Abstract
Objectives
The dermal exposure route is expected to become increasingly significant relative to total worker exposure as inhalational exposure limits continue to decrease. However, standardization of occupational exposure assessment methods and scientific consensus are needed. This is the first scoping review mapping the literature across all dermal exposure assessment methods and their targeted substances/chemicals in occupational settings.
Methods
Eligibility criteria broadly included studies reporting any noninvasive dermal exposure assessment method in an occupational setting. The literature search (Web of Science and MEDLINE) was restricted to peer-reviewed, primary literature published in the last 20 years (2002–2022). Titles/abstracts were dual independently screened. Data charting was performed by a single reviewer using standard template. All stages were pilot tested. The JBI (formerly, the Joanna Briggs Institute) scoping review methods and PRISMA-ScR checklist (Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews) were used.
Results
In total, 493 articles were data charted and categorized by 4 study types: methods development (22%), exposure assessment (51%), health outcomes (21%), and controls assessment (6%). Fourteen types of dermal exposure assessment methods were charted with biomarkers (51%), dosimeters (21%), and qualitative assessments such as questionnaires or surveys (17%) most common. Seventeen different chemicals/substances were charted; pesticides (28%) and polycyclic aromatic hydrocarbons (PAHs) (22%) associated with crude oil products and combustion were most common. Mapping between substances and exposure assessment method categories, pesticide dosimeters (11%), and PAH biomarker studies (14%) were most reported. Literature gaps were identified for cleaning agents, hair dyes, glycol ether, N,N-dimethylformamide/N-methyl-2-pyrrolidone, dioxins, and bisphenol A.
Conclusions
To foster scientific consensus, standardization across study reporting is needed for describing: (i) exposure assessment methods used, (ii) worker tasking/conditions, (iii) targeted substances and substance state, and (iv) targeted exposure routes. Overall, this review categorizes, maps, and defines the scope of literature for occupational dermal exposure assessment methods.
Keywords: exposure, industrial health, methodological study, occupational health, occupational safety, risk assessment, skin, standardization, workers, workplace
What’s Important About This Paper?
This study reviewed the last 20 years of literature across all dermal exposure assessment methods and substances/chemicals in occupational settings and identified the work to encompass 17 substances and 14 categories of methods. The substances and methods most studied included pesticides for all exposure assessment methods and polycyclic aromatic hydrocarbon biomarkers, but studies are lacking for many other substances. The study provides suggestions and language to move toward standardization of dermal exposure assessment study reporting.
Introduction
As occupational exposure limits are continuously decreased for airborne contaminants, the relative contributions of the dermal exposure route to total workers’ exposure may become increasingly significant (Fenske 1993; Harper 2004). However, standardization and scientific consensus on best practices are needed for dermal exposure assessment methodologies in occupational settings. This need for increased standardization is broadly apparent across different occupational environments and contaminants. Additionally, evidence suggests that practicing industrial hygienists would benefit from and wish to obtain further training and education on how to assess risks from dermal exposures to chemicals in the workplace (Gaskin et al. 2021; Sahmel et al. 2023).
Various methods have been described for assessing dermal exposures in occupational settings, although with some differences in terminologies used and ways in which methods have been categorized (McArthur 1992; Fenske 1993, 2005; Vermeulen et al. 2002; Semple 2004; Van de Sandt et al. 2007; Ng et al. 2014; Naylor et al. 2020). Dermal exposure assessment methods are commonly described and categorized as direct methods including skin wiping/swabbing/rinsing/washing or patch/pad/cloth dosimeters to quantify the amount of contaminant deposited or potentially deposited onto skin; this is in contrast to indirect methods which assess a proxy or correlate to dermal exposure levels, such as with biomarkers and surface wiping of high touch surfaces (OSHA 2022).
At present, there are no scoping reviews broadly summarizing what methods have been used to assess dermal exposures to different substances/chemicals in the occupational environment. There are systematic literature reviews with targeted scope, such as a focus on dermal exposure assessment methods appropriate for metals in the construction industry (Naylor et al. 2020), the use of biomonitoring to assess cumulative occupational exposures to polycyclic aromatic hydrocarbons (Louro et al. 2022) or biomonitoring via buccal micronuclei to assess worker exposures to carcinogens (Hopf et al. 2019). There are also several narrative style (i.e. nonsystematic) literature reviews reporting occupational dermal exposure assessment methods and their respective benefits and limitations (McArthur 1992; Fenske 1993; McDougal and Boeniger 2002; Vermeulen et al. 2002; Harper 2004; Semple 2004). To the best our knowledge, the nonsystematic literature reviews that do exist on this topic have been conducted about 20 years ago. There are also literature reviews (including some systematically performed and some recent publications) on health outcomes and responses of skin to occupational exposures (Spalt et al. 2009; Anderson and Meade 2014; Fitoussi et al. 2022; Jacobsen et al. 2022).
All the above example reviews make important contributions to the literature. Yet, it is still apparent that there is a need for further standardization for how to perform dermal exposure assessment in an occupational environment depending upon target chemical/substance (Kasiotis et al. 2020). The call for harmonization has been a recurring theme of the field for more than 20 years (Marquart et al. 2001). Furthermore, considering recent regulatory solicitations of dermal absorption and exposure assessment data, there is an urgent and present need to move the dermal exposure assessment field toward scientific consensus on best practices (Lynch et al. 2023). In doing so, the practical limitations and resource intensity of performing dermal exposure assessment in the field must be considered; a tiered approach utilizing a set of progressively refined methods balancing costs and benefits has been called for in previous research (Van de Sandt et al. 2007; Lynch et al. 2023). However, it must first be established the best practices and principles for which methods to utilize and how to best perform these methods.
Therefore, a scoping review is conducted with the following objectives: (i) explore breadth of literature across dermal exposure assessment methods in occupational settings; (ii) map and summarize literature on dermal exposure assessment methods by method type and chemical/substance targeted; (iii) illustrate trends in method use over time; and (iv) inform future in depth research cross comparing studies using similar methods to foster scientific consensus and standardization. As a result of accomplishing these goals, identification of knowledge gaps will also be achieved for potential skin contaminants that have sparse exposure assessment literature. This scoping review is based on one primary question and one sub-question. The primary question is: what methods have been used in the literature in occupational settings to assess worker dermal exposures? The sub-question is: for which chemicals/substances have these methods been used? A preliminary search for the existing scoping reviews on this topic has been conducted in PubMed and Web of Science with no similar scoping reviews found (searches performed on 20 September 2022).
Methods
The scoping review was conducted in accordance with the JBI methodology for scoping reviews (Peters et al. 2020a, 2020b) and reported following the PRISMA-ScR checklist (Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews) (Tricco et al. 2018). The checklist is provided in Supplementary Table S1.
Protocol and registration
The scoping review protocol was developed a priori and registered on the Open Science Framework (Therkorn and Laursen 2022).
Eligibility criteria
The scope for this review was developed using the PCC (population, concept, and context) elements of eligibility criteria (Peters et al. 2020b). The concept for this review broadly included noninvasive dermal exposure assessment methodologies in occupational settings and their respective targeted chemicals/substances. All concepts were broadly defined. Chemicals/substances included any contaminant that may come into contact with skin, such as chemicals in vapor/liquid form or dusts/aerosols. Studies focused on only acute dermal injuries, such as temperature burns, sharps accidents, or needle sticks, were out of scope. Additionally, radiation exposures were also deemed out of study scope. Dermal exposure assessment methods included any quantitative, qualitative, and/or semi-quantitative methods for assessing dermal exposure in an occupational setting. These were defined as follows: (i) qualitative methods provide a yes/no indication for whether dermal exposure to contaminant has likely occurred; (ii) quantitative methods provide information on the concentration or mass of contaminant on or absorbed into skin (including absorbed dose); (iii) semi-quantitative methods provide an ordered scaling or derivative scoring for dermal contamination levels although without concentration or mass units associated with quantitative measures (Vermeulen et al. 2002). While dermal exposure assessment methods were broadly considered, invasive methods such as skin punch biopsy were excluded as these are not appropriate for regular use in an occupational setting. Similarly, methods that assess skin sensitization and reaction to contaminants such as skin prick testing were also out of scope as these are considered invasive for an occupational setting and furthermore address health effects and symptoms postexposure.
The population for this review included workers and professionals. The context included occupational settings. Only studies that applied a dermal exposure assessment method in an occupational context were considered. This means that any study performed in solely a laboratory setting, such as methods development without validation or with an in vitro/animal design, was excluded. Occupational settings were broadly defined to include studies performed in actual occupational environments, simulated occupational tasks performed in a controlled setting, the reuse of previously collected datasets from occupational studies, and studies that were performed with unclear boundaries between occupational and residential setting (e.g. where farmers work and live within the same area). There were no limitations with respect to occupational type.
Types of sources
Only peer-reviewed, primary literature were included. Qualitative/narrative style reviews, editorials, conference abstracts, book chapters, or any documents existing in the grey literature were not considered. The timeframe for the literature search was limited to the past 20 years (i.e. from 2002 to 2022). Additionally, given logistical constraints, the literature was limited to English language articles only. Table 1 summarizes all exclusion criteria.
Table 1.
Summary of exclusion criteria.
| Exclusion criterion | Definition |
|---|---|
| Not a peer-reviewed, primary study | Abstract/article did not report on data collected within the described study (i.e. a review article where primary data not collected) |
| Non-English language | Self-explanatory |
| Missing abstract or full text | Self-explanatory |
| No dermal exposure assessment method used | Study did not include any dermal exposure assessment method for chemical/substance contaminants. Inclusion criteria were broadly defined to include any noninvasive quantitative, qualitative and/or semi-quantitative method (Vermeulen et al. 2002). However, assessment of radiation exposure and acute dermal injuries, such as temperature burns and sharps wounds, were not within scope of study. |
| Study not conducted in an occupational setting | Study not conducted in an occupational setting. Inclusion criteria were broadly defined to include human studies in actual occupational environments, simulated occupational tasks with human subjects, studies validating models with data collected from occupational studies, or studies performed in settings with unclear boundaries between occupational and residential environment (e.g. farm workers living and working in same area). |
Search strategy
The search strategy aimed to be as comprehensive as possible while considering both the study objectives and time/resources. The literature search strategy was developed and performed by a trained librarian. An initial search was performed in Web of Science to analyze identified articles’ key words in titles, abstracts, and index terms. The literature was iteratively explored to identify a broad array of articles. Additionally, the literature search strategy was developed with the data charting and literature mapping objectives in mind as described below.
The full search strategy implemented in the Web of Science searched title, abstract, and keywords of articles as follows (conducted on 21 September 2022):
- Primary topic: (skin or derm* or percutaneous or epidermis or stratum corneum) and (exposure or absorption or deposition or uptake or loading).
- AND topic: occupational or workplace or industrial or professional or job* or (work near/2 setting) or (work near/2 practice) or employ*.
- AND topic: wip* or patch or cloth or glove or tape or rinse or coveralls or glove or sampl* or pad or media or dosimeter or strip or swab or biomarker* or metabolite or biomonitor* or tool* or survey or questionnaire or inspection.
- AND topic: gas or vapor or vapour or liquid or solution or mixture or aerosol or dust or solid or mist or spray.
- NOT topic: drug delivery or in vitro or in vitro or in-vitro.
The same search strategy was also implemented in MEDLINE with the following change (conducted on 1 November 2022):
- Addition of MeSH (Medical Subject Headings) search terms: AND (Humans (MeSH Headings) AND Occupational Exposure (MeSH Headings) AND Skin (MeSH Headings)).
Both searches were limited to the last 20 years (2002–2022) and restricted to articles in English language, having full texts available and primary research (i.e. not reviews). Articles identified by both databases were merged and de-duplicated.
Title/abstract screening pilot test
After executing the literature search, the first phase of study selection included a pilot test of the abstract/title screening process across the review team. Identified articles were imported into Orbit Intellixir software (Questel Intelligence, Alexandria, Virginia) which is a data analytics software for exploring topical trends and bibliographic statistics. From Orbit Intellixir, the literature was automatically grouped into topic categories. Using these topic groups, a random sample of 25 articles were selected to perform a pilot test of the title/abstract screening process. This included 10 articles each from potentially relevant and nonrelevant topic categories (e.g. “dermal exposure” and “asthma”) and another 5 articles from the full list of identified references. This process ensured that a mix of both relevant and nonrelevant articles were included in the pilot. Following the pilot test, reviewers met to discuss any discrepancies. The team proceeded to screening the rest of the titles/abstracts once all reviewers were in 100% agreement for include/exclude status of the pilot test articles.
Title/abstract screening
Title/abstract screening utilized SWIFT Active Screener (Sciome, Durham, North Carolina) to increase efficiency. This web-based tool is a collaborative platform for conducting systematic reviews with text mining and machine learning to actively learn which articles should be excluded as the team screens and includes articles (Howard et al. 2020). This tool has been recommended and used in an increasing number of systematic literature reviews with success in decreasing work time while providing a comparative performance to human screeners reviewing all studies (Lam et al. 2019; Pelch et al. 2019; Cohen Hubal et al. 2020; Elmore et al. 2020; Hamel et al. 2021; Kim et al. 2022). References were uploaded into the web tool after screening for duplicates in Mendeley. In the web tool, the order in which articles are presented for screening is first randomized; as screening progresses, the tool prioritizes which articles are brought forward for review.
Dual independent screening was performed. Any discrepancies between reviewer decisions were flagged and resolved by discussion between the reviewers or with a third reviewer. If it was unclear whether a study met the eligibility criteria, it was moved forward to full text review and later confirmed. Screening was stopped when an estimated 95% recall was achieved; this is the point at which the SWIFT Active Screener tool predicted that ≥95% of all relevant articles were identified and included by the study team. This estimated recall level has been used in previous studies and has been shown to be a conservative estimate where the true recall is often greater than 95% (Lam et al. 2019; Howard et al. 2020; Kim et al. 2022). Furthermore, the titles of the articles marked for exclusion by SWIFT Active Screener were also screened by a single human reviewer to ensure there are no false negatives marked by the tool.
Full text screening and data charting
Articles included during title/abstract screening were retrieved in full text form. Articles were further assessed in detail against the eligibility criteria and included articles were data charted. The data charting tool was developed using an Excel spreadsheet and pilot tested across 2 reviewers for 5 articles. Then, 2 reviewers conducted the data charting process across 54 included full text articles (54/493, 11%) and iteratively updated the charting tool as needed to ensure that the data were most accurately and efficiently captured. A single reviewer completed data charting for the rest of the full texts.
In addition to each article’s bibliometric metadata, charted data included the study’s primary objective, chemical(s)/substance(s) targeted, dermal exposure assessment measurement type(s), and sampling method(s). As data charting commenced, it was observed that the biomarkers literature was often ambiguous regarding whether the dermal exposure route was targeted or not. Therefore, further contextual data were also charted to differentiate dermal exposure studies from studies with unclearly defined targeted exposure route(s): yes/no whether air sampling was performed and yes/no whether it was documented which study subjects wore respiratory protection sufficient to mitigate the inhalational exposure route. Consistent with established methods for a scoping review, quantitative meta-analysis was not conducted.
Further review of each included articles’ reference lists was conducted during the data charting process. Each article moved from abstract screening to full text review and subsequently included had its references list (titles) scanned for additional relevant articles. Any additional articles identified were screened as described above and included if relevant.
Risk of bias appraisal of individual sources of evidence
Consistent with established methods for a scoping review, risk of bias for individual sources of evidence was not conducted.
Deviations from the study protocol
Relative to the originally published study protocol (Therkorn and Laursen 2022), several modifications were made. First, it was deemed that radiation exposure was out of scope for this study as it may broadly encompass exposures from radiation contamination as well as ultraviolet radiation (i.e. sunlight); future further study on this topic alone may be warranted requiring additional targeting of these types of studies in the literature pull. Second, it was deemed prudent to screen the reference lists for all studies undergoing full text review to ensure broad capture of relevant literature. Third, the data charting tool underwent several iterations throughout data charting to ensure accurate yet efficient charting for included studies. Fourth, as described above, it was observed that the biomarkers literature was often ambiguous regarding whether the dermal exposure route was targeted or not. Therefore, these studies were moved to full text review for closer examination. During full text review, the article’s objectives, methods, discussion, and final conclusions all had to be screened to determine whether or not the article targeted the dermal exposure route whether intentionally or incidentally.
Results
Source of evidence selection
A total of 1,052 unique records were identified from databases (Fig. 1). A total of 768 records were then excluded during title/abstract and full text screening resulting in 284 articles included from database searches (1,052 − 768 = 284). The majority of the exclusions were attributed to not meeting the study definition for having a dermal exposure assessment method or not being conducted in an occupational setting (50% (385/768), across both phases of article screening). A total of 493 articles were subsequently data charted after including an additional 209 articles from included articles’ references lists (284 + 209 = 493).
Fig. 1.
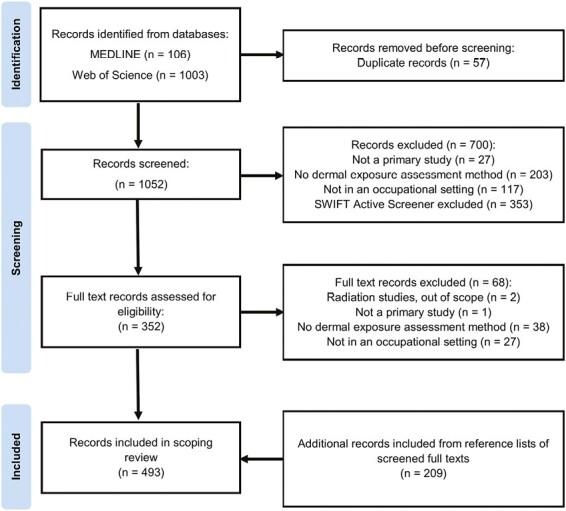
PRISMA flow diagram. Study flow illustrating article screening, inclusion, and exclusion.
Study characteristics
Three main types of data were charted for each included study: (i) the article’s primary objective or study type, (ii) the dermal exposure assessment method(s) used, and (iii) the targeted chemical(s)/substance(s) these method(s) were used for. Article’s primary objectives were determined based on explicitly stated aims/objectives and where necessary based on text in article introductions and conclusions. The primary objectives for the included articles covered a variety of study types encompassing method development/method validation studies (22%, 110/493), exposure assessment studies including those evaluating contributors to exposure variability (51%, 253/493), studies focused on health outcomes/risk assessment/epidemiology (21%, 103/493), and evaluations of controls and protective measures (6%, 27/493). The charted data from each included article is provided as a dataset in Supplementary Material.
Identified dermal exposure assessment methods
The number of methods charted exceeds the number of included articles because many studies used multiple methods. For example, one study described the use of patches (dosimeter) to capture fluorescent tracer (visual tracer) for subsequent analysis by video imaging performed by the study team (qualitative assessment) (Fenske et al. 2002). The most commonly reported method was biomarkers with 51% (252/493) of studies reporting their use (Table 2). Termed herein as biomarker plus studies (41%, 201/493), these were studies complying with at least one of the following criteria: (i) included air sampling to measure airborne contaminant concentrations, (ii) clearly documented whether workers were wearing respiratory protective equipment to mitigate the inhalational route, and/or (iii) included an additional complementary dermal exposure assessment method to characterize and add more context to the biomarker data. Fifty-one studies (10%, 51/493) not meeting any of these criteria were subset and categorized as biomarker-only studies.
Table 2.
Summary of identified dermal exposure assessment methods and description of how these were data charted (overall N = 493).
| Method | Description | Examples | N | % |
|---|---|---|---|---|
| Biomarker plus | Biomonitoring as an indirect measure of dermal exposure and dose via correlated biological indicator. Biomarker plus articles included more context to differentiate targeted exposure route(s) such as air sampling, respiratory protective equipment use to mitigate inhalation and/or a complementary dermal exposure assessment method. | Metabolites (urinary, blood) | 201 | 41 |
| Dosimeter | Dosimeter of specific, fixed dimensions to intercept and capture substances | Dermal patches/cloths/pads, including those fastened to gloves or coveralls | 102 | 21 |
| Qualitative | Qualitative assessments based on human self-report or third-party human observation | Questionnaire/survey administered to workers or industrial hygienists | 86 | 17 |
| Skin wipe | Substance removal by wipe to estimate amount of contaminant on skin | Gauze pad wipe | 74 | 15 |
| Surrogate skin | Surrogate skin to intercept capture of substances where collection surface area is variable depending upon the wearer’s dimensions and their tasking | Gloves to represent hands, headbands to represent forehead, silicone wristbands to represent wrist area | 73 | 15 |
| Surface proxy | Surface sampling as proxy indicator for high touch surfaces. This category was only data charted if it was linked to the dermal exposure route, such as by sampling high touch surfaces identified via site survey. For example, any studies only characterizing floor contamination throughout a facility would be out of scope. | Wiping or swabbing of high touch surfaces | 70 | 14 |
| Model | Modelling and simulation by statistical or deterministic approaches | RISKOFDERM (van Hemmen et al. 2003) | 52 | 11 |
| Biomarker only | Biomonitoring articles not meeting criteria described above for biomarker plus. | Metabolites (urinary, blood) | 51 | 10 |
| Whole body method (WBM) | The use of overalls, coveralls, or clothing fully covering top and bottom portions of body (arms and legs) subsequently cut into pieces to determine potential dermal exposure. Head covering also presumably or explicitly included. | Coveralls or overalls with head pad (WHO 1982) | 47 | 10 |
| Skin wash | Substance removal by wash, dip or rinse to estimate amount of contaminant on skin | Bag rinse method where hands are submerged and shaken into bag of rinsing liquid (Lind et al., 2004) | 47 | 10 |
| Skin tape | Removal of top layers of skin to estimate amount of substance on and absorbed into top layers of skin | Tape stripping | 33 | 7 |
| Visual tracer | Visualization techniques for simultaneous or post task observation of where substances have deposited | Fluorescent tracers | 27 | 6 |
| Skin swab | Substance removal by a swab to estimate amount of contaminant on skin | Nylon flocked swab | 12 | 2 |
| Vacuum | Skin vacuuming to remove contaminants | Small vacuuming sampler (Lundgren et al. 2006) | 1 | < 1 |
Identified chemicals/substances
The categories used for summarizing chemical/substance results were selected to represent a substance type and its product usage (Table 3). For example, the category of pesticides is representative of both a substance grouping and how the product is typically used (i.e. sprayed, mixed, loaded). Where it was unclear what the targeted chemical or substance was for the dermal exposure assessment, articles’ analytical methods were screened to discern the specific targeted analyte. If a paper assessed multiple substances not all attributed to the dermal route, then only those specific to the dermal route were data charted. The most targeted substances across the literature were pesticides (136/493, 28%) and polycyclic aromatic hydrocarbons (PAHs) (107/493, 22%) comprising about half of the included articles.
Table 3.
Summary of chemicals/substances categories used for data charting, their description, and key contributors to categories observed from the literature (overall N = 493).
| Chemical or substance category | Description | Observed key contributors to category | N | % |
|---|---|---|---|---|
| Pesticide | Pesticides, herbicides, fungicides, and biocides such as organophosphates, organochlorines, neonicotinoids, and carbamates | Organophosphates | 136 | 28 |
| PAH | Polycyclic aromatic hydrocarbons (PAHs) such as benzo(a)pyrene and contaminants commonly present with PAHs including naphthalene | Asphalt/bitumen, fuels, coke, coal tar, and burning materials associated with firefighting | 107 | 22 |
| Metal dust | Metals, metal dusts, and other inorganic dusts, such as glass | Copper, beryllium, cobalt, and chromium | 36 | 7 |
| Isocyanates | Isocyanates and diisocyanates, including 1,6-hexamethylene diisocyanate monomer, 1,6-hexamethylene diisocyanate isocyanurate, methylenediphenyl diisocyanate (MDI) and toluene diisocyanate (TDI) | Spray paint and polyurethane | 32 | 7 |
| Drug | Antineoplastic chemotherapy drugs, most commonly 5-fluorouracil, cyclophosphamide, doxorubicin, ifosfamide, and/or methotrexate. This category also includes one article each for two other drugs (anesthetic and corticosteroid). | Antineoplastic drugs | 29 | 6 |
| Organic dust | Organic dusts, biological materials and particulates, including organisms or pathogens, nicotine residues, wood dusts, cannabis residues, and carbon nanomaterials. Also includes studies utilizing fluorescent tracers specifically to simulate contact with pathogens. | Microorganisms | 29 | 6 |
| Nontargeted | Nontargeted to a specific substance or contaminant, such as the use of fluorescent tracers in simulated studies of various, nonspecific occupational settings or targeting chemicals crossing multiple contaminant categories, such as both cleaning agents and PAHs simultaneously. | Fluorescent tracers | 27 | 6 |
| Polymer/plastic | Polymeric and liquid polymeric substances, such as epoxy resins, methyl methacrylate, paints, phthalates and styrenes. | Epoxy resin systems (bisphenol A diglycidyl ether (BADGE)) | 16 | 3 |
| BTEX | Benzene, toluene, ethylbenzene and/or xylenes (BTEX) | BTEX from solvents | 15 | 3 |
| HFR | Halogenated flame retardants (HFR) including organophosphates and organobromines | Electronic waste | 14 | 3 |
| Nontargeted HC | Multiple chemicals/substances representing a hydrocarbon (HC) mixture or UVCB substance (unknown, variable, complex, and biological). For example, methods assessing multiple contaminants (i.e. not only PAHs) from the emissions of burning materials, asphalt/bitumen, or jet fuels. | Burning materials, oils/fuels, metalworking fluids | 12 | 2 |
| BPA/BPS | Bisphenol A (BPA), bisphenol S (BPS), and/or 4-hydroxyphenyl 4-isoprooxyphenylsulfone (BPSIP) | Thermal paper receipts | 9 | 2 |
| DMF/NMP | N,N-dimethylformamide (DMF) and N-methyl-2-pyrrolidone (NMP) | Peptide synthesis solvents | 9 | 2 |
| Cleaner | Substances and chemicals used for cleaning and disinfection purposes, including alcohol-based hand rubs, main ingredients in cleaning products such as ammonia and chlorine, and detergent enzymes | Alcohol-based hand rubs | 8 | 2 |
| Dioxin/PCB | Polychlorinated biphenyls (PCBs), polychlorinated dibenzo-p-dioxins and polychlorinated dibenzofurans (PCDD/Fs) | Electronic waste | 7 | 1 |
| Glycol ether | Glycol ether solvent (i.e. 2-butoxyethanol) | Glycol ether | 4 | 1 |
| Hair dye | Organic compounds associated with use of hair dyes, including para-phenylenediamine | Hair dye | 3 | 1 |
Trends in methods use over time
Over the timeline of evidence included in the present study’s scope (2002 to 2020), no apparent trends emerge for popularity in the use of different methods. These data are presented in Supplementry Figs. S1–S5 stratified by primary study types.
Mapping across methods and targeted substances
Mapping across the dermal exposure assessment method types and their targeted chemicals/substances illustrates (i) where the most publications reside on these combinations, versus and (ii) where the data gaps exist in the literature (Fig. 2). Overall, pesticides and PAHs were the most targeted substances; methods for assessing pesticides were spread across the different method categories whereas methods for assessing PAH exposures were more concentrated in categories of biomarkers, dosimeters, qualitative assessments, and skin wiping. Neither pesticides nor PAHs had any studies identified using skin swabbing methods. Alternatively, skin swabbing was the most common method in studies assessing skin contact with microorganisms charted here as part of the organic dusts category. Similarly, certain substances were more commonly assessed using specific methods likely reflecting methods tailored to targeted chemicals/substances, such as skin wiping for metal dusts and skin tape stripping for isocyanates. The drug category, largely representing dermal contact with antineoplastic drugs for healthcare workers, was most assessed by indirect measures including biomarkers and surface proxies (surface wiping). Modeling and qualitative assessments were the most chosen methods when a study targeted multiple substances reflecting the flexibility in these exposure assessment approaches. The category defined as biomarker plus was the only method charted across all substance categories.
Fig. 2.
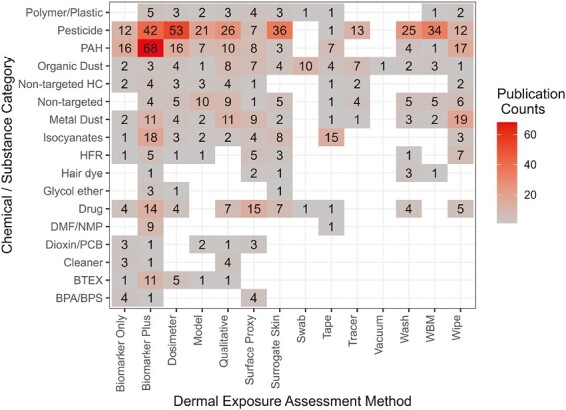
Heat map across chemical/substance categories (y-axis) and dermal exposure assessment methods (x-axis). The colors reflect publication counts where hotter colors reflect higher counts. The absence of number tiles reflects gaps in literature for a given substance/method combination. The definitions for dermal exposure assessment methods are presented in Table 2 while definitions for the chemical/substance categories are presented in Table 3. Abbreviations: PAH = polycyclic aromatic hydrocarbon; HC = hydrocarbon; HFR = halogenated flame retardant; DMF/DMP = N,N-dimethylformamide and N-methyl-2-pyrrolidone; PCB = polychlorinated bisphenol; BTEX = benzene, toluene, ethylbenzene, and xylene; BPA/BPS = bisphenol A, bisphenol S, and/or 4-hydroxyphenyl 4-isoprooxyphenylsulfone; WBM = whole body method.
Literature gaps
Converse to the highest number of publications counts, the empty spaces shown in Fig. 2 reflect gaps in the literature. Presuming the categories of chemicals reflects the contaminants of most concern for dermal exposure, there is a lack of evidence for cleaning agents, hair dyes, glycol ether, N,N-dimethylformamide/N-methyl-2-pyrrolidone (DMF/DMP), dioxins and polychlorinated bisphenols (PCBs), and bisphenol A/bisphenol S (BPA/BPS). See Table 3 for definitions and details on chemical/substance categories. Alternatively, considering chemicals with skin notation profiles from the National Institute for Occupational Safety and Health (NIOSH 2013), most of these chemicals overlap with the chemical/substance categories presented in Fig. 2. However, there are chemicals with skin notation profiles that are not represented by the data charted herein, including trichloroethylene, propargyl alcohol, diacetyl, explosives/propellants (e.g. cyclonite and nitroglycerin), and some chemicals commonly used as manufacturing precursors/intermediates or for laboratory purposes (e.g. formaldehyde, aniline, catechol, dimethyl sulfate, and phenol). It should be noted that skin notation profile chemicals are those that present health hazards if skin exposure occurs. Therefore, in determining the priority of pursuing further research on dermal exposure assessment methods for these chemicals in an occupational setting, it is important to first consider the potential for exposure to occur (Lynch et al. 2023).
Discussion
Fostering consensus and standardization
A total of 493 articles were data charted broadly comprising occupational dermal exposure assessment studies conducted over the last 20 years. These studies were mapped across 4 different major types of study designs, 17 different chemical/substance categories, and 14 different types of exposure assessment methods (including the stratification of biomarkers into 2 different categories). The major contributors to the dermal exposure assessment literature included pesticides for all charted methods (except swabs and vacuum) and PAH biomarker studies. Literature gaps were identified for nonbiomarker methods for DMF/DMP and for all methods for cleaning agents, hair dyes, glycol ether, dioxins/PCBs, and BPA/BPS.
Systematically mapping the literature has illustrated where detail and clarity in published studies would be beneficial to move the fields toward standardization and consensus of best practices. These recommendations, presented below, are intended to better enable future cross study comparisons for extracting best practices. General recommendations and best practices for exposure assessment, including establishing and reporting the exposure assessment plan (US EPA 2019), are clearly relevant as well.
Description and terminology for exposure assessment methods
Dermal exposure assessment methods were identified in each study and assessed against both what they were called and how they were described in the articles’ methods sections. Determinations were made as to how to classify each exposure assessment method using as much detail as possible. For example, a study may not make any mention to the terms whole body method or whole body dosimetry, but rather describe the use of overalls subsequently cut into sections and analyzed to assess pesticide exposure (Edwards et al. 2007). By definition (WHO 1982), this is the whole body method. Similarly, another study may not mention the whole body method within their methods section, yet they refer to the whole body method in both their introduction, results, and discussion (Atabila et al. 2017). Other studies may describe the use of patches/pads/dosimeters affixed to coveralls or overalls and subsequent extrapolation of dosimeter-based exposure estimates to represent whole body results (Baldi et al. 2006; Rubino et al. 2012; An et al. 2015). Table 2 provides a starting point to organizing naming and categorical conventions for methods used in future studies. Future work would be improved by accurately, clearly, and consistently describing exposure assessment methods.
Description of worker tasking, processes, and exposure scenarios
There was a lack of standardization and detail in how tasking information was presented across the different studies. For example, studies investigating PAH exposure associated with asphalt/bitumen may only include a generic description such as laying/paving of asphalt (Campo et al. 2006; Lotz et al. 2016; Xu et al. 2018). Asphalt paving crews, however, can include supervisors and inspectors not involved in manual work; operators of various vehicles and paving equipment; workers applying prime and tack coats; and workers conducting spreading/raking with hand tools (The Asphalt Institute 1978, 1983). Each of these different roles, their tasking, and modifying factors such as PPE represents different potential inhalational and dermal exposures to PAHs. Where it is feasible to collect data on detailed specific tasking, future research would be improved by supplying enough context to understand how exposures may occur.
Therefore, in the present study, the final binned categories used for summarizing substances were selected to represent a contaminant type and its typical product usage/tasking without extrapolating beyond what could be directly ascertained from each article. For example, the category of pesticides is representative of both a contaminant grouping and how a product is typically used (i.e. sprayed, mixed, and loaded) (Kuye et al. 2008; Basilicata et al. 2013; Illyassou et al. 2019). In an occupational regulatory context, the specific terminologies for exposure scenarios will vary, but the concepts and data types are consistent. For example, see Koivisto et al. (2021) for an overview of terminologies associated with exposure scenarios and conditions of use as organized under the European Union’s REACH (Registration, Evaluation, Authorization and restriction of Chemicals). Other past research efforts have been made to present best practices for reporting of exposure assessment studies, such as description of occupational exposure scenarios as described by Lynch et al. (2023), patterns/determinants of dermal exposure relevant to modeling as described by Marquart et al. (2003), Warren et al. (2006) and Rajan-Sithamparanadarajah et al. (2004), and work factors specific to exposures among pesticide sprayers (Garzia et al. 2018).
Description of chemicals/substances and their state
It was originally intended to data chart substances by their state (e.g. gas versus solid). However, many studies did not provide sufficient detail to chart this information with confidence. While it may be intuitive within a given field for what the assumed state of a contaminant is, this information should be clearly described to foster standardization across the dermal exposure assessment field where studies from different occupational settings may inform each other. For example, description of the substance’s state for antineoplastic drugs was rarely provided. But exposure to these drugs may broadly range from: pharmaceutical compounding and preparation of drugs presumably involving powders; administering intravenous solutions presumably involving liquids; disposing of patient urine and cleaning procedures presumably involving contact with potentially contaminated surfaces (Fransman et al. 2004, 2005; Hedmer et al. 2008; Koller et al. 2018). All these scenarios illustrate different potentials for dermal exposure that are not necessarily apparent outside of the healthcare domain. Additional guidance on detailed information that should be reported from dermal exposure studies is provided by Van de Sandt et al. (2007). Specifically, for studies generating data relevant to chemical risk assessment, an ideal set of study data would include information on concentration of chemical in contact media with state of media clearly described, as well as data on exposure timeframe and factors affecting contact of skin with contaminant (i.e. PPE and exposed skin surface area). Descriptions of these factors, including state of chemical/substance, would be beneficial to understanding the representativeness of a given exposure assessment method for its targeted substance.
A priori specification of targeted exposure route
It was observed that studies often reported the use of a biomarker to assess exposure without clearly differentiating which exposure route it was intended to represent. For example, a study may not specifically mention targeting of the dermal exposure route in their aims/objectives or methods, but generally discusses the importance of the dermal route with respect to the chemical/substance of interest in the introduction and discussion (Campo et al. 2010; Barbeau et al. 2014). Similarly, other studies may explicitly state they are inferring the dermal exposure route due to observed dermal contact with potentially contaminated surfaces or observed variabilities in biomarker levels across subjects with varying glove use (Chan et al. 2007; Serdar et al. 2012). Finally, other studies may explicitly state that their final results are unclear regarding whether it was the dermal and/or inhalational route which contributed to the observed biomarker levels (Viegas et al. 2015). It was challenging to determine which biomarker studies to include/exclude for this scoping review given this ambiguity. As described in the methods above, to discern whether a biomarker study should be included, the article’s objectives, methods, discussions, and final conclusions all had to be screened to determine whether or not the article should be included as a dermal study. Therefore, while a formal appraisal of study quality was not within present scope, a preliminary stratification of the biomarker studies was performed and used to present the results. This should not be taken to represent high- and low-quality categories of studies. Rather, these 2 different categories attempt to differentiate for data charting purposes the proportion of the literature for which it may be more possible to differentiate the dermal exposure route from other exposure routes. Future work investigating biomarkers of exposure with multiple potential exposure routes would benefit from clearly specifying targeted route(s) in their objectives and incorporating methods when feasible to differentiate and estimate different exposure route contributions to total exposure.
Limitations and future considerations
First and foremost, as is the nature of a scoping review, formal quality assessment of the included articles was not performed and should be addressed in future work. The availability of published literature does not necessarily indicate the availability of sufficiently useful information. The occupational dermal exposure assessment literature has been categorized into method types and targeted chemicals/substances for the purpose of mapping and characterizing what literature exists on the topic. Because a formal quality assessment has not been conducted for the included articles, categories into which each article has been mapped should be further explored to confirm the appropriateness and quality of the applied methods. Where different methods are available for a given method/substance combination (e.g. different approaches for skin wiping of metal dusts), best practices for a specific method/substance and comparability across methods must be determined (Geer et al. 2004; Ross et al. 2008; Aprea 2012; Galea et al. 2014; Ng et al. 2014).
As described in the methods, it was deemed prudent to screen the reference lists for all studies undergoing full text review to ensure broad capture of relevant literature. Due to limited time/resources, we could not go further through the references lists of each additional study found. Therefore, every single relevant study was likely not identified. However, as illustrated in remaking the heat map of Fig. 2 using only the articles in the original database pulls (Supplementary Fig. S6), the additional articles found in the reference lists contributed to completeness but did not alter the final results and conclusions. The exception to this is that 2 articles were found on radiation exposure in the original database pulls but subsequently excluded. Because radiation can refer to radioisotope contaminants and ultraviolet radiation, future research on these topics should specifically target these types of studies which were not sufficiently represented in the original database searches.
Regardless, because nearly half of the included articles in the present study were identified from references lists, this warrants closer examination of the literature search strategy. First, comparing across the types of articles found in references lists versus database pulls, there appears to be more journals and articles dedicated to healthcare (Supplementary Table S2). Healthcare worker exposure to microorganisms and antineoplastic drugs were identified across the literature and included in the final analyses. However, microorganisms were a relatively small category of contaminant substance identified and were included herein as a larger category of organic materials/organic dusts (Table 3). Future research should better target this contaminant category specifically. Second, as already discussed above in the Discussion section on a priori specification for targeted exposure route, the biomarker method type presented challenges in identifying relevant articles. Trends across articles identified via reference list screening further indicates biomarker studies without another type of dermal exposure assessment method presented challenges in identification (Supplementary Table S2). This further emphasizes suggestions presented above for future work to explicitly specify targeted exposure route(s) and utilize methods to differentiate contributions from different routes where applicable and feasible. Third, given the overall variety in different types of chemicals/substances charted from article reference lists, future work focused on a particular type of occupational environment and worker population should more specifically target these in the literature search strategy. For example, depending on the focus of future study, more targeted search terms could be included for healthcare workers, nurses, pesticide sprayers, agricultural workers, cashiers, their respective synonymous terms and any other key terms related to the specific exposure scenario of concern. Overall, given the intentionally broad scope of this scoping review, future work targeting specific worker types, substances and/or dermal exposure assessment method categories should tailor the literature search strategy accordingly. To help facilitate future targeted research, the data charted from the present study are provided as a dataset in Supplementary Material; this dataset can be used to filter the study types of interest to help tailor targeted literature search strategies with the guidance of a trained librarian.
Conclusions
This scoping review was based on a primary question: what methods have been used in the literature in occupational settings to assess worker dermal exposures? Additionally, this review had a subquestion: for which chemicals/substances have these methods been used? In answering the former question, this study provides a foundation and starting point to standardize language used to categorize dermal exposure assessment method types across 14 categories. In answering the latter question, this study defines a set of literature boundaries for 17 substance/chemical categories of concern for dermal exposure in the occupational setting. Mapping between exposure assessment method types and chemicals/substances highlights where most studies have thus far been published (pesticides and PAH biomarkers), and where further research is most required (cleaning agents, hair dyes, glycol ether, DMF/DMP, dioxins/PCBs, and BPA/BPS). As a result of mapping this broadly scoped literature, suggestions are provided to foster movement of the occupational dermal exposure assessment literature toward scientific consensus by standardizing reporting for: (i) terminology for exposure assessment methods, (ii) worker tasking/processes/exposure scenarios, (iii) targeted chemicals/substances and their state, and (iv) explicitly targeted exposure routes.
Supplementary Material
Acknowledgments
We would like to express our sincere gratitude to all who contributed to the completion of this research. We would especially like to thank Katy Goyak for her contributions to the development of the abstract review process. We also thank Erica Jones, Kate Serrano, Jennifer Shin, Maria Korre, and Lynn Rudd for their insights and support throughout this study. We are also thankful to the rest of our team members at ExxonMobil Biomedical Sciences Inc. for their constructive feedback and encouragement.
Contributor Information
Jennifer H Therkorn, ExxonMobil Biomedical Sciences, Inc., 1545 U.S. Highway 22 East, Annandale, NJ, United States.
Brittany A Mathewson, ExxonMobil Biomedical Sciences, Inc., 1545 U.S. Highway 22 East, Annandale, NJ, United States.
Christopher J Laursen, ExxonMobil Technology & Engineering, 22777 Springwoods Village Parkway, Spring, TX, United States.
Silvia Maberti, ExxonMobil Biomedical Sciences, Inc., 1545 U.S. Highway 22 East, Annandale, NJ, United States.
Vitaly Aizenberg, ExxonMobil Biomedical Sciences, Inc., 1545 U.S. Highway 22 East, Annandale, NJ, United States.
Brian T Dinkelacker, ExxonMobil Biomedical Sciences, Inc., 1545 U.S. Highway 22 East, Annandale, NJ, United States.
Saumitra Rege, ExxonMobil Biomedical Sciences, Inc., 1545 U.S. Highway 22 East, Annandale, NJ, United States.
Author contributions
The authors designed and executed the study, wrote the manuscript, and take responsibility for the content of the manuscript.
Funding
The authors are employees of ExxonMobil who provided funding for this study from ExxonMobil Biomedical Sciences Inc., a wholly owned subsidiary of ExxonMobil Corporation. The authors designed and executed the study and wrote the manuscript.
Conflict of interest statement
The authors are employed by ExxonMobil and this was an internally conducted study. The authors designed and executed the study and wrote the manuscript.
Data availability
The data charted from the present scoping review are provided as a dataset in the Excel format as given in Supplementary Material. Additionally, this dataset can be accessed via the Open Science Framework along with the study protocol (Therkorn and Laursen 2022).
References
- An X, Ji X, Jiang J, Wang Y, Wu C, Zhao X.. Potential dermal exposure and risk assessment for applicators of chlorothalonil and chlorpyrifos in cucumber greenhouses in China. Hum Ecol Risk Assess. 2015:21(4):972–985. 10.1080/10807039.2014.949165 [DOI] [Google Scholar]
- Anderson SE, Meade BJ.. Potential health effects associated with dermal exposure to occupational chemicals. Environ Health Insights. 2014:8(Supp 1):51-62. https://doi.org/10.4137%2FEHI.S15258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aprea MC. Environmental and biological monitoring in the estimation of absorbed doses of pesticides. Toxicol Lett. 2012:210(2):110–118. 10.1016/j.toxlet.2011.08.008 [DOI] [PubMed] [Google Scholar]
- The Asphalt Institute. 1978. Asphalt paving manual. 3rd ed. College Park, MD: The Asphalt Institute. [Google Scholar]
- The Asphalt Institute. 1983. Principles of construction of hot-mix asphalt pavements. Lexington, KY: The Asphalt Institute. [Google Scholar]
- Atabila A, Phung DT, Hogarh JN, Osei-Fosu P, Sadler R, Connell D, Chu C.. Dermal exposure of applicators to chlorpyrifos on rice farms in Ghana. Chemosphere. 2017:178: 350–358. 10.1016/j.chemosphere.2017.03.062 [DOI] [PubMed] [Google Scholar]
- Baldi I, Lebailly P, Jean S, Rougetet L, Dulaurent S, Marquet P.. Pesticide contamination of workers in vineyards in France. J Expo Sci Environ Epidemiol. 2006:16(2):115–124. 10.1038/sj.jea.7500443 [DOI] [PubMed] [Google Scholar]
- Barbeau D, Persoons R, Marques M, Hervé C, Laffitte-Rigaud G, Maitre A.. Relevance of urinary 3-hydroxybenzo(a)pyrene and 1-hydroxypyrene to assess exposure to carcinogenic polycyclic aromatic hydrocarbon mixtures in metallurgy workers. Ann Occup Hyg. 2014:58(5):579–590. 10.1093/annhyg/meu004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basilicata P, Simonelli A, Silvestre A, Lamberti M, Pedata P, Feola D, Acampora A, Pieri M, Sannolo N, Miraglia N.. Evaluation by environmental monitoring of pesticide absorption in farm workers of 18 Italian tomato cultivations. Int J Immunopathol Pharmacol. 2013:26(2):517–523. 10.1177/039463201302600226 [DOI] [PubMed] [Google Scholar]
- Campo L, Buratti M, Fustinoni S, Cirla PE, Martinotti I, Longhi O, Cavallo D, Foà V.. Evaluation of exposure to paths in asphalt workers by environmental and biological monitoring. Ann N Y Acad Sci. 2006:1076: 405–420. 10.1196/annals.1371.013 [DOI] [PubMed] [Google Scholar]
- Campo L, Rossella F, Pavanello S, Mielzynska D, Siwinska E, Kapka L, Bertazzi PA, Fustinoni S.. Urinary profiles to assess polycyclic aromatic hydrocarbons exposure in coke-oven workers. Toxicol Lett. 2010:192(1):72–78. 10.1016/j.toxlet.2008.12.018 [DOI] [PubMed] [Google Scholar]
- Chan XGH, Xu Y, Liang Y, Chen LX, Wu SC, Wong CKC, Leung CKM, Wong MH.. Body loadings and health risk assessment of polychlorinated dibenzo-p-dioxins and dibenzofurans at an intensive electronic waste recycling site in China. Environ Sci Technol. 2007:41(22):7668–7674. 10.1021/es071492j [DOI] [PubMed] [Google Scholar]
- Cohen Hubal EA, Frank JJ, Nachman R, Angrish M, Deziel NC, Fry M, Tornero-Velez R, Kraft A, Lavoie E.. Advancing systematic-review methodology in exposure science for environmental health decision making. J Expo Sci Environ Epidemiol. 2020:30(6):906–916. 10.1038/s41370-020-0236-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards JW, Lee S-G, Heath LM, Pisaniello DL.. Worker exposure and a risk assessment of malathion and fenthion used in the control of Mediterranean fruit fly in south Australia. Environ Res. 2007:103(1):38–45. 10.1016/j.envres.2006.06.001 [DOI] [PubMed] [Google Scholar]
- Elmore R, Schmidt L, Lam J, Howard BE, Tandon A, Norman C, Phillips J, Shah M, Patel S, Albert Tet al. . Risk and protectiv e factors in the covid-19 pandemic: a rapid evidence map. Front Public Health. 2020:8: 582205. 10.3389/fpubh.2020.582205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenske R, Birnbaum S, Methner M, Lu C, Nigg H.. Fluorescent tracer evaluation of chemical protective clothing during pesticide applications in central Florida citrus groves. J Agric Saf Health. 2002:8(3):319–331. 10.13031/2013.9056 [DOI] [PubMed] [Google Scholar]
- Fenske RA. Dermal exposure assessment techniques. Ann Occup Hyg. 1993:37(6):687–706. 10.1093/annhyg/37.6.687 [DOI] [PubMed] [Google Scholar]
- Fenske RA. State-of-the-art measurement of agricultural pesticide exposures. Scand J Work Environ Health. 2005:31(Suppl 1):67–73; discussion 63-65. [PubMed] [Google Scholar]
- Fitoussi R, Faure M-O, Beauchef G, Achard S.. Human skin responses to environmental pollutants: a review of current scientific models. Environ Pollut. 2022:306: 119316. 10.1016/j.envpol.2022.119316 [DOI] [PubMed] [Google Scholar]
- Fransman W, Vermeulen R, Kromhout H.. Occupational dermal exposure to cyclophosphamide in Dutch hospitals: a pilot study. Ann Occup Hyg. 2004:48(3):237–244. 10.1093/annhyg/meh017 [DOI] [PubMed] [Google Scholar]
- Fransman W, Vermeulen R, Kromhout H.. Dermal exposure to cyclophosphamide in hospitals during preparation, nursing and cleaning activities. Int Arch Occup Environ Health. 2005:78(5):403–412. 10.1007/s00420-004-0595-1 [DOI] [PubMed] [Google Scholar]
- Galea KS, McGonagle C, Sleeuwenhoek A, Todd D, Jiménez AS.. Validation and comparison of two sampling methods to assess dermal exposure to drilling fluids and crude oil. Ann Occup Hyg. 2014:58(5):591–600. 10.1093/annhyg/meu014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garzia NA, Spinelli JJ, Gotay CC, Teschke K.. Literature review: dermal monitoring data for pesticide exposure assessment of farm workers. J Agromedicine. 2018:23(3):187–214. 10.1080/1059924X.2018.1448734 [DOI] [PubMed] [Google Scholar]
- Gaskin S, Currie N, Cherrie JW.. What do occupational hygienists really know about skin exposure? Ann Work Expo Health. 2021:65(2):219–224. 10.1093/annweh/wxaa046 [DOI] [PubMed] [Google Scholar]
- Geer LA, Cardello N, Dellarco MJ, Leighton TJ, Zendzian RP, Roberts JD, Buckley TJ.. Comparative analysis of passive dosimetry and biomonitoring for assessing chlorpyrifos exposure in pesticide workers. Ann Occup Hyg. 2004:48(8):683–695. 10.1093/annhyg/meh056 [DOI] [PubMed] [Google Scholar]
- Hamel C, Hersi M, Kelly SE, Tricco AC, Straus S, Wells G, Pham B, Hutton B.. Guidance for using artificial intelligence for title and abstract screening while conducting knowledge syntheses. BMC Med Res Methodol. 2021:21(1):285. 10.1186/s12874-021-01451-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper M. Assessing workplace chemical exposures: the role of exposure monitoring. J Environ Monit. 2004:6(5):404–412. 10.1039/b314697a [DOI] [PubMed] [Google Scholar]
- Hedmer M, Tinnerberg H, Axmon A, Jönsson BAG.. Environmental and biological monitoring of antineoplastic drugs in four workplaces in a Swedish hospital. Int Arch Occup Environ Health. 2008:81(7):899–911. 10.1007/s00420-007-0284-y [DOI] [PubMed] [Google Scholar]
- Hopf NB, Bolognesi C, Danuser B, Wild P.. Biological monitoring of workers exposed to carcinogens using the buccal micronucleus approach: a systematic review and meta-analysis. Mutat Res Rev Mutat Res. 2019:781: 11–29. 10.1016/j.mrrev.2019.02.006 [DOI] [PubMed] [Google Scholar]
- Howard BE, Phillips J, Tandon A, Maharana A, Elmore R, Mav D, Sedykh A, Thayer K, Merrick BA, Walker Vet al. . Swift-active screener: accelerated document screening through active learning and integrated recall estimation. Environ Int. 2020:138: 105623. 10.1016/j.envint.2020.105623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illyassou KM, Adamou R, Schiffers B.. Exposure assessment of operators to pesticides in Kongou, a sub-watershed of Niger river valley. J Environ Sci Health B. 2019:54(3):176–186. 10.1080/03601234.2018.1536581 [DOI] [PubMed] [Google Scholar]
- Jacobsen G, Rasmussen K, Bregnhøj A, Isaksson M, Diepgen TL, Carstensen O.. Causes of irritant contact dermatitis after occupational skin exposure: a systematic review. Int Arch Occup Environ Health. 2022:95(1):35–65. 10.1007/s00420-021-01781-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasiotis KM, Spaan S, Tsakirakis AN, Franken R, Chartzala I, Anastasiadou P, Machera K, Rother D, Roitzsch M, Poppek Uet al. . Comparison of measurement methods for dermal exposure to hazardous chemicals at the workplace: The Sysdea project. Annals of Work Exposures and Health. 2020:64(1):55–70. 10.1093/annweh/wxz085 [DOI] [PubMed] [Google Scholar]
- Kim S, Hollinger H, Radke EG.. ‘Omics in environmental epidemiological studies of chemical exposures: a systematic evidence map. Environ Int. 2022:164: 107243. 10.1016/j.envint.2022.107243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koivisto AJ, Spinazzè A, Verdonck F, Borghi F, Löndahl J, Koponen IK, Verpaele S, Jayjock M, Hussein T, Lopez de Ipiña Jet al. . F V. Assessment of exposure determinants and exposure levels by using stationary concentration measurements and a probabilistic near-field/far-field exposure model. Open Res Europe. 2021:1(72). 10.12688/openreseurope.13752.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koller M, Böhlandt A, Haberl C, Nowak D, Schierl R.. Environmental and biological monitoring on an oncology ward during a complete working week. Toxicol Lett. 2018:298: 158–163. 10.1016/j.toxlet.2018.05.002 [DOI] [PubMed] [Google Scholar]
- Kuye RA, Donham KJ, Marquez SP, Sanderson WT, Fuortes LJ, Rautiainen RH, Jones ML, Culp KR.. Pesticide handling and exposures among cotton farmers in the Gambia. J Agromedicine. 2008:12(3):57–69. 10.1080/10599240801887876 [DOI] [PubMed] [Google Scholar]
- Lam J, Howard BE, Thayer K, Shah RR.. Low-calorie sweeteners and health outcomes: a demonstration of rapid evidence mapping (rem). Environ Int. 2019:123: 451–458. 10.1016/j.envint.2018.11.070 [DOI] [PubMed] [Google Scholar]
- Lotz A, Pesch B, Dettbarn G, Raulf M, Welge P, Rihs H-P, Breuer D, Gabriel S, Hahn J-U, Brüning Tet al. . Metabolites of the pah diol epoxide pathway and other urinary biomarkers of phenanthrene and pyrene in workers with and without exposure to bitumen fumes. Int Arch Occup Environ Health. 2016:89(8):1251–1267. 10.1007/s00420-016-1160-4 [DOI] [PubMed] [Google Scholar]
- Lind ML, Boman A, Surakka J, Sollenberg J, Meding B.. A method for assessing occupational dermal exposure to permanent hair dyes. Ann Occup Hyg. 2004:48(6):533-539. 10.1093/annhyg/meh047 [DOI] [PubMed] [Google Scholar]
- Louro H, Gomes BC, Saber AT, Iamiceli AL, Göen T, Jones K, Katsonouri A, Neophytou CM, Vogel U, Ventura Cet al. . The use of human biomonitoring to assess occupational exposure to pahs in Europe: A comprehensive review. Toxics. 2022:10(8):480. 10.3390/toxics10080480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundgren L, Skare L, Lidén C.. Measuring dust on skin with a small vacuuming sampler–a comparison with other sampling techniques. Ann Occup Hyg. 2006:50(1):95-103. 10.1093/annhyg/mei055 [DOI] [PubMed] [Google Scholar]
- Lynch HN, Gloekler LE, Allen LH, Maskrey JR, Bevan C, Maier A.. Analysis of dermal exposure assessment in the us environmental protection agency toxic substances control act risk evaluations of chemical manufacturing. Toxicol Ind Health. 2023:39(1):49–65. 10.1177/07482337221140946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquart H, Maidment S, McClaflin JL, Fehrenbacher MC.. Harmonization of future needs for dermal exposure assessment and modeling: a workshop report. Appl Occup Environ Hyg. 2001:16(2):218–227. 10.1080/104732201460361 [DOI] [PubMed] [Google Scholar]
- Marquart J, Brouwer DH, Gijsbers JH, Links IH, Warren N, van Hemmen JJ.. Determinants of dermal exposure relevant for exposure modelling in regulatory risk assessment. Ann Occup Hyg. 2003:47(8):599–607. 10.1093/annhyg/meg096 [DOI] [PubMed] [Google Scholar]
- McArthur B. Dermal measurement and wipe sampling methods: a review. Applied Occupational and Environmental Hygiene. 1992:7(9):599–606. 10.1080/1047322x.1992.10388051 [DOI] [Google Scholar]
- McDougal JN, Boeniger MF.. Methods for assessing risks of dermal exposures in the workplace. Crit Rev Toxicol. 2002:32(4):291–327. 10.1080/20024091064255 [DOI] [PubMed] [Google Scholar]
- Naylor CL, Davies B, Gopaldasani V.. Quantitative skin exposure assessment of metals: a systematic literature review of current approaches for risk assessment using the construction industry as an exposure scenario. Int Arch Occup Environ Health. 2020:93(7):789–803. 10.1007/s00420-020-01531-8 [DOI] [PubMed] [Google Scholar]
- Ng MG, de Poot S, Schmid K, Cowie H, Semple S, van Tongeren M.. A preliminary comparison of three dermal exposure sampling methods: rinses, wipes and cotton gloves. Environ Sci Process Impacts. 2014:16(1):141–147. 10.1039/c3em00511a [DOI] [PubMed] [Google Scholar]
- NIOSH. Skin notation (sk) profiles. The National Institute for Occupational Safety and Health (NIOSH), Centers for Disease Control and Prevention (CDC); 2013. [accessed 2023 Dec 15]. https://www.cdc.gov/niosh/topics/skin/skin-notation_profiles.html [Google Scholar]
- OSHA. Dermal exposure. United States Department of Labor, Occupational Safety and Health Administration (OSHA); 2022. [accessed 2022 Oct 06]. https://www.osha.gov/dermal-exposure/exposure-evaluation [Google Scholar]
- Pelch KE, Bolden AL, Kwiatkowski CF.. Environmental chemicals and autism: a scoping review of the human and animal research. Environ Health Perspect. 2019:127(4):46001. 10.1289/EHP4386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters M, Godfrey CM, McInerney P, Munn Z, Tricco AC, Khalil H.. Chapter 11: Scoping reviews (2020 version). JBI Manual for Evidence Synthesis; 2020a. [accessed 2022 Sep 01]. https://jbi-global-wiki.refined.site/space/MANUAL [Google Scholar]
- Peters MDJ, Marnie C, Tricco AC, Pollock D, Munn Z, Alexander L, McInerney P, Godfrey CM, Khalil H.. Updated methodological guidance for the conduct of scoping reviews. JBI Evidence Synthesis. 2020b:18(10):2119–2126. 10.11124/JBIES-20-00167 [DOI] [PubMed] [Google Scholar]
- Rajan-Sithamparanadarajah R, Roff M, Delgado P, Eriksson K, Fransman W, Gijsbers JH, Hughson G, Mäkinen M, van Hemmen JJ.. Patterns of dermal exposure to hazardous substances in European union workplaces. Ann Occup Hyg. 2004:48(3):285–297. 10.1093/annhyg/meh025 [DOI] [PubMed] [Google Scholar]
- Ross J, Chester G, Driver J, Lunchick C, Holden L, Rosenheck L, Barnekow D.. Comparative evaluation of absorbed dose estimates derived from passive dosimetry measurements to those derived from biological monitoring: Validation of exposure monitoring methodologies. J Expo Sci Environ Epidemiol. 2008:18(2):211–230. 10.1038/sj.jes.7500591 [DOI] [PubMed] [Google Scholar]
- Rubino FM, Mandic-Rajcevic S, Ariano E, Alegakis A, Bogni M, Brambilla G, De Paschale G, Firmi A, Minoia C, Micoli Get al. . Farmers’ exposure to herbicides in north Italy: assessment under real-life conditions in small-size rice and corn farms. Toxicol Lett. 2012:210(2):189–197. 10.1016/j.toxlet.2012.01.017 [DOI] [PubMed] [Google Scholar]
- Sahmel J, Arnold SF, Ramachandran G.. Accuracy of professional judgments for dermal exposure assessment using deterministic models. J Occup Environ Hyg. 2023:20(3-4):143–158. 10.1080/15459624.2023.2173365 [DOI] [PubMed] [Google Scholar]
- Semple S. Dermal exposure to chemicals in the workplace: Just how important is skin absorption? Occup Environ Med. 2004:61(4):376–382. 10.1136/oem.2003.010645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serdar B, Lee D, Dou Z.. Biomarkers of exposure to polycyclic aromatic hydrocarbons (pahs) and DNA damage: a cross-sectional pilot study among roofers in south Florida. BMJ Open. 2012:2(4):e001318. 10.1136/bmjopen-2012-001318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalt EW, Kissel JC, Shirai JH, Bunge AL.. Dermal absorption of environmental contaminants from soil and sediment: a critical review. J Expo Sci Environ Epidemiol. 2009:19(2):119–148. 10.1038/jes.2008.57 [DOI] [PubMed] [Google Scholar]
- Therkorn J, Laursen C.. Methods to assess worker dermal exposures in occupational settings: a scoping review. Charlottesville, VA: Open Science Framework; 2022. [accessed 2022 Nov 01]. osf.io/7csu4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, Moher D, Peters MDJ, Horsley T, Weeks Let al. . Prisma extension for scoping reviews (prisma-scr): Checklist and explanation. Ann Intern Med. 2018:169(7):467–473. 10.7326/M18-0850 [DOI] [PubMed] [Google Scholar]
- US EPA. Guidelines for human exposure assessment. Washington, DC: U.S. Environmental Protection Agency, Risk Assessment Forum; 2019. [accessed 2023 Sep 10]. https://www.epa.gov/risk/guidelines-human-exposure-assessment [Google Scholar]
- Van de Sandt JJM, Dellarco M, Van Hemmen JJ.. From dermal exposure to internal dose. J Expo Sci Environ Epidemiol. 2007:17(Suppl 1):S38–S47. 10.1038/sj.jes.7500579 [DOI] [PubMed] [Google Scholar]
- Vermeulen R, Stewart P, Kromhout H.. Dermal exposure assessment in occupational epidemiologic research. Scand J Work Environ Health. 2002:28(6):371–385. 10.5271/sjweh.689 [DOI] [PubMed] [Google Scholar]
- Viegas S, Veiga L, Almeida A, dos Santos M, Carolino E, Viegas C.. Occupational exposure to aflatoxin b1 in a Portuguese poultry slaughterhouse. Ann Occup Hyg. 2015:60(2):176–183. 10.1093/annhyg/mev077 [DOI] [PubMed] [Google Scholar]
- Warren N, Marquart H, Christopher Y, Laitinen J, Van Hemmen J.. Task-based dermal exposure models for regulatory risk assessment. Ann Occup Hyg. 2006:50(5):491–503. 10.1093/annhyg/mel014 [DOI] [PubMed] [Google Scholar]
- WHO. Field surveys of exposure to pesticides: standard protocol. Geneva: World Health Organization, Division of Vector, Biology, and Control; 1982. [accessed 2023 Mar 20]. https://iris.who.int/handle/10665/112732 [Google Scholar]
- Xu Y, Lindh CH, Jönsson BAG, Broberg K, Albin M.. Occupational exposure to asphalt mixture during road paving is related to increased mitochondria DNA copy number: a cross-sectional study. Environ Health. 2018:17(1):29. 10.1186/s12940-018-0375-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data charted from the present scoping review are provided as a dataset in the Excel format as given in Supplementary Material. Additionally, this dataset can be accessed via the Open Science Framework along with the study protocol (Therkorn and Laursen 2022).


