Abstract
Expression of many early viral genes during human cytomegalovirus (HCMV) infection is dependent on cellular transcription factors. Several immediate-early and early viral promoters contain DNA binding sites for cellular factors such as CREB, AP-1, serum response factor, and Elk-1, and these transcription factors can be activated by phosphorylation via the cellular mitogen-activated protein kinase (MAPK) signal transduction cascade. To determine if the extracellular signal-regulated MAPKs, ERK1 and ERK2, play a role in transcription factor activation during infection, we tested for ERK activity during viral infection. We found that HCMV infection resulted in the maintenance of previously activated ERK1 and ERK2 by a mechanism which appears to involve the inhibition of a cellular phosphatase activity. ERK phosphorylation and activity were sustained for at least 8 h after infection, whereas in mock-infected cells, ERK activity steadily declined by 1 h postinfection. The activity of at least one cellular substrate of the ERKs, the protein kinase RSK1, was also maintained during this period. UV inactivation experiments suggested that viral gene expression was required for sustained ERK activity. In turn, activation of the ERKs appeared to be important for viral gene expression, as evidenced by the observed decrease in the transcriptional activity of the HCMV UL112-113 promoter during infection in the presence of the MEK inhibitor PD98059. These data suggest that HCMV utilizes cellular signal transduction pathways to activate viral or cellular transcription factors involved in the control of early viral gene expression and DNA replication.
Human cytomegalovirus (HCMV), a member of the betaherpesvirus family, is an important opportunistic pathogen in immunocompromised individuals and is recognized as a major viral cause of birth defects (8). Like other herpesvirus genes, HCMV genes are expressed in a temporal pattern upon infection (14, 36, 59, 67, 68). The immediate-early (IE) class of genes are the first genes expressed after infection and do not require de novo protein synthesis, whereas the early class of RNAs depends on IE protein expression. The late genes require early protein production as well as viral DNA synthesis for their expression. Both IE gene expression and early gene expression require the host cell transcriptional machinery, including various host-encoded regulatory transcription factors. For example, the UL112-113 promoter is regulated by an ATF/CREB binding site (50, 54), and the promoter for the 1.2-kb class of RNAs is regulated by an AP-1 binding site (65). In addition, the major IE promoter (MIEP), which drives expression of the transcriptional regulatory proteins, IE86 and IE72, contains binding sites for several host transcription factors, including NF-κB, CREB, serum response factor (SRF), and the Ets family of transcription factors (12, 39).
Many of the host transcription factors involved in controlling HCMV gene expression are regulated by phosphorylation events. The prototypical mitogen-activated protein kinase (MAPK) pathway, the ERK (extracellular signal-regulated kinase) pathway, can regulate the activity of SRF/Ets, AP-1, and CREB (16, 21, 27, 46, 72, 73). The MAPK pathways are activated by extracellular signals and transmit the signals intracellularly to the nucleus (for recent reviews, see references 13, 23, 35, and 49). In the case of the ERK pathway, mitogen-receptor interaction leads to Ras-dependent, sequential activation of Raf, MEK (MAPK/ERK kinase), ERK and RSK (ribosomal S6 kinase). Activation of these kinases is transient, and cellular phosphatases begin to inactivate them within minutes to hours after stimulation, depending on the cell type and stimulus (23, 35). ERK1 and ERK2 can directly phosphorylate the AP-1 subunits Fos and Jun and the Ets family members Elk-1 and SAP1α (4, 24, 25, 34, 47). RSK1 can directly phosphorylate SRF, Fos, and Jun (4). In some cell types, CREB phosphorylation correlates with ERK activation, and all three RSK family members (RSK1, RSK2, and RSK3) are capable of phosphorylating CREB in vitro and in vivo at the regulatory site, Ser-133 (46, 72, 73). The ERK pathway has been implicated in the regulation of cell growth, and uncontrolled stimulation of the pathway leads to cellular transformation and oncogenesis (26, 37, 45, 52). Cellular proto-oncogenes such as c-fos, c-jun, and c-myc, which are up-regulated in response to mitogenic stimuli, are also stimulated by HCMV infection (5, 6, 18). Furthermore, 12-O-tetradecanoylphorbol-13-acetate (TPA), which stimulates the ERK pathway, has been shown to activate the HCMV MIEP (12).
The ERK pathway has been implicated in the regulation of viral gene expression for simian virus 40 (SV40), adenovirus, and hepatitis B virus. The SV40 small tumor antigen (small t) interacts with protein phosphatase 2A (PP2A) and alters its activity (for a review, see reference 43). Since PP2A can deactivate the ERKs, the small t-PP2A interaction leads to increased activation of the ERKs and, in turn, stimulates AP-1 activity in SV40-infected cells. ERK activity is stimulated upon infection with adenovirus, and the ERKs are capable of phosphorylating the adenovirus E1A protein (9, 70). This phosphorylation event is involved in the activation of the adenovirus E4 promoter. Furthermore, the human hepatitis B virus X protein induces ERK activity, which leads to increased AP-1 activity (3). Thus, several viruses have evolved various mechanisms to alter cellular signal transduction pathways which may ultimately be beneficial to viral replication.
To better understand the mechanisms that control early HCMV gene expression, we sought to determine the role of signal transduction pathways and phosphorylation events in regulating HCMV promoter activity. Since the ERK pathway can regulate the activity of several cellular transcription factors that are involved in HCMV early gene regulation, we asked if this pathway was involved in regulating viral promoter activity during infection. We find that if the ERK pathway is previously activated, HCMV infection results in a sustained activation of the ERKs through 8 h postinfection. Viral infection does not directly activate the ERK pathway but instead appears to result in inhibition of an ERK-specific phosphatase activity. Inhibition of the ERK pathway with the MEK inhibitor PD98059 results in decreased expression from the UL112-113 promoter. Taken together, these data suggest that the ERK pathway is involved in regulating HCMV early gene expression and that specific virus-host cell interactions alter the regulation of the pathway to benefit viral replication.
MATERIALS AND METHODS
Cells and virus.
Human foreskin fibroblasts (FFs) were maintained in minimum essential medium with Earle’s salts (MEM-Earle’s) containing 10% fetal bovine serum (FBS). HCMV Towne strain was obtained from the American Type Culture Collection. Methods for cell culture have been described elsewhere (61). All infections were performed with a multiplicity of infection (MOI) of 1 to 5. Cells were made quiescent by being grown to confluence and serum starved overnight in medium without serum. Where indicated, cells were stimulated with medium containing 10% FBS either 15 min before or at the time of infection with HCMV. Two hours after infection, cells were washed with phosphate-buffered saline (PBS) and incubated in medium without serum unless otherwise indicated. UV-inactivated virus was prepared as described previously (28).
For infections in the absence of serum, virus was first pelleted at 25,000 rpm in an SW27 rotor (Beckman) for 2 h at 4°C. The pellet was then gently washed in serum-free medium, resuspended in serum-free medium, and pelleted as described above for 1 h. The virus pellet was resuspended in serum-free medium, dimethyl sulfoxide (DMSO) was added to 1%, and the virus was stored at −80°C.
For infections in the presence of PD98059 (New England Biolabs), cells were pretreated with drug for 1 h prior to infection. Cells were then stimulated by dropwise addition of FBS and infected by dropwise addition of virus. Cells were maintained in PD98059 for the duration of the experiment. PD98059 was solubilized in DMSO, and all dilutions were made such that each dish of cells received an equal volume of DMSO.
For quantitation of UL112-113 promoter activity in the presence of PD98059, cells were infected with recombinant HCMV v358-CAT (50) at an MOI of 1. Cell lysates were analyzed for chloramphenical acetyltransferase (CAT) activity 8 h after infection as described previously (58). CAT levels were quantitated by phosphorimager analysis.
Western blot analysis.
To detect ERK phosphorylation by Western blotting mock- or virus-infected FFs were harvested at the indicated times by lysis directly on the plate in Laemmli sample buffer, and lysates were electrophoresed on 12.5% low-cross-linking polyacrylamide protein gels (32). After transfer to an Immobilon membrane (Millipore) and blocking in 5% milk, membranes were incubated with an antibody directed against ERK1 (sc-093; Santa Cruz Biotechnology), followed by incubation with a horseradish peroxidase (HRP)-coupled secondary antibody (Amersham) and detection with enhanced chemiluminescence (Pierce) according to standard methods. Polyclonal antiserum BSA 2-9 (71) was used to detect UL112-113 proteins, and IE proteins were detected by using the monoclonal antibody CH16.0 (a gift from L. Pereira).
To verify immunoprecipitated protein levels in immune complex kinase assays, immunoprecipitated kinases were electrophoresed on standard protein gels, transferred to Immobilon membranes, and blocked as described above. After incubation with primary antibody, membranes were incubated with a protein A/G-HRP conjugate (Pierce) and proteins were detected by chemiluminescence.
Immune complex kinase assays.
At the indicated time points, mock- or virus-infected cells were washed with PBS and lysed directly on the plate in radioimmunoprecipitation assay (RIPA) buffer (50 mM Tris-Cl [pH 8.0] 150 mM NaCl, 1.0% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate). For ERK assays, lysates were incubated with an agarose-conjugated antibody directed against ERK2 (sc-154 AC; Santa Cruz Biotechnology) for 2 to 3 h at 4°C. Immunoprecipitates were washed three times in RIPA buffer and twice in assay buffer (25 mM Tris-Cl [pH 7.4], 5 mM β-glycerophosphate, 2 mM dithiothreitol, 0.1 mM Na3VO4, 10 mM MgCl2, 1 mM phenylmethylsulfonyl fluoride). Reactions were initiated by resuspending immunoprecipitates in 30 μl of assay buffer containing 20 μM unlabeled ATP, 15 μCi of [γ-32P]ATP, and 7.5 μg of myelin basic protein (MBP; Sigma). Reaction mixtures were incubated at 30°C for 15 min, reactions were terminated by the addition of 12 μl of 4× Laemmli sample buffer, and reaction products were electrophoresed on a 15% polyacrylamide protein gel and transferred to an Immobilon membrane, which was then cut at the 30-kDa molecular mass marker. The bottom portion was exposed to film and quantitated by phosphorimager analysis; the top portion was subjected to Western blot analysis (as described above) to compare levels of immunoprecipitated proteins.
RSK1 assays were performed with an S6 kinase assay kit (Upstate Biotechnology). Lysates were incubated with antibody directed against RSK1 (sc-231; Santa Cruz Biotechnology) for 2 to 3 h at 4°C. Protein A-Sepharose beads were added, and incubation was allowed to continue for 1 h. Immunoprecipitates were washed three times in RIPA buffer, washed twice in S6 assay buffer, and resuspended in 40 μl of S6 assay buffer; 10 μl was used in a total reaction volume of 40 μl containing 10 μCi of [γ-32P]ATP and an S6 kinase recognition site peptide as specified by the manufacturer’s protocol. After 10 min at 30°C, reactions were terminated by spotting 20 μl onto P81 filter paper. Filters were washed five to six times in 0.75% phosphoric acid and once in acetone, and radioactivity incorporated into bound peptide was quantitated in a scintillation counter.
32P pulse-chase analysis.
Serum-starved FFs (1.3 × 106/100-mm-diameter dish) were pulsed for 45 min in 3 ml of phosphate-free, serum-free Dulbecco’s modified Eagle’s medium containing 1.0 mCi of [32P]orthophosphate per ml. Cells were then stimulated by the addition of 300 μl of FBS. After a 15-min incubation, the cells were washed in MEM-Earle’s containing 10% FBS and then mock or virus infected. At 2 h postinfection, the cells were washed with serum-free MEM-Earle’s, and further incubation was performed in serum-free medium. Cells were harvested at the indicated time points by washing with PBS and lysing in 1 ml of RIPA buffer directly on the plate. ERK2 was immunoprecipitated from the lysates by incubation with an agarose-conjugated antibody directed against ERK2, whereas MEK1 was immunoprecipitated by incubation with antibody directed against MEK1 (sc-219; Santa Cruz Biotechnology) followed by incubation with protein A-Sepharose beads. Immunoprecipitates were washed five times in RIPA buffer, resuspended in Laemmli sample buffer, and electrophoresed on 10% polyacrylamide protein gels. 32P-labeled proteins were detected by autoradiography and quantitated by phosphorimager analysis.
RESULTS
ERK phosphorylation and activity during HCMV infection.
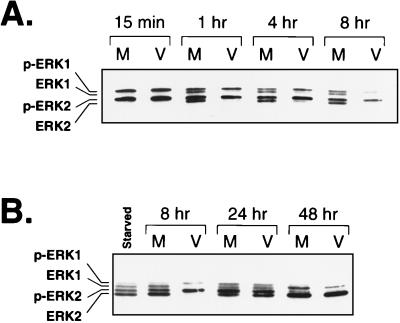
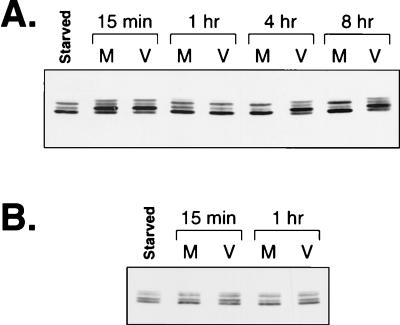
Many IE and early HCMV genes are activated by cellular transcription factors such as CREB, AP-1, NF-κB, and SRF/Ets (12, 50, 53, 54, 65). Since the activity of each of these transcription factors is regulated by various signal transduction pathways within the cell, we sought to determine whether cell signaling pathways were involved in regulating HCMV gene expression. We focused on the ERK (Raf-MEK-ERK) pathway since this pathway has been shown to regulate the activity of SRF/Ets, AP-1, and CREB (16, 21, 27, 46, 72, 73). To determine if the ERK pathway was involved in HCMV gene regulation, we first asked if this pathway was active during infection. Since ERK activity correlates with its phosphorylation on regulatory Thr and Tyr residues (48), we checked for ERK phosphorylation during HCMV infection by using a gel system capable of resolving phosphorylated ERK from nonphosphorylated ERK. Serum stimulation of cells followed by mock or virus infection resulted in complete phosphorylation of ERK1 and ERK2 by 15 min postinfection (Fig. 1A). At 1, 4, and 8 h after mock infection, about 50% of ERK1 and ERK2 was converted to the nonphosphorylated form. However, in the virus-infected samples, nearly 100% of the ERKs remained in the phosphorylated form at these time points. This effect was observed only at early times during the infection, since by 24 or 48 h postinfection the ERKs were dephosphorylated to similar extents in the virus- and mock-infected samples (Fig. 1B). These data suggest that HCMV infection results in the sustained phosphorylation of the ERKs early during infection.
FIG. 1.
Western blot analysis of phosphorylated ERK levels during HCMV infection. (A) ERK levels early during infection. Total protein from either mock (M)- or virus (V)-infected cells was harvested at the indicated times, separated on 12.5% low-cross-linking polyacrylamide gels, and transferred to Immobilon. Phosphorylated ERK (p-ERK1 and p-ERK2) and nonphosphorylated ERK (ERK1 and ERK2) were detected with antibody directed against ERK1. Time postinfection is indicated above the blot. (B) ERK levels late during infection. Total protein from mock- or virus-infected cells was harvested at the indicated times postinfection and analyzed by Western blotting as described above. “Starved” indicates a lysate from cells that were grown to confluence and serum starved for 24 h. Time postinfection is indicated above the blot.
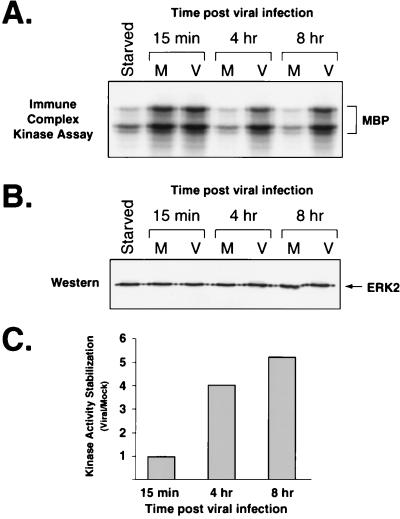
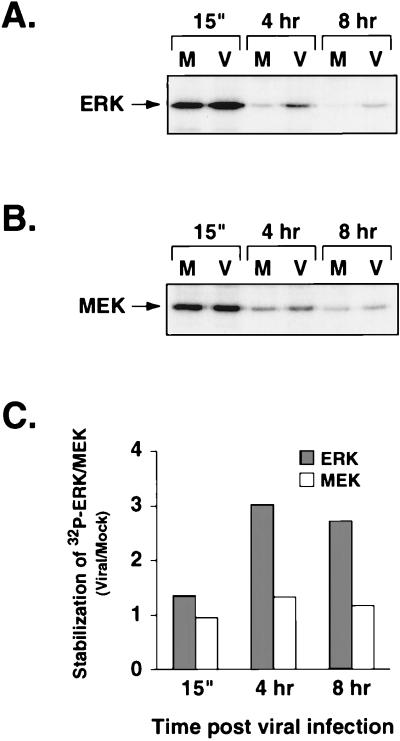
To determine if the ERKs were functionally active during the infection, we assayed for ERK activity at various time points after infection by using an immune complex kinase assay. After serum stimulation and mock or virus infection, ERK2 was immunoprecipitated from cell lysates prepared at 15 min, 4 h, and 8 h postinfection. The immunoprecipitates were incubated with [γ-32P]ATP and MBP as a substrate, and the reactions were resolved by polyacrylamide gel electrophoresis. At 15 min after infection, the mock- and virus-infected samples showed similar ERK activities, as demonstrated by equivalent levels of 32P-labeled MBP (Fig. 2A). However, by 4 h ERK activity had declined in the mock-infected samples, whereas it remained high in the virus-infected samples at least through 8 h after infection. Since, Western blot analysis demonstrated that equivalent levels of ERK were immunoprecipitated from each sample, the reduced level of phosphorylated MBP was due to decreased ERK activity rather than decreased ERK protein levels (Fig. 2B). Quantitation of the 32P incorporated into MBP in each reaction revealed that there was four- to fivefold more ERK activity in the virus-infected samples than in the mock-infected samples harvested at 4 and 8 h postinfection (Fig. 2C). These data demonstrate that ERK activity is also sustained early during HCMV infection.
FIG. 2.
ERK activity during HCMV infection, determined by an immune complex kinase assay. ERK was immunoprecipitated from starved cells (Starved) or from lysates at the indicated times after mock (M) or viral (V) infection. After incubation of immunoprecipitated ERK with [γ-32P]ATP and MBP, the reaction mixtures were run on a 15% polyacrylamide gel and the products were transferred to Immobilon. (A) Autoradiography of 32P-labeled MBP. The bottom portion of the blot (below 30 kDa) was exposed to film to detect 32P-labeled MBP. The bracket indicates MBP and breakdown products. (B) Western blot analysis of immunoprecipitated ERK. The top portion of the blot was treated with antibody to ERK1 followed by protein A/G-HRP secondary antibody (Pierce) and detection by chemiluminesence. The antibody to ERK2 (C-14) preferentially precipitates ERK2, which can be detected with the antibody to ERK1 (C-16). Immunoprecipitated ERK1 can be detected on longer exposures. (C) Quantitation of ERK activity in mock- and virus-infected cells. Kinase activity stabilization is a measure of ERK activity in virus-infected cells versus mock-infected cells at a specific time after infection. It is defined as the amount of radioactivity incorporated into MBP in an immune complex kinase assay from virus-infected samples divided by that in mock-infected samples. The values were determined by phosphorimager analysis of the blot from panel A.
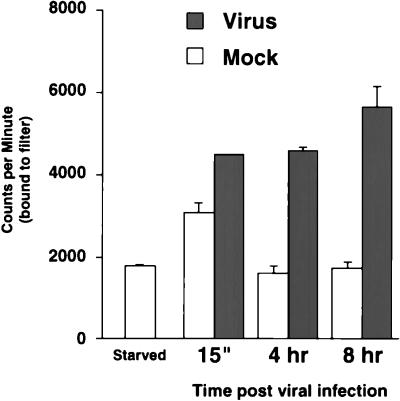
As an indication of whether the active ERKs were functioning normally in the infected cells, we assayed for the activity of RSK1, a normal cellular substrate of the ERKs. An immune complex kinase assay was performed with an S6 peptide substrate and RSK1 immunoprecipitated from lysates prepared at 15 min, 4 h, and 8 h after virus and mock infection. Quantitation of 32P-labeled peptide demonstrated that there was more active RSK1 in the virus-infected samples at 4 and 8 h postinfection than in the mock-infected samples (Fig. 3). Thus, RSK1 activity correlated with ERK activity at each time point tested, indicating that the ERKs were functioning normally early during HCMV infection. Collectively, these data suggest that the ERKs are active early after infection at a time when they could contribute to the regulation of transcription factors involved in early HCMV gene expression.
FIG. 3.
RSK1 activity during HCMV infection. RSK1 activity was determined by an immune complex kinase assay. RSK1 was immunoprecipitated from serum-starved cell lysates and mock- or virus-infected cell lysates at the indicated times postinfection. RSK1 activity was detected by using an S6 kinase assay kit (Upstate Biotechnology) and [γ-32P]ATP. Bars represent the average of two independent assays depicted as counts per minute of 32P-labeled peptide bound to filters as quantitated by scintillation counting; error bars represent half of the range of the two values.
ERK activity during infection with UV-inactivated virus.
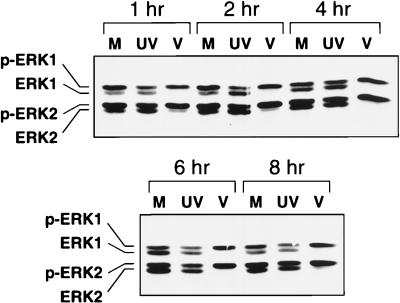
Since specific ligand-receptor interactions result in activation of the ERK pathway, we asked whether virus binding to the cell surface was sufficient to result in the sustained activation of the ERKs early during HCMV infection. To address this question, we used UV-inactivated virus, which can bind to and enter the cells but fails to undergo viral gene expression (22). At each time point after inoculation with UV-inactivated virus, the ERKs were only about 50% phosphorylated, which was similar to what was observed with mock-infected samples (Fig. 4). However, in non-UV-treated virus-infected samples, the ERKs remained 100% phosphorylated. Thus, sustained ERK activity during infection was not due simply to virus binding to the cell surface. Furthermore, since viral gene expression does not occur in cells inoculated with UV-inactivated virus, these results suggest that a newly synthesized viral gene product is involved in the maintenance of ERK activity early after HCMV infection.
FIG. 4.
Western blot analysis of phosphorylated ERK levels during inoculation with UV-inactivated HCMV. FFs were inoculated with UV-inactivated HCMV (UV), mock infected (M), or HCMV infected (V). Cell lysates were harvested at the indicated times after infection, and Western blot analysis was performed as described in the legend to Fig. 1.
Inhibition of a cellular phosphatase activity during infection.
In the previously described experiments, conditions were such that the ERKs were completely active at the time of infection. To determine if HCMV infection resulted in the activation of a kinase which could directly or indirectly lead to ERK activation, we infected cells with virus in the absence of serum. Cells were grown to confluence and serum starved for 18 to 24 h in an attempt to infect the cells when the ERKs were inactive. This treatment resulted in substantial but not complete inactivation of ERK activity. At 15 min and 1 h after infection of starved cells, there were low levels of phosphorylated ERK which were identical between mock- and virus-infected samples (Fig. 5A), suggesting that the events occurring in the initial stages of the infection did not directly activate the ERKs. The low level of ERK activation at 15 min was probably due to the presence of DMSO in the inoculum since in a separate serum starvation experiment, in which DMSO was not present, there was no ERK activation at the early time points (Fig. 5B). At 4 and 8 h after infection, more phosphorylated ERK was detected in virus-infected samples than in mock-infected samples. Although this result may be due to direct activation of the ERK pathway or other effects from serum starvation, it could also be obtained by an inhibition of cellular phosphatase activity coupled with low-level activity of the ERK pathway. Indeed, elimination of phosphatase activity eventually results in the accumulation of activated ERKs (20, 43, 60).
FIG. 5.
Western blot analysis of phosphorylated ERK levels during HCMV infection of serum-starved cells. (A) FFs were grown to confluence, starved in medium without serum for 24 h, and then infected with virus which had been pelleted and resuspended in medium without serum. Mock infections were performed with serum-free medium containing equivalent concentrations of DMSO as virus-infected samples. Lysates were prepared at the indicated time points and subjected to Western blot analysis as described in the legend to Fig. 1. (B) Serum-starved FFs were mock or virus infected in the absence of DMSO. Western blot analysis was performed as described above.
To determine if the sustained ERK activation observed during HCMV infection was due to inhibition of phosphatase activity, we performed a pulse-chase analysis to monitor phosphorylated ERK during the infection. Cells were pulsed with [32P]orthophosphate for 1 h prior to infection. After serum stimulation to incorporate 32P into the ERKs, the labeling medium was removed and the cells were infected. Cells were harvested at 15 min, 4 h, and 8 h postinfection, the ERKs were immunoprecipitated and electrophoresed on a polyacrylamide gel, and 32P-labeled ERK was detected by autoradiography (Fig. 6A). After a 4- or 8-h chase period, we observed more 32P-labeled ERK in the virus-infected cells than in the mock-infected cells, suggesting that phosphatase activity was compromised during infection. To determine if the dephosphorylation of other members of the ERK pathway was affected, we also assayed for phosphorylation of MEK1, the kinase that phosphorylates and activates ERK. Immunoprecipitation of MEK1 from the same 32P-labeled lysates as used in the assay described above demonstrated that MEK1 dephosphorylation in virus-infected cells occurred at a similar rate as in mock-infected cells (Fig. 6B). Quantitation of the immunoprecipitates by phosphorimager analysis revealed that there was about three- to fourfold more 32P-labeled ERK in the virus-infected cells than in the mock-infected cells at 4 or 8 h after infection, whereas at each time point, the level of phosphorylated MEK was the same in mock- and virus-infected samples (Fig. 6C). Taken together, these results suggest that an ERK-specific phosphatase may be preferentially inhibited during HCMV infection.
FIG. 6.
32P pulse-chase analysis of phosphorylated ERK and MEK. (A) Maintenance of phosphorylated ERK during infection. ERK2 was immunoprecipitated from 32P-labeled, mock (M)- or virus (V)-infected cell lysates after the indicated chase periods and resolved on a 10% polyacrylamide gel. 32P-labeled ERK2 was detected by autoradiography. (B) Phosphorylated levels of MEK during infection. MEK1 was immunoprecipitated from 32P-labeled, mock (M)- or virus (V)-infected cell lysates after the indicated chase periods and resolved on a 10% polyacrylamide gel. 32P-labeled MEK1 was detected by autoradiography. (C) Quantitation of 32P-labeled ERK and MEK during infection. Stabilization of 32P-labeled ERK and MEK is a measure of phosphorylated ERK and MEK remaining in virus-infected samples versus mock-infected samples after the indicated chase times. It is defined as the amount of 32P-labeled ERK or MEK in virus-infected samples divided by that in mock-infected samples. Quantitation of the gels in panels A and B was performed by phosphorimager analysis.
Role of the ERK pathway during early HCMV gene expression.
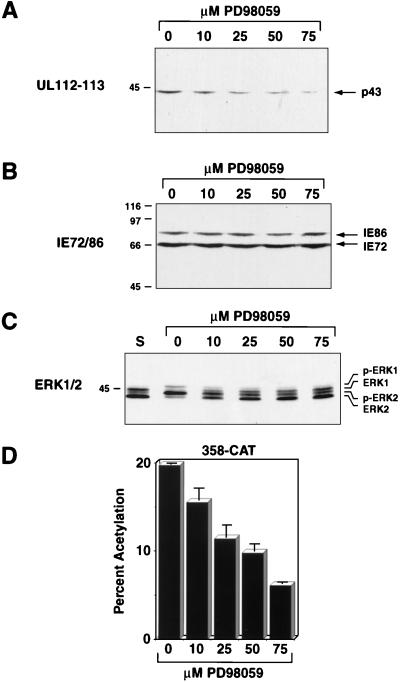
To determine if the ERK pathway is involved in regulating viral gene expression, we analyzed steady-state viral protein levels in HCMV-infected cells treated with the specific MEK inhibitor PD98059 (1, 15, 33, 44). Serum-starved FFs were pretreated for 1 h with 0, 10, 25, 50, or 75 μM PD98059, serum stimulated, and infected with HCMV at an MOI of 1. Steady-state viral protein levels were determined 8 h postinfection by Western blot analysis. The levels of the 43-kDa UL112-113 protein were lower after infection in the presence of 75 μM PD98059 than after infection in the absence of drug, suggesting that early viral gene expression was affected by specific inhibition of the ERK pathway (Fig. 7A). This concentration of drug did not inhibit overall protein synthesis, as determined by [35S]methionine labeling after infection (data not shown). A stepwise increase in the concentration of drug from 10 to 75 μM resulted in a sequential decrease in UL112-113 protein levels. However, this drug had little if any effect on the steady-state levels of IE72 and IE86, indicating that expression from the MIEP was less affected by inhibition of ERK activity (Fig. 7B). Increasing concentrations of PD98059 also resulted in decreased maintenance of phosphorylated ERKs, suggesting that decreased UL112-113 protein levels correlated with decreased ERK activity (Fig. 7C).
FIG. 7.
Effect of inhibition of the ERK pathway on early viral gene expression. (A) Western blot analysis of UL112-113 protein levels during wild-type HCMV infection in the presence of PD98059. FFs were grown to confluence and serum starved for 24 h. After a 1-h pretreatment with the indicated concentrations of PD98059, the cells were serum stimulated and infected. Cell lysates were prepared 8 h after infection and subjected to Western blot analysis. The UL112-113 43-kDa protein (indicated on the right) was detected by using antibody BSA 2-9 followed by HRP-conjugated secondary antibody and chemiluminescence. (B) Western blot analysis of IE86 and IE72 protein levels during wild-type HCMV infection in the presence of PD98059. The blot from panel A was stripped and probed with antibody CH16.0 followed by HRP-conjugated secondary antibody and detection by chemiluminescence. IE86 and IE72 are indicated by the arrows on the right. (C) Western blot analysis of phosphorylated ERK levels during wild-type HCMV infection in the presence of PD98059. Lysates from the experiment described for panel A were electrophoresed on 12.5% low-cross-linking polyacrylamide gels and subjected to Western blotting as described in the legend to Fig. 1A. Phosphorylated (p-ERK1 and p-ERK2) and nonphosphorylated forms of ERK1 and ERK2 are indicated on the right. (D) Analysis of CAT protein expression from the UL112-113 promoter during infection with v358-CAT in the presence of PD98059. FFs were grown to confluence and serum starved for 24 h. After a 1-h pretreatment with the indicated concentrations of PD98059, the cells were serum stimulated and infected with the HCMV recombinant v358-CAT. Lysates were prepared 8 h postinfection and assayed for CAT activity. Solid bars represent the average of two independent infections; error bars represent half of the range of the two values.
To determine if the lower level of UL112-113 protein in the presence of PD98059 was a result of decreased transcription, UL112-113 promoter activity was quantitated by using the recombinant virus v358-CAT, which contains the CAT gene under control of the UL112-113 promoter (50). Infection with v358-CAT in the presence of 75 μM PD98059 resulted in a threefold decrease in promoter activity when assayed 8 h after infection compared to infection in the absence of drug (Fig. 7D). Taken together, these data suggest that there is a correlation between the level of ERK activity and UL112-113 gene expression and that the ERKs are involved in regulating early viral gene expression.
DISCUSSION
Since CREB and AP-1 can both be activated by the ERK pathway and both are involved in regulating early viral promoter activity (16, 46, 50, 54, 65, 72, 73), we have focused on the role of the ERK pathway in the regulation of early HCMV gene expression. The ERK pathway can also regulate the activity of SRF and the Ets family of transcription factors, which can potentially regulate the activity of the HCMV MIEP (12). Since IE gene expression is required for viral replication, signal transduction pathways may be involved in ensuring a productive viral infection or reactivation from the latent state.
ERK activity during HCMV infection.
We have demonstrated here that regulation of the ERK pathway is altered during HCMV infection. Normally, in response to extracellular signals, the ERKs are stimulated by sequential activation of a series of protein kinases and then rapidly attenuated by cellular phosphatases. However, our data suggest that phosphatase activity is compromised during the infection, resulting in the sustained phosphorylation and activation of the ERKs. The maintenance of ERK activity results in the sustained activation of at least one ERK substrate, RSK1, demonstrating that the ERKs are functioning normally in infected cells. Therefore, it is possible that other ERK substrates, such as transcription factors, also remain in the active form longer in infected cells. This sustained transcription factor activity presumably would lead to higher levels of viral gene expression, which would be beneficial for enhancing viral replication.
Our results suggest that one or more viral gene products are involved in inhibiting an ERK-specific phosphatase activity during infection. First, since little if any viral gene expression occurs in cells inoculated with UV-inactivated virus, we have demonstrated that viral gene expression is required to sustain ERK activity through 8 h postinfection. The observation that the sustained ERK activity can be detected as early as 1 h postinfection suggests that an IE function may be involved. Therefore, it is possible that the virus-encoded transcriptional regulatory proteins, IE86 and IE72, are involved in phosphatase inhibition, but we cannot rule out the involvement of other early viral functions. One example of a viral protein affecting phosphatase activity is demonstrated by the inhibition of PP2A activity by SV40 small t (43). This interaction results in the activation of the ERKs and of the AP-1 transcription factor.
The evidence for phosphatase inhibition is provided by the 32P pulse-chase experiment. In this experiment, the 32P was incorporated into the ERKs before infection. Since more 32P remained incorporated into the ERKs after the 8-h chase period in infected cells than in mock-infected cells, it seems likely that phosphatase activity was compromised. An alternative explanation is that ERK protein half-life is increased during infection, resulting in less turnover of phosphorylated ERK. However, 35S pulse-chase experiments reveal no difference in ERK turnover rates between mock- and virus-infected cells (51).
Current evidence suggests that the ERKs can be dephosphorylated by at least PP2A, MKP-1, MKP-2, MKP-3, MKP-4, and PAC1 (17, 23, 38, 40–42, 60, 66). PP2A is a cytoplasmic phosphatase which can also act on the MEKs (23), whereas MKP-1, MKP-2, MKP-4, and PAC1 are dual-specificity phosphatases which reside in the nucleus and show some specificity for the ERKs. Of particular interest is MKP-3, a dual-specificity phosphatase that is located in the cytoplasm and is highly selective for inactivation of the ERKs (17, 40, 42). It has also recently been shown that MKP-3 is activated by direct binding to ERK2 (11). Our result that MEK1 dephosphorylation occurs normally in infected cells suggests that inhibition of PP2A is probably not responsible for the observed effects. However, at this point, we do not know which, if any, of the above-mentioned dual-specificity phosphatases might be affected by HCMV infection.
There is evidence that receptor-mediated signaling cascades may be initiated by contact between the virus and the host cell. For example, the interaction of human immunodeficiency virus type 1 envelope glycoproteins with cell surface CD4 causes signaling events resulting in the activation of the ERK pathway (7, 31). To rule out virus-receptor interaction as the cause of sustained ERK activity during HCMV infection, we infected cells with UV-inactivated virus, which can bind to and enter cells but will not express its genes. Since our results show that treatment of cells with UV-inactivated virus does not result in sustained ERK activity, we believe that receptor-mediated signaling is not responsible for the sustained ERK activity in HCMV-infected cells. Moreover, in most of our experiments serum was removed 2 h after infection. Since we still observed high levels of ERK activity up to 8 h postinfection in the absence of stimuli, it is unlikely that the ERK activity at 8 h is due to recent stimulation of receptors. The more likely explanation is that previously activated ERK remains in its active form throughout the first 8 h of the infection.
Role of the ERK pathway in HCMV gene expression.
To determine if the sustained ERK activity played a role in early viral gene expression, we focused on the effect of active ERK on the HCMV UL112-113 promoter. This promoter is predominantly regulated by a CREB site early in infection, although IE86 is also required for promoter activity (2, 30, 50, 54, 55). We used the specific MEK inhibitor PD98059 to ascertain viral promoter activity when the ERKs were inactive. Our results show that as the concentration of PD98059 was increased, UL112-113 promoter activity decreased. We observed a decrease in the steady-state UL112-113 protein levels at 8 h after infection with wild-type virus, as well as lower CAT activity from the UL112-113 promoter during infection with the HCMV recombinant v358-CAT. The latter result demonstrates that the inhibition was at the transcriptional level. At the concentrations used, PD98059 did not affect overall protein synthesis (data not shown). PD98059 also did not have a major effect on the MIEP, suggesting that the drug is not a general inhibitor of transcription and does not affect viral entry or uncoating. Although the MIEP contains an SRF/Ets site, the presence of several other transcription factor binding sites within the promoter and enhancer may overcome the loss of ERK-stimulated SRF/Ets activity. Further support for this possibility comes from a recent study which indicates that the MIEP can function normally in the absence of the SRF/Ets sites (10). Since the levels of ERK phosphorylation and UL112-113 promoter activity both decrease with increasing concentration of PD98059, there is a correlation between ERK activity and transcriptional regulation of at least one viral promoter.
At this point, it is not clear how the ERKs are involved in regulating UL112-113 activity. Inhibition of ERK activity with PD98059 could mean that CREB phosphorylation and activity is reduced. We have shown that a CREB binding site is important for high levels of UL112-113 promoter activity early during infection (50). However, Xing et al. have shown that nearly complete inhibition of ERK activity with PD98059 reduces but does not eliminate CREB phosphorylation, implicating other signaling pathways that are not affected by PD98059 as contributing to CREB phosphorylation (73). This most likely explains why UL112-113 activity is reduced rather than eliminated in our experiments. Alternatively, our preliminary experiments have demonstrated that ERK can phosphorylate IE86 and IE72 in vitro (51), suggesting that ERK may regulate the activity of these viral proteins during infection, in turn affecting those promoters responsive to IE86 and IE72. In this regard, we have also noted that PD98059 inhibits the activity of the promoter for the 1.2-kb class of RNAs, which is responsive to AP-1 as well as IE86 (51). After this report was submitted for publication, Harel and Alwine (19) published results demonstrating that ERK2 phosphorylates several domains of IE86 in vitro. In addition, they showed that substitution of alanines for specific serines or threonines found in ERK consensus sites within IE86 prevented ERK2-specific phosphorylation of those motifs in vitro and in vivo.
Although we have not yet investigated in depth the overall effects of ERK inhibition on viral replication, we do note that at 8 h postinfection in the presence of drug, some cytopathic effects are evident and viral gene expression is occurring, albeit at reduced levels. Thus, inhibition of ERK activity may delay the infection only until sufficient levels of early gene products are produced. Complete ERK inhibition was not achieved at the concentrations of drug used in this study, as demonstrated by the low levels of ERK phosphorylation at 8 h postinfection in the presence of 75 μM PD98059. A more complete ERK inhibition, preferably without the use of drugs, would be beneficial in determining the role of the ERK pathway in HCMV gene regulation.
Sustained ERK activity may also affect cellular gene expression in the initial phases of HCMV infection. The mRNA levels of the proto-oncogenes c-myc, c-jun, and c-fos as well as the mRNA for the p105/p50 and p65 subunits of NF-κB are all induced after HCMV infection (2, 29, 30, 50, 54, 55, 74). In addition, c-fos promoter activity is regulated by the SRF/Elk-1 complex, which is activated by the ERK pathway (for a review, see reference 64). Thus, keeping the ERKs active during infection may lead to higher levels of cellular transcription factors such as AP-1, NF-κB, and Myc, resulting in enhanced activation of both viral and cellular promoters.
Another aspect of HCMV biology that may be regulated by the ERKs is reactivation from latency. In healthy HCMV carriers, monocytes have been identified as one site of persistence of the HCMV genome (62). It has been reported that monocytes of healthy seropositive individuals do not produce viral RNAs and that in culture, the monocyte cell line THP-1 is nonpermissive for viral infection (57, 63, 69). However, THP-1 cells become permissive for HCMV infection upon differentiation by treatment with TPA (69). Furthermore, human teratocarcinoma cells, which are normally nonpermissive for HCMV infection, become permissive when differentiation is induced by expression of oncogenic ras (56). Since, TPA and oncogenic ras expression also activate the ERK pathway, these data suggest that reactivation from latency in response to cellular differentiation may be linked to activation of the ERK pathway.
The results presented here indicate that the ERK pathway, and possibly other signal transduction pathways, plays a role in the regulation of viral gene expression and initiating a productive viral infection. It would be advantageous for the virus to maintain activity of specific regulatory kinases, such as ERK1 and ERK2, to ensure that viral and cellular transcription factors involved in stimulating early viral genes remain in their active form. In addition, signal transduction pathways may be an important factor involved in reactivation of HCMV from latency. Understanding how the ERK pathway is involved in the initial stages of a productive viral infection, during either primary infection or reactivation from latency, may prove to be beneficial in preventing HCMV disease in immunocompromised hosts.
ACKNOWLEDGMENTS
We thank Charles Clark, Roopashree Dwarakanath, Elizabeth Fortunato, Anita McElroy, Chris Morello, and Bryan Salvant for helpful discussions and critical reviews of the manuscript. We also thank Michael David and John Blenis for helpful discussions.
This investigation was supported by NIH grant CA 34729 (D.H.S.) and NIH AIDS training grant AI-07384 (S.M.R.).
REFERENCES
- 1.Alessi D R, Cuenda A, Cohen P, Dudley D T, Saltiel A R. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem. 1995;270:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- 2.Arlt H, Lang D, Gebert S, Stamminger T. Identification of binding sites for the 86-kilodalton IE2 protein of human cytomegalovirus within an IE2-responsive viral early promoter. J Virol. 1994;68:4117–4125. doi: 10.1128/jvi.68.7.4117-4125.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benn J, Su F, Doria M, Schneider R J. Hepatitis B virus HBx protein induces transcription factor AP-1 by activation of extracellular signal-regulated and c-Jun N-terminal mitogen-activated protein kinases. J Virol. 1996;70:4978–4985. doi: 10.1128/jvi.70.8.4978-4985.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blenis J. Signal transduction via the MAP kinases: proceed at your own RSK. Proc Natl Acad Sci USA. 1993;90:5889–5892. doi: 10.1073/pnas.90.13.5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boldogh I, AbuBakar S, Albrecht T. Activation of proto-oncogenes: an immediate early event in human cytomegalovirus infection. Science. 1990;247:561–564. doi: 10.1126/science.1689075. [DOI] [PubMed] [Google Scholar]
- 6.Boldogh I, AbuBakar S, Deng C Z, Albrecht T. Transcriptional activation of cellular oncogenes fos, jun, and myc by human cytomegalovirus. J Virol. 1991;65:1568–1571. doi: 10.1128/jvi.65.3.1568-1571.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Briant L, Robert-Hebmann V, Sivan V, Brunet A, Pouyssegur J, Devaux C. Involvement of extracellular signal-regulated kinase module in HIV-mediated CD4 signals controlling activation of nuclear factor-kappa B and AP-1 transcription factors. J Immunol. 1998;160:1875–1885. [PubMed] [Google Scholar]
- 8.Britt W, Alford C. Cytomegalovirus. In: Fields B N, et al., editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 2493–2523. [Google Scholar]
- 9.Bruder J T, Kovesdi I. Adenovirus infection stimulates the Raf/MAPK signaling pathway and induces interleukin-8 expression. J Virol. 1997;71:398–404. doi: 10.1128/jvi.71.1.398-404.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cahill M A, Nordheim A, Janknecht R. Co-occurrence of CArG boxes and TCF sites within viral genomes. Biochem Biophys Res Commun. 1994;205:545–551. doi: 10.1006/bbrc.1994.2699. [DOI] [PubMed] [Google Scholar]
- 11.Camps M, Nichols A, Gillieron C, Antonsson B, Muda M, Chabert C, Boschert U, Arkinstall S. Catalytic activation of the phosphatase MKP-3 by ERK2 mitogen-activated protein kinase. Science. 1998;280:1262–1265. doi: 10.1126/science.280.5367.1262. [DOI] [PubMed] [Google Scholar]
- 12.Chan Y J, Chiou C J, Huang Q, Hayward G S. Synergistic interactions between overlapping binding sites for the serum response factor and ELK-1 proteins mediate both basal enhancement and phorbol ester responsiveness of primate cytomegalovirus major immediate-early promoters in monocyte and T-lymphocyte cell types. J Virol. 1996;70:8590–8605. doi: 10.1128/jvi.70.12.8590-8605.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cobb M H, Hepler J E, Cheng M, Robbins D. The mitogen-activated protein kinases, ERK1 and ERK2. Semin Cancer Biol. 1994;5:261–268. [PubMed] [Google Scholar]
- 14.DeMarchi J M. Human cytomegalovirus DNA: restriction enzyme cleavage maps and map locations for immediate- early, early, and late RNAs. Virology. 1981;114:23–28. doi: 10.1016/0042-6822(81)90249-x. [DOI] [PubMed] [Google Scholar]
- 15.Dudley D T, Pang L, Decker S J, Bridges A J, Saltiel A R. A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc Natl Acad Sci USA. 1995;92:7686–7689. doi: 10.1073/pnas.92.17.7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frost J A, Geppert T D, Cobb M H, Feramisco J R. A requirement for extracellular signal-regulated kinase (ERK) function in the activation of AP-1 by Ha-Ras, phorbol 12-myristate 13-acetate, and serum. Proc Natl Acad Sci USA. 1994;91:3844–3848. doi: 10.1073/pnas.91.9.3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Groom L A, Sneddon A A, Alessi D R, Dowd S, Keyse S M. Differential regulation of the MAP, SAP and RK/p38 kinases by Pyst1, a novel cytosolic dual-specificity phosphatase. EMBO J. 1996;15:3621–3632. [PMC free article] [PubMed] [Google Scholar]
- 18.Hagemeier C, Walker S M, Sissons P J G, Sinclair J H. The 72K IE1 and 80K IE2 proteins of human cytomegalovirus independently trans-activate the c-fos, c-myc and hsp70 promoters via basal promoter elements. J Gen Virol. 1992;73:2385–2393. doi: 10.1099/0022-1317-73-9-2385. [DOI] [PubMed] [Google Scholar]
- 19.Harel N Y, Alwine J C. Phosphorylation of the human cytomegalovirus 86-kilodalton immediate-early protein IE2. J Virol. 1998;72:5481–5492. doi: 10.1128/jvi.72.7.5481-5492.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haystead T A, Weiel J E, Litchfield D W, Tsukitani Y, Fischer E H, Krebs E G. Okadaic acid mimics the action of insulin in stimulating protein kinase activity in isolated adipocytes. The role of protein phosphatase 2a in attenuation of the signal. J Biol Chem. 1990;265:16571–16580. [PubMed] [Google Scholar]
- 21.Hill C S, Treisman R. Transcriptional regulation by extracellular signals: mechanisms and specificity. Cell. 1995;80:199–211. doi: 10.1016/0092-8674(95)90403-4. [DOI] [PubMed] [Google Scholar]
- 22.Hirai K, Maeda F, Watanabe Y. Expression of early virus functions in human cytomegalovirus infected HEL cells: effect of ultraviolet light-irradiation of the virus. J Gen Virol. 1977;38:121–133. doi: 10.1099/0022-1317-38-1-121. [DOI] [PubMed] [Google Scholar]
- 23.Hunter T. Protein kinases and phosphatases: the yin and yang of protein phosphorylation and signaling. Cell. 1995;80:225–236. doi: 10.1016/0092-8674(95)90405-0. [DOI] [PubMed] [Google Scholar]
- 24.Janknecht R, Ernst W H, Nordheim A. SAP1α is a nuclear target of signaling cascades involving ERKs. Oncogene. 1995;10:1209–1216. [PubMed] [Google Scholar]
- 25.Janknecht R, Ernst W H, Pingoud V, Nordheim A. Activation of ternary complex factor Elk-1 by MAP kinases. EMBO J. 1993;12:5097–5104. doi: 10.1002/j.1460-2075.1993.tb06204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson G L, Vaillancourt R R. Sequential protein kinase reactions controlling cell growth and differentiation. Curr Opin Cell Biol. 1994;6:230–238. doi: 10.1016/0955-0674(94)90141-4. [DOI] [PubMed] [Google Scholar]
- 27.Karin M. Signal transduction from the cell surface to the nucleus through the phosphorylation of transcription factors. Curr Opin Cell Biol. 1994;6:415–424. doi: 10.1016/0955-0674(94)90035-3. [DOI] [PubMed] [Google Scholar]
- 28.Koval V, Clark C, Vaishnav M, Spector S A, Spector D H. Human cytomegalovirus inhibits human immunodeficiency virus replication in cells productively infected by both viruses. J Virol. 1991;65:6969–6978. doi: 10.1128/jvi.65.12.6969-6978.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kowalik T F, Wing B, Haskill J S, Azizkhan J C, Baldwin A S, Jr, Huang E-S. Multiple mechanisms are implicated in the regulation of NF-κB activity during human cytomegalovirus infection. Proc Natl Acad Sci USA. 1993;90:1107–1111. doi: 10.1073/pnas.90.3.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lang D, Gebert S, Arlt H, Stamminger T. Functional interaction between the human cytomegalovirus 86-kilodalton IE2 protein and the cellular transcription factor CREB. J Virol. 1995;69:6030–6037. doi: 10.1128/jvi.69.10.6030-6037.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lannuzel A, Barnier J V, Hery C, Huynh V T, Guibert B, Gray F, Vincent J D, Tardieu M. Human immunodeficiency virus type 1 and its coat protein gp120 induce apoptosis and activate JNK and ERK mitogen-activated protein kinases in human neurons. Ann Neurol. 1997;42:847–856. doi: 10.1002/ana.410420605. [DOI] [PubMed] [Google Scholar]
- 32.Lavoie J N, L’Allemain G, Brunet A, Muller R, Pouyssegur J. Cyclin D1 expression is regulated positively by the p42/p44MAPK and negatively by the p38/HOGMAPK pathway. J Biol Chem. 1996;271:20608–20616. doi: 10.1074/jbc.271.34.20608. [DOI] [PubMed] [Google Scholar]
- 33.Lazar D F, Wiese R J, Brady M J, Mastick C C, Waters S B, Yamauchi K, Pessin J E, Cuatrecasas P, Saltiel A R. Mitogen-activated protein kinase kinase inhibition does not block the stimulation of glucose utilization by insulin. J Biol Chem. 1995;270:20801–20807. doi: 10.1074/jbc.270.35.20801. [DOI] [PubMed] [Google Scholar]
- 34.Marais R, Wynne J, Treisman R. The SRF accessory protein Elk-1 contains a growth factor-regulated transcriptional activation domain. Cell. 1993;73:381–393. doi: 10.1016/0092-8674(93)90237-k. [DOI] [PubMed] [Google Scholar]
- 35.Marshall C J. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80:179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- 36.McDonough S H, Spector D H. Transcription in human fibroblasts permissively infected by human cytomegalovirus strain AD169. Virology. 1983;125:31–46. doi: 10.1016/0042-6822(83)90061-2. [DOI] [PubMed] [Google Scholar]
- 37.Meloche S. Cell cycle reentry of mammalian fibroblasts is accompanied by the sustained activation of p44mapk and p42mapk isoforms in the G1 phase and their inactivation at the G1/S transition. J Cell Physiol. 1995;163:577–588. doi: 10.1002/jcp.1041630319. [DOI] [PubMed] [Google Scholar]
- 38.Misra-Press A, Rim C S, Yao H, Roberson M S, Stork P J. A novel mitogen-activated protein kinase phosphatase. Structure, expression, and regulation. J Biol Chem. 1995;270:14587–14596. doi: 10.1074/jbc.270.24.14587. [DOI] [PubMed] [Google Scholar]
- 39.Mocarski E S. Cytomegaloviruses and their replication. In: Fields B N, Knipe D M, Howley P M, et al., editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 2447–2492. [Google Scholar]
- 40.Muda M, Boschert U, Dickinson R, Martinou J C, Martinou I, Camps M, Schlegel W, Arkinstall S. MKP-3, a novel cytosolic protein-tyrosine phosphatase that exemplifies a new class of mitogen-activated protein kinase phosphatase. J Biol Chem. 1996;271:4319–4326. doi: 10.1074/jbc.271.8.4319. [DOI] [PubMed] [Google Scholar]
- 41.Muda M, Boschert U, Smith A, Antonsson B, Gillieron C, Chabert C, Camps M, Martinou I, Ashworth A, Arkinstall S. Molecular cloning and functional characterization of a novel mitogen-activated protein kinase phosphatase, MKP-4. J Biol Chem. 1997;272:5141–5151. doi: 10.1074/jbc.272.8.5141. [DOI] [PubMed] [Google Scholar]
- 42.Muda M, Theodosiou A, Rodrigues N, Boschert U, Camps M, Gillieron C, Davies K, Ashworth A, Arkinstall S. The dual specificity phosphatases M3/6 and MKP-3 are highly selective for inactivation of distinct mitogen-activated protein kinases. J Biol Chem. 1996;271:27205–27208. doi: 10.1074/jbc.271.44.27205. [DOI] [PubMed] [Google Scholar]
- 43.Mumby M. Regulation by tumour antigens defines a role for PP2A in signal transduction. Semin Cancer Biol. 1995;6:229–237. doi: 10.1006/scbi.1995.0030. [DOI] [PubMed] [Google Scholar]
- 44.Pang L, Sawada T, Decker S J, Saltiel A R. Inhibition of MAP kinase kinase blocks the differentiation of PC-12 cells induced by nerve growth factor. J Biol Chem. 1995;270:13585–13588. doi: 10.1074/jbc.270.23.13585. [DOI] [PubMed] [Google Scholar]
- 45.Patarca R. Protein phosphorylation and dephosphorylation in physiologic and oncologic processes. Crit Rev Oncog. 1996;7:343–432. doi: 10.1615/critrevoncog.v7.i5-6.20. [DOI] [PubMed] [Google Scholar]
- 46.Pende M, Fisher T L, Simpson P B, Russell J T, Blenis J, Gallo V. Neurotransmitter- and growth factor-induced cAMP response element binding protein phosphorylation in glial cell progenitors: role of calcium ions, protein kinase C, and mitogen-activated protein kinase/ribosomal S6 kinase pathway. J Neurosci. 1997;17:1291–1301. doi: 10.1523/JNEUROSCI.17-04-01291.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Price M A, Rogers A E, Treisman R. Comparative analysis of the ternary complex factors Elk-1, SAP-1a and SAP-2 (ERP/NET) EMBO J. 1995;14:2589–2601. doi: 10.1002/j.1460-2075.1995.tb07257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ray L B, Sturgill T W. Insulin-stimulated microtubule-associated protein kinase is phosphorylated on tyrosine and threonine in vivo. Proc Natl Acad Sci USA. 1988;85:3753–3757. doi: 10.1073/pnas.85.11.3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Robinson M J, Cobb M H. Mitogen-activated protein kinase pathways. Curr Opin Cell Biol. 1997;9:180–186. doi: 10.1016/s0955-0674(97)80061-0. [DOI] [PubMed] [Google Scholar]
- 50.Rodems S M, Clark C L, Spector D H. Separate DNA elements containing ATF/CREB and IE86 binding sites differentially regulate the human cytomegalovirus UL112-113 promoter at early and late times in the infection. J Virol. 1998;72:2697–2707. doi: 10.1128/jvi.72.4.2697-2707.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rodems, S. M., and D. H. Spector. Unpublished data.
- 52.Ruderman J V. MAP kinase and the activation of quiescent cells. Curr Opin Cell Biol. 1993;5:207–213. doi: 10.1016/0955-0674(93)90104-x. [DOI] [PubMed] [Google Scholar]
- 53.Sambucetti L C, Cherrington J M, Wilkinson G W G, Mocarski E S. NF-κB activation of the cytomegalovirus enhancer is mediated by a viral transactivator and by T cell stimulation. EMBO J. 1989;8:4251–4258. doi: 10.1002/j.1460-2075.1989.tb08610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schwartz R, Helmich B, Spector D H. CREB and CREB-binding proteins play an important role in the IE2 86-kilodalton protein- mediated transactivation of the human cytomegalovirus 2.2-kilobase RNA promoter. J Virol. 1996;70:6955–6966. doi: 10.1128/jvi.70.10.6955-6966.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schwartz R, Sommer M H, Scully A, Spector D H. Site-specific binding of the human cytomegalovirus IE2 86-kilodalton protein to an early gene promoter. J Virol. 1994;68:5613–5622. doi: 10.1128/jvi.68.9.5613-5622.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shelbourn S L, Sissons J G, Sinclair J H. Expression of oncogenic ras in human teratocarcinoma cells induces partial differentiation and permissiveness for human cytomegalovirus infection. J Gen Virol. 1989;70:367–374. doi: 10.1099/0022-1317-70-2-367. [DOI] [PubMed] [Google Scholar]
- 57.Sinclair J H, Baillie J, Bryant L A, Taylor-Wiedeman J A, Sissons J G. Repression of human cytomegalovirus major immediate early gene expression in a monocytic cell line. J Gen Virol. 1992;73:433–435. doi: 10.1099/0022-1317-73-2-433. [DOI] [PubMed] [Google Scholar]
- 58.Staprans S I, Rabert D K, Spector D H. Identification of sequence requirements and trans-acting functions necessary for regulated expression of a human cytomegalovirus early gene. J Virol. 1988;62:3463–3473. doi: 10.1128/jvi.62.9.3463-3473.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stinski M F. Sequence of protein synthesis in cells infected by human cytomegalovirus: early and late virus-induced polypeptides. J Virol. 1978;26:686–701. doi: 10.1128/jvi.26.3.686-701.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sun H, Charles C H, Lau L F, Tonks N K. MKP-1 (3CH134), an immediate early gene product, is a dual specificity phosphatase that dephosphorylates MAP kinase in vivo. Cell. 1993;75:487–493. doi: 10.1016/0092-8674(93)90383-2. [DOI] [PubMed] [Google Scholar]
- 61.Tamashiro J C, Hock L J, Spector D H. Construction of a cloned library of the EcoRI fragments from the human cytomegalovirus genome (strain AD169) J Virol. 1982;42:547–557. doi: 10.1128/jvi.42.2.547-557.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Taylor-Wiedeman J, Sissons J G, Borysiewicz L K, Sinclair J H. Monocytes are a major site of persistence of human cytomegalovirus in peripheral blood mononuclear cells. J Gen Virol. 1991;72:2059–2064. doi: 10.1099/0022-1317-72-9-2059. [DOI] [PubMed] [Google Scholar]
- 63.Taylor-Wiedeman J, Sissons P, Sinclair J. Induction of endogenous human cytomegalovirus gene expression after differentiation of monocytes from healthy carriers. J Virol. 1994;68:1597–1604. doi: 10.1128/jvi.68.3.1597-1604.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Treisman R. Ternary complex factors: growth factor regulated transcriptional activators. Curr Opin Genet Dev. 1994;4:96–101. doi: 10.1016/0959-437x(94)90097-3. [DOI] [PubMed] [Google Scholar]
- 65.Wade E J, Klucher K M, Spector D H. An AP-1 binding site is the predominant cis-acting regulatory element in the 1.2-kilobase early RNA promoter of human cytomegalovirus. J Virol. 1992;66:2407–2417. doi: 10.1128/jvi.66.4.2407-2417.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ward Y, Gupta S, Jensen P, Wartmann M, Davis R J, Kelly K. Control of MAP kinase activation by the mitogen-induced threonine/tyrosine phosphatase PAC1. Nature. 1994;367:651–654. doi: 10.1038/367651a0. [DOI] [PubMed] [Google Scholar]
- 67.Wathen M W, Stinski M F. Temporal patterns of human cytomegalovirus transcription: mapping the viral RNAs synthesized at immediate early, early, and late times after infection. J Virol. 1982;41:462–477. doi: 10.1128/jvi.41.2.462-477.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wathen M W, Thomsen D R, Stinski M F. Temporal regulation of human cytomegalovirus transcription at immediate early and early times after infection. J Virol. 1981;38:446–459. doi: 10.1128/jvi.38.2.446-459.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Weinshenker B G, Wilton S, Rice G P. Phorbol ester-induced differentiation permits productive human cytomegalovirus infection in a monocytic cell line. J Immunol. 1988;140:1625–1631. [PubMed] [Google Scholar]
- 70.Whalen S G, Marcellus R C, Whalen A, Ahn N G, Ricciardi R P, Branton P E. Phosphorylation within the transactivation domain of adenovirus E1A protein by mitogen-activated protein kinase regulates expression of early region 4. J Virol. 1997;71:3545–3553. doi: 10.1128/jvi.71.5.3545-3553.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wright D A, Staprans S I, Spector D H. Four phosphoproteins with common amino termini are encoded by human cytomegalovirus AD169. J Virol. 1988;62:331–340. doi: 10.1128/jvi.62.1.331-340.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xing J, Ginty D D, Greenberg M E. Coupling of the RAS-MAPK pathway to gene activation by RSK2, a growth factor-regulated CREB kinase. Science. 1996;273:959–963. doi: 10.1126/science.273.5277.959. [DOI] [PubMed] [Google Scholar]
- 73.Xing J, Kornhauser J M, Xia Z, Thiele E A, Greenberg M E. Nerve growth factor activates extracellular signal-regulated kinase and p38 mitogen-activated protein kinase pathways to stimulate CREB serine 133 phosphorylation. Mol Cell Biol. 1998;18:1946–1955. doi: 10.1128/mcb.18.4.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yurochko A D, Kowalik T F, Huong S M, Huang E S. Human cytomegalovirus upregulates NF-κB activity by transactivating the NF-κB p105/p50 and p65 promoters. J Virol. 1995;69:5391–5400. doi: 10.1128/jvi.69.9.5391-5400.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]