Abstract
Herpes simplex virus type 1 (HSV-1) causes chronic blepharitis and conjunctivitis as well as keratitis in humans. The pathogenesis of these inflammatory ocular and dermal lesions is not well understood. We have examined the persistence of HSV-1 DNA and its relationship to inflammatory lesions in the conjunctiva and eyelid skin of mice which were inoculated with HSV-1 by the corneal route. Viral DNA was detected by in situ PCR in the conjunctiva and eyelid tissue of infected mice at 5, 11, 23, and 37 days postinfection (p.i.). This DNA was localized in the epithelial cells of the conjunctiva and hair follicles and in the epidermal cells of the eyelid skin. Viral proteins were not detected in the conjunctiva or the eyelid skin after 5 days p.i., even though histopathological lesions were found at 23 and 37 days p.i. in both tissues. The DNA-containing cells were adjacent to sites of inflammation in the chronic lesions in both the conjunctiva and the eyelid skin. A similar temporal and spatial relationship between HSV-1 DNA and inflammatory lesions has been previously reported for the cornea. Our data suggest that the lesions in the cornea, conjunctiva, and eyelid skin progress similarly. Further studies are required to determine whether the long-term presence of HSV-1 is involved in the mechanism by which these chronic inflammatory lesions develop. The presence of HSV-1 DNA in these extraocular tissues for extended periods may constitute persistent viral infection of nonneuronal cells.
Herpes simplex virus type 1 (HSV-1) causes a number of important diseases in humans, including chronic ocular disease (37). Although stromal keratitis is perhaps the best-studied ocular disease induced by HSV (11), chronic eyelid and conjunctival disease are also well-defined clinical entities (20). The mouse model has been widely used for studies of the pathogenesis of chronic inflammatory lesions of the cornea induced by HSV-1 (12, 16, 17, 23, 24, 27, 31). The pathogenesis of chronic herpes-induced stromal keratitis has been shown to be immunopathological, but the molecular details have not been fully characterized (11). The pathogenesis of HSV-1-induced disease of the conjunctiva and eyelid has received little attention in animal models and is therefore less well characterized. However, there is a reasonable possibility that these inflammatory lesions are caused by a mechanism similar to that which functions in herpes-induced keratitis.
HSV-1 replicates acutely in epithelial cells of the conjunctiva, eyelid skin, and cornea before being transported to neurons, where a latent infection occurs (19, 35). Chronic ocular diseases, including conjunctivitis, keratitis, and blepharitis, may result from periodic reactivation of the latent neuronal infection. It has been suggested that in chronic herpetic keratitis, the initial HSV infection results in the exposure of antigens found only in the cornea, which then cause chronic keratitis by an immune mechanism (4). More recently, it has been suggested that an epitope in the protein encoded by the HSV-1 UL 6 gene can mimic a corneal antigen and may initiate an immune system-mediated attack on corneal antigens (38). Other reports support the idea that the continued presence of HSV-1 in the cornea may be necessary to induce chronic, immune system-mediated keratitis (5, 27).
There is evidence that HSV can persist in peripheral nonneural tissues of chronically infected animals. Some studies have reported that long after infection HSV can be isolated from skin or other peripheral tissues at the site of inoculation in mice and guinea pigs (1, 9, 18, 32). In these experiments, reactivation by release of virus from the associated ganglia was unlikely as a possible cause of virus detection. The isolation of infectious HSV and the detection of viral antigens in corneas and eyelids after immunosuppression and UV irradiation of chronically infected mice have also been reported (33). In mice HSV-1 DNA has been found in the epithelial cells of the cornea up to 4 months following infection (27) and in keratinocytes in footpad skin for up to 2 weeks postinoculation (p.i.) (34). Claoué et al. (8) demonstrated infectious virus in the iris after explanation of the anterior segment of the eye of chronically infected mice. HSV DNA or antigen has also been demonstrated in numerous tissues of humans with chronic lesions, including the cornea (10), skin (3, 6, 7, 28), blood (7), and gingiva (2). Thus, HSV-1 DNA or in some cases infectious virus has been shown to remain for extended periods in a number of tissues, including skin and ocular tissues.
In the course of experiments to examine keratitis in the mouse model, we observed histological lesions in the conjunctiva and eyelid skin that were very similar to those in the cornea. A spatial and temporal relationship between the presence of viral DNA and the corneal lesions was demonstrated in the previous study (27). Here we present data which point to a similar relationship between HSV-1 DNA and lesions in the conjunctiva and eyelid skin. It is important to understand whether the long-term presence of HSV-1 is involved in the mechanism by which these chronic lesions develop. The long-term presence of HSV-1 DNA in extraocular tissues may constitute persistent viral infection of nonneuronal cells.
MATERIALS AND METHODS
Animal infections.
HSV-1 strain F was grown in Vero cells and virus stocks were prepared as previously described (25). Groups of 7- to 9-week-old, female BALB/c mice were anesthetized with methoxyflurane, and each cornea was scratched 10 times with a 26-gauge needle. Each animal received either 5 μl of minimum essential medium containing fetal calf serum (mock-inoculated mice) or 5 μl of medium containing 107 PFU of HSV-1 strain F on each cornea. Groups of mice were killed at 5, 11, 23, and 37 days p.i., and tissues were harvested for analysis by in situ PCR, immunohistochemistry, or viral culture. In one group of mice, the eyes with the attached periocular skin were removed from each mouse, fixed in formalin, and embedded in paraffin. In a second group, the eyelid skin and conjunctiva were dissected free from other ocular tissues under a dissecting microscope and immediately frozen at −70°C for viral culture. All animals used in this study were maintained and handled in accordance with the Association for Research in Vision and Ophthalmology statement for the use of animals in ophthalmic and vision research, and all experimental procedures were approved by the University of Missouri’s Animal Care and Use Committee.
Scoring of clinical lesions.
Eyes were examined at ×3 or ×10 magnification with a focal light source before inoculation and at 1, 3, 5, 11, 18, 23, 29, and 37 days p.i. Conjunctival hyperemia, conjunctival swelling, and blepharitis were graded from 1 (least severe) to 4 (most severe) with previously published scoring systems with minor modifications (13, 30). Eyes without detectable lesions were scored as 0. Each clinical sign was graded by specific criteria. Conjunctival hyperemia was scored as follows: 1, pale pink conjunctiva; 2, dark pink conjunctiva; 3, red conjunctiva; and 4, frank hemorrhage. Conjunctival swelling was scored as follows: 1, swollen conjunctiva observed only after eversion of the eyelids or partial prolapse of the globe; 2, conjunctiva visible without eyelid eversion or partial prolapse of globe but obscuring less than 25% of the cornea; 3, conjunctiva obscuring 25 to 75% of the cornea; and 4, conjunctiva obscuring more than 75% of the cornea. Blepharitis was scored as follows: 1, noticeably puffy eyelids; 2, puffy eyelids with moderate crusting; 3, eyelid swollen half shut with severe crusting; and 4, eyelid crusted and totally shut. Mean disease scores (MDS) for conjunctivitis or blepharitis were calculated for each group of mice on each day of observation. For conjunctivitis, the MDS was calculated from summed conjunctival swelling and hyperemia scores. Four HSV-1-infected mice and three mock-inoculated mice were examined throughout the experiment.
In situ PCR.
Paraffin-embedded sections of eyes and periocular skin from HSV-1-infected or control mice were deparaffinized with xylene and ethanol, and HSV-1 DNA localization was evaluated by in situ PCR as previously described (15, 27). After the slides were heated to 82°C for 2 min, the reaction mixture (also at 82°C) was added, and in situ PCR was performed for 15 cycles of 1 min at 96°C, 1 min at 59°C, and 1 min at 72°C in a thermocycling oven. The reaction mixture contained 10 μM (each) dATP, dCTP, and dGTP, 3.5 μM dTTP, 6.5 μM digoxigenin-dUTP, 10% glycerol, 10% salmon sperm DNA, 2.5 mM MgCl2, 4 U of native Taq polymerase (Stoffel fragment), 10 mM Tris-HCl (pH 8.3), 10 mM KCl, and 0.25 μM of each primer. Oligonucleotide primers (5′TACCCGAGCCGATGACTTAC3′ and 5′GCGCTTGTCATTACCACCGC3′) (22, 27) were used to amplify a 130-bp fragment from the thymidine kinase gene of HSV-1. The slides were washed three times at room temperature in a mixture of 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 1.5 mM MgCl2, and 0.001% gelatin for 5 min each time, three times in 2× SSC–50% formamide (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) at 37°C for 10 min each time, and twice at room temperature in 2× SSC for 15 min each time. The in situ PCR product was visualized by using alkaline phosphatase-conjugated antidigoxigenin antibody and an enzyme color reaction as described in the literature accompanying the DIG nucleic acid detection kit (Boehringer Mannheim).
At least two sections from each of the eyes from five mice infected with HSV-1 were tested at each time point. In addition, in several eyes serial sections from the same location were tested for labeling. As negative controls, primers which had been used in earlier studies for the in situ PCR detection of a 92-bp fragment of the feline herpesvirus type 1 thymidine kinase gene (5′TTGCTTGATAGTGGGCGGTG3′ and 5′TGTCGGTGGTATCTATGCCG3′) (21, 29) were substituted for the HSV-1 primers in selected sections obtained from adjacent to or near sections which had been labeled for HSV-1 DNA. Additionally, in each group of slides sections of eyes from mock-inoculated mice were always tested with the HSV-1 primers.
Histopathology and immunocytochemistry.
Sections from the eyes that were assayed by in situ PCR were also evaluated for evidence of lesions by light-microscopic evaluation of hematoxylin- and eosin-stained sections. Other sections from the same eyes were deparaffinized and assayed for the presence of viral antigen by the avidin-biotin-peroxidase method (Vector) with HSV-1 antiserum (Dako) at a 1:1,000 dilution (25, 26). As controls, uninfected mouse eyes were reacted with the HSV-1 antiserum and infected eyes were reacted with control rabbit serum in the same assays.
Viral culture.
Sections of eyelid skin and conjunctiva which had been dissected free from other ocular tissues were homogenized, and each homogenate was assayed for HSV-1 on Vero cells overlaid with methylcellulose in a standard plaque assay (26). Culture results were expressed as the number of eyes from which virus could be cultured from combined eyelid skin and conjunctiva. Tissues from 10 eyes were cultured at each time point except at 11 days p.i., when only 6 eyes from mice infected with HSV-1 were assayed.
RESULTS
Clinical lesions in mice infected with HSV-1.
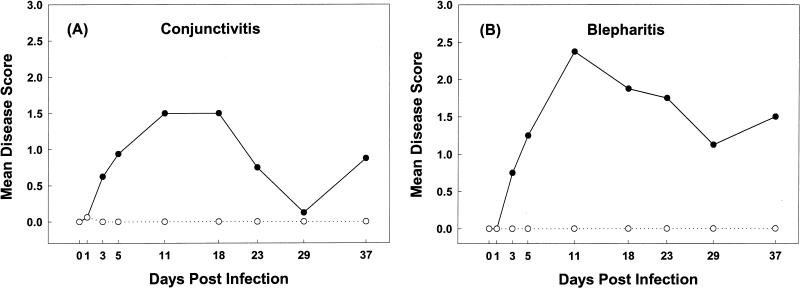
All HSV-1 strain F infected mice that were examined developed clinical evidence of moderate to severe conjunctivitis and blepharitis. Conjunctivitis was evident in all mice by 3 days p.i. The MDS for conjunctivitis was at its maximum level at 11 and 18 days p.i. and remained at >0 for the duration of the experiment (Fig. 1A). Clinical evidence of blepharitis was first detected at 3 days p.i. The MDS was greatest at 11 days p.i. and remained at >0 until the termination of the study (Fig. 1B). One mock-inoculated mouse developed mild conjunctival swelling (score = 1) in one eye at 1 day p.i. No other clinical evidence of conjunctivitis or blepharitis was detected in any of the other mock-inoculated mice examined during the experiment.
FIG. 1.
Mean clinical disease scores for conjunctivitis and blepharitis (eyelid skin inflammation) in mice infected with HSV-1 (•) and mock-inoculated mice (○). Eyes were examined at ×3 magnification and with a focal light source before inoculation and at 1, 3, 5, 11, 18, 23, 29, and 37 days p.i. Clinical signs of conjunctivitis (A) and blepharitis (B) were scored and the MDS were calculated for each group of mice on each day of observation.
Histopathological lesions and viral antigen distribution in mice infected with HSV-1.
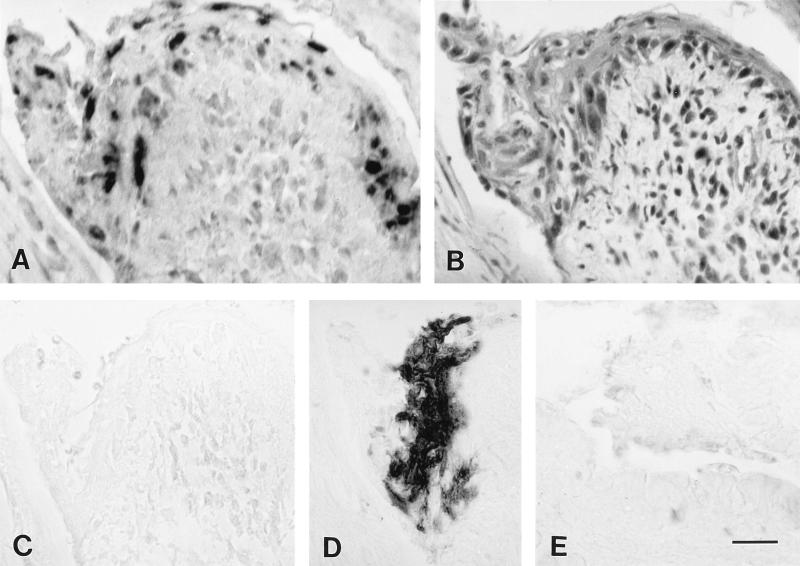
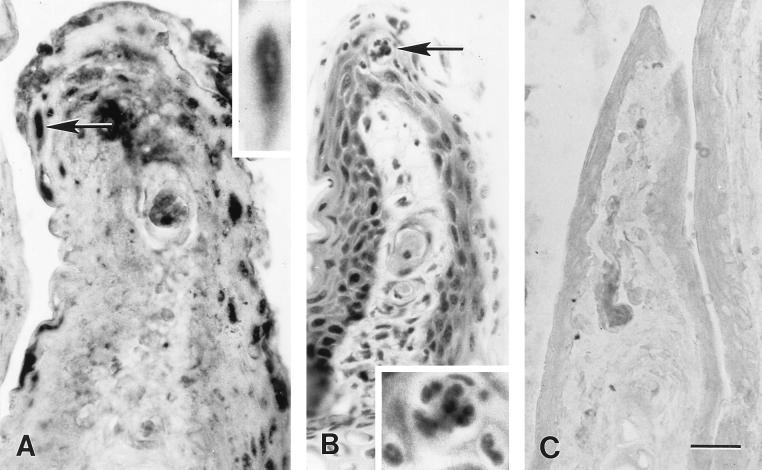
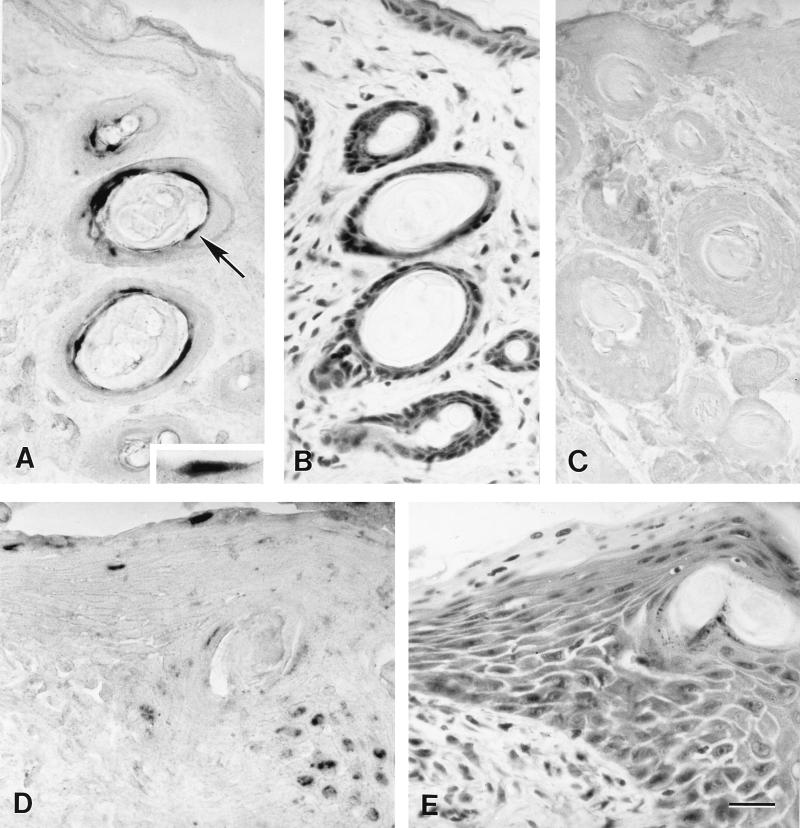
Histologic lesions in the conjunctiva and eyelid skin were present in all eyes at all times p.i. and were located in the follicular epithelium, epidermis, conjunctival epithelium, and subjacent tissues. At 5 days p.i., inflammatory infiltrates were comprised predominantly of macrophages and neutrophils (Fig. 2B and results not shown). Necrosis of the conjunctival, epidermal, and follicular epithelial cells and edema of the dermis and conjunctival substantia propria were also common. Beginning at 11 days p.i. and continuing through 37 days p.i., there were multifocal areas of moderate to severe inflammation in the conjunctival and follicular epithelium, the epidermis, and the subjacent dermis and substantia propria. At 23 and 37 days p.i., the inflammation was of a chronic active nature and the infiltrate consisted predominantly of macrophages, lymphocytes, and neutrophils (Fig. 3B and 4B and E). Hyperplasia of the epidermis, follicular epithelium, and conjunctival epithelium was also present in some sections obtained at later times p.i.
FIG. 2.
In situ PCR and immunoperoxidase labeling for HSV-1 and histological lesions in sections of acutely infected conjunctivae. (A) In situ PCR labeling of HSV-1 DNA in conjunctival cells at 5 days p.i. with HSV-1. The sections shown in panels B to D were in the same series and obtained from sites near the section shown in panel A. (B) Hematoxylin- and eosin-stained section. Note the inflammatory cell infiltration of the epithelium and substantia propria and the necrosis of the epithelium. (C) In situ PCR testing with primers for a feline herpesvirus type 1 gene. Note the absence of labeling. (D) Immunoperoxidase labeling of HSV-1 antigen. (E) In situ PCR testing of a section of conjunctiva from a mock-inoculated mouse at 5 days p.i. Note the absence of labeling. Bar, 19.6 μm.
FIG. 3.
In situ PCR labeling of HSV-1 DNA and histological lesions in sections of chronically infected conjunctiva of the third eyelid. (A) In situ PCR labeling of HSV-1 DNA in conjunctival cells at 23 days p.i. with HSV-1. Inset shows, at higher magnification, the epithelial cell marked by an arrow in the main photomicrograph. (B) Hematoxylin- and eosin-stained section in the same series obtained from a site near the section shown in panel A. Note the inflammatory cell infiltration of the epithelium and substantia propria. Inset shows, at higher magnification, the focus of neutrophils and macrophages indicated by an arrow in the main photomicrograph. (C) In situ PCR testing of a section of the conjunctiva of the third eyelid from a mock-inoculated mouse at 11 days p.i. Note the absence of labeling. Bar, 19.6 μm in the main photomicrographs and 11.5 μm in the insets.
FIG. 4.
In situ PCR labeling of HSV-1 DNA in sections of chronically infected eyelid skin. (A) In situ PCR labeling of HSV-1 DNA in follicular epithelial cells at 37 days p.i. with HSV-1. The epithelial cell indicated by an arrow is shown at higher magnification in the inset. (B) Hematoxylin- and eosin-stained section in the same series obtained from near the section shown in panel A. Note the inflammatory cell infiltration of follicular epithelium and surrounding dermis. (C) In situ PCR testing of a section of eyelid skin including both epidermal and follicular epithelial cells from a mock-inoculated mouse at 11 days p.i.; note the absence of labeling. (D) In situ PCR labeling of HSV-1 DNA in epidermal cells of eyelid skin at 37 days p.i. with HSV-1. (E) Hematoxylin- and eosin-stained section in the same series obtained from near the section shown in panel D. Note the inflammatory cell infiltration in the epidermis and dermis. Bar, 19.6 μm in the main photomicrographs and 11.5 μm in the inset.
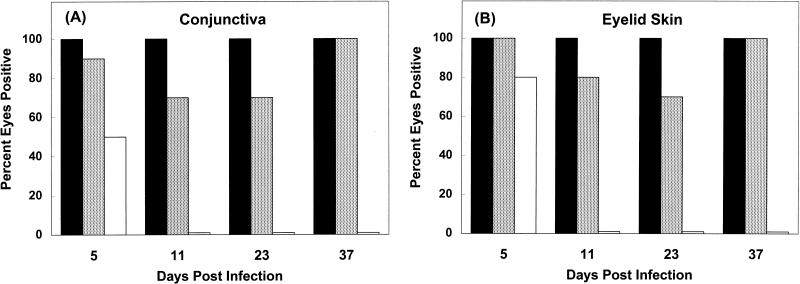
At 5 days p.i., viral antigens were detected in the conjunctiva in 5 of 10 eyes (Fig. 2D and 5A) and in the eyelid skin in 8 of 10 eyes (Fig. 5B). The areas of the conjunctiva and eyelid skin in which viral antigens were detected corresponded to but were not confined to the areas with histologic lesions. Viral antigen was not detected in conjunctiva or eyelid skin after 5 days p.i. in any of the samples (Fig. 5).
FIG. 5.
Percentages of eyes in which viral DNA (░⃞), histological lesions (■), and viral antigen (□) could be detected in the conjunctiva (A) and eyelid skin (B) at different times p.i. with HSV-1. All groups included 10 eyes.
Localization of HSV-1 DNA.
HSV-1 DNA was localized predominantly within epithelial cell nuclei of the conjunctiva, epidermis, and hair follicles of the eyelid skin; its location was related to acute and chronic inflammatory lesions. HSV-1 DNA was detected by in situ PCR in the conjunctiva and eyelid skin of virus-infected mice at all times p.i. (Fig. 5). Viral DNA was detected in the conjunctivae of 9 of 10 eyes examined at 5 days p.i., 7 of the total of 10 eyes examined at 11 and 23 days p.i., and all 10 eyes examined at 37 days p.i. (Fig. 2A, 3A, and 5A). Conjunctival samples from uninfected control mice were negative for viral DNA (Fig. 2E and 3C). HSV-1-infected conjunctiva was negative when tested by in situ PCR with primers specific for feline herpesvirus type 1 (Fig. 2C). Viral DNA was detected in the eyelid skin of 10, 8, 7, and 10, respectively, of the 10 eyes examined at 5, 11, 23, and 37 days p.i. each (Fig. 4A and D and 5B). Skin from uninfected control mice was negative for viral DNA by in situ PCR (Fig. 4C). HSV-1 DNA was localized predominantly in the nuclei of conjunctival and follicular epithelial cells, as well as in the nuclei of epidermal cells of the eyelid skin (Fig. 2A, 3A, and 4A and D). In both the conjunctiva and skin, the DNA-containing cells were found in regions adjacent to sites of acute (Fig. 2A and B) or chronic (Fig. 3A and B and 4A, B, D, and E) inflammation.
Apparent absence of infectious virus in conjunctiva and skin of chronically infected mice.
At 5 days p.i., HSV-1 was cultured from homogenates of combined skin and conjunctiva from 8 of 10 eyes (five of five mice). Virus cultures were negative for skin and conjunctiva taken from all eyes at 11, 23, and 37 days following infection with HSV-1.
DISCUSSION
In this study, we have demonstrated that HSV-1 infection results in chronic inflammatory lesions in the conjunctiva and eyelid skin and that viral DNA persists in these tissues in a mouse model. The HSV-1 DNA was found in epithelial cells of the conjunctiva and hair follicles and in epidermal cells. It was usually located in or near inflammatory lesions. In chronically infected mice HSV DNA has been previously detected in cell types other than latently infected neurons. HSV-1 DNA has been detected in corneal epithelial cells up to 4 months p.i. (27), and HSV-2 DNA has been shown to remain for a long time in the astrocytes in the brain (14). Replicating virus has been recovered from skin and other tissues of SCID mice chronically infected with a VP16-negative mutant of HSV-1 (36). In this study we have detected HSV-1 DNA in two other nonneuronal cell types in chronically infected mice.
Further experiments are required to determine whether the presence of HSV-1 DNA is mechanistically associated with progression of chronic inflammatory lesions in the conjunctiva and eyelid skin. The observations presented in this report are important for understanding the mechanism by which HSV-1 causes chronic lesions of the eyelid skin and conjunctiva as well as the cornea. In a study in humans (20), eyelid or conjunctival disease was present in 54% of patients examined for their first episode of ocular herpes simplex. Although the mechanism by which HSV-1 induces chronic inflammatory lesions in extraocular tissues is poorly understood, it may be similar to the mechanism involved in chronic corneal inflammatory lesions.
It has been previously shown that herpetic stromal keratitis in the mouse model is an immune system-mediated, chronic inflammatory lesion mediated primarily by CD4+ T lymphocytes (12). Experiments were initiated to determine whether the inflammatory lesions of the eyelid and conjunctiva which we observed in mice infected with HSV-1 were the result of an immunopathological response, as has been previously shown for the corneal lesions. We attempted to determine whether CB17 SCID mice, which do not possess T or B lymphocytes, could develop the chronic inflammatory lesions after infection with HSV-1 strain F. The data from these experiments were not definitive. SCID mice inoculated with 105 PFU of HSV-1 per eye died by day 11 (9 of 11 mice) or day 12 (2 of 11 mice) p.i., thus precluding an analysis of the chronic inflammation. Control mice and SCID mice inoculated with 104 PFU of HSV-1 strain F per eye survived, but none developed lesions. In all of the other experiments presented in this report, mice received 107 PFU of HSV-1 strain F per eye. Further experiments with other strains of HSV-1 are necessary to examine whether chronic lesions of conjunctiva and eyelid skin develop in SCID mice after infection.
The continued presence of HSV-1 appears to be related to the progression of chronic inflammatory lesions within the cornea (5, 27). An unanswered question is whether an autoantigen found only in the cornea is involved in the progression of stromal keratitis. Some recent data suggest the existence of a corneal autoantigen (4, 38). It has also been suggested that the mechanisms by which HSV-1 induces inflammation in the cornea and in the skin differ (16, 17). We speculate that the continued presence of HSV-1 may be central to the progression of lesions in the cornea, conjunctiva, and eyelid skin. Further studies comparing the mechanisms of lesion progression in the conjunctiva, eyelid skin, and cornea should help to better clarify the pathogenesis of inflammation in the cornea as well as the eyelid skin and conjunctiva. Initial experiments have not demonstrated the presence of HSV-1 mRNA in chronic lesions of the eyelid and conjunctiva. Further experiments to examine whether HSV-1 RNA is present in these chronic lesions are in progress. It is possible that persistent infection of these tissues by HSV-1 could result in the presence of low levels of viral antigen which cannot be detected by immunocytochemistry but which are recognized by the immune system and therefore result in immunopathological lesions. The presence of HSV-1 DNA in epithelial cells of these tissues for protracted periods following inoculation may be an example of persistent infection of nonneuronal cells by HSV-1.
ACKNOWLEDGMENTS
This work was partially supported by a grant from the National Institutes of Health (R01-EY11855) and by a University of Missouri Research Board grant.
We thank Robert Myers and Brandon Reinbold for excellent technical assistance.
REFERENCES
- 1.Al-Saadi S A, Gross P, Wildy P. Herpes simplex virus type 2 latency in the footpad of mice: effect of acycloguanosine on the recovery of virus. J Gen Virol. 1988;69:433–438. doi: 10.1099/0022-1317-69-2-433. [DOI] [PubMed] [Google Scholar]
- 2.Amit R, Morag A, Ravid Z, Hochman N, Ehrlich J, Zakay-Rones Z. Detection of herpes simplex virus in gingival tissue. J Periodontol. 1992;63:502–506. doi: 10.1902/jop.1992.63.6.502. [DOI] [PubMed] [Google Scholar]
- 3.Aslanzadeh J, Helm K F, Espy M J, Muller S A, Smith T F. Detection of HSV-specific DNA in biopsy tissue of patients with erythema multiforme by polymerase chain reaction. Br J Dermatol. 1992;126:19–23. doi: 10.1111/j.1365-2133.1992.tb08397.x. [DOI] [PubMed] [Google Scholar]
- 4.Avery A C, Zhao Z, Rodriguez A, Bikoff E K, Soheilian M, Foster C S, Cantor H. Resistance to herpes stromal keratitis conferred by an IgG2a-derived peptide. Nature. 1995;376:431–434. doi: 10.1038/376431a0. [DOI] [PubMed] [Google Scholar]
- 5.Babu J S, Thomas J, Kanangat S, Morrison L A, Knipe D M, Rouse B T. Viral replication is required for induction of ocular immunopathology by herpes simplex virus. J Virol. 1996;70:101–107. doi: 10.1128/jvi.70.1.101-107.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brice S L, Krzemien D, Weston W L, Huff J C. Detection of herpes simplex virus DNA in cutaneous lesions of erythema multiforme. J Investig Dermatol. 1989;93:183–187. doi: 10.1111/1523-1747.ep12277397. [DOI] [PubMed] [Google Scholar]
- 7.Brice S L, Leahy M A, Ong L, Krecji S, Stockert S S, Huff J C, Weston W L. Examination of non-involved skin, previously involved skin, and peripheral blood for herpes simplex virus DNA in patients with recurrent herpes-associated erythema multiforme. J Cutan Pathol. 1994;21:408–412. doi: 10.1111/j.1600-0560.1994.tb00281.x. [DOI] [PubMed] [Google Scholar]
- 8.Claoué C M P, Hodges T J, Darville J M, Hill T J, Blyth W A, Easty D L. Possible latent infection with herpes simplex virus in the mouse eye. J Gen Virol. 1990;71:2385–2390. doi: 10.1099/0022-1317-71-10-2385. [DOI] [PubMed] [Google Scholar]
- 9.Clements G B, Subak-Sharpe J H. Herpes simplex virus type 2 establishes latency in the mouse footpad. J Gen Virol. 1988;69:375–383. doi: 10.1099/0022-1317-69-2-375. [DOI] [PubMed] [Google Scholar]
- 10.Crouse C A, Pflugfelder S C, Pereira I, Cleary T, Rabinowitz S, Atherton S S. Detection of herpesviral genomes in normal and diseased corneal epithelium. Curr Eye Res. 1990;9:569–581. doi: 10.3109/02713689008999597. [DOI] [PubMed] [Google Scholar]
- 11.Doymaz M Z, Rouse B T. Immunopathology of herpes simplex virus infections. Curr Top Microbiol Immunol. 1992;179:121–136. doi: 10.1007/978-3-642-77247-4_8. [DOI] [PubMed] [Google Scholar]
- 12.Doymaz M Z, Rouse B T. Herpetic stromal keratitis: an immunopathologic disease mediated by CD4+T lymphocytes. Investig Ophthalmol Vis Sci. 1992;33:2165–2173. [PubMed] [Google Scholar]
- 13.Grau D R, Visalli R J, Brandt C R. Herpes simplex virus stromal keratitis is not titer-dependent and does not correlate with neurovirulence. Investig Ophthalmol Vis Sci. 1989;30:2474–2480. [PubMed] [Google Scholar]
- 14.Gressens P, Martin J R. HSV-2 DNA persistence in astrocytes of the trigeminal root entry zone: double labeling by in situPCR and immunohistochemistry. J Neuropathol Exp Neurol. 1994;53:127–135. doi: 10.1097/00005072-199403000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Gressens P, Martin J R. In situpolymerase chain reaction: localization of HSV-2 DNA sequences in infections of the nervous system. J Virol Methods. 1994;46:61–83. doi: 10.1016/0166-0934(94)90017-5. [DOI] [PubMed] [Google Scholar]
- 16.Hendricks, R. L., and T. M. Tumpey. 1991. Concurrent regeneration of T lymhocytes and susceptibility to HSV-1 corneal stromal disease. Curr. Eye Res. 10(Suppl.):47–53. [DOI] [PubMed]
- 17.Hendricks R L, Tumpey T M, Finnegan A. IFN-γ and IL-2 are protective in the skin but pathologic in the corneas of HSV-1 infected mice. J Immunol. 1992;149:3023–3028. [PubMed] [Google Scholar]
- 18.Hill T J, Harbour D J, Blyth W A. Isolation of herpes simplex virus from the skin of clinically normal mice during latent infection. J Gen Virol. 1980;47:205–207. doi: 10.1099/0022-1317-47-1-205. [DOI] [PubMed] [Google Scholar]
- 19.Hill T J. Herpes simplex virus latency. In: Roizman B, editor. The herpesviruses. Vol. 3. New York. N.Y: Plenum Press; 1985. pp. 175–240. [Google Scholar]
- 20.Liesegang, T. J. 1991. A community study of ocular herpes simplex. Curr. Eye Res. 10(Suppl.):111–115. [DOI] [PubMed]
- 21.Maggs, D. J., W. J. Mitchell, and M. P. Nasisse. Unpublished data.
- 22.McKnight S L. The nucleotide sequence and transcript map of the herpes simplex virus thymidine kinase gene. Nucleic Acid Res. 1980;8:5949–5964. doi: 10.1093/nar/8.24.5949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mercadal C M, Bouley D M, DeStephano D, Rouse B T. Herpetic stromal keratitis in the reconstituted scidmouse model. J Virol. 1993;67:3404–3408. doi: 10.1128/jvi.67.6.3404-3408.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Metcalf J F, Hamilton D S, Reichert R W. Herpetic keratitis in athymic (nude) mice. Infect Immun. 1979;26:1164–1171. doi: 10.1128/iai.26.3.1164-1171.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mitchell W J, Martin J R. Herpes simplex virus type 1 replicates in the lens and induces cataracts in mice. Lab Investig. 1992;66:32–38. [PubMed] [Google Scholar]
- 26.Mitchell W J, De Santo R J, Zhang S-D, Odenwald W F, Arnheiter H. Herpes simplex virus pathogenesis in transgenic mice is altered by the homeodomain protein Hox 1.3. J Virol. 1993;67:4484–4491. doi: 10.1128/jvi.67.8.4484-4491.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mitchell W J, Gressens P, Martin J R, De Santo R. Herpes simplex virus type 1 DNA persistence, progressive disease and transgenic immediate early gene promoter activity in chronic corneal infections in mice. J Gen Virol. 1994;75:1201–1210. doi: 10.1099/0022-1317-75-6-1201. [DOI] [PubMed] [Google Scholar]
- 28.Miura S, Smith C C, Burnett J W, Aurelian L. Detection of viral DNA within skin of healed recurrent herpes simplex infection and erythema multiforme lesions. J Investig Dermatol. 1992;98:68–72. doi: 10.1111/1523-1747.ep12495372. [DOI] [PubMed] [Google Scholar]
- 29.Nunberg J H, Wright D K, Cole G E, Petrovskis E A, Post L E, Compton T, Gilbert J H. Identification of the thymidine kinase gene of feline herpesvirus: use of degenerate oligonucleotides in the polymerase chain reaction to isolate herpesvirus gene homologs. J Virol. 1989;63:3240–3249. doi: 10.1128/jvi.63.8.3240-3249.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roze M, Thomas E, Davot J L. Tolfenamic acid in the control of ocular inflammation in the dog: pharmacokinetics and clinical results obtained in an experimental model. J Small Anim Pract. 1996;37:371–375. doi: 10.1111/j.1748-5827.1996.tb02418.x. [DOI] [PubMed] [Google Scholar]
- 31.Russell R G, Nasisse M P, Larsen H S, Rouse B T. Role of T-lymphocytes in the pathogenesis of herpetic stromal keratitis. Investig Ophthalmol Vis Sci. 1984;25:938–944. [PubMed] [Google Scholar]
- 32.Scriba M. Extraneural localization of herpes simplex virus in latently infected guinea pigs. Nature. 1977;267:529–531. doi: 10.1038/267529a0. [DOI] [PubMed] [Google Scholar]
- 33.Shimeld C, Hill T J, Blyth W A, Easty D L. Reactivation of latent infection and induction of recurrent herpetic eye disease in mice. J Gen Virol. 1990;71:397–404. doi: 10.1099/0022-1317-71-2-397. [DOI] [PubMed] [Google Scholar]
- 34.Simmons A, Bowden R, Slobedman B. Retention of herpes simplex virus DNA sequences in the nuclei of mouse footpad keratinocytes after recovery from primary infection. J Gen Virol. 1997;78:867–871. doi: 10.1099/0022-1317-78-4-867. [DOI] [PubMed] [Google Scholar]
- 35.Stevens J G. Latent herpes simplex virus and the nervous system. Curr Top Microbiol Immunol. 1975;70:31–50. doi: 10.1007/978-3-642-66101-3_2. [DOI] [PubMed] [Google Scholar]
- 36.Valyi-Nagy T, Deshmane S L, Raengsakulrach B, Nicosia M, Gesser R M, Wysocka M, Dillner A, Fraser N W. 1814 establishes a unique, slowly progressing infection in SCID mice. J. Virol. 66:7336–7345. 1992. Herpes simplex virus type 1 mutant strain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Whitley R J. Epidemiology of herpes simplex viruses. In: Roizman B, editor. The herpesviruses. Vol. 3. New York, N.Y: Plenum Press; 1985. pp. 1–44. [Google Scholar]
- 38.Zhao Z, Granucci F, Yeh L, Schaffer P A, Cantor H. Molecular mimicry by herpes simplex virus-type 1: autoimmune disease after viral infection. Science. 1998;279:1344–1347. doi: 10.1126/science.279.5355.1344. [DOI] [PubMed] [Google Scholar]