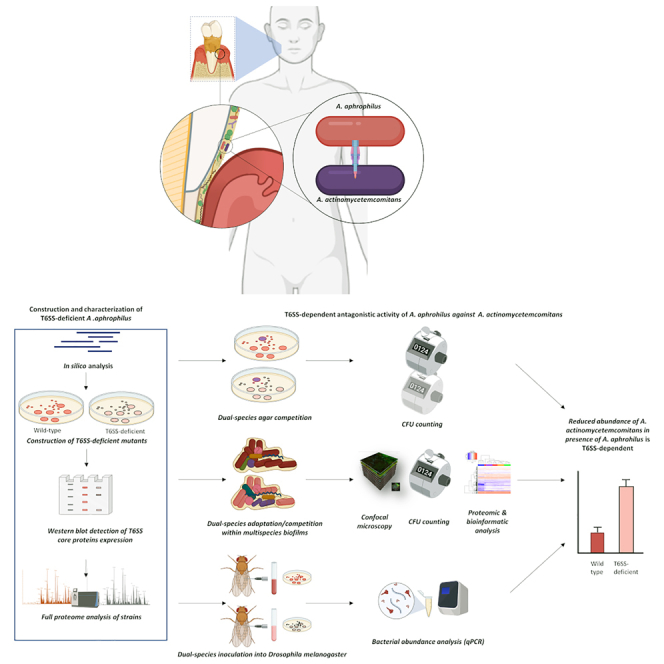
Summary
Microbial ecosystems experience spatial and nutrient restrictions leading to the coevolution of cooperation and competition among cohabiting species. To increase their fitness for survival, bacteria exploit machinery to antagonizing rival species upon close contact. As such, the bacterial type VI secretion system (T6SS) nanomachinery, typically expressed by pathobionts, can transport proteins directly into eukaryotic or prokaryotic cells, consequently killing cohabiting competitors. Here, we demonstrate for the first time that oral symbiont Aggregatibacter aphrophilus possesses a T6SS and can eliminate its close relative oral pathobiont Aggregatibacter actinomycetemcomitans using its T6SS. These findings bring nearer the anti-bacterial prospects of symbionts against cohabiting pathobionts while introducing the presence of an active T6SS in the oral cavity.
Subject areas: Bacteriology, Microbial flora, Microbial interactions
Graphical abstract
Highlights
-
•
Discovery that oral symbiont A. aphrophilus expresses type VI secretion system (T6SS)
-
•
A. aphrophilus uses the T6SS to kill its pathobiont relative A. actinomycetemcomitans
-
•
This T6SS-dependent killing was lost in mutants lacking the T6SS tube protein, Hcp
Bacteriology; Microbial flora; Microbial interactions
Introduction
Bacteria utilize secretion systems that enable them to exert their influence. The type VI secretion system (T6SS) is present in approximately 25% of Gram-negative bacterial species.1,2,3 The T6SS enables bacteria to suppress or eliminate vulnerable prokaryotic or eukaryotic cells through the direct delivery of toxic effector proteins into the target cells, causing cell death via diverse mechanisms. Cognate immunity proteins also play an important role in protecting bacteria against their own secreted T6SS effectors.2 T6SS-dependent bacterial antagonism has been shown to promote the persistence of Pseudomonas aeruginosa in the lungs of cystic fibrosis patients,4 and the establishment of Bacteroides species, and Salmonella Typhimurium as members of the gut microbiota.5,6
Although Aggregatibacter aphrophilus has been reported in some cases of infectious endocarditis and brain abscesses,7,8 it is frequently found in the oral cavity with no association with periodontitis, which is the most prevalent oral disease and the leading cause of adult tooth loss.9 Its genome encodes a potential T6SS,10 which has not been further confirmed or studied. A. aphrophilus is commonly found within the oral microbial community11 and is considered commensal due to the lack of association with oral disease. In contrast, Aggregatibacter actinomycetemcomitans, closely associated with A. aphrophilus and sharing approximately 80% gene content,9 is strongly associated with infective endocarditis12 and aggressive forms of periodontitis in young individuals.13 While A. actinomycetemcomitans expresses unique virulence factors in the oral microbiome, such as a leukotoxin, and a cytolethal distending toxin,14 the presence of a T6SS has neither been confirmed nor speculated within the oral microbiome.
The natural co-habitat of A. aphrophilus and A. actinomycetemcomitans is multi-species biofilms that form on the tooth surface (dental plaque), and potentially interacting with the juxtaposed oral epithelium.15,16,17 Dysbiotic shifts in the microbial composition of the biofilms can permit the establishment of oral infection.18 Dental biofilms provide an ideal environment for studying interspecies interactions and potential roles of T6SS in these.5 As a putative endogenous pathogen, A. actinomycetemcomitans exerts ecological pressure on other biofilm co-habitant species with potentially deleterious effects on the host.19,20 We hypothesize that A. aphrophilus acts as a niche competitor by expressing a functional T6SS, which may regulate behavior and virulence of the cogenerate oral pathogen A. actinomycetemcomitans upon co-existence. Hence, our work aimed to validate the presence of a T6SS in A. aphrophilus and investigate potential antagonism toward A. actinomycetemcomitans in various model systems.
Results
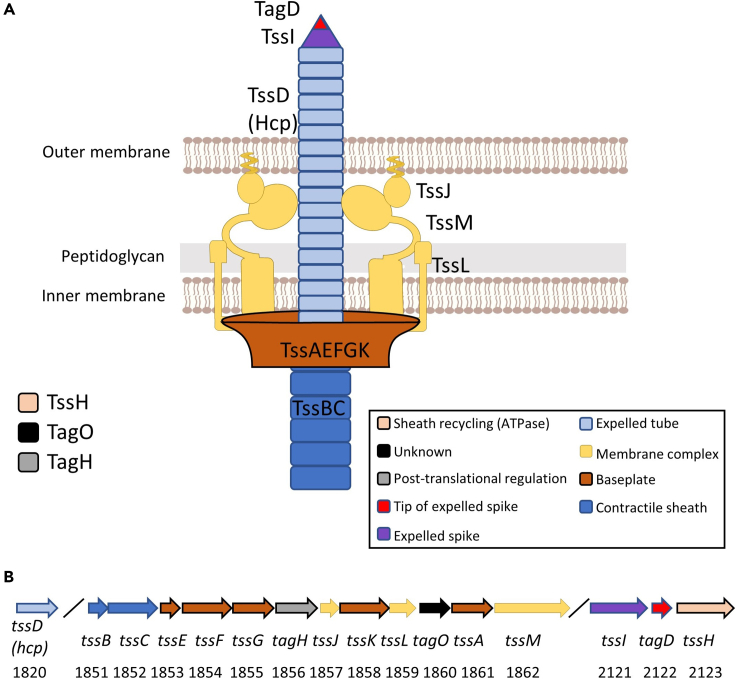
Prevalence of T6SS and its gene organization in A. aphrophilus
To confirm and assess the prevalence of the T6SS in A. aphrophilus, we searched whole genome sequences (n = 18) of 17 strains (National Center for Biotechnology; NCBI) using BLAST. Using Tss (tssA-M) nomenclature for core components, and Tag (tagA-P) for accessory proteins,1,21 we identified most of the 13 conserved T6SS core components and two accessory proteins (TssM is absent in strain C2008000870, and TagO is lacking in ATCC 7901) in all of the sequenced strains (Figure 1A, Tables S1 and S2). Ten core and two accessory genes were typically clustered in one major T6SS operon, whereas tssI, tagD, and tagH were grouped into a separate, auxiliary gene cluster (Figure 1B). Based on these findings together, we concluded that genes encoding a Type VI secretion system are present in all known A. aphrophilus strains. In contrast, according to BLAST search, we found no evidence of a T6SS gene cluster present in any of the genome-sequenced A. actinomycetemcomitans strains (data not shown).
Figure 1.
A. aphrophilus T6SS core components
(A) Overview of predicted functions of the respective components, according to in silico search, and which are color-coded in both panels. More details including their alternative names and UniProtKB ID are listed in Table S2.
(B) Schematic map of the T6SS-encoding main and auxiliary gene clusters, respectively, in A. aphrophilus reference strain NJ8700, with their respective gene numbers.10
Active expression of T6SS in A. aphrophilus during growth, in mono-species biofilms
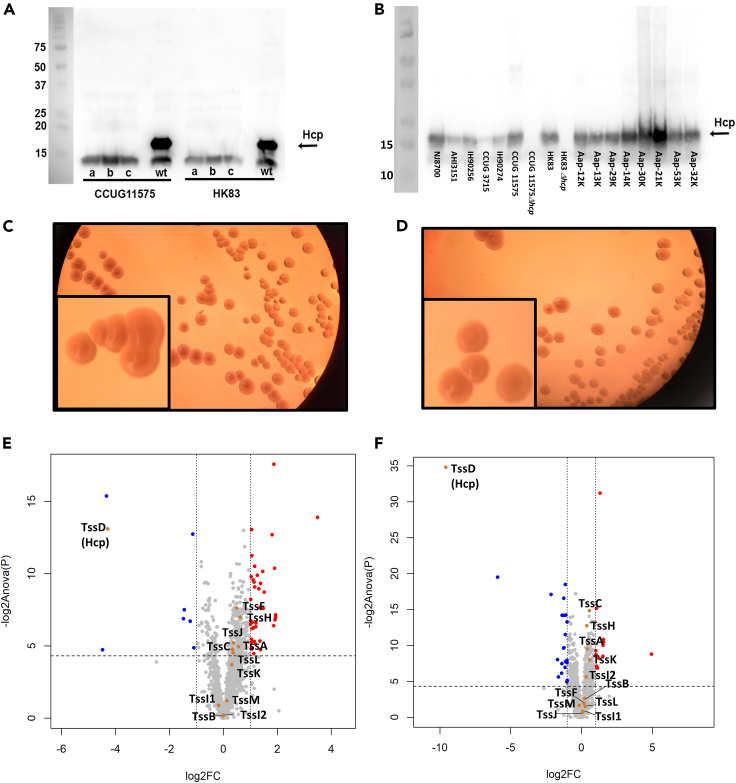
To initially assess the functional expression of Type VI secretion in A. aphrophilus strains, western blot was used to monitor the production of the “tube” core protein, hemolysin co-regulated protein (Hcp; TssD), a hallmark of T6SS activity,22 in mono-species biofilms. Mutant derivatives with an allelic replacement of hcp were generated in strains HK83 and CCUG 11575, as negative controls. This revealed the expression of Hcp in all wild-type (n = 15), but not in the hcp mutant (n = 2) strains (Figures 2A and 2B). This is consistent with presence of active T6SS secretion during bacterial growth. As an additional negative control, also A. actinomycetemcomitans strain D7SS was assessed, revealing no Hcp-specific reactive band in western blot (data not shown). Moreover, inactivation of the hcp gene had no apparent effect on bacterial growth as evidenced via colony morphology on blood agar (Figures 2C and 2D).
Figure 2.
Expression of Hcp in A. aphrophilus strains, and regulatory trends in protein expression profiles upon gene replacement of hcp in mono-species biofilms
(A) Western blot detection of Hcp expression in A. aphrophilus wildtype (wt) CCUG11575 and HK83 strains, and three independently isolated, isogenic hcpkan gene replacement mutants of each strain (a, b, and c). (B) Western blot detection of Hcp expression in A. aphrophilus strains. Similar colony morphology appearance of A. aphrophilus strain HK83. (C) and its hcp mutant derivative, HK83 Δhcp. (D) Cultivated on blood agar. The scale bar lengths correspond to 0.5 cm in all figures. (E) The Log2 fold change (FC) of label-free quantified full protein profiles in HK83 Δhcp compared with HK83 (n = 6 each). Upregulated [log2 (FC) ≥ 1, p ≤ 0.05] and downregulated [log2 (FC) ≤ −1, p ≤ 0.05] proteins were plotted in red and blue, respectively, while unregulated proteins were plotted in gray. T6SS core proteins were indicated in yellow. Vertical dashed lines represented |log2FC| = 1, and the horizontal dashed line represented p = 0.05. (F) The Log2FC of label-free quantified full protein profiles in CCUG 11575 hcp compared with CCUG 11575 (n = 6 each). Two TssI proteins (A0A3M6NIH8_AGGAP and A0A3M6PR51_AGGAP) recorded in Uniprot have been identified by us and are here indicated as Tssl1 and Tssl2.
Detection of T6SS core proteins and identification of a hcp-affected proteins in A. aphrophilus, in mono-species biofilms
To assess the expression of additional T6SS core proteins in A. aphrophilus and their potential regulation upon hcp inactivation, we analyzed proteome profiles of HK83 and CCUG 11575, and their respective hcp mutant derivatives, respectively, cultivated in mono-species biofilms (n = 6, each). We detected 1517 proteins with a protein false discovery rate of 0.73% (Table S3). When applying a 2-fold change (FC) and a significance cutoff of p < 0.05 on protein abundance, only 3% of the proteins were significantly regulated in the A. aphrophilus Δhcp strains, relative to their respective parental strains (39 up-regulated ↑, and 8 down-regulated ↓ in HK83 biofilms (Figure 2E), whereas 12↑ and 23↓ in CCUG 11575 biofilms (Figure 2F). Gene ontology (GO) analysis of the T6SS-regulated revealed specific gathered functions (i.e., maximally two proteins share the same biological process) (Tables S4 and S5).
In addition to Hcp, we also detected ten additional of the 12 T6SS core proteins, including both contractile sheath proteins (TssB and TssC), the sheath recycling ATPase, TssH, the “spike protein”, TssI, three baseplate proteins (TssA, TssK, and TssF), and all three membrane complex proteins (TssJ, TssL, and TssM) (indicated as brown dots in Figures 2E and 2F)(Table S3). Of note, the expressions of other T6SS core proteins did not exhibit significant differences between the wild types and their respective hcp mutations (Figures 2E and 2F). In addition to our in-silico analysis, this finding further confirms the presence of T6SS-core proteins in A. aphrophilus and suggests that they are affected by the deletion of hcp, at least in monoculture conditions.
A. aphrophilus exhibits a T6SS-dependent anti-bacterial activity against A. actinomycetemcomitans in vitro
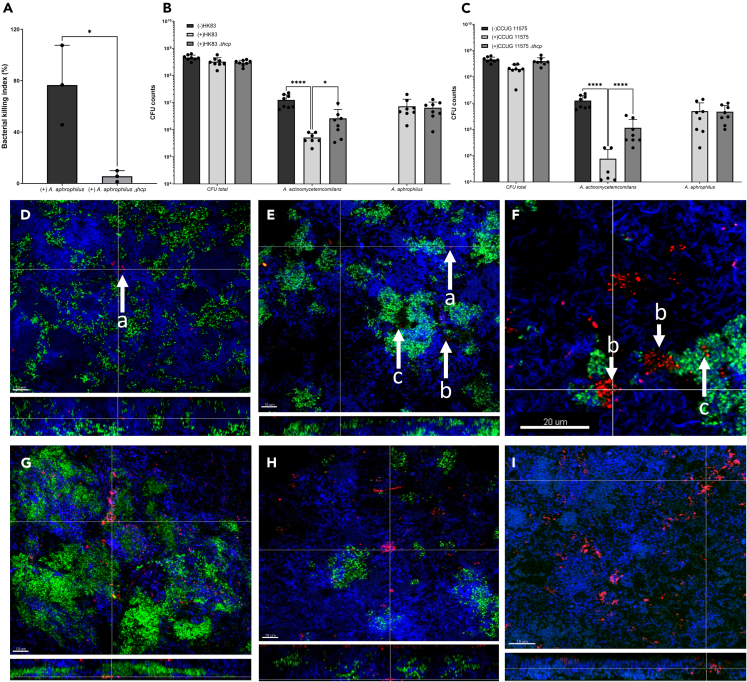
To test the importance of the T6SS in contact-dependent killing of competitor species, bacterial competition assays with A. aphrophilus strain HK83 or its hcp mutant were conducted on agar, with A. actinomycetemcomitans strain D7SS as prey. When quantifying the reduction of A. actinomycetemcomitans cell numbers using the bacterial killing index (based on ratio of A. aphrophilus CFU numbers in co-culture divided by those when in monoculture), a significant decrease (p = 0.017) was observed when co-incubated with A. aphrophilus HK83, in comparison to using HK83 Δhcp as a donor (Figure 3A). This reduction was substantial, with a mean ± SD of 76.6% ± 25.2% for the former and 5.6% ± 3.5% for the latter. Hence, we concluded that A. aphrophilus HK83 could indeed kill A. actinomycetemcomitans cells in vitro, whereas this property was lost in the hcp mutant, confirming the role of the T6SS in this anti-bacterial activity.
Figure 3.
T6SS-dependent anti-bacterial activity of A. aphrophilus against A. actinomycetemcomitans
The bacterial killing index (based on ratio of A. aphrophilus CFU numbers in co-culture divided by those when in monoculture) in an A. aphrophilus and A. actinomycetemcomitans co-culture environment on agar using either A. aphrophilus HK83 or HK83 Δhcp, and A. actinomycetemcomitans strain D7SS as prey (A). The relative cell abundances of A. aphrophilus and A. actinomycetemcomitans strain JP2, respectively, in the multi-species biofilms containing either A. aphrophilus strain HK83 or HK83 Δhcp (B), or CCUG11575 or CCUG11575 Δhcp (C). Quantification was performed using colony forming unit (CFU) counting from 8 biological replicates (ANOVA test: ∗p < 0.05). Localization of cells of A. actinomycetemcomitans strain JP2 (in red) and A. aphrophilus strains (in green) was achieved using Cyanine 3 (Cy3)-labelled A. actinomycetemcomitans 16S rRNA oligonucleotide probe Act639 and FAM-labeled A. aphrophilus 16S rRNA oligonucleotide probe Aaph639, respectively. This staining was performed within the multi-species biofilms: HK83 (D), CCUG11575 (E), CCUG11575 (F), HK83 Δhcp (G), and CCUG 11575 Δhcp (H). Panel (I) represents a multi-species biofilm that did not include A. aphrophilus. The letters on the arrows in (D) represent: a) Microcolonies of A. actinomycetemcomitans JP2 growing when A. aphrophilus was absent; b) Microcolonies of A. actinomycetemcomitans JP2 encounter with macrocolonies of A. aphrophilus in the vicinity; c) Single cells or small aggregates of A. actinomycetemcomitans JP2 can be identified within the biomass, embedded among microcolonies of A. aphrophilus. Panels D, E, G, H and I show a representative area of one disc, respectively. Panel (F) represents a magnified screenshot from an A. aphrophilus strain CCUG 11575-inclusive multi-species biofilm. This zoomed-in view aims to provide a closer depiction of the physical interaction between A. aphrophilus and A. actinomycetemcomitans. The scale bars for panels D, E, G, H, and I were 10, 10, 20, 10, 10, and 15 μm, respectively. Bacterial cells of additional species (blue) were counterstained with YoPro-1 iodide and Sytox Green, and their CFU data are listed in Table S6.
A. aphrophilus specifically kills A. actinomycetemcomitans in a T6SS-dependent manner in oral multi-species biofilms in vitro
As genetically similar, these bacterial species may compete within the same oral niche. To investigate this, we utilized in vitro multi-species biofilm models to mimic the natural habitat for assessing the killing activity of A. aphrophilus against A. actinomycetemcomitans. This multispecies biofilm also included six additional species, representing both early and late stages of oral biofilm development. These species are Actinomyces oris, Candida albicans, Fusobacterium nucleatum subsp. nucleatum, Streptococcus oralis, Streptococcus mutans, and Veillonella dispar. Both A. aphrophilus HK83 (7.33E06 ± 5.95E06) and HK83 Δhcp (2.64E6 ± 2.76E6) survived in high numbers within the multi-species biofilms (Figure 3B and Table S6). However, when A. aphrophilus HK83 was present, there were significant reductions (p < 0.05) in the abundance of the A. actinomycetemcomitans strain (5.14E5 ± 2.10E5) compared to the biofilm without A. aphrophilus (Figure 3B) (1.26E7± 6.86E6) or one containing HK83 Δhcp (2.64E6 ± 2.76E6). Interestingly, HK83 Δhcp, which lacks Hcp expression, exhibited reduced killing of the A. actinomycetemcomitans prey strain (p < 0.05), with the recovered A. actinomycetemcomitans numbers not significantly difference to those in the biofilm without A. aphrophilus. Notably, neither HK83 nor HK83 Δhcp had a significant impact on the growth of the other species within the biofilm. The only exceptional species was V. dispar, which levels were reduced by 6.51 times, as compared with 24.4 times for A. actinomycetemcomitans in the HK83 Δhcp biofilm compared with the control biofilm (Table S6). Similar patterns of T6SS-dependent fitness reduction of A. actinomycetemcomitans were observed within CCUG 11575-containing biofilms (Figure 3C and Table S6).
Using confocal laser scanning microscopy (CLSM) in combination with in situ hybridization (FISH)-stained biofilms, we determined the spatial localization of selected species within the biofilms (Figures 3D–3H). In biofilms containing wildtype A. aphrophilus, A. actinomycetemcomitans was found in low abundance and primarily detected as dispersed microcolonies when A. aphrophilus was absent from their vicinity (Figures 3D and 3E). In contrast, densely aggregated colonies of A. aphrophilus were abundantly distributed throughout the entire biofilm. In addition, we observed microcolonies of A. actinomycetemcomitans embedded within a matrix, primarily located at the forefront of A. aphrophilus accumulations, which indicates the potential presence of contact-dependent stress (Figure 3E). Moreover, when trapped within larger A. aphrophilus communities, A. actinomycetemcomitans mainly formed single or small aggregates (Figure 3F), suggesting a contact-specific elimination mechanism employed by A. aphrophilus against A. actinomycetemcomitans within the biofilm. However, in the presence of Δhcp A. aphrophilus strains (Figures 3G and 3H), or in biofilms without A. aphrophilus (Figure 3I), A. actinomycetemcomitans numbers were significantly higher and the biofilm structure appeared more tightly packed, resulting in a spatially more uniform distribution of A. actinomycetemcomitans.
T6SS-dependent metaproteome dynamics within the multi-species oral biofilms
We next assessed potential T6SS-dependent metaproteome dynamics in the co-habiting species within the multi-species biofilms containing A. aphrophilus, with or without Hcp expression (n = 4 for each group, biological replicates), to gain a concept of the potential interplay between bacteria in the oral ecosystem using our multi-species biofilm model, which is designed to replicate the composition and structures of natural dental biofilms.19,23,24
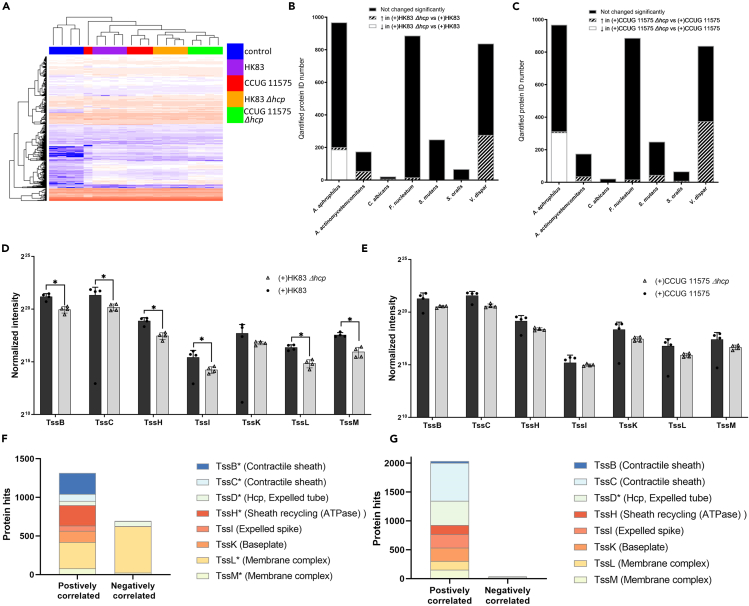
Label-free quantitative proteomics identified and quantified 3286 proteins (pro-FDR: 0.091%, Table S7). Unsupervised clustering analysis based on quantified protein abundance revealed that biofilms containing an A. aphrophilus Δhcp strain clustered together, irrespective of strain derivative used (Figure 4A), indicating a similar influence on the overall proteome composition of the biofilm. Furthermore, comparing protein abundances in biofilms containing a Δhcp strain instead of the isogenic wildtype, revealed a significant regulation of 564 proteins (abs(FC) > 2, p < 0.05) in HK83 biofilms, predominantly from A. aphrophilus (202) and V. dispar (277). In CCUG11575 biofilms, 795 proteins were significantly regulated, with 313 from A. aphrophilus and 375 from V. dispar (Figures 4B and 4C, Table S8). Unlike in mono-culture biofilm, A. aphrophilus proteins represented 21% of the regulated proteins in the HK83 Δhcp vs. the HK83 biofilms, and 32.2% in the CCUG11575 Δhcp vs. the CCUG 11575 biofilms (Figures 4B and 4C). This regulation in proteome composition seemed to also extend beyond known T6SS-related proteins, impacting cellular signaling such as carbohydrate processing, protein folding, and translation (Tables S8 and S9). Taken together, absence of Hcp expression in both A. aphrophilus strains not only altered the overall proteome composition of the entire biofilm, particularly impacting V. dispar but also changed various biological processes beyond the T6SS within A. aphrophilus itself.
Figure 4.
Metaproteomic shifts in multi-species biofilms
(A) Protein profiles from different biofilms were compared using heatmaps based on unsupervised clustering of label-free quantitation data. The multi-species biofilms included a non-A. aphrophilus control (blue), or an A. aphrophilus strain as follows: HK83 (purple), CCUG 11575 (red), and the hcp mutant strains HK83 Δhcp (yellow), and CCUG 11575 Δhcp (green) as indicated.
(B) The number of regulated proteins in HK83 Δhcp compared with HK83–included multi-species biofilms.
(C) Numbers of regulated proteins in CCUG 11575 Δhcp compare with CCUG 11575 multi-species biofilms. Actinomyces oris proteins were excluded from panels B and C due to the low number of proteins identified from this species (n = 3). In the HK83–containing biofilms, one protein was regulated, i.e., upregulated in HK83 Δhcp biofilms. Conversely, no A. oris protein was regulated in the CCUG 11575-containing biofilms. The normalized abundance of identified T6SS core proteins (excluding Hcp) in multi-species biofilms containing HK83 Δhcp or HK83 (D), and CCUG 11575 Δhcp or CCUG (E), respectively. The number of proteins correlated to T6SS core proteins in HK83 Δhcp or HK83- included in multi-species biofilms (F).
(G) CCUG 11575 Δhcp or CCUG 11575 included in multi-species biofilms.
The results are expressed as means ± standard deviations. The asterisk (∗) denotes the core proteins that have significant differences (p-value<0.05 and abs(log2FC)≥2) between the Δhcp mutated and wild-type-included multi-species biofilms.
Identification of T6SS core proteins within the multi-species biofilms, and their associated proteins
In addition to TssD (Hcp), seven out of 12 other core T6SS proteins (TssB, TssC, TssH, TssI, TssK, TssL, and TssM) were identified as part of the biofilm metaproteome. When HK83 was used, a significant upregulation of five T6SS-associated proteinswas observed. In contrast, although similar regulatory patterns were observed when strain CCUG 11575 Δhcp was used in the biofilm compared to when the wild-type CCUG 11575, the differences were not reached definition of significant (Figures 4D and 4E).
We aimed to investigate the regulatory impact of T6SS on the entire biofilm. Since there was no inter-species functional analysis tool available, our approach relied on establishing correlations between eight core proteins with other proteins (n = 3201) based on their abundance changes among individual samples, without necessarily inferring a cause-and-effect relationship. We found significantly positive correlations (r > 0.7, p-value<0.05) of the T6SS core proteins with 582 proteins, primarily from A. aphrophilus, and strong negative correlations (r < −0.7, p-value<0.05) with 643 proteins, mainly from V. dispar (Table S10). All eight core proteins demonstrated robust correlations with multiple proteins, with TssB, TssH, and TssL sharing more than half of the positive correlations. TssL was particularly associated with the majority of negative correlations (Figure 4F), mainly those originating from V. dispar. Similar patterns were observed in the biofilms containing A. aphrophilus CCUG 11575 biofilms (Figure 4G).
Besides core proteins, other factors may influence T6SS expression. For instance, lipopolysaccharide (LPS) can alter the bacterial surface composition, leading to a substantial regulation of surface proteins including T6SS core proteins. Therefore, we conducted a search for all proteins identified throughout the biofilm with “lipoprotein localization to outer membrane” GO terms. Notably, we found only three proteins meeting these criteria, all exclusively A. aphrophilus proteins (Tables S7, S8, and S9). These proteins demonstrated a significantly decreased in HK83 Δhcp biofilms and display strongly positive correlations with numerous T6SS core proteins (Table S10). These proteins are outer-membrane lipoprotein LolB (A0A3M6P2B3_AGGAP), outer-membrane lipoprotein carrier protein (A0A3M6P269_AGGAP, coded by lolA), and the lipoprotein-releasing system transmembrane subunit LolE (A0A3M6PJD7_AGGAP). Similarly, two non-T6SS bacterial secretion system-related proteins, TatA (A0A0K1N244_AGGAP) and VWA domain-containing protein (A0A3M6NR98_AGGAP), were also down-regulated and highly positively correlated with different T6SS core proteins. According to the computational results from the KEGG database), A0A0K1N244_AGGAP is involved in the bacterial secretion system as an inner membrane protein for twin arginine targeting, while A0A3M6NR98_AGGAP is one of the regulatory proteins for the T6SS system (Tables S7 and S10). In CCUG 11575-containing biofilms, we also observed similar patterns in non-T6SS core secretion proteins (Table S8). This suggests that Hcp not only significantly altered abundances of T6SS-related proteins but also exerted a regulatory effect on other secretion-related and lipoprotein-localized proteins in microbial communities.
The T6SS-dependent anti-bacterial activity of A. aphrophilus against A. actinomycetemcomitans in the Drosophila melanogaster infection model
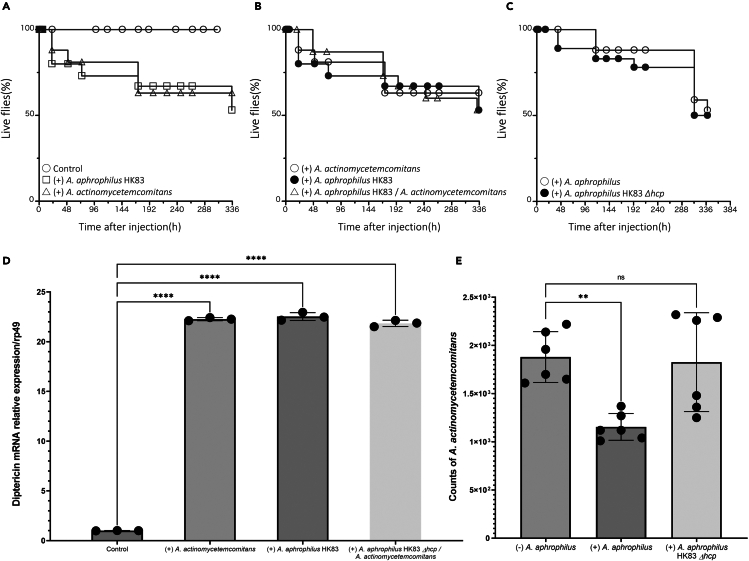
D. melanogaster relies on humoral and cell-mediated innate rather than adaptive immunity to defend against pathogens,25 making it a good model to mimic the cohabiting properties of the oral environment, where innate immunity plays a unique role by triggering a crucial systemic response to protect the host and maintain homeostasis.26 The T6SS-dependent antagonism of A. aphrophilus toward A. actinomycetemcomitans was next assessed in a D. melanogaster bacterial infection model. Under our experimental conditions, the A. aphrophilus and A. actinomycetemcomitans strains tested killed approximately 20% of the flies within two days, whereas around 50% remained alive even after 14 days (Figure 5A). Notably, the survival rate of D. melanogaster was similarly reduced regardless both bacterial species were co-injected, or upon mono-infection (Figure 5B). Neither did lack of Hcp expression in HK83 Δhcp affect the fly viability (Figure 5C). As humoral immune responses in Drosophila against Gram-negative bacteria are often induced through activation of the Imd pathway, inducing production of antimicrobial peptides, including diptericin, this was monitored. We observed a significant elevation of mRNA levels of diptericin in flies infected with the A. aphrophilus strains tested, but no significant difference between flies infected with either strain alone or with A. aphrophilus HK83 and A. actinomycetemcomitans JP2 combined (Figure 5D).
Figure 5.
The T6SS-dependent anti-bacterial activity of A. aphrophilus against A. actinomycetemcomitans in the D. melanogaster model
The survival (%) of flies was assessed as displayed in panels A-C as follows. Controls represent inoculation with PBS. D. melanogaster inoculated with the wild-type A. aphrophilus strain HK83, or with A. actinomycetemcomitans strain JP2 in monoinfection (A). Flies inoculated with A. actinomycetemcomitans JP2, and A. aphrophilus HK83 in monoinfection, and in co-infection with both strains (B). Flies inoculated with A. aphrophilus HK83 or with HK83 Δhcp in monoinfection (C). Panel D displays the abundances of A. actinomycetemcomitans in D. melanogaster 5 min after being inoculated, either with A. actinomycetemcomitans alone or in combination with A. aphrophilus HK83 or HK83 Δhcp. Panel E illustrates diptericin mRNA expressions in D. melanogaster across different groups: the none-inoculation group (control), A. actinomycetemcomitans alone, A. aphrophilus HK83 alone, or both HK83 Δhcp and A. actinomycetemcomitans. The mRNA expression levels were calculated using 2–ΔΔCt compared to the ribosomal protein rp49. The results are expressed as means ± standard deviations. Dunn’s multiple comparison test was performed to identify differences between individual time points (∗p < 0.001, ∗∗∗p < 0.00001).
To determine the infection level in flies with A. aphrophilus HK83 and A. actinomycetemcomitans JP2 alone or combined, we randomly selected viable flies and quantified the abundance of A. actinomycetemcomitans JP2 within the flies by qPCR. Lysates from uninfected flies did not yield detectable signals, suggesting endogenous bacteria did not affect the detection of these two bacteria. While levels of A. actinomycetemcomitans JP2 (mean ± SD 1155 ± 127) were significantly declined (p < 0.05) from flies once A. aphrophilus HK83 was co-injected, as compared with A. actinomycetemcomitans JP2 mono-inject group (1880 ± 241). Additionally, the Δhcp strains did not effectively eliminate A. actinomycetemcomitans JP2 (1827 ± 469) from flies, unlike the parental strain (Figure 5E). This establishes that A. aphrophilus effectively utilizes the T6SS to outcompete A. actinomycetemcomitans within this in vivo D. melanogaster model.
Discussion
Here we discovered and assessed the expression of a functional T6SS in A. aphrophilus, an oral commensal bacterium. According to the present results, A. aphrophilus, as a symbiont, uses this system to specifically target a pathobiont, i.e., its close relative A. actinomycetemcomitans, a species implicated in infective endocarditis and periodontitis among young individuals.13 To the best of our knowledge, this is the first commonly carried oral bacterium found to possess the T6SS secretion system. The comprehensive investigation of symbiont-pathobiont antagonism suggest that the A. aphrophilus T6SS serves a crucial role in the competitive activity of this species against A. actinomycetemcomitans in niches where the two related, yet strongly antagonistic, species may coexist.
Although there are free-living bacteria with the T6SS, T6SS is more common among complex microbial communities.27 Oral biofilms are highly diverse multi-species microbial communities encompassing more than 700 different bacterial species.28 A. aphrophilus and A. actinomycetemcomitans, show high genetic similarity (approximately 80% in gene content),9 suggesting their evolution from a common ancestor to establish a competitive relationship within the oral ecological habitat. Supporting this speculation, our recent assessment of the Maasai population supports this notion as, bacterial loads of A. actinomycetemcomitans and A. aphrophilus were inversely related in dental biofilms.29 Multi-species biofilm model was designed to replicate the composition and structures of natural dental biofilms,19,23,24 and demonstrated the proximity of these two species within the microbial community. Both Aggregatibacter species possess strong aggregative properties,30,31 which may facilitate the activity of a contact-dependent T6SS.32
The T6SS nanomachine delivers effector proteins targeting neighboring “prey” bacterial cells.33 The T6SS and its components were first named in Vibrio cholerae,22 however, T6SS-encoding genes were discovered earlier34 and believed to be widely distributed in nearly 25% of Gram-negative bacteria.33,35 A T6SS unit typically consists of 13 core protein components and a few accessory proteins, named using a unifying “Tss” and “Tag” nomenclature.1,21 Our in silico searches revealed genes encoding all 13 core components of the T6SS, along with three major accessory proteins, in most A. aphrophilus genomes available in the NCBI database. Evidently, expressing a T6SS is energetically expensive and tightly regulated in bacteria.36 In V. cholerae, the expression of TssD was halted at stationary phase37 as Hcp accumulation inhibits its own synthesis, and that of other T6SS components.38 However, except Hcp, we did not observe a complete or significant reduction in the expression of any of the other T6SS core components during mono-culture conditions. This discrepancy may be attributed to the apparent absence of the bacterial enhancer binding protein, VasH in A. aphrophilus genomes. In V. cholerae, VasH is encoded within the large virulence-associated secretion (VAS) cluster operon, which also encompasses several T6SS core components. Some of these core proteins, particularly TssM (VasK), have been shown to inhibit T6SS activity and the eradication of certain bacterial competitors, as well as host cells, in Drosophila models.39 Therefore, VasH is crucial for the Hcp-dependent regulation of T6SS component expression.38 VasH is a σ54-dependent-transcriptional activator in V. cholerae,40 suggesting the possibility that the T6SS of A. aphrophilus might be controlled within such a regulon as well. This remains to be experimentally assessed. Interestingly, in contrast to the mono-culture biofilms, we observed elevated levels of six out of eight T6SS core proteins in multi-species biofilms containing a wild-type A. aphrophilus strain compared to those with hcp mutant derivatives. Moreover, many non-T6SS related proteins were also associated with T6SS core proteins in multi-species biofilms, including A0A3M6NR98_AGGAP and all three lipoproteins A0A3M6P269_AGGAP, A0A3M6P2B3_AGGAP, and A0A3M6PJD7_AGGAP. The former one is homologous to PpKA in P. aeruginosa, known for its crucial role in T6SS regulation.41 Earlier studies have demonstrated that the depletion of lipopolysaccharide can alter the composition of the bacterial surface and subsequently lead to a substantial decrease in the expression of the Hcp homolog.42 Importantly, our mono-species experiment showed no regulation of these proteins, in contrast to the results observed in the multi-species model where regulation and association were evident. This suggests that the presence of other species in the multi-species environment can crucial for triggering Hcp-dependent regulation in T6SS core components and other proteins, potentially through quorum sensing mechanisms. In V. cholerae, quorum-sensing factor regulators such as LuxO,43 HapR,37 and quorum-regulatory small RNAs44 exhibited an apparent regulation on the T6SS expression. Although A. aphrophilus lacks the LuxO-HapR cascade-related quorum sensing regulation found in V, cholera, our studies discovered the LuxS production and other transport system ATP-binding proteins related to LuxS/AI-2 quorum sensing. Overall, the regulation of T6SS core proteins emphasizes the significance of T6SS regulation in a multi-species environment, likely mediated by quorum sensing mechanisms.
Furthermore, we used the D. melanogaster infection model to test whether T6SS-mediated pathobiont-symbiont interactions take place in vivo.26,39,45 It is a well-equipped model to study many aspects of host-bacterial interactions. Interestingly, we found that inoculated flies with all tested species, either alone or in combination with A. aphrophilus, was sufficient to kill the host independent of T6SS. This may imply that both species may use additional virulence factors to kill the host in a T6SS-independent manner. Finally, we noted that the antimicrobial response of Drosophila is nonspecific across both species, as evidenced by the absence of differential expression in diptericin. This finding aligns with previous studies by where non-specific Gram-negative bacteria-induced diptericin upregulation was documented. Despite, potent antimicrobial response, the elimination of A. actinomycetemcomitans by A. aphrophilus in Drosophila strongly support a role of A. aphrophilus T6SS as a highly functional molecular tool that enhances the fitness and competitive advantage of this species within their co-existing niches.
In summary, we demonstrate first time that oral symbiont Aggregatibacter aphrophilus possesses a T6SS and can eliminate its close relative oral pathobiont Aggregatibacter actinomycetemcomitans using its T6SS. The potential effect this system may have on V. dispar will be investigated in further studies. These findings bring newer the anti-bacterial prospects of symbionts against cohabiting pathobionts while introducing presence of an active T6SS in the oral cavity. Apart from being a seminal discovery in the field of oral ecology and beyond, it may represent an important step toward a more thorough understanding of bacterial competition strategies within complex ecosystems where they are present. Future studies on potential antibacterial effectors of the T6SS of A. aphrophilus will be pursued, and it is plausible that exploitation of the T6SS of A. aphrophilus could be used in future oral microbiome therapies as well.
Limitations of the study
A limitation of the present study is that we do not have data on the role of T6SS on the regulation of the host response, but we plan to address that in future studies.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Rabbit polyclonal antiserum specific for V. cholerae Hcp | Ishikawa et al.37 | RRID: AB_2313773 |
| Anti-rabbit horseradish peroxidase - conjugate | Jackson Immuno Research, Newmarket, UK | RRID: AB_2313773 |
| Bacterial and virus strains | ||
| A. aphrophilus strains HK83 | Culture Collection University of Gothenburg (CCUG) | CCUG 49494 |
| A. aphrophilus strains CCUG 11575 | CCUG | CCUG 11575 |
| A. aphrophilus strains NJ8700 | CCUG | NJ8700 |
| A. aphrophilus strains Aap-4K | Isolated from a patient29,46 | Aap-4K |
| A. aphrophilus strains Aap-12K | Isolated from a patient29,46 | Aap-12K |
| A. aphrophilus strains Aap-13K | Isolated from a patient29,46 | Aap-13K |
| A. aphrophilus strains Aap-21K | Isolated from a patient29,46 | Aap-21K |
| A. aphrophilus strains Aap-29K | Isolated from a patient29,46 | Aap-29K |
| A. aphrophilus strains Aap-30K | Isolated from a patient29,46 | Aap-30K |
| A. aphrophilus strains Aap-32K | Isolated from a patient29,46 | Aap-32K |
| A. aphrophilus strains Aap-53K | Isolated from a patient29,46 | Aap-53K |
| A. aphrophilus strains AHI-3151 | Isolated from a patient47,48 | AHI-3151 |
| A. aphrophilus strains IH-90256 | Isolated from a patient47,48 | IH-90256 |
| A. aphrophilus strains IH-90274 | Isolated from a patient47,48 | IH-90274 |
| A. actinomycetemcomitans strain D7SS | Isolated from a patient49 | D7SS |
| A. actinomycetemcomitans strain JP2 | Collection from division of Clinical Oral Microbiology and Immunology, university of Zurich (OMZ) | OMZ 295 |
| Actinomyces oris | OMZ | OMZ 745 |
| Candida albicans | OMZ | OMZ 110 |
| Fusobacterium nucleatum subsp. nucleatum KP-F2 | OMZ | OMZ 598 |
| Streptococcus oralis SK248 | OMZ | OMZ 607 |
| Streptococcus mutans UA159 | OMZ | OMZ 918 |
| V. dispar ATCC 17748T | OMZ | OMZ 493 |
| Chemicals, peptides, and recombinant proteins | ||
| Clarity™ Western ECL Substrate | Bio-Rad | Cat#1705062 |
| Deposited data | ||
| Proteomic raw files | ProteomeXchange | PXD042723 |
| Experimental models: Organisms/strains | ||
| Drosophila melanogaster | Kyorin-Fly, Kyorin University, Tokyo, Japan | Line Oregon R |
| Oligonucleotides | ||
| The A. actinomycetemcomitans-specific primers 5’-GAACCTTACCTACTCTTGACATCCGAA-3(forward) and 5’-TGCAGCACCTGTCTCAAAGC-3’ (reverse) | Choi et al.50 | N/A |
| Cyanine 3 -labelled A. actinomycetemcomitans 16S rRNA oligonucleotide probe Act639 5’-CTCCAGACCCCCAGTATG-3’ | Thurnheer and Belibasakis24 | Act639 |
| FAM-labelled A. aprophilus 16S rRNA oligonucleotide 5’-CTCTAGACCCCCAGTCTG-3’ | This work | Aaph639 |
| Software and algorithms | ||
| Quantable packages | https://github.com/protViz/quantable | |
| Progenesis QI for proteomics | https://www.nonlinear.com/progenesis/qi-for-proteomics/ | |
Resource availability
Lead contact
Further information and requests for reagents may be directed to and will be fulfilled by the lead contact, Jan Oscarsson (jan.oscarsson@umu.se).
Materials availability
All A. aphrophilus strains generated in this study are available upon request.
Data and code availability
Data: The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE51 partner repository with the dataset identifier PXD042723.
Experimental model and study participant details
A. aphrophilus strains were collected from Umeå University. Mutant derivatives were constructed there, and mono-species biofilm-related experiments were also performed in Umeå University. Multi-species biofilm-related experiments took place at University of Zurich, while Drosophila-related experiments were conducted at Sapporo Medical University. Protein extraction and proteomic experiments were carried out at the Functional Genomics Center Zurich. For additional details, please refer to the method details section.
Method details
Ethics considerations
All procedures were conducted in accordance with the guidelines of the local ethics committee at the Medical Faculty of Umeå University, which are in compliance with the Declaration of Helsinki (64th WMA General Assembly, Fortaleza, October 2013). The Drosophila experiment plan was approved by the ethics review committee of Kanazawa University (#6-1790).
Screening for Type VI secretion systems encoded in Aggregatibacter aphrophilus genomes
To identify T6SS component protein sequences, BLAST searches52 were conducted against whole genome sequence assemblies of A. aphrophilus strains (n=18 in December 2022) available in the NCBI database. These previously sequenced genomes and their GenBank accession numbers are listed in Table S1. Selected additional, A. aphrophilus strains were assessed by PCR, using the primer pairs hcp_F1 (5′-CCTACACCAGCGTTTATTTC-3′) and hcp_R1 (5’-CTGAGGTTTACGCCAGTC-3’), amplifying an internal fragment of the hcp gene.
Bacterial strains and growth conditions
The naturally genetic competent A. aphrophilus strains HK83 (CCUG 49494), and CCUG 11575 were originally sampled from saliva, and brain abscesses, respectively,53 and thereafter transformed into a V factor-independent growth phenotype.46 Mutant derivatives HK83 hcp::kan [Kmr] and CCUG 11575 hcp::kan [Kmr] were generated in the present work. CCUG 3715 and NJ8700 are reference strains of A. aphrophilus.10,53 Strains AHI-3151, IH-90256, and IH-90274 are part of the collection of clinical isolates of A. aphrophilus in our laboratory, made by Dr. Sirkka Asikainen.47,48 The A. aphrophilus strains Aap-4K, Aap-12K, Aap-13K, Aap-21K, Aap-29K, Aap-30K, Aap-32K, and Aap-53K were collected in a field study.29,46 A. actinomycetemcomitans strain D7SS is a naturally genetic competent, smooth-colony derivative of D7S (serotype a), which was originally isolated from a patient with aggressive periodontal disease.49 The A. actinomycetemcomitans and A. aphrophilus strains were routinely cultivated in air supplemented with 5% CO2 at 37°C as previously described,54 on blood agar plates (5% defibrinated horse blood, 5 mg hemin/l, 10 mg Vitamin K/l, Columbia agar base). Alternatively, for transformation assays, the A. aphrophilus strains were grown on Trypticase soy broth (TSB) supplemented with 0.1% yeast extract, 5% heat-inactivated horse serum, and 1.5% agar (sTSB agar). Escherichia coli laboratory strain DH5α55 was used for maintenance of the plasmid pUC4K, and was cultured aerobically at 37°C in Luria-Bertani (LB) broth, or on LB broth solidified with 1.5% (w/v) agar. When needed, growth media was supplemented with 100 μg/ml (final concentration) kanamycin, rifampicin, or streptomycin.
Construction of A. aphrophilus gene replacement mutant derivatives
A PCR-based approach following standard cloning procedures56 was used to construct the hcp gene replacement mutants in naturally competent strains of A. aphrophilus, i.e., HK83 and CCUG 11575. Whole genome sequence data of these strains were available via GenBank (assembly accession numbers GCA_003130375.1, and GCA_003703745.1, respectively), and used as references in oligonucleotide synthesis for gene replacement. In brief, PCR fragments flanking the hcp gene were amplified. An upstream, 856-bp fragment, was generated using the primers hcp_F2 (5’-CGAGCGCAGGATTATAGCAGCT-3’) and hcp_R2 (5’- AAACGCTGGTGGATCCATAGAATTCTC-3’), and a downstream, 1,024-bp fragment was generated using the primers hcp_F3 (5’-GATGACTGGCGGATCCCTCAGGTT-3’) and hcp_R3 (5’-CACCGCTTGTGTATTGGCAGTGGC -3’). The PCR primers contained a BamHI restriction site where indicated (underlined bold text), allowing ligation of the PCR fragments to flank the kanamycin resistance gene from pUC4K.57 Ligation products were then used to transform HK83 and CCUG 11575 on agar plates using procedures described earlier.49 Confirmation of allelic replacements and the orientation of the inserted resistance cassette were done by DNA sequencing and PCR. For this we used the hcp upstream and downstream oligonucleotide primers, respectively, in combination with a primer specific for the kanamycin resistance cassette (H7R: 5ʹ-GGACGGCGGCTTTGTTGAATAAATCG-3ʹ).
SDS-PAGE and western blot analysis
The SDS-Page and Western blot were used to detect expression of Hcp in the mono-species biofilm on the blood agar plates as described previously.58,59 For the Western blot, we used a rabbit polyclonal antiserum specific for V. cholerae Hcp37 (final dilution 1:5,000). The Hcp proteins of V. cholerae and A. aphrophilus exhibit approximately 70 % amino acid sequence identity. As a secondary antibody, anti-rabbit horseradish peroxidase (HRP)-conjugate was used (Jackson ImmunoResearch, Newmarket, UK) (1:10,000). Immunoreactive bands were visualized using Clarity™ Western ECL Substrate (Bio-Rad) and the ChemiDoc™ XRS+ System (Bio-Rad).
Bacterial killing assay of interbacterial virulence
Competition experiments on agar were carried out essentially as described earlier.60 Spontaneous rifampicin- and streptomycin-resistant derivatives of the A. aphrophilus and A. actinomycetemcomitans model strains, respectively, and A. aprophilus hcp mutants, were isolated for these experiments. Donor bacterial cells (OD600 = 1.7 in TSB) were mixed with recipient bacterial cells (OD600 = 1.3 in TSB) at a ratio of 3:1. Aliquots of 40 μl were spotted on blood agar plates and incubated overnight at 37°C (5% CO2). Cells were then harvested and competition analyzed. Colony-forming units (CFUs) of the donor and recipient were enumerated on blood agar plates supplemented with rifampin and streptomycin, respectively.
Multi-species biofilm formation and harvesting
A seven-species biofilm was cultivated as previously described.61 It contained A. actinomycetemcomitans strain JP2 (OMZ 295), Actinomyces oris (OMZ 745), Candida albicans (OMZ 110) Fusobacterium nucleatum subsp. nucleatum KP-F2 (OMZ 598), Streptococcus oralis SK248 (OMZ 607), Streptococcus mutans UA159 (OMZ 918), and V. dispar ATCC 17748T (OMZ 493) . Two modified biofilms, with an additional A. aphrophilus strain, i.e., HK83 (CCUG 49494) or HK83 Δhcp, were also developed in parallel. Briefly, 200 μl of each species at densities of OD550nm = 1.0 ( ± 0.05) were aliquoted on the surface of the hydroxyapatite (HA) dish and anaerobically incubated for 64 h. During the incubation, the cultivated medium was replenished at 16 h and 40 h, while the HA dishes were dip-washed in 0.9% w/v NaCl at 16 h, 20 h, 40 h, 44 h, 48 h, and 64 h. After the incubation, biofilms were collected in 0.9% w/v NaCl and processed for CFU count or incubated at -80°C for proteomic analysis. Biofilm supernatant was filtered with a 0.2 μm syringe filter (Acrodisc) before being stored at -80°C for further usage.
Protein extraction and clean up
Proteins from biofilm (n=4 for each) were extracted and lysed in Microcon YM-30 centrifugal filter unit (Millipore) following the protocol described previously.20 In brief, 20 μg of biofilm extracts were denatured with 8 M urea buffer (in 100 mM Tris/HCl buffer, pH 8.2), alkylated with 0.05 M iodoacetamide, washed by 0.5M NaCl, and digested by trypsin (Sigma-Aldrich) in an enzyme/protein ratio = 1:50 w/w overnight at room temperature. The digested solutions were then purified with StageTips (200 μL tip with a C18 disk core (Thermo Scientific)). Proteins from co-cultured samples were digested and cleaned using the in-StageTip (iST) sample preparation kit (PreOmic). Cells seeded in 24-well plates collected from co-cultured assay were washed with ice-cold phosphate-buffered saline (PBS), lysed in lysis buffer from the iST sample, and then removed by cell scraper. 50 μg of cured extracted proteins were collected and processed following the manufactoring protocol of the iST kit. Two snap-frozen flies were homogenized in 140 μL 8 M urea buffer (in 100 mM Tris/HCl buffer, pH 8.2) by a 5 mm stainless steel bead (Qiagen). The bead milling was repeated three times, each for five minutes at 30 Hz in a Tissuelyser II lysis (Qiagen). Then, 50 ug of extracted Drosophila proteins were digested using the iST sample preparation kit (PreOmic). The purified peptides were dried in a Speedvac (Thermo Savant SPD121P, Thermo Scientific), reconstituted in 50mM NH4FA (pH 10 with NH4OH) stock solution, then loaded onto StageTips to clean up the salt. The bound peptides were then eluted with 5, 10 and 30 % of acetonitrile (ACN) solutions (in 50mM NH4FA), respectively. Both purified and fractionated peptides were eventually dried in a Speedvac (Thermo Savant SPD121P, Thermo Scientific) and stored at –20°C until further usage.
LC-MS/MS analysis
Frozen peptides were reconstituted in 3% ACN and 0.1% formic acid. A pooled sample was created by mixing 1 μL of each sample for every experiment. All samples in the same experiment were subjected together in a random order to an Orbitrap Fusion mass spectrometer (Thermo Fisher Scientific) for proteomic analysis as described previously62 with the modification described below. In brief, peptides were first separated using a Thermo Scientific EASY-nLC 1200 system (Thermo Fisher Scientific) coupled to a 15 cm-long and 75 μm-diameter silica emitter as well as a ReproSil-Pur C18-AQ 120 A and 1.9 μm resin (Dr Maisch HPLC GmbH). A three-liner gradient of acetonitrile/water (containing 0.1% formic acid, at a flow rate of 300 nL/minute), first from 2% to 30% acetonitrile in 60 min, second from 30% to 97% in 10 minutes, then 97% for 10 min, was applied. The mass spectrometer was set in a data-dependent manner with an automatic switch between MS and MS/MS using the Xcalibur software package (Thermo Fisher Scientific). A mass range of 300–1500 m/z was selected for the Orbitrap analyzer.
Database generation
Two separate in-house databases were constructed, i.e., one for the mono-species biofilm (https://fgcz-ms.uzh.ch/FASTA/p2953_db5_d_Aaphro_20210803.fasta) and another for the multi-species biofilm (https://fgcz-ms.uzh.ch/FASTA/p2953_db5_d_Aaphro_20210803.fasta). Each database had a 260-sequences MS contaminants database (https://fgcz-proteomics.uzh.ch/fasta/fgcz_contaminants_20100901.fasta), and non-redundant databases containing all strains belonging to the target species sourced (i.e., A. aphrophilus for mono-species biofilms, and A. oris, A. aphrophilus, A. actinomycetemcomitans, C. albicans, F. nucleatum, Homo sapiens, S. cerevisiae, S. mutans, S. oralis, and V. dispar for mono-species biofilms.) from Uniprot. In addition, reverse sequences were included as decoys to facilitate the calculation of the false discovery rate. The Uniprot proteome identifiers (UPID) and the NCBI taxon identifiers for each database were listed in Table S11.
Functional analysis of proteins in biofilms
The Gene Ontology (GO) information of all identified proteins was downloaded from UniProt (accessed on September 13th, 2020, for multispecies biofilm and August 31st, 2021, for mono-species biofilm) to summarize the regulated proteins. The predicted function of A. aphrophilus was constructed based on the computational results from the KEGG database (accessed on December 19th, 2022).
Protein quantification
Progenesis QI for proteomics (version 4.1 Nonlinear Dynamics) was used for label-free quantification as described previously.62 In brief, all raw files from an experiment were aligned with its corresponding pooled sample as an alignment reference. All alignment results were thoroughly reviewed to verify that the alignment scores exceeded 50% before proceeding with peak picking. After peak picking, the normalization results were assessed to ensure that the difference between the largest and smallest values was within a 10-fold range. Any samples that failed to meet the expected alignment scores or normalization criteria were considered outliers and excluded from further analysis (not included in the experiment). Subsequently, peptides with charges 2+, 3+, or 4+ were selected for export as a mascot generic file (mgf). The top 5 MS/MS spectra per feature were chosen for export, and a maximum limit of 200 ions was enforced to control the fragment ion count. Deisotoping and charge deconvolution were included as essential steps in the data processing pipeline. The resulting mgf files were exported and searched using Mascot (version 2.4.1, Matrix Science) using the following parameters precursor tolerance: ± 10 ppm; fragment ion tolerance: ± 0.6 Da; Instrument type: LTQ-ORBI-Default; enzyme: trypsin; maximum missed cleavages: 2; fixed medication: Carbamidomethyl (C); variable modification: deamidated (NQ), oxidation (M) and acetyl (Protein N-term) against their corresponding databases.
The spectrum reports of the search result were generated by Scaffold v4.0 (Proteome Software) with a threshold of protFDR of 1%, minimal one peptide and pepFDR of 0.5% for biofilm and co-culture experiment, while the threshold for drosophila experiment was protFDR of 5%, minimal one peptide and pepFDR of 5%. These reports were imported in Progenesis QI for Proteomics for identifying the quantified proteins. For Drosophila experiments, each of the three fractions (i.e. Eluted from 5, 10 and 30 % of ACN) was first analysed separately and later recombined using the “combine analysed fractions” feather in Progenesis QI for Proteomics.
Only proteins with at least two peptides identified were considered in the study.
Data clustering and heat maps for regulated proteins
The R software (R: A Language and Environment for Statistical Computing, R Development Core Team) in particular the Quantable packages (https://github.com/protViz/quantable) were used to generate to heatmaps and unsupervised clustering analysis of quantified proteins.
Quantification and statistical analysis
The protein quantification data are derived from normalized protein abundances between the given two conditions within each experiment. The significance of differences for a specific protein between strains was calculated using a two-tailed Student's t-test in Progenesis QI. Proteins exhibiting an absolute log2-fold change >1 and a Student's t-test p-value < 0.05 were considered as truly regulated. Additionally, multiple comparison (q-value) and power analysis for each protein were provided using Progenesis QI, and these results are included in the corresponding supplementary tables.
Image processing
Images for Fig.s were assembled using Adobe Photoshop CS6 or Microsoft PowerPoint. The correlations between T6SS core proteins and other regulated proteins were generated with the R software in particular the corrplot packages (https://cran.r-project.org/web/packages/corrplot/index.html). The significance levels of the correlations were set to p<0.05.
Confocal laser scanning microscopy and image analysis
Confocal laser scanning microscopy (CLSM) and image analysis were employed to evaluate the localization pattern of A. aphrophilus and A. actinomycetemcomitans within the biofilm structure. In this study, biofilm-containing discs were prepared and subjected to fluorescence in situ hybridization (FISH) using a Cyanine 3 (Cy3)-labelled A. actinomycetemcomitans 16S rRNA oligonucleotide probe Act639 (5’-CTCCAGACCCCCAGTATG-3’),24 and a FAM-labelled A. aprophilus 16S rRNA oligonucleotide probe Aaph639 (5’-CTCTAGACCCCCAGTCTG-3’) (this work). The FISH-stained discs were counterstained with YoPro-1 iodide and Sytox Green, following the previously described protocol.63 Visualization of the stained samples was performed using a Leica SP-5 microscope equipped with a resonant scanner system (8000 Hz), an argon laser (excitation wavelengths: 458 nm, 476 nm, 488 nm, 496 nm, and 514 nm), and a helium-neon laser (excitation wavelengths: 561 nm, 594 nm, and 633 nm). Filters were set to detect green fluorescence from the YoPro-1 iodide and Sytox Green mixture (500-540 nm) and Cy3 (570-630 nm). A glycerol immersion objective with a numerical aperture (NA) of 1.3 and 63x magnification was used for image acquisition. The acquired images were further processed using Imaris 7.4.0 software (Bitplane) to reconstruct the biofilm structure virtually. This processing allowed for a comprehensive analysis of the localization pattern of A. actinomycetemcomitans within the biofilm.
D. melanogaster stocks and bacterial infection assays
The Drosophila lines Oregon R (Kyorin-Fly, Kyorin University, Tokyo, Japan) was used in this study and microinjection of the bacterial strains into a hemocoel was carried out as we reported previously.64 Briefly, male flies, 3∼7 days after eclosion (15 to 20 flies per vial, 1 to 3 vials in each experiment), were anaesthetized with CO2 and injected with 100 nL of PBS with or without (as control) 1/500 loop of bacteria using a nitrogen gas-operated microinjector (Narishige, Tokyo, Japan). Flies that had received infections were maintained at 29°C with the usual food until they were subjected to the analysis. The number of dead flies was counted to evaluate the virulence of the bacteria.
Real-time quantitative PCR (qPCR)
The abundance of A. actinomycetemcomitans within the flies was determined by qPCR as described previously.65 In brief, bacteria-injected flies were first homogenized with micromixer pestles and their genomic DNA was extracted using the GenElute bacterial genomic DNA kit (Sigma-Aldrich). The extracted DNA was subjected to quantitative PCR using THUNDERBIRD SYBR qPCR Mix (Toyobo, Osaka, Japan) and Mx5005p (Agilent, CA). The A. actinomycetemcomitans-specific primers 5’-GAACCTTACCTACTCTTGACATCCGAA-3’ (forward) and 5’-TGCAGCACCTGTCTCAAAGC-3’ (reverse)50 targeting the 16S ribosomal RNA were used. The bacterial numbers per fly were calculated using standard curves generated with A. actinomycetemcomitans DNA extracted from known cell numbers as described previously.62
Acknowledgments
This work was funded by grants from Swedish Research Council (2017-01198; 2021-03528, to N.B.), strategic funds from Karolinska Institutet (to G.N., and N.B.), TUA grants from Region Västerbotten, Sweden (7002667; to J.O.), and by funds from Insamlingsstiftelsen, Medical Faculty, Umeå University (to J.O.). We would like to thank Dr. Marek Basler, and Dr. Karina Persson for valuable discussions, and Manuela Flury, Elisabeth Granström, and Carina Öhman for their excellent technical advice and assistance.
Author contributions
All authors have made substantial contributions to the conception and design of the study. J.O., K.B., G.N.B., and N.B. were responsible for the study concept and design. J.O., K.B., A.S., J.G., W.W., K.M.A., M.L., A.J., F.R.M., and N.B. have been involved in data collection and data analysis. All authors have been involved in data interpretation, drafting the manuscript, and revising it critically and have given final approval of the version to be published.
Declaration of interests
The authors declare no competing interests.
Published: March 29, 2024
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2024.109650.
Contributor Information
Jan Oscarsson, Email: jan.oscarsson@umu.se.
Nagihan Bostanci, Email: nagihan.bostanci@ki.se.
Supplemental information
References
- 1.Cianfanelli F.R., Monlezun L., Coulthurst S.J. Aim, Load, Fire: The Type VI Secretion System, a Bacterial Nanoweapon. Trends Microbiol. 2016;24:51–62. doi: 10.1016/j.tim.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 2.Russell A.B., Peterson S.B., Mougous J.D. Type VI secretion system effectors: poisons with a purpose. Nat. Rev. Microbiol. 2014;12:137–148. doi: 10.1038/nrmicro3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singh R.P., Kumari K. Bacterial type VI secretion system (T6SS): an evolved molecular weapon with diverse functionality. Biotechnol. Lett. 2023;45:309–331. doi: 10.1007/s10529-023-03354-2. [DOI] [PubMed] [Google Scholar]
- 4.Mougous J.D., Cuff M.E., Raunser S., Shen A., Zhou M., Gifford C.A., Goodman A.L., Joachimiak G., Ordoñez C.L., Lory S., et al. A virulence locus of Pseudomonas aeruginosa encodes a protein secretion apparatus. Science. 2006;312:1526–1530. doi: 10.1126/science.1128393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Russell A.B., Wexler A.G., Harding B.N., Whitney J.C., Bohn A.J., Goo Y.A., Tran B.Q., Barry N.A., Zheng H., Peterson S.B., et al. A type VI secretion-related pathway in Bacteroidetes mediates interbacterial antagonism. Cell Host Microbe. 2014;16:227–236. doi: 10.1016/j.chom.2014.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sana T.G., Flaugnatti N., Lugo K.A., Lam L.H., Jacobson A., Baylot V., Durand E., Journet L., Cascales E., Monack D.M. Salmonella Typhimurium utilizes a T6SS-mediated antibacterial weapon to establish in the host gut. Proc. Natl. Acad. Sci. USA. 2016;113:E5044–E5051. doi: 10.1073/pnas.1608858113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kommedal Ø., Wilhelmsen M.T., Skrede S., Meisal R., Jakovljev A., Gaustad P., Hermansen N.O., Vik-Mo E., Solheim O., Ambur O.H., et al. Massive parallel sequencing provides new perspectives on bacterial brain abscesses. J. Clin. Microbiol. 2014;52:1990–1997. doi: 10.1128/JCM.00346-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Norskov-Lauritsen N. Classification, identification, and clinical significance of Haemophilus and Aggregatibacter species with host specificity for humans. Clin. Microbiol. Rev. 2014;27:214–240. doi: 10.1128/CMR.00103-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kittichotirat W., Bumgarner R.E., Chen C. Evolutionary Divergence of Aggregatibacter actinomycetemcomitans. J. Dent. Res. 2016;95:94–101. doi: 10.1177/0022034515608163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Di Bonaventura M.P., DeSalle R., Pop M., Nagarajan N., Figurski D.H., Fine D.H., Kaplan J.B., Planet P.J. Complete genome sequence of Aggregatibacter (Haemophilus) aphrophilus NJ8700. J. Bacteriol. 2009;191:4693–4694. doi: 10.1128/JB.00447-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tempro P.J., Slots J. Selective medium for the isolation of Haemophilus aphrophilus from the human periodontium and other oral sites and the low proportion of the organism in the oral flora. J. Clin. Microbiol. 1986;23:777–782. doi: 10.1128/jcm.23.4.777-782.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Winkelhoff A.J., Slots J. Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis in nonoral infections. Periodontol 2000. 1999;20:122–135. doi: 10.1111/j.1600-0757.1999.tb00160.x. [DOI] [PubMed] [Google Scholar]
- 13.Haubek D., Ennibi O.K., Poulsen K., Vaeth M., Poulsen S., Kilian M. Risk of aggressive periodontitis in adolescent carriers of the JP2 clone of Aggregatibacter (Actinobacillus) actinomycetemcomitans in Morocco: a prospective longitudinal cohort study. Lancet. 2008;371:237–242. doi: 10.1016/S0140-6736(08)60135-X. [DOI] [PubMed] [Google Scholar]
- 14.Belibasakis G.N., Maula T., Bao K., Lindholm M., Bostanci N., Oscarsson J., Ihalin R., Johansson A. Virulence and Pathogenicity Properties of Aggregatibacter actinomycetemcomitans. Pathogens. 2019;8 doi: 10.3390/pathogens8040222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fine D.H., Markowitz K., Furgang D., Velliyagounder K. Aggregatibacter actinomycetemcomitans as an early colonizer of oral tissues: epithelium as a reservoir? J. Clin. Microbiol. 2010;48:4464–4473. doi: 10.1128/JCM.00964-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dabdoub S.M., Tsigarida A.A., Kumar P.S. Patient-specific analysis of periodontal and peri-implant microbiomes. J. Dent. Res. 2013;92:168S–175S. doi: 10.1177/0022034513504950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paster B.J., Boches S.K., Galvin J.L., Ericson R.E., Lau C.N., Levanos V.A., Sahasrabudhe A., Dewhirst F.E. Bacterial diversity in human subgingival plaque. J. Bacteriol. 2001;183:3770–3783. doi: 10.1128/JB.183.12.3770-3783.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lamont R.J., Koo H., Hajishengallis G. The oral microbiota: dynamic communities and host interactions. Nat. Rev. Microbiol. 2018;16:745–759. doi: 10.1038/s41579-018-0089-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bao K., Bostanci N., Selevsek N., Thurnheer T., Belibasakis G.N. Quantitative proteomics reveal distinct protein regulations caused by Aggregatibacter actinomycetemcomitans within subgingival biofilms. PLoS One. 2015;10 doi: 10.1371/journal.pone.0119222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bao K., Bostanci N., Thurnheer T., Grossmann J., Wolski W.E., Thay B., Belibasakis G.N., Oscarsson J. Aggregatibacter actinomycetemcomitans H-NS promotes biofilm formation and alters protein dynamics of other species within a polymicrobial oral biofilm. NPJ Biofilms Microbiomes. 2018;4:12. doi: 10.1038/s41522-018-0055-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shalom G., Shaw J.G., Thomas M.S. In vivo expression technology identifies a type VI secretion system locus in Burkholderia pseudomallei that is induced upon invasion of macrophages. Microbiology. 2007;153:2689–2699. doi: 10.1099/mic.0.2007/006585-0. [DOI] [PubMed] [Google Scholar]
- 22.Pukatzki S., Ma A.T., Sturtevant D., Krastins B., Sarracino D., Nelson W.C., Heidelberg J.F., Mekalanos J.J. Identification of a conserved bacterial protein secretion system in Vibrio cholerae using the Dictyostelium host model system. Proc. Natl. Acad. Sci. USA. 2006;103:1528–1533. doi: 10.1073/pnas.0510322103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ammann T.W., Belibasakis G.N., Thurnheer T. Impact of early colonizers on in vitro subgingival biofilm formation. PLoS One. 2013;8 doi: 10.1371/journal.pone.0083090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thurnheer T., Belibasakis G.N. Integration of non-oral bacteria into in vitro oral biofilms. Virulence. 2015;6:258–264. doi: 10.4161/21505594.2014.967608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tzou P., De Gregorio E., Lemaitre B. How Drosophila combats microbial infection: a model to study innate immunity and host-pathogen interactions. Curr. Opin. Microbiol. 2002;5:102–110. doi: 10.1016/s1369-5274(02)00294-1. [DOI] [PubMed] [Google Scholar]
- 26.Yu J.C., Khodadadi H., Baban B. Innate immunity and oral microbiome: a personalized, predictive, and preventive approach to the management of oral diseases. EPMA J. 2019;10:43–50. doi: 10.1007/s13167-019-00163-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Unni R., Pintor K.L., Diepold A., Unterweger D. Presence and absence of type VI secretion systems in bacteria. Microbiology (Read.) 2022;168 doi: 10.1099/mic.0.001151. [DOI] [PubMed] [Google Scholar]
- 28.Hajishengallis G., Lambris J.D. Complement and dysbiosis in periodontal disease. Immunobiology. 2012;217:1111–1116. doi: 10.1016/j.imbio.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lindholm M., Claesson R., Kemoli A., Mulli T., Oscarsson J., Haubek D., Johansson A. Aggregatibacter actinomycetemcomitans and Aggregatibacter aphrophilus in a Kenyan Maasai adolescent population and inhibition of leukotoxic activity by herbal plants used as part of oral hygiene procedures. J. Clin. Med. 2021;10 doi: 10.3390/jcm10225402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kachlany S.C., Planet P.J., DeSalle R., Fine D.H., Figurski D.H. Genes for tight adherence of Actinobacillus actinomycetemcomitans: from plaque to plague to pond scum. Trends Microbiol. 2001;9:429–437. doi: 10.1016/s0966-842x(01)02161-8. [DOI] [PubMed] [Google Scholar]
- 31.Rempe K.A., Spruce L.A., Porsch E.A., Seeholzer S.H., Nørskov-Lauritsen N., St Geme J.W., 3rd Unconventional N-linked glycosylation promotes trimeric autotransporter function in Kingella kingae and Aggregatibacter aphrophilus. mBio. 2015;6 doi: 10.1128/mBio.01206-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin L., Capozzoli R., Ferrand A., Plum M., Vettiger A., Basler M. Subcellular localization of Type VI secretion system assembly in response to cell-cell contact. EMBO J. 2022;41 doi: 10.15252/embj.2021108595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ho B.T., Dong T.G., Mekalanos J.J. A view to a kill: the bacterial type VI secretion system. Cell Host Microbe. 2014;15:9–21. doi: 10.1016/j.chom.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Folkesson A., Löfdahl S., Normark S. The Salmonella enterica subspecies I specific centisome 7 genomic island encodes novel protein families present in bacteria living in close contact with eukaryotic cells. Res. Microbiol. 2002;153:537–545. doi: 10.1016/s0923-2508(02)01348-7. [DOI] [PubMed] [Google Scholar]
- 35.Lin L., Lezan E., Schmidt A., Basler M. Abundance of bacterial Type VI secretion system components measured by targeted proteomics. Nat. Commun. 2019;10:2584. doi: 10.1038/s41467-019-10466-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gutierrez J.M., Feizi A., Li S., Kallehauge T.B., Hefzi H., Grav L.M., Ley D., Baycin Hizal D., Betenbaugh M.J., Voldborg B., et al. Genome-scale reconstructions of the mammalian secretory pathway predict metabolic costs and limitations of protein secretion. Nat. Commun. 2020;11:68. doi: 10.1038/s41467-019-13867-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ishikawa T., Rompikuntal P.K., Lindmark B., Milton D.L., Wai S.N. Quorum sensing regulation of the two hcp alleles in Vibrio cholerae O1 strains. PLoS One. 2009;4 doi: 10.1371/journal.pone.0006734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Manera K., Caro F., Li H., Pei T.T., Hersch S.J., Mekalanos J.J., Dong T.G. Sensing of intracellular Hcp levels controls T6SS expression in Vibrio cholerae. Proc. Natl. Acad. Sci. USA. 2021;118 doi: 10.1073/pnas.2104813118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fast D., Kostiuk B., Foley E., Pukatzki S. Commensal pathogen competition impacts host viability. Proc. Natl. Acad. Sci. USA. 2018;115:7099–7104. doi: 10.1073/pnas.1802165115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seibt H., Aung K.M., Ishikawa T., Sjöström A., Gullberg M., Atkinson G.C., Wai S.N., Shingler V. Elevated levels of VCA0117 (VasH) in response to external signals activate the type VI secretion system of Vibrio cholerae O1 El Tor A1552. Environ. Microbiol. 2020;22:4409–4423. doi: 10.1111/1462-2920.15141. [DOI] [PubMed] [Google Scholar]
- 41.Mougous J.D., Gifford C.A., Ramsdell T.L., Mekalanos J.J. Threonine phosphorylation post-translationally regulates protein secretion in Pseudomonas aeruginosa. Nat. Cell Biol. 2007;9:797–803. doi: 10.1038/ncb1605. [DOI] [PubMed] [Google Scholar]
- 42.Henry R., Vithanage N., Harrison P., Seemann T., Coutts S., Moffatt J.H., Nation R.L., Li J., Harper M., Adler B., Boyce J.D. Colistin-resistant, lipopolysaccharide-deficient Acinetobacter baumannii responds to lipopolysaccharide loss through increased expression of genes involved in the synthesis and transport of lipoproteins, phospholipids, and poly-beta-1,6-N-acetylglucosamine. Antimicrob. Agents Chemother. 2012;56:59–69. doi: 10.1128/AAC.05191-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zheng J., Shin O.S., Cameron D.E., Mekalanos J.J. Quorum sensing and a global regulator TsrA control expression of type VI secretion and virulence in Vibrio cholerae. Proc. Natl. Acad. Sci. USA. 2010;107:21128–21133. doi: 10.1073/pnas.1014998107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shao Y., Bassler B.L. Quorum regulatory small RNAs repress type VI secretion in Vibrio cholerae. Mol. Microbiol. 2014;92:921–930. doi: 10.1111/mmi.12599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fast D., Petkau K., Ferguson M., Shin M., Galenza A., Kostiuk B., Pukatzki S., Foley E. Vibrio cholerae-Symbiont Interactions Inhibit Intestinal Repair in Drosophila. Cell Rep. 2020;30:1088–1100.e5. doi: 10.1016/j.celrep.2019.12.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lindholm M., Min Aung K., Nyunt Wai S., Oscarsson J. Role of OmpA1 and OmpA2 in Aggregatibacter actinomycetemcomitans and Aggregatibacter aphrophilus serum resistance. J. Oral Microbiol. 2019;11 doi: 10.1080/20002297.2018.1536192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Doğan B., Asikainen S., Jousimies-Somer H. Evaluation of two commercial kits and arbitrarily primed PCR for identification and differentiation of Actinobacillus actinomycetemcomitans, Haemophilus aphrophilus, and Haemophilus paraphrophilus. J. Clin. Microbiol. 1999;37:742–747. doi: 10.1128/jcm.37.3.742-747.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Paju S., Carlson P., Jousimies-Somer H., Asikainen S. Actinobacillus actinomycetemcomitans and Haemophilus aphrophilus in systemic and nonoral infections in Finland. APMIS. 2003;111:653–657. doi: 10.1034/j.1600-0463.2003.1110608.x. [DOI] [PubMed] [Google Scholar]
- 49.Wang Y., Goodman S.D., Redfield R.J., Chen C. Natural transformation and DNA uptake signal sequences in Actinobacillus actinomycetemcomitans. J. Bacteriol. 2002;184:3442–3449. doi: 10.1128/JB.184.13.3442-3449.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Choi H., Kim E., Kang J., Kim H.J., Lee J.Y., Choi J., Joo J.Y. Real-time PCR quantification of 9 periodontal pathogens in saliva samples from periodontally healthy Korean young adults. J. Periodontal Implant Sci. 2018;48:261–271. doi: 10.5051/jpis.2018.48.4.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Perez-Riverol Y., Bai J., Bandla C., García-Seisdedos D., Hewapathirana S., Kamatchinathan S., Kundu D.J., Prakash A., Frericks-Zipper A., Eisenacher M., et al. The PRIDE database resources in 2022: a hub for mass spectrometry-based proteomics evidences. Nucleic Acids Res. 2022;50:D543–D552. doi: 10.1093/nar/gkab1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Altschul S.F., Madden T.L., Schäffer A.A., Zhang J., Zhang Z., Miller W., Lipman D.J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Norskov-Lauritsen N., Kilian M. Reclassification of Actinobacillus actinomycetemcomitans, Haemophilus aphrophilus, Haemophilus paraphrophilus and Haemophilus segnis as Aggregatibacter actinomycetemcomitans gen. nov., comb. nov., Aggregatibacter aphrophilus comb. nov. and Aggregatibacter segnis comb. nov., and emended description of Aggregatibacter aphrophilus to include V factor-dependent and V factor-independent isolates. Int. J. Syst. Evol. Microbiol. 2006;56:2135–2146. doi: 10.1099/ijs.0.64207-0. [DOI] [PubMed] [Google Scholar]
- 54.Karched M., Ihalin R., Eneslätt K., Zhong D., Oscarsson J., Wai S.N., Chen C., Asikainen S.E. Vesicle-independent extracellular release of a proinflammatory outer membrane lipoprotein in free-soluble form. BMC Microbiol. 2008;8:18. doi: 10.1186/1471-2180-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hanahan D. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 1983;166:557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 56.Sambrook J.E., Fritsch E.F., Maniatis T. Cold Spring Harbor Laboratory Press; 1989. Molecular Cloning: A Laboratory Manual. [Google Scholar]
- 57.Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982;19:259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- 58.Laemmli U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 59.Paul-Satyaseela M., Karched M., Bian Z., Ihalin R., Borén T., Arnqvist A., Chen C., Asikainen S. Immunoproteomics of Actinobacillus actinomycetemcomitans outer-membrane proteins reveal a highly immunoreactive peptidoglycan-associated lipoprotein. J. Med. Microbiol. 2006;55:931–942. doi: 10.1099/jmm.0.46470-0. [DOI] [PubMed] [Google Scholar]
- 60.Ishikawa T., Sabharwal D., Bröms J., Milton D.L., Sjöstedt A., Uhlin B.E., Wai S.N. Pathoadaptive conditional regulation of the type VI secretion system in Vibrio cholerae O1 strains. Infect. Immun. 2012;80:575–584. doi: 10.1128/IAI.05510-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thurnheer T., van der Ploeg J.R., Giertsen E., Guggenheim B. Effects of Streptococcus mutans gtfC deficiency on mixed oral biofilms in vitro. Caries Res. 2006;40:163–171. doi: 10.1159/000091065. [DOI] [PubMed] [Google Scholar]
- 62.Bao K., Bostanci N., Thurnheer T., Belibasakis G.N. Proteomic shifts in multi-species oral biofilms caused by Anaeroglobus geminatus. Sci. Rep. 2017;7:4409. doi: 10.1038/s41598-017-04594-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bao K., Belibasakis G.N., Thurnheer T., Aduse-Opoku J., Curtis M.A., Bostanci N. Role of Porphyromonas gingivalis gingipains in multi-species biofilm formation. BMC Microbiol. 2014;14:258. doi: 10.1186/s12866-014-0258-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hashimoto Y., Tabuchi Y., Sakurai K., Kutsuna M., Kurokawa K., Awasaki T., Sekimizu K., Nakanishi Y., Shiratsuchi A. Identification of lipoteichoic acid as a ligand for draper in the phagocytosis of Staphylococcus aureus by Drosophila hemocytes. J. Immunol. 2009;183:7451–7460. doi: 10.4049/jimmunol.0901032. [DOI] [PubMed] [Google Scholar]
- 65.Ammann T.W., Bostanci N., Belibasakis G.N., Thurnheer T. Validation of a quantitative real-time PCR assay and comparison with fluorescence microscopy and selective agar plate counting for species-specific quantification of an in vitro subgingival biofilm model. J. Periodontal. Res. 2013;48:517–526. doi: 10.1111/jre.12034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data: The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE51 partner repository with the dataset identifier PXD042723.