Highlights
-
•
Recent RCTs of doxycycline PEP have failed to consider four factors that could lead to AMR.
-
•
The studies have all used proportion resistant as the outcome measure.
-
•
These RCTs have not considered population-level pathways of AMR selection.
-
•
The relationship between antimicrobial consumption and resistance may be saturated.
-
•
Genetic linkage of AMR means that tetracyclines could select for AMR to other antimicrobials.
Keywords: Doxycycline PEP, Tetracycline, MIC, Proportion resistant, AMR, PrEP, Gonorrhoea
Abstract
Two recently published randomized trials of doxycycline post exposure prophylaxis (PEP) have concluded that this intervention is highly effective at reducing the incidence of bacterial sexually transmitted infections (STIs) and has little or no risk of promoting the spread of antimicrobial resistance (AMR). In this perspective piece, we review four types of evidence that suggest that the risk of promoting AMR has been inadequately assessed in these studies. 1) The studies have all used proportion resistant as the outcome measure. This is a less sensitive measure of resistogenicity than MIC distribution. 2) These RCTs have not considered population-level pathways of AMR selection. 3) In populations with very high antimicrobial consumption such as PrEP cohorts, the relationship between antimicrobial consumption and resistance may be saturated. 4) Genetic linkage of AMR means that increased tetracycline use may select for AMR to not only tetracyclines but also other antimicrobials in STIs and other bacterial species. We recommend novel study designs to more adequately assess the AMR-inducing risk of doxycycline PEP.
Graphical abstract
Introduction
Doxycycline post exposure prophylaxis (doxycycline PEP) involves the ingestion of a single oral dose of 100 mg or 200 mg of doxycycline within 72 h following unprotected anal, vaginal or oral sex (Kong et al., 2023). Four randomized controlled trials (RCTs) have found that doxycycline post exposure prophylaxis (PEP) can reduce the incidence of chlamydia and syphilis in men who have sex with men (MSM) (Molina et al., 2018; Bolan et al., 2015; Luetkemeyer et al., 2023; Molina et al., 2023; Luetkemeyer and Luetkemeyer, 2023). Two of these RCTs (DoxyPEP and IPERGAY) assessed the effect of doxycycline PEP on tetracycline resistance in Neisseria gonorrhoeae (Molina et al., 2018; Luetkemeyer and Luetkemeyer, 2023). Both found no statistically significant effect. The Bolan et al., study did not evaluate the effect on tetracycline resistance and the DOXYVACC study antimicrobial resistance results have yet to be published (Bolan et al., 2015; Molina et al., 2023). One of these RCTs (DoxyPEP) evaluated the effect of doxycycline PEP on the prevalence of doxycycline resistance in Staphylococcus aureus and commensal Neisseria species (Luetkemeyer et al., 2023; Luetkemeyer and Luetkemeyer, 2023). They found no association between doxycycline PEP and resistance in these species and concluded that the risk of antimicrobial resistance (AMR) is small or non-existent (Molina et al., 2018; Luetkemeyer et al., 2023; Luetkemeyer and Luetkemeyer, 2023). These findings have, in turn, resulted in a number of health care providers to recommend and offer doxycycline PEP to a range of persons attending STI clinics (Population Health Division San Francisco Department of Health 2022; Mårdh et al., 2023).
In this opinion piece, we argue that these AMR conclusions are premature. We base this argument on four recent insights as to how best to measure the resistogenicity of doxycycline PEP.
-
1.
Assessing resistance induction: Minimum inhibitory concentration (MIC) distribution is a more sensitive indicator than the proportion of MIC resistant strains
Both the DoxyPEP and IPERGAY studies used the relatively insensitive MIC proportions method to evaluate the emergence of AMR (Molina et al., 2018; Luetkemeyer et al., 2023; Luetkemeyer and Luetkemeyer, 2023). This method involves the application of breakpoints (CLSI or EUCAST) to the MICs of N. gonorrhoeae isolates to classify them as susceptible or resistant ot tetracycline. Statistical tests are then conducted to compare the proportion of gonococcal infectionsmin both arms with tetracycline resistance (Zawack et al., 2016; Fedorov et al., 2009). A key problem with this proportion method is that it dichotomizes all MICs into susceptible or resistant, which results in a loss of information and reduces the probability of detecting various types of shifts in MIC values (Zawack et al., 2016; Fedorov et al., 2009). Studies have, therefore, found that testing the MICs of individual colonies is a more sensitive method to ascertain the effects of antimicrobials on resistance than testing the proportion of colonies with resistance (Zawack et al., 2016; Harrison et al., 1979). Indeed, tetracycline PEP studies provide further supporting evidence of the greater sensitivity of the MIC method compared with the proportion method. Only one tetracycline PEP study has used the MIC distribution method. This was the Harrison et al. an RCT from 1979, found that minocycline PEP reduced the incidence of gonorrhoea by 54 %. This lower incidence was driven by a reduction in the incidence of gonococcal infections with low minocycline MICs (Harrison et al., 1979). Minocycline PEP had no effect on the incidence of infections with higher tetracycline MICs. The authors concluded that minocycline PEP would very likely select for gonococcal strains with high tetracycline MICs and was thus not advisable. If the proportion method used in the DoxyPEP and IPERGAY studies is applied to the Harrison et al. results, then the use of tetracycline is no longer associated with tetracycline resistance (Fig. 1) (Vanbaelen et al., 2023a). A parsimonious explanation for these contrasting findings as to the resistogenicity of doxycycline PEP, is the different methods these studies used to assess AMR. The two studies that found no effect on resistance used proportion resistance, whereas the study that found an effect used MIC distribution as the outcome measure (Harrison et al., 1979).
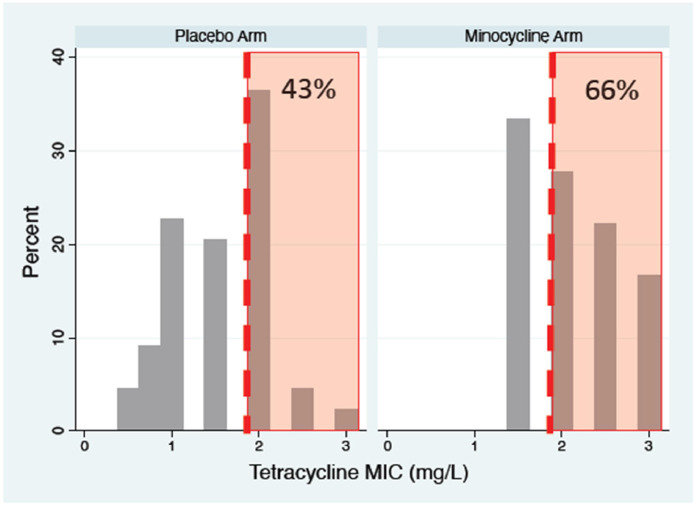
Fig. 1.
Tetracycline MICs of Neisseria gonorrhoeae isolates from the placebo and minocycline PEP arms of the Harrison et al. randomized controlled trial of minocycline PEP conducted in 1979. The MIC distribution curves reveal that the effectiveness of minocycline at preventing infection varied between 0 % and 100 % for high and low tetracycline MIC infections, respectively (P = 0.002; Mann–Whitney test). When this data is transformed into proportion resistant to tetracycline (≥2 mg/L; red shaded area) then 43 % and 66 % are classified as resistant in the placebo and minocycline arms, respectively – a nonsignificant difference, P = 0.168; Chi-square test).
These findings provide a cogent rationale to measure MIC distributions in studies assessing the resistogenicity of doxycycline PEP.
-
2.
Population level selection
There are at least five population-level pathways through which the consumption of antimicrobials can select for AMR (Lipsitch et al., 2002; Samore et al., 2006; Kenyon et al., 2018). These population-level effects may be large and can be missed if only individual-level pathways are evaluated – as was the case for doxycycline PEP studies (Bell et al., 2014; Goossens et al., 2007). Two of these pathways are illustrated in Fig. 2 for the effect of doxycycline use on doxycycline resistance in oral streptococci. Epidemiological studies have provided evidence for each of these five pathways. For example, a serial cross-sectional study of nasopharyngeal pneumococcal carriage found that the receipt of cephalosporin by the participant (individual-level selection) or their sibling (population-level selection) were independent risk factors for carriage of beta-lactam-resistant pneumococci (Samore et al., 2006). The use of penicillins was, however, not associated with increased carriage of beta-lactam-resistant pneumococci. Rather, it was strongly associated with a decreased carriage of susceptible pneumococci (population-level selection). These findings could explain the very strong country-level association between beta-lactam consumption and reduced susceptibility to penicillin in pneumococci (Goossens et al., 2005). Further examples of these population-level effects come from studies that have found that residing in areas with higher consumption of fluoroquinolones is an independent risk factor for fluoroquinolone resistant urinary tract infections, that travelling to areas with a high prevalence of antimicrobial resistance is an independent risk factor for colonization by resistant bacteria, and that consumption of antimicrobials by parents increases the probability of their children acquiring resistant bacteria (Murray et al., 1990; Low et al., 2019; Gottesman et al., 2020).
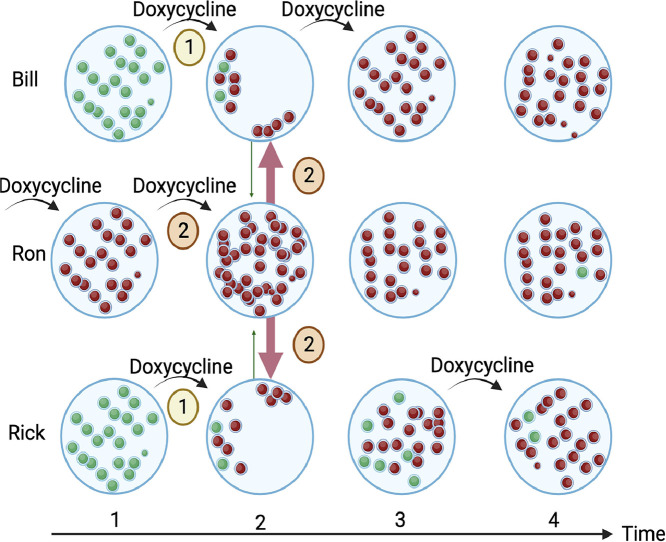
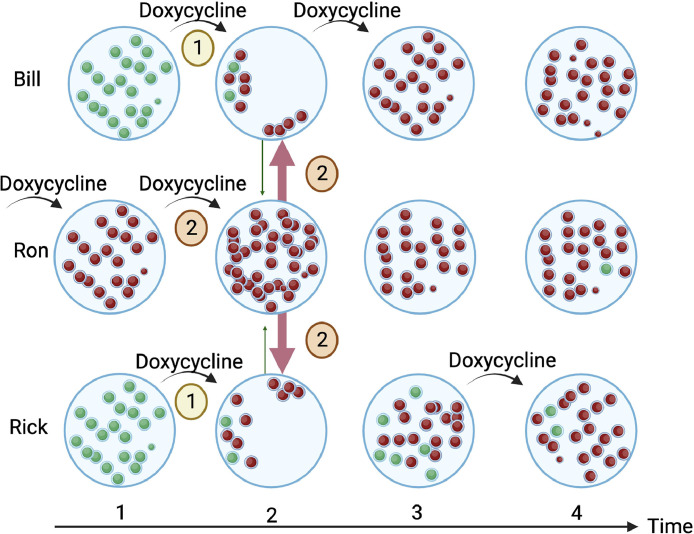
Fig. 2.
A schematic illustration of two population-level pathways through which antimicrobial consumption can facilitate the spread of antimicrobial resistance in oral streptococci. 1) Doxycycline reduces the abundance of doxycycline susceptible streptococci in Bill and Rick. This means they are less likely to transmit susceptible streptococci to others, creating a vacant niche that can be colonized by streptococci from contacts at time point two. 2) The use of doxycycline by Ron increases the abundance of doxycycline resistant streptococci at time point two. Ron's resistant streptococci can then colonize the vacant niches of Bill and Rick. By timepoint four, the predominance of doxycycline resistance will reduce the probability that a study will find a positive association between doxycycline use and resistance).
Tetracyclines, including doxycycline, have been shown to directly select for resistance in a range of aerobic and anaerobic bacterial species (Mättö et al., 2008; Heimdahl et al., 1983; Vanbaelen et al., 2023b; Rashid et al., 2013). Various types of evidence also point to a population-level selection of tetracycline AMR (Messele et al., 2023; Jia et al., 2022; Grossman, 2016). One of these factors is that tetracyclines have been found to reduce the abundance of susceptible bacteria (Luetkemeyer and Luetkemeyer, 2023; Mättö et al., 2008; Jia et al., 2022; Moura et al., 2022; Moura et al., 2019). For instance, the DoxyPEP study found that doxycycline PEP was associated with both an 8 % absolute increase in the prevalence of doxycycline resistant S.aureus and a 21 % absolute reduction in the prevalence of doxycycline susceptible S. aureus colonization in the doxyPEP arm (Luetkemeyer and Luetkemeyer, 2023).
-
3.
Saturation of antimicrobial resistance
In populations with high levels of antimicrobial usage, bacteria may be so resistant to antimicrobials that additional antibiotic exposure may result in little or no additional AMR (Bell et al., 2014; Kenyon, 2019). In other words, the association between antimicrobial consumption and resistance is saturated (Vanbaelen et al., 2023c). Studies have documented that antimicrobial consumption in PrEP cohorts is up to 7-fold higher than thresholds associated with AMR induction and spread in Streptococcus pneumoniae, Treponema pallidum and Mycoplasma genitalium (Kenyon, 2021; Kenyon et al., 2020; Vanbaelen et al., 2021). High levels of antimicrobial consumption are likely explanations for why AMR in STIs has frequently emerged in higher-risk groups such as MSM taking PrEP (Kenyon et al., 2018; Lewis, 2013). The prevalence of M. genitalium and T. pallidum macrolide resistance in MSM in Belgium is now 100 %, meaning that future studies would find it extremely difficult to find an association between macrolide consumption and resistance in these two species (Fig. 2) (Mikalova et al., 2017; De Baetselier et al., 2022). A similar, although less-pronounced, degree of saturation has been described in other bacterial species in MSM. The ResistAZM study, for example, was a randomized controlled trial of ceftriaxone versus ceftriaxone plus azithromycin 2 g for the treatment of N. gonorrhoeae in MSM (Vanbaelen et al., 2023c). The primary outcome was the abundance of macrolide resistance determinants in the anorectum 14 days post treatment. Surprisingly, no difference was found between the two arms. This was in contrast to the MORDOR study, which found that an equivalent dose of azithromycin was followed by a 7-fold increase in macrolide resistance determinants (Doan et al., 2020). The MORDOR study was performed in a population in Niger with a low background consumption of antimicrobials, whereas 43 % of the ResistAZM participants reported antimicrobial consumption in the prior year (Vanbaelen et al., 2023c; Doan et al., 2020). Unsurprisingly, the abundance of macrolide resistance determinants was 10-fold greater in the ResistAZM than in the MORDOR participants (Unpublished data). The phenotypic assessments of AMR were similar. In ResistAZM, the receipt of azithromycin was not associated with a significant increase in the proportion of oral streptococci or commensal Neisseria spp. resistant to azithromycin (Vanbaelen et al., 2023c). This may be related to the fact that all the individuals in the ResistAZM study had at least one streptococcal colony that was resistant to azithromycin (>1 mg/L) at baseline (Vanbaelen et al., 2023c). In contrast, in MORDOR, only 3 % of individuals harbored azithromycin-resistant oral streptococci at baseline (Doan et al., 2020). In MORDOR, the receipt of azithromycin was associated with an increase in the proportion of individuals with azithromycin resistance in oral streptococci and other bacteria (Doan et al., 2019). Likewise, a study in Belgian students who had not consumed antibiotics in the preceding 3 months found that the receipt of 1.5 g azithromycin was associated with an increase in the proportion of individuals with oral streptococcal resistance to macrolides from 30 % to 80 % (Malhotra-Kumar et al., 2007). In the ResistAZM study, the equivalent increase was 65–80 %, which was not statistically significant (Vanbaelen et al., 2023c). It is possible that the higher baseline proportion of macrolide resistance in ResistAZM compared to the students might have resulted in the proportion method being incapable of detecting a significant effect of the azithromycin. Further evidence to this effect comes from a reanalysis of the samples from the ResistAZM study using individual colony MICs as outcome measure. This reanalysis revealed that the receipt of azithromycin was associated with a significant increase in streptococcal azithromycin MICs (Vanbaelen et al., 2023b). These findings provide further evidence that MIC distribution is a more appropriate measure of resistogenicity than proportion resistant in populations with high antimicrobial exposure.
The combined effect of saturation and population-level selection
Antimicrobial resistance frequently emerges first in commensal Neisseria and streptococcal species and is then transferred to pathogenic species such as N. gonorrhoeae (Goytia et al., 2022). This provides the rationale to evaluate the prevalence of AMR in the commensals in populations at risk of AMR emergence, such as MSM on PrEP (Goytia et al., 2022). One such study compared the oral commensal Neisseria MIC distributions of azithromycin, ciprofloxacin and ceftriaxone in three populations: the general population and two groups of MSM taking PrEP- one group that had- and one group that had-not taken antimicrobials in the preceding 6 months. This study found that the Neisseria azithromycin and ciprofloxacin MICs were significantly higher in the two MSM groups, but there was no difference between the two MSM groups (Laumen et al., 2022). The abundance of resistance genes (including tetracyclines) in the oro-pharynx was similarly higher in the two groups of MSM than in the general population, without a difference apparent between the MSM groups (Van Dijck et al., 2022). In addition, there was no individual-level association between consumption of antimicrobials in the prior 6 months and the MICs of the commensal Neisseria or abundance of resistance genes (Vanbaelen et al., 2023d). These findings are compatible with both saturation and population-level effects. The considerably higher consumption of antimicrobials in the MSM PrEP group than the general population (Kenyon, 2021; Vanbaelen et al., 2021) could explain the higher AMR in the two MSM groups, and the combination of saturation and population-level spread of AMR could explain why this AMR is fairly evenly spread between MSM with and without recent antimicrobial consumption.
-
1.
Co-selection of resistance
Antimicrobial resistance frequently spreads in particular clades of bacteria that carry resistance mechanisms to multiple antimicrobials (Klemm et al., 2018). If a particular clade has resistance to both tetracyclines and another antimicrobial class, then the use of tetracyclines could result in the selection of resistance to this other antimicrobial. Recent studies have provided evidence of this association between tetracyclines and antimicrobials such as cephalosporins, macrolides, fluoroquinolones, aminoglycosides and lincomycins for a range of pathogens, including N. gonorrhoeae, S. aureus, S. pneumoniae, Shigella spp., Salmonella spp. and Klebsiella pneumoniae (Gestels et al., 2023; Mortimer et al., 2023; Whiley et al., 2023). Considering that doxycycline PEP would result in a 26–90-fold increase in doxycycline consumption in a typical PrEP cohort, it is possible that this could select for resistance to tetracyclines and other antimicrobials in this list of priority pathogens (Gestels et al., 2023; Whiley et al., 2023; Vanbaelen et al., 2023e). These considerations imply that studies need to include assessing the effect of doxycycline PEP on a broader range of bacteria-antimicrobial combinations before concluding that it is safe.
Conclusions
Each of these four insights describes a limitation in how the resistogenicity of doxycycline PEP has been measured thus far. These limitations mean that, based on the published doxycycline PEP studies, we cannot conclude that doxycycline PEP will not select for AMR. As we have argued, we need more evidence of safety before we can reach this conclusion. In particular, we need to expand the number of bug-drug combinations assessed for AMR and include assessments of MIC distribution and not just the proportion resistant. We need to include RCTs and observational studies that evaluate population-level effects (such as effects on the microbiota of contacts) and study designs that can deal with saturated populations.
We acknowledge that doxycycline PEP will likely reduce the consumption of broad-spectrum antimicrobials such as ceftriaxone by approximately two-fold (Vanbaelen et al., 2023e). However, empirical studies are required to assess the net effect on the resistome of this reduction in ceftriaxone use versus the up to 90-fold increase in doxycycline consumption (Vanbaelen et al., 2023e). These insights should also serve as a reminder to clinicians to send culture and antimicrobial susceptibility tests for all suspected gonococcal infections prior to treatment.
Outstanding questions
-
•
Doxycycline PEP will increase the consumption of tetracyclines but reduce the consumption of broad-spectrum antimicrobials such as ceftriaxone. It is unknown what the net effect of these changes will be on AMR.
-
•
Studies are required to assess the effects of doxycycline PEP on antimicrobial susceptibility to tetracyclines and other antimicrobials in a broader range of bacterial species
-
•
We need to include studies that evaluate population-level effects (such as effects on the microbiota of contacts) and study designs that can deal with saturated populations.
-
•
We have insufficient evidence on the effects of doxycycline PEP on the microbiome.
Glossary
Doxycycline PEP: Doxycycline post exposure prophylaxis. This refers to taking doxycycline (typically 200 mg) as soon after unprotected sex as possible. The rationale is to reduce the incidence of bacterial STIs.
Authors’ contributions
CK, TV and SMB conceptualized the study. CK was responsible for writing the first draft. All authors read and approved the final draft.
Consent for publication
Not applicable.
Funding
Nil.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Chris Kenyon reports financial support was provided by Institute of Tropical Medicine. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
Nil.
Data availability
No data was used for the research described in the article.
References
- Bell B.G., et al. A systematic review and meta-analysis of the effects of antibiotic consumption on antibiotic resistance. BMC Infect. Dis. 2014:14. doi: 10.1186/1471-2334-14-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolan R.K., et al. Doxycycline prophylaxis to reduce incident syphilis among HIV-infected men who have sex with men who continue to engage in high risk sex: a randomized, controlled pilot study. Sex Transm. Dis. 2015;42(2) doi: 10.1097/OLQ.0000000000000216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Baetselier I., et al. Worryingly high prevalence of resistance-associated mutations to macrolides and fluoroquinolones in Mycoplasma genitalium among men who have sex with men with recurrent sexually transmitted infections. Int. J. STD AIDS. 2022;33(4) doi: 10.1177/09564624211070704. [DOI] [PubMed] [Google Scholar]
- Doan T., et al. Macrolide resistance in MORDOR I—aA cluster-randomized trial in Niger. N. Engl. J. Med. 2019;380(23) doi: 10.1056/NEJMc1901535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doan T., et al. Macrolide and nonmacrolide resistance with mass azithromycin distribution. N. Engl. J. Med. 2020;383(20) doi: 10.1056/NEJMoa2002606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorov V., et al. Consequences of dichotomization. Pharm.Stat.: J. Appl. Stat. Pharm. Ind. 2009;8(1) doi: 10.1002/pst.331. [DOI] [PubMed] [Google Scholar]
- Gestels Z., et al. Doxycycline post exposure prophylaxis could select for cross-resistance to other antimicrobials in various pathogens: an in silico analysis. Int. J. STD AIDS. 2023 doi: 10.1177/09564624231190108. [DOI] [PubMed] [Google Scholar]
- Goossens H., et al. Outpatient antibiotic use in Europe and association with resistance: a cross-national database study. Lancet. 2005;365(9459) doi: 10.1016/S0140-6736(05)17907-0. [DOI] [PubMed] [Google Scholar]
- Goossens H., et al. Comparison of outpatient systemic antibacterial use in 2004 in the United States and 27 European countries. Clin. Infect. Dis. 2007;44(8) doi: 10.1086/512810. [DOI] [PubMed] [Google Scholar]
- Gottesman B.-S., et al. Quinolone consumption by mothers increases their children's risk of acquiring quinolone-resistant bacteriuria. Clin. Infect. Dis. 2020;71(3) doi: 10.1093/cid/ciz858. [DOI] [PubMed] [Google Scholar]
- Goytia M., et al. Canary in the coal mine: how resistance surveillance in commensals could help curb the spread of AMR in pathogenic neisseria. MBio. 2022;13(5) doi: 10.1128/mbio.01991-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman T.H. Tetracycline antibiotics and resistance. Cold Spring Harb. Perspect. Med. 2016;6(4) doi: 10.1101/cshperspect.a025387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison W.O., et al. A trial of minocycline given after exposure to prevent gonorrheal. N. Engl. J. Med. 1979;300(19) doi: 10.1056/NEJM197905103001903. [DOI] [PubMed] [Google Scholar]
- Heimdahl A., et al. Influence of doxycycline on the normal human flora and colonization of the oral cavity and colon. Scand. J. Infect. Dis. 1983;15(3) doi: 10.3109/inf.1983.15.issue-3.10. [DOI] [PubMed] [Google Scholar]
- Jia S., et al. The pass-on effect of tetracycline-induced honey bee (Apis mellifera) gut community dysbiosis. Front. Microbiol. 2022:12. doi: 10.3389/fmicb.2021.781746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon C. Prevalence of macrolide resistance in Treponema pallidum is associated with macrolide consumption. J. Med. Microbiol. 2019;68(2) doi: 10.1099/jmm.0.000885. [DOI] [PubMed] [Google Scholar]
- Kenyon C., et al. Screening for STIs in PrEP cohorts results in high levels of antimicrobial consumption. Int. J. STD AIDS. 2020;31(12) doi: 10.1177/0956462420957519. [DOI] [PubMed] [Google Scholar]
- Kenyon C. Dual azithromycin/ceftriaxone therapy for gonorrhoea in PrEP cohorts results in levels of macrolide consumption that exceed resistance thresholds by up to 7-fold. J. Infect. Dis. 2021 doi: 10.1093/infdis/jiab178. [DOI] [PubMed] [Google Scholar]
- Kenyon C.R., et al. Effects of sexual network connectivity and antimicrobial drug use on antimicrobial resistance in Neisseria gonorrhoeae. Emerg. Infect. Dis. 2018;24(7) doi: 10.3201/eid2407.172104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemm E.J., et al. Emergence of dominant multidrug-resistant bacterial clades: lessons from history and whole-genome sequencing. Proc. Natl. Acad. Sci. 2018;115(51) doi: 10.1073/pnas.1717162115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong F.Y.S., et al. Important considerations regarding the widespread use of doxycycline chemoprophylaxis against sexually transmitted infections. J. Antimicrob. Chemother. 2023 doi: 10.1093/jac/dkad129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laumen J.G.E., et al. Antimicrobial susceptibility of commensal Neisseria in a general population and men who have sex with men in Belgium. Sci. Rep. 2022;12(1) doi: 10.1038/s41598-021-03995-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis D.A. The role of core groups in the emergence and dissemination of antimicrobial-resistant N gonorrhoeae. Sex Transm. Infect. 2013;89(4) doi: 10.1136/sextrans-2013-051020. [DOI] [PubMed] [Google Scholar]
- Lipsitch M., et al. Antimicrobial use and antimicrobial resistance: a population perspectivel. Emerg. Infect. Dis. 2002;8(4) doi: 10.3201/eid0804.010312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low M., et al. Association between urinary community-acquired fluoroquinolone-resistant Escherichia coli and neighbourhood antibiotic consumption: a population-based case-control study. Lancet Infect. Dis. 2019;19(4) doi: 10.1016/S1473-3099(18)30676-5. [DOI] [PubMed] [Google Scholar]
- Luetkemeyer A, et al., editors. Doxy-PEP and antimicrobial resistance in S. aureus, N. gonorrhoeae, and commensal Neisseria. Conference on Retroviruses and Opportunistic Infections; Seattle, Washington, USA; 2023. [Google Scholar]
- Luetkemeyer A.F., et al. Postexposure doxycycline to prevent bacterial sexually transmitted infections. N. Engl. J. Med. 2023;388(14) doi: 10.1056/NEJMoa2211934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra-Kumar S., et al. Effect of azithromycin and clarithromycin therapy on pharyngeal carriage of macrolide-resistant streptococci in healthy volunteers: a randomised, double-blind, placebo-controlled study. Lancet. 2007;369(9560) doi: 10.1016/S0140-6736(07)60235-9. [DOI] [PubMed] [Google Scholar]
- Mårdh O., et al. Using doxycycline for prophylaxis of bacterial sexually transmitted infections: considerations for the European Union and European Economic Area. Eurosurveillance. 2023;28(46) doi: 10.2807/1560-7917.ES.2023.28.46.2300621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mättö J., et al. Influence of oral doxycycline therapy on the diversity and antibiotic susceptibility of human intestinal bifidobacterial population. J. Appl. Microbiol. 2008;105(1) doi: 10.1111/j.1365-2672.2008.03792.x. [DOI] [PubMed] [Google Scholar]
- Messele Y.E., et al. Meta-analysis on the global prevalence of tetracycline resistance in Escherichia coli isolated from beef cattle. Vet. Sci. 2023;10(7) doi: 10.3390/vetsci10070479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikalova L., et al. Molecular typing of syphilis-causing strains among human immunodeficiency virus-positive patients in antwerp, Belgium. Sex Transm. Dis. 2017;44(6) doi: 10.1097/OLQ.0000000000000600. [DOI] [PubMed] [Google Scholar]
- Molina J.-M., et al. Post-exposure prophylaxis with doxycycline to prevent sexually transmitted infections in men who have sex with men: an open-label randomised substudy of the ANRS IPERGAY trial. Lancet Infect. Dis. 2018;18(3) doi: 10.1016/S1473-3099(17)30725-9. [DOI] [PubMed] [Google Scholar]
- Molina J.-M., B. Bercot, L. Assoumou, A.-G. Michele, E. Rubenstein, G. Pialoux, C. Katlama, L. Surgers, C. Bebear, N. Dupin, J.-P. Viard, J. Pavie, C. Duvivier, J. Ghosn, D. Costagliola,. ANRS 174 DOXYVAC: an open-label randomized trial to prevent STIs in MSM on PrEPl. CROI 2023, Seattle, Washington.
- Mortimer T.D., et al. A genomic perspective on the near-term impact of doxycycline post-exposure prophylaxis on Neisseria gonorrhoeae antimicrobial resistance. medRxiv. 2023 doi: 10.1093/cid/ciad279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moura I.B., et al. Omadacycline gut microbiome exposure does not induce Clostridium difficile proliferation or toxin production in a model that simulates the proximal, medial, and distal human colon. Antimicrob. Agents Chemother. 2019;63(2) doi: 10.1128/AAC.01581-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moura I.B., et al. Profiling the effects of systemic antibiotics for acne, including the narrow-spectrum antibiotic sarecycline, on the human gut microbiota. Front. Microbiol. 2022;13 doi: 10.3389/fmicb.2022.901911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray B.E., et al. Emergence of resistant fecal Escherichia coli in travelers not taking prophylactic antimicrobial agentsl. Antimicrob. Agents Chemother. 1990;34(4) doi: 10.1128/aac.34.4.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Population Health Division San Francisco Department of Health. Doxycycline post-exposure prophylaxis reduces incidence of sexually transmitted infections. 2022.
- Rashid M.-U., et al. Ecological impact of doxycycline at low dose on normal oropharyngeal and intestinal microfloral. Int. J. Antimicrob. Agents. 2013;41(4) doi: 10.1016/j.ijantimicag.2012.11.014. [DOI] [PubMed] [Google Scholar]
- Samore M.H., et al. Mechanisms by which antibiotics promote dissemination of resistant pneumococci in human populationsl. Am. J. Epidemiol. 2006;163(2) doi: 10.1093/aje/kwj021. [DOI] [PubMed] [Google Scholar]
- Vanbaelen T., et al. Screening for STIs is one of the main drivers of macrolide consumption in PrEP users. Int. J. STD AIDS. 2021 doi: 10.1177/09564624211025940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanbaelen T., et al. Total antimicrobial consumption in doxycycline postexposure prophylaxis cohorts and the intensity of screening for bacterial sexually transmitted infections. Clin. Infect. Dis. 2023 doi: 10.1093/cid/ciad553. [DOI] [PubMed] [Google Scholar]
- Vanbaelen T., et al., 2023, No association between antimicrobial consumption and antimicrobial resistance in a PrEP population: a cross sectional study. Preprintsorg.
- Vanbaelen T., et al. Effect on the resistome of dual- vs monotherapy for the treatment of Neisseria gonorrhoeae: results from a randomized controlled trial (ResistAZM Trial) Open Forum Infect. Dis. 2023 doi: 10.1093/ofid/ofad462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanbaelen T., et al., 2023. Doxcycline post-exposure prophylaxis: are we using the correct method to assess for effect on antimicrobial resistance?. Preprintsorg.
- Vanbaelen T., et al. 2023, 45 years of tetracycline post exposure prophylaxis for STIs and the risk of tetracycline resistance: a systematic review and meta-analysis. preprintsorg. [DOI] [PMC free article] [PubMed]
- Van Dijck C., et al., 2022, The oropharynx of men using HIV pre-exposure prophylaxis is enriched with antimicrobial resistance genes. ECCMID, Lisbon. 2022.
- Whiley D.M., et al. Selection of Neisseria gonorrhoeae ceftriaxone resistance using doxycycline post-exposure prophylaxis. Lancet Infect. Dis. 2023 doi: 10.1016/S1473-3099(23)00359-6. [DOI] [PubMed] [Google Scholar]
- Zawack K., et al. Monitoring antimicrobial resistance in the food supply chain and its implications for FDA policy initiatives. Antimicrob. Agents Chemother. 2016;60(9) doi: 10.1128/AAC.00688-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.