Cancer Immunol Immunother. 1998;45:234. doi: 10.1007/s002620050438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shortman Nature Rev Immunol. 2002;3:151. doi: 10.1038/nri746. [DOI] [PubMed] [Google Scholar]
- 34.Siena Blood. 1991;77:400. [PubMed] [Google Scholar]
- 35.Siena Exp Hematol. 1995;23:1463. [PubMed] [Google Scholar]
- 36.Steinman Ann Rev Immunol. 1991;9:271. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 37.Stocchi Bone Marrow Transpl. 2001;27:123. [Google Scholar]
- 38.Szabolcs J Immunol. 1995;154:5851. [PubMed] [Google Scholar]
- 39.Tarte Leukemia. 1999;13:653. [Google Scholar]
- 40.To LB, Juttner CA (1993) Stem cell mobilization by myelosuppressive chemotherapy. In: Wunder EW, Henon PR (eds) Peripheral blood stem cell autografts. Springer, Berlin Heidelberg New York, p 132
- 41.Upham Cytometry. 2000;40:50. doi: 10.1002/(SICI)1097-0320(20000501)40:1<50::AID-CYTO7>3.0.CO;2-P. [DOI] [Google Scholar]
- 42.Venturini Cancer. 1994;74:2300. doi: 10.1002/1097-0142(19941015)74:8<2300::aid-cncr2820740814>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]


