Abstract
Background
During the COVID-19 pandemic, we developed a digital research platform to longitudinally investigate COVID-19-related outcomes in patients with rheumatic diseases and healthy controls. We used home finger-prick testing in order to collect serum samples remotely and increase the overall efficiency of the platform. The aim of the present study was to evaluate the success rate of the finger prick and patients’ perspective towards the finger prick.
Methods
Serum samples were collected up to five times during follow-up, either via a venepuncture at the research institute or a finger prick from participants’ home. Participants were asked to complete a digital evaluation questionnaire of the finger prick after their attempts.
Results
A total of 2135 patients and 899 controls performed at least one finger prick and were included in this study. The first finger prick was successfully done by 92% (95% CI: 90% to 93%) of patients, 94% (95% CI: 92% to 95%) of controls, 93% (95% CI: 92% to 94%) of all participants aged ≤70 years and 89% (95% CI: 86% to 92%) of all participants aged >70 years. Sex did not impact these success rates. Repeated failure occurred in 11/439 (0.8%) patients and 4/712 (0.6%) controls. Both patients and controls were less willing to perform a finger prick for individual healthcare compared with scientific research.
Conclusion
The vast majority of participants, among which elderly and patients with rheumatic diseases, were able to successfully draw the required amount of blood for serological analyses. This shows that finger-prick testing is suitable for a high-throughput implementation to monitor patients remotely.
Keywords: epidemiology, COVID-19, autoimmune diseases
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT.
A small number of studies have used a finger prick as the primary method for blood withdrawal and showed that it is an efficient way to collect blood samples remotely, but the use of finger pricks in both research and healthcare settings is still uncommon.
Data on blood levels from a finger prick are directly comparable to that obtained by a venepuncture.
WHAT DOES THIS STUDY ADD
We showed that the vast majority of participants, among which elderly and patients with rheumatic diseases of whom hand function may be impaired by their underlying disease, was able to successfully draw the required amount of blood for serological analyses using a home finger prick.
A considerable number of patients with rheumatic diseases indicated restraint towards using the finger prick, especially for healthcare purposes, which could mainly be attributed to lack of confidence in the serological analyses performed in capillary blood compared with the golden standard of venous blood.
HOW MIGHT THIS IMPACT ON CLINICAL PRACTICE OR FUTURE DEVELOPMENTS
Finger-prick testing is suitable for a high-throughput implementation to monitor patients remotely, and can therefore contribute to improving the efficiency and cost-effectiveness of both healthcare and scientific research.
Education of patients by healthcare professionals about the reliability of serological analyses performed in capillary blood is important to improve patients’ willingness to use a finger prick for healthcare and research purposes.
Introduction
During the COVID-19 pandemic, healthcare and scientific research requiring patients to visit a healthcare centre was seriously hampered due to the national COVID-19 regulations. In order to answer important questions regarding the SARS-CoV-2 virus, we developed a large digital research platform for patients with rheumatic diseases and healthy controls.1 The digital platform was complemented with serum collection via a home finger prick in order to collect samples without the need of visits to the research institute. The combination of the digital platform and the finger prick provided a high degree of adaptability and high-throughput, allowing fast and efficient data collection and blood processing. This enabled us to rapidly answer new and relevant clinical research questions about COVID-19 severity, seroprevalence and immunogenicity after SARS-CoV-2 vaccination in patients with rheumatic diseases.2–4
Previous studies have shown that using finger prick as the primary method for blood withdrawal is an efficient way to collect blood samples remotely,5 6 and data on blood levels from a finger prick are directly comparable to that obtained by a venepuncture.7 8 However, there is limited data on peoples’ willingness and ability to successfully use the finger prick at home, especially in patients with rheumatic diseases for whom a finger prick might be more difficult to manage without assistance. In addition, the use of finger pricks in a healthcare setting is still uncommon and not yet tested on a large scale. Therefore, we investigated the feasibility of finger-prick testing in combination with a digital research platform in our prospective cohort study by evaluating the success rate and patients’ perspective towards the use of the finger prick. The results of this study may contribute to a widespread implementation of finger-prick tests in rheumatology for scientific research and healthcare purposes.
Methods
Here, we describe the role of the finger prick in a large ongoing prospective cohort study (Netherlands Trial Register, Trial ID NL8513), and evaluated success rates and participants’ perspective on the use of a finger-prick test. The protocol of the study has been described previously.1–4 Briefly, all adult patients with a rheumatic immune-mediated inflammatory disease (IMID) from the Amsterdam Rheumatology and immunology Centre, Amsterdam, the Netherlands, were invited to participate in the study between April 2020 and March 2021. All patients were asked (but not obliged) to register an adult control participant of the same sex and similar age (<5 years difference) who was not diagnosed with an IMID and did not receive immunosuppressive treatment. Demographic and clinical data were collected via online questionnaires distributed via email. Serum samples were collected up to five times during follow-up via blood withdrawal by venepuncture at the local research institute or via a finger prick (Sanquin, Amsterdam, The Netherlands) that could be performed at home by participants themselves. For this option, participants received a package at home that included a contact-activated lancet (BD Microtainer), a collection device for serum (Greiner Minicollect 450533), a set of instructions and a return envelope to send the blood sample to the central laboratory. Participants were instructed to collect three drops of blood falling down by gravity, which would yield approximately 40–80 μL of serum after clotting. Cross-sectional sampling of a large part of the cohort was performed three times during follow-up: in January 2021 to screen for SARS-CoV-2 antibodies prior to vaccination (first), in May 2021 to assess antibody development after vaccination (second) and in October 2021 to identify asymptomatic COVID-19 cases via analyses of nucleocapsid antibodies (third).
All participants who received a finger-prick test before 26 June 2021 were asked to complete a digital evaluation questionnaire of the finger prick after their attempt (online supplemental appendix, pp6–7). In addition, in August 2021, preferences for a certain method of blood withdrawal in different settings (healthcare and scientific research) were explored in all study participants, including those who did not receive a finger-prick test during the conduct of the study. All questions were multiple choice, and answer options were based on information provided by participants who had contacted the researchers during the conduct of the study, but prior to the development of the evaluation questionnaires.
rmdopen-2023-003933supp001.pdf (451.2KB, pdf)
The data on the success rate of the first, second and third execution of the finger prick were stratified for having a rheumatic disease, sex and age above 70, and the 95% CI was calculated. All participants who made an attempt to execute the finger prick were included in these analyses. The finger prick was defined as failed when less than 10 µL of serum could be recovered from the collection device, or if no sample was returned to the laboratory and participants indicated in the questionnaires that they did not succeed in collecting the required amount of serum.
Results
Between April 2020 and March 2021, 3080 consecutive patients with a rheumatic IMID and 1102 healthy controls were included in the study. Most serum samples collected during the conduct of the study were collected via finger pricks; 3300 (68%) of 4833 samples collected between January and March 2021, 1108 (71%) of 1554 samples collected between May and June 2021 and 1606 (81%) of 1980 samples collected between October and December 2021. A total of 3034 participants (2135 (69%) patients and 899 (82%) controls) attempted to execute at least one finger prick during the conduct of the study and were included for analyses. Of these, 1439 (67%) patients and 712 (21%) controls executed multiple finger pricks. The evaluation questionnaire of the finger prick was completed by 2251 participants (1500 patients and 751 controls), and 2131 participants (1575 patients and 556 controls) completed the survey in which their preference for sampling methods was evaluated.
Participant characteristics are shown in table 1. The mean age of patients and controls was 58 years (SD 13) and 56 years (SD 13), respectively, and the majority were female (66% of patients and 68% of controls). The most common rheumatic diseases were rheumatoid arthritis (53%), psoriatic arthritis (15%) and ankylosing spondylitis (16%). The majority of all patients received immunosuppressive treatment (77%).
Table 1.
Characteristics of patients with rheumatic IMIDs compared with healthy controls
| Patients (n=2135) |
Healthy controls (n=899) |
|||
| Patient characteristics | ||||
| Mean age (years) | 58±13 | 56±13 | ||
| Female sex, no. (%) | 1402 | (66) | 612 | (68) |
| Caucasian race, no. (%)* | 1814 | (89) | 795 | (92) |
| Education level, no. (%)* | ||||
| High | 961 | (48) | 488 | (59) |
| Medium | 622 | (31) | 225 | (27) |
| Low | 430 | (21) | 121 | (15) |
| Comorbidity, no. (%) | ||||
| Chronic pulmonary disease | 257 | (12) | 49 | (6) |
| Cardiovascular disease | 289 | (14) | 65 | (7) |
| Diabetes | 127 | (6) | 28 | (3) |
| Obesity | 354 | (17) | 86 | (10) |
| Anticoagulant medication, no. (%) | 295 | (14) | 80 | (9) |
| Currently smoking, no. (%) | 233 | (11) | 57 | (6) |
| Autoimmune disease type, no. (%) | ||||
| Rheumatoid arthritis | 1108 | (53) | NA | |
| Psoriatic arthritis | 308 | (15) | NA | |
| Ankylosing spondylitis | 333 | (16) | NA | |
| Axial or peripheral spondyloarthritis | 54 | (3) | NA | |
| Juvenile idiopathic arthritis | 32 | (2) | NA | |
| Systemic lupus erythematosus | 108 | (5) | NA | |
| Vasculitis | 68 | (3) | NA | |
| Polymyalgia rheumatica | 85 | (4) | NA | |
| Sjogren’s disease | 129 | (6) | NA | |
| Raynaud’s disease | 52 | (3) | NA | |
| Gout | 87 | (4) | NA | |
| MCTD | 16 | (0.8) | NA | |
| Sarcoidosis | 6 | (0.3) | NA | |
| Scleroderma | 49 | (2) | NA | |
| Other rheumatic diseases | 88 | (4) | NA | |
| Immunosuppressive medication, no. (%) | ||||
| No immunosuppressive medication | 483 | (23) | NA | |
| csDMARDs | 1072 | (52) | NA | |
| Methotrexate | 788 | (38) | NA | |
| Biologicals | 785 | (38) | NA | |
| TNF inhibitor | 317 | (15) | NA | |
| Anti-CD20 therapy | 49 | (2) | NA | |
| Oral glucocorticoids | 83 | (4) | NA | |
Data are mean±SD or n (%). Educational levels were based on the International Standard Classification of Education (ISCED). Other rheumatic diseases included myositis, dermatomyositis, polymyositis, reactive arthritis, relapsing polychondritis, remitting seronegative symmetrical synovitis with pitting oedema, IgG4-mediated diseases, SAPHO syndrome, eosinophilic fasciitis and diffuse idiopathic skeletal hyperostosis. Other bDMARDs were ustekinumab, secukinumab, anakinra, ixekizumab and sarilumab. Other csDMARDs were leflunomide, azathioprine, ciclosporine and gold injections. One person can be diagnosed with more than one autoimmune disease and receive more than one immunosuppressive drug
*There were missing values, percentage are displayed as valid percentages.
bDMARDs, biological disease-modifying antirheumatic drugs; csDMARDs, conventional synthetic disease-modifying drugs; IMID, immune-mediated inflammatory disease; NA, not applicable; TNF, tumour necrosis factor.
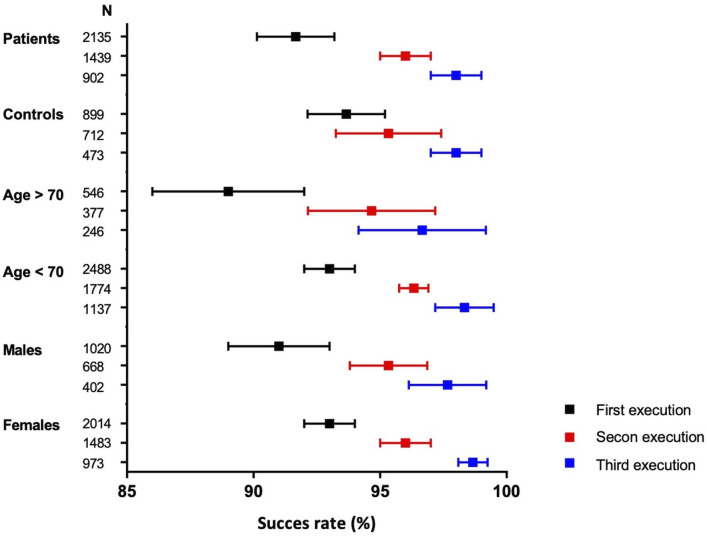
The first finger prick was successfully executed by 1959 of 2135 (92%, 95% CI: 90% to 93%) patients with a rheumatic disease and by 842 of 899 (94%, 95% CI: 92% to 95%) healthy controls (figure 1). Success rates for the first finger prick were similar across age groups and remained high among participants aged 70 years and older (89%, 95% CI: 86% to 92%, figure 1, online supplemental appendix, p5). Sex did not impact success rates (figure 1). Repeated failure (twice) occurred in 11 of 1439 (0.8%) patients and 4 of 712 (0.6%) controls (online supplemental appendix, p2). A first failed attempt therefore might not imply repeated failure. The success rate increased for the second and third execution, with an overall success rate of 96% for the second execution and 98% for the third execution (online supplemental appendix, p2). Characteristics of the participants who executed the finger prick at least one, two or three time(s) are comparable (online supplemental appendix, p3).
Figure 1.
Success rates for the first, second and third execution of the finger prick stratified for participant status, age and sex.
In total, 89 (52%) of 170 participants who failed to perform a correct finger prick indicated that they did collect the required minimum three drops of blood as described in the instruction form (online supplemental appendix, p4). The two most common reasons for perceived failure of the finger prick were related to insufficient bleeding after applying the finger prick. Forty-seven of 124 (38%) participants who failed the finger prick and 465 of 1845 (25%) who succeeded sought assistance for the execution. Overall, participant experience with the finger prick was largely positive. As might be expected, participants who were successful in their attempt to collect enough serum were more frequently positive towards the finger prick compared with participants who were unsuccessful (57% vs 27%, respectively).
Finally, participants were questioned about their preference for a particular sampling method for individual healthcare and for scientific research (table 2). A total of 492 of 1575 (31%) patients and 339 of 556 (61%) controls were willing to perform a finger prick for scientific research, and 300 of 1575 (19%) patients and 214 of 556 (39%) controls preferred a finger prick for healthcare. Thus, both patients and controls seemed less willing to perform a finger prick for individual healthcare compared with scientific research. Participants displayed more confidence in the execution and laboratory measurements when blood was drawn via a venepuncture compared with a finger prick (online supplemental appendix, p5). The most important reason for participants to choose for a finger prick was absence of travelling time and not being dependent on opening hours or appointments of a healthcare centre.
Table 2.
Preference of serum sampling methods in different settings
| Patients (n=1575) |
Controls (n=556) |
|||||
| Preference for scientific research purposes | N | Proportion (%) | (95% CI) | N | Proportion (%) | (95% CI) |
| Finger prick at home | 492 | 31 | (29 to 34) | 339 | 61 | (57 to 65) |
| Venepuncture at healthcare institute | 559 | 36 | (33 to 38) | 56 | 10 | (8 to 13) |
| No preference | 523 | 33 | (31 to 36) | 161 | 29 | (25 to 33) |
| Preference for healthcare purposes | ||||||
| Finger prick at home | 300 | 19 | (17 to 21) | 214 | 39 | (34 to 43) |
| Venepuncture at healthcare institute | 681 | 43 | (41 to 46) | 103 | 19 | (15 to 22) |
| No preference | 594 | 38 | (35 to 40) | 239 | 43 | (39 to 47) |
Data are n and proportion with corresponding 95% CI.
Discussion
During the COVID-19 pandemic, we longitudinally collected clinical and serological data of more than 3000 patients with rheumatic diseases and 1000 healthy controls by using digital questionnaires and finger pricks to facilitate remote data collection. At three separate timepoints during follow-up, we needed to collect serum samples of the entire cohort within a short timeframe. This study demonstrates that over two-third of these serum samples were collected via home finger pricks, thereby indicating that large-scale implementation of a finger prick is feasible, at least in a research setting. We also demonstrated that the vast majority of participants, among which elderly and patients with a rheumatic disease of whose hand function may be impaired, were able to successfully draw the required amount of blood for serological analyses. In case of a failed attempt, a second attempt was almost always successful. This shows that the finger-prick testing is suitable for a high-throughput implementation to monitor patients with rheumatic diseases remotely, which will likely contribute to improving the efficiency and cost-effectiveness of both healthcare and scientific research.
Finger-prick testing can potentially be used for many applications that are widely used within rheumatology, for example measuring therapeutic biologicals,9 10 or blood inflammatory markers such as C reactive protein,7 especially since current assays are very sensitive and do not require large volumes of blood. However, a considerable number of patients with rheumatic diseases indicated restraint towards using the finger prick, especially for healthcare purposes. This reluctance was for an important part caused by lack of confidence in the serological analyses performed in capillary blood compared with the golden standard of venous blood. This might be improved by proper education by their healthcare professional and additional validation studies for certain laboratory tests.
A limitation of the use of the finger prick is the relative low blood volume obtained, allowing only limited analyses to be performed. However, it is possible to obtain between on average more than 100 µL of serum/plasma from a single finger prick, which is sufficient to perform multiple routine ISO15189 validated diagnostic tests in rheumatic diseases, such as antinuclear antibodies, anticitrullinated protein antibody and rheumatoid factor testing (unpublished data). Of note, even though high volumes of blood are normally obtained from a venipuncture only a small amount of blood is actually required due to the high sensitivity and specificity of the diagnostic tests. Another limitation is that we currently cannot verify whether the intended patient collected the sample or if someone else did. To solve this, digital tools could be used to add a form of verification using an application on the mobile phone. Third, the finger prick could potentially be lost by the postal service, but this could be tackled by using digital track-and-trace. Fourth, we have no data on the presence of joint deformities, and can therefore not assess how this affects the performance of the finger prick. However, joint deformities are becoming much rarer due to improved treatment options for rheumatic diseases, so we expect this will not impair widespread implementation of the finger prick in rheumatology. Lastly, external validation studies are needed to confirm whether widespread implementation of the finger prick for healthcare and research is possible.
In conclusion, we demonstrated that the combination of a digital research platform and the use of the finger prick facilitates fast and efficient data and blood sample collection. Our data might help to further improve the process of serum collection via finger pricks and increase patients’ willingness to use a finger-prick test. This study will hopefully also raise awareness of the ease and potential of the use of home finger-prick testing for healthcare and scientific research purposes both within and outside the borders of rheumatology.
Acknowledgments
Data included in this manuscript have been presented on a poster at the EULAR 2023 conference in Milan.
Footnotes
Contributors: YRB and LB wrote the first draft of the manuscript; all other authors revised the manuscript for important intellectual content. YRB, LB and FH performed the statistical analyses. TR, MS, SK and JK performed serological assays; all other authors contributed in data acquisition. All authors met the criteria for authorship set by the International Committee of Medical Journal Editors.
Funding: ZonMw and Reade Foundation provided funding for the study.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
We intend to share deidentified participant level data on request after we have published all data on our predefined research objectives. Researchers who are interested in doing additional analyses using these data can contact the corresponding author. Data can only be used for scientific research without conflict of interests. After data collection has been completed and data on our predefined research objectives has been published, there will be no end date before which researchers can request access to the data. Additional documents that will be made available on request are the protocol (including all amendments), and informed consent forms.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by The research protocol was approved by the medical ethical committee of the VU University medical centre (registration number 2020-169). Participants gave informed consent to participate in the study before taking part.
References
- 1.Boekel L, Hooijberg F, Vogelzang EH, et al. Spinning straw into gold: description of a disruptive rheumatology research platform inspired by the COVID-19 pandemic. Arthritis Res Ther 2021;23:207. 10.1186/s13075-021-02574-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boekel L, Hooijberg F, Vogelzang EH, et al. Antibody development and disease severity of COVID-19 in non-Immunised patients with rheumatic immune-mediated inflammatory diseases: data from a prospective cohort study. RMD Open 2022;8:e002035. 10.1136/rmdopen-2021-002035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boekel L, Stalman EW, Wieske L, et al. Breakthrough SARS-Cov-2 infections with the Delta (B.1.617.2) variant in vaccinated patients with immune-mediated inflammatory diseases using immunosuppressants: a Substudy of two prospective cohort studies. Lancet Rheumatol 2022;4:e417–29. 10.1016/S2665-9913(22)00102-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boekel L, Besten YR, Hooijberg F, et al. SARS-Cov-2 breakthrough infections in patients with immune-mediated inflammatory diseases during the Omicron dominant period [published online ahead of print]. The Lancet Rheumatology 2022;4:e747–50. 10.1016/S2665-9913(22)00221-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raptis CE, Andrey DO, Polysopoulos C, et al. Op0175 type of mRNA Covid-19 vaccine and treatment influence antibody Kinetics in patients with inflammatory rheumatic diseases. Ann Rheum Dis 2022;81:115. 10.1136/annrheumdis-2022-eular.1796 [DOI] [Google Scholar]
- 6.Pichler J, Zilbauer M, Torrente F, et al. Feasibility of a finger prick-based self-testing kit in First- and second-degree relatives of children with coeliac disease. World J Gastroenterol 2011;17:1840–3. 10.3748/wjg.v17.i14.1840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knitza J, Tascilar K, Vuillerme N, et al. Accuracy and tolerability of self-sampling of capillary blood for analysis of inflammation and Autoantibodies in rheumatoid arthritis patients-results from a randomized controlled trial. Arthritis Res Ther 2022;24. 10.1186/s13075-022-02809-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vogelzang EH, Loeff FC, Derksen NIL, et al. Development of a SARS-Cov-2 total antibody assay and the dynamics of antibody response over time in hospitalized and Nonhospitalized patients with COVID-19. J Immunol 2020;205:3491–9. 10.4049/jimmunol.2000767 [DOI] [PubMed] [Google Scholar]
- 9.Kneepkens EL, Pouw MF, Wolbink GJ, et al. Dried blood spots from finger prick facilitate therapeutic drug monitoring of Adalimumab and anti-Adalimumab in patients with inflammatory diseases. Br J Clin Pharmacol 2017;83:2474–84. 10.1111/bcp.13371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berends SE, D’Haens GRAM, Schaap T, et al. Dried blood samples can support monitoring of Infliximab concentrations in patients with inflammatory bowel disease: A clinical validation. Br J Clin Pharmacol 2019;85:1544–51. 10.1111/bcp.13939 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2023-003933supp001.pdf (451.2KB, pdf)
Data Availability Statement
We intend to share deidentified participant level data on request after we have published all data on our predefined research objectives. Researchers who are interested in doing additional analyses using these data can contact the corresponding author. Data can only be used for scientific research without conflict of interests. After data collection has been completed and data on our predefined research objectives has been published, there will be no end date before which researchers can request access to the data. Additional documents that will be made available on request are the protocol (including all amendments), and informed consent forms.