Abstract
Background
There is no consensus on the second-line treatment of patients with progressive high-grade neuroendocrine neoplasms (NENs G3) and large-cell lung neuroendocrine carcinoma. These patients generally have poor performance status and low tolerance to combination therapy. In this trial, we aim to evaluate the efficacy and safety of temozolomide given every other week in patients with advanced platinum-pretreated NENs G3.
Patients and methods
This trial is an open-label, non-randomized, phase II trial. Patients with platinum-pretreated metastatic neuroendocrine carcinoma were treated with 75 mg/m2/day of temozolomide for 7 days, followed by 7 days of no treatment (regimen one week on/one week off). The primary endpoint was the overall response rate. Secondary endpoints included progression-free survival (PFS), overall survival (OS), safety and tolerability.
This study is registered with ClinicalTrials.gov, NCT04122911.
Results
From 2017 to 2020, 38 patients were enrolled. Among the patients with determined Ki67, 12 out of 36 (33.3%) had a Ki67 index <55% and the remaining 24 out of 36 (66.6%) had an index ≥55%. Overall response rate was 18% (7/38), including one complete response and six partial responses. The median PFS was 5.86 months [95% confidence interval (CI) 4.8 months-not applicable) and the median OS was 12.1 months (95% CI 5.6-20.4 months). The 1-year PFS rate was 37%. No statistically significant difference in median PFS [hazard ratio 1.3 (95% CI 0.6-2.8); P = 0.44] and median OS [hazard ratio 1.1 (95% CI 0.5-2.4); P = 0.77] was observed among patients with Ki67 <55% versus ≥55%. Only G1-G2 adverse events were registered, the most common being G1 nausea, diarrhea and abdominal pain.
Conclusion
One week on/one week off temozolomide shows promising activity in patients with poorly differentiated NEN. The good safety profile confirmed the possibility of using this scheme in patients with poor performance status.
Key words: neuroendocrine tumors, temozolomide, NEC, performance status, metronomic
Highlights
-
•
Currently, there is no consensus on the second-line regimen for patients (pts) with metastatic NECs.
-
•
Pts with progressed metastatic NEC, generally have a low PS and are not candidates for combination therapies.
-
•
TMZ showed activity at the standard dose in pretreated pts with NENs as both monotherapy and combination therapy.
-
•
Here, one week on/one week off TMZ showed a promising activity and a good safety profile in NENs-G3 pts.
Introduction
Neuroendocrine carcinomas (NECs) represent a highly malignant subgroup of neuroendocrine neoplasms (NENs) found in various body locations.1 The classification of NENs as NECs has evolved over the past decade, with the site of origin playing a role. In the 2010 World Health Organization (WHO) classification for gastroenteropancreatic (GEP) NENs, the two-group division included neuroendocrine tumors (NETs) and NECs.2 This clinical trial was designed under this classification. The 2019 WHO classification, however, highlighted that a group of well-differentiated NETs could exhibit high-grade features, such as a mitotic count >20/10 high-power fields (HPF) or a Ki67 LI >20%. This subgroup is now classified as NETs G3 or NECs according to their morphological appearance and genetic phenotype.
The 2015 WHO classification has already divided lung NENs into four variants based on mitotic count, presence of necrosis and local/distant invasion. Two variants are considered low-grade tumors: typical carcinoid (TC) and atypical carcinoid (AC), while the other two are high-grade tumors: small-cell lung carcinoma (SCLC) and large-cell NEC (LCNEC).3
In 2021, WHO and the International Association for the Study of Lung Cancer (IASLC) suggested a standard classification system for all thoracic NENs that includes the evaluation of the mitotic rate, expressed as mitoses per 10 HPF or 2 mm2, and levels of necrosis to differentiate low-, intermediate- and high-grade pulmonary NEN. The classification proposed by WHO 2021 defines the subgroup of carcinoid tumors as well-differentiated NENs and includes typical and atypical carcinoids in both low- and intermediate-grade tumors. Instead, poorly differentiated NENs include SCLC and LCNEC.4,5
Furthermore, according to the WHO classification, LCNEC can be divided into pure LCNEC and combined LCNEC; the latter is composed of components of adenocarcinoma, squamous cell carcinoma and other rare subtypes, which together with small-cell lung cancer are not included in this clinical study.4,5
Nonetheless, NECs originating from GEP and lung sites share common characteristics, including a high proliferation rate, clinical aggressiveness and elevated Ki67 LI. This commonality makes the clinical management of NECs complex, with limited treatment options beyond first-line therapy.6
In fact, while the front-line treatment of high-grade plurimetastatic NECs is well established and based on systemic platinum-based chemotherapies,7 the second-line treatment is still a debated hot topic. Combinations of drugs have been evaluated as second-line treatment of NECs, with highly variable response rates ranging between 17% and 70%.
Most studies, however, are retrospective and small (<25 patients) due to the low incidence of the disease, and current evidence does not allow consensus on optimal second-line regimens at disease progression.6,8
Notably, patients who have progressed during first-line platinum-based chemotherapy generally have a poor performance status (PS), resulting in poor tolerability of combination chemotherapy.9 Therefore, a low-toxicity regimen is necessary, and a low-dose monotherapy may be preferred.
Temozolomide (TMZ) is an orally active alkylating agent used in the therapy of malignant glioma and malignant melanoma that can inhibit angiogenesis at low, non-toxic doses.10 TMZ 150 mg/day every other week has been used with bevacizumab in locally advanced or metastatic NETs, achieving a high response rate in pancreatic NETs. Nevertheless, the combination was associated with substantial toxicity, including lymphopenia and thrombocytopenia.11
Interestingly, in 2012, Koumarianou et al.12 discovered that metronomic TMZ, when combined with bevacizumab and long-acting octreotide, proved effective in treating advanced grade II NETs. Furthermore, intermittent dosing (treatment every other week) of TMZ in glioma has been proposed to reduce the frequency and severity of hematologic toxicity compared with more prolonged dosing regimens while maintaining effectiveness.13,14
Our research group conducted a retrospective study to assess the safety and efficacy of TMZ administered every other week as a second-line treatment of advanced NETs, including G2 NETs and NECs. The results showed an overall response rate (ORR) of 27% and a disease control rate (DCR) of 87% in patients with NEC, an ORR of 19% and a DCR of 92% in the overall study population.8
Building upon these promising findings, we have designed a multicenter, open-label, single-arm prospective phase II trial to systematically investigate the clinical benefits of administering TMZ every other week to patients with metastatic NEC who have progressed after receiving first-line platinum-based treatment.
Patients and methods
Study design and participants
The trial was conducted between 2017 and 2020 at four distinct centers within the ENETS Center of Excellence in Naples, Italy. This study was designed as a single-arm, open-label, phase II clinical trial aimed at assessing the antitumor efficacy and safety of TMZ administered every other week as a second-line treatment of patients with NENs G3, including NET-G3, NEC and large-cell lung NECs, who had progressed following initial platinum-based therapy. Inclusion criteria also comprised patients aged >18 years, Eastern Cooperative Oncology Group (ECOG) PS of 0-3, a life expectancy exceeding 3 months, and the presence of at least one evaluable disease site measurable according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1, and satisfactory hematological, renal and hepatic function. Exclusion criteria encompassed active systemic infections, coagulation disorders, or decompensated chronic illnesses. Before the commencement of the study treatment, written informed consent was obtained from all participating patients.
Procedures
TMZ was given orally once daily at 75 mg/m2 for 7 days, followed by 7 days of suspension and further treatment for 7 days and suspension for 7 days (every other week treatment) every 4 weeks in a cycle.
The treatment continued until progressive disease or intolerable toxicity. By RECIST version 1.1, responses were assessed by an independent, experienced radiologist at each site. Tumor assessment was carried out using computed tomography or magnetic resonance imaging within 4 weeks before treatment started at baseline and then every 9 weeks from the first dosing.15
The incidence and the severity of adverse events (AEs) or laboratory test abnormalities were recorded every 3 weeks. AEs were graded according to the National Cancer Institute Common Terminology Criteria for AEs (NCI-CTCAE version 4).
Physical examination, complete blood count and blood chemistry tests were done twice during each treatment cycle and at follow-up safety visits.
Outcomes
The primary endpoint of the study was the ORR [complete responses (CRs) + partial responses (PRs)]; secondary endpoints included DCR [CRs + PRs + stable diseases (SDs)]; second-line progression-free survival (PFS), defined as the time from first dosing to the first documented disease progression or death from any cause, whichever occurred first; 1-year overall survival (OS), defined as the time from first dosing to death from any cause; safety and tolerability.
Statistical analysis
Demographic and clinical data were summarized using descriptive statistics, continuous variables were described by median and range (minimum and maximum) and categorical variables were described by absolute and relative frequencies. A waterfall plot was used to visualize the maximum percent change in tumor measurements according to RECIST criteria from baseline. Median PFS and OS curves were generated using the Kaplan–Meier method. Exploratory subgroup analyses were conducted using the log-rank test. Data were censored for patients who were still alive at their last follow-up for the OS, had not experienced disease progression and were still alive at their last follow-up for the PFS. Treatment-related AEs were presented as the number and percentage of patients who experienced AEs that were assessed by the investigator as at least possibly related to the treatment.
An α level of 0.10 and a power of 80% has been adopted. An ORR <5% was considered an unacceptable rate, and an ORR ≥19% was considered acceptable. Given these hypotheses, the sample size of this trial was set at 30 patients, obtained using the ph2single function from the clinfun package in R. Therefore, predicting a drop rate of 20%, we have estimated a sample size of 38 patients. The study will be successful if a response is recorded in more than three patients.
Ethics statement
The study protocol was reviewed and approved by the Ethics Committees of the National Cancer Institute of Naples with act n. 293, following the principles of the Declaration of Helsinki and Good Clinical Practice guidelines. Written informed consent was obtained from all patients before study enrollment.
Role of the funding source
There was no external funding source. This work was (partially) supported by Italian Ministry of Health Ricerca Corrente.
Results
Patients and tumor characteristics
Between 2017 and 2020, a total of 38 patients were recruited. All enrolled patients were included in the efficacy and safety analysis. Patient and disease characteristics are summarized in Table 1. Overall, the median age of the enrolled patients was 61.5 years (range: 25-77 years). Before starting second-line treatment, 2 patients (5.3%) had an ECOG PS of 0, 22 patients (58%) had a PS of 1, 13 patients (34%) had a PS of 2 and 1 patient (3%) had a PS of 3. Among patients with a determined Ki67 index (percentage of cell immunohistochemical staining), 14 out of 36 (38.9%) had a Ki67 index <55%, while the remaining 22 (61.1%) had an index ≥55%. Since patients were enrolled before the 2019 WHO classification, we retrospectively revised the histological features of the tumor samples. We found that 8 out of the 38 enrolled patients, previously classified as NEC patients, had a high-grade NET-G3 with well-differentiated morphology, whereas 28 patients have been confirmed to have a poorly differentiated NEC. The evaluation was not possible for two patients because of the paucity of tumor tissue. NENs of the lung were all LCNEC.
Table 1.
Demographic and clinical data
| Characteristics | n = 38, n (%) |
|---|---|
| Age (years), median (range) | 61.5 (25-77) |
| Performance status: | |
| 0 | 2 (5) |
| 1 | 22 (58) |
| 2 | 13 (34) |
| 3 | 1(3) |
| Site of the primary tumor: | |
| Pancreas | 5 (13) |
| Lung | 8 (21) |
| Head and neck | 1 (3) |
| Gastrointestinal tract | 6 (16) |
| Gallbladder | 4 (10) |
| Skin | 1(3) |
| Endometrium | 3 (8) |
| Kidney | 1 (3) |
| Unknown | 9 (24) |
| Metastatic sites: | |
| 1 | 20 (53) |
| 2 | 14 (37) |
| ≥3 | 4 (10) |
| Ki67 expression: | |
| <55% | 12 (31.6) |
| ≥55% | 24 (63.2) |
The primary tumor sites were predominantly the pancreas and lungs; 13 patients (34%) had metastases in a single site, 12 patients (31%) had metastases in two different sites and 6 patients (15%) had metastases in three or more sites.
Survival outcomes
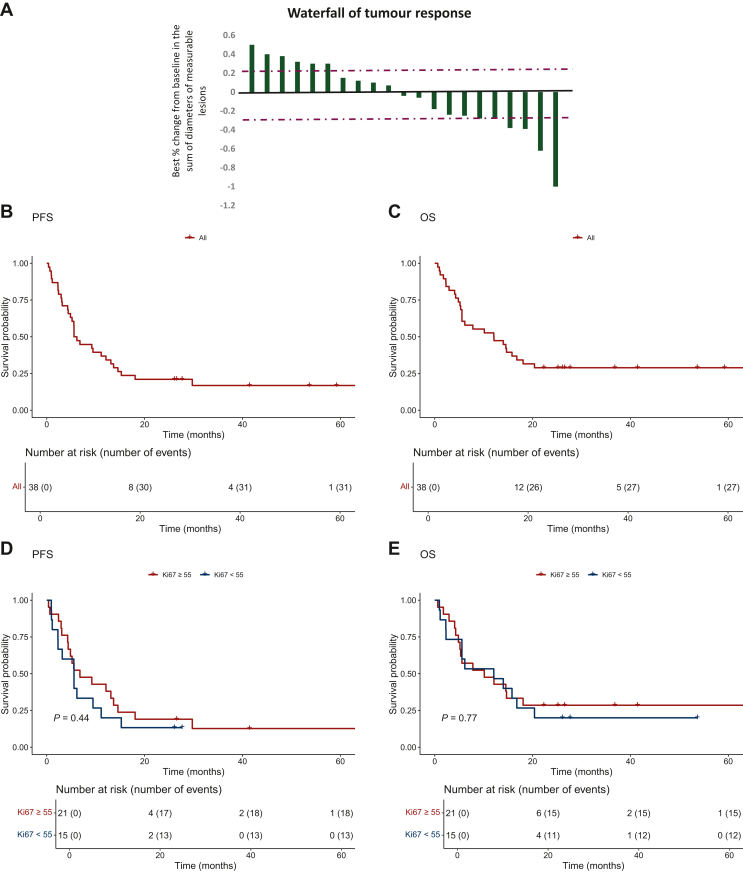
Among the 38 enrolled patients treated with TMZ one week on/one week off, 1 (3.8%) patient had a confirmed CR, and 6 (15.7%) had a confirmed PR, yielding an ORR of 18% (n = 7), according to RECIST version 1.1 by blinded independent central review (BICR). The DCR was 71% (n = 27, with one CR, six PRs, and 20 with SD for at least 24 weeks) (Figure 1A). The median PFS was 5.86 months [95% confidence interval (CI) 4.8 months-not applicable (NA)], and the median OS was 12.1 months (95% CI 5.6-20.4 months) (Figure 1B and C). The 1-year PFS rate was 37%, and seven patients were on treatment at the time of data censoring. No statistically significant difference in median OS [hazard ratio 1.1 (95% CI 0.5-2.4); P = 0.77] and median PFS [hazard ratio 1.3 (95% CI 0.6-2.8); P = 0.44] was observed among patients with Ki67 <55% versus ≥55% (Figure 1D and E).
Figure 1.
Response to treatment. (A) The best percentage change in target lesion size relative to baseline. The values represent the largest percentage change in the sum of the longest diameters for each patient with a measurable tumor. The data were available for 21 patients. Kaplan–Meier curves of PFS (B) and OS (C) in the overall sample of patients, and PFS (D) and OS (E) according to Ki67 % expression. ORR, overall response rate; OS, overall survival; PFS, progression-free survival.
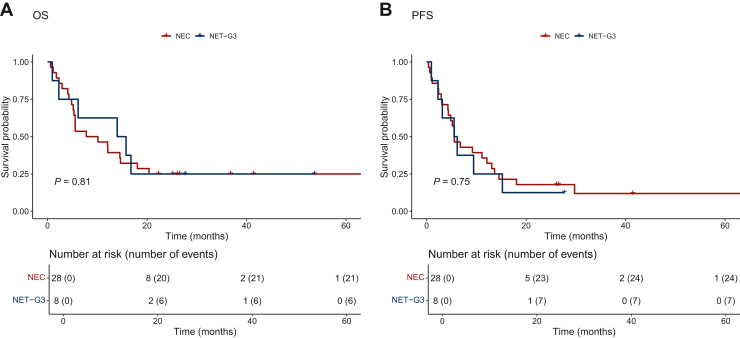
Patients with NETs-G3 had a longer median OS (14.9 months, 95% CI 6.1 months-NA) than patients with NECs (8.96 months, 95% CI 5.3 months-NA). The difference between the two groups, however, was not significant (Figure 2A). The median PFS was similar in the two groups, NETs-G3 and NECs (5.8 versus 5.6 months, respectively; P = 0.75) (Figure 2B). According to primary sites, patients with pancreatic NENs had longer survival outcomes (median OS and median PFS) than NENs from other sites (Supplementary Figure S1 and Table S1, available at https://doi.org/10.1016/j.esmoop.2024.103003). The same trend is also noticed upon removal of the 8 patients classified as NET-G3. Patients with pancreatic NEC had longer, even if not statistically significant, survival outcomes compared with gastrointestinal NEC and pulmonary LCNEC (Supplementary Figure S2 and Table S2, available at https://doi.org/10.1016/j.esmoop.2024.103003).
Figure 2.
Response to treatment by 2019 WHO classification. Kaplan–Meier curves of OS (A) and PFS (B) according to the 2019 WHO classification. NEC, neuroendocrine carcinoma; NET, neuroendocrine tumor; OS, overall survival; PFS, progression-free survival.
Safety
Ten (26%) patients experienced AEs of any grade, with no patients experiencing grade 3 or 4 AEs. The most common AEs of any grade were nausea (n = 5, 13%), diarrhea (n = 3, 8%) and abdominal pain (n = 3, 8%) (Table 2).
Table 2.
Incidence of adverse events (AEs) for all patients
| Adverse events | (n = 38), n (%) |
|---|---|
| All AEs | 10 (26) |
| Grade 3 or 4 | 0 (0) |
| Serious AEs | 0 (0) |
| Grade 1 reported AEs: | |
| Nausea | 5 (13) |
| Diarrhea | 2 (5) |
| Abdominal pain | 3 (8) |
| Decreased appetite | 1 (3) |
| Anemia | 1 (3) |
| Grade 2 reported AEs: | |
| Nausea | 0 (0) |
| Diarrhea | 1 (3) |
| Abdominal pain | 0 (0) |
| Decreased appetite | 0 (0) |
| Anemia | 0 (0) |
Discussion
This prospective, single-arm, phase II study demonstrated that low-dose TMZ administered on an alternating-week schedule yielded favorable efficacy outcomes with minimal toxicity. Additionally, there were no statistically significant differences in OS and PFS among patients with NENs G3 based on their Ki67 index. This finding suggests that even individuals with a poorer prognosis14 may benefit from TMZ treatment when administered the appropriate schedule. Over the past two decades, research has explored the clinical advantages of prolonged, regular and low-dose TMZ administration, indicating that such dosing could reduce toxicity and improve quality of life.16, 17, 18, 19, 20 The choice of a low-dose, alternate-week schedule may have facilitated the favorable tolerability observed in our study. This scheduling approach allowed rest periods between treatment cycles, activating protective systems that mitigate drug-induced damage while preserving drug exposure.18 Consequently, this schedule may be better tolerated by patients with a low PS, who often struggle with extensive high-dose and combination treatments. Moreover, the proposed TMZ schedule may offer efficacy benefits beyond safety advantages. Metronomic TMZ,21 instead of conventional dosing, can activate antiangiogenic and immune-mediated pathways.10,12,22 Since NECs are highly vascularized tumors and express numerous proangiogenic molecules and receptors,12,23 the low-dose every-other-week schedule may be a promising option for second-line treatment, as it shares some characteristics of metronomic administration (i.e. low dosage and prolonged drug exposure) with a predominant antiangiogenic effect. In addition to its antiangiogenic potential, metronomic TMZ can stimulate immune-modulating activity by enhancing dendritic cell function and selectively depleting CD4+CD25+Foxp3+ regulatory T cells (Tregs). Treg cells can suppress antitumor immunity, hindering the body’s ability to surveil and respond to neoplasia, thereby promoting tumor growth and progression.24 These biological mechanisms could explain the higher ORR, median PFS and median OS observed in our trial compared with the study by Olsen et al.,25 where TMZ was administered using the conventional schedule as second- or third-line treatment of NECs. As a limitation of the trial, however, we have not explored these biomarkers. Significantly, in our TENEC trial, where the majority of patients had a PS of 1 or 2, we observed superior DCR and median PFS compared with the DCR and median PFS reported in trials involving more physically fit patients for regimens such as TMZ plus capecitabine (CAPTEM), 5-fluorouracil and oxaliplatin (FOLFOX) and 5-fluorouracil and irinotecan (FOLFIRI) +/− bevacizumab.26, 27, 28, 29, 30 Furthermore, the clinical outcomes (ORR, median OS and median PFS) achieved in our trial with TMZ administered every other week were superior to those reported by McNamara et al.30 for both liposomal irinotecan (nal-IRI)/5-fluorouracil/folinic acid and docetaxel in patients with extrapulmonary NEC. While comparing results from different studies is methodologically challenging, these findings are noteworthy, particularly considering the potential role of reduced toxicity and mechanisms enhancing the immune response and antiangiogenic effects associated with prolonged low-dose therapy.26, 27, 28, 29, 30 It is crucial to note that the safety profile was more favorable in the TENEC trial than in the other trials. For instance, Kunz et al. reported 45% grade 3/4 toxicity with the CAPTEM combination, which may be excessive for patients with pretreated advanced or metastatic NECs.27 In contrast, our study confirms that the TMZ one week on/one week off regimen is a safe treatment approach. The safety outcomes align with previously published results of this schedule in glioblastoma regarding gastrointestinal AEs, although they differ in terms of hematological toxicity.13,20 This variation may be partially explained by the fact that we used a TMZ dose of 75 mg/m2 here, whereas in trials in patients with glioblastoma, the TMZ dose was 150 mg/m2. The results presented in this trial are subject to inherent limitations associated with phase II non-randomized clinical study designs. The relevance of the findings may be affected by the small number of enrolled patients due to the rare incidence of the disease and the absence of control groups, as well as by the fact that the trial is a single-arm study with no comparison possible with other treatments. Despite these limitations, our data suggest that TMZ administered every other week could be a viable option for patients when the benefit–risk balance does not favor more aggressive treatments. Given these results, TMZ every other week is the Naples ENETs Centre of Excellence treatment of choice for unfit patients with metastatic NECs progressive to platinum-based chemotherapy. Furthermore, the encouraging results from this prospective study support the feasibility of a phase III trial, which aims to compare TMZ administered every other week with physician choice as a second-line treatment of G3 NENs, which is currently under development at our institution.
Conclusion
To our knowledge, this is the first prospective study that included patients with extrapulmonary and pulmonary LCNEC. The results of this study showed that TMZ given every other week in patients with NENs G3 is effective and safe, and even if a direct comparison is not possible, one week on/one week off TMZ showed, in these groups of patients, similar clinical outcomes to other therapies. In particular, the study also provides valuable insight into the safety and efficacy of this TMZ schedule in patients unsuitable for combination therapies. This group of patients is considered orphans in terms of therapeutic options, particularly after first-line therapy.
Acknowledgements
We thank the Italian Ministry of Health that partially supported this project by Ricerca corrente and the non-profit organization Lega Italiana Per La Lotta Contro i Tumori (LILT) of Naples. Many thanks to Dr Alessandra Trocino, Librarian at the Library of Istituto Nazionale Tumori Fondazione ‘G Pascale’, Naples, Italy, for her excellent bibliographic service and assistance.
Funding
The study was based on internal funds.
Disclosure
The authors have declared no conflicts of interest.
Data sharing
R.O.I. data of the present trial are available from the corresponding author.
Ethics approval
All subjects provided informed written consent before enrollment in the study. All procedures carried out were in accordance with the 1964 Helsinki Declaration and its later amendments. The present study was approved by the Scientific Directorate of the National Cancer Institute of Naples approved with act number 293; EudraCT Number: 005238-31; ClinicalTrials.gov Identifier: NCT04122911.
Consent to participate
All subjects provided informed written consent to participate before enrollment in the study.
Consent for publication
All subjects provided informed consent to the publication of anonymous data.
Statement of translational relevance
This study shows that metronomic temozolomide has an encouraging efficacy and safety profile as a second-line treatment in poorly differentiated G3 NENs. This result provides a therapeutic opportunity for this setting of patients, who generally have a poor performance status and low tolerance to combination therapy. As there is no consensus on the second-line regimen in this setting, data from this prospective study may be essential to guide therapeutic decisions and to suggest here TMZ one week on/one week off as second-line standard therapy.
Supplementary data
References
- 1.Popa O., Taban S.M., Pantea S., et al. The new WHO classification of gastrointestinal neuroendocrine tumors and immunohistochemical expression of somatostatin receptor 2 and 5. Exp Ther Med. 2021;22(4):1179. doi: 10.3892/etm.2021.10613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rindi G., Wiedenmann B. Neuroendocrine neoplasms of the gut and pancreas: new insights. Nat Rev Endocrinol. 2011;8(1):54–64. doi: 10.1038/nrendo.2011.120. Erratum in: Nat Rev Endocrinol. 2012;8(2):66. [DOI] [PubMed] [Google Scholar]
- 3.Travis W.D., Brambilla E., Nicholson A.G., et al. WHO Panel. The 2015 World Health Organization Classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol. 2015;10(9):1243–1260. doi: 10.1097/JTO.0000000000000630. [DOI] [PubMed] [Google Scholar]
- 4.Noonan K., Derks J., Laskin J., Dingemans A. Neuroendocrine tumors of the lung other than small cell lung cancer. IASLC Thorac Oncol. 2018:555–568.e6. [Google Scholar]
- 5.Nicholson A.G., Tsao M.S., Beasley M.B., et al. The 2021 WHO Classification of lung tumors: impact of advances since 2015. J Thorac Oncol. 2021;17:362–387. doi: 10.1016/j.jtho.2021.11.003. [DOI] [PubMed] [Google Scholar]
- 6.Mollazadegan K., Welin S., Crona J. Systemic treatment of gastroenteropancreatic neuroendocrine carcinoma. Curr Treat Options Oncol. 2021;22(8):68. doi: 10.1007/s11864-021-00866-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strosberg J.R., Halfdanarson T.R., Bellizzi A.M., et al. The North American Neuroendocrine Tumor Society Consensus Guidelines for surveillance and medical management of midgut neuroendocrine tumors. Pancreas. 2017;46(6):707–714. doi: 10.1097/MPA.0000000000000850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tafuto S., von Arx C., Capozzi M., et al. Safety and activity of metronomic temozolomide in second- line treatment of advanced neuroendocrine neoplasms. J Clin Med. 2019;8(8):1224. doi: 10.3390/jcm8081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prigerson H.G., Bao Y., Shah M.A., et al. Chemotherapy use, performance status, and quality of life at the end of life. JAMA Oncol. 2015;1(6):778–784. doi: 10.1001/jamaoncol.2015.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kurzen H., Schmitt S., Näher H., Möhler T. Inhibition of angiogenesis by non-toxic doses of temozolomide. Anticancer Drugs. 2003;14(7):515–522. doi: 10.1097/00001813-200308000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Chan J.A., Stuart K., Earle C.C., et al. Prospective study of bevacizumab plus temozolomide in patients with advanced neuroendocrine tumors. J Clin Oncol. 2012;30(24):2963–2968. doi: 10.1200/JCO.2011.40.3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koumarianou A., Antoniou S., Kanakis G., et al. Combination treatment with metronomic temozolomide, bevacizumab and long-acting octreotide for malignant neuroendocrine tumours. Endocr Relat Cancer. 2012;19(1):L1–L4. doi: 10.1530/ERC-11-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galldiks N., Berhorn T., Blau T., Dunkl V., Fink G.R., Schroeter M. “One week on-one week off”: efficacy and side effects of dose-intensified temozolomide chemotherapy: experiences of a single center. J Neurooncol. 2013;112(2):209–215. doi: 10.1007/s11060-013-1048-z. [DOI] [PubMed] [Google Scholar]
- 14.Taal W., Segers-van Rijn J.M., Kros J.M., et al. Dose dense 1 week on/1 week off temozolomide in recurrent glioma: a retrospective study. J Neurooncol. 2012;108(1):195–200. doi: 10.1007/s11060-012-0832-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eisenhauer E.A., Therasse P., Bogaerts J., et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 16.Fidler I.J., Ellis L.M. Chemotherapeutic drugs--more really is not better. Nat Med. 2000;6(5):500–502. doi: 10.1038/74969. [DOI] [PubMed] [Google Scholar]
- 17.Della Monica R., Cuomo M., Visconti R., et al. Evaluation of MGMT gene methylation in neuroendocrine neoplasms. Oncol Res. 2022;28(9):837–845. doi: 10.3727/096504021X16214197880808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gatenby R.A., Silva A.S., Gillies R.J., Frieden B.R. Adaptive therapy. Cancer Res. 2009;69(11):4894–4903. doi: 10.1158/0008-5472.CAN-08-3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Divitiis C., von Arx C., Grimaldi A.M., et al. European Neuroendocrine Tumor Society (ENETS) Center of Excellence-Multidisciplinary Group for Neuroendocrine Tumors in Naples (Italy) Metronomic temozolomide as second line treatment for metastatic poorly differentiated pancreatic neuroendocrine carcinoma. J Transl Med. 2016;14(1):113. doi: 10.1186/s12967-016-0857-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wick W., Platten M., Weller M. New (alternative) temozolomide regimens for the treatment of glioma. Neuro Oncol. 2009;11(1):69–79. doi: 10.1215/15228517-2008-078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun C., Yu Y., Wang L., et al. Additive antiangiogenesis effect of ginsenoside Rg3 with low-dose metronomic temozolomide on rat glioma cells both in vivo and in vitro. J Exp Clin Cancer Res. 2016;35:32. doi: 10.1186/s13046-015-0274-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lambrescu I., Fica S., Martins D., et al. IEO ENETS Center of Excellence for GEP NET. Metronomic and metronomic-like therapies in neuroendocrine tumors - rationale and clinical perspectives. Cancer Treat Rev. 2017;55:46–56. doi: 10.1016/j.ctrv.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 23.Woo J.Y., Yang S.H., Lee Y.S., Lee S.Y., Kim J., Hong Y.K. Continuous low-dose temozolomide chemotherapy and microvessel density in recurrent glioblastoma. J Korean Neurosurg Soc. 2015;58(5):426–431. doi: 10.3340/jkns.2015.58.5.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Togashi Y., Shitara K., Nishikawa H. Regulatory T cells in cancer immunosuppression - implications for anticancer therapy. Nat Rev Clin Oncol. 2019;16(6):356–371. doi: 10.1038/s41571-019-0175-7. [DOI] [PubMed] [Google Scholar]
- 25.Olsen I.H., Sørensen J.B., Federspiel B., et al. Temozolomide as second or third line treatment of patients with neuroendocrine carcinomas. ScientificWorldJ. 2012;2012 doi: 10.1100/2012/170496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Welin S., Sorbye H., Sebjornsen S., Knappskog S., Busch C., Oberg K. Clinical effect of temozolomide-based chemotherapy in poorly differentiated endocrine carcinoma after progression on first-line chemotherapy. Cancer. 2011;117(20):4617–4622. doi: 10.1002/cncr.26124. [DOI] [PubMed] [Google Scholar]
- 27.Kunz P.L., Graham N.T., Catalano P.J., et al. Randomized study of temozolomide or temozolomide and capecitabine in patients with advanced pancreatic neuroendocrine tumors (ECOG-ACRIN E2211) J Clin Oncol. 2023;41(7):1359–1369. doi: 10.1200/JCO.22.01013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hadoux J., Malka D., Planchard D., et al. Post-first-line FOLFOX chemotherapy for grade 3 neuroendocrine carcinoma. Endocr Relat Cancer. 2015;22(3):289–298. doi: 10.1530/ERC-15-0075. [DOI] [PubMed] [Google Scholar]
- 29.Hentic O., Hammel P., Couvelard A., et al. FOLFIRI regimen: an effective second-line chemotherapy after failure of etoposide-platinum combination in patients with neuroendocrine carcinomas grade 3. Endocr Relat Cancer. 2012;19(6):751–757. doi: 10.1530/ERC-12-0002. [DOI] [PubMed] [Google Scholar]
- 30.McNamara M.G., Swain J., Craig Z., et al. NET-02: a randomised, noncomparative, phase II trial of nal-IRI/5-FU or docetaxel as second-line therapy in patients with progressive poorly differentiated extra-pulmonary neuroendocrine carcinoma. EClinicalMedicine. 2023;60 doi: 10.1016/j.eclinm.2023.102015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.