Abstract
Purpose
To evaluate the effect of low-concentration (0.01% and 0.05%) atropine eyedrops on ocular surface characteristics in young adults.
Methods
Twenty-six myopic students aged 18 to 30 years were randomly assigned to receive either 0.01% or 0.05% atropine once nightly for 14 days, followed by cessation, with a ≥14-day interval between each administration. Assessments were conducted one, two, seven, and 14 days after using atropine with corresponding timepoints after atropine cessation. Tear meniscus height and first and average noninvasive keratograph tear film breakup time (NIKBUT-first, NIKBUT-average) were measured using Keratograph 5M, whereas the objective scatter index (OSI) was measured by OQAS II devices; the ocular surface disease index (OSDI) score was also obtained.
Results
The mean OSI peaked after two days of administration of 0.05% atropine (β = 0.51, P = 0.001), accompanied by significant decreases in NIKBUT-first (β = −7.73, P < 0.001) and NIKBUT-average (β = −8.10, P < 0.001); the OSDI peaked after 14 days (β = 15.41, P < 0.001). The above parameters returned to baseline one week after atropine discontinuation (all P > 0.05). NIKBUT-first and NIKBUT-average reached their lowest points after 14 days of 0.01% atropine administration (NIKBUT-first: β = −4.46, P = 0.005; NIKBUT-average: β = −4.42, P = 0.001), but those significant changes were diminished once atropine treatment stopped.
Conclusions
Young adult myopes experienced a significant but temporary impact on the ocular surface with 0.05% atropine administration, whereas 0.01% atropine had a minimal effect.
Translational Relevance
The investigation of the ocular surface effects of different concentrations of atropine may inform evidence-based clinical decisions regarding myopia control in young adults.
Keywords: myopia control, ocular surface, atropine eyedrops, adult myopia
Introduction
The prevalence of myopia has been increasing globally, particularly in East and Southeast Asia.1–4 High myopia significantly elevates the risk of serious complications such as glaucoma, macular degeneration, and retinal detachment.5–7 Therefore it is crucial to implement effective measures for myopia control. Although the focus of myopia control has primarily been on children and adolescents, a recent report has indicated that early adulthood (ages 18–25 years) may also be a vulnerable period for the onset and progression of myopia, possibly because of prolonged near-work hours and limited outdoor activities during this stage.8–10 Consequently, monitoring myopia progression in this age group has become necessary, along with implementing appropriate interventions.
Currently, clinical interventions for myopia control primarily involve optical and pharmaceutical approaches. Among these interventions, atropine eye drops have been considered the most effective strategy for myopia control. 11–14 A recent meta-analysis comparing eight atropine concentrations (1% to 0.01%) demonstrated that 0.05% was considered the most beneficial concentration in terms of efficacy and safety for myopia control in children.15 In addition, a five-year randomized controlled study (LAMP2) confirmed that 0.05% atropine could effectively reduce myopia incidence and myopic shift in children.16 Despite the demonstrated efficacy in halting myopia development, it is critical to acknowledge the potential side effects associated with atropine eye drops.
Topical atropine, a nonselective muscarinic antagonist, induces cycloplegic effects, pupil dilation, decreased accommodation amplitude, allergic conjunctivitis, and eyelid dermatitis related to allergy.17–19 A previous review identified ocular allergy as a risk factor for dry eye in children and adults.20 Zhao et al.21 hypothesized that low-concentration atropine eye drops may lead to dry eye in children. However, studies have shown no observed dry-eye symptoms when using 0.01% atropine eye drops for myopia control in children.22,23 Conversely, higher concentrations of atropine (e.g., 0.1% and 0.5%) have been associated with common adverse effects such as allergic conjunctivitis and dermatitis in children.23 Nevertheless, the effects of different concentrations of atropine on ocular surface symptoms in older children or young adults remain unclear.
Advancing age has been acknowledged as a significant risk factor for dry eye disease,24 with adults exhibiting more pronounced dry-eye symptoms compared to pediatric patients experiencing similar stages of ocular surface damage,25 suggesting heightened sensitivity to ocular symptoms in young adults. Therefore, before implementing atropine eyedrops for myopia control in young adults, it is necessary to comprehend their potential impact on ocular surface characteristics within this demographic. This study aimed to assess the effects of the application and cessation of 0.01% and 0.05% atropine eyedrops on the ocular surface among university students over time.
Methods
Subjects
Twenty-six myopic adults aged 18 to 30 years, with spherical equivalent refraction ranging from −0.50 diopters (D) to −5.75 D, astigmatism < 1.00 D, anisometropia < 1.50 D, and best-corrected visual acuity of 0.00 logMAR or better in each eye, were enrolled. None of the subjects had a history of continuous use of myopia control treatment for more than one month, any ocular or systemic conditions that could affect vision outcomes, or a fluorescein breakup time less than five seconds. Throughout the experiment, each subject was fully corrected using a trial frame, whereas the measurements were taken exclusively from the right eye, with the left eye occluded. This study adhered to the tenets of the Declaration of Helsinki and received approval from the Ethics Committee of the Eye Hospital of Wenzhou Medical University (no. 2022-140-K-109-01). Written informed consent was obtained from each subject before the study.
Study Design
Each subject received 0.01% atropine (5 mL-unit concentration, preservative-free, hospital preparation) and 0.05% atropine (0.4 mL-unit concentration, preservative-free, Aier Eye Institute) in random order, with a minimum interval of 14 days between the two doses. Each eye drop was administered once nightly (after 8:00 PM) in both eyes for 14 days, after which atropine administration was discontinued. Follow-up assessments were conducted on day 1, day 2, day 7, and day 14 after initiating atropine and at the corresponding timepoints after its cessation.
Ophthalmic Parameters and Questionnaires
The objective scattering index (OSI) was measured using an Optical Quality Analysis System (OQAS; Visiometrics SL, Terrassa, Spain) for a 4.0-mm pupil diameter. During the measurement, subjects were instructed to maintain steady fixation without blinking for 20 seconds. Throughout this period, OQAS continually captured 40 OSI images at intervals of 0.5 seconds and calculated the mean and standard deviation of these images for analysis. Keratograph 5M Topographer inspection (Oculus Optikgerate GmbH, Wetzlar, Germany) was used to measure tear meniscus height (TMH), the first and average noninvasive Keratograph tear film break-up time (NIKBUT-first, NIKBUT-average), bulbar redness (BR), and meibography. The degree of meibomian gland dropout, referred to as the meiboscore (MS), was classified as follows: Grade 0, no loss of the meibomian gland; Grade 1, loss of less than one third of the entire gland area; Grade 2, loss of one third to two thirds of the entire gland area; and Grade 3, loss of more than two thirds of the total gland area. The MS for each eye was determined by summing the scores from both the upper and lower eyelids and was assessed only at baseline and 14 days after the application and discontinuation of atropine.26 The severity of subjects’ dry eyes was assessed at each visit using the Ocular Surface Disease Index (OSDI) questionnaire.27
Statistical Analysis
Statistical analysis was conducted using R software (version 4.1.3). Generalized estimating equations were used to evaluate the effects of time, concentration, and the interaction between the two, as well as the difference between the two concentrations. In cases where a significant difference was observed, Bonferroni-corrected alpha for multiple comparisons was used to identify significant differences in post hoc pairwise comparisons. A P value < 0.05 was considered indicative of statistical significance.
Results
Twenty-six participants were initially enrolled in the study. However, one participant discontinued the use of 0.01% atropine eyedrops on the first day because of photophobia, whereas three others dropped out because of relocation to other cities. Consequently, data from the 22 participants who completed all the experiments were included for analysis. The demographic and ocular characteristics at baseline are presented in the Table.
Table.
Baseline Demographics and Ocular Characteristics of Participants Who Completed the Experiments
| Clinical Value | |
|---|---|
| Age (yr), mean (SD) | 22.1 ± 2.4 |
| Sex | |
| Male | 10 (45.5%) |
| Female | 12 (54.5%) |
| Refractive error (SER), D | |
| Right eye, mean (SD) | −3.80 ± 1.44 |
| Left eye, mean (SD) | −3.50 ± 1.53 |
| IOP (mm Hg) | |
| Right eye, mean (SD) | 17.18 ± 1.59 |
| Left eye, mean (SD) | 17.23 ± 1.93 |
IOP, intraocular pressure; SER, spherical equivalent refraction.
OSI
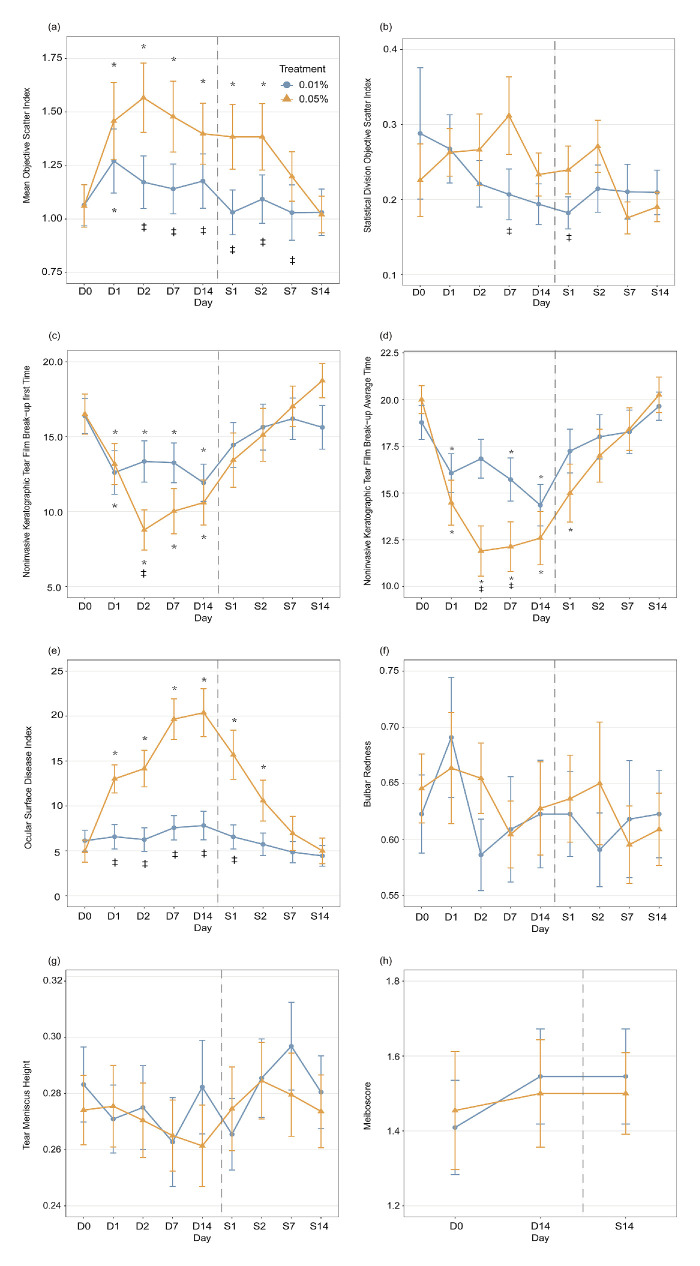
The mean OSI did not differ significantly between the two concentrations at baseline (P > 0.05). However, after two days of using atropine, there were significant changes in OSI observed for the interaction of time and concentration (β = 0.40, P = 0.001), as well as after cessation of atropine after seven days (β = 0.17, P = 0.04). The use of 0.05% atropine after one day resulted in a significant increase in OSI (β = 0.40, P = 0.01, Fig. a), which peaked after two days (β = 0.51, P = 0.001); the significance of OSI persisted for two days after discontinuing the use of 0.05% atropine (β = 0.32, P = 0.004). In contrast, a significant change in OSI was observed only on the first day after the use of 0.01% atropine (β = 0.21, P = 0.002). The differences between the two concentrations remained significant from two days after usage until seven days after cessation (all P < 0.05, Fig. a).
Figure.
Changes in ocular surface parameters across time with 0.05% (orange) and 0.01% (blue) atropine use and cessation. (a) Mean OSI, (b) standard deviation OSI, (c) NIKBUT-first , (d) NIKBUT-average, (e) OSDI, (f) bulb redness, (g) TMH, and (h) meiboscore. D0 indicates the baseline measurement, whereas D1/D2/D7/D14 represent one/two/seven and 14 days of applying atropine, and S1/S2/S7/S14 indicate one/two/seven and 14 days after stopping the use of atropine. The gray line is the boundary between applying atropine and stopping its use. Error bars: standard errors of the mean. *P < 0.05, for data compared with baseline; ‡P < 0.05 for data compared between the two concentrations of atropine.
The interactions between time and concentrations in OSI variations (i.e., OSI standard deviations) during application and cessation were nonsignificant (all P > 0.05). Additionally, significant differences in OSI variations between the two concentrations were observed seven days after use (β = 0.11, P = 0.047, Fig. b) and one day after cessation (β = 0.06, P = 0.04).
NIKBUT-First and NIKBUT-Average
The baseline NIKBUT-first and NIKBUT-average showed no significant differences at either concentration (P > 0.05). However, there was a significant interaction between time and concentration in the NIKBUT-first when atropine was applied for seven days (β = −4.67, P = 0.007). When using 0.05% atropine, a significant decrease in NIKBUT-first was found after one day (β = −3.33, P = 0.03, Fig. c), reaching its lowest point after two days (β = −7.73, P < 0.001). However, after discontinuation of 0.05% atropine, the significance diminished (β = −3.07, P = 0.14). For 0.01% atropine, significant changes in NIKBUT-first were observed one day after application (β = −3.78, P < 0.001), and the changes remained stable in the following days (pairwise comparisons between subsequent days, all P > 0.05). Moreover, once the application of 0.01% atropine stopped, the significance diminished (all P > 0.05). To compare the differences between these two concentrations, a difference was only evident after two days of application (β = −4.57, P = 0.004).
After using atropine for two (β = −6.16, P < 0.001) and seven days (β = −4.81, P = 0.003), the interaction between time and concentrations for NIKBUT-average became significant. A significant decrease in NIKBUT-average was found after one day (β = −5.52, P < 0.001, Fig. d) when using 0.05% atropine, and the significance reached its lowest point after two days (β = −8.10, P < 0.001). The significance diminished two days after stopping 0.05% atropine treatment (β = −3.00, P = 0.05). With 0.01% atropine, significant changes in NIKBUT-average were observed one (β = −2.97, P < 0.001), seven (β = −3.05, P = 0.007), and 14 (β = −4.42, P = 0.001) days after application. The significance disappeared once the application of 0.01% atropine stopped. Significant differences existed between these two concentrations after two (β = −4.94, P = 0.003) and seven (β = −3.59, P = 0.04) days of application.
OSDI
The baseline OSDI between the two concentrations showed no significant differences (P > 0.05). However, a significant interaction was observed between time and concentrations in OSDI after one day of atropine application (β = 7.60, P < 0.001) and two days after stopping atropine (β = 6.02, P < 0.001). When using 0.05% atropine, the OSDI increased significantly after one day (β = 8.05, P < 0.001, Fig. e) and reached its peak after fourteen days (β = 15.41, P < 0.001). Notably, the significance of OSDI persisted even after two days (β = 5.62, P = 0.002) of discontinuing the use of 0.05% atropine. With 0.01% atropine, no difference was found after application and cessation (all P > 0.05). To compare the differences between these two concentrations, there were differences from one day after application (β = 6.44, P = 0.001) to one day after cessation (β = 9.13, P = 0.002).
BR, TMH, and MS
No significant differences were observed in BR, TMH, and MS between the two concentrations at baseline, during application, and cessation (all P > 0.05, Figs. f–h). Furthermore, there were no significant interactions between time and concentration for BR, TMH, and MS during application or cessation (all P > 0.05).
Discussion
The concentration-dependent effect of atropine has been demonstrated for both myopia control efficacy and its side effects.18,28 However, there are limited studies on the effect of low-concentration atropine on the ocular surface. This finding revealed that using 0.05% atropine eyedrops had significant effects on the ocular surface in young adult myopes after the first use, but these effects gradually disappeared within seven days of stopping the eyedrops after two weeks of continuous use. In contrast, 0.01% atropine eye drops had a negligible effect on the ocular surface.
Temporal changes in the OSI have been recognized as a prospective indicator of real-time variations in ocular scatter, representing changes in the optical quality of the tear film.29 The study by Guo et al.30 demonstrated that wearing orthokeratology lenses for one month led to a significant increase in OSI in children (from 0.572 ± 0.29 to 1.212 ± 0.97). Similarly, this study showed that the OSI increased after the first day of 0.05% atropine eye drops but recovered gradually within seven days after 14 days of use. Ju31 reported that TMH and NIKBUT decreased significantly on the first day after cataract surgery but recovered one month post-surgery, while OSDI increased throughout the study. Li et al.32 revealed that the secretion of aqueous tears decreased significantly after 14 days of using 0.1% topical benzalkonium chloride and recovered 14 days after cessation. A similar trend was observed in this study.
Atropine reduces aqueous production and affects tear stability by antagonizing muscarinic receptors in the lacrimal gland. Stern et al.33 showed that the ocular surface (including the cornea, conjunctiva, and accessory lacrimal glands), the Meibomian glands, and the main lacrimal gland were interconnected through neural reflex loops to maintain the tear system's functionality. Previous studies have reported that anticholinergic agents such as atropine could impede these neural reflex loops in a rabbit dry-eye model.34–37 This study also revealed that 0.05% atropine had significant effects on NIKBUT-first, NIKBUT-average, and OSDI, with the latter exceeding the normal upper limit of 12. However, 0.01% atropine eyedrops had minimal impact on ocular surface characteristics. These findings reveal a concentration-dependent relationship between atropine and changes in these ocular surface characteristics, and the effects of 0.05% atropine on these parameters were temporary and reversible but require further investigation for longer-term effects.
Previous studies on the use of 0.05% atropine for myopia treatment in children did not report any symptoms of dry eye.16,18,38,39 The difference between children and young adults may be attributed to age-related decline in tear production and quality, as reduced tear secretion in adults could lead to unstable tear films and increased dry eye risk.40 Several lifestyle and environmental factors, such as excessive screen time, air conditioning, pollution, and stress, can also exacerbate dry eye symptoms in adults by increasing tear evaporation and reducing blinking.41–43 In addition, adults are more sensitive than children and can easily recognize and report symptoms like eye discomfort.
This study has a few limitations. First, the follow-up period was relatively short, and a longer study period is necessary to thoroughly evaluate the safety of atropine eyedrops on the ocular surface. Second, this study did not include a questionnaire on eye behavior but based on communication with the subjects, of whom 54% (12/22) reported experiencing dry-eye symptoms while using 0.05% atropine, whereas only 4.5% (1/22) of subjects reported experiencing dry-eye symptoms while using 0.01% atropine. It is also important to consider factors such as seasonal variation and humidity, which can affect the ocular surface.44,45 In this study, the indoor temperature averaged 23.97°C ± 1.4°C, with a humidity level of approximately 50%. Last, further studies are needed to assess additional factors, such as visual performance, to determine the safety of atropine eye drops in young adults.
Conclusions
In summary, this study found that using 0.05% atropine eye drops has a significant yet temporary impact on the tear film in young adults, without affecting other ocular surface parameters, such as bulbar redness. However, using 0.01% atropine eye drops had minimal effects. Further studies are needed to determine the long-term effects of different concentrations of atropine and to ascertain the efficacy and safety of low-concentration atropine eye drops in myopia control in this specific population.
Acknowledgments
Supported by the Key Research and Development Program of Zhejiang Province (grant number 2020C03111).
Disclosure: Y. Luo, None; Z. Yin, None; J. Zhang, None; W. Wang, None; Y. Huang, None; X. Li, None; H. Chen, None; F. Lu, None; J. Bao, None
References
- 1. Dolgin E. The myopia boom. Nature. 2015; 519(7543): 276–278. [DOI] [PubMed] [Google Scholar]
- 2. Morgan IG, He M, Rose KA. Epidemic of pathologic myopia: what can laboratory studies and epidemiology tell us? Retina. 2017; 37: 989–997. [DOI] [PubMed] [Google Scholar]
- 3. Morgan IG, French AN, Ashby RS, et al.. The epidemics of myopia: aetiology and prevention. Prog Retin Eye Res. 2018; 62: 134–149. [DOI] [PubMed] [Google Scholar]
- 4. Baird PN, Saw SM, Lanca C, et al.. Myopia. Nat Rev Dis Primers. 2020; 6: 99. [DOI] [PubMed] [Google Scholar]
- 5. Marcus MW, de Vries MM, Junoy Montolio FG, Jansonius NM. Myopia as a risk factor for open-angle glaucoma: a systematic review and meta-analysis. Ophthalmology. 2011; 118: 1989–1994.e2. [DOI] [PubMed] [Google Scholar]
- 6. Asakuma T, Yasuda M, Ninomiya T, et al.. Prevalence and risk factors for myopic retinopathy in a Japanese population: the Hisayama Study. Ophthalmology. 2012; 119: 1760–1765. [DOI] [PubMed] [Google Scholar]
- 7. Morgan IG, Ohno-Matsui K, Saw SM. Myopia. Lancet. 2012; 379(9827): 1739–1748. [DOI] [PubMed] [Google Scholar]
- 8. Kinge B, Midelfart A.. Refractive changes among Norwegian university students—a three-year longitudinal study. Acta Ophthalmol Scand. 1999; 77: 302–305. [DOI] [PubMed] [Google Scholar]
- 9. Jacobsen N, Jensen H, Goldschmidt E.. Does the level of physical activity in university students influence development and progression of myopia? A 2-year prospective cohort study. Invest Ophthalmol Vis Sci. 2008; 49: 1322–1327. [DOI] [PubMed] [Google Scholar]
- 10. Bullimore MA, Lee SSY, Schmid KL, et al.. IMI—onset and progression of myopia in young adults. Invest Ophthalmol Vis Sci. 2023; 64(6): 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Huang J, Wen D, Wang Q, et al.. Efficacy comparison of 16 interventions for myopia control in children: a network meta-analysis. Ophthalmology. 2016; 123: 697–708. [DOI] [PubMed] [Google Scholar]
- 12. Lawrenson JG, Shah R, Huntjens B, et al.. Interventions for myopia control in children: a living systematic review and network meta-analysis. Cochrane Database Syst Rev. 2023; 2023(2): CD014758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Walline JJ, Lindsley KB, Vedula SS, et al.. Interventions to slow progression of myopia in children. Cochrane Database Syst Rev. 2020; 1(1): CD004916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wildsoet CF, Chia A, Cho P, et al.. IMI—Interventions Myopia Institute: interventions for controlling myopia onset and progression report. Invest Ophthalmol Vis Sci. 2019; 60(3): M106–M131. [DOI] [PubMed] [Google Scholar]
- 15. Ha A, Kim SJ, Shim SR, Kim YK, Jung JH.. Efficacy and safety of 8 atropine concentrations for myopia control in children: a network meta-analysis. Ophthalmology. 2022; 129: 322–333. [DOI] [PubMed] [Google Scholar]
- 16. Yam JC, Zhang XJ, Zhang Y, et al.. Effect of low-concentration atropine eyedrops vs placebo on myopia incidence in children. JAMA. 2023; 329: 472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chia A, Lu QS, Tan D.. Five-year clinical trial on atropine for the treatment of myopia 2: myopia control with atropine 0.01% eyedrops. Ophthalmology. 2016; 123: 391–399. [DOI] [PubMed] [Google Scholar]
- 18. Yam JC, Jiang Y, Tang SM, et al.. Low-Concentration Atropine for Myopia Progression (LAMP) Study: a randomized, double-blinded, placebo-controlled trial of 0.05%, 0.025%, and 0.01% atropine eye drops in myopia control. Ophthalmology. 2019; 126: 113–124. [DOI] [PubMed] [Google Scholar]
- 19. Shih KC, Chan TCY, Ng ALK, et al.. Use of atropine for prevention of childhood myopia progression in clinical practice. Eye Contact Lens-Sci Clin Pra. 2016; 42: 16–23. [DOI] [PubMed] [Google Scholar]
- 20. Garcia Del Valle I, Alvarez-Lorenzo C. Atropine in topical formulations for the management of anterior and posterior segment ocular diseases. Expert Opin Drug Deliv. 2021; 18: 1245–1259. [DOI] [PubMed] [Google Scholar]
- 21. Zhao F, Ma JX.. Will the long-term use of atropine eye drops in children increase the risk of dry eye? Med Hypotheses. 2019; 132: 109331. [DOI] [PubMed] [Google Scholar]
- 22. Cheng J, Yang Y, Kong X, et al.. The effect of 0.01% atropine eye drops on the ocular surface in children for the control of myopia-the primary results from a six-month prospective study. Ther Clin Risk Manag. 2020; 16: 735–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chia A, Chua WH, Cheung YB, et al.. Atropine for the treatment of childhood myopia: safety and efficacy of 0.5%, 0.1%, and 0.01% doses (Atropine for the Treatment of Myopia 2). Ophthalmology. 2012; 119: 347–354. [DOI] [PubMed] [Google Scholar]
- 24. Wolffsohn JS, Wang MTM, Vidal-Rohr M, et al.. Demographic and lifestyle risk factors of dry eye disease subtypes: a cross-sectional study. Ocul Surf. 2021; 21: 58–63. [DOI] [PubMed] [Google Scholar]
- 25. Han SB, Yang HK, Hyon JY, Hwang JM.. Children with dry eye type conditions may report less severe symptoms than adult patients. Graefes Arch Clin Exp Ophthalmol. 2013; 251: 791–796. [DOI] [PubMed] [Google Scholar]
- 26. Arita R, Itoh K, Maeda S, et al.. Proposed diagnostic criteria for obstructive meibomian gland dysfunction. Ophthalmology. 2009; 116: 2058–2063.e1. [DOI] [PubMed] [Google Scholar]
- 27. Wolffsohn JS, Arita R, Chalmers R, et al.. TFOS DEWS II Diagnostic Methodology report. Ocul Surf. 2017; 15: 539–574. [DOI] [PubMed] [Google Scholar]
- 28. Gong Q, Janowski M, Luo M, et al.. Efficacy and adverse effects of atropine in childhood myopia: a meta-analysis. JAMA Ophthalmol. 2017; 135: 624–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Benito A, Pérez GM, Mirabet S, et al.. Objective optical assessment of tear-film quality dynamics in normal and mildly symptomatic dry eyes. J Cataract Refract Surg. 2011; 37: 1481–1487. [DOI] [PubMed] [Google Scholar]
- 30. Guo HC, Jin WQ, Pan AP, Wang QM, Qu J, Yu AY.. Changes and diurnal variation of visual quality after orthokeratology in myopic children. J Ophthalmol. 2018; 2018: 3174826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ju RH. Changes in ocular surface status and dry eye symptoms following femtosecond laser-assisted cataract surgery. Int J Ophthalmol. 2019; 12: 1122–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li C, Song Y, Luan S, et al.. Research on the stability of a rabbit dry eye model induced by topical application of the preservative benzalkonium chloride. PLoS ONE. 2012; 7(3): e33688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stern ME, Beuerman RW, Fox RI, Gao J, Mircheff AK, Pflugfelder SC.. The pathology of dry eye. Cornea. 1998; 17: 584. [DOI] [PubMed] [Google Scholar]
- 34. Schoenwald RD, Vidvauns S, Wurster DE, Barfknecht CF. The influence of tear proteins on the film stability of rabbit tear extracts. J Ocul Pharmacol Therap. 1998; 14(1): 15–29. [DOI] [PubMed] [Google Scholar]
- 35. Bucolo C, Musumeci M, Salomone S, et al.. Effects of topical fucosyl-lactose, a milk oligosaccharide, on dry eye model: an example of nutraceutical candidate. Front Pharmacol. 2015; 6: 164166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Burgalassi S, Panichi L, Chetoni P, Saettone MF, Boldrini E.. Development of a simple dry eye model in the albino rabbit and evaluation of some tear substitutes. Ophthalmic Res. 1999; 31: 229–235. [DOI] [PubMed] [Google Scholar]
- 37. Sánchez-Ríos A, Correa-Gallegos EY, Medina-Espinoza JM, et al.. Validation of a preclinical dry eye model in New Zealand white rabbits during and following topical instillation of 1% ophthalmic atropine sulfate. Animal Model Exp Med. 2022; 5: 266–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yam JC, Li FF, Zhang X, et al.. Two-year clinical trial of the Low-Concentration Atropine for Myopia Progression (LAMP) Study. Ophthalmology. 2020; 127: 910–919. [DOI] [PubMed] [Google Scholar]
- 39. Yam JC, Zhang XJ, Zhang Y, et al.. Three-year clinical trial of Low-Concentration Atropine for Myopia Progression (LAMP) Study: continued versus washout: phase 3 report. Ophthalmology. 2022; 129: 308–321. [DOI] [PubMed] [Google Scholar]
- 40. Cho P, Yap M.. Age, gender, and tear break-up time. IJMS. 1993; 70(10): 828–831. [DOI] [PubMed] [Google Scholar]
- 41. Wang MTM, Muntz A, Mamidi B, Wolffsohn JS, Craig JP.. Modifiable lifestyle risk factors for dry eye disease. Contact Lens and Anterior Eye. 2021; 44(6): 101409. [DOI] [PubMed] [Google Scholar]
- 42. Klein BEK, Klein R.. Lifestyle exposures and eye diseases in adults. Am J Ophthalmol. 2007; 144: 961–969.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mandell JT, Idarraga M, Kumar N, Galor A.. Impact of air pollution and weather on dry eye. JCM. 2020; 9: 3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dermer H, Galor A, Hackam AS, Mirsaeidi M, Kumar N.. Impact of seasonal variation in meteorological conditions on dry eye severity. OPTH. 2018; 12: 2471–2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wolkoff P. Indoor air humidity, air quality, and health—an overview. Int J Hyg Environ Health. 2018; 221: 376–390. [DOI] [PubMed] [Google Scholar]