Abstract
Large retrorectal tumors are rare and often a diagnostic and surgical challenge due to their anatomical location. We report the case of a 55-year-old patient with weight loss and changed bowel habits, where digital rectal examination revealed a retrorectal mass raising suspicion of a tumor. Magnetic resonance imaging (MRI) and computed tomography (CT) showed a large retrorectal tumor and histopathology after surgical resection showed undifferentiated spindle cell sarcoma. This tumor type has not been previously reported as the etiology of large retrorectal tumors. We discuss the implications of diagnostic imaging, especially MRI, in the approach to diagnosis and surgical treatment of retrorectal tumors with reference to the scientific literature and previously reported cases of retrorectal tumors.
Keywords: Retrorectal tumor, Presacral tumor, Sarcoma, MRI, CT, Colorectal cancer
Introduction
Retrorectal tumors are very rare and have been reported to account for 1 out of every 40,000 hospital admissions [1]. Most of the tumors are benign and they are classified based on their origin into congenital, neurogenic, osseous, or miscellaneous tumors [1,2]. They are often asymptomatic and diagnosed incidentally on diagnostic imaging performed for other purposes or by digital rectal examination [3]. In symptomatic cases, the most common symptoms are related to mass effect and include sacral pain, constipation, lower-back pain, and neurological symptoms such as fecal and urine incontinence. Recurrent anal fistula and perirectal abscesses have also been reported as the main symptoms of retrorectal masses [2,3].
Magnetic resonance imagining (MRI) is the modality of choice for the diagnosis and evaluation of retrorectal masses. MRI is superior to computed tomography (CT) and endorectal ultrasound (ERUS) in identifying and discriminating the anatomical planes and spaces crucial to selecting the surgical management of the tumor and in some cases presurgical chemoradiation therapy [2]. Additionally, invasive growth is better evaluated on MRI compared to CT and ERUS [2].
Presurgical biopsy is generally not recommended, mainly because of the difficult access to the area and high risk of complications [1,2]. Surgical resection is the preferred therapy for retrorectal tumors [4], but the surgical approach is debated due to their infrequency and limited experience in the clinical setting, but also because of limited access when using an abdominopelvic approach and poor vascular control with a posterior approach, contributing to a high risk of intra- and postoperative complications [1,5].
In this article we report the case of a very rare, malignant etiology of a large retrorectal tumor. Sarcomas are rare malignant tumors originating from mesenchymal tissue. Spindle cell sarcoma comprises a very rare subgroup of a larger group classified as undifferentiated soft-tissue sarcomas, and they are characterized by their spindle shaped appearance on microscopical examination [6].
Case description
A 55-year-old male was referred to a colonoscopy at an outpatient endoscopic department at the local hospital due to newly diagnosed anemia, borborygmi, and problems with passing stool for more than 2 years. He reported a 7 kg weight loss over an unknown period. The patient had diabetes mellitus type 2 for which he was treated with metformin. His weight was normal with a BMI of 23.8, and he had no history of tobacco use or previous known cancers. Initial routine laboratory tests were unremarkable except for mild iron deficiency anemia.
Digital rectal examination on the day of endoscopic examination found a large, firm, and solid mass compressing the rectum posteriorly, raising suspicion of a retrorectal tumor. The colonoscopy was not completed as the patient's colon was not sufficiently cleaned out for the procedure. Therefore, the patient was referred to a rectal MRI and CT thorax-abdomen with intravenous contrast.
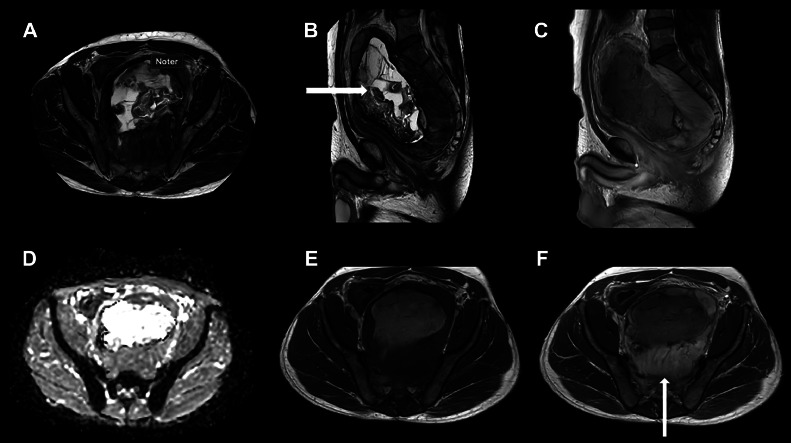
A prone position MRI scan was performed, including T2-weighted sequences in coronal, sagittal, and axial planes and a diffusion-weighted sequence including 5 b-values ranging from 0 to 1000. A T1-weighted sequence without and with intravenous gadolinium contrast was also included. The MRI images (Fig. 1) showed a large, well-demarcated retrorectal lesion measuring 14 × 9.5 × 17.5 cm (anterior-posterior [AP] x laterolateral [LL] x craniocaudal [CC]).
Fig. 1.
MRI scan showing the well-demarcated retrorectal tumor with a central cystic appearance (horizontal arrow) and a solid periphery with contrast enhancement (vertical arrow). Axial T2w (A), Sagittal T2w (B), sagittal gd enhanced T1w (C), axial ADC map (D), axial T1w -/+ gd (E, F) sequences.
The lesion exhibited heterogeneous signaling with central cystic and solid components including fatty tissue and calcifications (Fig. 1). Some peripheral areas had marginally increased diffusion weighted imaging (DWI) values and marginally decreased apparent diffusion coefficients (ADC) in soft-tissue areas without diffusion restriction in the center (Fig. 1D). The post-contrast sagittal and axial T1-weighted images exhibited enhancement of the tumor, except for the central part (Figs. 1C, E, and F).
The lesion exerted severe pressure on the surrounding pelvic organs, with external obstruction and anterior and lateral displacement of the rectum. The urinary bladder was also compressed and displaced superiorly. No osseous destructions were observed.
A sarcoma was suspected, but a schwannoma was considered as a differential diagnosis due to the relation to the sacral plexus.
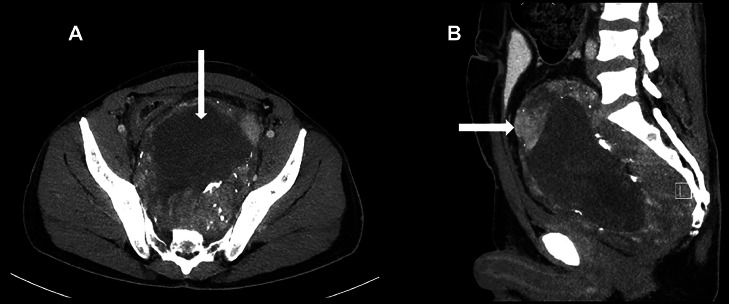
On the contrast-enhanced CT thorax-abdomen from the same day, the tumor had a homogenous, hypodense center without enhancement and a heterogeneously enhanced dense periphery. It could not be determined if the heterogeneity of the peripheral part of the tumor represented calcifications or contrast-filled blood vessels (Fig. 2).
Fig. 2.
Contrast-enhanced CT scan showing the large retrorectal tumor with a homogenous, hypodense center (vertical arrow) and a hyperdense peripheral part with heterogenous contrast enhancement (horizontal arrow). Axial (A) and sagittal (B) reconstructions.
There was no ascites and no signs of metastatic disease or enlarged lymph nodes.
Three weeks after the MRI and CT, the patient underwent surgical resection of the tumor and part of the rectum with the creation of a left colostomy. The patient was not treated with chemotherapy or radiotherapy.
On macroscopical pathology examination, the excised tumor was 14 × 9 × 24 cm. On cross-section, the tumor had a cystic appearance with a solid 2 cm wall. The rectal mucosa was normal. Microscopically, the tumor was heterogeneous with some components comprising spindle cells. There was no tumor cell infiltration of the fibrous capsule. The rectal wall muscle was microscopically hypertrophic and fibrotic but showed no signs of malignancy. The surgical margins were microscopically negative for malignant infiltration. Two lymph nodes from the mesorectal fatty tissue were normal.
Based on the above histopathological findings, gastrointestinal stromal tumor (GIST) (wild type) and dedifferentiated liposarcoma were considered, but the immunohistochemical reactions were found too unspecific to support these diagnoses. Therefore, it was concluded that the findings were representative of an undifferentiated spindle cell sarcoma grade 2.
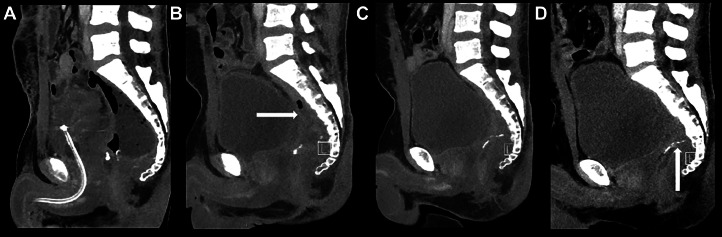
Concurrent with the diagnostic work-up and treatment for the retrorectal tumor, the patient was investigated for 2 small lung noduli found on the initial CT scan. Four weeks after the surgery, a renewed CT thorax-abdomen found a retrorectal lesion measuring 11 × 8 × 5 cm with a thickened wall encapsulating liquid and gaseous areas suspicious of a postsurgical abscess, which was subsequently managed by means of a trans-rectal Foley catheter with repeated irrigation. A PET-CT was performed 1 week later to ensure that the noduli did not represent metastatic disease, and it supported the diagnosis of a retrorectal abscess with high FDG-uptake in the periphery of the lesion. There were no PET or CT signs of metastatic disease. Sequential follow-up CT scans after 1, 2, 3, and 6 months showed gradual size reduction of the retrorectal abscess and no signs of metastatic disease (Fig. 3).
Fig. 3.
Contrast-enhanced CT scans after 1, 2, 3, and 6 months (A, B, C, D) showed further reduction of the pelvic cavity/abscess (horizontal arrow). The clips at the remaining rectal stump are visible (vertical arrow).
Discussion
We reported a case in which a large peripherally enhancing retrorectal tumor with diffusion restriction was detected on MRI. Histopathological examination revealed a spindle cell sarcoma.
Weight loss and changes in bowel habits should prompt further investigation, and colonoscopy is the first-line examination in most cases. In this case, a complete colonoscopy was not possible, but a digital examination revealed a bulging lump behind the rectum. This prompted a rectal MRI that demonstrated a large enhancing tumor with diffusion restriction and a central non-enhancing area. The presence of weight loss, the size of the enhancing tumor, and the diffusion restriction raised suspicion of malignancy. MRI of the rectum and pelvis as well as CT of the thorax and abdomen were performed on the same day to prevent diagnostic delay, as per standard protocol. The tumor did not appear typical of a rectal adenocarcinoma, and a sarcoma was suspected.
Radiographic findings are rarely reported in detail in the existing literature on retrorectal lesions, but our case is generally consistent with previous reports. Li and colleagues reported 33 cases of retrorectal tumors ranging from 2.4 to 21.4 cm with a mean of 9.3 cm [5], illustrating the large size variation exhibited by these tumors, presumably due to their often asymptomatic presentation and incidental diagnosis. The tumor in our case was up to 17.5 cm and we assumed it was present but asymptomatic for an extended period of time. Other authors have reported CT findings of bladder and rectum displacement [6], which was also seen in our case, and heterogeneous, hypodense tumors [6,7], consistent with the heterogeneous peripheral areas of the tumor in our case, although we found a homogeneous, hypodense center without enhancement.
The scientific literature on retrorectal tumor imaging is consistent in highlighting the importance of MRI, especially in determining anatomical resection planes when planning the surgical approach, but also with respect to diagnostic accuracy and assessment of involvement of adjacent structures such as nerve root and bone marrow involvement [2,3,6,8]. However, MRI-findings are rarely described in detail in previous case reports. Tarchouli and colleagues reported heterogeneous signaling on T1- and T2-weighted imaging in their case of a large retrorectal leiomyosarcoma [6]. The possibility of malignant transformation must be considered in the presence of a heterogeneous tumor [9]. These MRI characteristics are consistent with the findings in our case. This serves to illustrate the fact that radiological examinations cannot differentiate between tumor types but, as mentioned above, they play a crucial role in determining the optimal surgical approach, which is often both diagnostic and curative.
The main strength of our case report is the use of both CT and MRI, including gadolinium contrast-enhanced MRI with multiple sequences. With regard to the latter, contrast-enhanced MRI is not routinely used or recommended as standard in rectal MRI [10], but may add valuable information to the clinician when planning the surgical approach of large retrorectal tumors. Standardized imaging protocols also allow for more accurate and reproducible interpretations, which merits the widespread use of this technique. Intravenous contrast may be used in select cases [[11], [12]].
The main weakness of this report is the lack of more cases, both in our report and in the scientific literature, for contextualization and comparison.
Conclusion
Our case underscores the significance of detecting large retrorectal tumors on MRI, which can be effectively examined using diffusion-weighted imaging (DWI) and intravenous contrast. While the underlying etiology can only be established by histopathological examination, MRI is important in planning the surgical approach to the tumor, which is independent of the tumor type.
Author contributions
All authors contributed to this work. All authors have read and approved the final version of the manuscript.
Ethics approval and consent to participate
Our institution does not require ethical approval for reporting individual cases or case series.
Patient consent
Written informed consent for publication was obtained from the patient. The authors will provide a copy of the written consent upon request from the journal.
Footnotes
Competing Interests: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Wolpert A, Beer-Gabel M, Lifschitz O, Zbar AP. The management of presacral masses in the adult. Tech Coloproctol. 2002;6(1):43–49. doi: 10.1007/s101510200008. [DOI] [PubMed] [Google Scholar]
- 2.Hosseini-Nik H, Hosseinzadeh K, Bhayana R, Jhaveri KS. MR imaging of the retrorectal-presacral tumors: an algorithmic approach. Abdom Imaging. 2015;40(7):2630–2644. doi: 10.1007/s00261-015-0404-1. [DOI] [PubMed] [Google Scholar]
- 3.Balci B, Yildiz A, Leventoğlu S, Mentes B. Retrorectal tumors: a challenge for the surgeons. World J Gastrointest Surg. 2021;13(11):1327–1337. doi: 10.4240/wjgs.v13.i11.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reiter MJ, Schwope RB, Bui-Mansfield LT, Lisanti CJ, Glasgow SC. Surgical management of retrorectal lesions: what the radiologist needs to know. AJR Am J Roentgenol. 2015;204(2):386–395. doi: 10.2214/AJR.14.12791. [DOI] [PubMed] [Google Scholar]
- 5.Li GD, Chen K, Fu D, Ma XJ, Sun MX, Sun W, et al. Surgical strategy for presacral tumors: analysis of 33 cases. Chin Med J (Engl) 2011;124(23):4086–4091. [PubMed] [Google Scholar]
- 6.Doyle LA. Sarcoma classification: an update based on the 2013 World Health Organization classification of tumors of soft tissue and bone. Cancer. 2014;120(12):1763–1774. doi: 10.1002/cncr.28657. [DOI] [PubMed] [Google Scholar]
- 7.Tarchouli M, Zentar A, Ratbi MB, Bensal A, Khmamouche MR, Ali AA, et al. Perineal approach for surgical treatment in a patient with retro-rectal tumor: a case report and review of the literature. BMC Res Notes. 2015;8:470. doi: 10.1186/s13104-015-1457-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eriguchi N, Aoyagi S, Hara M, Tanaka E, Hashimoto M. A case of giant leiomyosarcoma of the rectum. Kurume Med J. 1998;45(1):137–141. doi: 10.2739/kurumemedj.45.137. [DOI] [PubMed] [Google Scholar]
- 9.Murphy A, O'Sullivan H, Stirling A, Fenlon H, Cronin C. Integrated multimodality and multi-disciplinary team approach to pre-sacral lesions. Clin Imaging. 2020;67:255–263. doi: 10.1016/j.clinimag.2020.08.011. [DOI] [PubMed] [Google Scholar]
- 10.Yang BL, Gu YF, Shao WJ, Chen HJ, Sun GD, Jin HY, et al. Retrorectal tumors in adults: magnetic resonance imaging findings. World J Gastroenterol. 2010;16(46):5822–5829. doi: 10.3748/wjg.v16.i46.5822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beets-Tan RGH, Lambregts DMJ, Maas M, Bipat S, Barbaro B, Curvo-Semedo L, et al. Magnetic resonance imaging for clinical management of rectal cancer: updated recommendations from the 2016 European Society of Gastrointestinal and Abdominal Radiology (ESGAR) consensus meeting. Eur Radiol. 2018;28(4):1465–1475. doi: 10.1007/s00330-017-5026-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horvat N, Carlos Tavares Rocha C, Clemente Oliveira B, Petkovska I, Gollub MJ. MRI of rectal cancer: tumor staging, imaging techniques, and management. Radiographics. 2019;39(2):367–387. doi: 10.1148/rg.2019180114. [DOI] [PMC free article] [PubMed] [Google Scholar]