Abstract
Introduction
Subjective cognitive decline means a decline in the subjective perception of self-cognitive function, which is likely to evolve into mild cognitive impairment and dementia. The number of elderly with subjective cognitive decline has increased, bringing huge burdens and challenges to caregivers and society. With the increase in research on art therapies, some of them have gradually been proven to be effective for cognitive function. Therefore, this study aims to summarise the evidence and identify the best art therapy for elderly with subjective cognitive decline.
Methods and analysis
We will include published randomised controlled trials written in English and Chinese if the intervention is one of the art therapies and applied in people aged 60 and above with subjective cognitive decline. Eight electronic databases, including the Cochrane Central Register of Controlled Trials, PubMed, Web of Science, Elsevier, China BioMedical Literature Database, China National Knowledge Infrastructure, VIP Database and Wanfang Database, will be searched from January 2013 to December 2023. Art therapies will mainly include music therapy, reminiscence therapy, painting therapy, dance therapy, reading therapy, horticultural therapy, museum therapy, calligraphy therapy and so on. The outcome will be cognitive function. Study selection, data extraction and quality assessment will be performed by two reviewers. The risk of bias will be evaluated according to the Cochrane Collaboration’s risk-of-bias tool, and the evidence quality will be assessed with the Grading of Recommendations Assessment, Development and Evaluation. Standard pairwise meta-analysis and Bayesian network meta-analysis will be conducted. The probabilities of each art therapy will be ranked based on the surface under the cumulative ranking curve.
Ethics and dissemination
Ethical approval is not required for reviewing published studies. To provide important evidence for clinicians and guideline developers, the findings of this study will be submitted to a peer-reviewed journal.
PROSPERO registration number
CRD42023443773.
Keywords: Delirium & cognitive disorders, Nursing Care, Systematic Review, Protocols & guidelines
STRENGTHS AND LIMITATIONS OF THIS STUDY.
This network meta-analysis will integrate direct evidence with indirect evidence and allow the comparison of multiple art therapies in one model.
Network meta-analysis will promote precision of intervention and provide evidence for the decisions of intervention and the development of guidelines.
On account of the retrospective nature of this study, the findings may be influenced by the quantity and quality of the included studies.
Introduction
As a global public health problem that urgently needs to be addressed, dementia leads to the loss of reasoning, memory, language and ultimately basic self-care skills, which places an enormous burden on families and the healthcare system. The development of dementia, such as Alzheimer’s disease, is insidious, with onset of clinical symptoms preceded years earlier by perceived and/or objective cognitive decline,1 which is also the early stage of mild cognitive impairment.2 Subjective cognitive decline refers to the decline in the subjective perception of self-cognitive function compared with the previous normal state, mainly memory decline, but objective neuropsychological tests are not abnormal, and this decline is not related to other acute events.3 Previous studies have suggested that subjective cognitive decline is a preclinical symptom of mild cognitive impairment and dementia.4–7 The risk of developing dementia in the elderly with subjective cognitive decline was 2.48 times higher than that in the elderly without cognitive abnormalities, and the risk of developing mild cognitive impairment was 1.83 times higher than that in the elderly without cognitive abnormalities.7 Moreover, the findings of a long-term observational study over 10 years indicate that subjective cognitive decline can occur 10 years before the diagnosis of Alzheimer’s disease.8 Therefore, the therapeutic window for addressing Alzheimer’s disease can be moved forward, and subjective cognitive decline can be regarded as an important gateway for early prevention and treatment of Alzheimer’s disease, which may help alleviate the major public health problem, although there are no approved treatments for Alzheimer’s disease and even mild cognitive impairment.9
The efficacy of pharmacological interventions is limited. To avoid the side effects of antipsychotics, more attention is given to the application of non-pharmacological interventions.10 Art therapy is an emerging non-pharmacological intervention with distinctive features that integrates psychology, art and medicine. Art therapy can take a variety of forms, such as music, singing, dancing, reading and poetry groups, museum/gallery art and collections, creative writing, life story narrative reminiscence, painting, printmaking, collage, pottery, sewing, knitting, woodwork and gardening.11 As a kind of non-pharmacological intervention, art therapy has been widely proven to be beneficial in the care of patients with cognitive impairment and has been used in clinical practice in Europe and the USA.12 It is helpful to reduce the mental and behavioural abnormalities of patients and slow down the progress of cognitive impairment, to achieve great effects in treatment and care. It is suggested that music listening can help to improve both subjective memory function and objective cognitive performance in adults with subjective cognitive decline.13 Several randomised controlled trials have demonstrated that dance therapy can improve cognitive function, especially episodic memory and processing speed.14–16 Moreover, painting therapy has been proven to improve cognitive function in patients with mild cognitive impairment.17 A narrow review has summarised the positive effects of therapeutic gardens on the health, spanning physical, social, psychological and cognitive effects.18 Additionally, spiritual reminiscence programmes using expressive arts therapy are conducive to improving cognitive function.19
With the increase in relevant research, art therapy has gradually been proven to be of great significance in alleviating cognitive decline. However, the advantages and disadvantages of the effects of different art therapies on elderly with subjective cognitive decline are still inconclusive at present, and studies directly comparing the differences in various art therapies are lacking. Network meta-analysis is capable of summarising direct and indirect evidence, thus evaluating and comparing the relative efficacy of multiple treatments.20 More importantly, network meta-analysis can provide the ranking of intervention options in accordance with their effectiveness. Therefore, this study will evaluate the effects of different art therapies on cognitive function in elderly (aged 60 and above) with subjective cognitive decline through a network meta-analysis, with the aim of identifying the most effective art therapy for this patient group. These findings may provide important evidence for care decision-making in elderly with subjective cognitive decline.
Methods and analysis
This network meta-analysis protocol was registered on the PROSPERO platform (CRD42023443773). The timeline of this work is planned between 28 June 2023 and 31 May 2024. The results of this network meta-analysis will be reported following the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols.21
Eligibility criteria
Type of participants
Elderly diagnosed with subjective cognitive decline will be included. Inclusion criteria will be: (1) diagnosed as subjective cognitive declined according to the criteria published by the Subjective Cognitive Decline Initiative22 23 and (2) aged 60 years and above. The exclusion criteria will be: (1) complicated with nervous system diseases which may cause subjective cognitive decline, such as dementia, Parkinson’s disease, stroke, cephalomeningitis and craniocerebral trauma; (2) complicated with major diseases that may affect subjective cognitive function, such as severe metabolic diseases and severe cardiopulmonary diseases; (3) complicated with severe mental illnesses, such as schizophrenia, bipolar disorder, severe depression and severe anxiety.
Type of studies
Only randomised controlled trials written in English or Chinese will be included. Cluster randomised controlled trials and cross-over randomised controlled trials will be excluded. Trials without a control group will be excluded. If the control group did not receive art therapy or usual care, the trial will also be excluded.
Type of intervention
Any art therapy that is combined with usual care and implemented in elderly with subjective cognitive decline will be included. However, multicomponent interventions that two or more art interventions or art therapies combined with other types of treatment (in addition to usual care) are applied in a experimental group will be excluded. These art therapies may be aimed at improving cognitive function. The types of art therapies for elderly with subjective cognitive decline may include: (1) music therapy, (2) dance therapy, (3) reading therapy, (4) painting therapy, (5) horticultural therapy, (6) reminiscence therapy, (7) calligraphy therapy and (8) museum therapy.
Comparison
Comparator will be considered a usual care of subjective cognitive decline (such as guidance of drugs, diet, rehabilitation and complication prevention) or another art therapy combined with usual care.
Type of outcomes
The outcome will focus on cognitive function, which might be measured by a validated cognitive outcome measure, for example, Subjective Cognitive Decline Questionnaire, the Mini-Mental State Examination, Montreal Cognitive Assessment, Addenbrooke’s Cognitive Examination-III, Mini-Addenbrooke’s Cognitive Examination, Memory Impairment Screen, Quick Screen for Mild Cognitive Impairment, Clock-Drawing Test or Repeatable Battery for the Assessment of Neuropsychological Status. Subjective cognitive decline will be reported by using the original scores after intervention in the original study.
Data sources and search strategy
The search used a combination of Medical Subject Headings and free words for professional searches. The search items will include subjective cognitive decline or SCD, art therapy (music* or singing or danc* or painting or drawing or collage or clay or theatre or drama or reading or poetry or woodwork or garden* or horticultural or handwriting or penmanship or calligraphy or ceramics or pottery or writing or sculpture or carving or narrative or reminiscence or printmaking or sewing or knitting or museum or gallery) and randomised controlled trial. Searches will be undertaken in electronic databases to identify published studies, including the Cochrane Central Register of Controlled Trials, PubMed, Web of Science, Elsevier, China BioMedical Literature Database, China National Knowledge Infrastructure, VIP Database and Wanfang Database. A draft search strategy is summarised in online supplemental material 1. The retrieval time will be from January 2013 to December 2023. In addition to the database search, the references of the included studies will be scanned to identify additional eligible studies.
bmjopen-2023-079146supp002.pdf (110.1KB, pdf)
Study selection
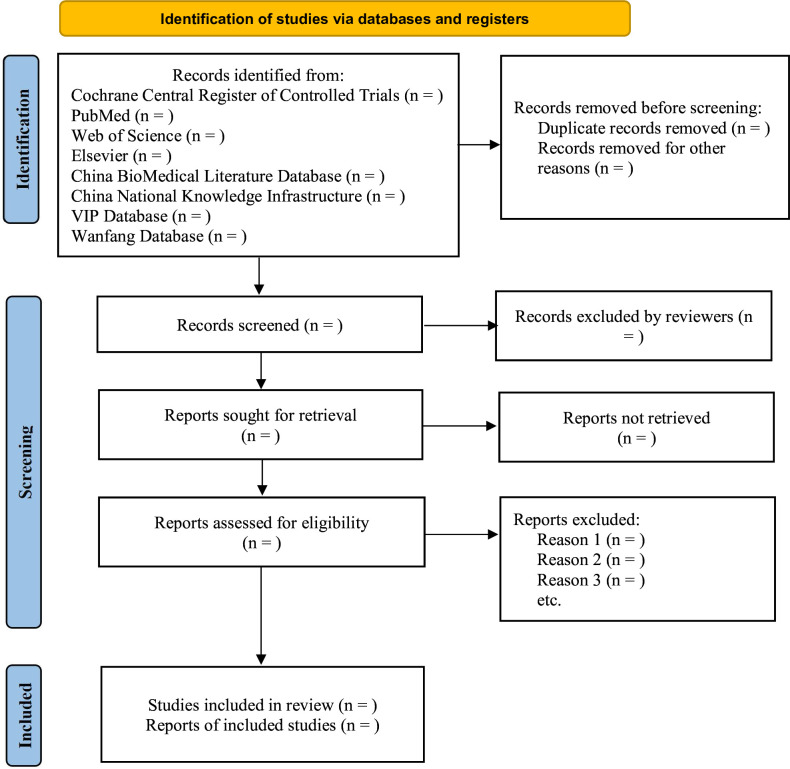
All investigators will receive appropriate training prior to study selection. All retrieved studies will be imported into NoteExpress software to download references. Duplicate studies will be removed. The titles and abstracts of selected studies will be screened independently by two reviewers to exclude studies that obviously do not meet the inclusion criteria. The preliminary results will be cross-checked. Then, the remaining full-text studies will be examined independently by the same two reviewers to determine eligibility. If there are disagreements, the third reviewer will be asked to evaluate the full text. Any discrepancies will be resolved through group discussions. We will record the excluded studies and reasons for exclusion. Figure 1 shows the process of study selection.
Figure 1.
Study flow diagram. The processes of study selection.
Data extraction
All researchers of this study will discuss and design a standard form for data extraction. Then, two reviewers will independently extract data in accordance with the standard form, including author (s), year of publication, sample size, characteristics of patients (gender and age), intervention (type, frequency and duration) and measurements of outcome. After completing data extraction, the results of two reviewers will be crosschecked to ensure that there is no mistake. Team discussion will be used to resolve the disagreements.
Risk-of-bias assessment
The revised version of the Cochrane tool (RoB V.2) will be used to assess the risk of bias for all included studies.24 This tool will assess five domains, including (1) bias arising from the randomization process, (2) bias due to deviations from intended interventions, (3) bias due to missing outcome data, (4) bias in the measurement of the outcome and (5) bias in the selection of the reported result. The assessment of each domain will be rated as ‘low risk of bias’, ‘some concerns’ and ‘high risk of bias’. The response options for an overall risk-of-bias judgement are the same as those for individual domains. If all domains are rated as ‘low risk of bias’, this trial will be judged to be at low risk of bias. If at least one domain is rated as ‘some concerns’ and no domain is rated as ‘high risk of bias’, this trial will be judged to be ‘some concerns’. If at least one domain is rated as ‘high risk of bias’ or multiple domains are rated as ‘some concerns’ in a way that substantially lowers confidence in the result, this trial will be judged to be at high risk of bias. Two reviewers will independently evaluate the risk of bias for each study and then cross-check the results. Differences will be resolved through team discussion with the third reviewer.
Statistical analysis
Pairwise meta-analysis
We will use Review Manager (V.5.3) to conduct a pairwise meta-analysis of direct evidence. For continuous outcomes, standardised mean differences with 95% CIs will be used. The χ2 test will be used to assess the heterogeneity across all included trials. A fixed effects model will be used to synthesise the standardised mean difference if the p value is ≥0.1. Conversely, if the p value is <0.1, a random effects model will be used.
Network meta-analysis
Considering the expected heterogeneity between studies, the effects of different art therapies will be compared by conducting a random effects network meta-analysis within a Bayesian framework using Markov Chains Monte Carlo in R software (V.4.1.3). Brooks-Gelman-Rubin diagnosis and potential scale reduction factor will be used to ensure the convergence of the model.25 Moreover, the surface under the cumulative ranking curve with its 95% CI and rank-heat plot will be used to evaluate the hierarchy of each art therapy.26 27
Dealing with missing data
If there is a lack of some information, the missing data will be obtained by contacting the corresponding authors whenever possible. We will try to calculate the missing data based on availability factors if there is no reply. Sensitivity analysis will be used to examine the potential impact of missing data on the results of this study.
Assessment of publication bias
If more than nine studies are included in the analysis, funnel plots, Begg’s rank correlation and Egger’s regression tests will be used to evaluate the presence of publication bias in Stata software (V.15.0).28–30
Assessment of inconsistency and subgroup analysis
Based on a loop-special method within each loop of the network,31 the local inconsistency and global inconsistency will be measured in Stata software (V.15.0).32 If there is heterogeneity or inconsistency, the sources of heterogeneity will be explored by network meta-regression. Subgroup analysis will be performed in accordance with age, gender or duration of intervention.
Sensitivity analysis
We will perform a sensitivity analysis for primary outcomes to verify the robustness of the findings. In the sensitivity analysis, trials judged to be at high risk of bias, trials with missing data and trials with the smallest sample size will be excluded. Then, to examine whether the results change and whether the transitivity (consistency and model fit) is affected, the same methods used to conduct the network meta-analysis will be repeated.
Quality of evidence
We will also evaluate the quality of evidence conducing to primary outcomes based on the Grading of Recommendations Assessment, Development and Evaluation framework, in accordance with limitations of study, imprecision, heterogeneity, inconsistency, indirectness and publication bias.33
Patient and public involvement
This study is based on published data, so patients or the public were not involved in the design, conduct, reporting and dissemination plans of our research.
Discussion
According to a cohort study, the prevalence of subjective cognitive decline is currently slightly high, ranging from 7.8% to 52.7% which is based on estimated based on age and gender standardisation in the population.34 This means that subjective cognitive decline is gradually becoming a health issue in elderly who requires special attention. Subjective cognitive decline is an important risk factor for mild cognitive impairment and Alzheimer’s disease,6 which may cause adverse effects on the quality of life of elderly and bring enormous burdens to caregivers and society, and obstruct the realisation of healthy ageing and active ageing. The effects of pharmacological intervention are limited to some extent. Non-pharmacological interventions have become the preferred approach to treat and care for patients with cognitive impairment because of their simplicity, easy operation and high safety.35
At present, art therapies such as music therapy, dance therapy, painting therapy, reading therapy, horticultural therapy, reminiscence therapy, calligraphy therapy and museum therapy have been applied to improve cognitive function in patients with subjective cognitive decline. Music, as an artistic manifestation, offers a profound impact on the emotional, cognitive and physiological aspects of personal experiences.36 Some studies have shown that music therapy has a positive impact on individuals with decreased cognitive function, helping improve objective and subjective cognitive functions, such as subjective memory function.37 Reminiscence therapy is also one of the common methods used to improve cognitive function in the elderly, which helps stimulate autobiographical memory and simultaneously improve mental health.38 Horticultural therapy can promote active contact and interaction between human and natural elements, thereby improving the cognitive function and mental health of the elderly.39 In addition, some scholars believe that horticultural therapy may improve cognition function through metabolic biomarkers such as tryptophan, kynurenine and serotonin.40 From the current research results, it can be seen that arious art therapies have a positive effect on improving cognitive function through different means. However, to date, no network meta-analysis has been performed to evaluate the comparative efficacy of all available art therapies. Consequently, it is necessary to conduct a network meta-analysis to identify the effects of art therapies. To the best of our knowledge, this is the first network meta-analysis to analyse the effects of art therapies in elderly with subjective cognitive decline. Based on the comparative effectiveness evidence, this network meta-analysis is expected to find the best art therapy for improving cognitive function in elderly with subjective cognitive decline. The results could help patients and clinicians choose the best intervention. In addition, we hope that the results of this study could provide evidence for the recommendations of guidelines.
Ethics and dissemination
This study is based on published data, so ethical approval is not a requirement. We plan to publish the findings of this study in a peer-reviewed journal. This work is now in progress, and preparations have started on 28 June 2023. We are searching the relevant studies. The expected end time is 31 May 2024. The results will be reported based on the PRISMA-compliant guidelines.
bmjopen-2023-079146supp001.pdf (110.8KB, pdf)
Supplementary Material
Footnotes
Contributors: QL designed this study with oversight by XH. QL drafted the protocol, and the draft was modified by FW and LL. QL and LT will search, select and identify studies and extract data independently, while LL will be the third reviewer for study selection and data extraction. QL will be responsible for the methodology. All authors have approved the publication of this protocol.
Funding: This study is funded by a project from the West China Hospital of Sichuan University and University of Electronic Science and Technology of China (Grant No. HXDZ21003) and the Institutional Research Fund from Sichuan University (2022SCUH0030).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1. Alzheimer’s Association . Alzheimer’s disease facts and figures. Alzheimers Dement 2015;11:332–84. 10.1016/j.jalz.2015.02.003 [DOI] [PubMed] [Google Scholar]
- 2. Jack CR, Bennett DA, Blennow K, et al. NIA-AA research framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement 2018;14:535–62. 10.1016/j.jalz.2018.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jessen F, Amariglio RE, van Boxtel M, et al. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimers Dement 2014;10:844–52. 10.1016/j.jalz.2014.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lin Y, Shan P-Y, Jiang W-J, et al. Subjective cognitive decline: preclinical manifestation of Alzheimer’s disease. Neurol Sci 2019;40:41–9. 10.1007/s10072-018-3620-y [DOI] [PubMed] [Google Scholar]
- 5. Slot RER, Sikkes SAM, Berkhof J, et al. Subjective cognitive decline and rates of incident Alzheimer’s disease and non-Alzheimer’s disease dementia. Alzheimers Dement 2019;15:465–76. 10.1016/j.jalz.2018.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Colijn MA, Grossberg GT. Amyloid and tau biomarkers in subjective cognitive impairment. J Alzheimers Dis 2015;47:1–8. 10.3233/JAD-150180 [DOI] [PubMed] [Google Scholar]
- 7. Pike KE, Cavuoto MG, Li L, et al. Subjective cognitive decline: level of risk for future dementia and mild cognitive impairment, a meta-analysis of longitudinal studies. Neuropsychol Rev 2022;32:703–35. 10.1007/s11065-021-09522-3 [DOI] [PubMed] [Google Scholar]
- 8. Verlinden VJA, van der Geest JN, de Bruijn R, et al. Trajectories of decline in cognition and daily functioning in preclinical dementia. Alzheimers Dement 2016;12:144–53. 10.1016/j.jalz.2015.08.001 [DOI] [PubMed] [Google Scholar]
- 9. Sperling RA, Jack CR, Aisen PS. Testing the right target and right drug at the right stage. Sci Transl Med 2011;3:111. 10.1126/scitranslmed.3002609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Abraha I, Rimland JM, Trotta FM, et al. Systematic review of systematic reviews of non-pharmacological interventions to treat behavioural disturbances in older patients with dementia. BMJ Open 2017;7:e012759. 10.1136/bmjopen-2016-012759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Roe B. Arts for health initiatives: an emerging international agenda and evidence base for older populations. J Adv Nurs 2014;70:1–3. 10.1111/jan.12216 [DOI] [PubMed] [Google Scholar]
- 12. Camic PM, Zeilig H, Crutch SJ. The arts and dementia: emerging directions for theory, research and practice. Dementia (London) 2018;17:641–4. 10.1177/1471301218772972 [DOI] [PubMed] [Google Scholar]
- 13. Innes KE, Selfe TK, Khalsa DS, et al. Meditation and music improve memory and cognitive function in adults with subjective cognitive decline: a pilot randomized controlled trial. J Alzheimers Dis 2017;56:899–916. 10.3233/JAD-160867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhu Y, Wu H, Qi M, et al. Effects of a specially designed aerobic dance routine on mild cognitive impairment. Clin Interv Aging 2018;13:1691–700. 10.2147/CIA.S163067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Doi T, Verghese J, Makizako H, et al. Effects of cognitive leisure activity on cognition in mild cognitive impairment: results of a randomized controlled trial. J Am Med Dir Assoc 2017;18:686–91. 10.1016/j.jamda.2017.02.013 [DOI] [PubMed] [Google Scholar]
- 16. Borges E da S, Vale R de S, Pernambuco CS, et al. Effects of dance on the postural balance, cognition and functional autonomy of older adults. Rev Bras Enferm 2018;71:2302–9. 10.1590/0034-7167-2017-0253 [DOI] [PubMed] [Google Scholar]
- 17. Zhao J, Li H, Lin R, et al. Effects of creative expression therapy for older adults with mild cognitive impairment at risk of Alzheimer’s disease: a randomized controlled clinical trial. Clin Interv Aging 2018;13:1313–20. 10.2147/CIA.S161861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Uwajeh PC, Iyendo TO, Polay M. Therapeutic gardens as a design approach for optimising the healing environment of patients with Alzheimer’s disease and other dementias: a narrative review. Explore (NY) 2019;15:352–62. 10.1016/j.explore.2019.05.002 [DOI] [PubMed] [Google Scholar]
- 19. Ho YV. A pilot study of spiritual reminiscence programme using expressive arts therapy for the older adults with mild cognitive impairment staying in nursing home. Hong Kong: The University of Hong Kong, 2017. [Google Scholar]
- 20. Li J, Zhong D, Ye J, et al. Rehabilitation for balance impairment in patients after stroke: a protocol of a systematic review and network meta-analysis. BMJ Open 2019;9:e026844. 10.1136/bmjopen-2018-026844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ 2015;350:g7647. 10.1136/bmj.g7647 [DOI] [PubMed] [Google Scholar]
- 22. Molinuevo JL, Rabin LA, Amariglio R, et al. Implementation of subjective cognitive decline criteria in research studies. Alzheimers Dement 2017;13:296–311. 10.1016/j.jalz.2016.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Song YH, Liu YS, Yang Q, et al. Correlation of subjective cognitive decline with multimorbidity among elderly people [in Chinese]. Chinese General Practice 2023;26:1241–9. [Google Scholar]
- 24. Sterne JAC, Savović J, Page MJ, et al. Rob 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898. 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 25. Brooks SP, Gelman A. Alternative methods for monitoring convergence of Iterative simulations. J Comput Graph Stat 1998;7:434. 10.2307/1390675 [DOI] [Google Scholar]
- 26. Salanti G, Ades AE, Ioannidis JPA. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and Tutorial. J Clin Epidemiol 2011;64:163–71. 10.1016/j.jclinepi.2010.03.016 [DOI] [PubMed] [Google Scholar]
- 27. Veroniki AA, Straus SE, Fyraridis A, et al. The rank-heat plot is a novel way to present the results from a network meta-analysis including multiple outcomes. J Clin Epidemiol 2016;76:193–9. 10.1016/j.jclinepi.2016.02.016 [DOI] [PubMed] [Google Scholar]
- 28. Collaboration TC . Cochrane handbook for systematic reviews of interventions version 5.1.0. 2011. [Google Scholar]
- 29. Begg CB, Mazumdar M. Operating characteristics of a RANK correlation test for publication bias. Biometrics 1994;50:1088–101. [PubMed] [Google Scholar]
- 30. Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Du Y, Wu F, Lu S, et al. Efficacy of pressure ulcer prevention interventions in adult intensive care units: a protocol for a systematic review and network meta-analysis. BMJ Open 2019;9:e026727. 10.1136/bmjopen-2018-026727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Higgins JPT, Jackson D, Barrett JK, et al. Consistency and inconsistency in network meta-analysis: concepts and models for multi-arm studies. Res Synth Methods 2012;3:98–110. 10.1002/jrsm.1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Puhan MA, Schünemann HJ, Murad MH, et al. A GRADE working group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ 2014;349:g5630. 10.1136/bmj.g5630 [DOI] [PubMed] [Google Scholar]
- 34. Röhr S, Pabst A, Riedel-Heller SG, et al. Estimating prevalence of subjective cognitive decline in and across international cohort studies of aging: a COSMIC study. Alzheimers Res Ther 2020;12:167. 10.1186/s13195-020-00734-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Calsolaro V, Antognoli R, Okoye C, et al. The use of antipsychotic drugs for treating behavioral symptoms in Alzheimer’s disease. Front Pharmacol 2019;10:1465. 10.3389/fphar.2019.01465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gómez-Carballa A, Navarro L, Pardo-Seco J, et al. Music compensates for altered gene expression in age-related cognitive disorders. Sci Rep 2023;13:21259. 10.1038/s41598-023-48094-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kim J, Cuevas H, Wood ST. Effect of music interventions on cognitive function in older adults with mild cognitive impairment: a systematic review. Res Gerontol Nurs 2023;16:259–68. 10.3928/19404921-20230609-01 [DOI] [PubMed] [Google Scholar]
- 38. Villasán-Rueda A, Sánchez-Cabaco A, Mejía-Ramírez M, et al. Transcultural pilot study of the efficacy of reminiscence therapy for Mexican and Spanish older adults with different levels of cognitive decline. J Cross Cult Gerontol 2023;38:371–88. 10.1007/s10823-023-09486-2 [DOI] [PubMed] [Google Scholar]
- 39. Wang M, Wu J, Yan H. The effect of horticultural therapy on older adults in pension institutions: a systematic review. Geriatr Nurs 2023;51:25–32. 10.1016/j.gerinurse.2023.02.006 [DOI] [PubMed] [Google Scholar]
- 40. Park S-A, Son SY, Lee A-Y, et al. Metabolite profiling revealed that a gardening activity program improves cognitive ability correlated with BDNF levels and serotonin metabolism in the elderly. Int J Environ Res Public Health 2020;17:541. 10.3390/ijerph17020541 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2023-079146supp002.pdf (110.1KB, pdf)
bmjopen-2023-079146supp001.pdf (110.8KB, pdf)