Abstract
Background
Post-surgery blood-based biomarkers may be useful for guiding treatment and surveillance decisions among colorectal cancer (CRC) patients. However, most candidate biomarkers provide little if any predictive value beyond stage at diagnosis. We aimed to investigate the independent prognostic value of post-operative serum C-reactive protein (CRP), a highly sensitive biomarker of inflammation, for long-term CRC outcomes in two large patient cohorts.
Materials and methods
CRP levels were measured from serum samples of CRC patients collected ≥1 month post-surgery in the German DACHS (n = 1416) and the UK Biobank (n = 1149) cohorts. Associations of post-operative CRP with overall survival (OS) and CRC-specific survival (CSS) were assessed using Cox regression and presented as hazard ratios (HRs) with 95% confidence intervals (CIs), adjusted for key sociodemographic and clinical covariates.
Results
In both cohorts, consistent strong dose–response relationships between post-operative CRP and both OS and CSS were observed. Adjusted HRs (95% CI) for CRP >10 versus <3 mg/l were 1.93 (1.58-2.35) and 2.70 (2.03-3.59) in the DACHS cohort, and 2.70 (1.96-3.71) and 2.61 (1.83-3.72) in the UK Biobank cohort, respectively. Associations between post-operative CRP and OS were particularly strong among younger patients (<65 years at diagnosis; P value for interaction by age <0.01).
Conclusions
Serum CRP determined a month or more after surgery may be useful as a strong independent prognostic biomarker for guiding therapeutic decisions and for surveillance of the course of disease of CRC patients, particularly those <65 years of age at diagnosis.
Key words: colorectal, C-reactive protein, survival, systemic inflammation, prognosis
Highlights
-
•
Patients were treated with surgery for CRC.
-
•
Post-operative CRP (poCRP) was measured from blood collected ≥1 month after surgery.
-
•
Associations of poCRP with long-term survival were assessed using Cox regression.
-
•
Elevated poCRP was independently associated with poorer survival.
-
•
poCRP assessed ≥1 month after surgery may be valuable for CRC surveillance.
Introduction
Colorectal cancer (CRC) is the second leading cause of cancer death and the third most diagnosed cancer globally.1 Prognosis of patients with CRC strongly depends on stage at diagnosis, with 5-year relative survival ranging from >90% for patients with localised disease to <15% for patients with distant metastases,2 which underlines the large potential of reducing the burden of the disease by effective screening programmes.3 Nevertheless, there is considerable heterogeneity in survival outcomes also for CRC patients within the same stage.4 Besides major variation of prognosis according to stage, site and molecular features of the tumour,5 additional patient factors may be informative to guide treatment and surveillance decisions. Although many candidate biomarkers have been proposed and evaluated, most of them provide very limited prognostic value beyond stage at diagnosis.
Inflammation is a notable hallmark of cancer with strong links to both tumorigenesis and tumour progression.6, 7, 8 Circulating C-reactive protein (CRP) is a well-established and highly sensitive biomarker of systemic inflammation that has been shown to be associated with survival of patients with various diseases including cancer.9 Elevated post-surgery serum levels of CRP among CRC patients have been linked to shorter survival, independent of tumour stage at diagnosis.10,11 However, pertinent evidence is mostly based on small studies with partly conflicting results.12,13 Since systemic inflammation is highly variable and strongly triggered by the acute care interventions or its potential complications in the immediate period following surgery,14 this study seeks to thoroughly investigate the association of serum CRP concentrations assessed a month or more after surgery with survival outcomes of CRC patients in two independent cohorts, the German DACHS study and the UK Biobank.
Materials and methods
Study populations and study design
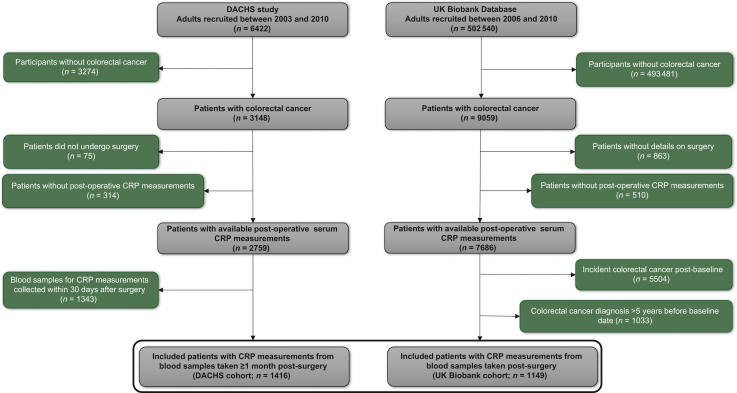
Figure 1 shows the details of patient selection criteria. We retrospectively analysed data from two population-based cohort studies prospectively recruiting participants in Germany (DACHS study) and in the UK (UK Biobank), both following the guidelines of the Declaration of Helsinki. The DACHS study is a population-based case-control study with long-term follow-up of patients (≥30 years of age, no upper age limit) who were recruited after a first diagnosis of CRC from 22 clinics in the Rhine-Neckar region in south-west Germany in 2003-2021. Details of the study design, recruitment, data collection and follow-up procedures have been reported elsewhere.15, 16, 17, 18, 19
Figure 1.
Study participant selection flow diagram for the DACHS and UK Biobank cohorts. CRP, C-reactive protein.
Briefly, standardised questionnaires were used to collect sociodemographic, lifestyle history and medical information from CRC patients by personal interviews conducted during hospital stay for surgery or within weeks to months after discharge. Medical data on tumour stage and site, and therapy were obtained from hospital charts. Blood samples were collected after personal interviews and serum aliquots were stored at −80°C until analysis. Mortality follow-up was conducted through record linkage with population registries, and cause of death was obtained from health authorities through death certificates using the 10th revision of the International Statistical Classification of Diseases (ICD-10) to identify mortality due to CRC (C18-C20). The study was approved by the ethics committees of the University of Heidelberg (#310/2001, 06 December 2001) and the state medical boards of Baden-Württemberg and Rhineland-Palatinate. All participants provided written informed consent. For the current analysis, we included a total of 1416 patients with CRC recruited in 2003-2010 for whom blood samples taken at least 1 month post-surgery and long-term follow-up data with respect to survival were available.
The UK Biobank is a prospective cohort study that recruited over 500 000 men and women aged between 40 and 69 years in 2006-2010. Biomedical information was collected from the 22 assessment centres across England, Scotland and Wales through questionnaires, verbal interviews, physical and medical assessments. Biological specimens such as blood, urine, stool and hair were collected at the initial assessment visit (baseline date).20 Data on health outcomes were gathered through linkages to health care records, including the UK National Health Service (NHS) data, primary care data, cancer screening data and disease-specific registers.21 Information on the dates and causes of death was obtained from the NHS for the period of time from enrolment to 12 November 2021. The ICD-10 was used to identify causes of death. The UK Biobank was approved by the North West Multi-Center Research Ethics Committee (MREC) as a research tissue bank (approval renewed in 2021: 21/NW/0157). The UK Biobank study was approved by the North West Haydock Research Ethics Committee (#16/NW/0274, 13 May 2016). For the current analysis, we included 1149 patients with a CRC diagnosis ≤5 years before the baseline date, for whom CRP measurements and follow-up details of survival outcomes of interest were available. From the available UK Biobank data, all participants had their blood samples collected at least 9 months after surgery; therefore, complete exclusion of patients with CRC surgery <1 month before recruitment or blood sampling was not necessary.
Post-operative CRP measurements
For the DACHS cohort, the ADVIA XPT Siemens Healthineers instrument (Siemens Healthineers AG, Erlangen, Germany) was used to measure CRP in 300 μl post-operative serum samples by immunoturbidimetric method. Measurements for serum CRP were conducted over a time span of 3 months in 2022 at the Central Laboratory of the Heidelberg University Clinics. In the UK Biobank cohort, CRP was measured at baseline within 24 h of the blood draw during recruitment. CRP was measured using the immunoturbidimetric method with the Beckman Coulter AU5800 instrument (Beckman Coulter, Inc., Brea, CA).21 In both cohorts, CRP assays were conducted blinded to the survival endpoints. CRP levels <3 mg/l are generally known to be reflective of good health and disease control22; therefore, CRP was converted into a variable with four categories comparing patients with CRP levels of 3-5 mg/l, 5-10 mg/l and ≥10 mg/l to the reference group of patients with CRP levels <3 mg/l.
Outcomes
Endpoints for overall survival (OS) and CRC-specific survival (CSS) were defined as death from any cause or death from CRC, respectively. In addition, for the DACHS cohort, we also estimated the relapse-free survival (RFS). Follow-up times for survival outcome endpoints were calculated in days beginning from the date of blood sampling (≥1 month and up to 52.6 months after surgery in the DACHS cohort and up to 60 months after surgery in the UK Biobank cohort) to the date of having the event. Patients who did not reach a specific endpoint were censored at the time when they were last known to have been alive.
Covariates
For our analyses, we considered the following factors as covariates for both cohorts: sex, age at diagnosis, body mass index (BMI), alcohol consumption, history of smoking, physical exercise, history of cardiovascular disease, history of diabetes, history of hypertension, vitamin D status and season of blood draw. Details on TNM (tumour–node–metastasis) stage, chemotherapy use and molecular characteristics [microsatellite stability (MS), BRAF and KRAS status] were only available in the DACHS cohort at the time of analysis.
Statistical analyses
Descriptive statistics were used to analyse population characteristics. Continuous variables of patient characteristics are presented as median values with interquartile ranges, while categorical variables are presented as numbers with percentages. For missing values, multiple imputation with five imputed datasets and 30 iterations using the R software ‘mice package’ was used to fill in missing values except for CRP, time to event and survival outcomes.23
The distribution of post-operative serum CRP by time after surgery is presented as boxplots. Survival estimates were calculated using Kaplan–Meier methodology and log-rank testing for different categories of CRP. Associations of CRP with survival outcomes were assessed using Cox proportional hazards (PH) models to calculate hazard ratios and 95% confidence intervals, adjusting for all other cohort-specific covariates. The PH assumption was tested by plotting the smoothed Schoenfeld residuals for all covariates against time, and visually assessed for any systematic trends over time. In order to explore the relevance of adjustment for stage, which was not included in the UK Biobank dataset, the analyses of the DACHS data were conducted both with (model 2) and without (model 1) inclusion of stage among the covariates.
We also carried out subgroup analyses by sex, age at diagnosis, BMI, vitamin D status and additionally for the DACHS cohort, cancer stage at diagnosis, chemotherapy use, tumour site (right: cecum to transverse colon; left: splenic flexure to rectum), MS and BRAF/KRAS status. In the UK Biobank cohort, blood-cell count variables were available and Cox regressions were additionally adjusted for lymphocyte/monocyte ratio, platelet/lymphocyte ratio and neutrophil/lymphocyte ratio. We assessed potential interactions between CRP and covariates with respect to survival by adding their product term to the Cox PH models, using Wald test statistics for these product terms. We also carried out sensitivity analyses in which follow-up was restricted to 5 years from blood sampling. Statistical analyses were carried out using R statistical software (version 4.2, R Core Team 2023, Vienna, Austria). Two-sided P values <0.05 were considered statistically significant for all analyses.
Results
Patient characteristics
The main characteristics of patients in the DACHS and UK Biobank cohorts are summarised in Table 1. A total of 1416 and 1149 patients with CRC were included in the DACHS and UK Biobank cohorts, respectively. After a median follow-up of 9.9 years for the DACHS cohort, 690 (48.7%) patients had died, of whom 332 had died from CRC. The median follow-up time in the UK Biobank cohort was 12.2 years during which 317 (27.6%) deaths were reported, 242 of these due to CRC. Both cohorts included more male (61.0% and 59.2%, respectively) than female patients. Median age and median post-operative CRP concentrations were higher in the DACHS cohort (69 years; 2.6 mg/l) than in the UK patient cohort (63 years; 2.2 mg/l). Approximately one half (46.7%) and slightly more than a third (38.1%) of DACHS and UK Biobank patients had post-operative serum CRP concentrations ≥3 mg/l, respectively. Post-operative serum CRP levels after surgery were significantly elevated among patients whose blood samples were collected within the first month following surgery, who were excluded from our analysis on the prognostic value of CRP; thereafter lower and stable median values of CRP were observed for all the successive time periods (Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2024.102982). Moreover, details of cancer stage were only available for the DACHS cohort in which the majority of patients had stage II (32.2%) or stage III (33.4%) CRC at diagnosis.
Table 1.
Main characteristics of the study population from the DACHS and UK Biobank cohorts
| Characteristic | DACHS cohort (ntotal = 1416) |
UK Biobank cohort (ntotal = 1149) |
P valuea |
|---|---|---|---|
| n (%) | n (%) | ||
| Sex | |||
| Female | 553 (39.0) | 469 (40.8) | 0.18 |
| Male | 863 (61.0) | 680 (59.2) | |
| Age (years) | |||
| Median (IQR) | 69 (61-75) | 63 (58-66) | |
| <60 | 290 (20.7) | 356 (31.0) | <0.001 |
| 60-64 | 246 (17.6) | 422 (36.8) | |
| ≥65 | 864 (61.7) | 370 (32.2) | |
| C-reactive protein (mg/l) | |||
| Median (IQR) | 2.6 (1.0-7.5) | 2.2 (1.1-4.6) | |
| <3 | 754 (53.3) | 712 (62.0) | <0.001 |
| 3-5 | 165 (11.7) | 181 (15.8) | |
| 5-10 | 223 (15.8) | 150 (13.1) | |
| ≥10 | 274 (19.4) | 106 (9.2) | |
| Body mass index (kg/m2) | |||
| Median (IQR) | 26.1 (23.6-29.0) | 27.3 (24.0-29.9) | |
| Normal (18.5-<25) | 536 (37.9) | 292 (25.4) | <0.001 |
| Overweight (25-<30) | 608 (42.9) | 530 (46.1) | |
| Obese (≥30) | 272 (19.2) | 327 (28.5) | |
| Alcohol consumption | |||
| Yes | 1016 (71.8) | 762 (66.3) | 0.01 |
| No | 400 (28.2) | 387 (33.7) | |
| History of smoking | |||
| Yes | 797 (56.3) | 654 (56.9) | 0.11 |
| No | 619 (43.7) | 495 (43.1) | |
| Physical exercise | |||
| Yes | 931 (65.8) | 896 (78.0) | <0.001 |
| No | 485 (34.2) | 253 (22.0) | |
| Cardiovascular disease | |||
| Yes | 334 (24.1) | 113 (9.8) | <0.001 |
| No | 1053 (75.9) | 1136 (90.2) | |
| History of diabetes | |||
| Yes | 258 (18.2) | 114 (9.9) | <0.001 |
| No | 1158 (81.8) | 1035 (90.1) | |
| History of hypertension | |||
| Yes | 746 (52.7) | 408 (35.5) | <0.001 |
| No | 670 (47.3) | 741 (64.5) | |
| Serum vitamin D (nmol/l) | |||
| Median (IQR) | 29.3 (17.4-45.2) | 43.9 (31.3-57.0) | |
| <30 | 728 (51.4) | 264 (23.0) | <0.001 |
| 30-49 | 408 (28.8) | 494 (43.0) | |
| ≥50 | 280 (19.8) | 391 (34.0) | |
| Season of blood draw | |||
| Autumn | 334 (24.2) | 269 (23.4) | 0.72 |
| Spring | 396 (28.7) | 329 (28.6) | |
| Summer | 348 (25.2) | 311 (27.1) | |
| Winter | 301 (21.8) | 240 (20.9) | |
| UICC cancer stage (TNM) | |||
| I | 330 (23.3) | NA | NA |
| II | 456 (32.2) | NA | |
| III | 473 (33.4) | NA | |
| IV | 157 (11.1) | NA | |
| Median follow-up (years) | |||
| Median (IQR) | 9.9 (4.8-10.7) | 12.2 (11.0-13.2) | |
| Survival | |||
| Alive | 726 (51.3) | 832 (72.4) | <0.001 |
| Dead | 690 (48.7) | 317 (27.6) | |
| CRC death | 332 (23.5) | 242 (21.1) |
Missing values were excluded from percentage calculations. Missing data at baseline for age at diagnosis (DACHS = 9, UK Biobank = 2); BMI (UK Biobank = 6); history of cardiovascular disease (DACHS = 29); season of blood draw (DACHS = 137). Season of blood draw: spring: ‘March-May’; summer: ‘June-August’; autumn: ‘September-November’; winter: ‘December-February’.
BMI, body mass index; IQR, interquartile range; NA, not available; TNM, tumour–node–metastasis; UICC, Union for International Cancer Control.
P values based on Pearson chi-square test (bold values are statistically significant).
Post-operative serum CRP levels and survival outcomes
Survival curves for different categories of post-operative serum CRP levels are presented in Supplementary Figure S2, available at https://doi.org/10.1016/j.esmoop.2024.102982. OS and CSS were lower for all categories in which CRP was >3 mg/l compared to CRP <3 mg/l in both cohorts (log-rank P value <0.0001), with clear dose–response patterns. Multivariable Cox PH regression results confirmed these strong dose–response patterns in both cohorts (Table 2), which were only slightly attenuated and remained highly statistically significant (Ptrend < 0.001 for both OS and CSS) after additional adjustment for stage at diagnosis in the DACHS cohort. Post-operative CRP was also a strong and independent prognostic factor for RFS in the DACHS cohort (Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2024.102982). Besides, additional adjustment for blood-cell count-based inflammatory biomarkers in the UK Biobank showed only slight attenuation for the association of post-operative CRP with both OS and CSS (Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2024.102982). Schoenfeld tests showed no violations of PH assumptions for all variables (all P values >0.05).
Table 2.
Multivariable Cox regression results for the association of C-reactive protein with survival outcomes for the DACHS and UK Biobank cohorts
| Outcome | DACHS cohort |
Ptrend | UK Biobank cohort |
Ptrend | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C-reactive protein serum level |
C-reactive protein serum level |
|||||||||
| <3 mg/l | 3-5 mg/l | 5-10 mg/l | ≥10 mg/l | <3 mg/l | 3-5 mg/l | 5-10 mg/l | ≥10 mg/l | |||
| Overall survival | ||||||||||
| No. at risk/events | 754/315 | 165/73 | 223/129 | 274/171 | 712/159 | 181/50 | 150/51 | 106/51 | ||
| Model 1 HR (95% CI)a | Ref | 1.26 (0.97-1.63) | 1.76 (1.42-2.18) | 2.01 (1.65-2.45) | <0.001 | Ref | 1.26 (0.92-1.74) | 1.69 (1.23-2.31) | 2.70 (1.96-3.71) | <0.001 |
| Model 2 HR (95% CI)b | Ref | 1.08 (0.83-1.40) | 1.66 (1.34-2.07) | 1.93 (1.58-2.35) | <0.001 | — | — | — | — | — |
| CRC-specific survival | ||||||||||
| No. at risk/events | 754/126 | 165/38 | 223/63 | 274/105 | 712/130 | 181/32 | 150/35 | 106/41 | ||
| Model 1 HR (95% CI)a | Ref | 1.50 (1.03-2.18) | 1.98 (1.44-2.72) | 2.92 (2.22-3.86) | <0.001 | Ref | 1.00 (0.67-1.47) | 1.47 (1.02-2.12) | 2.61 (1.83-3.72) | <0.001 |
| Model 2 HR (95% CI)b | Ref | 1.17 (0.81-1.71) | 1.79 (1.30-2.47) | 2.70 (2.03-3.59) | <0.001 | — | — | — | — | — |
Values shown in bold are statistically significant (P value < 0.05).
CI, confidence interval; HR, hazard ratio; Ref, reference; TNM, tumour–node–metastasis.
Model 1: Adjusted for sex, age, body mass index, alcohol consumption, smoking status, physical exercise, history of cardiovascular disease (heart failure, myocardial infarction, angina pectoris, stroke), history of diabetes, history of hypertension, vitamin D status and season of blood draw.
Model 2: Adjusted for model 1 + TNM stage.
Subgroup analyses
Subgroup analyses are presented in Table 3. Associations of higher post-operative CRP levels with lower OS and CSS were rather consistently observed across subgroups defined by sex, age at diagnosis, BMI, vitamin D status and CRC stage (for the DACHS cohort). However, the association of post-operative CRP with OS was much stronger among younger patients (<65 years) than among older patients (P value for interaction <0.01 in both cohorts). There was also no statistically significant variation of the prognostic value of post-operative CRP among DACHS cohort patients with stage II or III according to use of adjuvant chemotherapy (all P values for interaction >0.05; Supplementary Table S3, available at https://doi.org/10.1016/j.esmoop.2024.102982). Similarly, associations of post-operative CRP with survival outcomes showed no significant variation by tumour location, MS and BRAF/KRAS status (all P values for interaction >0.05; Supplementary Table S4, available at https://doi.org/10.1016/j.esmoop.2024.102982).
Table 3.
Multivariable Cox regression results for the association of C-reactive protein levels with survival among subgroups (C-reactive protein <3 mg/l as reference)
| Outcome | n/events | DACHS cohort |
Pinteraction | n/events | UK Biobank cohort |
Pinteraction | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| 3-5 mg/l | 5-10 mg/l | ≥10 mg/l | 3-5 mg/l | 5-10 mg/l | ≥10 mg/l | |||||
| Overall survival | ||||||||||
| Male | 863/434 | 1.20 (0.87-1.66) | 1.96 (1.49-2.57) | 2.14 (1.67-2.75) | 0.47 | 680/218 | 1.37 (0.93-2.01) | 2.14 (1.49-3.06) | 3.13 (2.14-4.58) | 0.44 |
| Female | 553/251 | 0.96 (0.61-1.50) | 1.41 (0.95-2.08) | 1.87 (1.32-2.65) | 469/93 | 1.12 (0.62-2.02) | 0.88 (0.44-1.75) | 2.88 (1.52-5.46) | ||
| <60 years | 290/92 | 0.81 (0.37-1.76) | 1.95 (1.02-3.71) | 3.15 (1.74-5.73) | <0.01 | 356/85 | 1.44 (0.75-2.77) | 1.47 (0.76-2.82) | 3.61 (2.01-6.48) | <0.01 |
| 60-64 years | 246/68 | 0.76 (0.32-1.81) | 2.80 (1.57-5.00) | 2.54 (1.41-4.58) | 422/104 | 1.53 (0.83-2.81) | 2.45 (1.40-4.26) | 4.27 (2.53-7.20) | ||
| ≥65 years | 864/497 | 1.20 (0.89-1.62) | 1.46 (1.13-1.89) | 1.63 (1.28-2.06) | 370/122 | 0.99 (0.62-1.59) | 1.32 (0.80-2.17) | 1.25 (0.65-2.41) | ||
| Normal weight | 536/262 | 1.08 (0.66-1.74) | 1.79 (1.22-2.61) | 2.53 (1.83-3.51) | 0.48 | 292/80 | 1.38 (0.68-2.80) | 3.17 (1.60-6.28) | 5.63 (2.89-11.0) | <0.01 |
| Overweight | 608/296 | 1.05 (0.72-1.53) | 1.55 (1.11-2.17) | 1.38 (1.00-1.90) | 530/125 | 1.40 (0.87-2.25) | 1.60 (0.94-2.72) | 1.91 (1.08-3.36) | ||
| Obese | 272/127 | 1.34 (0.69-2.60) | 2.10 (1.26-3.51) | 2.43 (1.45-4.07) | 327/106 | 0.90 (0.50-1.62) | 1.41 (0.83-2.40) | 2.49 (1.46-4.23) | ||
| Vitamin D deficient | 728/284 | 1.34 (0.69-2.60) | 2.10 (1.26-3.51) | 2.43 (1.45-4.07) | 0.12 | 264/93 | 1.38 (0.77-2.49) | 1.80 (1.02-3.19) | 3.56 (1.95-6.50) | 0.23 |
| Vitamin D insufficient | 408/168 | 1.45 (0.82-2.56) | 1.85 (1.20-2.84) | 2.30 (1.51-3.50) | 494/131 | 1.26 (0.76-2.08) | 1.87 (1.19-2.95) | 2.65 (1.60-4.39) | ||
| Vitamin D sufficient | 280/117 | 1.49 (0.85-2.61) | 2.64 (1.51-4.61) | 2.38 (1.32-4.29) | 391/87 | 1.19 (0.65-2.19) | 0.92 (0.41-2.05) | 2.57 (1.38-4.80) | ||
| CRC stage I | 330/107 | 1.61 (0.83-3.16) | 1.73 (1.01-2.95) | 2.14 (1.24-3.68) | 0.30 | — | — | — | — | — |
| CRC stage II | 456/191 | 1.35 (0.81-2.26) | 2.03 (1.37-3.01) | 1.92 (1.31-2.81) | — | — | — | — | — | |
| CRC stage III | 473/243 | 1.10 (0.73-1.66) | 1.79 (1.20-2.66) | 1.53 (1.06-2.19) | — | — | — | — | — | |
| CRC stage IV | 157/138 | 0.79 (0.40-1.54) | 1.83 (1.06-3.16) | 2.78 (1.70-4.54) | — | — | — | — | — | |
| CRC-specific survival | ||||||||||
| Male | 863/192 | 1.07 (0.63-1.80) | 2.36 (1.54-3.60) | 3.11 (2.12-4.57) | 0.01 | 680/167 | 1.15 (0.73-1.82) | 1.90 (1.26-2.89) | 3.21 (2.10-4.91) | 0.59 |
| Female | 553/139 | 1.34 (0.75-2.39) | 1.68 (0.98-2.86) | 3.06 (1.98-4.90) | 469/71 | 0.79 (0.37-1.71) | 0.64 (0.28-1.49) | 3.03 (1.48-6.22) | ||
| <60 years | 290/66 | 0.37 (0.11-1.27) | 1.76 (0.77-3.98) | 3.35 (1.75-6.42) | <0.01 | 356/73 | 1.27 (0.63-2.57) | 1.22 (0.58-2.53) | 2.82 (1.47-5.43) | 0.02 |
| 60-64 years | 246/57 | 0.83 (0.28-2.49) | 2.86 (1.33-6.18) | 3.34 (1.45-7.72) | 422/83 | 1.53 (0.77-3.04) | 2.23 (1.19-4.21) | 4.47 (2.53-7.91) | ||
| ≥65 years | 864/204 | 1.47 (0.94-2.31) | 1.49 (0.98-2.26) | 2.43 (1.69-3.50) | 370/82 | 0.54 (0.27-1.07) | 1.08 (0.58-2.01) | 1.25 (0.58-2.68) | ||
| Normal weight | 536/123 | 1.28 (0.63-2.60) | 1.84 (1.00-3.37) | 3.66 (2.22-6.01) | 0.48 | 292/65 | 1.21 (0.53-2.76) | 2.71 (1.24-5.91) | 5.72 (2.82-11.6) | 0.02 |
| Overweight | 608/130 | 1.23 (0.70-2.14) | 2.16 (1.35-3.45) | 2.17 (1.38-3.41) | 530/96 | 1.02 (0.56-1.86) | 1.43 (0.77-2.64) | 2.22 (1.20-4.11) | ||
| Obese | 272/69 | 0.78 (0.29-2.08) | 1.31 (0.60-2.85) | 2.12 (1.04-4.33) | 327/77 | 0.77 (0.39-1.55) | 1.16 (0.62-2.18) | 1.99 (1.06-3.74) | ||
| Vitamin D deficient | 728/199 | 0.96 (0.56-1.65) | 1.59 (1.03-2.47) | 2.66 (1.84-3.85) | 0.54 | 264/65 | 1.12 (0.54-2.31) | 1.46 (0.73-2.93) | 2.58 (1.24-5.36) | 0.81 |
| Vitamin D insufficient | 408/75 | 2.25 (1.00-5.06) | 2.59 (1.30-5.14) | 4.42 (2.34-8.34) | 494/110 | 1.09 (0.61-1.95) | 1.73 (1.04-2.86) | 2.81 (1.65-4.77) | ||
| Vitamin D sufficient | 280/58 | 0.98 (0.41-2.35) | 3.64 (1.70-7.78) | 3.98 (1.69-9.35) | 391/63 | 0.82 (0.36-1.87) | 0.68 (0.24-1.91) | 2.74 (1.36-5.49) | ||
| CRC stage I | 330/21 | 1.05 (0.11-9.88) | 3.87 (1.04-14.4) | 10.0 (3.05-33.4) | 0.90 | — | — | — | — | — |
| CRC stage II | 456/57 | 1.77 (0.72-4.34) | 2.10 (0.98-4.52) | 2.03 (0.99-4.17) | — | — | — | — | — | |
| CRC stage III | 473/124 | 1.41 (0.82-2.50) | 2.54 (1.49-4.35) | 2.44 (1.49-4.00) | — | — | — | — | — | |
| CRC stage IV | 157/126 | 0.77 (0.38-1.53) | 1.48 (0.83-2.64) | 2.51 (1.51-4.15) | — | — | — | — | — | |
Cox regression analyses for both cohorts were adjusted for sex, age, body mass index, alcohol consumption, smoking status, physical exercise, history of cardiovascular disease (heart failure, myocardial infarction, angina pectoris, stroke), history of diabetes, history of hypertension, vitamin D status and season of blood draw. DACHS cohort analyses were additionally adjusted for TNM stage. Cox regression results are presented as HRs and 95% CIs. Values shown in bold are statistically significant (P value < 0.05).
CI, confidence interval; HR, hazard ratio; TNM, tumour–node–metastasis.
Sensitivity analyses
Sensitivity analyses in which follow-up time was restricted to a maximum of 5 years after blood draw showed even stronger dose–response relationships between elevated post-operative CRP concentrations and both survival outcomes (Supplementary Table S5, available at https://doi.org/10.1016/j.esmoop.2024.102982).
Discussion
Our results showed strong and independent dose–response relationships between elevated serum levels of CRP assessed a month or more after surgery and worse OS and CSS. The association of CRP with OS was particularly strong among male patients and also younger patients <65 years of age.
CRP is a highly sensitive marker of systemic inflammation and it is primarily produced by liver hepatocytes in response to elevated inflammatory cytokines such as interleukin-6.24 The exact mechanism by which CRP is related to the prognosis of patients with CRC remains obscure, but mechanistic studies have suggested that elevated CRP correlates with increased expression of oncogenes resulting in DNA damage.25 Consequently, elevated circulating CRP has been widely reported as a marker of poor prognosis,11,26 infectious complications27 and compromised treatment response22 in patients with various disease conditions including cancer. Indeed, we observed positive associations between post-operative serum CRP levels and disease relapse, which might explain the shorter OS and CSS in our study, particularly for patients with CRP >5 mg/l.
While the clinical relevance of modestly increased CRP detectable by high-sensitivity CRP measurements (e.g. CRP between 3 and 10 mg/l) remains elusive for cancer patients, levels <3 mg/l are generally known to be reflective of good health and disease control.22 Very few and mostly smaller studies have previously assessed the associations of post-operative CRP levels and prognosis among CRC patients. A most recent systematic review and meta-analysis of these studies likewise found elevated levels of CRP in blood samples taken 1 or more months after surgery to be associated with poorer OS and CSS.28 However, due to the low number and strong heterogeneity in the design of the studies, and their limited sample size, meta-analyses did not allow comprehensive analyses of dose–response patterns which are clearly addressed in our study.
In the subgroup analyses, our results showed stronger associations of post-operative CRP with OS for younger compared to older patients. More aggressive CRC has been reported in younger populations partly due to late-stage diagnosis linked to a lack of early detection guidelines for younger populations.29 The recent increase in the global burden of early-onset CRC underscores the need for targeted early detection and intervention programmes in younger populations.30, 31, 32 In addition, the association between elevated CRP and survival in older patients could be confounded by several factors including age-related comorbidities.33,34 Male compared to female sex was generally a risk factor for worse prognosis among CRC patients with elevated post-operative CRP levels. Male patients may have poor health outcomes compared to female patients partly attributed to more frequent high-risk behaviour, for example, excessive smoking, high alcohol consumption and poor health care seeking behaviour.35 The ‘obesity paradox’ is confirmed by our findings in which we showed post-diagnostic obesity as a predictor of better survival outcomes among CRC patients.36,37
We did not find any significant interaction between post-operative CRP and vitamin D status in the prediction of survival outcomes although preclinical evidence consistently reports the role of vitamin D in immune-inflammatory modulation.38,39 The prognostic role of post-operative serum vitamin D levels for both OS and CSS has been shown to be independent of CRP levels.40 Although previous studies have reported chemotherapy-induced inflammation in cancer patients,41,42 we did not observe any significant interaction between chemotherapy use and CRP levels, probably due to low statistical power. Moreover, CRP showed prognostic value for survival irrespective of tumour location and BRAF/KRAS status.
Limitations and future research
Despite the large overall sample size of the patient cohorts, case numbers were low for some of the subgroup analyses. Furthermore, there was substantial heterogeneity in the timing of blood draw for CRP measurements after surgery (mostly within a few weeks to months after surgery). However, the consistency of our results despite this variation suggests that CRP assessed a month or more after surgery may be a relevant prognostic marker regardless of the exact timing of assessment. While there is emerging evidence on the prognostic value of circulating tumour DNA (ctDNA) among resected CRC patients,43, 44, 45 ctDNA was not assessed in our cohorts. Nonetheless, we think that CRP, which showed similar or even stronger prognostic value compared to reported prognostic value of ctDNA,46 could be a rather routine and cheaper prognostic biomarker to apply in clinical settings as compared to the more expensive ctDNA, especially for low-resource settings. Variables for post-operative complications were also not available in our study cohorts, but a previous study demonstrated that CRP assessed within a month after surgery was a prognostic factor for CSS, independent of post-operative complications.47 Future studies should establish the prognostic potential of post-operative CRP in combination with these emerging or established prognostic factors and provide risk-stratifying criteria for surgical CRC patients.
Conclusions
Serum CRP determined a month or more after surgery may be useful as a prognostic biomarker and for surveillance of the course of disease of CRC patients, particularly younger patients <65 years of age. Future studies should validate our findings and evaluate the potential use of post-operative serum CRP in surveillance and treatment decisions in the long-term care of CRC patients.
Acknowledgments
Funding
The DACHS study was supported by the German Research Council [grant numbers BR 1704/6-1, BR 1704/6-3, BR 1704/6-4, CH 117/1-1, HO 5117/2-1, HE 5998/2-1, KL 2354/3-1, RO 2270/8-1, BR 1704/17-1], the Interdisciplinary Research Program of the National Center for Tumor Diseases (NCT), Germany, and the German Federal Ministry of Education and Research [grant numbers 01KH0404, 01ER0814, 01ER0815, 01ER1505A, 01ER1505B, 01KD2104A]. The UK Biobank study has been conducted using the UK Biobank Resource under Application Number ‘62848’. UK Biobank was established by the Wellcome Trust, Medical Research Council, Department of Health, Scottish Government and Northwest Regional Development Agency. It has also received funding from the Welsh assembly government, British Heart Foundation, Cancer Research UK and Diabetes UK. UK Biobank is supported by the National Health Service (NHS). The sponsors had no role in data acquisition or the decision to publish the data. The authors assume full responsibility for analyses and interpretation of these data.
Disclosure
The authors have declared no conflicts of interest.
Supplementary data
References
- 1.Sung H., Ferlay J., Siegel R.L., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R.L., Wagle N.S., Cercek A., Smith R.A., Jemal A. Colorectal cancer statistics, 2023. CA Cancer J Clin. 2023;73(3):233–254. doi: 10.3322/caac.21772. [DOI] [PubMed] [Google Scholar]
- 3.Lieberman D., Ladabaum U., Brill J.V., et al. Reducing the burden of colorectal cancer: AGA position statements. Gastroenterology. 2022;163(2):520–526. doi: 10.1053/j.gastro.2022.05.011. [DOI] [PubMed] [Google Scholar]
- 4.Li C., Pei Q., Zhu H., et al. Survival nomograms for stage III colorectal cancer. Medicine (Baltimore) 2018;97(49) doi: 10.1097/MD.0000000000013239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ugai T., Akimoto N., Haruki K., et al. Prognostic role of detailed colorectal location and tumor molecular features: analyses of 13,101 colorectal cancer patients including 2994 early-onset cases. J Gastroenterol. 2023;58(3):229–245. doi: 10.1007/s00535-023-01955-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balkwill F., Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357(9255):539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 7.Wen Y., Zhu Y., Zhang C., et al. Chronic inflammation, cancer development and immunotherapy. Front Pharmacol. 2022;13 doi: 10.3389/fphar.2022.1040163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pęczek P., Gajda M., Rutkowski K., Fudalej M., Deptała A., Badowska-Kozakiewicz A.M. Cancer-associated inflammation: pathophysiology and clinical significance. J Cancer Res Clin Oncol. 2023;149(6):2657–2672. doi: 10.1007/s00432-022-04399-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Y., Zhong X., Cheng G., et al. Hs-CRP and all-cause, cardiovascular, and cancer mortality risk: a meta-analysis. Atherosclerosis. 2017;259:75–82. doi: 10.1016/j.atherosclerosis.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 10.Hua X., Kratz M., Malen R.C., et al. Association between post-treatment circulating biomarkers of inflammation and survival among stage II-III colorectal cancer patients. Br J Cancer. 2021;125(6):806–815. doi: 10.1038/s41416-021-01458-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lehtomäki K., Mustonen H., Kellokumpu-Lehtinen P.L., et al. Lead time and prognostic role of serum CEA, CA19-9, IL-6, CRP, and YKL-40 after adjuvant chemotherapy in colorectal cancer. Cancers (Basel) 2021;13(15):3892. doi: 10.3390/cancers13153892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hermunen K., Soveri L.M., Boisen M.K., et al. Postoperative serum CA19-9, YKL-40, CRP and IL-6 in combination with CEA as prognostic markers for recurrence and survival in colorectal cancer. Acta Oncol. 2020;59(12):1416–1423. doi: 10.1080/0284186X.2020.1800086. [DOI] [PubMed] [Google Scholar]
- 13.Wesselink E., Balvers M.G.J., Kok D.E., et al. Levels of inflammation markers are associated with the risk of recurrence and all-cause mortality in patients with colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2021;30(6):1089–1099. doi: 10.1158/1055-9965.EPI-20-1752. [DOI] [PubMed] [Google Scholar]
- 14.Watt D.G., McSorley S.T., Park J.H., Horgan P.G., McMillan D.C. A postoperative systemic inflammation score predicts short- and long-term outcomes in patients undergoing surgery for colorectal cancer. Ann Surg Oncol. 2017;24(4):1100–1109. doi: 10.1245/s10434-016-5659-4. [DOI] [PubMed] [Google Scholar]
- 15.Brenner H., Chang-Claude J., Seiler C.M., Rickert A., Hoffmeister M. Protection from colorectal cancer after colonoscopy: a population-based, case-control study. Ann Intern Med. 2011;154(1):22–30. doi: 10.7326/0003-4819-154-1-201101040-00004. [DOI] [PubMed] [Google Scholar]
- 16.Brenner H., Chang-Claude J., Jansen L., Knebel P., Stock C., Hoffmeister M. Reduced risk of colorectal cancer up to 10 years after screening, surveillance, or diagnostic colonoscopy. Gastroenterology. 2014;146(3):709–717. doi: 10.1053/j.gastro.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 17.Carr P.R., Jansen L., Walter V., et al. Associations of red and processed meat with survival after colorectal cancer and differences according to timing of dietary assessment. Am J Clin Nutr. 2016;103(1):192–200. doi: 10.3945/ajcn.115.121145. [DOI] [PubMed] [Google Scholar]
- 18.Walter V., Jansen L., Ulrich A., et al. Alcohol consumption and survival of colorectal cancer patients: a population-based study from Germany. Am J Clin Nutr. 2016;103(6):1497–1506. doi: 10.3945/ajcn.115.127092. [DOI] [PubMed] [Google Scholar]
- 19.Maalmi H., Walter V., Jansen L., et al. Relationship of very low serum 25-hydroxyvitamin D(3) levels with long-term survival in a large cohort of colorectal cancer patients from Germany. Eur J Epidemiol. 2017;32(11):961–971. doi: 10.1007/s10654-017-0298-z. [DOI] [PubMed] [Google Scholar]
- 20.Elliott P., Peakman T.C. The UK Biobank sample handling and storage protocol for the collection, processing and archiving of human blood and urine. Int J Epidemiol. 2008;37(2):234–244. doi: 10.1093/ije/dym276. [DOI] [PubMed] [Google Scholar]
- 21.Sudlow C., Gallacher J., Allen N., et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12(3) doi: 10.1371/journal.pmed.1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hart P.C., Rajab I.M., Alebraheem M., Potempa L.A. C-reactive protein and cancer-diagnostic and therapeutic insights. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.595835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sterne J.A., White I.R., Carlin J.B., et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393. doi: 10.1136/bmj.b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boras E., Slevin M., Alexander M.Y., et al. Monomeric C-reactive protein and Notch-3 co-operatively increase angiogenesis through PI3K signalling pathway. Cytokine. 2014;69(2):165–179. doi: 10.1016/j.cyto.2014.05.027. [DOI] [PubMed] [Google Scholar]
- 25.Tuomisto A.E., Mäkinen M.J., Väyrynen J.P. Systemic inflammation in colorectal cancer: underlying factors, effects, and prognostic significance. World J Gastroenterol. 2019;25(31):4383–4404. doi: 10.3748/wjg.v25.i31.4383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Osterman E., Mezheyeuski A., Sjöblom T., Glimelius B. Beyond the NCCN risk factors in colon cancer: an evaluation in a Swedish population-based cohort. Ann Surg Oncol. 2020;27(4):1036–1045. doi: 10.1245/s10434-019-08148-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nouvenne A., Ticinesi A., Folesani G., et al. The association of serum procalcitonin and high-sensitivity C-reactive protein with pneumonia in elderly multimorbid patients with respiratory symptoms: retrospective cohort study. BMC Geriatr. 2016;16:16. doi: 10.1186/s12877-016-0192-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gwenzi T., Zhu A., Schrotz-King P., et al. Prognostic value of post-operative C-reactive protein-based inflammatory biomarkers in colorectal cancer patients: systematic review and meta-analysis. Clin Epidemiol. 2023;15:795–809. doi: 10.2147/CLEP.S415171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sebbag G., Lantsberg L., Arish A., Levi I., Hoda J. Colon carcinoma in the adolescent. Pediatr Surg Int. 1997;12(5-6):446–448. doi: 10.1007/BF01076965. [DOI] [PubMed] [Google Scholar]
- 30.Xu C., Zhang F., Cheng W., Zhu Y. Prediction models for overall and cancer-specific survival in patients with metastatic early-onset colorectal cancer: a population-based study. Int J Colorectal Dis. 2023;38(1):99. doi: 10.1007/s00384-023-04369-x. [DOI] [PubMed] [Google Scholar]
- 31.Spaander M.C.W., Zauber A.G., Syngal S., et al. Young-onset colorectal cancer. Nat Rev Dis Primers. 2023;9(1):21. doi: 10.1038/s41572-023-00432-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fritz C.D.L., Otegbeye E.E., Zong X., et al. Red-flag signs and symptoms for earlier diagnosis of early-onset colorectal cancer. J Natl Cancer Inst. 2023;115:909–916. doi: 10.1093/jnci/djad068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dolin T.G., Christensen I.J., Johansen A.Z., et al. Pre- and perioperative inflammatory biomarkers in older patients resected for localized colorectal cancer: associations with complications and prognosis. Cancers (Basel) 2021;14(1):161. doi: 10.3390/cancers14010161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dolin T.G., Christensen I.J., Lund C.M., et al. Preoperative plasma vitamin D in patients with localized colorectal cancer: age-dependent association with inflammation, postoperative complications, and survival. Eur J Surg Oncol. 2023;49(1):244–251. doi: 10.1016/j.ejso.2022.08.040. [DOI] [PubMed] [Google Scholar]
- 35.Baraibar I., Ros J., Saoudi N., et al. Sex and gender perspectives in colorectal cancer. ESMO Open. 2023;8(2) doi: 10.1016/j.esmoop.2023.101204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Y., Li C., Wu G., et al. The obesity paradox in patients with colorectal cancer: a systematic review and meta-analysis. Nutr Rev. 2022;80(7):1755–1768. doi: 10.1093/nutrit/nuac005. [DOI] [PubMed] [Google Scholar]
- 37.Lim D.M., Lee H., Eom K., Kim Y.H., Kim S. Bioinformatic analysis of the obesity paradox and possible associated factors in colorectal cancer using TCGA cohorts. J Cancer. 2023;14(3):322–335. doi: 10.7150/jca.80977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Harten-Gerritsen A.S., Balvers M.G., Witkamp R.F., Kampman E., van Duijnhoven F.J. Vitamin D, inflammation, and colorectal cancer progression: a review of mechanistic studies and future directions for epidemiological studies. Cancer Epidemiol Biomarkers Prev. 2015;24(12):1820–1828. doi: 10.1158/1055-9965.EPI-15-0601. [DOI] [PubMed] [Google Scholar]
- 39.Colotta F., Jansson B., Bonelli F. Modulation of inflammatory and immune responses by vitamin D. J Autoimmun. 2017;85:78–97. doi: 10.1016/j.jaut.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 40.Vaughan-Shaw P.G., Zgaga L., Ooi L.Y., et al. Low plasma vitamin D is associated with adverse colorectal cancer survival after surgical resection, independent of systemic inflammatory response. Gut. 2020;69(1):103–111. doi: 10.1136/gutjnl-2018-317922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vyas D., Laput G., Vyas A.K. Chemotherapy-enhanced inflammation may lead to the failure of therapy and metastasis. Onco Targets Ther. 2014;7:1015–1023. doi: 10.2147/OTT.S60114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brown T., Sykes D., Allen A.R. Implications of breast cancer chemotherapy-induced inflammation on the gut, liver, and central nervous system. Biomedicines. 2021;9(2):189. doi: 10.3390/biomedicines9020189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Malla M., Loree J.M., Kasi P.M., Parikh A.R. Using circulating tumor DNA in colorectal cancer: current and evolving practices. J Clin Oncol. 2022;40(24):2846–2857. doi: 10.1200/JCO.21.02615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fan X., Zhang J., Lu D. CtDNA's prognostic value in patients with early-stage colorectal cancer after surgery: a meta-analysis and systematic review. Medicine (Baltimore) 2023;102(6) doi: 10.1097/MD.0000000000032939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Loft M., To Y.H., Gibbs P., Tie J. Clinical application of circulating tumour DNA in colorectal cancer. Lancet Gastroenterol Hepatol. 2023;8(9):837–852. doi: 10.1016/S2468-1253(23)00146-2. [DOI] [PubMed] [Google Scholar]
- 46.Taieb J., Taly V., Henriques J., et al. Prognostic value and relation with adjuvant treatment duration of ctDNA in stage III colon cancer: a post hoc analysis of the PRODIGE-GERCOR IDEA-France trial. Clin Cancer Res. 2021;27(20):5638–5646. doi: 10.1158/1078-0432.CCR-21-0271. [DOI] [PubMed] [Google Scholar]
- 47.McSorley S.T., Watt D.G., Horgan P.G., McMillan D.C. Postoperative systemic inflammatory response, complication severity, and survival following surgery for colorectal cancer. Ann Surg Oncol. 2016;23(9):2832–2840. doi: 10.1245/s10434-016-5204-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.