Key Points
-
•
Age-dependent effects on plasma VWF levels in type 1 VWD define distinct subgroups with important differences in underlying pathogenesis.
-
•
Low VWF does not constitute a discrete clinical-pathological entity; rather, it is part of an age-dependent type 1 VWD evolving phenotype.
Visual Abstract
Abstract
There is significant ongoing debate regarding type 1 von Willebrand disease (VWD) defintion. Previous guidelines recommended patients with von Willebrand factor (VWF) levels <30 IU/dL be diagnosed type 1 VWD, whereas patients with significant bleeding and VWF levels from 30 to 50 IU/dL be diagnosed with low VWF. To elucidate the relationship between type 1 VWD and low VWF in the context of age-induced increases in VWF levels, we combined data sets from 2 national cohort studies: 162 patients with low VWF from the Low VWF in Ireland Cohort (LoVIC) and 403 patients with type 1 VWD from the Willebrand in The Netherlands (WiN) studies. In 47% of type 1 VWD participants, VWF levels remained <30 IU/dL despite increasing age. Conversely, VWF levels increased to the low VWF range (30-50 IU/dL) in 30% and normalized (>50 IU/dL) in 23% of type 1 VWD cases. Crucially, absolute VWF antigen (VWF:Ag) levels and increase of VWF:Ag per year overlapped between low VWF and normalized type 1 VWD participants. Moreover, multiple regression analysis demonstrated that VWF:Ag levels in low VWF and normalized type 1 VWD patients would not have been different had they been diagnosed at the same age (β = 0.00; 95% confidence interval, −0.03 to 0.04). Consistently, no difference was found in the prevalence of VWF sequence variants; factor VIII activity/VWF:Ag or VWF propeptide/VWF:Ag ratios; or desmopressin responses between low VWF and normalized type 1 VWD patients. In conclusion, our findings demonstrate that low VWF does not constitute a discrete clinical or pathological entity. Rather, it is part of an age-dependent type 1 VWD evolving phenotype. Collectively, these data have important implications for future VWD classification criteria.
von Willebrand disease (VWD) is the most common inherited bleeding disorder, but diagnosis and accurate subtyping of VWD are complex and have been controversial. Atiq et al analyzed data from 2 large cohort studies to illuminate the natural history of patients with reduced (30-50 IU/dL) von Willebrand factor (VWF) who did not meet the strict definition of type 1 VWD (VWF levels <30 IU/dL). The authors’ data support the hypothesis that low VWF is an age-dependent evolution of type 1 VWD rather than its own discrete clinical entity. This finding reinforces the general principle that bleeding phenotype should be the key determinant of management decisions in patients with VWD.
Introduction
Von Willebrand disease (VWD) results from a reduction in plasma von Willebrand factor (VWF) levels and constitutes the most common inherited bleeding disorder.1 VWD is classified according to whether the underlying VWF deficiency is quantitative and/or qualitative in nature.2 Type 1 VWD is characterized by a partial quantitative reduction in plasma VWF antigen (VWF:Ag) and accounts for ∼75% of cases.1 Over recent years, a number of consensus guidelines on VWD diagnosis have been published.2, 3, 4, 5, 6 Initial guidelines proposed that patients with partial quantitative VWF deficiency be subgrouped into 2 categories.2, 3, 4, 5 First, the guidelines proposed that individuals with plasma VWF:Ag <30 IU/dL should be diagnosed with type 1 VWD.2, 3, 4, 5 Second, the guidelines recommended that patients who had personal bleeding histories coupled with plasma VWF:Ag in the range of 30 to 50 IU/dL should be diagnosed with low VWF.2, 3, 4, 5 The recommendation that low VWF should be considered a distinct entity was predicated upon previous studies that highlighted important differences between patients with VWF levels <30 IU/dL compared with those with VWF levels in the 30 to 50 IU/dL range.7, 8, 9, 10 For example, VWF pathological sequence variants were significantly more prevalent in patients with plasma VWF levels <30 IU/dL than in those with low VWF.11, 12, 13, 14, 15 In addition, mucocutaneous bleeding in the type 1 VWD group was more common and correlated inversely with residual plasma VWF levels.11,16 Conversely, accumulating data have demonstrated that many individuals with mild-to-moderate reduction of plasma VWF levels in the 30 to 50 IU/dL range do not have significant bleeding tendencies.10,17, 18, 19, 20 Furthermore, bleeding risk in this low VWF group does not correlate with plasma VWF levels.17,19
Classification of patients with partial quantitative reductions in plasma VWF levels was recently revised in American Society of Hematology (ASH)/International Society on Thrombosis and Haemostasis (ISTH)/World Federation of Hemophilia (WFH)/National Hemophilia Foundation (NHF) expert consensus guidelines.6 Based on a systematic review that noted low certainty in the available evidence, the panel strongly recommended that VWF levels of <30 IU/dL (and VWF activity [VWF:Act]/VWF:Ag ratio >0.7) were sufficient to confirm type 1 VWD, irrespective of whether the patient had a personal bleeding history.6 In contrast to previous guidelines, the ASH/ISTH/WFH/NHF panel further recommended that patients with a significant bleeding history and plasma VWF levels in the 30 to 50 IU/dL range should also be diagnosed with type 1 VWD.2,4,6 The ASH/ISTH/WFH/NHF panel advocated that for individuals with VWF levels in the 30 to 50 IU/dL range and bleeding symptoms, a diagnosis of type 1 VWD rather than low VWF might better facilitate access to clinical care in some countries.21 Nevertheless, the decision to merge the low VWF cohort into the type 1 VWD category has been challenged and remains the focus of ongoing debate.21,22
The question of whether low VWF constitutes a discrete clinical entity is further complicated by the fact that plasma VWF:Ag is known to increase with aging.19,23, 24, 25, 26 This has been demonstrated in healthy individuals as well as patients with VWD.19,23, 24, 25, 26 Although the biology underpinning this age-induced increase in VWF levels remains poorly defined, it has been attributed to both increased VWF biosynthesis and reduced plasma VWF clearance rates.27, 28, 29 As highlighted in the recent ASH/ISTH/WFH/NHF expert consensus guidelines, significant clinical challenges remain with respect to diagnosis and management of patients with mild-to-moderate reductions in plasma VWF levels.6 In this study, we specifically investigated the critical question of whether low VWF is indeed a discrete clinical entity or whether it is instead part of an age-dependent type 1 VWD evolving phenotype. To gain novel insights into the relationships between aging, low VWF, and type 1 VWD, we utilized datasets for patients with low VWF included in the Low VWF in Ireland Cohort (LoVIC) combined with patients with type 1 VWD included in the Willebrand in The Netherlands (WiN) study.16,19 Collectively, our findings demonstrate that low VWF does not constitute a discrete clinical or pathological entity. Rather, it is part of an age-dependent type 1 VWD evolving phenotype.
Materials and methods
LoVIC and WiN studies
The methods of the LoVIC and WiN studies have previously been described in detail.19,30 In brief, both studies were national cross-sectional studies performed in Ireland and The Netherlands, respectively. The WiN study was initiated in 2007, whereas the LoVIC study was initiated in 2014. For the LoVIC study, inclusion criteria required that individuals had historically lowest plasma VWF:Ag and/or VWF:Act in the 30 to 50 IU/dL range, confirmed on 2 occasions measured at least 3 months apart. The LoVIC study was approved by the St James’ Hospital Research Ethics Committee, and written informed consent was obtained from all participants. For the WiN study, inclusion criteria required that patients had either a personal bleeding history or positive family history, combined with historically lowest plasma VWF:Ag, VWF:Act, or VWF collagen binding (VWF:CB) ≤30 IU/dL. The WiN study was approved by the Medical Ethics Committee of all participating centers, and written informed consent was obtained from all participants. Both studies determined bleeding history using bleeding assessment tools (BATs). In the LoVIC study, both Condensed Molecular and Clinical Markers for the Diagnosis and Management of Type 1 VWD (Condensed MCMDM-1VWD) and International Society of Thrombosis and Haemostasis Bleeding Assessment Tool (ISTH BAT) scores were assessed for each patient. In the WiN study, Condensed MCMDM-1VWD was self-administered at inclusion in the study. Extensive BAT data on both the LoVIC and WiN cohorts have previously been reported in detail.16,19,31,32
Laboratory studies
Hemostasis testing was repeated on patients at enrollment into both the LoVIC and WiN studies. This included plasma VWF:Ag, VWF ristocetin cofactor, VWF:CB, and FVIII activity (FVIII:C) as previously described.19,30 VWF propeptide (VWFpp) was measured in both studies with an enzyme-linked immunoassay, in which antibody CLB-Pro 35 was used for coating and antibody CLB-Pro 14.3 for detection.19,33 In addition, previously recorded laboratory hemostasis results for all patients were collected from electronic patient files. Finally, a subset of patients in the LoVIC and WiN studies were followed up over time with serial VWF and FVIII levels. For studies investigating the effect of age on plasma VWF levels, only LoVIC and WiN patients with a follow-up period >5 years were included.
Desmopressin response
Desmopressin trials were performed in a subset of patients as previously detailed.34,35 Briefly, desmopressin was administered IV at a dosage of 0.3 μg/kg body weight (maximum dose capped at 27 μg) in 50 mL of sodium chloride 0.9% and infused over 30 minutes or intranasally at a total dosage of 300 μg. Subsequently, blood samples were taken for VWF and FVIII levels at predetermined time points. Complete desmopressin response was defined according to the 2021 ASH/ISTH/WFH/NHF guidelines (ie, an increase of twofold or more from baseline levels and sustained VWF and FVIII levels >50 IU/dL for at least 4 hours).36,37 In patients with baseline VWF:Act >50 IU/dL, response was defined as VWF:Act >100 IU/dL for at least 4 hours, as used in previous studies.38
Genetic analysis
The genetic analysis in the WiN and LoVIC studies have previously been described.14,19 In short, in the LoVIC study a custom genetic array was used to sequence VWF. TruSeq Custom Amplicon v1.5 Library Prep Kit (Illumina, San Diego, CA) was used to prepare the libraries. MiSeq sequencer (Illumina) using the 300-bp paired-end sequencing was used to analyze the pooled libraries. In the WiN study, all 52 exones of the VWF gene ± 20 bp exon-intron boundaries were analyzed using ion semiconductor sequencing (Ion-Torrent). If no mutation was found, multiplex ligation-dependent probe amplification was performed to detect large deletions or duplications.
Statistical analysis
Continuous data are presented as mean ± standard deviation, whereas categorical data are presented as number (percentage). Because we had >30 individuals in each group, parametric tests were used to compare continuous variables. Independent t test was used to compare continuous data between 2 groups, whereas analysis of variance was used to compare >2 groups. Bonferroni correction was applied to take multiple testing in to account. Categorical data were compared between groups using χ2tests. Again, Bonferroni correction was applied if multiple groups where compared with χ2 tests. Regression analysis was used to assess whether VWF:Ag would be different if patients were diagnosed at the same age. Regression analysis was also used to investigate whether desmopressin response changes with aging, in which we included age groups as independent variable in the analysis. Outcomes of regression analysis are presented as β (difference) and 95% confidence interval (CI). Kaplan-Meier curves with log-rank test were used to determine whether there were differences in time-to-normalization of VWF levels. Cox regression analysis were used to investigate which variable is independently associated with time-to-normalization of VWF levels. Outcomes of Cox regression analysis are presented as hazard rate (HR) and 95% CI. A P value <.05 was defined as significant. Statistical analysis were performed with SPSS Statistics version 25 (IBM Corp, Armonk, NY).
Results
In total, 565 patients were included in the combined analysis (403 WiN patients with type 1 VWD and 162 LoVIC patients with low VWF). For the WiN cohort, plasma VWF levels had been assessed at the time of first local diagnosis in The Netherlands (defined as historical levels) and then subsequently repeated at the time of enrollment into the national WiN study (defined as levels at inclusion). Based on VWF levels at inclusion in the WiN study, patients with type 1 VWD were categorized into 3 groups: (1) 188 patients with persistent plasma VWF:Ag and/or VWF:Act levels <30 IU/dL; (2) 121 patients with partial correction in plasma VWF levels into the 30 to 50 IU/dL range; and (3) 94 patients with normalization of plasma VWF levels >50 IU/dL at inclusion in the study (Table 1).
Table 1.
Patient characteristics
| WiN persistent <30 IU/dL n = 188 |
WiN partially corrected 30-50 IU/dL n = 121 |
WiN normalized >50 IU/dL n = 94 |
LoVIC n = 162 |
P | |
|---|---|---|---|---|---|
| Age at diagnosis, y | 23.3 ± 19.0 | 26.5 ± 17.3 | 25.1 ± 17.7 | 32.5 ± 13.5 | <.001 |
| Age at enrollment, y | 39.3 ± 19.9 | 39.4 ± 18.5 | 41.2 ± 20.3 | 39.2 ± 13.5 | .532 |
| Time between diagnosis and enrollment | 16.0 ± 14.3 | 13.0 ± 10.0 | 15.5 ± 11.2 | 6.9 ± 6.9 | <.001 |
| Female sex | 119 (63.3%) | 84 (69.4%) | 65 (69.1%) | 144 (88.9%) | <.001 |
| Blood group O | 115 (61.8%) | 92 (76.0%) | 68 (73.1%) | 145 (91.8%) | <.001 |
| Reason for referral | |||||
| Positive family history | 109 (62.6%) | 37 (32.2%) | 22 (25.6%) | 40 (24.0%) | <.001 |
| Bleeding history | 62 (35.6%) | 70 (60.9%) | 61 (70.9%) | 78 (46.7%) | |
| Both family history and bleeding | 12 (7.2%) | ||||
| Incidental findings | 3 (1.7%) | 8 (7.0%) | 3 (3.5%) | 37 (22.2%) | |
| VWF sequence variants | 120 (93.0%) | 39 (53.4%) | 14 (22.6%) | 44 (36.4%) | <.001 |
| Age at desmopressin trial, y | 31.8 ± 16.1 | 36.0 ± 13.9 | 41.9 ± 13.8 | 32.9 ± 10.7 | <.001 |
Data are presented as mean ± standard deviation or number (%).
P values are outcomes of analysis of variance or χ2 tests.
Overall, we observed that female sex (88.9%) and blood group O (91.8%) were significantly more common in LoVIC patients than those in the WiN groups (P < .001). In contrast, WiN patients with persistent VWF levels <30 IU/dL were less often female (63.3%) and less frequently had blood group O (61.8%) compared with those in the other WiN groups (Table 1; P < .001). No significant differences in VWF parameters (including FVIII:C/VWF:Ag and VWFpp/VWF:Ag ratios) were observed between females and males nor between blood group O and non-O (data not shown). In addition, WiN patients with persistent VWF levels <30 IU/dL were more often referred because of a positive family history, whereas family history was significantly less common in WiN patients in whom VWF levels had normalized >50 IU/dL over time or in LoVIC patients (P < .001). Interestingly, the number of patients who were referred because of a positive family history was similar (P = .775) between the WiN-normalized cohort (25.6%) and LoVIC cohort (24.0%). Importantly, the MCMDM-1VWD bleeding score and bleeding requiring treatment in the year before inclusion in the WiN study were not different in males and females with persistent VWF levels <30 IU/dL, partially corrected VWF levels, and normalized levels >50 IU/dL (P > .05; supplemental Figure 1, available on the Blood website).
Effect of aging on diagnosis of type 1 VWD and low VWF
Mean age at diagnosis was significantly higher in the LoVIC cohort than the 3 WiN subgroups (32.5 years vs 23.3 years, 26.5 years, and 25.1 years, respectively; P < .001; Table 1). Conversely, there was no difference in age for the LoVIC cohort or WiN subgroups at time of enrollment into both national studies (P = .532). To assess the impact of this age difference, we first investigated how plasma VWF:Ag varied over time in WiN patients with type 1 VWD compared with the LoVIC cohort.
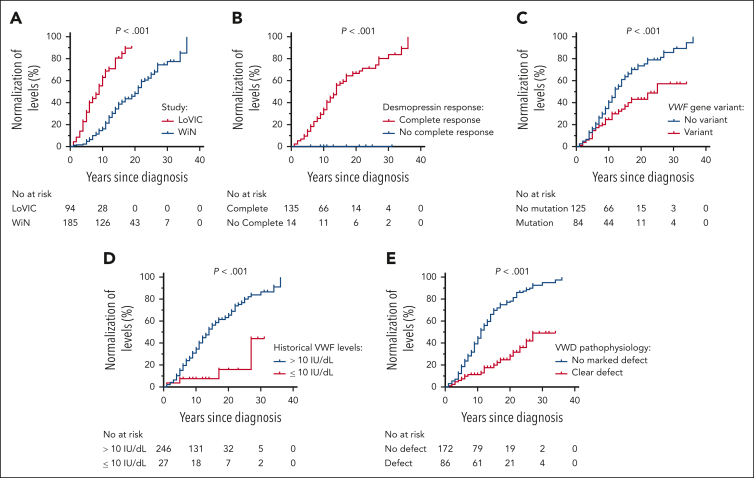
In line with previous studies, we observed an age-dependent increase in plasma VWF levels in half of the type 1 VWD and the majority of patients with low VWF (Figure 1A). Among the total WiN cohort, 47% of patients had VWF levels that remained <30 IU/dL despite advancing age. Conversely, in 30% of patients with type 1 VWD, plasma VWF levels increased into the low VWF range (30-50 IU/dL) over time, whereas 23% had age-dependent increments that led to complete normalization in VWF levels >50 IU/dL. Finally, in the LoVIC cohort, plasma VWF levels normalized >50 IU/dL over time in 39% of patients. It should be noted that the time between diagnosis and enrollment was much shorter for the LoVIC cohort (6.9 years) than for the WiN subgroups (>13 years; P < .001; Table 1).
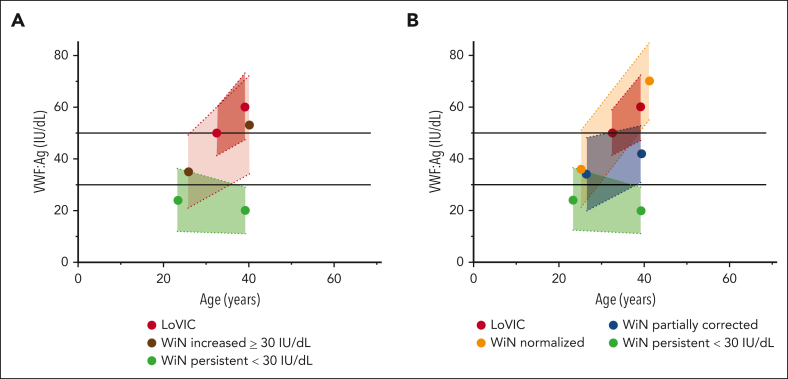
Figure 1.
Effects of age on plasma VWF:Ag levels in patients with low VWF compared with those in type 1 VWD subgroups. Plasma VWF:Ag levels were assessed in patients with low VWF and type 1 VWD at the time of first diagnosis and then subsequently repeated at time of enrollment into the national studies. The left dot depicts the mean plasma VWF:Ag and the corresponding mean age at time of original diagnosis and the right dot depicts mean plasma VWF:Ag and the corresponding mean age at trial enrollment. Broken lines with colored areas depict the standard deviations for separate groups. (A) Age-dependent increases in plasma VWF:Ag aligns for LoVIC participants and WiN patients whose plasma VWF levels increased ≥30 IU/dL. (B) Age-dependent increases in plasma VWF:Ag completely overlap for LoVIC patients compared with WiN patients whose plasma VWF levels normalized ≥50 IU/dL.
Importantly, we observed that VWF:Ag in patients with low VWF clearly overlapped with VWF:Ag in patients with type 1 VWD whose plasma VWF levels had progressively increased >30 IU/dL as they got older (Figure 1A). Furthermore, when the WiN cohort were subcategorized into 3 groups based upon time-dependent effect on VWF levels, the LoVIC cohort appeared as a subgroup within the WiN–normalized >50 IU/dL (Figure 1B). Additionally, the increase of VWF:Ag per year was not different between LoVIC patients and WiN normalized >50 IU/dL patients (2.6 ± 8.5 IU/dL vs 2.2 ± 2.1 IU/dL; P = .652). To further investigate this concept, we proceeded to perform multiple regression analysis. Importantly, we observed that plasma VWF:Ag in the LoVIC cohort and the WiN–normalized >50 IU/dL subgroup would not have been significantly different had they been diagnosed at the same age (difference of β = 0.00; 95% CI, −0.03 to 0.04). Cumulatively, these results indicate that significant heterogeneity exists among patients with type 1 VWD with respect to the effect of aging on their plasma VWF levels. In addition, our findings demonstrate that because of age-dependent increases in plasma VWF:Ag, the majority of patients with low VWF would have been diagnosed with type 1 VWD had they undergone assessment earlier in life.
Low VWF and normalized type 1 VWD patients share similar pathophysiology
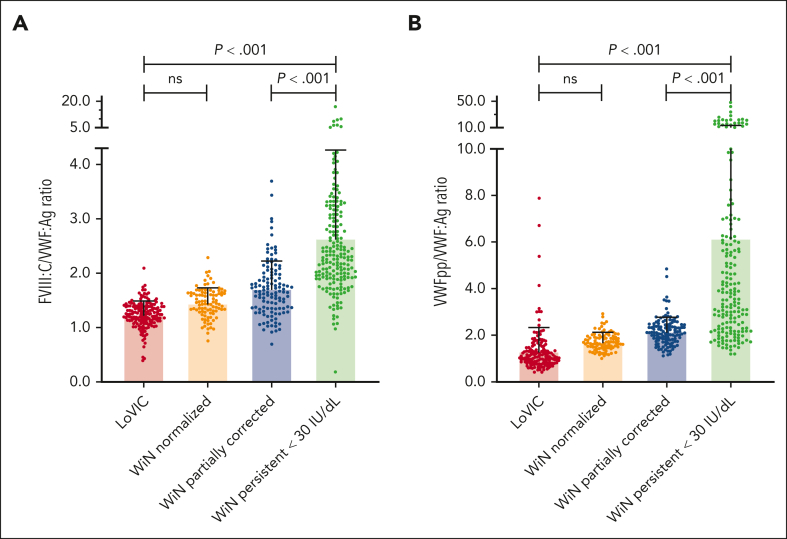
To further address the hypothesis that low VWF and normalized type 1 VWD represent the same condition assessed at different time points, we next investigated underlying pathophysiology in the LoVIC and WiN subgroups. Elevated plasma FVIII:C/VWF:Ag ratios have been used to identify VWD patients with reduced VWF synthesis and/or secretion.39 Importantly, we observed similar FVIII:C/VWF:Ag ratios in WiN-normalized (1.46 ± 0.27) and LoVIC patients (1.24 ± 0.24) (Figure 2A; P = .444). In contrast, WiN patients with persistent VWF levels <30 IU/dL had significantly higher FVIII:C/VWF:Ag ratios (2.65 ± 1.62) than both WiN-normalized and LoVIC patients (Figure 2A; P < .001). Previous studies have demonstrated that elevated VWFpp/VWF:Ag ratios can identify VWD patients with enhanced VWF clearance.40,41 Although VWFpp/VWF:Ag ratios were again similar (P = 1.000) in WiN-normalized (1.75 ± 0.38) and LoVIC patients (1.36 ± 0.99), they were markedly elevated in WiN patients with persistent VWF levels <30 IU/dL (6.14 ± 7.05) compared to the other groups (P < .001; Figure 2B). Together, these results suggest that the pathophysiological mechanisms in patients with low VWF and normalized type 1 VWD are similar. Furthermore, the data highlight that patients with type 1 VWD who fail to correct plasma VWF levels with increasing age have more marked VWF biosynthesis/secretion and/or VWF clearance defects as the cause of their VWD.
Figure 2.
Pathophysiological mechanisms in patients with low VWF compared with type 1 VWD subgroups. FVIII:C/VWF:Ag and VWFpp/VWF:Ag ratios were assessed in patients with low VWF and type 1 VWD to assess underlying pathophysiology. (A) Significantly increased plasma FVIII:C/VWF:Ag ratios suggesting marked reductions in VWF synthesis/secretion were observed in the WiN persistent <30 IU/dL subgroup. In contrast, FVIII:C/VWF:Ag ratios were the same in LoVIC and WiN-normalized patients. (B) Similarly, significantly increased plasma VWFpp/VWF:Ag ratios suggesting markedly enhanced VWF clearance were also observed in the WiN persistent <30 IU/dL subgroup. Again, VWFpp/VWF:Ag ratios were not significantly different between LoVIC and WiN-normalized patients. Ns, not significant.
Desmopressin responses in low VWF compared with WiN type 1 VWD subgroups
We next investigated how desmopressin responses varied among different WiN type 1 VWD subgroups compared with patients with low VWF. Importantly, a complete response to desmopressin was observed in 100% of both the LoVIC patients and WiN patients who had increased ≥30 IU/dL (Figure 3). Conversely however, only 58.1% of WiN patients with persistent VWF levels <30 IU/dL demonstrated a complete response to desmopressin (P < .001). Consistent with the FVIII:C/VWF:Ag and VWFpp/VWF:Ag ratio data, this subgroup demonstrated both a significantly attenuated initial VWF response at 1 hour after desmopressin (P < .001) as well as a more rapid falloff in plasma VWF levels over time (Figure 3; P < .001).
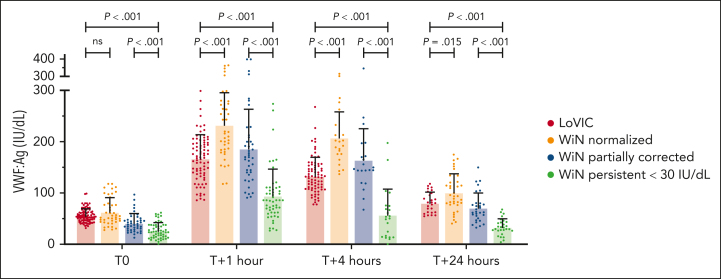
Figure 3.
Desmopressin responses in low VWF compared with WiN type 1 VWD subgroups. Plasma VWF:Ag were determined prior to desmopressin administration (T0), and at 1 (T + 1), 4 (T + 4), and 24 (T + 24) hours after desmopressin administration. The mean and standard deviations are depicted; n = 226 before desmopressin administration; n = 217 at 1 hour; n = 146 at 4 hours; and n = 126 at 24 hours. Significantly reduced VWF responses were observed at all postdesmopressin time points in the WiN persistent <30 IU/dL subgroup. Plasma VWF:Ag levels at 1, 4, and 24 hours after desmopressin were significantly higher in WiN-normalized than in LoVIC patients. ns, not significant.
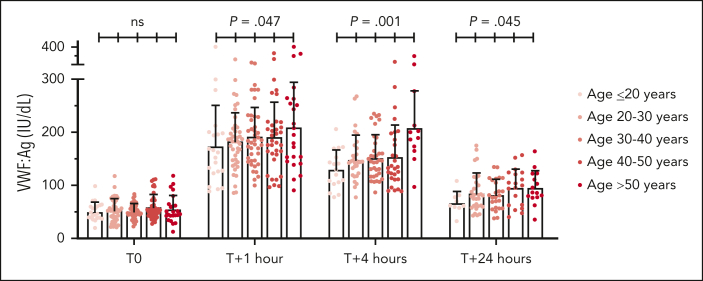
Unexpectedly, plasma VWF:Ag at 1, 4, and 24 hours after desmopressin were significantly higher in WiN-normalized than in LoVIC patients (Figure 3; P < .001). Because the WiN-normalized cohort were significantly older at the time of their desmopressin trial than the LoVIC group (41.9 ± 13.8 years vs 32.9 ± 10.7 years; P = .002), we hypothesized that the difference in VWF responses might be age-dependent. To investigate this concept, desmopressin responses were assessed in LoVIC and WiN patients who had increased ≥30 IU/dL, divided into 5 different age groups (Figure 4). Interestingly, an age-dependent effect on VWF responses after desmopressin administration was observed. Plasma VWF:Ag responses at 1, 4, and 24 hours after desmopressin were significantly higher in the advancing age groups (Figure 4; P = .047; P = .001; and P = .045, respectively). Collectively, these results further support the idea that patients with low VWF and normalized type 1 VWD share similar pathogenesis and consequently exhibit comparable complete VWF responses after desmopressin administration. Our findings further suggest that in older patients initial VWF response is better and VWF half-life is longer after desmopressin administration.
Figure 4.
Effect of aging on desmopressin-induced VWF responses. The effects of increasing age on plasma VWF:Ag levels after desmopressin administration were assessed in combined subgroups of patients with low VWF and type 1 VWD in whom plasma VWF levels increased ≥30 IU/dL. Plasma VWF:Ag were determined prior to desmopressin administration (T0), and at 1 (T + 1), 4 (T + 4), and 24 (T + 24) hours after desmopressin administration. The mean and standard deviations are depicted; n = 171 before desmopressin administration; n = 164 at 1 hour; n = 123 at 4 hours; and n = 98 at 24 hours after desmopressin administration. Significantly increased VWF responses were observed at 1, 4, and 24 hours after desmopressin administration with increasing age at the time of desmopressin trial. P values are outcomes of linear regression analysis with the presented age groups included as independent variable. ns, not significant.
Determinants of age-induced VWF level normalization in low VWF and type 1 VWD
To investigate determinants of age-induced VWF level normalization in low VWF and type 1 VWD, we performed a retrospective cohort study that included all LoVIC (n = 94) and WiN (n = 185) patients who had been followed up for >5 years. Importantly, there was no difference in the VWF:Ag increase per decade between LoVIC patients (21 ± 26 IU/dL per decade) and WiN-normalized patients (18 ±31 IU/dL per decade; P = .434). As expected, normalization in plasma VWF levels with aging was significantly more common in patients with low VWF than in patients with type 1 VWD (Figure 5A; P = .025). From the LoVIC cohort, 62 patients (66%) achieved plasma VWF levels >50 IU/dL over time (mean follow-up, 9.7 ± 3.9 years). In contrast, despite a longer follow-up period (mean follow-up, 16.9 ±7.4 years), only 96 patients (51.9%) of the WiN type 1 VWD cohort attained VWF levels in the normal range. Interestingly, none of the patients who had an incomplete response to desmopressin reached plasma VWF levels >50 IU/dL during follow-up, whereas the median time of normalization was 13 years (interquartile range 7-26) in patients with complete response to desmopressin (Figure 5B; P < .001). Thus, an incomplete desmopressin response had a 100% negative predictive value for lack of normalization of VWF levels with aging.
Figure 5.
Determinants of age-induced VWF level normalization in low VWF and type 1 VWD. Normalization of plasma VWF levels (defined as increase of VWF:Ag, VWF:Act, VWF:CB and FVIII:C >50 IU/dL with aging) was assessed in LoVIC and WiN patients with more than 5 years of retrospective follow-up. Kaplan-Meier curves were used to illustrate determinants of normalization of VWF levels with aging. P values are outcomes of log-rank tests. (A) Normalization in plasma VWF levels during follow-up was significantly higher in LoVIC than in WiN patients. (B) Normalization in plasma VWF levels during follow-up was significantly increased in patients with low VWF and type 1 VWD who demonstrated complete desmopressin responses (defined according to the 2021 ASH/ISTH/WFH/NHF guideline). (C) Age-induced normalization in plasma VWF levels was significantly higher in patients with low VWF and type 1 VWD who did not have a pathological VWF sequence variant. (D) Normalization in plasma VWF levels with aging was markedly reduced in patients with historically lowest levels VWF levels <10 IU/dL. (E) Age-induced normalization in plasma VWF levels was significantly decreased in patients with marked underlying pathophysiological defects (defined as FVIII:C/VWF:Ag ratio ≥1.9 and/or VWFpp/VWF:Ag ratio ≥2.2).
Pathological VWF gene sequence variants were significantly more common in the WiN subgroup of patients with persistent VWF levels <30 IU/dL than in either the WiN–partially corrected or WiN-normalized groups (93.0% vs 53.4% and 22.6%, respectively; Table 1; supplemental Figure 2; P < .001). There was no significant difference in the percentage of patients with VWF gene variants between LoVIC (36.4%) and WiN–partially corrected (P = 1.000) or WiN–normalized >50 IU/dL subgroups (P = 1.000). The presence or absence of pathological VWF sequence variants was shown to significantly influence the likelihood of age-induced plasma VWF level normalization (Figure 5C; P < .001). Likewise, presence of a type 1C VWF gene variant was associated with a significantly reduced chance of normalization of VWF levels with aging (P < .001). Similarly, historically lowest VWF levels ≤10 IU/dL was strongly associated with significantly reduced normalization of VWF levels over time (Figure 5D; P < .001). Finally, underlying pathogenic mechanisms (marked reduced synthesis and/or increased clearance of VWF) were also identified as significant determinants of a lower chance of VWF normalization over time (Figure 5E; P < .001). Because these determinants are strongly correlated, we used a Cox regression model to investigate which factors are independently associated with the normalization of plasma VWF levels over time. Historically lowest levels ≤10 IU/dL (HR, 0.27; 95% CI, 0.10-0.76) and pathogenesis (HR, 0.37; 95% CI, 0.22-0.61) remained strongly associated with a lower chance of normalization over time, whereas presence of VWF sequence variants was no longer significantly associated (HR, 0.76; 95% CI, 0.50-1.16). Additional analysis revealed that reduced synthesis (HR, 0.43; 95% CI, 0.19-0.99) and increased clearance (HR, 0.49; 95% CI, 0.27-0.88) were both associated with a lower chance of normalization of VWF levels during follow-up, independent of VWF variants and historically lowest levels.
Discussion
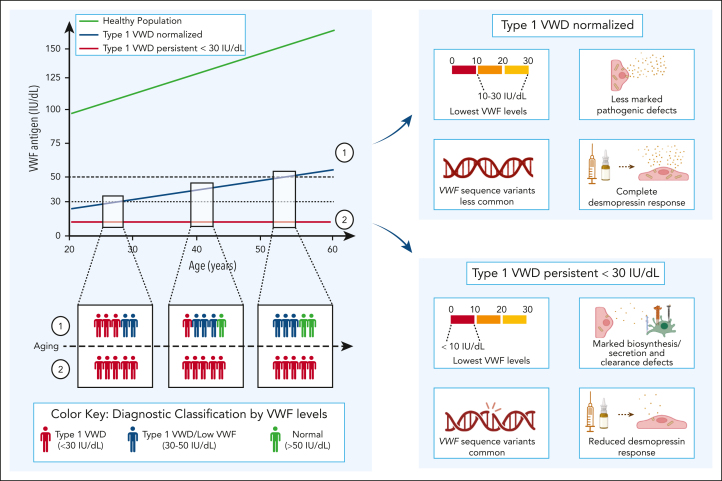
For many years, there has been an ongoing debate about how to optimally diagnose type 1 VWD.2,3,6,7,21,22,42 This is complicated by (1) the wide variation in plasma VWF levels in healthy individuals (∼50-200 IU/dL); (2) the effect of ABO blood group on VWF levels; (3) the effect of aging on plasma VWF levels; and (4) the prevalence of bleeding symptoms that are estimated to be present in at least 25% of the healthy general population.7, 8, 9,42 Consequently, establishing the relationship between partial quantitative VWF deficiency and bleeding is difficult. Furthermore, defining diagnostic criteria to delineate healthy from diseased has presented an ongoing challenge, particularly for patients with mild-to-moderate reductions in plasma VWF in the 30 to 50 IU/dL range.8,10 The cumulative findings from the WiN and LoVIC studies presented herein provide important insights in this context. By combining data from these 2 deeply phenotyped and genotyped cohorts of patients with low VWF and type 1 VWD, respectively, we have specifically investigated the relationship between age, low VWF, and type 1 VWD. Our data demonstrate that type 1 VWD (as defined by historical plasma VWF levels <30 IU/dL and VWF:Act/VWF:Ag ratio >0.7) is a heterogeneous disorder. Almost half of patients (47%) with type 1 VWD will continue to have persistently reduced VWF levels <30 IU/dL that do not change as they age. Conversely, in a majority of individuals diagnosed with type 1 VWD at younger ages (53%), plasma VWF levels progressively increase over time. Our data clearly demonstrate that as these patients get older, their plasma VWF levels increase into the low VWF range (30-50 IU/dL), before ultimately reaching the normal range (>50 IU/dL) in many cases. Importantly however, the rate at which plasma VWF levels increases varies between different patients with type 1 VWD. Finally, it is important to note that the majority of enrolled patients were Caucasian. Therefore, the results may not be applicable to patients from other ethnic backgrounds, because the rate at which their VWF levels increase with aging may be different.
The dynamic influence of age on plasma VWF levels has major implications in relation to defining thresholds for diagnostic guidelines. Previous guidelines recommended that patients with plasma VWF levels in the 30 to 50 IU/dL range should be diagnosed with low VWF rather than type 1 VWD.2, 3, 4, 5 Our data highlight that these criteria mean that the registered diagnosis for many patients will critically depend upon the age at which they first undergo VWF laboratory testing. Importantly, recent studies have demonstrated that there is often a major delay to VWD diagnosis, notably in women with heavy menstrual bleeding (HMB).43, 44, 45 Consequently, many of these patients do not have plasma VWF assessment until they are aged 20 to 30 years. Our findings suggest that had these individuals undergone testing earlier in life, their plasma VWF levels would have been <30 IU/dL in many cases and, therefore, would have led to a type 1 VWD diagnosis. In line, it was recently demonstrated that VWF:Ag levels steadily increase an average of 20 IU/dL from early childhood to late adolescence in healthy children from the general population.46 Thus, the delay in VWF testing in the setting of HMB likely explains the overrepresentation of women diagnosed with low VWF in the LoVIC cohort, together with the observation that HMB constitutes the most common bleeding domain in low VWF cohort studies.32
Importantly, our findings clearly demonstrate that low VWF does not constitute a discrete clinic-pathological entity. Rather, patients with VWF in the 30 to 50 IU/dL range have plasma VWF levels that are dynamically evolving over time. Consequently, first VWF testing is only a snapshot taken at a single time point on a progressive age-dependent gradient. Nevertheless, this initial VWF level critically affects ultimate VWD diagnosis for the patient. Consistent with the idea that low VWF is really a subgroup within the heterogeneous type 1 VWD, our findings demonstrate that genetic background, underlying pathophysiological mechanisms, and desmopressin response are the same for patients with low VWF and those with normalized type 1 VWD. Additionally, the number of patients with a positive family history were similar between low VWF and normalized type 1 VWD patients. Furthermore, patients with low VWF and type 1 VWD are frequently seen across different generations of a single family. Collectively, our findings, therefore, support the decision of the recent ASH/ISTH/WFH/NHF guidelines, which recommended that individuals with VWF levels of 30 to 50 IU/dL and a personal bleeding phenotype should be classified as type 1 VWD as opposed to low VWF.6
In striking contrast, we further demonstrate that patients with type 1 VWD with persistent VWF levels that remain <30 IU/dL despite aging represent a distinct entity compared with patients with low VWF and normalized type 1 VWD. In particular, pathological VWF sequence variants, marked pathophysiological defects, and reduced responses to desmopressin were markedly more prevalent in the subgroup of patients with type 1 VWD with persistent VWF levels <30 IU/dL over time. In addition, these patients had more often a positive family history of VWD compared with patients with type 1 VWD whose VWF levels increased with aging. These findings suggest that any future subcategorization of type 1 VWD seeking to focus according to underlying biology should be based upon time-dependent progression in plasma VWF levels rather than simply applying a single VWF threshold cutoff level (Figure 6).
Figure 6.
Overview of the relationship between low VWF, type 1 VWD, and progressive aging.
It has been debated for years whether age-induced increases in plasma VWF levels in patients with partial quantitative VWF deficiency lead to a reduced bleeding phenotype.36,47 Based upon bleeding scores, as well as specific bleeding episodes requiring treatment in the year before inclusion in the WiN study, we observed no difference in bleeding severity among WiN patients with type 1 VWD with persistent VWF levels <30 IU/dL, WiN–partially corrected, or WiN-normalized subgroups of patients. Similarly, significant bleeding was still observed in LoVIC patients even though their plasma VWF levels had increased >50 IU/dL over time.19 Together, these clinical data suggest that mild-to-moderate reductions in plasma VWF levels do not explain all of the bleeding observed in these patients, but rather, other pathological modifiers may also be contributing to their bleeding phenotype.8 Consistently, previous studies have reported similar BAT scores in patients with VWF levels in the 10 to 50 IU/dL range.13,16,17,19 This hypothesis is further supported by evidence demonstrating that most individuals with mild reductions in plasma VWF levels do not demonstrate significant bleeding.7,10,42 With respect to clinical management, these data suggest that increases in plasma VWF levels >50 IU/dL will therefore not necessarily be associated with a complete correction in bleeding risk for all patients with partial quantitative VWD. However, it should be noted that these results are based on interindividual differences. It remains unknown whether the bleeding phenotype changes intraindividually when VWF levels normalize with aging. Further prospective studies are needed to investigate this specific question that is of direct clinical significance.
From a clinical perspective, an important finding from our study is that desmopressin responses are better in older patients with VWD. This age-dependent improved response involves 2 components (Figure 4). First, there is a significant increase in initial VWF secretion after desmopressin infusion. Second, the clearance of VWF released in patients after desmopressin is also significantly reduced in older patients with VWD (Figure 4). These findings are consistent with previous murine data that reported that both increased synthesis and reduced clearance contributed to age-dependent elevations in plasma VWF levels in mice.28 However, desmopressin is less widely used in older patients in many countries (eg, with an upper age limit of 60-70 years) because of concerns regarding potential cardiovascular risks.48 Given our data, future studies assessing the clinical efficacy and safety of using attenuated desmopressin doses should be considered. Alternatively, developing additional therapies that can be safely used to trigger secretion of enhanced Weibel-Palade body stores of VWF in older patients may provide novel treatment opportunities.
In conclusion, this study demonstrates that low VWF does not constitute a discrete clinical or pathological entity. Rather, it is part of an age-dependent type 1 VWD evolving phenotype. In contrast, patients with type 1 VWD with persistent VWF levels <30 IU/dL despite aging represent a distinct entity compared with patients with low VWF and age-dependent normalized type 1 VWD. As such, our findings have important implications for VWD diagnosis in the clinic; the definition of diagnostic thresholds in future VWD guidelines; and for futures studies investigating pathogenic mechanisms underpinning heterogeneous type 1 VWD.
Conflict-of-interest disclosure: F.A. received research support from CSL Behring, Takeda, Octapharma, and Sobi; and also received travel grants from Sobi. F.W.G.L. has received unrestricted grants/research funding from CSL Behring, uniQure, Sobi, and Takeda; consultancy fees from BioMarin, CSL Behring, Takeda, and uniQure (all fees to the institution); and served as a data safety and monitoring board member for a study sponsored by Roche. J.S.O. has served on the speaker’s bureau for Baxter, Bayer, Novo Nordisk, Sobi, Boehringer Ingelheim, Leo Pharma, Takeda, and Octapharma; served on the advisory boards of Baxter, Sobi, Bayer, Octapharma CSL Behring, Daiichi Sankyo, Boehringer Ingelheim, Takeda, and Pfizer; and has also received research grant funding awards from 3M, Baxter, Bayer, Pfizer, Shire, Takeda, 3M, and Novo Nordisk. D.D. has received honoraria from Takeda and educational support sponsorship from NovoNordisk and Amgen. M.L. has received consultancy fees from Sobi, Band Therapeutics, and CSL Behring; honoraria from CSL Behring and Pfizer; and indirect funding for development of educational content from Takeda. J.G.v.d.B. received research funding from Novo Nordisk. N.M.O. served on advisory boards for Sobi, F. Hoffman-La Roche Ltd, uniQure, CSL Behring, AstraZeneca, and Freeline and on the speaker’s bureau for Novo Nordisk, Sobi, CSL Behring, Bayer, and Takeda. S.E.M.S. has received research funding from Bayer. R.I.B.’s institution has received research support/clinical trial funding from Bayer, Takeda, Pfizer, Daiichi Sankyo, CSL Behring, Roche, Amgen, AstraZeneca, AbbVie, Sanofi, Acerta Pharma, Jansen-Cileg, Bristol Myers Squibb, Boehringer Ingelheim, Werfen, and Technoclone, unrelated to this study. K.M. reports speaker fees from Alexion, Bayer, and CSL Behring; participation in trial steering committees for Bayer and AstraZeneca; consulting fees from uniQure and Therini; and participation in data monitoring and end point adjudication committee for Octapharma (all fees paid to the institution). P.J. receives research funding from Bayer and consultancy fees from Band/Guardian Therapeutics, Star/Vega Therapeutics, and Roche. K.F. has received unrestricted grants/research funding from CSL Behring, Sobi, and Takeda for research unrelated to this study and consultancy fees from SOBI, Sanofi, Takeda, Novo Nordisk, and Roche (all fees to the institution). K.P.M.v.G. has received an unrestricted research grant from Octapharma. The remaining authors declare no competing financial interests.
Acknowledgments
The graphical abstract was made with BioRender.com.
F.A. is supported by a Rubicon grant (452022310) from the Netherlands Organization for Health Research and Development (ZonMw). J.S.O. is supported by a Science Foundation Ireland Frontiers for the Future Award (20/FFP-A/8952). The WiN study was supported in part by research funding from the Dutch Hemophilia Foundation (Stichting Haemophilia), Shire (Takeda), and CSL Behring (unrestricted grant).
Authorship
Contribution: F.A., F.W.G.L., and J.S.O. designed the research and wrote the article; F.A. and R.B. performed statistical analysis; F.A., R.B., C.B.v.K., D.D., M.L., J.G.v.d.B., N.M.O., J.d.M., K.R., S.E.M.S., M.B., F.C.J.I.H.-M., K.P.M.v.G., M.H.C., K.F., K.M., J.E., F.W.G.L., and J.S.O. contributed to patient enrollment, literature review, interpretated data, final draft writing, and critical revision; R.J.S.P., R.I.B., P.J., and J.D.P. contributed to literature review, data interpretation, and final draft writing and critical revision; and all authors participated sufficiently in this work, take public responsibility for the content, and have given consent to the final version of the article.
Footnotes
F.W.G.L. and J.S.O. contributed equally to this study.
The data that support the findings of this study are available upon reasonable request from the corresponding author, Ferdows Atiq (ferdowsatiq@rcsi.ie).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Supplementary Material
References
- 1.Leebeek FW, Eikenboom JC. Von Willebrand's disease. N Engl J Med. 2016;375(21):2067–2080. doi: 10.1056/NEJMra1601561. [DOI] [PubMed] [Google Scholar]
- 2.Nichols WL, Hultin MB, James AH, et al. von Willebrand disease (VWD): evidence-based diagnosis and management guidelines, the National Heart, Lung, and Blood Institute (NHLBI) Expert Panel report (USA) Haemophilia. 2008;14(2):171–232. doi: 10.1111/j.1365-2516.2007.01643.x. [DOI] [PubMed] [Google Scholar]
- 3.Sadler JE, Budde U, Eikenboom JC, et al. Update on the pathophysiology and classification of von Willebrand disease: a report of the Subcommittee on von Willebrand Factor. J Thromb Haemost. 2006;4(10):2103–2114. doi: 10.1111/j.1538-7836.2006.02146.x. [DOI] [PubMed] [Google Scholar]
- 4.Laffan MA, Lester W, O'Donnell JS, et al. The diagnosis and management of von Willebrand disease: a United Kingdom Haemophilia Centre Doctors Organization guideline approved by the British Committee for Standards in Haematology. Br J Haematol. 2014;167(4):453–465. doi: 10.1111/bjh.13064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castaman G, Goodeve A, Eikenboom J, European Group on von Willebrand Disease Principles of care for the diagnosis and treatment of von Willebrand disease. Haematologica. 2013;98(5):667–674. doi: 10.3324/haematol.2012.077263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.James PD, Connell NT, Ameer B, et al. ASH ISTH NHF WFH 2021 guidelines on the diagnosis of von Willebrand disease. Blood Adv. 2021;5(1):280–300. doi: 10.1182/bloodadvances.2020003265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sadler JE. Low von Willebrand factor: sometimes a risk factor and sometimes a disease. Hematology Am Soc Hematol Educ Program. 2009;2009(1):106–112. doi: 10.1182/asheducation-2009.1.106. [DOI] [PubMed] [Google Scholar]
- 8.O'Donnell JS, Baker RI. Low von Willebrand disease: a bleeding disorder of unknown cause? Hamostaseologie. 2023;43(1):44–51. doi: 10.1055/a-1980-8198. [DOI] [PubMed] [Google Scholar]
- 9.Lavin M, O'Donnell JS. How I treat low von Willebrand factor levels. Blood. 2019;133(8):795–804. doi: 10.1182/blood-2018-10-844936. [DOI] [PubMed] [Google Scholar]
- 10.Tosetto A, Castaman G, Rodeghiero F. Evidence-based diagnosis of type 1 von Willebrand disease: a Bayes theorem approach. Blood. 2008;111(8):3998–4003. doi: 10.1182/blood-2007-08-105940. [DOI] [PubMed] [Google Scholar]
- 11.Flood VH, Christopherson PA, Gill JC, et al. Clinical and laboratory variability in a cohort of patients diagnosed with type 1 VWD in the United States. Blood. 2016;127(20):2481–2488. doi: 10.1182/blood-2015-10-673681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.James PD, Paterson AD, Notley C, et al. Genetic linkage and association analysis in type 1 von Willebrand disease: results from the Canadian type 1 VWD study. J Thromb Haemost. 2006;4(4):783–792. doi: 10.1111/j.1538-7836.2006.01860.x. [DOI] [PubMed] [Google Scholar]
- 13.Flood VH, Abshire TC, Christopherson PA, et al. Von Willebrand disease in the United States: perspective from the Zimmerman program. Ann Blood. 2018;3:7. doi: 10.21037/aob.2017.12.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Atiq F, Boender J, van Heerde WL, et al. Importance of genotyping in von Willebrand disease to elucidate pathogenic mechanisms and variability in phenotype. HemaSphere. 2022;6(6) doi: 10.1097/HS9.0000000000000718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eikenboom J, Van Marion V, Putter H, et al. Linkage analysis in families diagnosed with type 1 von Willebrand disease in the European study, molecular and clinical markers for the diagnosis and management of type 1 VWD. J Thromb Haemost. 2006;4(4):774–782. doi: 10.1111/j.1538-7836.2006.01823.x. [DOI] [PubMed] [Google Scholar]
- 16.de Wee EM, Sanders YV, Mauser-Bunschoten EP, et al. Determinants of bleeding phenotype in adult patients with moderate or severe von Willebrand disease. Thromb Haemost. 2012;108(4):683–692. doi: 10.1160/TH12-04-0244. [DOI] [PubMed] [Google Scholar]
- 17.Atiq F, Wuijster E, de Maat MPM, Kruip M, Cnossen MH, Leebeek FWG. Criteria for low von Willebrand factor diagnosis and risk score to predict future bleeding. J Thromb Haemost. 2021;19(3):719–731. doi: 10.1111/jth.15227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O'Donnell JS. Low VWF: insights into pathogenesis, diagnosis, and clinical management. Blood Adv. 2020;4(13):3191–3199. doi: 10.1182/bloodadvances.2020002038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lavin M, Aguila S, Schneppenheim S, et al. Novel insights into the clinical phenotype and pathophysiology underlying low VWF levels. Blood. 2017;130(21):2344–2353. doi: 10.1182/blood-2017-05-786699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doherty D, Lavin M, O'Sullivan JM, et al. Management of elective procedures in low von Willebrand factor patients in the LoVIC study. J Thromb Haemost. 2021;19(3):701–710. doi: 10.1111/jth.15220. [DOI] [PubMed] [Google Scholar]
- 21.James PD, Connell NT, Flood VH, Mustafa RA. Response to “The 2021 von Willebrand disease guidelines: clarity and controversy.”. Haemophilia. 2022;28(3):371–372. doi: 10.1111/hae.14528. [DOI] [PubMed] [Google Scholar]
- 22.Makris M, Hermans C. The 2021 von Willebrand disease guidelines: clarity and controversy. Haemophilia. 2022;28(1):1–3. doi: 10.1111/hae.14465. [DOI] [PubMed] [Google Scholar]
- 23.Tofler GH, Massaro J, Levy D, et al. Relation of the prothrombotic state to increasing age (from the Framingham Offspring Study) Am J Cardiol. 2005;96(9):1280–1283. doi: 10.1016/j.amjcard.2005.06.072. [DOI] [PubMed] [Google Scholar]
- 24.Vischer UM, Herrmann FR, Peyrard T, Nzietchueng R, Benetos A. Plasma von Willebrand factor and arterial aging. J Thromb Haemost. 2005;3(4):794–795. doi: 10.1111/j.1538-7836.2005.01242.x. [DOI] [PubMed] [Google Scholar]
- 25.Rydz N, Grabell J, Lillicrap D, James PD. Changes in von Willebrand factor level and von Willebrand activity with age in type 1 von Willebrand disease. Haemophilia. 2015;21(5):636–641. doi: 10.1111/hae.12664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanders YV, Giezenaar MA, Laros-van Gorkom BA, et al. von Willebrand disease and aging: an evolving phenotype. J Thromb Haemost. 2014;12(7):1066–1075. doi: 10.1111/jth.12586. [DOI] [PubMed] [Google Scholar]
- 27.Atiq F, Meijer K, Eikenboom J, et al. Comorbidities associated with higher von Willebrand factor (VWF) levels may explain the age-related increase of VWF in von Willebrand disease. Br J Haematol. 2018;182(1):93–105. doi: 10.1111/bjh.15277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Michels A, Dwyer CN, Mewburn J, et al. von Willebrand factor is a critical mediator of deep vein thrombosis in a mouse model of diet-induced obesity. Arterioscler Thromb Vasc Biol. 2020;40(12):2860–2874. doi: 10.1161/ATVBAHA.120.314690. [DOI] [PubMed] [Google Scholar]
- 29.Atiq F, van de Wouw J, Sorop O, et al. Endothelial dysfunction, atherosclerosis, and increase of von Willebrand factor and factor VIII: a randomized controlled trial in swine. Thromb Haemost. 2021;121(5):676–686. doi: 10.1055/s-0040-1722185. [DOI] [PubMed] [Google Scholar]
- 30.de Wee EM, Mauser-Bunschoten EP, Van Der Bom JG, et al. Health-related quality of life among adult patients with moderate and severe von Willebrand disease. J Thromb Haemost. 2010;8(7):1492–1499. doi: 10.1111/j.1538-7836.2010.03864.x. [DOI] [PubMed] [Google Scholar]
- 31.De Wee EM, Knol HM, Mauser-Bunschoten EP, et al. Gynaecological and obstetric bleeding in moderate and severe von Willebrand disease. Thromb Haemost. 2011;106(5):885–892. doi: 10.1160/TH11-03-0180. [DOI] [PubMed] [Google Scholar]
- 32.Lavin M, Aguila S, Dalton N, et al. Significant gynecological bleeding in women with low von Willebrand factor levels. Blood Adv. 2018;2(14):1784–1791. doi: 10.1182/bloodadvances.2018017418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanders YV, Groeneveld D, Meijer K, et al. von Willebrand factor propeptide and the phenotypic classification of von Willebrand disease. Blood. 2015;125(19):3006–3013. doi: 10.1182/blood-2014-09-603241. [DOI] [PubMed] [Google Scholar]
- 34.Doherty D, Michelle L, Byrne M, et al. Enhanced VWF clearance in low VWF pathogenesis: limitations of the VWFpp/VWF:Ag ratio and clinical significance. Blood Adv. 2023;7(3):302–308. doi: 10.1182/bloodadvances.2022007340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Atiq F, Schütte LM, Looijen AEM, et al. von Willebrand factor and factor VIII levels after desmopressin are associated with bleeding phenotype in type 1 VWD. Blood Adv. 2019;3(24):4147–4154. doi: 10.1182/bloodadvances.2019000863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Connell NT, Flood VH, Brignardello-Petersen R, et al. ASH ISTH NHF WFH 2021 guidelines on the management of von Willebrand disease. Blood Adv. 2021;5(1):301–325. doi: 10.1182/bloodadvances.2020003264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Connell NT, James PD, Brignardello-Petersen R, et al. von Willebrand disease: proposing definitions for future research. Blood Adv. 2021;5(2):565–569. doi: 10.1182/bloodadvances.2020003620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Atiq F, Heijdra JM, Snijders F, et al. Desmopressin response depends on the presence and type of genetic variants in patients with type 1 and type 2 von Willebrand disease. Blood Adv. 2022;6(18):5317–5326. doi: 10.1182/bloodadvances.2021006757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eikenboom J, Federici AB, Dirven RJ, et al. VWF propeptide and ratios between VWF, VWF propeptide, and FVIII in the characterization of type 1 von Willebrand disease. Blood. 2013;121(12):2336–2339. doi: 10.1182/blood-2012-09-455089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haberichter SL, Castaman G, Budde U, et al. Identification of type 1 von Willebrand disease patients with reduced von Willebrand factor survival by assay of the VWF propeptide in the European study: molecular and clinical markers for the diagnosis and management of type 1 VWD (MCMDM-1VWD) Blood. 2008;111(10):4979–4985. doi: 10.1182/blood-2007-09-110940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haberichter SL, Balistreri M, Christopherson P, et al. Assay of the von Willebrand factor (VWF) propeptide to identify patients with type 1 von Willebrand disease with decreased VWF survival. Blood. 2006;108(10):3344–3351. doi: 10.1182/blood-2006-04-015065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sadler JE. Von Willebrand disease type 1: a diagnosis in search of a disease. Blood. 2003;101(6):2089–2093. doi: 10.1182/blood-2002-09-2892. [DOI] [PubMed] [Google Scholar]
- 43.Atiq F, Saes JL, Punt MC, et al. Major differences in clinical presentation, diagnosis and management of men and women with autosomal inherited bleeding disorders. EClinicalMedicine. 2021;32 doi: 10.1016/j.eclinm.2021.100726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kirtava A, Crudder S, Dilley A, Lally C, Evatt B. Trends in clinical management of women with von Willebrand disease: a survey of 75 women enrolled in haemophilia treatment centres in the United States. Haemophilia. 2004;10(2):158–161. doi: 10.1046/j.1351-8216.2003.00832.x. [DOI] [PubMed] [Google Scholar]
- 45.Ragni MV, Bontempo FA, Hassett AC. von Willebrand disease and bleeding in women. Haemophilia. 1999;5(5):313–317. doi: 10.1046/j.1365-2516.1999.00342.x. [DOI] [PubMed] [Google Scholar]
- 46.Gill JC, Conley SF, Johnson VP, et al. Low VWF levels in children and lack of association with bleeding in children undergoing tonsillectomy. Blood Adv. 2020;4(1):100–105. doi: 10.1182/bloodadvances.2019000992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Seaman CD, Ragni MV. The effect of age on von Willebrand factor and bleeding symptoms in von Willebrand disease. Thromb Haemost. 2020;120(8):1159–1165. doi: 10.1055/s-0040-1713636. [DOI] [PubMed] [Google Scholar]
- 48.Federici AB. The use of desmopressin in von Willebrand disease: the experience of the first 30 years (1977-2007) Haemophilia. 2008;14(suppl 1):5–14. doi: 10.1111/j.1365-2516.2007.01610.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.