Abstract
Background
Periodontitis is a prevalent inflammatory periodontal disease that has an impact on the overall quality of life. Although several studies have indicated an association between individual vitamin intake and periodontitis risk, the associations of the multivitamins with periodontitis risk remain unclear.
Aim
This study aimed to explore the joint effect of multivitamins (including vitamin A, vitamin B1, vitamin B2, vitamin B6, vitamin B12, vitamin C, vitamin D, vitamin E, and vitamin K) on periodontitis.
Methods
For this cross-sectional study, data were collected from participants aged ≥ 30 years in the National Health and Nutrition Examination Surveys 2009–2014 (n = 9,820). We employed weighted multivariate logistic regression models to evaluate the single association between individual vitamin intakes and periodontitis, and Bayesian kernel machine regression (BKMR), weighted quantile sum (WQS) regression, and quantile g-computation (qgcomp) models to assess the joint effect of nine vitamins on periodontitis.
Results
The overall prevalence of periodontitis was approximately 35.97%. After adjustment of covariates, vitamin B6 [odds ratio (OR) = 0.82, 95% confidence interval (CI): 0.72–0.94] and vitamin E (OR = 0.79, 95%CI: 0.69–0.92) were negatively related to the likelihood of developing periodontitis, respectively. The result of three models indicated that, mixture of vitamin A, vitamin B1, vitamin B2, vitamin B6, vitamin B12, vitamin C, vitamin D, vitamin E, and vitamin K had a significant negative combined effect on the risk of periodontitis. In the BKMR model, when all remaining vitamins were at their median levels, the periodontitis risk decreased with increased concentration levels of vitamin E and vitamin B2. WQS analysis indicated the highest weighted chemical was vitamin E, followed by vitamin B12 and vitamin D. In the qgcomp model, vitamin E received the highest negative weights for the periodontitis risk, followed by vitamin B2 and vitamin D, respectively.
Conclusion
Both dietary vitamin B6 and vitamin E were associated with decreased odds of periodontitis. Additionally, the mixture-exposed analyses consistently showed the negative correlations between nine dietary vitamins mixtures and periodontitis.
Keywords: multiple vitamins co-exposure, periodontitis, Bayesian kernel machine regression, weighted quantile sum, quantile g-computation model
Introduction
Periodontitis is a prevalent inflammatory periodontal disease that constitutes the primary etiology of tooth loss in adults (1). In addition to its impact on oral health, periodontitis has been linked to an increased susceptibility to chronic conditions such as cardiovascular disease (CVD) (2), prediabetes (3), Alzheimer’s disease (4), and pre-eclampsia (5). Therefore, it holds immense significance to identify modifiable influencing factors for the prevention and management of periodontitis.
Nutrition is recognized as a key modifiable factor for periodontitis, and maintaining a well-balanced nutrition is crucial for promoting periodontal health (6). Vitamin, which is crucial element for maintaining normal physiological functions in the human body, can be obtained from the diet and nutritional supplements (7). A number of epidemiological studies have found the association between vitamin and periodontitis risk. For example, vitamin C and vitamin E, can actively participate in the oxidative stress process within the body and exert an anti-inflammatory effect by eliminating reactive oxygen species. As a result, they effectively prevent the occurrence and progression of periodontitis (8, 9). A survey based on National Health and Nutrition Examination Survey (NHANES) data revealed a non-linear relationship between dietary vitamin C intake and periodontitis risk, and both too low and high vitamin C intake were associated with an elevated periodontitis (10). A recent study investigated the association between different vitamin intake and periodontitis risk, revealing a negative correlation between levels of vitamin A and vitamin B2 intake and periodontitis risk. Conversely, excessive consumption of vitamin B1 may elevate the risk of developing periodontitis (11). However, to the best of our knowledge, most current nutritional epidemiological studies solely evaluate the impact of individual vitamin intake on periodontitis (9–11), rather than considering co-intakes of all vitamins. In general, populations are typically exposed to multiple dietary vitamins in combination, which may result in synergistic, or antagonistic effects (12).
Therefore, this study aims to explore the joint effect of multivitamins on periodontitis based on NHANES database, including vitamin A, vitamin B1, vitamin B2, vitamin B6, vitamin B12, vitamin C, vitamin D, vitamin E, vitamin K, which provided certain references for the diet management of periodontal health.
Methods
Study participants
This cross-sectional study used data from NHANES database 2009–2014. NHANES is a nationally representative survey of the non-institutionalized U.S. population, gathering data through self-reported questionnaires, laboratory assessments and clinical examinations (13). The NHANES only collects periodontal examination data for individuals aged over 30 years between 2009 and 2014. The NHANES database was reviewed and approved by the Ethics Review committees of both the Centers for Disease Control (CDC) and the National Center for Health Statistics (NCHS) (Protocol Number: NHANES 2009–2010: Protocol #2005–06; NHANES 2011–2014: Protocol #2011–17.). The requirement of ethical approval for this was waived by the Institutional Review Board of Liuzhou People’s Hospital, because the data was accessed from NHANES (a publicly available database). The need for written informed consent was waived by the Institutional Review Board of Liuzhou People’s Hospital due to retrospective nature of the study.
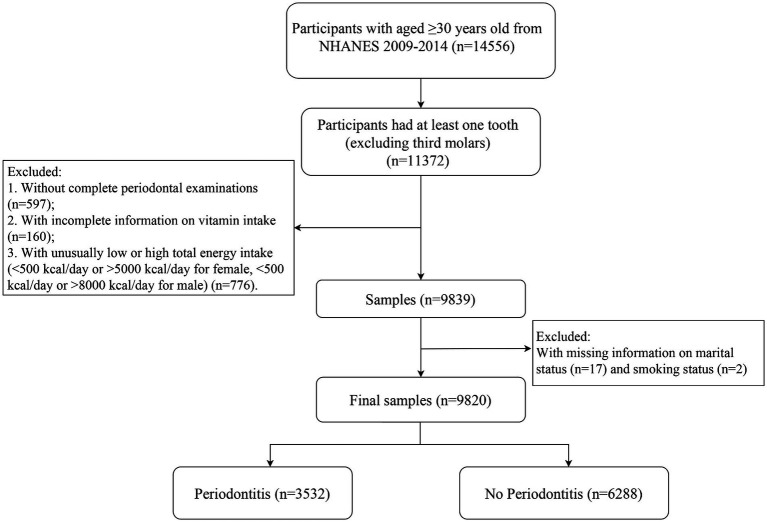
For the purpose of this study, we included participants aged≥30 years old who had at least one tooth (excluding third molars) (n = 11372). Notably, the exclusion criteria were listed as follows: (1) Participants who did not undergo complete periodontal examinations. (2) Participants with incomplete information on vitamin intake. (3) Participants with unusually low or high total energy intake (<500 kcal/day or > 5000 kcal/day for female, <500 kcal/day or > 8000 kcal/day for male). (4) Participants with missing information on key co-variables. The analysis ultimately encompassed a total of 9820 subjects. The detailed flow chart of this study is depicted in Figure 1.
Figure 1.
Flow chart for the selection of participants in the study.
Exposure and outcome variables
For each NHANES participant, the NHANES database employs a 24 h dietary recall method to gather information on participants’ diets (12). In this study, vitamins included vitamin A, vitamin B1, vitamin B2, vitamin B6, vitamin B12, vitamin C, vitamin D, vitamin E, and vitamin K. Vitamins intake levels were assessed by both dietary intake and dietary supplement intake. The total daily intake of vitamins was obtained by summing the first 24 h dietary recall interviews and the daily intake of dietary supplements. Each dietary vitamin in this study is divided into two categories according to the weighted median.
Periodontitis was considered as the primary outcome in this study. Clinical examinations of periodontal and dental status were conducted by dental examiners for NHANES participants aged ≥30 years between 2009 and 2014. Used the criteria proposed by Eke et al. (14), periodontitis was categorized into three groups: mild, moderate, and severe. Periodontitis was regarded as mild if there were ≥ 2 interproximal sites with attachment loss (AL) ≥3 mm, and ≥ 2 interproximal sites with pocket depth (PD) ≥4 mm (not on the same tooth) or one site with PD ≥5 mm. The definition of moderate periodontitis was established as the presence of 2 interproximal sites with AL ≥ 4 mm, not on the same tooth, or the presence of at least 2 interproximal sites with PD ≥5 mm, not on the same tooth. Severe periodontitis was defined as 2 interproximal sites with AL ≥ 6 mm (not on the same tooth), or ≥ 1 interproximal sites with PD ≥ 5 mm (not on the same tooth). In this study, mild, moderate, and severe periodontitis were classified as having periodontitis.
Covariates
Possible covariates in this study were as follows: age (years), gender (male/female), race/ethnicity (white, black, or other race), education level (<high school, or ≥ high school), marital status (married & living with a partner, or never married& divorced& separated& widowed), poverty-income ratio (PIR, <1, ≥1, or unknown), smoking status (never smoked, former smoker, or current smoker), alcohol consumption (never drinker, moderate drinker, heavy drinker, or unknown), body mass index (BMI, kg/m2, <25, 25–30, or ≥ 30), physical activity [metabolic equivalent (MET)·min/week, <450, or ≥ 450], hypertension (No/Yes), diabetes (No/Yes), dyslipidemia (No/Yes), CVD (No/Yes), nonsteroidal anti-inflammatory agents (No/Yes), anti-infectives (No/Yes), serum vitamin D (<50 nmol/L, or ≥ 50 nmol/L), white blood cell (WBC, 1000 cells/uL), decayed teeth (No/Yes), dental floss (No/Yes), total energy (kcal/day), total sugars (g/day), and forms of vitamin A [including Alpha-carotene (μg), Beta-carotene (μg), Beta-cryptoxanthin (μg), Lycopene (μg), Lutein + zeaxanthin (μg)]. Alcohol consumption was determined based on drinking frequency: never drinkers (0 drinks per day), moderate drinkers (<2 drinks per day for males and < 1 drink per day for females), and heavy drinkers (≥2 drinks per day for males and ≥ 1 drink per day for females). BMI category was defined as three levels according to World Health Organization (WHO) guidelines: BMI < 25 kg/m2 (underweight & normal), 25 ≤ BMI < 30 kg/m2 (overweight), and BMI ≥ 30 kg/m2 (obesity).
Statistical analysis
Three weighted variables were adopted: WTDRD1, SDMVPSU, and SDMVSTRA. The measurement data were reported as Mean ± standard error (Mean ± S.E), and group comparisons were conducted T-test or F-test. Counting data were presented as the number of cases and proportion of components, with differences between groups assessed Chi-square test. The missing values in this study were imputed using multiple interpolation methods, and a sensitivity analysis was performed on the data before and after interpolation (Supplementary Table S1). First, taking periodontitis as the outcome, we developed weighted univariate logistic regression to identify significant variables (p < 0.05), which was used to perform the multivariate logistic regression analysis, and the backward stepwise regression method was employed to further screen the covariates (Supplementary Table S2). Second, we used weighted univariate and multivariate logistic regression models to evaluate the association between individual vitamin intakes and periodontitis. Odds ratio (OR) with 95% confidence interval (CI) were calculated. Lastly, we employed three mixture analysis methods: Bayesian kernel machine regression (BKMR), weighted quantile sum (WQS) regression, and quantile g-computation (qgcomp) models, to assess the joint effect of nine vitamins on periodontitis.
BKMR model-integrating Bayesian and statistical learning approaches to estimate the nonlinear and/or interactive effect in the association between exposure and outcome. Pearson correlation analysis was used to calculate the correlation coefficient between the nine vitamins. Nine vitamins were grouped according to the correlation coefficient diagram. The Group Posterior Inclusion Probability (GroupPIP) and Conditional Posterior Inclusion Probability (CondPIP) quantify the probability of each group, and vitamin of each group being included in the model, indicating their respective contributions to the overall effect. We used R packages “bkmr” to assess the joint effects of nine vitamins on periodontitis risk. Meanwhile, the exposure of single vitamin and periodontitis risk were also obtained.
WQS regression model-assessing the combined effects of nine vitamins co-exposure, and the contributing effects of individual vitamin (15). We used the R package “WQS” to calculate the WQS index comprised of weighted sums of individual vitamin concentrations. The weight assigned to each vitamin in the WQS index reflects its individual contribution to the overall impact.
Quantile g-computation (qgcomp) model-assessing the change in periodontitis risk for a simultaneous one quantile increase in the nine vitamins, irrespective of the direction of correlation between exposures and results (16). The sum of the positive and negative weights is 2. R package “qgcomp” was used to perform the analysis.
In addition, SAS 9.4 and R 4.2.3 software were used for statistical analyses. p < 0.05 was considered as statistically significant difference.
Results
Basic characteristics of the study subjects
A total of 9,820 subjects were included in this study, with a mean age of 50.95 ± 0.26 years and a male population accounting for 48.91%. Table 1 presents the basic characteristics of the included subjects. 37.52% subjects were obesity. The average total energy for all population was 2158.68 ± 14.42 kcal/day. The overall prevalence of periodontitis is approximately 35.97% (n = 3,532) in this study. Compared with the non- periodontitis group, periodontitis group was characterized by a higher age, lower education level, higher rates of hypertension and diabetes history, higher WBC level, total energy, and total sugars (p < 0.05).
Table 1.
Basic characteristics of the study subjects.
| Variables | Total (n = 9,820) | Non-periodontitis (N = 6,288) | Periodontitis (N = 3,532) | p |
|---|---|---|---|---|
| Age, years, Mean ± S. E | 50.95 ± 0.26 | 50.61 ± 0.29 | 51.80 ± 0.41 | 0.009 |
| Gender, n (%) | <0.001 | |||
| Female | 4,967 (51.09) | 3,591 (56.01) | 1,376 (39.03) | |
| Male | 4,853 (48.91) | 2,697 (43.99) | 2,156 (60.97) | |
| Race/ethnicity, n (%) | <0.001 | |||
| White | 4,348 (69.14) | 3,161 (74.64) | 1,187 (55.67) | |
| Black | 2004 (10.46) | 1,071 (7.94) | 933 (16.64) | |
| Others | 3,468 (20.40) | 2056 (17.42) | 1,412 (27.69) | |
| Education level, n (%) | <0.001 | |||
| Less than high school | 2,212 (14.73) | 1,081 (10.77) | 1,131 (24.44) | |
| High school or above | 7,608 (85.27) | 5,207 (89.23) | 2,401 (75.56) | |
| Marital status, n (%) | <0.001 | |||
| Married & Living with partner | 6,400 (68.79) | 4,201 (71.09) | 2,199 (63.15) | |
| Never married &Divorced &Separated &Widowed | 3,420 (31.21) | 2087 (28.91) | 1,333 (36.85) | |
| PIR, n (%) | <0.001 | |||
| <1 | 1,688 (11.12) | 875 (8.47) | 813 (17.60) | |
| ≥1 | 7,360 (82.70) | 4,975 (86.02) | 2,385 (74.57) | |
| Unknown | 772 (6.18) | 438 (5.51) | 334 (7.83) | |
| Smoking status, n (%) | <0.001 | |||
| Never smoked | 5,519 (55.98) | 3,828 (60.42) | 1,691 (45.10) | |
| Former smoker | 2,481 (26.67) | 1,600 (26.90) | 881 (26.09) | |
| Current smoker | 1820 (17.36) | 860 (12.68) | 960 (28.80) | |
| Drinking status, n (%) | 0.004 | |||
| Never drinker | 2,418 (19.35) | 1,608 (19.74) | 810 (18.39) | |
| Moderate drinker | 879 (9.50) | 558 (9.88) | 321 (8.58) | |
| Heavy drinker | 4,893 (57.12) | 3,164 (57.59) | 1729 (55.95) | |
| Unknown | 1,630 (14.03) | 958 (12.79) | 672 (17.08) | |
| Physical activity, MET· min/week, n (%) | 0.003 | |||
| <450 | 3,510 (32.26) | 2,221 (31.20) | 1,289 (34.87) | |
| ≥450 | 6,310 (67.74) | 4,067 (68.80) | 2,243 (65.13) | |
| Hypertension, n (%) | <0.001 | |||
| No | 4,106 (44.71) | 2,782 (46.86) | 1,324 (39.43) | |
| Yes | 5,714 (55.29) | 3,506 (53.14) | 2,208 (60.57) | |
| Diabetes, n (%) | <0.001 | |||
| No | 7,973 (85.84) | 5,239 (87.67) | 2,734 (81.37) | |
| Yes | 1847 (14.16) | 1,049 (12.33) | 798 (18.63) | |
| Dyslipidemia, n (%) | 0.710 | |||
| No | 2,530 (25.54) | 1,644 (25.69) | 886 (25.18) | |
| Yes | 7,290 (74.46) | 4,644 (74.31) | 2,646 (74.82) | |
| CVD, n (%) | 0.156 | |||
| No | 7,943 (83.45) | 5,096 (83.89) | 2,847 (82.39) | |
| Yes | 1877 (16.55) | 1,192 (16.11) | 685 (17.61) | |
| Nonsteroidal anti-inflammatory agents, n (%) | 0.295 | |||
| No | 8,569 (87.71) | 5,465 (87.99) | 3,104 (87.03) | |
| Yes | 1,251 (12.29) | 823 (12.01) | 428 (12.97) | |
| Anti-infectives, n (%) | 0.032 | |||
| No | 9,368 (94.87) | 5,965 (94.47) | 3,403 (95.85) | |
| Yes | 452 (5.13) | 323 (5.53) | 129 (4.15) | |
| BMI, kg/m2, n (%) | <0.001 | |||
| <25 | 2,593 (27.20) | 1757 (28.55) | 836 (23.89) | |
| 25–30 | 3,393 (35.28) | 2,181 (35.61) | 1,212 (34.46) | |
| ≥30 | 3,834 (37.52) | 2,350 (35.84) | 1,484 (41.66) | |
| Serum vitamin D, nmol/L, n (%) | <0.001 | |||
| <50 | 2,908 (22.39) | 1,608 (18.63) | 1,300 (31.60) | |
| ≥50 | 6,912 (77.61) | 4,680 (81.37) | 2,232 (68.40) | |
| WBC, 1000 cells/uL, Mean ± S. E | 7.13 ± 0.04 | 6.97 ± 0.04 | 7.52 ± 0.07 | <0.001 |
| Decayed teeth, n (%) | <0.001 | |||
| No | 6,822 (75.47) | 4,860 (81.97) | 1962 (59.56) | |
| Yes | 2,998 (24.53) | 1,428 (18.03) | 1,570 (40.44) | |
| Dental floss, n (%) | <0.001 | |||
| No | 3,130 (27.34) | 1,665 (23.19) | 1,465 (37.51) | |
| Yes | 6,690 (72.66) | 4,623 (76.81) | 2067 (62.49) | |
| Total energy, kcal/day, Mean ± S. E | 2158.68 ± 14.42 | 2127.75 ± 15.90 | 2234.46 ± 24.92 | <0.001 |
| Total sugars, g/day, Mean ± S. E | 112.34 ± 1.18 | 110.53 ± 1.51 | 116.77 ± 1.49 | 0.003 |
| Vitamin A, μg, n (%) | <0.001 | |||
| <528.00 | 5,298 (49.96) | 3,240 (47.74) | 2058 (55.38) | |
| ≥528.00 | 4,522 (50.04) | 3,048 (52.26) | 1,474 (44.62) | |
| Vitamin B1, μg, n (%) | 0.005 | |||
| <1884.00 | 5,343 (49.99) | 3,366 (48.89) | 1977 (52.70) | |
| ≥1884.00 | 4,477 (50.01) | 2,922 (51.11) | 1,555 (47.30) | |
| Vitamin B2, μg, n (%) | <0.001 | |||
| <2464.00 | 5,582 (49.97) | 3,446 (48.19) | 2,136 (54.33) | |
| ≥2464.00 | 4,238 (50.03) | 2,842 (51.81) | 1,396 (45.67) | |
| Vitamin B6, μg, n (%) | <0.001 | |||
| <2455.00 | 5,340 (49.99) | 3,293 (47.90) | 2047 (55.09) | |
| ≥2455.00 | 4,480 (50.01) | 2,995 (52.10) | 1,485 (44.91) | |
| Vitamin B12, μg, n (%) | <0.001 | |||
| <6.99 | 5,415 (49.98) | 3,344 (48.20) | 2071 (54.34) | |
| ≥6.99 | 4,405 (50.02) | 2,944 (51.80) | 1,461 (45.66) | |
| Vitamin C, mg, n (%) | <0.001 | |||
| <89.40 | 5,132 (49.97) | 3,137 (47.77) | 1995 (55.37) | |
| ≥89.40 | 4,688 (50.03) | 3,151 (52.23) | 1,537 (44.63) | |
| Vitamin D, μg, n (%) | <0.001 | |||
| <6.60 | 5,256 (49.66) | 3,207 (46.97) | 2049 (56.26) | |
| ≥6.60 | 4,564 (50.34) | 3,081 (53.03) | 1,483 (43.74) | |
| Vitamin E, mg, n (%) | <0.001 | |||
| <7.48 | 5,404 (49.94) | 3,314 (47.63) | 2090 (55.59) | |
| ≥7.48 | 4,416 (50.06) | 2,974 (52.37) | 1,442 (44.41) | |
| Vitamin K, μg, n (%) | <0.001 | |||
| <82.70 | 5,345 (49.98) | 3,261 (47.61) | 2084 (55.80) | |
| ≥82.70 | 4,475 (50.02) | 3,027 (52.39) | 1,448 (44.20) | |
| Alpha-carotene, μg, Mean ± S. E | 467.94 ± 24.48 | 501.15 ± 27.76 | 386.57 ± 24.84 | <0.001 |
| Beta-carotene, μg, Mean ± S. E | 2574.78 ± 92.75 | 2763.62 ± 116.01 | 2112.12 ± 102.61 | <0.001 |
| Beta-cryptoxanthin, μg, Mean ± S. E | 89.33 ± 2.28 | 88.53 ± 2.86 | 91.30 ± 4.93 | 0.652 |
| Lycopene, μg, Mean ± S. E | 5445.93 ± 159.91 | 5443.55 ± 188.35 | 5451.76 ± 215.51 | 0.974 |
| Lutein+ zeaxanthin, μg, Mean ± S. E | 1956.90 ± 78.89 | 2083.75 ± 96.07 | 1646.11 ± 113.30 | 0.003 |
PIR, poverty-income ratio; BMI, body mass index; MET, metabolic equivalent; CVD, cardiovascular disease; WBC, white blood cell.
Single vitamin exposure and periodontitis risk
The relationship between single dietary vitamin intake and periodontitis is presented in Table 2. In the weighted univariate logistic regression model (Model 1), higher level of nine dietary vitamins were all inversely associated with the odds of periodontitis risk. After adjusting age, gender, race/ethnicity, educational level, marital status, PIR, smoking status, anti-infectives, serum vitamin D, WBC, decayed teeth, dental floss, we found that higher intake levels of vitamin B6 (OR = 0.82, 95%CI: 0.72–0.94, p = 0.009) and vitamin E (OR = 0.79, 95%CI: 0.69–0.92, p = 0.004) were related to a decreased likelihood of developing periodontitis compared to lower intake levels, respectively.
Table 2.
Single dietary vitamin intake and periodontitis risk.
| Variables | Model 1 | Model 2 | ||
|---|---|---|---|---|
| OR (95%CI) | p | OR (95%CI) | p | |
| Vitamin A, μg | ||||
| <528.00 | Ref | Ref | ||
| ≥528.00 | 0.74 (0.65–0.83) | <0.001 | 0.88 (0.76–1.02) | 0.094 |
| Vitamin B1, μg | ||||
| <1884.00 | Ref | Ref | ||
| ≥1884.00 | 0.86 (0.78–0.95) | 0.005 | 0.94 (0.83–1.06) | 0.323 |
| Vitamin B2, μg | ||||
| <2464.00 | Ref | Ref | ||
| ≥2464.00 | 0.78 (0.70–0.88) | <0.001 | 0.89 (0.77–1.03) | 0.132 |
| Vitamin B6, μg | ||||
| <2455.00 | Ref | Ref | ||
| ≥2455.00 | 0.75 (0.67–0.84) | <0.001 | 0.82 (0.72–0.94) | 0.009 |
| Vitamin B12, μg | ||||
| <6.99 | Ref | Ref | ||
| ≥6.99 | 0.78 (0.71–0.86) | <0.001 | 0.93 (0.82–1.04) | 0.209 |
| Vitamin C, mg | ||||
| <89.40 | Ref | Ref | ||
| ≥89.40 | 0.74 (0.66–0.82) | <0.001 | 0.93 (0.82–1.04) | 0.218 |
| Vitamin D, μg | ||||
| <6.60 | Ref | Ref | ||
| ≥6.60 | 0.69 (0.62–0.77) | <0.001 | 0.87 (0.76–1.00) | 0.054 |
| Vitamin E, mg | ||||
| <7.48 | Ref | Ref | ||
| ≥7.48 | 0.73 (0.63–0.84) | <0.001 | 0.79 (0.69–0.92) | 0.004 |
| Vitamin K, μg | ||||
| <82.70 | Ref | Ref | ||
| ≥82.70 | 0.72 (0.62–0.84) | <0.001 | 0.87 (0.73–1.04) | 0.128 |
OR, odds ratio; CI, confidence interval. Model 1 was crude model; Model 2 was adjusted for age, gender, race/ethnicity, educational level, marital status, poverty-income ratio, smoking status, anti-infectives, serum vitamin D, white blood cell, decayed teeth, dental floss.
Multi-vitamin exposures and periodontitis risk
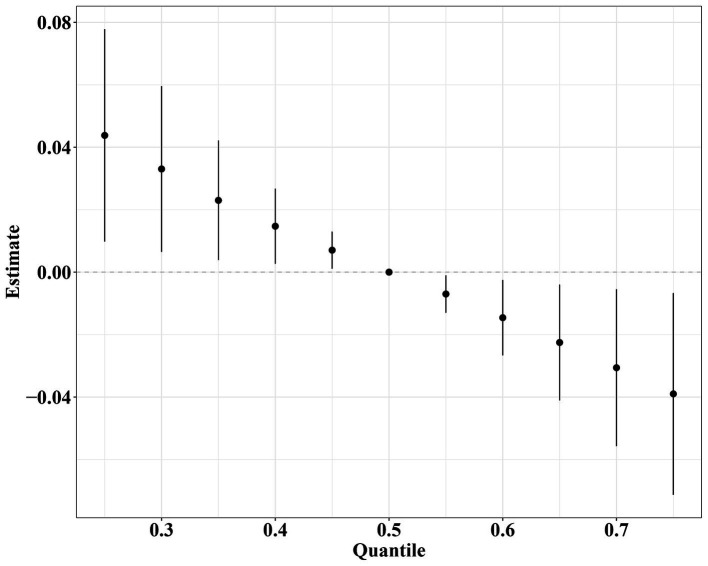
The BKMR model was employed to estimate the combined effect of nine dietary vitamins on periodontitis. In the fully adjusted BKMR model, the overall impact of nine dietary vitamins on periodontitis showed a downward trend, and an increase in the total level of vitamins mixture was associated with a decreased risk of periodontitis (Figure 2). Supplementary Figure S1 reveals the correlation of nine dietary vitamins. Vitamin B1 exhibited correlations with vitamin B2 (r = 0.87), vitamin B6 (r = 0.75), and vitamin B12 (r = 0.56). Additionally, notable correlations were observed between vitamin D and vitamin B12 (r = 0.55) as well as between vitamin D and vitamin B2 (r = 0.53), a substantial association was found between vitamin B6 and both vitamin B2 (r = 0.79) and vitamin B12 (r = 0.64). Lastly, vitamin E and vitamin K was correlated as well (r = 0.53). The nine dietary vitamins were grouped based on the correlations analysis. The GroupPIP and CondPIP derived from the BKMR model for nine dietary vitamins are summarized in Supplementary Table S3. The GroupPIP of group four (vitamin E and K: 1) was higher than other three groups (vitamin A: 0.302; vitamin B1, B2, B6, B12 and D: 0.292; vitamin C: 0.226). Vitamin E (CondPIP = 0.998) contributed most to the BKMR model for the periodontitis risk. Additionally, when all remaining vitamins were at their median levels, the periodontitis risk decreased with increased concentration levels of vitamin E and vitamin B2 (Supplementary Figure S2).
Figure 2.
Combined effects of nine dietary vitamins mixtures and periodontitis by BKMR analysis. Model was adjusted for age, gender, race/ethnicity, educational level, marital status, poverty-income ratio, smoking status, anti-infectives, serum vitamin D, white blood cell, decayed teeth, and dental floss.
As shown in Table 3, WQS regression analysis indicated a negative association between nine dietary vitamins co-exposure and periodontitis risk (OR = 0.88, 95%CI: 0.81–0.95, p = 0.001). The estimated weights of nine dietary vitamins for the WQS model was calculated (Supplementary Figure S3). The highest weighted chemical in the WQS model was vitamin E, followed by vitamin B12 and vitamin D.
Table 3.
The combined effect of nine dietary vitamins on periodontitis by WQS model.
| Model | OR (95% CI) | p |
|---|---|---|
| WQS model | 0.88 (0.81–0.95) | 0.001 |
WQS, weighted quantile sum; OR, odds ratio, CI, confidence interval; Model was adjusted for age, gender, race/ethnicity, educational level, marital status, poverty-income ratio, smoking status, anti-infectives, serum vitamin D, white blood cell, decayed teeth, dental floss.
Similar to the WQS model, an increase in the qgcomp index was related to decreased risk of periodontitis (Table 4, OR = 0.90, 95%CI: 0.84–0.96, p = 0.001). In the qgcomp model, vitamin E received the highest negative weights for the periodontitis risk, followed by vitamin B2 and vitamin D, respectively (Supplementary Figure S4).
Table 4.
The combined effect of nine dietary vitamins on periodontitis by qgcomp model.
| Model | OR (95% CI) | p |
|---|---|---|
| Qgcomp model | 0.90 (0.84–0.96) | 0.001 |
Qgcomp, Quantile g-computation; OR, odds ratio, CI, confidence interval; Model was adjusted for age, gender, race/ethnicity, educational level, marital status, poverty-income ratio, smoking status, anti-infectives, serum vitamin D, white blood cell, decayed teeth, dental floss.
Discussion
In the present study, we observed that dietary vitamin B6 and vitamin E were associated with decreased odds of periodontitis, respectively. Furthermore, we used three statistical approaches, namely BKMR, WQS regression, and qgcomp model, to evaluate the associations of nine dietary vitamins mixtures with periodontitis risk. Three models suggested that the mixtures of nine dietary vitamins showed inverse overall associations with periodontitis risk, and vitamin E made the most contribution in the association between nine dietary vitamins mixtures and periodontitis risk.
Consistent with previous research findings (8, 17), this study indicated a correlation between higher intakes of vitamins B6 and E and a reduced risk of periodontal, respectively. Some studies have indicated that an excessive formation of reactive oxygen species (ROS) occurs in the periodontal tissues, leading to a reduction in antioxidant capacity (18). Oxidative stress is believed to play a role in the pathogenesis of periodontitis (19). Vitamin E, as an antioxidant, can effectively eliminate ROS and contribute significantly to the repair of damaged cells (20). Furthermore, vitamin E has been reported to exert antioxidant effects by reducing lipid peroxidation and increasing the level of superoxide dismutase (21, 22). Given that chronic inflammation, mediated by immune cells, is accountable for the degradation of periodontal tissue, the anti-inflammatory properties of vitamin E could significantly inhibit the expression of inducible nitric oxide synthase (iNOS) in periodontal tissues, reduce the levels of gingival fibroblast production of [interleukin (IL)-1β and IL-6], and increase the levels of human β-defensins, which may help improve host anti-inflammatory environment and maintain oral health (8, 23–25).
Nevertheless, after controlling for all potential confounding factors, no evidence of significant associations between vitamin A, vitamin B1, vitamin B2, vitamin B12, vitamin C, vitamin D, and vitamin K and periodontitis risk was found in our current investigation, which is inconsistent with previous studies that reported these dietary vitamins and periodontitis (6, 10, 26). We speculated that the observed association may be to differences in sample size and adjusted confounding variables. Prospective studies with large sample size are warranted to further verify the relationship of single dietary vitamin and periodontitis.
Due to the diverse nutritional composition of food, individuals are simultaneously exposed to multiple vitamins in their daily life, an increasing number of researchers are advocating to explore the relationship of mixed vitamin exposures and human health (27). In general, BKMR, WQS regression, and qgcomp models are commonly employed to estimate the overall impact of mixed chemical exposure (28, 29). For instance, a cross-sectional study conducted by Tang et al., they found that multivitamins mixed intake was negatively associated with the obesity risk among children and adolescents via using the BKMR analysis (12). In the study of Peng et al., WQS regression model indicated an association of serum multivitamin levels and reduced risk of non-alcoholic fatty liver disease (30). To our knowledge, there is little evidence to present the association between mixed dietary vitamins and periodontitis risk so far. Therefore, in this study we used three methods to capture the association between mixed dietary vitamins and periodontitis risk the general population of the U.S. Remarkably, the findings of BKMR, WQS regression, and qgcomp models consistently revealed that mixture of vitamin A, vitamin B1, vitamin B2, vitamin B6, vitamin B12, vitamin C, vitamin D, vitamin E, and vitamin K had a significant negative combined effect on the risk of periodontitis. In addition, we also found that vitamin E contributes greatly and negatively to the association of mixed dietary vitamins and periodontitis. These results also indicated that adequate intake of vegetables, lean meat, milk, eggs, etc. into our daily diet can effectively mitigate the development of periodontitis. However, the possible mechanism related to the overall effect between mixed dietary vitamins and periodontitis remains unclear. Further exploration is needed regarding the potential mechanisms in the association.
The present study possesses several advantages. Firstly, this is the first study to explore the joint impact of dietary vitamins mixtures on periodontitis among adults in the United States, which might provide significant evidence for exploring the effects of dietary vitamins on periodontitis. Secondly, three different statistical methods were adopted to assess the relationship association between nine dietary vitamins mixtures and periodontitis risk. The study, however, does possess certain limitations that must be acknowledged. Firstly, the cross-sectional study design precludes us from establishing a definitive causal relationship between dietary vitamins and the risk of periodontitis. Secondly, dietary vitamins intake in this study was based on 24 h dietary recall interviews, and thereby there may be an inherent bias. Lastly, NHANES database lacked the information on the specific forms of vitamins B1, B2, B6, B12, C, D, E and K. Further prospective studies are needed to verify our findings and clarify the underlying mechanisms.
Conclusion
In summary, dietary vitamin B6 and vitamin E were found to be associated with decreased odds of periodontitis, respectively. Additionally, the mixture-exposed analyses consistently showed the negative correlations between nine dietary vitamins mixtures and periodontitis, with a highest contribution vitamin E towards the combined effect. Our findings provide evidence for exploring the effects of multiple dietary vitamins exposures on periodontitis.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: NHANES database, https://wwwn.cdc.gov/nchs/nhanes/.
Ethics statement
The requirement of ethical approval was waived by Department of Stomatology, Liuzhou People’s Hospital for the studies involving humans because the data was accessed from a publicly available database. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board also waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because retrospective nature of the study.
Author contributions
FL: Conceptualization, Formal analysis, Project administration, Supervision, Writing – original draft, Writing – review & editing. ML: Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing. YZ: Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing.
Funding Statement
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2024.1347712/full#supplementary-material
References
- 1.Cao R, Li C, Geng F, Pan Y. J-shaped association between systemic immune-inflammation index and periodontitis: results from NHANES 2009–2014. J Periodontol. (2023) 1–10. doi: 10.1002/jper.23-0260, PMID: [DOI] [PubMed] [Google Scholar]
- 2.Liccardo D, Cannavo A, Spagnuolo G, Ferrara N, Cittadini A, Rengo C, et al. Periodontal disease: a risk factor for diabetes and cardiovascular disease. Int J Mol Sci. (2019) 20:1414. doi: 10.3390/ijms20061414, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tan L, Liu J, Liu Z. Association between periodontitis and the prevalence and prognosis of prediabetes: a population-based study. J Transl Med. (2023) 21:484. doi: 10.1186/s12967-023-04340-y, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sadrameli M, Bathini P, Alberi L. Linking mechanisms of periodontitis to Alzheimer's disease. Curr Opin Neurol. (2020) 33:230–8. doi: 10.1097/wco.0000000000000797 [DOI] [PubMed] [Google Scholar]
- 5.Gare J, Kanoute A, Meda N, Viennot S, Bourgeois D, Carrouel F. Periodontal conditions and pathogens associated with pre-eclampsia: a scoping review. Int J Environ Res Public Health. (2021) 18:7194. doi: 10.3390/ijerph18137194, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martinon P, Fraticelli L, Giboreau A, Dussart C, Bourgeois D, Carrouel F. Nutrition as a key modifiable factor for periodontitis and Main chronic diseases. J Clin Med. (2021) 10:197. doi: 10.3390/jcm10020197, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang H, Yin Y, Wu CR, Liu Y, Guo F, Li M, et al. Dietary vitamin and carotenoid intake and risk of age-related cataract. Am J Clin Nutr. (2019) 109:43–54. doi: 10.1093/ajcn/nqy270 [DOI] [PubMed] [Google Scholar]
- 8.Shadisvaaran S, Chin KY, Shahida MS, Ima-Nirwana S, Leong XF. Effect of vitamin E on periodontitis: evidence and proposed mechanisms of action. J Oral Biosci. (2021) 63:97–103. doi: 10.1016/j.job.2021.04.001, PMID: [DOI] [PubMed] [Google Scholar]
- 9.Tada A, Miura H. The relationship between vitamin C and periodontal diseases: a systematic review. Int J Environ Res Public Health. (2019) 16:2472. doi: 10.3390/ijerph16142472, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li W, Song J, Chen Z. The association between dietary vitamin C intake and periodontitis: result from the NHANES (2009–2014). BMC Oral Health. (2022) 22:390. doi: 10.1186/s12903-022-02416-7, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li W, Shang Q, Yang D, Peng J, Zhao H, Xu H, et al. Abnormal micronutrient intake is associated with the risk of periodontitis: a dose-response association study based on NHANES 2009–2014. Nutrients. (2022) 14:2466. doi: 10.3390/nu14122466, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang W, Zhan W, Wei M, Chen Q. Associations between different dietary vitamins and the risk of obesity in children and adolescents: a machine learning approach. Front Endocrinol (Lausanne). (2021) 12:816975. doi: 10.3389/fendo.2021.816975, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aldosari M, Helmi M, Kennedy EN, Badamia R, Odani S, Agaku I, et al. Depression, periodontitis, caries and missing teeth in the USA, NHANES 2009–2014. Fam Med Community Health. (2020) 8:e000583. doi: 10.1136/fmch-2020-000583, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eke PI, Page RC, Wei L, Thornton-Evans G, Genco RJ. Update of the case definitions for population-based surveillance of periodontitis. J Periodontol. (2012) 83:1449–54. doi: 10.1902/jop.2012.110664, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen L, Zhao Y, Liu F, Chen H, Tan T, Yao P, et al. Biological aging mediates the associations between urinary metals and osteoarthritis among U.S. adults. BMC Med. (2022) 20:207. doi: 10.1186/s12916-022-02403-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li W, Li X, Su J, Chen H, Zhao P, Qian H, et al. Associations of blood metals with liver function: analysis of NHANES from 2011 to 2018. Chemosphere. (2023) 317:137854. doi: 10.1016/j.chemosphere.2023.137854, PMID: [DOI] [PubMed] [Google Scholar]
- 17.Watson S, Woodside JV, Winning L, Wright DM, Srinivasan M, McKenna G. Associations between self-reported periodontal disease and nutrient intakes and nutrient-based dietary patterns in the UK biobank. J Clin Periodontol. (2022) 49:428–38. doi: 10.1111/jcpe.13604, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Georgiou AC, Cornejo Ulloa P, Van Kessel GMH, Crielaard W, Van der Waal SV. Reactive oxygen species can be traced locally and systemically in apical periodontitis: a systematic review. Arch Oral Biol. (2021) 129:105167. doi: 10.1016/j.archoralbio.2021.105167 [DOI] [PubMed] [Google Scholar]
- 19.Sczepanik FSC, Grossi ML, Casati M, Goldberg M, Glogauer M, Fine N, et al. Periodontitis is an inflammatory disease of oxidative stress: we should treat it that way. Periodontol. (2020) 84:45–68. doi: 10.1111/prd.12342, PMID: [DOI] [PubMed] [Google Scholar]
- 20.Miyazawa T, Burdeos GC, Itaya M, Nakagawa K, Miyazawa T. Vitamin E: regulatory redox interactions. IUBMB Life. (2019) 71:430–41. doi: 10.1002/iub.2008, PMID: [DOI] [PubMed] [Google Scholar]
- 21.Carvalho R, de Souza CM, Neves JC d S, Holanda-Pinto SA, Pinto LMS, Brito GAC, et al. Vitamin E does not prevent bone loss and induced anxiety in rats with ligature-induced periodontitis. Arch Oral Biol. (2013) 58:50–8. doi: 10.1016/j.archoralbio.2012.04.020 [DOI] [PubMed] [Google Scholar]
- 22.Singh N, Chander Narula S, Kumar Sharma R, Tewari S, Kumar Sehgal P. Vitamin E supplementation, superoxide dismutase status, and outcome of scaling and root planing in patients with chronic periodontitis: a randomized clinical trial. J Periodontol. (2014) 85:242–9. doi: 10.1902/jop.2013.120727, PMID: [DOI] [PubMed] [Google Scholar]
- 23.Derradjia A, Alanazi H, Park HJ, Djeribi R, Semlali A, Rouabhia M. α-Tocopherol decreases interleukin-1β and −6 and increases human β-defensin-1 and -2 secretion in human gingival fibroblasts stimulated with Porphyromonas gingivalis lipopolysaccharide. J Periodontal Res. (2016) 51:295–303. doi: 10.1111/jre.12308, PMID: [DOI] [PubMed] [Google Scholar]
- 24.Hatipoglu M, Alptekin NÖ, Avunduk MC. Effects of alpha-tocopherol on gingival expression of inducible nitric oxide synthase in the rats with experimental periodontitis and diabetes. Niger J Clin Pract. (2016) 19:480–5. doi: 10.4103/1119-3077.183301 [DOI] [PubMed] [Google Scholar]
- 25.Lewis ED, Meydani SN, Wu D. Regulatory role of vitamin E in the immune system and inflammation. IUBMB Life. (2019) 71:487–94. doi: 10.1002/iub.1976, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alzahrani AAH, Alharbi RA, Alzahrani MSA, Sindi MA, Shamlan G, Alzahrani FA, et al. Association between periodontitis and vitamin D status: a case-control study. Saudi J Biol Sci. (2021) 28:4016–21. doi: 10.1016/j.sjbs.2021.04.006, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang R, Huang Q, Su G, Wei M, Cui Y, Zhou H, et al. Association between multiple vitamins and bone mineral density: a cross-sectional and population-based study in the NHANES from 2005 to 2006. BMC Musculoskelet Disord. (2023) 24:113. doi: 10.1186/s12891-023-06202-6, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gu L, Wang Z, Pan Y, Wang H, Sun L, Liu L, et al. Associations between mixed urinary phenols and parabens metabolites and bone mineral density: four statistical models. Chemosphere. (2023) 311:137065. doi: 10.1016/j.chemosphere.2022.137065, PMID: [DOI] [PubMed] [Google Scholar]
- 29.Nguyen HD, Oh H, Kim MS. Mixtures modeling identifies vitamin B1 and B3 intakes associated with depression. J Affect Disord. (2022) 301:68–80. doi: 10.1016/j.jad.2021.12.133, PMID: [DOI] [PubMed] [Google Scholar]
- 30.Peng H, Wang M, Pan L, Cao Z, Yao Z, Chen Q, et al. Associations of serum multivitamin levels with the risk of non-alcoholic fatty liver disease: a population-based cross-sectional study in U.S. adults. Front Nutr. (2022) 9:962705. doi: 10.3389/fnut.2022.962705, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found here: NHANES database, https://wwwn.cdc.gov/nchs/nhanes/.