Abstract
Objective
Grifolin is a natural secondary metabolite isolated from edible fruiting bodies of the mushroom Albatrellus confluens. Grifolin has antitumor activities in several types of cancer. We aimed to determine the effects of grifolin on lung cancer.
Methods
We determined the proliferation, migration, invasion, and apoptosis of lung cancer cells using 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, Ethynyl deoxyuridine, colony formation, wound scratch, transwell, flow cytometry, and xenograft mouse assays. Molecular docking evaluated the binding relation between grifolin and phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA). The levels of PIK3CA, AKT, and p-AKT were measured by western blot.
Results
Grifolin (10, 20, or 40 μM) inhibited the proliferation, migration, and invasion of lung cancer cells, and induced cell cycle arrest and apoptosis. Grifolin also decreased CDK4, CDK6, and CyclinD1 expression and significantly decreased PIK3CA and p-AKT expression in lung cancer cells. These anticancer effects were abolished by 740Y-P.
Conclusions
Grifolin regulates the PI3K/AKT pathway, thus inhibiting lung cancer progression.
Keywords: Grifolin, Lung cancer, PI3K/AKT
1. Introduction
Lung cancer is the primary cause of death among human malignancies in China, and the survival rate is very poor [1,2]. Some systemic lung cancer treatment options have been described [[3], [4], [5], [6]], and imaging and examinations continuously advance. However, the onset of lung cancer and typical symptoms and signs in early stage patients are not obvious [7]. Chemotherapy is the mainstay of treatment for advanced lung cancer [8], but it has obvious toxic side effects that negatively affect the quality of life of patients [9]. Some patients develop drug resistance, which can decrease or eliminate the therapeutic effects [10]. Therefore, new anticancer agents with high efficiency and little or no toxic side effects are urgently needed. Many fungal extracts inhibit tumor growth and metastasis [[11], [12], [13]]. Grifolin (C22H32O2; molecular weight: 328) is a terpenoid compound extracted from edible fruiting bodies of the mushroom Albatrellus confluens [14]. It inhibits the progression of nasopharyngeal carcinoma [15] and gastric cancer [16]. Grifolin inhibits the phosphatidylinositol 3-kinase (PI3K)/AKT pathway, and then induces cell apoptosis in osteosarcoma [17]. Additionally, grifolin influences autophagy by suppressing the AKT/mTOR/S6K pathway in ovarian cancer [18]. However, whether grifolin exerts anticancer effects in lung cancer remains unknown.
Here, we found that grifolin inhibited the progression of lung cancer by inducing G1 phase arrest and promoting apoptosis. Phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA) and p-AKT were markedly downregulated in lung cancer cells following grifolin treatment. Therefore, grifolin has potential for treating lung cancer.
2. Materials and methods
2.1. Cell culture
A549 cells (CL-0016, Procell, Wuhan, China) and H1299 cells (CL-0165, Procell) were cultured in Ham's F-12K and Roswell Park Memorial Institute (RPMI)-1640 that were supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin G, and 0.1 mg/mL streptomycin (HyClone, Laboratories Inc., South Logan, UT, USA) at 37 °C under a 5% CO2 atmosphere. Grifolin was purchased from Shanghai Yuanye Biotechnology Co., Ltd (China). A549 and H1299 cells were treated with grifolin (10, 20, and 40 μM) for 24 h. Cells were pretreated with 740Y–P (30 μΜ, Selleck, USA) for 2 h to activate PI3K.
2.2. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay
Cells (2 × 104) were seeded in 96-well plates and incubated with grifolin for 12, 24, and 48 h, followed by MTT solution (20 μL/well, 5 mg/mL) for 4 h at 37 °C. Formazan crystals were dissolved using DMSO, then absorbance at 570 nm was measured using a microplate reader (Bio-Rad, Laboratories Inc., Hercules, CA, USA).
2.3. Ethynyl deoxyuridine (EdU) incorporation assay
Cell proliferative ability was detected using the EdU Kit (RiboBio, Guangzhou, China). Cells were incubated with EdU solution (50 μM) for 2 h, then fixed in 4% paraformaldehyde. Apollo was added in cells for 30 min. Nuclei were stained with Hoechst 33,342. Cells were photographed using a microscope.
2.4. Colony formation assay
Cells (500 cells/well) were incubated in 6-well plates at 37 °C for 14 days. Colonies were stained with 0.1% crystal violet solution and manually counted.
2.5. Flow cytometry
For cell cycle assay, cells were fixed with 70% ethanol and stained with propidium iodide (PI). For apoptosis assay, treated cells were collected and resuspended in binding buffer. Annexin V-FITC and PI were added to cell suspensions for 15 min in the dark. Samples were analyzed using a flow cytometer (BD Biosciences, San Jose, CA, USA).
2.6. Scratch assay
Confluent cells in 6-well plates were scratched using a pipette tip, then incubated with grifolin at 37 °C for 24 h. Cells were visualized 0 and 24 h, and the wound distance was measured in images.
2.7. Transwell assay
Cells (2 × 104 cells/well) in serum-free media were added to the upper of Matrigel-coated Transwell chamber with or without grifolin and the PI3K activator 740 Y-P. Medium containing 10% FBS was added to the lower chambers. After 24 h incubation, the invaded cells were fixed with methanol for 15 min and stained with 0.1% crystal for 20 min. The invaded cells were counted using a microscope.
2.8. Western blot
This part refers to a previous experimental methods [19]. Cells were disrupted in the lysis buffer. Protein concentrations were detected using bicinchoninic acid protein assay kit (Beyotime, China). Proteins were separated on 10% SDS-PAGE gels and transferred onto polyvinylidene fluoride membrane. After blocking in 5% non-fat milk, membranes were incubated at 4 °C overnight with the anti-PIK3CA, anti-cyclin dependent kinase 4 (CDK4), anti-cyclin dependent kinase 6 (CDK6), anti-Cyclin D1 (Proteintech), anti-Bcl-2, anti-cleaved caspase 3, anti-AKT, anti-phospho-AKT, anti-β-actin (Cell signaling), and anti-Bax (GeneTex) antibody, followed by the secondary antibody for 1 h. Enhanced chemiluminescence reagent (Beyotime, China) was used to observe immunoreactive bands.
2.9. Molecular docking
Common targets between grifolin and lung cancer were analyzed using Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis. The 3D structure of grifolin and PIK3CA were downloaded from the PubChem and PDB databases. Grifolin and PIK3CA were docked using molecular operating environment software (Chemical Computing Group, Montreal, PQ, Canada) [20].
2.10. Animal model
We prepared subcutaneous xenograft models by injecting 0.1 mL containing A549 cells into the flanks of six-week-old, male BALB/c nude mice (GemPharmatech, Nanjing, China). The mice were intraperitoneally injectreated with grifolin (15 and 30 mg/kg) or phosphate buffered saline daily. On day 30, tumors were excised, weighed, and processed for western blot, immunohistochemistry (IHC), and TdT-mediated dUTP nick end labeling (TUNEL) staining. The experimental protocol of our study was performed in accordance with the Guide for the Care and Use of Laboratory Animals and approved by the Affiliated Jiangning Hospital of Nanjing Medical University (20230115).
2.11. Statistical analysis
Data are presented as means ± standard deviation. The data were analyzed using GraphPad Prism 8.0 software (GraphPad Software Inc., San Diego, CA, USA). Differences between two groups were assessed using Student's t-test and those among multiple groups were evaluated using one-way analyses of variance followed by Tukey's post hoc test. Values with p < 0.05 were considered statistically significant.
3. Results
3.1. Grifolin inhibits cell proliferation
We incubated A549 and H1299 cells with grifolin (0, 10, 20, or 40 μM) for 24 h, then assessed cell proliferation using MTT, EdU, and colony formation assays. Grifolin suppressed A549 and H1299 cell proliferation (Fig. 1A–C).
Fig. 1.
Grifolin inhibits cell proliferation. Cells were treated with grifolin (10, 20, and 40 μM) for 24 h. (A) Cell proliferation was detected by MTT (A), EdU (B), and colony formation assays (C). n = 3, *P < 0.05.
3.2. Grifolin promotes the apoptosis of cells
Grifolin significantly induced apoptosis (Fig. 2A). Bax, Bcl-2, and cleaved caspase-3 are markers of apoptosis [21]. Grifolin downregulated Bcl-2 expression, upregulated the protein expression of Bax and cleaved caspase 3 in A549 and H1299 cells (Fig. 2B).
Fig. 2.
Grifolin promotes the apoptosis of cells. (A) Apoptosis was detected by flow cytometry. (B) Bcl-2, Bax, and cleaved caspase 3 levels were detected by western blot. Full and non-adjusted images are available in the supplementary material (multimedia component 1). n = 3, *P < 0.05.
3.3. Grifolin induces cell cycle arrest at G1 phase
Grifolin arrested the cell cycle at the G1 phase (Fig. 3A), and decreased the levels of cell cycle-related proteins CDK4, 6, and CyclinD1 (Fig. 3B).
Fig. 3.
Grifolin induces cell cycle arrest at G1 phase. (A) Cells cycle was detected by flow cytometry. (B) CDK4, CDK6, and Cyclin D1 levels were measured by western blot. Full and non-adjusted images are available in the supplementary material (multimedia component 1). n = 3, *P < 0.05.
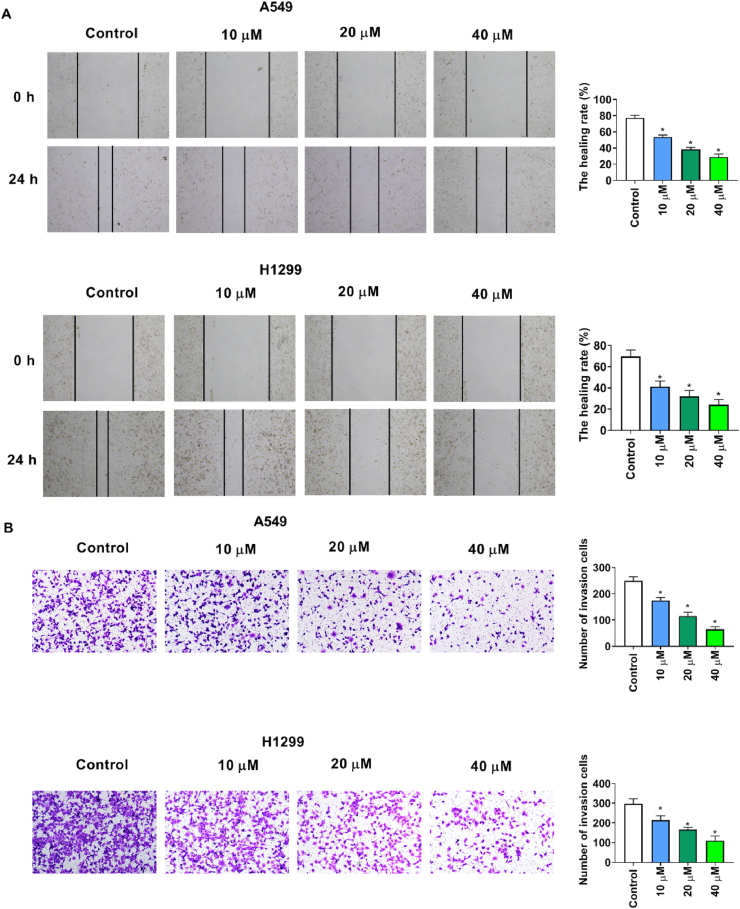
3.4. Grifolin inhibits cell migration and invasion
We next investigated whether grifolin affects lung cancer cell migration and invasion. Grifolin markedly suppressed the wound healing ability of cells (Fig. 4A), and significantly inhibited invasion ability of cells (Fig. 4B).
Fig. 4.
Grifolin inhibits cell migration and invasion. Cell migration and invasion were detected by wound healing assay (A) and transwell assay (B). n = 3, *P < 0.05.
3.4.1. Grifolin binds to PI3KCA to inhibit PI3K/AKT pathway
The KEGG findings showed that PI3K/AKT pathway was enriched (Fig. 5A). Next, we used molecular docking to explore how grifolin interactes with the PI3K/AKT pathway and found that it binds to PI3KCA. Grifolin showed an interaction with Ser 474 via H-donor at a distance of 2.76 Å. The binding energy of interaction was −0.5 kcal/mol. Grifolin also showed an interaction with Arg 481 via H-acceptor at a distance of 2.99 Å. The binding energy of interaction was −0.6 kcal/mol (Fig. 5B and C). Grifolin decreased PIK3CA and p-AKT expression (Fig. 5D).
Fig. 5.
Grifolin binds to PI3KCA and inhibits the PI3K/AKT pathway. (A) Diagram of KEGG pathway enrichment. (B) Docking complex of PI3KCA and grifolin. (C) Ligand interaction between PI3KCA and grifolin. (D) Protein expression of PI3KCA, P-AKT, and AKT was detected by western blot. Full and non-adjusted images are available in the supplementary material (multimedia component 1). n = 3, *P < 0.05.
3.5. Grifolin inhibits cell proliferation and invasion by suppressing the PI3K/AKT pathway
We reconfirmed the effects of PI3K/AKT pathway in the biological function of grifolin using cell rescue experiments. The EdU (Fig. 6A), apoptosis (Fig. 6B), and transwell (Fig. 6C) findings showed that 740 Y-P reversed the roles of grifolin in A549 and H1299 cells.
Fig. 6.
Grifolin inhibits cell proliferation and invasion by suppressing the PI3K/AKT pathway. Cells were treated with grifolin (40 μM) or 740 Y-P (30 μM) for 24 h. Rescue experiments in cells using EdU assay (A), apoptosis (B), and transwell assays (C). n = 3, *P < 0.05, #P < 0.05.
3.6. Grifolin inhibits growth of lung cancer xenograft tumors
We administered 0.1 mL grifolin (15 and 30 mg/kg/day; i.p.) to nude mice harboring A549 xenografts to validate the roles of grifolin in vivo. Grifolin inhibited A549 xenograft tumor growth in vivo (Fig. 7A and B). Xenograft tumors were observably lighter in mice injected with grifolin than PBS (Fig. 7C). The IHC staining for Ki67 found that grifolin decreased A549 cell proliferation (Fig. 7D), and TUNEL staining showed that it increased cell apoptosis (Fig. 7E). Xenografts from mice injected with grifolin had noticeably decreased PIK3CA and p-AKT values (Fig. 7F).
Fig. 7.
Grifolin inhibits growth of lung cancer xenograft tumors. (A) Tumors were excised at day 30. (B) Tumor growth was measured every 5 days. (C) Tumors were weighed on day 30. Representative image of (D) Ki67 staining and (E) TUNEL staining. (F) PIK3CA, p-AKT, and AKT levels in subcutaneous tumors were measured by western blot. Full and non-adjusted images are available in the supplementary material (multimedia component 1). n = 5, *P < 0.05.
4. Discussion
The global mortality of lung cancer remains high despite remarkable progress in treatment strategies [22]. Surgical and chemotherapy are routine treatments for lung cancer [23]. But, chemotherapeutic approaches have shortcomings such as toxicity and drug resistance that cannot be overcome [24]. Therapeutic agents with little or no side-effects are required. Edible mushrooms are implicated in reducing the side effects of chemotherapy and improving life quality of patients [25,26]. Therefore, discovering active ingredients and critical mechanisms remains challenging. We therefore integrated the effects of grifolin on lung cancer.
The terpenoid compound grifolin is inhibitory in many types of cancer [27]. We first assessed the anti-proliferative effect of grifolin in human lung cancer A549 and H1299 cell lines. Grifolin (10, 20, and 40 μM) obviously inhibited cell proliferation, which was consistent with the concentration required to inhibit proliferation of other types of cancer cells [28,29]. Grifolin (40 μM) has the anti-invasive effects on 5–8F, MDA-MB-231 and MGC-803 cells, and 32 mg/kg/day prevents 5–8F-Z cell metastasis to the lung [30]. In this study, we found that grifolin (40 μM) inhibited the migration and invasion of lung cancer cells and 15 and 30 mg/kg/day inhibited A549 xenograft tumor growth. Mushrooms exert anti-cancer effects by inducing cell cycle arrest [[31], [32], [33]]. Our results showed that grifolin induced G1 arrest, which also does in the gastric cancer cell lines GC823 and SGC7901 [16]. Different CDKs and cyclins control the rapid cyclical growth of cancer cells [34]. The Cyclin D1/CDK4/6 complex is activated in G1-phase progression and Cyclin E/CDK2 complex is needed for the G1/S transition [35]. Grifolin treatment leads to G1 phase cell cycle arrest, with decreasing Cyclin D1, Cyclin E, and CDK4 levels [28]. We found that grifolin substantially decreased CDK4, CDK6, and Cyclin D1 expression in lung cancer cells. Pro-apoptotic BAX and anti-apoptotic Bcl-2 proteins belong to the BCL-2 family [36], and increased Bax/Bcl-2 ratio is characteristic of apoptotic cells [37]. Caspase 3 is also an important apoptotic mediator [38]. Grifolin induces apoptosis and affects apoptosis-related proteins, such as Bax, Bcl-2, and cleaved-caspase-3 in the ovarian cancer A2780 cells [39]. In this study, grifolin treatment induced lung cancer cell apoptosis. Grifolin dramatically increased Bax/Bcl-2 ratio and cleaved caspase-3 expression, indicating that grifolin induced lung cancer cell apoptosis.
The PI3K/AKT pathway and the cell cycle are closely associated [40,41]. Moreover, the PI3K/AKT pathway triggers a series of downstream effectors in tumor cells, including apoptosis [42,43]. The PI3K/AKT pathway is abnormally activated in lung cancer [44]. Grifolin induces apoptosis by inhibiting the PI3K/Akt pathway in osteosarcoma cells (U2OS and MG63) [17]. PIK3CA, a catalytic PI3K subunit, that is frequently activated and mutated in lung cancer [45]. PI3K stimulates AKT phosphorylation, and drives tumorigenesis in lung cancer [46]. We investigated how grifolin inhibits PI3K/AKT pathway in lung cancer cells. Molecular docking revealed that grifolin tightly bound to PI3KCA. Our experimental results verified the binding ability predicted by molecular docking. We found that grifolin suppressed PIK3CA expression and AKT phosphorylation. The activator 740 Y-P of the PI3K/AKT pathway partially reversed the ability of grifolin to suppress cell proliferation and invasion and induce apoptosis in lung cancer cells. These findings indicated that the PI3K/AKT pathway is significantly involved in the effects of grifolin against lung cancer.
Although we introduced the antitumor function of grifolin in lung cancer, this study has some limitations. The specific reasons for PI3K/AKT inhibition and the specific role of PIK3CA in lung cancer cells treated with grifolin remain unclear. Thus, more extensive studies are necessary to completely clarify the mechanisms by which grifolin exerts anticancer effects in lung cancer cells. More investigation is needed to determine which individual or combined treatments would the most effective against lung cancer.
5. Conclusions
Grifolin inhibited the progression of lung cancer cells by suppressing the PI3K/AKT pathway
Funding
This work was supported by the Nanjing Health Science and Technology Development Special fund project (grant no. YKK22220).
Ethics approval and informed consent
The experimental protocol of our study was performed in accordance with the Guide for the Care and Use of Laboratory Animals and approved by the Affiliated Jiangning Hospital of Nanjing Medical University (20230115).
Consent for publication
No.
Availability of data and materials
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
CRediT authorship contribution statement
Li Wang: Conceptualization. Yongjun Wang: Formal analysis, Data curation. Zexu Wang: Software, Methodology, Data curation. Xiuwei Zhang: Software, Investigation, Data curation. Huayong Chen: Visualization, Methodology, Data curation. Qiuqi Lin: Methodology, Investigation, Data curation. Xin Wang: Investigation, Formal analysis, Data curation. Yuting Wen: Project administration, Investigation, Data curation. Xia Pan: Validation, Investigation, Data curation, Conceptualization. Zhongliang Guo: Conceptualization. Bing Wan: Writing – review & editing, Writing – original draft, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e29447.
Contributor Information
Zhongliang Guo, Email: gzl@tongji.edu.cn.
Bing Wan, Email: bingwan76@njmu.edu.cn.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Xia C., Dong X., Li H., et al. Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin. Med. J. 2022;135(5):584–590. doi: 10.1097/CM9.0000000000002108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maomao C., He L., Dianqin S., et al. Current cancer burden in China: epidemiology, etiology, and prevention. Cancer Biol Med. 2022;19(8):1121–1138. doi: 10.20892/j.issn.2095-3941.2022.0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rizzo A., Cusmai A., Giovannelli F., et al. Impact of proton pump inhibitors and histamine-2-receptor antagonists on non-small cell lung cancer immunotherapy: a systematic review and meta-analysis. Cancers. 2022;14(6) doi: 10.3390/cancers14061404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rizzo A. Identifying optimal first-line treatment for advanced non-small cell lung carcinoma with high PD-L1 expression: a matter of debate. Br. J. Cancer. 2022 Nov;127(8):1381–1382. doi: 10.1038/s41416-022-01929-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Santoni M., Rizzo A., Mollica V., et al. The impact of gender on the efficacy of immune checkpoint inhibitors in cancer patients: the MOUSEION-01 study. Crit. Rev. Oncol. Hematol. 2022;170(103596):12. doi: 10.1016/j.critrevonc.2022.103596. [DOI] [PubMed] [Google Scholar]
- 6.Mollica V., Rizzo A., Marchetti A., et al. The impact of ECOG performance status on efficacy of immunotherapy and immune-based combinations in cancer patients: the MOUSEION-06 study. Clin. Exp. Med. 2023;23(8):5039–5049. doi: 10.1007/s10238-023-01159-1. [DOI] [PubMed] [Google Scholar]
- 7.Nooreldeen R., Bach H. Current and future development in lung cancer diagnosis. Int. J. Mol. Sci. 2021;22(16) doi: 10.3390/ijms22168661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duma N., Santana-Davila R., Molina J.R. Non-Small cell lung cancer: epidemiology, screening, diagnosis, and treatment. Mayo Clin. Proc. 2019;94(8):1623–1640. doi: 10.1016/j.mayocp.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 9.Morita S., Kobayashi K., Eguchi K., et al. Influence of clinical parameters on quality of life during chemotherapy in patients with advanced non-small cell lung cancer: application of a general linear model. Jpn. J. Clin. Oncol. 2003;33(9):470–476. doi: 10.1093/jjco/hyg083. [DOI] [PubMed] [Google Scholar]
- 10.Zaman A., Bivona T.G. Targeting AXL in NSCLC. Lung Cancer. 2021;12:67–79. doi: 10.2147/LCTT.S305484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hetland G., Tangen J.M., Mahmood F., et al. Antitumor, anti-inflammatory and antiallergic effects of agaricus blazei mushroom extract and the related medicinal basidiomycetes mushrooms, hericium erinaceus and grifola frondosa: a review of preclinical and clinical studies. Nutrients. 2020;12(5) doi: 10.3390/nu12051339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shamsaei S., Getso M., Ahmadikia K., et al. Recent findings on the role of fungal products in the treatment of cancer. Clin. Transl. Oncol. 2021;23(2):197–204. doi: 10.1007/s12094-020-02428-1. [DOI] [PubMed] [Google Scholar]
- 13.Fritz H., Kennedy D.A., Ishii M., et al. Polysaccharide K and Coriolus versicolor extracts for lung cancer: a systematic review. Integr. Cancer Ther. 2015;14(3):201–211. doi: 10.1177/1534735415572883. [DOI] [PubMed] [Google Scholar]
- 14.Luo X.J., Li W., Yang L.F., et al. DAPK1 mediates the G1 phase arrest in human nasopharyngeal carcinoma cells induced by grifolin, a potential antitumor natural product. Eur. J. Pharmacol. 2011;670(2–3):427–434. doi: 10.1016/j.ejphar.2011.08.026. [DOI] [PubMed] [Google Scholar]
- 15.Luo X.J., Li L.L., Deng Q.P., et al. Grifolin, a potent antitumour natural product upregulates death-associated protein kinase 1 DAPK1 via p53 in nasopharyngeal carcinoma cells. Eur. J. Cancer. 2011;47(2):316–325. doi: 10.1016/j.ejca.2010.09.021. [DOI] [PubMed] [Google Scholar]
- 16.Wu Z., Li Y. Grifolin exhibits anti-cancer activity by inhibiting the development and invasion of gastric tumor cells. Oncotarget. 2017;8(13):21454–21460. doi: 10.18632/oncotarget.15250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jin S., Pang R.P., Shen J.N., Huang G., Wang J., Zhou J.G. Grifolin induces apoptosis via inhibition of PI3K/AKT signalling pathway in human osteosarcoma cells. Apoptosis. 2007;12(7):1317–1326. doi: 10.1007/s10495-007-0062-z. [DOI] [PubMed] [Google Scholar]
- 18.Che X., Yan H., Sun H., et al. Grifolin induces autophagic cell death by inhibiting the Akt/mTOR/S6K pathway in human ovarian cancer cells. Oncol. Rep. 2016;36(2):1041–1047. doi: 10.3892/or.2016.4840. [DOI] [PubMed] [Google Scholar]
- 19.Gao P., Wang H., Li H., et al. miR-21-5p inhibits the proliferation, migration, and invasion of glioma by targeting S100A10. J. Cancer. 2023;14(10):1781–1793. doi: 10.7150/jca.84030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang S., Xiao J., Wu J., et al. Tizoxanide promotes apoptosis in glioblastoma by inhibiting CDK1 activity. Front. Pharmacol. 2022;13 doi: 10.3389/fphar.2022.895573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Permatasari H.K., Wewengkang D.S., Tertiana N.I., et al. Anti-cancer properties of Caulerpa racemosa by altering expression of Bcl-2, BAX, cleaved caspase 3 and apoptosis in HeLa cancer cell culture. Front. Oncol. 2022;12(964816) doi: 10.3389/fonc.2022.964816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schabath M.B., Cote M.L. Cancer progress and priorities: lung cancer. Cancer Epidemiol. Biomarkers Prev. 2019;28(10):1563–1579. doi: 10.1158/1055-9965.EPI-19-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller M., Hanna N. Advances in systemic therapy for non-small cell lung cancer. Bmj. 2021;9(375) doi: 10.1136/bmj.n2363. [DOI] [PubMed] [Google Scholar]
- 24.Islam K.M., Anggondowati T., Deviany P.E., et al. Patient preferences of chemotherapy treatment options and tolerance of chemotherapy side effects in advanced stage lung cancer. BMC Cancer. 2019;19(1):19–6054. doi: 10.1186/s12885-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neergheen V.S., Hip Kam A., Pem Y., Ramsaha S., Bahorun T. Regulation of cancer cell signaling pathways as key events for therapeutic relevance of edible and medicinal mushrooms. Semin. Cancer Biol. 2022;80:145–156. doi: 10.1016/j.semcancer.2020.03.004. [DOI] [PubMed] [Google Scholar]
- 26.Li X., He Y., Zeng P., et al. Molecular basis for Poria cocos mushroom polysaccharide used as an antitumour drug in China. J. Cell Mol. Med. 2019;23(1):4–20. doi: 10.1111/jcmm.13564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bouyahya A., El Allam A., Zeouk I., et al. Pharmacological effects of grifolin: focusing on anticancer mechanisms. Molecules. 2022;27(1) doi: 10.3390/molecules27010284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ye M., Luo X., Li L., et al. Grifolin, a potential antitumor natural product from the mushroom Albatrellus confluens, induces cell-cycle arrest in G1 phase via the ERK1/2 pathway. Cancer Lett. 2007;258(2):199–207. doi: 10.1016/j.canlet.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 29.Ye M., Liu J.K., Lu Z.X., et al. Grifolin, a potential antitumor natural product from the mushroom Albatrellus confluens, inhibits tumor cell growth by inducing apoptosis in vitro. FEBS Lett. 2005;579(16):3437–3443. doi: 10.1016/j.febslet.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 30.Luo X., Yang L., Xiao L., et al. Grifolin directly targets ERK1/2 to epigenetically suppress cancer cell metastasis. Oncotarget. 2015;6(40):42704–42716. doi: 10.18632/oncotarget.5678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nandi S., Adhikary A., Acharya K. Anti-cancer effect of astrakurkurol from a folklore tribal mushroom on human hepatocellular carcinoma cells via mediating cell cycle inhibition, apoptosis, and migration. J. Food Biochem. 2022;46(1):22. doi: 10.1111/jfbc.14021. [DOI] [PubMed] [Google Scholar]
- 32.Lee H.S., Kim E.J., Kim S.H. Ethanol extract of Innotus obliquus (Chaga mushroom) induces G1 cell cycle arrest in HT-29 human colon cancer cells. Nutr. Res. Prac. 2015;9(2):111–116. doi: 10.4162/nrp.2015.9.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Din S.R.U., Zhong M., Nisar M.A., et al. Latcripin-7A, derivative of Lentinula edodes C(91-3), reduces migration and induces apoptosis, autophagy, and cell cycle arrest at G(1) phase in breast cancer cells. Appl. Microbiol. Biotechnol. 2020;104(23):10165–10179. doi: 10.1007/s00253-020-10918-z. [DOI] [PubMed] [Google Scholar]
- 34.Dang F., Nie L., Wei W. Ubiquitin signaling in cell cycle control and tumorigenesis. Cell Death Differ. 2021;28(2):427–438. doi: 10.1038/s41418-020-00648-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rahimi Lifshagerd M., Safari F. Therapeutic effects of hAMSCs secretome on proliferation of MDA-MB-231 breast cancer cells by the cell cycle arrest in G1/S phase. Clin. Transl. Oncol. 2023;25(6):1702–1709. doi: 10.1007/s12094-022-03067-4. [DOI] [PubMed] [Google Scholar]
- 36.Rahmani M., Nkwocha J., Hawkins E., et al. Cotargeting BCL-2 and PI3K induces BAX-dependent mitochondrial apoptosis in AML cells. Cancer Res. 2018;78(11):3075–3086. doi: 10.1158/0008-5472.CAN-17-3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yakop F., Abd Ghafar S.A., Yong Y.K., et al. Silver nanoparticles Clinacanthus Nutans leaves extract induced apoptosis towards oral squamous cell carcinoma cell lines. Artif. Cells, Nanomed. Biotechnol. 2018;46(sup2):131–139. doi: 10.1080/21691401.2018.1452750. [DOI] [PubMed] [Google Scholar]
- 38.Zhang C.C., Li C.G., Wang Y.F., et al. Chemotherapeutic paclitaxel and cisplatin differentially induce pyroptosis in A549 lung cancer cells via caspase-3/GSDME activation. Apoptosis. 2019;24(3–4):312–325. doi: 10.1007/s10495-019-01515-1. [DOI] [PubMed] [Google Scholar]
- 39.Yan H., Che X., Lv Q., et al. Grifolin induces apoptosis and promotes cell cycle arrest in the A2780 human ovarian cancer cell line via inactivation of the ERK1/2 and Akt pathways. Oncol. Lett. 2017;13(6):4806–4812. doi: 10.3892/ol.2017.6092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang M., Liu Y., Yin Y., et al. UBE2S promotes the development of ovarian cancer by promoting PI3K/AKT/mTOR signaling pathway to regulate cell cycle and apoptosis. Mol Med. 2022;28(1):22–489. doi: 10.1186/s10020-022-00489-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang L., Li C., Marhaba A., Zhu R., Jiapaer Z. ITF2357 induces cell cycle arrest and apoptosis of meningioma cells via the PI3K-Akt pathway. Med. Oncol. 2022;40(1):22–1883. doi: 10.1007/s12032-w. [DOI] [PubMed] [Google Scholar]
- 42.Noorolyai S., Shajari N., Baghbani E., Sadreddini S., Baradaran B. The relation between PI3K/AKT signalling pathway and cancer. Gene. 2019;698:120–128. doi: 10.1016/j.gene.2019.02.076. [DOI] [PubMed] [Google Scholar]
- 43.Fresno Vara J.A., Casado E., de Castro J., Cejas P., Belda-Iniesta C., González-Barón M. PI3K/Akt signalling pathway and cancer. Cancer Treat Rev. 2004;30(2):193–204. doi: 10.1016/j.ctrv.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 44.Tan A.C. Targeting the PI3K/Akt/mTOR pathway in non-small cell lung cancer (NSCLC) Thorac Cancer. 2020;11(3):511–518. doi: 10.1111/1759-7714.13328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krop I.E., Jegede O.A., Grilley-Olson J.E., et al. Phase II study of taselisib in PIK3CA-mutated solid tumors other than breast and squamous lung cancer: results from the NCI-match ECOG-ACRIN trial (EAY131) subprotocol I. JCO Precis Oncol. 2022;6(10) doi: 10.1200/PO.21.00424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xun G., Hu W., Li B. PTEN loss promotes oncogenic function of STMN1 via PI3K/AKT pathway in lung cancer. Sci. Rep. 2021;11(1):21–93815. doi: 10.1038/s41598-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.