Abstract
The strong association between lipoprotein (a) [Lp(a)] and atherosclerotic cardiovascular disease has led to considerations of Lp(a) being a potential target for mitigating residual cardiovascular risk. While approximately 20 % of the population has an Lp(a) level greater than 50 mg/dL, there are no currently available pharmacological lipid-lowering therapies that have demonstrated substantial reduction in Lp(a). Novel therapies to lower Lp(a) include antisense oligonucleotides and small-interfering ribonucleic acid molecules and have shown promising results in phase 2 trials. Phase 3 trials are currently underway and will test the causal relationship between Lp(a) and ASCVD and whether lowering Lp(a) reduces cardiovascular outcomes. In this review, we summarize emerging insights related to Lp(a)’s role as a risk-enhancing factor for ASCVD, association with calcific aortic stenosis, effects of existing therapies on Lp(a) levels, and variations amongst patient populations. The evolving therapeutic landscape of emerging therapeutics is further discussed.
Keywords: Dyslipidemia, Lipoprotein(a), Atherosclerotic cardiovascular disease, Calcific aortic stenosis
1. Introduction
Lipoprotein(a) [Lp(a)] has garnered significant interest within the cardiovascular community over the past several decades given its association with atherosclerotic cardiovascular disease (ASCVD). The recent emergence of potential therapeutics targeting Lp(a) have brought this biomarker further into the limelight [1]. Approximately 14–20 % of the population has an Lp(a) level greater than 50 mg/dL [2,3]. While the lowering of low-density lipoprotein cholesterol (LDL-C) has traditionally been the predominant focus for ASCVD risk reduction, several studies have underscored the residual cardiovascular risk that remains despite adequate control of other risk factors [4,5]. Therefore, novel therapeutic targets are needed to address the pressing challenge of this residual risk [6].
In this review, we summarize emerging insights related to Lp(a) including its unique atherogenic structure, role as a risk-enhancing factor for ASCVD, association with calcific aortic stenosis, and variation amongst patient populations. The existing lipid-lowering therapies and effects on Lp(a) and the emerging therapeutics landscape of pharmacological therapies for lowering Lp(a) that are in various phases of clinical trials are also reviewed.
2. Unique structure of Lp(a) and association with ASCVD events
Similar to LDL-C, Lp(a) contains a core of triglycerides and cholesterol esters surrounded by an outer membrane of phospholipids and an apolipoprotein-B100 (apo-B100) component [7]. The unique properties of Lp(a) are related to its apolipoprotein(a) [apo(a)] portion which is covalently linked by a disulfide bond to apo-B100 [7]. Plasma Lp(a) concentration is largely genetically determined by the LPA gene which encodes apo(a) and is comprised of repeating kringle domains with a variable number of repeats in the kringle IV type 2 (KIV-2) domain. This copy number variation affects the size of the apo(a) protein, leading to substantial size heterogeneity, with an inverse correlation between the number of KIV-2 repeats and Lp(a) levels [8]. Lp(a) can be measured either in mass units (mg/dL) or in molar units (nmol/L) with differences in standardization across assays [9].
Proposed mechanisms for the role of Lp(a) in the mediation of ASCVD include a contribution to atherosclerosis through the lipoprotein moiety and a potential contribution to thrombosis through the apo(a) moiety which bears structural similarities to plasminogen [10]. Lp(a) may have other potential prothrombotic actions by upregulating proteins that inhibit fibrinolysis and inactivate tissue factor pathway inhibitor thereby promoting blood coagulation [11,12]. Finally, apo(a) contains binding sites for oxidized phospholipids that can potentially trigger inflammation and endothelial dysfunction which further potentiate the atherogenicity of Lp(a) [13]. Evidence supporting the causal relationship between elevated Lp(a) and ASCVD originates from genetic studies. In a Mendelian randomization study of individuals from Copenhagen, a doubling of genetically elevated Lp(a) level was associated with a 22 % higher risk of myocardial infarction (MI) [14]. The proposed causal role of Lp(a) in coronary artery disease was later corroborated by several other genetic studies and large observational epidemiological studies demonstrating strong continuous association between Lp(a) and ASCVD [15], [16], [17], [18], [19], [20]. The association between Lp(a) levels and several cardiovascular outcomes have been reported in recent years, with risk being greatest for MI and aortic valve stenosis (3 to 4-fold increase in risk in Copenhagen General Population Study) as compared to 1.6-fold for ischemic stroke [14,21,22]. While Mendelian randomization studies have provided evidence for causality between elevated Lp(a) and ASCVD, randomized cardiovascular outcome trials are necessary to demonstrate that lowering Lp(a) reduces adverse cardiovascular outcomes.
3. Lp(a) as a risk enhancing factor for ASCVD
While LDL-C has been the predominant focus for primary and secondary prevention of ASCVD to date, data support utilizing Lp(a) as a biomarker to enhance CVD risk prediction. In the JUPITER (Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin) trial, Lp(a) was a significant determinant of residual risk in patients treated with potent statin therapy [5]. In a recent analysis of more than 400,000 participants from the United Kingdom (UK) Biobank, adding Lp(a) as a continuous variable to a prediction model of traditional CVD risk factors modestly improved discrimination and reclassification. A more sizeable improvement in reclassification was seen among the intermediate (5–7.49 %) 10-year ASCVD risk group [23]. This highlights that the incorporation of Lp(a) in this patient population may help guide treatment decisions. Furthermore, in this primary prevention cohort, Lp(a) above 100 nmol/L accounted for 5.8 % of the composite CVD outcome, while Lp(a) above 175 nmol/L accounted for 3 % [23]. These results are consistent with a prior analysis demonstrating improved CVD risk prediction with the addition of Lp(a) to the Reynolds Risk Score, particularly in intermediate-risk groups [24].
The utility of measuring Lp(a) was also seen in an analysis examining approximately 2000 participants from the Framingham Offspring study; in individuals with LDL-C ≥ 135 mg/dL, the added presence of Lp(a) ≥ 100 nmol/L was associated with a 43 % increase in cardiovascular risk after adjusting for other key cardiovascular risk factors [25]. Moreover, the presence of high Lp(a) (≥100 nmol/L) with moderate LDL-C levels (135–159 mg/dL) yielded absolute risks equivalent to those with LDL-C ≥ 160 mg/dL [25], a threshold cited by guidelines to consider earlier lipid-lowering therapy [26]. Similarly, in an analysis of participants from the Multi-Ethnic Study of Atherosclerosis (MESA), risk of coronary heart disease (CHD) events was increased with elevated Lp(a) (≥ 50 mg/dL) regardless of baseline LDL-C [27]. While most of the aforementioned studies were conducted in primary prevention cohorts, Lp(a) has also been shown to be a strong prognostic biomarker in secondary prevention with associations with future coronary events in those with known CHD [28].
Elevated Lp(a) may be a strong marker of risk when there is significant atherosclerosis as demonstrated by coronary artery calcium (CAC) score. In an analysis of the MESA and Dallas Heart Study, elevated Lp(a) and CAC score were independently associated with ASCVD risk. However, there was an additive joint association between Lp(a) and CAC with participants with elevated Lp(a) and CAC ≥100 having the highest risk (HR 4.71; 95 % CI 3.01–7.40) for ASCVD [29]. These findings underscore the importance of aggressive risk factor and lifestyle modifications to reduce ASCVD risk in patients with concurrent Lp(a) and CAC elevation. Whether or not Lp(a) has an impact on CAC is still uncertain. One analysis from the Heinz Nixdorf Recall study suggested Lp(a) to be a causal risk factor for CAC by demonstrating a significant association between a single nucleotide polymorphism at the Lp(a) locus and CAC [30]. However, more recent data from MESA demonstrated that Lp(a) was not associated with baseline CAC volume or density and was only modestly associated with volume progression [31]. Apart from CAC, advanced plaque composition assessment by coronary computed tomography angiography (CCTA) has shown that among patients with advanced stable CAD, Lp(a) is associated with accelerated progression of low-attenuation plaque (a quantitative marker of necrotic core and high risk plaque) [32]. This progression of vulnerable plaque phenotypes may provide an explanation for the association between high Lp(a) and residual risk of atherosclerotic events, further substantiating the importance of Lp(a) as a treatment target [32].
3.1. Guideline recommendations related to Lp(a)
Despite the available evidence indicating the utility of Lp(a) in risk stratification, the majority of guidelines and scientific statements do not yet support widespread screening for elevation of Lp(a) (Table 1). The 2018 American College of Cardiology/American Heart Association Cholesterol guidelines list Lp(a) ≥ 50 mg/dL or 125 nmol/L as a risk enhancing factor [26]. The 2019 National Lipid Association scientific statement on Lp(a) has more specific indications where measurement of Lp(a) is or may be reasonable, as summarized in Table 1 [33]. The 2019 European Atherosclerosis Society/European Society of Cardiology guidelines are the first to recommend measurement of Lp(a) measurement at least once in each adult's lifetime to identify those with very high inherited levels [34]. In contrast, the HEART UK Consensus statement from 2019 advocated for measurement of Lp(a) in a targeted population and to manage Lp(a) associated risk in those with levels > 90 nmol/L [35]. The stated rationale in the UK statement is that although the European guidelines aim to identify those with Lp(a) >430 nmol/L, the risk conferred by Lp(a) occurs at a much lower Lp(a) threshold. The 2021 Canadian Guidelines, on the other hand, concur with the European guidelines in recommending a one-time screening of Lp(a) in all adults [36]. At this time, repeated measurements of Lp(a) is not currently recommended as the clinical value of serial measurements has not been established, and current evidence suggests Lp(a) levels are relatively stable given their predominant genetic basis [10].
Table 1.
Summary of major society guidelines and scientific statement recommendations on Lp(a) testing.
| When to measure Lp(a) | Class/LOE | Threshold of Lp(a) | Class/LOE | |
|---|---|---|---|---|
| 2018 ACC/AHA Cholesterol Guidelines | Family history of premature ASCVD or personal history of ASCVD | N/A | Lp(a) ≥ 50 mg/dL or 125 nmol/L as a risk enhancing factor that favor initiation of statin therapy in adults 40 to 75 years of age without diabetes mellitus and 10-year ASCVD risk of 7.5 %−19.9 % | N/A |
| 2019 NLA Scientific Statement on Use of Lipoprotein(a) in Clinical Practice | Reasonable to refine risk assessment for ASCVD events in 1) individuals with a family history of first-degree relatives with premature ASCVD), 2) individuals with premature ASCVD in absence of traditional risk factors, 3) primary severe hypercholesterolemia or suspected FH, 4) individuals at very-high-risk of ASCVD to better define those who are more likely to benefit from PCSK9 inhibitor therapy. | IIa/B-NR for all indications (except LOE C-LD for #1) | When Lp(a) values are used for ASCVD risk assessment in Caucasian patients, it is reasonable to use measured values ≥ 50 mg/dL or 100 nmol/L as levels suggesting increased risk | IIa/B-R |
| May be reasonable for individuals with: 1) intermediate 10-y ASCVD risk when decision to use a statin is uncertain, to improve risk stratification in primary prevention, 2) borderline 10-y ASCVD risk when the decision to use a statin is uncertain, to improve risk stratification in primary prevention, less-than-anticipated LDL-C lowering, despite good adherence to therapy, 4) a family history of elevated Lp(a), 5) calcific valvular aortic stenosis, 6) recurrent or progressive ASCVD despite optimal lipid-lowering therapy | IIb for all B-NR for # 1 and 2 C-LD for #3–6 |
– | – | |
| 2019 ESC/EAS Guidelines for Management of Dyslipidemias | Lp(a) measurement should be considered at least once in each adult person's lifetime to identify those with very high inherited Lp(a) levels >180 mg/dL (>430 nmol/L) who may have a lifetime risk of ASCVD equivalent to the risk associated with heterozygous FH. | IIa/C | – | – |
| Lp(a) should be considered in selected patients with a family history of premature CVD, and for reclassification in people who are borderline between moderate and high risk | IIa/C | – | – | |
| 2019 HEART UK Consensus Statement on Lipoprotein(a) | 1) Personal or family history of premature atherosclerotic CV disease, first degree relatives 2) with raised serum Lp(a) levels (> 200 nmol/l), familial hypercholesterolemia or other genetic dyslipidemias, 3) calcified aortic valve stenosis, 4) a borderline increased (but <15 %) 10-year risk of CV event | N/A | CV risk conferred by Lp(a) is 32–90 nmol/L minor, 90–200 nmol/L moderate, 200–400 nmol/l high, >400 nmol/l very high. The management of raised Lp(a) levels (>90 nmol/L) should include reducing overall atherosclerotic risk, controlling hyperlipidemia, considering lipoprotein apheresis. |
N/A |
| 2021 Canadian Cardiovascular Society Guidelines for the Management of Dyslipidemia | Once in patient's lifetime, with initial screening. | N/A | Presence of risk modifier, including Lp(a) ≥ 50 mg/dL (≥ 100 nmol/L), in intermediate-risk individuals favors the use of statins. | N/A |
LOE=level of evidence, ACC=American College of Cardiology, AHA=American Heart Association, ASCVD=atherosclerotic cardiovascular disease, NLA=National Lipid Association, FH=familial hypercholesterolemia, ESC=European Society of Cardiology, EAS=European Atherosclerosis Society, CV=cardiovascular.
4. Lp(a) and calcific aortic stenosis
Calcific aortic valve stenosis (AS) is a common valvular disease with increasing prevalence with advanced age [37]. The pathophysiology leading to the development of AS is complex, resulting from the interplay of endothelial dysfunction, inflammation, and lipoprotein oxidation leading to aortic valve calcification as well as additional risk factors such as smoking and hypertension [38], [39], [40]. Lp(a) is thought to play a significant role in the development of AS, as a major lipoprotein carrier of the pro-inflammatory and pro-calcific oxidized phospholipids which can induce osteogenic differentiation of valvular interstitial cells [41,42]. Enzymes such as autotaxin have also been associated with calcific AS and concomitant elevations in Lp(a) and oxidized phospholipids strongly predict risk of calcific AS suggesting interconnected mechanisms that require further investigation [43].
The association between Lp(a) and increased risk of calcific AS has been captured by genome wide association studies, including evidence that genetic variations in the LPA locus, the predominant determinant of Lp(a) levels, are associated with aortic valve calcification [44]. In the EPIC—Norfolk Study which evaluated the association between genetic variants, Lp(a) levels and AS development, Lp(a) > 50 mg/dL was associated with an increased risk of developing AS (HR 1.98 [1.25–3.09]), even after adjustment for traditional risk factors and LDL [45]. A Mendelian randomization study of Danish patients quantified this risk demonstrating elevated Lp(a) and corresponding genotypes were associated with AS risk, with a threefold increase in risk among those with Lp(a) >90 mg/dl [22]. Further confirmation exists in case-control, and cross-sectional studies, including a large meta-analysis of eight studies with 52,931 participants demonstrating a nearly 2-fold increase in AS in those with Lp(a) >50 mg/dl [46].
Beyond increasing the likelihood of development of AS, Lp(a) may also predict disease progression and the risk of requiring aortic valve replacement (AVR). In a secondary analysis of the ASTRONOMER trial which evaluated the effects of intensive lipid lowering with rosuvastatin on AS progression, the top tertile of Lp(a) (>58.5 mg/dL) was associated with faster AS progression and a two-fold risk of AVR compared with the bottom two tertiles, independent of baseline AS severity [47]. Similarly, Zheng et al. demonstrated faster progression in aortic valve calcium score, as assessed via repeat CT calcium scoring scans, and faster hemodynamic progression by echocardiography in patients with elevated Lp(a). After adjustment for baseline calcium score and traditional risk factors, the highest Lp(a) tertile was an independent predictor of annualized progression in AV calcium score (144 AU/year, 95 % CI 52–239 AU/year) [27]. A secondary analysis of the FOURIER trial with PCSK9 inhibition found that elevated Lp(a) levels were associated with an increased risk of AS-related events (including AVR) [48]. On the other hand, in the Rotterdam study of 922 patients and mean follow up of 14.0 years, Lp(a) was strongly associated with development of AS, but not with AS progression [49]. Given these findings, there is significant interest in further understanding whether targeted Lp(a) reduction may impact the natural history of AS development and progression. Such interest is further amplified by data showing that treatment with evolocumab has been associated with a lower risk of AS-related events [48], whereas statin therapy has not demonstrated benefit in AS progression in clinical trials [50].
5. Lp(a) and the association of cerebrovascular and peripheral vascular outcomes
While several large, population-based cohort studies have associated Lp(a) with ischemic stroke [51], the association has not been as consistent or robust as with MI [52]. In a prospective study of 5888 adults older than age 65, men in the highest Lp(a) quintile had three times the unadjusted risk of stroke as compared with those in the lowest quintile though there was no significant association in women [53]. In contrast, in the Atherosclerosis Risk in Communities (ARIC) study, increased levels of Lp(a), particularly ≥ 300 µg/mL vs. < 100 µg/mL, were associated with ischemic stroke in Black (RR 1.84, 95 % CI 1.05–3.07) and White women (RR 2.42, 95 % CI 1.30–4.53) but not in men [54]. In a more contemporary analysis from the REGARDS (Reasons for Geographic and Racial Differences in Stroke) study, Lp(a) in the highest quartile was only weakly associated with ischemic stroke after adjustment (HR 1.45, 95 % CI 0.96–2.19) with notable racial differences [55].
The association of Lp(a) with peripheral arterial disease (PAD) is similarly not as robust. Smaller studies have demonstrated that Lp(a) is a potential risk factor for progression of PAD [56,57]. Furthermore, in the large prospective EPIC—Norfolk cohort, Lp(a) was associated with development of PAD (HR 1.37, 95 % CI 1.25–1.50) [58]. However, in a nested case-control study of 14,916 males, there was no difference in Lp(a) levels in those with vs. without incident PAD and no risk gradient when evaluating increasing quartiles of Lp(a) [59]. Studies in patients with established PAD have demonstrated that high Lp(a), particularly ≥30 mg/dL, is associated with higher rates of lower limb peripheral revascularizations (HR 1.33; 95 % CI 1.06–1.66) though no differences were seen in risk of major adverse cardiovascular outcomes or mortality in this particular analysis [60]. Similarly, in a large retrospective study utilizing administrative data from a center in Paris, the 1-year incidence of major adverse limb events was 4.5 % in those with Lp(a) ≥134 mg/dL vs. 2.4 % in overall population [61]. Further large prospective randomized controlled clinical trials will hopefully continue to explore the impact of Lp(a) on both the incidence and the progression of PAD.
6. Lp(a) across patient populations
6.1. Racial and ethnic differences
Significant racial and ethnic differences exist in Lp(a) levels across the population. In an analysis from the Coronary Artery Risk Development in Young Adults (CARDIA) study in 1994 of 4,125 young adults, Black participants had three-fold higher Lp(a) levels than White participants [62]. More recent studies – including data from the National Health and Nutrition Examination Survey III, Dallas Heart Study, and the Atherosclerosis Risk in Communities Study – also report higher median Lp(a) among Black participants [3,63,64]. Findings from the Mediators of Atherosclerosis in South Asians Living in America (MASALA) cohort found that median Lp(a) level in South Asian participants was lower than Black participants but higher than other ethnic groups including White, Hispanic, and Chinese participants [65]. Differences in Lp(a) concentrations are likely related to differences in Lp(a) isoform size that occur due to variations in kringle copy numbers. Importantly, however, a study from INTERHEART demonstrated that the risk of MI increased similarly with increasing Lp(a) concentrations across multiple ethnic groups, independent of Lp(a) isoform size [66]. There are also other genetic variants that likely contribute to differences across ethnicities with variations in the promoter region of the apo (a) gene and other single nucleotide polymorphisms [67,68].
A contemporary study compiling data from over 450,000 participants of the UK Biobank revealed that while there are substantial differences in Lp(a) concentrates according to race and ethnic background, hazard ratio for ASCVD per 50 nmol/L increase in Lp(a) was similar across racial groups [69]. Likewise, in data from MESA, Lp(a) concentrations were continuously associated with CHD risk in both Black and White participants [70]. In another analysis from MESA, the conventional 30 mg/dL Lp(a) cutoff was associated with aortic valve calcifications in White participants but was only borderline significant in Black participants [71]. Further data is needed to best elucidate the interplay between ethnic heritage, which may reflect genetic variance, and the effect of Lp(a) on ASCVD.
6.2. Sex differences
Studies have demonstrated notable sex differences in Lp(a) concentration. Specifically, in an analysis of 126,634 participants from 36 prospective studies, women had 12 % higher Lp(a) concentrations compared to men. In this analysis, there was a continuous association of Lp(a) with risk of CHD that did not vary by sex [19]. However, other studies highlight the complexity of the relationship between Lp(a) and CVD in women. In an analysis of three cohorts of women (Women's Health Study, Women's Health Initiative Observational Study, and the JUPITER trial), increased CVD risk among those with elevated Lp(a) was only present among women with total cholesterol > 220 mg/dL while such an interaction with total cholesterol did not exist in men [72]. Further study of the differential association of Lp(a) on ASCVD outcomes by sex is necessary to better elucidate this important relationship.
Differences based on the use of hormone replacement therapy and menopausal status for Lp(a) are described – although the data are conflicting. In a study from 2010, in women without hormone replacement therapy, those in the highest quintile of Lp(a) were 1.77 times more likely to develop cardiovascular events compared to the lowest quintile. In contrast, the relationship of Lp(a) and CVD was no longer statistically significant in those taking hormone replacement therapy [73]. The mechanism is unclear although hypothesized that the direct biological effects of estrogen may induce increased uptake of Lp(a) by the LDL receptor and reduce Lp(a) production by the liver [74]. Conversely, a study of postmenopausal patients from the UK Biobank showed no evidence of lower Lp(a)-associated risk in those using hormone replacement therapy as compared to nonusers [75]. Differences between these two studies may be due to variations in hormonal use patterns over time. Furthermore, other studies do demonstrate changes in Lp(a) concentrations based on menopausal status [76]. In a large systematic review and meta-analysis that combined seventeen studies, Lp(a) concentrations were lower in premenopausal than in postmenopausal women [76]. Similarly, in data from 37,545 women from the Copenhagen General Population study, Lp(a) levels were 27 % higher after menopause, with women having an increase in levels around age 50 [77]. While there were differences in Lp(a) levels based on sex, elevated Lp(a) was associated with increased risk of MI, ischemic heart disease, and aortic valve stenosis in sex-stratified multivariable adjusted models [77].
6.3. Lp(a) and inflammation
Prior data has implicated that inflammation may play an important role for Lp(a) in the mediation of CVD as oxidized phospholipids are a large contributor to pro-inflammatory effects and resultant atherogenicity [13]. Mechanistically, patients with high Lp(a) exhibit an enhanced arterial wall inflammation and macrophage activation and this effect can be removed by inactivated oxidized phospholipids on Lp(a) [13]. In an exploratory post hoc analysis of the Assessment of Clinical Effects of Cholesteryl Ester Transfer Protein Inhibition with Evacetrapib in Patients at a High Risk for Vascular Outcomes (ACCELERATE) trial, which included patients with established vascular disease, increasing quintiles of Lp(a) were significantly associated with greater risks of death, MI, and stroke in those with high-sensitivity C-reactive protein (hsCRP) greater than 2 mg/L, but not in those with hsCRP less than 2 mg/L [78]. This is consistent with analyses of a primary prevention population from the MESA study that demonstrated the association of elevated Lp(a) with a significantly higher risk of incident CVD events was only present among those with elevated hsCRP (>2 mg/L) (HR 1.62, 95 %CI 1.25–2.10) [79].
Other reports have demonstrated that Lp(a)-associated ASCVD risk is modulated by the presence of a proinflammatory IL-1 genotype; patients that carry this pro-inflammatory IL-1 genotype (presumed to be associated with higher rates of inflammation) and Lp(a) above the median had worse event-free survival compared to IL-1 genotype negative patients (HR 3.59, 95 % CI 1.07–12.03) [80]. While these findings pose the question of whether individuals with certain markers of inflammation may derive more benefit from future Lp(a)-lowering therapies, further mechanistic studies are still needed.
Lp(a) is also linked to IL-6, with the LPA gene having several response elements to IL-6 which can work to promote apo(a) production [81]. There are several lines of evidence that highlight the role of IL-6. For instance, the IL-6 monoclonal antibody tocilizumab inhibits IL-6 induced expression of LPA in human hepatocytes in vitro (both at the mRNA and protein level) and can inhibit IL-6 response elements in the promoter region of the LPA gene [82]. These results appear specific to IL-6 as prior studies have shown that TNF inhibition does not significantly influence Lp(a) levels [82]. Post-hoc analyses of randomized controlled trials investigating IL-6 inhibitors, such as tocilizumab or sarilumab, and more recently ziltivekimab, demonstrate a reduction in Lp(a) between 16 and 41 % though most did not investigate relationship with major adverse cardiovascular events [83], [84], [85]. Therefore, inhibition of the IL-6 signaling pathway may be a potential therapeutic strategy to target Lp(a)-associated ASCVD risk, but trials designed to test this question are still pending.
7. Existing medications and effects on Lp(a)
7.1. Statins
Studies have demonstrated conflicting findings regarding the extent to which statin therapy affects Lp(a) levels. In an older meta-analysis of randomized controlled trials of atorvastatin through 2011, results demonstrated lower Lp(a) concentrations in the atorvastatin than control group; however, there was significant trial heterogeneity [86]. In contrast, in a meta-analysis of 5,256 patients from six randomized trials, there was an increase in Lp(a) from baseline ranging from 11.6 % to 20.4 % in the pravastatin group and 18.7 % to 24.2 % in the atorvastatin group [87]. This specific study used an inclusion criterion of the same Lp(a) assay resulting in a smaller number of trials being included; however, the homogeneity in Lp(a) assays may have led to more accurate results.
Given the various forms and dosages of statins used in these studies which could result in inconsistency, a larger pairwise and a network meta-analysis of 23,065 participants was performed and demonstrated that statins did not have a clinically significant effect on Lp(a) concentrations [88]. Relatedly, another more contemporary systematic review and meta-analysis specifically examined the effect of statins as compared to placebo on levels of Lp(a) and observed little to no differences in Lp(a) level [89]. The study had high heterogeneity and since not all analyses were pre-specified, the results should be considered explorative. Overall, while statins may possibly lead to minor increases in Lp(a), this should not impact the utilization of these agents for ASCVD risk reduction.
7.2. Ezetimibe
In a meta-analysis of 10 randomized placebo-controlled clinical trials, ezetimibe therapy did not have effect on plasma Lp(a) concentration. There was no difference based on ezetimibe monotherapy or addition of ezetimibe to statin therapy [90].
7.3. Niacin
Niacin has previously been suggested to be a therapy that can lower Lp(a) by ≤ 30 %; and can downregulate the transcriptional activity of the LPA promoter [91]. In the HPS2-THRIVE trial of patients with vascular disease randomized to 2 g of extended-release niacin and 40 mg of laropiprant or matching placebo, there was an absolute difference of −9.5 (p = 0.006) in Lp(a) levels; however, the trial did not show any benefit in regard to cardiovascular outcomes [92] and thus niacin is not considered to be a first-line treatment strategy for high Lp(a).
7.4. PCSK9i
Out of all current lipid lowering therapies, proprotein convertase subtilisin/kexin type 9 (PCSKK9) inhibitors provide the greatest reduction in Lp(a), approximately 20–25 % [93]; however, whether this degree of Lp(a) reduction leads to direct cardiovascular benefit beyond that attributable to LDL-C lowering is not entirely clear.
In the LAPLACE-TIMI 57 trial of 631 patients, evolocumab reduced Lp(a) by 18 % with low dose and 32 % with high dose at 12 weeks. Patients with higher levels of Lp(a) at baseline had larger absolute reductions but smaller percent reductions in Lp(a) [94]. In the FOURIER (Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects with Elevated Risk) trial, evolocumab significantly reduced Lp(a) by a median of 26.9 %. Patients with higher baseline Lp(a) experienced greater absolute reduction in Lp(a) and derived greater ASCVD reduction from PCSK9 inhibition. Evolocumab reduced risk of CHD death, MI, and urgent revascularization by 23 % in pts with baseline Lp(a) > median vs 7 % in those Lp(a) <median [95]. In the ODYSSEY outcomes trial, which randomized patients on high-intensity statin therapy to alirocumab or placebo, alirocumab reduced Lp(a) by 5 mg/dL and this reduction of Lp(a) independently predicted lower risk of major adverse cardiovascular events (MACE) [96]. In a post-hoc analysis of this trial that evaluated the benefit of adding a PCSK9 inhibitor in patients with LDL-C near 70 mg/dL, alirocumab provided incremental benefit only when Lp(a) was at least mildly elevated [97].
Ranges of Lp(a) lowering with inclisran, a small interfering RNA against PCSK9, are similar. In the ORION-1 (Trial to Evaluate the Effect of ALN-PCSSC Treatment on Low Density Lipoprotein Cholesterol) trial, median Lp(a) levels decreased by 15–25 % from baseline in the single and 2-dose groups but did not reach statistical significance in any group as there was significant interindividual variation. 90 % of participants in the 300-mg two-dose group had lower Lp(a), and the 26 % median reduction at day 180 was similar to other PCSK9i trials [98]. In the ORION-11 primary prevention trial, the placebo-corrected percent reduction in Lp(a) levels from baseline to day 450 was 28.5 % with twice-yearly dosing of inclisran [99].
The exact mechanisms by which PCSK9 inhibition leads to a reduction in Lp(a) is not known but recent evidence suggest that they may enhance Lp(a) clearance and reduce production [100], [101], [102]. PCSK9 inhibitors do not significantly alter inflammation along the hsCRP pathway in patients with elevated Lp(a); in a randomized study of patients receiving evolocumab and median baseline Lp(a) of 200 nmol/L, arterial wall inflammation was not altered and PCSK9 inhibition did not lead to a significant reduction in hsCRP [103]. It is important to note that Lp(a) was only reduced by 14 % in this study, and whether a more significant reduction in Lp(a) would alter the inflammatory response is currently unknown.
7.5. Bempedoic acid
Bempedoic acid, an ATP citrate lyase inhibitor, has not been shown to reduce Lp(a). In a secondary analysis of the CLEAR Harmony trial that randomized patients with ASCVD and/or familial hypercholesterolemia on maximally tolerated statin therapy to bempedoic acid to placebo, placebo-corrected median percent change in Lp(a) from baseline to 12 weeks was only 2.4 % [104].
7.6. Antiplatelet agents
Since Lp(a) may play a role in thrombosis and fibrinolysis [11,12], there have been proposed hypotheses that aspirin may benefit individuals with elevated Lp(a). In the Women's Health Study which compared aspirin 100 mg every other day with placebo in healthy women older than age 45, women with elevated Lp(a) (>44 mg/dL) were 1.47 times more likely to develop CVD events than women with low Lp(a) [105]. Notably, in women carrying the genetic variant associated with high Lp(a), there was a twofold reduction in CVD risk in those randomized to aspirin. This was further validated in an analysis of the ASPREE (ASPirin in Reducing Events in the Elderly) trial in which participants who carried genotypes associated with elevated Lp(a), particularly rs3798220-C carriers, were found to have a reduction in MACE by 11.4 per 1000 person years with aspirin as compared to 1.7 events per 1000 person-years amongst the entire population of participants [106]. In a recent propensity-matched analysis from MESA, aspirin use was associated with a reduction in coronary heart disease events in those with Lp(a) > 50 mg/dL (HR 0.54; 95 %CI 0.32–0.94). Furthermore, those with aspirin use and Lp(a) > 50 mg/dL had similar event rates as participants with Lp(a) ≤ 50 mg/dL, while participants with Lp(a) > 50 mg/dL and no aspirin use had the highest event rates [107]. These studies suggest that aspirin may be a potential therapeutic agent for individuals with elevated Lp(a) for primary prevention, but further studies with larger sample sizes and data from randomized trials are needed.
There are limited investigations regarding other antiplatelet agents and their relationship with Lp(a). Notably, in a recent analysis from the PEGASUS-TIMI 54 trial that randomized patients 1 to 3 years after MI, the effect of prolonged or intensified DAPT was investigated as a function of baseline Lp(a) concentration. Patients with high Lp(a) randomized to placebo had 48 % greater risk of MACE compared with patients with low Lp(a), defined as <200 nmol/L (HR 1.48, p = 0.036). In those randomized to ticagrelor, the risk due to having high Lp(a) was attenuated (HR 1.18, p = 0.27), suggesting that ticagrelor may partially mitigate risk conferred by higher Lp(a), though these results are only hypothesis generating [108]. Clinical trials of standard vs. prolonged DAPT in patients with acute coronary syndrome and elevated Lp(a) undergoing PCI are needed to further assess whether there is improvement in outcomes [109].
8. Lipoprotein apheresis
Lipoprotein apheresis is effective in reducing Lp(a) but burdensome and requires weekly or biweekly sessions ranging from two to four hours in duration [110,111], and it is infrequently used to treat isolated elevation in Lp(a) [110]. In a prospective study in Germany, lipoprotein apheresis reduced Lp(a) by 68.1 % on average [112]. However, in other studies when averaged over time, the reduction in Lp(a) achieved by apheresis is only approximately 30 % [110]. While there are several observational studies comparing cardiovascular event rates in patient's pre-apheresis to post, data from randomized clinical trials are still pending. In the German Lipoprotein Apheresis Registry, there was a 90 % decrease in major adverse coronary events with lipoprotein apheresis which reduced both LDL-C and Lp(a) levels [113]. In another prospective observational multicenter study, mean annual rates for major adverse coronary events declined from 0.41 for 2 years pre-apheresis to 0.09 for 2 years post-apheresis [114]. The ongoing randomized MultiSELECt trial (Effect of Lipoprotein(a) Elimination by Lipoprotein Apheresis on Cardiovascular Outcomes) is investigating the effects of Lp(a) reduction by apheresis on CV outcomes (NCT02791802) [115].
9. Lifestyle modifications
Lifestyle modifications are a core component of ASCVD risk modification; however, most dietary changes display minimal impact on Lp(a) levels. Replacement of saturated fat with carbohydrates or unsaturated fats was studied in the two DELTA trials (Dietary Effects in Lipoproteins and Thrombogenic Activity) which demonstrated opposite changes in Lp(a) and LDL-C in response to dietary SFA replacement, Lp(a) increased by approximately 15 % whereas LDL-C was reduced by 7–11 % as predicted [116], [117], [118]. Furthermore, the Omni Heart randomized, controlled feeding study assessed the effect of the DASH diet (Dietary Approaches to Stop Hypertension) with differing macronutrient compositions, including high carbohydrate diet, high-protein diet, and diet high in unsaturated fat, and found that Lp(a) increased by 8–18 % with all three diets; the high-protein diet led to the largest increase in Lp(a) and the high-unsaturated fat diet led to the least [119]. Studies examining the effect of low-fat, high carbohydrate diets vs. high-fat, low carbohydrate diet have shown increases in Lp(a) with low-fat, high carbohydrate diet [120].
Similar to diet, the role of physical activity has been examined after an early report suggested a possible benefit in healthy young to middle-aged men after an 8-day cross-country skiing regimen [121]. However, when examined further in various cohorts, Lp(a) did not change consistently with exercise [122,123]. Although there may be unique populations (such as diabetic or younger) based on the available data [124], the totality of the data is that modulation of physical activity does not largely impact Lp(a) levels [125]. Overall, while lifestyle modifications continue to be an important pillar of ASCVD prevention, there is no evidence to suggest a role in lowering Lp(a).
10. Therapeutics in clinical trials
10.1. Antisense oligonucleotides
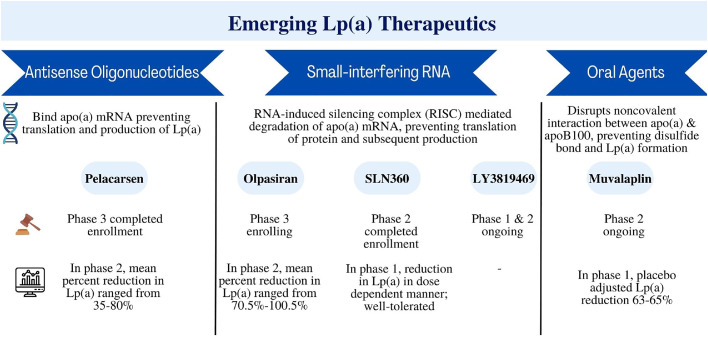
Emerging therapies to lower Lp(a) focus on inhibiting apo(a) synthesis (Fig. 1). Antisense oligonucleotides are a short single-stranded synthetic analogue of nucleic acids which are targeted to bind messenger RNA in a sequence-specific manner via Watson-Crik base-pair interaction, ultimately inhibiting the synthesis of the target protein in a highly specific manner, making it an ideal technology for Lp(a) lowering [126]. In phase I and II studies, anti-sense oligonucleotides, like IONIS-APO(a)-LRx, effectively decreased Lp(a) without serious adverse effects [127,128]. In the phase 1 trial, this pharmaceutical agent decreased plasma Lp(a) by 40–78 %. A more targeted version of this antisense oligonucleotide, pelacarsen, is a N-acetylgalactosamine (GalNac), which is a conjugated version of IONAIS-APO(a)-LRx [129], previously known as AKCEA-APO(a)-L(Rx). The GalNac addition allows for specific targeting to the hepatocyte. Trials have shown that this conjugated version is roughly 30 times more potent than the original version, with mean percentage reduction in Lp(a) of 92.4 % in a phase 1/2 investigation [128]. A randomized controlled trial demonstrated that pelacarsen reduced Lp(a) in a dose dependent manner in patients with established CVD and elevated Lp(a) (>60 mg/dL, 150 nmol/L), with mean percent decreases ranging from 35 to 80 % [130]. At the highest cumulative dose regimen, 98 % of patients achieved an Lp(a) level of lower than 50 mg/dL or 125 nmol/L. Pelacarsen was well-tolerated with the most common adverse events reported as mild injection-site reactions [130].
Fig. 1.
Summary of emerging Lp(a) therapeutics
RNA=ribonucleic acid, mRNA=messenger RNA.
The Lp(a) HORIZON trial (NCT 04023552) is a current phase 3 cardiovascular outcomes trial investigating pelacarsen, with estimated study completion in 2025. The trial enrolled patients with established ASCVD defined as a history of MI, ischemic stroke, or symptomatic peripheral arterial disease who are already on LDL-C lowering therapy and have an Lp(a) > 70 mg/dL (175 nmol/L). Patients were randomized to 80 mg pelacarsen subcutaneously monthly versus placebo and the primary outcome is MACE which consists of cardiovascular death, nonfatal MI, nonfatal stroke, and urgent coronary revascularization. Additionally, there is another planned phase 3 trial of pelacarsen in patients with Lp(a) >125 and mild or moderate calcific aortic stenosis (NCT 05646381).
10.2. Small-interfering RNA
Olpasiran is a GalNac-conjugated small-interfering ribonucleic acid (siRNA) molecule that degrades apo(a) mRNA and prevents translation of the protein [131]. siRNAs function by using the RNA-induced silencing complex (RISC) to cleave target mRNA [131]. Olpasiran was studied in a phase 1 trial that enrolled predominately Japanese participants; mean percent decreases in Lp(a) ranged from 56 % to 99 % with no serious adverse events [132]. The randomized, double-blind, placebo-controlled phase 2 trial, OCEAN(a)-DOSE trial, enrolled patients with established ASCVD and Lp(a) >150 nmol/L and randomized patients to one of four doses of olpasiran – 10 mg every 12 weeks, 75 mg every 12 weeks, 225 mg every 12 weeks, or 225 mg every 24 weeks or matching placebo. The primary endpoint was percent change in Lp(a) concentration from baseline at week 36. The trial demonstrated significant reduction in Lp(a) in a dose dependent manner; a 97.4 % reduction was seen with the 75 mg dose [133,134]. The percent lowering of Lp(a) concentration was consistent across prespecified subgroups including LDL-C concentrations. The phase 3 OCEAN(a)-Outcomes trial is currently enrolling patients with Lp(a) ≥ 200 nmol/L and history of ASCVD [defined as MI and/or coronary revascularization with percutaneous coronary intervention (PCI) and at least 1 additional risk factor (which includes age greater than 65 years, diabetes mellitus, ischemic stroke, PAD, and multivessel PCI)] (NCT 05581303). The primary outcome is time to CHD death, MI, or urgent coronary revascularization.
Another siRNA in development is SLN360, also covalently linked to a GALNAc moiety, thereby leading to selective uptake in hepatocytes [135]. The phase 1 APOLLO trial of 32 participants was well tolerated after a single dose and led to a dose-dependent reduction in Lp(a) concentration persisting up to 150 days [136]. There is another phase 1 trial of SLN360 that has been completed in patients with Lp(a) >125 nmol/L and high ASCVD risk with results pending (NCT 04606602). Furthermore, a phase 2 trial of SLN360 targeting enrollment of 160 participants in this same population is ongoing (NCT 05537571). Lastly, a novel therapeutic siRNA for Lp(a) lowering is LY3819469, now named lepodisiran - a GALNAc-conjugated mixed 2-I-me, 2-fluoro and unmodified dicer siRNA. A phase 1 study has been completed though results are still pending (NCT 04914546). There is also an ongoing phase 2 trial that is expected to be completed by October 2024 (NCT 05565742).
10.3. Oral agents
Muvalaplin is an orally administered medication that inhibits Lp(a) formation by disrupting the noncovalent interaction between apo(a) and apoB100, preventing the disulfide bond and Lp(a) formation. In a phase 1 trial of 114 participants at one site in the Netherlands, muvalaplin was well tolerated. Maximum placebo adjusted Lp(a) reduction was 63–65 % [137].
11. Future directions
As new therapeutic agents for Lp(a) lowering are being investigated, it is crucial to raise awareness amongst healthcare providers of the utility of this biomarker given that Lp(a) testing remains relatively infrequent across the United States [138]. Further work on how to best incorporate Lp(a) into commonly used prediction and risk stratification tools will be important to guide clinicians on therapeutic decision making.
Results of phase 3 cardiovascular outcome trials of Lp(a) lowering agents are eagerly awaited. Given the global burden of cardiovascular disease and the residual CV risk that exists despite control of other risk factors, new therapeutic agents have the potential to be pivotal in CV risk reduction (Fig. 1). A key question is how much Lp(a) lowering is required to decrease CHD outcomes. Data from Mendelian randomization studies suggest that Lp(a) lowering by 65.7 mg/dL is required to attain the same effect on clinical outcomes as a reduction in LDL-C by 38.67 mg/dL – a reduction that has been shown to lower cardiovascular risk by 20–25 % [139]. This is consistent with data from the Copenhagen General population study which estimated that to achieve 20 and 40 % risk reduction in MACE for those with a history of ASCVD, plasma Lp(a) would need to be lowered by 50 mg/dL and 99 mg/dL for 5 years, respectively [140]. Thus, it is likely that large absolute reductions in Lp(a) will be necessary to show significant cardiovascular benefit.
Current considerations for ongoing investigation include the optimal recruitment criteria for enrollment in trials (Table 2). While current trials are focused on high-risk secondary prevention populations, future studies will need to assess the potential role of Lp(a) lowering therapies in high-risk primary prevention populations, and possible patients with lower Lp(a) thresholds than used in HORIZON or OCEAN(a) Outcomes. Ultimately, the results will shed light onto which patients will derive the most benefit with Lp(a) lowering. Another important consideration in Lp(a) lowering trials is to define what threshold of elevated Lp(a) to use for trial eligibility. Challenges include the absence of standardized assays, variable reporting according to mass and molar quantity, and no standardization of conversion factors. The threshold for enrollment in the Lp(a) HORIZON trial (NCT 04023552) is above 70 mg/dL (175 nmol/L) whereas the OCEAN(a)-Outcomes trial (NCT 05581303) requires a threshold of 200 nmol/L. In an observational cohort from the Mass General Brigham Lp(a) Registry resembling OCEAN(a)-Outcomes’ main enrolment criteria, the primary composite outcome occurred more frequently in those with Lp(a) ≥ 200 nmol/L (HR 1.3, 95 % CI 1.09–1.53) [141]. These findings support the threshold of 200nmo/L for identifying patients who have a higher risk of various cardiovascular outcomes. Another noteworthy difference between these two trials is their respective clinical endpoints (Table 2). Namely, OCEAN(a)-Outcomes’ primary outcome is a composite of CHD death, MI, or urgent revascularization and does not include ischemic stroke, an outcome which has demonstrated inconsistent associations with Lp(a) levels [55]
Table 2.
Comparison between ongoing phase 3 cardiovascular outcomes trials.
| HORIZON (Pelacarsen) | OCEAN(a)-Outcomes (Olpasiran) | |
|---|---|---|
| Sponsor (Phase) | Novartis (P3) | Amgen (P3) |
| Study Design | Randomized double-blind, placebo-controlled, multicenter trial | Randomized double-blind, placebo-controlled, multicenter trial |
| Population | Age 18 to 80 years N = 7680 |
Age 18 to 85 years N∼6000 |
| Mechanism | Antisense oligonucleotides (ASO) | Small interfering RNA (siRNA) |
| Intervention | SC injection Q4 weeks | SC injection Q12 weeks |
| Key Inclusion Criteria | Lp(a) ≥ 70 mg/dLCVD, as evidence by either:
|
Lp(a) > 200 nmol/LASCVD, as evidence by either:
|
| Primary Endpoint | Cardiovascular death, nonfatal MI, non-fatal stroke and urgent coronary revascularization requiring hospitalization (∼4.25 years) | CHD death, MI, urgent coronary revascularization |
| Status | Enrollment completed | Enrollment ongoing |
P3=phase 3, SC=subcutaneous, CVD=cardiovascular disease, MI=myocardial infarction, PAD=peripheral arterial disease, PCI=percutaneous coronary intervention.
While current trials are focused on secondary prevention, future trials in primary prevention will be the next frontier but necessarily present unique challenges in terms of enrollment of larger populations and determination of ideal outcomes. Exploration of coronary atherosclerosis imaging – whether by AI based techniques or dedicated cardiac imaging – may be useful for selecting high risk populations who are more likely to benefit from Lp(a) therapies [142].
CRediT authorship contribution statement
Gurleen Kaur: Writing – original draft, Conceptualization. Khaled Abdelrahman: Writing – original draft. Adam N. Berman: Writing – review & editing. David W. Biery: Writing – review & editing, Writing – original draft. Arthur Shiyovich: Writing – review & editing. Daniel Huck: Writing – review & editing. Michael Garshick: Writing – review & editing. Ron Blankstein: Writing – review & editing. Brittany Weber: Writing – review & editing, Writing – original draft, Conceptualization.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
RB reports consulting/advisory fees from Amgen, and Novartis. MG reports consulting fees from BMS, Aepha, Horizon Therapeutics, Kiniksa Pharmaceuticals Corp. BW reports advisory/consulting from Novo Nordisk, BMS, Aepha, Horizon Therapeutics, and Kiniksa Pharmaceuticals.
Acknowledgments
Disclosures
Dr Blankstein receives Research support and consulting for Amgen Inc and Novartis Inc.
Dr. Garshick receives grants from the NIH-NHLBI (K23-HL152013) Pfizer and personal fees from BMS, Agepha, Horizon Therapeutics (now part of Amgen), and Kiniksa Pharmaceuticals outside the submitted work. Dr. Weber reports personal fees from NovoNordisk, BMS, Agepha, Horizon Therapeutics (now part of Amgen), and Kiniksa Pharmaceuticals (all scientific advisory board member roles) outside of the submitted work. Drs. Kaur, Abdelrahman, Berman, Biery, Shiyovich, and Huck have no disclosures to report.
Funding
Dr. Huck receives funding from American Heart Association Career Development Award (grant number 23CDA1037589) Dr. Weber is funded in part by NHLBI K23 HL159276-01, AHA 21CDA851511
References
- 1.Berman A.N., Blankstein R. Current and future role of Lipoprotein(a) in preventive cardiology. Curr Opin Cardiol. 2019;34(5):514–518. doi: 10.1097/HCO.0000000000000661. [DOI] [PubMed] [Google Scholar]
- 2.Varvel S., McConnell J.P., Tsimikas S. Prevalence of elevated Lp(a) mass levels and patient thresholds in 532 359 patients in the United States. Arterioscler Thromb Vasc Biol. 2016;36(11):2239–2245. doi: 10.1161/ATVBAHA.116.308011. [DOI] [PubMed] [Google Scholar]
- 3.Brandt E.J., et al. Lipoprotein(a) levels and association with myocardial infarction and stroke in a nationally representative cross-sectional US cohort. J Clin Lipidol. 2020;14(5):695–706. doi: 10.1016/j.jacl.2020.06.010. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lieb W., et al. Residual cardiovascular risk in individuals on lipid-lowering treatment: quantifying absolute and relative risk in the community. Open Heart. 2018;5(1) doi: 10.1136/openhrt-2017-000722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khera A.V., et al. Lipoprotein(a) concentrations, rosuvastatin therapy, and residual vascular risk: an analysis from the JUPITER Trial (Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin) Circulation. 2014;129(6):635–642. doi: 10.1161/CIRCULATIONAHA.113.004406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berman A.N., Blankstein R. Optimizing dyslipidemia management for the prevention of cardiovascular disease: a focus on risk assessment and therapeutic options. Curr Cardiol Rep. 2019;21(9):110. doi: 10.1007/s11886-019-1175-z. [DOI] [PubMed] [Google Scholar]
- 7.Schmidt K., et al. Structure, function, and genetics of lipoprotein (a) J Lipid Res. 2016;57(8):1339–1359. doi: 10.1194/jlr.R067314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jawi M.M., Frohlich J., Chan S.Y. Lipoprotein(a) the insurgent: a new insight into the structure, function, metabolism, pathogenicity, and medications affecting Lipoprotein(a) molecule. J Lipid. 2020;2020 doi: 10.1155/2020/3491764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kronenberg F. Lipoprotein(a) measurement issues: are we making a mountain out of a molehill? Atherosclerosis. 2022;349:123–135. doi: 10.1016/j.atherosclerosis.2022.04.008. [DOI] [PubMed] [Google Scholar]
- 10.Reyes-Soffer G., et al. Lipoprotein(a): a genetically determined, causal, and prevalent risk factor for atherosclerotic cardiovascular disease: a scientific statement from the American Heart Association. Arterioscler Thromb Vasc Biol. 2022;42(1):e48–e60. doi: 10.1161/ATV.0000000000000147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buechler C., et al. Lipoprotein (a) up-regulates the expression of the plasminogen activator inhibitor 2 in human blood monocytes. Blood. 2001;97(4):981–986. doi: 10.1182/blood.v97.4.981. [DOI] [PubMed] [Google Scholar]
- 12.Caplice N.M., et al. Lipoprotein (a) binds and inactivates tissue factor pathway inhibitor: a novel link between lipoproteins and thrombosis. Blood. 2001;98(10):2980–2987. doi: 10.1182/blood.v98.10.2980. [DOI] [PubMed] [Google Scholar]
- 13.van der Valk F.M., et al. Oxidized phospholipids on Lipoprotein(a) elicit arterial wall inflammation and an inflammatory monocyte response in humans. Circulation. 2016;134(8):611–624. doi: 10.1161/CIRCULATIONAHA.116.020838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kamstrup P.R., et al. Genetically elevated Lipoprotein(a) and increased risk of myocardial infarction. JAMA. 2009;301(22):2331–2339. doi: 10.1001/jama.2009.801. [DOI] [PubMed] [Google Scholar]
- 15.Clarke R., et al. Genetic variants associated with Lp(a) lipoprotein level and coronary disease. N Engl J Med. 2009;361(26):2518–2528. doi: 10.1056/NEJMoa0902604. [DOI] [PubMed] [Google Scholar]
- 16.Kamstrup P.R., et al. Extreme Lipoprotein(a) levels and risk of myocardial infarction in the general population: the Copenhagen City Heart Study. Circulation. 2008;117(2):176–184. doi: 10.1161/CIRCULATIONAHA.107.715698. [DOI] [PubMed] [Google Scholar]
- 17.Waldeyer C., et al. Lipoprotein(a) and the risk of cardiovascular disease in the European population: results from the BiomarCaRE consortium. Eur Heart J. 2017;38(32):2490–2498. doi: 10.1093/eurheartj/ehx166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Willeit P., et al. Baseline and on-statin treatment Lipoprotein(a) levels for prediction of cardiovascular events: individual patient-data meta-analysis of statin outcome trials. Lancet. 2018;392(10155):1311–1320. doi: 10.1016/S0140-6736(18)31652-0. [DOI] [PubMed] [Google Scholar]
- 19.Erqou S., et al. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA. 2009;302(4):412–423. doi: 10.1001/jama.2009.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berman A.N., et al. Atherosclerotic cardiovascular disease risk and elevated Lipoprotein(a) among young adults with myocardial infarction: the Partners YOUNG-MI Registry. Eur J Prev Cardiol. 2021;28(8):e12–e14. doi: 10.1177/2047487320931296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Langsted A., Nordestgaard B.G., Kamstrup P.R. Elevated Lipoprotein(a) and risk of ischemic stroke. J Am Coll Cardiol. 2019;74(1):54–66. doi: 10.1016/j.jacc.2019.03.524. [DOI] [PubMed] [Google Scholar]
- 22.Kamstrup P.R., Tybjærg-Hansen A., Nordestgaard B.G. Elevated Lipoprotein(a) and risk of aortic valve stenosis in the general population. J Am Coll Cardiol. 2014;63(5):470–477. doi: 10.1016/j.jacc.2013.09.038. [DOI] [PubMed] [Google Scholar]
- 23.Welsh P., et al. Lipoprotein(a) and cardiovascular disease: prediction, attributable risk fraction, and estimating benefits from novel interventions. Eur J Prev Cardiol. 2022;28(18):1991–2000. doi: 10.1093/eurjpc/zwaa063. [DOI] [PubMed] [Google Scholar]
- 24.Willeit P., et al. Discrimination and net reclassification of cardiovascular risk with Lipoprotein(a): prospective 15-year outcomes in the Bruneck Study. J Am Coll Cardiol. 2014;64(9):851–860. doi: 10.1016/j.jacc.2014.03.061. [DOI] [PubMed] [Google Scholar]
- 25.Afshar M., et al. Risks of incident cardiovascular disease associated with concomitant elevations in Lipoprotein(a) and low-density lipoprotein cholesterol-The Framingham Heart Study. J Am Heart Assoc. 2020;9(18) doi: 10.1161/JAHA.119.014711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grundy Scott M., et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation. 2019;139(25):e1082–e1143. doi: 10.1161/CIR.0000000000000625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rikhi R., et al. Relationship of low-density lipoprotein-cholesterol and Lipoprotein(a) to cardiovascular risk: the Multi-Ethnic Study of Atherosclerosis (MESA) Atherosclerosis. 2022;363:102–108. doi: 10.1016/j.atherosclerosis.2022.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nestel P.J., et al. Plasma Lipoprotein(a) concentration predicts future coronary and cardiovascular events in patients with stable coronary heart disease. Arterioscler Thromb Vasc Biol. 2013;33(12):2902–2908. doi: 10.1161/ATVBAHA.113.302479. [DOI] [PubMed] [Google Scholar]
- 29.Mehta A., et al. Independent association of Lipoprotein(a) and coronary artery calcification with atherosclerotic cardiovascular risk. J Am Coll Cardiol. 2022;79(8):757–768. doi: 10.1016/j.jacc.2021.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pechlivanis S., et al. Association between Lipoprotein(a) (Lp(a)) levels and Lp(a) genetic variants with coronary artery calcification. BMC Med Genet. 2020;21(1):62. doi: 10.1186/s12881-020-01003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jackson C.L., et al. Lipoprotein(a) and coronary artery calcium in comparison with other lipid biomarkers: the multi-ethnic study of atherosclerosis. J Clin Lipidol. 2023;17(4):538–548. doi: 10.1016/j.jacl.2023.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaiser Y., et al. Association of Lipoprotein(a) with atherosclerotic plaque progression. J Am Coll Cardiol. 2022;79(3):223–233. doi: 10.1016/j.jacc.2021.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilson D.P., et al. Use of Lipoprotein(a) in clinical practice: a biomarker whose time has come. A scientific statement from the National Lipid Association. J Clin Lipidol. 2019;13(3):374–392. doi: 10.1016/j.jacl.2019.04.010. [DOI] [PubMed] [Google Scholar]
- 34.Mach F., et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41(1):111–188. doi: 10.1093/eurheartj/ehz455. [DOI] [PubMed] [Google Scholar]
- 35.Cegla J., et al. HEART UK consensus statement on Lipoprotein(a): a call to action. Atherosclerosis. 2019;291:62–70. [Google Scholar]
- 36.Pearson G.J., et al. 2021 Canadian Cardiovascular Society guidelines for the management of dyslipidemia for the prevention of cardiovascular disease in adults. Can J Cardiol. 2021;37(8):1129–1150. doi: 10.1016/j.cjca.2021.03.016. [DOI] [PubMed] [Google Scholar]
- 37.Coffey S., Cox B., Williams M.J. The prevalence, incidence, progression, and risks of aortic valve sclerosis: a systematic review and meta-analysis. J Am Coll Cardiol. 2014;63(25):2852–2861. doi: 10.1016/j.jacc.2014.04.018. Pt. [DOI] [PubMed] [Google Scholar]
- 38.Lindman B.R., et al. Calcific aortic stenosis. Nat Rev Dis Prim. 2016;2:16006. doi: 10.1038/nrdp.2016.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goody P.R., et al. Aortic valve stenosis: from basic mechanisms to novel therapeutic targets. Arterioscler Thromb Vasc Biol. 2020;40(4):885–900. doi: 10.1161/ATVBAHA.119.313067. [DOI] [PubMed] [Google Scholar]
- 40.Hsieh G., et al. The current landscape of Lipoprotein(a) in calcific aortic valvular disease. Curr Opin Cardiol. 2021;36(5):542–548. doi: 10.1097/HCO.0000000000000901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zheng K.H., et al. Lipoprotein(a) and oxidized phospholipids promote valve calcification in patients with aortic stenosis. J Am Coll Cardiol. 2019;73(17):2150–2162. doi: 10.1016/j.jacc.2019.01.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nsaibia M.J., et al. Implication of lipids in calcified aortic valve pathogenesis: why did statins fail? J Clin Med. 2022;11(12) doi: 10.3390/jcm11123331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nsaibia M.J., et al. Autotaxin interacts with Lipoprotein(a) and oxidized phospholipids in predicting the risk of calcific aortic valve stenosis in patients with coronary artery disease. J Intern Med. 2016;280(5):509–517. doi: 10.1111/joim.12519. [DOI] [PubMed] [Google Scholar]
- 44.Thanassoulis G., et al. Genetic associations with valvular calcification and aortic stenosis. N Engl J Med. 2013;368(6):503–512. doi: 10.1056/NEJMoa1109034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arsenault B.J., et al. Lipoprotein(a) levels, genotype, and incident aortic valve stenosis: a prospective Mendelian randomization study and replication in a case-control cohort. Circ Cardiovasc Genet. 2014;7(3):304–310. doi: 10.1161/CIRCGENETICS.113.000400. [DOI] [PubMed] [Google Scholar]
- 46.Guddeti R.R., et al. Lipoprotein(a) and calcific aortic valve stenosis: a systematic review. Prog Cardiovasc Dis. 2020;63(4):496–502. doi: 10.1016/j.pcad.2020.06.002. [DOI] [PubMed] [Google Scholar]
- 47.Capoulade R., et al. Oxidized phospholipids, Lipoprotein(a), and progression of calcific aortic valve stenosis. J Am Coll Cardiol. 2015;66(11):1236–1246. doi: 10.1016/j.jacc.2015.07.020. [DOI] [PubMed] [Google Scholar]
- 48.Bergmark B.A., et al. An exploratory analysis of proprotein convertase Subtilisin/Kexin Type 9 inhibition and aortic stenosis in the Fourier trial. JAMA Cardiol. 2020;5(6):709–713. doi: 10.1001/jamacardio.2020.0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kaiser Y., et al. Lipoprotein(a) is associated with the onset but not the progression of aortic valve calcification. Eur Heart J. 2022;43(39):3960–3967. doi: 10.1093/eurheartj/ehac377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cowell S.J., et al. A randomized trial of intensive lipid-lowering therapy in calcific aortic stenosis. N Engl J Med. 2005;352(23):2389–2397. doi: 10.1056/NEJMoa043876. [DOI] [PubMed] [Google Scholar]
- 51.Nguyen T.T., et al. Predictive value of electrophoretically detected Lipoprotein(a) for coronary heart disease and cerebrovascular disease in a community-based cohort of 9936 men and women. Circulation. 1997;96(5):1390–1397. doi: 10.1161/01.cir.96.5.1390. [DOI] [PubMed] [Google Scholar]
- 52.Ridker P.M., Stampfer M.J., Hennekens C.H. Plasma concentration of Lipoprotein(a) and the risk of future stroke. JAMA. 1995;273(16):1269–1273. [PubMed] [Google Scholar]
- 53.Ariyo A.A., Thach C., Tracy R. Lp(a) lipoprotein, vascular disease, and mortality in the elderly. N Engl J Med. 2003;349(22):2108–2115. doi: 10.1056/NEJMoa001066. [DOI] [PubMed] [Google Scholar]
- 54.Ohira T., et al. Lipoprotein(a) and incident ischemic stroke: the Atherosclerosis Risk in Communities (ARIC) study. Stroke. 2006;37(6):1407–1412. doi: 10.1161/01.STR.0000222666.21482.b6. [DOI] [PubMed] [Google Scholar]
- 55.Arora P., et al. Lipoprotein(a) and Risk of Ischemic Stroke in the REGARDS Study. Arterioscler Thromb Vasc Biol. 2019;39(4):810–818. doi: 10.1161/ATVBAHA.118.311857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aboyans V., et al. Risk factors for progression of peripheral arterial disease in large and small vessels. Circulation. 2006;113(22):2623–2629. doi: 10.1161/CIRCULATIONAHA.105.608679. [DOI] [PubMed] [Google Scholar]
- 57.Dieplinger B., et al. Increased serum Lipoprotein(a) concentrations and low molecular weight phenotypes of apoLipoprotein(a) are associated with symptomatic peripheral arterial disease. Clin Chem. 2007;53(7):1298–1305. doi: 10.1373/clinchem.2007.088013. [DOI] [PubMed] [Google Scholar]
- 58.Gurdasani D., et al. Lipoprotein(a) and risk of coronary, cerebrovascular, and peripheral artery disease: the EPIC-Norfolk prospective population study. Arterioscler Thromb Vasc Biol. 2012;32(12):3058–3065. doi: 10.1161/ATVBAHA.112.255521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ridker P.M., Stampfer M.J., Rifai N. Novel risk factors for systemic atherosclerosis: a comparison of C-reactive protein, fibrinogen, homocysteine, Lipoprotein(a), and standard cholesterol screening as predictors of peripheral arterial disease. JAMA. 2001;285(19):2481–2485. doi: 10.1001/jama.285.19.2481. [DOI] [PubMed] [Google Scholar]
- 60.Golledge J., et al. Association of serum Lipoprotein (a) with the requirement for a peripheral artery operation and the incidence of major adverse cardiovascular events in people with peripheral artery disease. J Am Heart Assoc. 2020;9(6) doi: 10.1161/JAHA.119.015355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Guédon A.F., et al. Association of Lipoprotein(a) levels with incidence of major adverse limb events. JAMA Netw Open. 2022;5(12) doi: 10.1001/jamanetworkopen.2022.45720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Howard B.V., et al. Concentrations of Lp(a) in black and white young adults: relations to risk factors for cardiovascular disease. Ann Epidemiol. 1994;4(5):341–350. doi: 10.1016/1047-2797(94)90067-1. [DOI] [PubMed] [Google Scholar]
- 63.Lee S.R., et al. LPA gene, ethnicity, and cardiovascular events. Circulation. 2017;135(3):251–263. doi: 10.1161/CIRCULATIONAHA.116.024611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Virani S.S., et al. Associations between Lipoprotein(a) levels and cardiovascular outcomes in black and white subjects: the Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2012;125(2):241–249. doi: 10.1161/CIRCULATIONAHA.111.045120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Makshood M., et al. Lipoprotein (a) and aortic valve calcium in South Asians compared to other race/ethnic groups. Atherosclerosis. 2020;313:14–19. doi: 10.1016/j.atherosclerosis.2020.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Paré G., et al. Lipoprotein(a) Levels and the risk of myocardial infarction among 7 ethnic groups. Circulation. 2019;139(12):1472–1482. doi: 10.1161/CIRCULATIONAHA.118.034311. [DOI] [PubMed] [Google Scholar]
- 67.Rubin J., et al. Apo[a] size and PNR explain African American-Caucasian differences in allele-specific apo[a] levels for small but not large apo[a] J Lipid Res. 2006;47(5):982–989. doi: 10.1194/jlr.M500359-JLR200. [DOI] [PubMed] [Google Scholar]
- 68.Rubin J., et al. The apoLipoprotein(a) gene: linkage disequilibria at three loci differs in African Americans and Caucasians. Atherosclerosis. 2008;201(1):138–147. doi: 10.1016/j.atherosclerosis.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Patel A.P., et al. Lp(a) (Lipoprotein[a]) concentrations and incident atherosclerotic cardiovascular disease: new insights from a large National Biobank. Arterioscler Thromb Vasc Biol. 2021;41(1):465–474. doi: 10.1161/ATVBAHA.120.315291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Guan W., et al. Race is a key variable in assigning Lipoprotein(a) cutoff values for coronary heart disease risk assessment: the Multi-Ethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol. 2015;35(4):996–1001. doi: 10.1161/ATVBAHA.114.304785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cao J., et al. Lipoprotein(a) levels are associated with subclinical calcific aortic valve disease in white and black individuals: the multi-ethnic study of atherosclerosis. Arterioscler Thromb Vasc Biol. 2016;36(5):1003–1009. doi: 10.1161/ATVBAHA.115.306683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cook N.R., Mora S., Ridker P.M. Lipoprotein(a) and cardiovascular risk prediction among women. J Am Coll Cardiol. 2018;72(3):287–296. doi: 10.1016/j.jacc.2018.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Suk Danik J., et al. Lipoprotein(a), hormone replacement therapy, and risk of future cardiovascular events. J Am Coll Cardiol. 2008;52(2):124–131. doi: 10.1016/j.jacc.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sacks F.M., McPherson R., Walsh B.W. Effect of postmenopausal estrogen replacement on plasma Lp(a) lipoprotein concentrations. Arch Intern Med. 1994;154(10):1106–1110. [PubMed] [Google Scholar]
- 75.Honigberg M.C., Trinder M., Natarajan P. Lipoprotein(a), menopausal hormone therapy, and risk of coronary heart disease in postmenopausal individuals. JAMA Cardiol. 2022;7(5):565–568. doi: 10.1001/jamacardio.2022.0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Anagnostis P., et al. The effect of menopause on lipoprotein (a) concentrations: a systematic review and meta-analysis. Maturitas. 2023;167:39–45. doi: 10.1016/j.maturitas.2022.09.012. [DOI] [PubMed] [Google Scholar]
- 77.Simony S.B., et al. Sex differences of Lipoprotein(a) levels and associated risk of morbidity and mortality by age: the Copenhagen General Population Study. Atherosclerosis. 2022;355:76–82. doi: 10.1016/j.atherosclerosis.2022.06.1023. [DOI] [PubMed] [Google Scholar]
- 78.Puri R., et al. Effect of C-reactive protein on Lipoprotein(a)-associated cardiovascular risk in optimally treated patients with high-risk vascular disease: a prespecified secondary analysis of the ACCELERATE Trial. JAMA Cardiol. 2020;5(10):1136–1143. doi: 10.1001/jamacardio.2020.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang W., et al. High-sensitivity c-reactive protein modifies the cardiovascular risk of Lipoprotein(a): multi-ethnic study of atherosclerosis. J Am Coll Cardiol. 2021;78(11):1083–1094. doi: 10.1016/j.jacc.2021.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Naka K.K., et al. Interleukin-1 genotypes modulate the long-term effect of Lipoprotein(a) on cardiovascular events: the Ioannina Study. J Clin Lipidol. 2018;12(2):338–347. doi: 10.1016/j.jacl.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 81.Berthold H.K., et al. Association between the interleukin-6 promoter polymorphism -174 G/C and serum Lipoprotein(a) concentrations in humans. PLoS ONE. 2011;6(9):e24719. doi: 10.1371/journal.pone.0024719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Müller N., et al. IL-6 blockade by monoclonal antibodies inhibits apolipoprotein (a) expression and lipoprotein (a) synthesis in humans. J Lipid Res. 2015;56(5):1034–1042. doi: 10.1194/jlr.P052209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.García-Gómez C., et al. Lipoprotein(a) concentrations in rheumatoid arthritis on biologic therapy: results from the CARdiovascular in rheuMAtology study project. J Clin Lipidol. 2017;11(3):749–756. doi: 10.1016/j.jacl.2017.02.018. e3. [DOI] [PubMed] [Google Scholar]
- 84.Lee J.S., et al. Remodeling of plasma lipoproteins in patients with rheumatoid arthritis: interleukin-6 receptor-alpha inhibition with tocilizumab. Proteomics Clin Appl. 2016;10(2):183–194. doi: 10.1002/prca.201500036. [DOI] [PubMed] [Google Scholar]
- 85.Ridker P.M., et al. IL-6 inhibition with ziltivekimab in patients at high atherosclerotic risk (RESCUE): a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet. 2021;397(10289):2060–2069. doi: 10.1016/S0140-6736(21)00520-1. [DOI] [PubMed] [Google Scholar]
- 86.Takagi H., Umemoto T. Atorvastatin decreases Lipoprotein(a): a meta-analysis of randomized trials. Int J Cardiol. 2012;154(2):183–186. doi: 10.1016/j.ijcard.2011.09.060. [DOI] [PubMed] [Google Scholar]
- 87.Tsimikas S., et al. Statin therapy increases Lipoprotein(a) levels. Eur Heart J. 2020;41(24):2275–2284. doi: 10.1093/eurheartj/ehz310. [DOI] [PubMed] [Google Scholar]
- 88.Wang X., et al. Effect of different types and dosages of statins on plasma Lipoprotein(a) levels: a network meta-analysis. Pharmacol Res. 2021;163 doi: 10.1016/j.phrs.2020.105275. [DOI] [PubMed] [Google Scholar]
- 89.de Boer L.M., et al. Statin therapy and Lipoprotein(a) levels: a systematic review and meta-analysis. Eur J Prev Cardiol. 2022;29(5):779–792. doi: 10.1093/eurjpc/zwab171. [DOI] [PubMed] [Google Scholar]
- 90.Sahebkar A., et al. Impact of ezetimibe on plasma Lipoprotein(a) concentrations as monotherapy or in combination with statins: a systematic review and meta-analysis of randomized controlled trials. Sci Rep. 2018;8(1):17887. doi: 10.1038/s41598-018-36204-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chennamsetty I., et al. Nicotinic acid inhibits hepatic APOA gene expression: studies in humans and in transgenic mice. J Lipid Res. 2012;53(11):2405–2412. doi: 10.1194/jlr.M029769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Landray M.J., et al. Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med. 2014;371(3):203–212. doi: 10.1056/NEJMoa1300955. [DOI] [PubMed] [Google Scholar]
- 93.Farmakis I., et al. Lipoprotein(a) reduction with proprotein convertase Subtilisin/Kexin type 9 inhibitors: a systematic review and meta-analysis. J Cardiovasc Pharmacol. 2021;77(3):397–407. doi: 10.1097/FJC.0000000000000963. [DOI] [PubMed] [Google Scholar]
- 94.Desai N.R., et al. AMG145, a monoclonal antibody against proprotein convertase subtilisin kexin type 9, significantly reduces Lipoprotein(a) in hypercholesterolemic patients receiving statin therapy: an analysis from the LDL-C Assessment with Proprotein Convertase Subtilisin Kexin Type 9 Monoclonal Antibody Inhibition Combined with Statin Therapy (LAPLACE)-Thrombolysis in Myocardial Infarction (TIMI) 57 trial. Circulation. 2013;128(9):962–969. doi: 10.1161/CIRCULATIONAHA.113.001969. [DOI] [PubMed] [Google Scholar]
- 95.O'Donoghue M.L., et al. Lipoprotein(a), PCSK9 inhibition, and cardiovascular risk. Circulation. 2019;139(12):1483–1492. doi: 10.1161/CIRCULATIONAHA.118.037184. [DOI] [PubMed] [Google Scholar]
- 96.Bittner V.A., et al. Effect of alirocumab on Lipoprotein(a) and cardiovascular risk after acute coronary syndrome. J Am Coll Cardiol. 2020;75(2):133–144. doi: 10.1016/j.jacc.2019.10.057. [DOI] [PubMed] [Google Scholar]
- 97.Schwartz G.G., et al. Lipoprotein(a) and benefit of PCSK9 inhibition in patients with nominally controlled LDL cholesterol. J Am Coll Cardiol. 2021;78(5):421–433. doi: 10.1016/j.jacc.2021.04.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ray K.K., et al. Effect of an siRNA therapeutic targeting PCSK9 on atherogenic lipoproteins: prespecified secondary end points in ORION 1. Circulation. 2018;138(13):1304–1316. doi: 10.1161/CIRCULATIONAHA.118.034710. [DOI] [PubMed] [Google Scholar]
- 99.Ray K.K., et al. Effect of inclisiran on lipids in primary prevention: the ORION-11 trial. Eur Heart J. 2022;43(48):5047–5057. doi: 10.1093/eurheartj/ehac615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Reyes-Soffer G., et al. Effects of PCSK9 inhibition with alirocumab on lipoprotein metabolism in healthy humans. Circulation. 2017;135(4):352–362. doi: 10.1161/CIRCULATIONAHA.116.025253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Villard E.F., et al. PCSK9 modulates the secretion but not the cellular uptake of Lipoprotein(a) ex vivo: an effect blunted by alirocumab. JACC Basic Transl Sci. 2016;1(6):419–427. doi: 10.1016/j.jacbts.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Watts G.F., et al. Controlled study of the effect of proprotein convertase subtilisin-kexin type 9 inhibition with evolocumab on Lipoprotein(a) particle kinetics. Eur Heart J. 2018;39(27):2577–2585. doi: 10.1093/eurheartj/ehy122. [DOI] [PubMed] [Google Scholar]
- 103.Stiekema L.C.A., et al. Persistent arterial wall inflammation in patients with elevated Lipoprotein(a) despite strong low-density lipoprotein cholesterol reduction by proprotein convertase subtilisin/kexin type 9 antibody treatment. Eur Heart J. 2019;40(33):2775–2781. doi: 10.1093/eurheartj/ehy862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ridker P.M., et al. Effects of bempedoic acid on CRP, IL-6, fibrinogen and Lipoprotein(a) in patients with residual inflammatory risk: a secondary analysis of the CLEAR harmony trial. J Clin Lipidol. 2023;17(2):297–302. doi: 10.1016/j.jacl.2023.02.002. [DOI] [PubMed] [Google Scholar]
- 105.Chasman D.I., et al. Polymorphism in the apoLipoprotein(a) gene, plasma Lipoprotein(a), cardiovascular disease, and low-dose aspirin therapy. Atherosclerosis. 2009;203(2):371–376. doi: 10.1016/j.atherosclerosis.2008.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lacaze P., et al. Aspirin for primary prevention of cardiovascular events in relation to Lipoprotein(a) genotypes. J Am Coll Cardiol. 2022;80(14):1287–1298. doi: 10.1016/j.jacc.2022.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bhatia H.S., et al. Aspirin and cardiovascular risk in individuals with elevated Lipoprotein(a): the multi-ethnic study of atherosclerosis. J Am Heart Assoc. 2024 doi: 10.1161/JAHA.123.033562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Patel S B.M., Morrow D., Palazzolo M., Jarolim P., Gabriel Steg P., Bhatt D., Storey R., Sabatine M., O'Donoghue M. Lipoprotein(a) and benefit of antiplatelet therapy: insights from the PEGASUS-TIMI 54 trial. JACC Adv. 2023;9 doi: 10.1016/j.jacadv.2023.100675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bhatia H.S., et al. Lipoprotein(a), platelet function and cardiovascular disease. Nat Rev Cardiol. 2023 doi: 10.1038/s41569-023-00947-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Waldmann E., Parhofer K.G. Lipoprotein apheresis to treat elevated Lipoprotein (a) J Lipid Res. 2016;57(10):1751–1757. doi: 10.1194/jlr.R056549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Moriarty P.M., Gray J.V., Gorby L.K. Lipoprotein apheresis for Lipoprotein(a) and cardiovascular disease. J Clin Lipidol. 2019;13(6):894–900. doi: 10.1016/j.jacl.2019.09.010. [DOI] [PubMed] [Google Scholar]
- 112.Roeseler E., et al. Lipoprotein apheresis for Lipoprotein(a)-associated cardiovascular disease: prospective 5 years of follow-up and Apolipoprotein(a) characterization. Arterioscler Thromb Vasc Biol. 2016;36(9):2019–2027. doi: 10.1161/ATVBAHA.116.307983. [DOI] [PubMed] [Google Scholar]
- 113.Schettler V.J., et al. Impact of the German lipoprotein apheresis registry (DLAR) on therapeutic options to reduce increased Lp(a) levels. Clin Res Cardiol Suppl. 2015;10(Suppl 1):14–20. doi: 10.1007/s11789-015-0073-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Leebmann J., et al. Lipoprotein apheresis in patients with maximally tolerated lipid-lowering therapy, Lipoprotein(a)-hyperlipoproteinemia, and progressive cardiovascular disease: prospective observational multicenter study. Circulation. 2013;128(24):2567–2576. doi: 10.1161/CIRCULATIONAHA.113.002432. [DOI] [PubMed] [Google Scholar]
- 115.Hohenstein B., et al. Rationale and design of MultiSELECt: a European Multicenter Study on the effect of Lipoprotein(a) elimination by lipoprotein apheresis on cardiovascular outcomes. Atheroscler Suppl. 2017;30:180–186. doi: 10.1016/j.atherosclerosissup.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 116.Enkhmaa B., et al. Diet and Lp(a): does dietary change modify residual cardiovascular risk conferred by Lp(a)? Nutrients. 2020;(7):12. doi: 10.3390/nu12072024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ginsberg H.N., et al. Effects of reducing dietary saturated fatty acids on plasma lipids and lipoproteins in healthy subjects: the DELTA Study, protocol 1. Arterioscler Thromb Vasc Biol. 1998;18(3):441–449. doi: 10.1161/01.atv.18.3.441. [DOI] [PubMed] [Google Scholar]
- 118.Berglund L., et al. Comparison of monounsaturated fat with carbohydrates as a replacement for saturated fat in subjects with a high metabolic risk profile: studies in the fasting and postprandial states. Am J Clin Nutr. 2007;86(6):1611–1620. doi: 10.1093/ajcn/86.5.1611. [DOI] [PubMed] [Google Scholar]
- 119.Haring B., et al. Healthy dietary interventions and lipoprotein (a) plasma levels: results from the Omni Heart Trial. PLoS ONE. 2014;9(12) doi: 10.1371/journal.pone.0114859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Faghihnia N., et al. Changes in Lipoprotein(a), oxidized phospholipids, and LDL subclasses with a low-fat high-carbohydrate diet. J Lipid Res. 2010;51(11):3324–3330. doi: 10.1194/jlr.M005769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hellsten G., et al. Lipids and endurance physical activity. Atherosclerosis. 1989;75(1):93–94. doi: 10.1016/0021-9150(89)90211-6. [DOI] [PubMed] [Google Scholar]
- 122.MacAuley D., et al. Physical activity, lipids, apolipoproteins, and Lp(a) in the Northern Ireland Health and Activity Survey. Med Sci Sports Exerc. 1996;28(6):720–736. doi: 10.1097/00005768-199606000-00011. [DOI] [PubMed] [Google Scholar]
- 123.Sponder M., et al. Effect of long-term physical activity on PCSK9, high- and low-density lipoprotein cholesterol, and Lipoprotein(a) levels: a prospective observational trial. Pol Arch Intern Med. 2017;127(7–8):506–511. doi: 10.20452/pamw.4044. [DOI] [PubMed] [Google Scholar]
- 124.Hubinger L., et al. Acute effects of treadmill running on Lipoprotein(a) levels in males and females. Med Sci Sports Exerc. 1997;29(4):436–442. doi: 10.1097/00005768-199704000-00003. [DOI] [PubMed] [Google Scholar]
- 125.Enkhmaa B., Berglund L. Non-genetic influences on Lipoprotein(a) concentrations. Atherosclerosis. 2022;349:53–62. doi: 10.1016/j.atherosclerosis.2022.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Visser M.E., et al. Antisense oligonucleotides for the treatment of dyslipidaemia. Eur Heart J. 2012;33(12):1451–1458. doi: 10.1093/eurheartj/ehs084. [DOI] [PubMed] [Google Scholar]
- 127.Tsimikas S., et al. Antisense therapy targeting apoLipoprotein(a): a randomised, double-blind, placebo-controlled phase 1 study. Lancet. 2015;386(10002):1472–1483. doi: 10.1016/S0140-6736(15)61252-1. [DOI] [PubMed] [Google Scholar]
- 128.Viney N.J., et al. Antisense oligonucleotides targeting apoLipoprotein(a) in people with raised Lipoprotein(a): two randomised, double-blind, placebo-controlled, dose-ranging trials. Lancet. 2016;388(10057):2239–2253. doi: 10.1016/S0140-6736(16)31009-1. [DOI] [PubMed] [Google Scholar]
- 129.Karwatowska-Prokopczuk E., et al. Efficacy and safety of pelacarsen in lowering Lp(a) in healthy Japanese subjects. J Clin Lipidol. 2023;17(1):181–188. doi: 10.1016/j.jacl.2022.12.001. [DOI] [PubMed] [Google Scholar]
- 130.Tsimikas S., et al. Lipoprotein(a) reduction in persons with cardiovascular disease. N Engl J Med. 2020;382(3):244–255. doi: 10.1056/NEJMoa1905239. [DOI] [PubMed] [Google Scholar]
- 131.Koren M.J., et al. Preclinical development and phase 1 trial of a novel siRNA targeting Lipoprotein(a) Nat Med. 2022;28(1):96–103. doi: 10.1038/s41591-021-01634-w. [DOI] [PubMed] [Google Scholar]
- 132.Sohn W., et al. Pharmacokinetics, pharmacodynamics, and tolerability of olpasiran in healthy Japanese and non-Japanese participants: results from a phase I, single-dose, open-label Study. Clin Ther. 2022;44(9):1237–1247. doi: 10.1016/j.clinthera.2022.07.008. [DOI] [PubMed] [Google Scholar]
- 133.O'Donoghue M.L., et al. Small interfering RNA to reduce Lipoprotein(a) in cardiovascular disease. N Engl J Med. 2022;387(20):1855–1864. doi: 10.1056/NEJMoa2211023. [DOI] [PubMed] [Google Scholar]
- 134.O'Donoghue M.L., et al. Study design and rationale for the olpasiran trials of cardiovascular events and lipoproteiN(a) reduction-DOSE finding study (OCEAN(a)-DOSE) Am Heart J. 2022;251:61–69. doi: 10.1016/j.ahj.2022.05.004. [DOI] [PubMed] [Google Scholar]
- 135.Rider D.A., et al. Pre-clinical assessment of SLN360, a novel siRNA targeting LPA, developed to address elevated lipoprotein (a) in cardiovascular disease. Atherosclerosis. 2022;349:240–247. doi: 10.1016/j.atherosclerosis.2022.03.029. [DOI] [PubMed] [Google Scholar]
- 136.Nissen S.E., et al. Single ascending dose study of a short interfering RNA targeting Lipoprotein(a) production in individuals with elevated plasma Lipoprotein(a) levels. JAMA. 2022;327(17):1679–1687. doi: 10.1001/jama.2022.5050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Nicholls S.J., et al. Muvalaplin, an oral small molecule inhibitor of Lipoprotein(a) formation: a randomized clinical trial. JAMA. 2023;330(11):1042–1053. doi: 10.1001/jama.2023.16503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kelsey M.D., et al. Contemporary patterns of Lipoprotein(a) testing and associated clinical care and outcomes. Am J Prev Cardiol. 2023;14 doi: 10.1016/j.ajpc.2023.100478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lamina C., Kronenberg F. Estimation of the required Lipoprotein(a)-lowering therapeutic effect size for reduction in coronary heart disease outcomes: a Mendelian randomization analysis. JAMA Cardiol. 2019;4(6):575–579. doi: 10.1001/jamacardio.2019.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Madsen C.M., et al. Lipoprotein(a)-lowering by 50 mg/dL (105 nmol/L) may be needed to reduce cardiovascular disease 20 % in secondary prevention: a population-based study. Arterioscler Thromb Vasc Biol. 2020;40(1):255–266. doi: 10.1161/ATVBAHA.119.312951. [DOI] [PubMed] [Google Scholar]
- 141.Shiyovich A., et al. Cardiovascular outcomes in patients with coronary artery disease and elevated Lipoprotein(a): implications for the OCEAN(a)-outcomes trial population. Eur Heart J Open. 2023;3(4):oead077. doi: 10.1093/ehjopen/oead077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Malick W.A., et al. Clinical trial design for Lipoprotein(a)-lowering therapies: JACC focus seminar 2/3. J Am Coll Cardiol. 2023;81(16):1633–1645. doi: 10.1016/j.jacc.2023.02.033. [DOI] [PubMed] [Google Scholar]