Abstract
Objective
Colonoscopy is useful in diagnosing intestinal tuberculosis. However, the terminal ileum is generally not examined during routine colonoscopy. Therefore, even with colonoscopy, the diagnosis can be missed in patients with lesions confined to the terminal ileum. Herein, we report the case of an asymptomatic patient with intestinal tuberculosis, in whom a colonoscope insertion into the terminal ileum led to the diagnosis.
Patient
An asymptomatic 71-year-old man visited our hospital for a colonoscopy after a positive fecal occult blood test.
Results
Colonoscopy revealed diffuse edematous and erosive mucosa in the terminal ileum. Mycobacterium tuberculosis was detected by polymerase chain reaction and culture of biopsy specimens from the erosions, leading to the diagnosis of intestinal tuberculosis. The patient was treated with antitubercular agents for 6 months, and a follow-up colonoscopy revealed healing of the lesions.
Conclusion
Asymptomatic intestinal tuberculosis may occasionally be detected on colonoscopy following a positive fecal occult blood test and is sometimes confined to the terminal ileum. Therefore, clinicians should consider intestinal tuberculosis in the differential diagnosis of the causes of positive fecal occult blood test results and perform colonoscopies, including observation of the terminal ileum.
Keywords: colonoscopy, fecal occult blood test, intestinal tuberculosis
Introduction
Tuberculosis is an infectious disease caused by Mycobacterium tuberculosis and is the thirteenth leading cause of death worldwide1). Despite a steady decline in the worldwide incidence of tuberculosis, its prevalence remains high, especially in developing countries. One-quarter of the global population is estimated to have latent tuberculosis1). In addition, the World Health Organization reported that 10.6 million individuals worldwide were diagnosed with tuberculosis in 2021, of whom 1.6 million died from the disease2). Tuberculosis is a major infectious disease worldwide.
Tuberculosis is primarily a lung infection but can affect other body organs. Extrapulmonary tuberculosis accounts for approximately 20% of tuberculosis cases, and intestinal tuberculosis accounts for about 10% of extrapulmonary tuberculosis cases1). A resurgence of intestinal tuberculosis occurred in the last decade following an increase in immigrants to Western countries3). Patients with intestinal tuberculosis commonly present with nonspecific clinical manifestations such as fever, abdominal pain, diarrhea, weight loss, and fatigue4, 5). However, asymptomatic cases of intestinal tuberculosis are occasionally observed5,6,7,8,9), and the lack of typical manifestations may delay diagnosis5, 8). Confirming intestinal tuberculosis in asymptomatic individuals is challenging and may lead to incorrect or missed diagnosis1, 5).
Colonoscopy is useful in diagnosing intestinal tuberculosis5), and the terminal ileum and cecum are the most frequently affected8). However, the terminal ileum is generally unexamined during routine colonoscopy5, 9). Therefore, even with colonoscopy, the diagnosis can be missed in patients with lesions confined to the terminal ileum. Herein, we report a case of an asymptomatic patient with intestinal tuberculosis in whom a colonoscope insertion into the terminal ileum led to the diagnosis. We also discuss the key points in diagnosing asymptomatic intestinal tuberculosis based on a review of the relevant literature.
Case Presentation
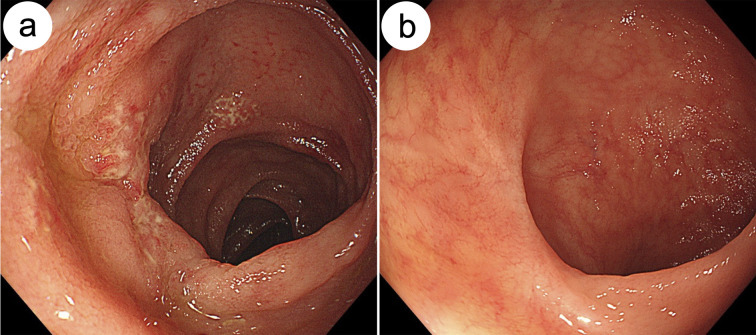
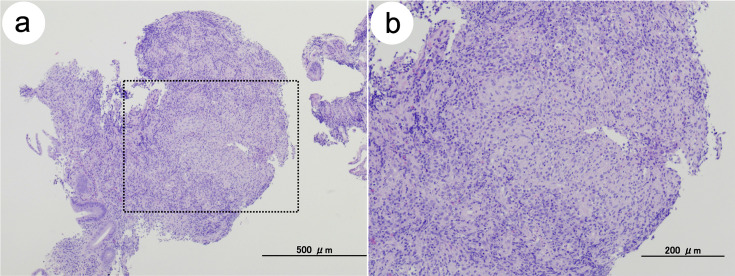
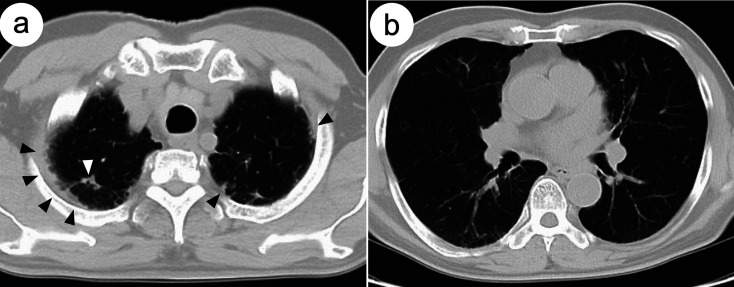
An asymptomatic 71-year-old man visited our hospital for a colonoscopy after obtaining a positive fecal occult blood test (FOBT) during a routine medical checkup. The colonoscopy revealed diffuse edematous mucosa and multiple erosions with scars in the terminal ileum (Figure 1a). Biopsy specimens of these erosions showed epithelioid granulomas without caseating necrosis (Figure 2a and 2b). Ziehl–Neelsen staining for acid-fast bacilli yielded a negative result. However, Mycobacterium tuberculosis was detected in biopsy samples using polymerase chain reaction (PCR), and a tuberculosis-specific interferon-γ release assay (IGRA) (T-SPOT.TB) was positive (Table 1). In addition, cultures of biopsy specimens taken from these erosions revealed the presence of Mycobacterium tuberculosis after 2 weeks. Based on these findings, the patient was diagnosed with intestinal tuberculosis. He had a history of pulmonary tuberculosis. Chest computed tomography revealed inflammatory nodules and pleural thickening in the pulmonary apex regions (Figure 3a and 3b), consistent with the previous pulmonary tuberculosis. To exclude active pulmonary tuberculosis, sputum examinations were performed thrice. Sputum smears and culture tests were negative, and PCR revealed the absence of Mycobacterium tuberculosis deoxyribonucleic acid in the sputum specimens. Thus, lesions found in the terminal ileum on colonoscopy were considered secondary intestinal tuberculosis, arising from pulmonary tuberculosis.
Figure 1.
Endoscopic findings of the terminal ileum before (a) and after (b) the antitubercular treatment.
(a) Colonoscopy showing edema, indicated by the lack of visible vascular pattern and mucosal erosions with scars in the terminal ileum. (b) Colonoscopy after completion of antitubercular treatment showing a lack of lesions in the terminal ileum.
Figure 2.
Pathological findings before the initiation of antitubercular treatment.
(a) Hematoxylin and eosin staining of the biopsy specimen showing granulation tissue formation, consistent with an epithelioid granuloma. Caseating necrosis is absent. Scale bar, 500 μm. (b) An enlarged image of the regions enclosed within the dotted line shown in (a). Scale bar, 200 μm.
Table 1. Laboratory data at the time of diagnosis of intestinal tuberculosis.
| Parameter | Data | N.R. | Parameter | Data | N.R. |
|---|---|---|---|---|---|
| WBC (cells/μL) | 7,290 | 3,300–8,600 | Na (mmol/L) | 135 | 138–145 |
| Neut (%) | 54.0 | 38–74 | K (mmol/L) | 4.5 | 3.6–4.8 |
| Lym (%) | 25.8 | 16.5–49.5 | Cl (mmol/L) | 103 | 101–108 |
| RBC (×106/μL) | 4.63 | 4.35–5.55 | T.Bil (mg/dL) | 0.5 | 0.4–1.5 |
| Hb (g/dL) | 14.2 | 13.7–16.8 | AST (U/L) | 30 | 13–30 |
| Ht (%) | 40.7 | 40.7–50.1 | ALT (U/L) | 28 | 10–42 |
| Plt (×103/μL) | 194 | 158–348 | ALP (U/L) | 265 | 106–322 |
| PT (%) | 100 | 70–130 | γ-GTP (U/L) | 49 | 13–64 |
| TP (g/dL) | 7.9 | 6.6–8.1 | ChE (IU/L) | 282 | 240–486 |
| Alb (g/dL) | 4.2 | 4.1–5.1 | Amy (U/L) | 83 | 44–132 |
| TC (mg/dL) | 156 | 142–248 | CRP (mg/dL) | 1.07a | <0.14 |
| HDL-C (mg/dL) | 40 | 38–90 | ESR 30 min (mm) | 5 | 2–10 |
| LDL-C (mg/dL) | 88 | 65–163 | 60 min (mm) | 28a | 2–10 |
| TG (mg/dL) | 139 | 40–243 | CEA (ng/mL) | 3.0 | <5.0 |
| FBS (mg/dL) | 83 | 73–109 | CA19-9 (U/mL) | 14.5 | <37.0 |
| HbA1c (%) | 5.0 | 4.9–6.0 | T-SPOT.TB | (+) | (–) |
| UN (mg/dL) | 11.9 | 8.0–20.0 | HBs-Ag | (–) | (–) |
| Cr (mg/dL) | 0.93 | 0.65–1.07 | HCV-Ab | (–) | (–) |
aIncreased compared with normal range. Alb: albumin; ALP: alkaline phosphatase; ALT: alanine aminotransferase; Amy: amylase; AST: aspartate aminotransferase; CA19-9: CA19-9 antigen; CEA: carcinoembryonic antigen; ChE: cholinesterase; Cl: chloride; Cr: creatinine; CRP: C-reactive protein; ESR: erythrocyte sedimentation rate; FBS: fasting blood glucose; γ-GTP: gamma-glutamyl transpeptidase; Hb: hemoglobin; HbA1c: hemoglobin A1c; HBs-Ag: hepatitis B surface antigen; HCV-Ab: hepatitis C virus antibody; HDL-C: high-density lipoprotein cholesterol; Ht: hematocrit; K: potassium; LDL-C: low-density lipoprotein cholesterol; Lym: lymphocyte; Na: sodium; Neut: neutrophil; N.R.: normal range; Plt: platelet; PT: prothrombin; RBC: red blood cell; T.Bil: total bilirubin; TC: total cholesterol; TG: triglyceride; T-SPOT.TB: tuberculosis specific interferon-γ releasing assay; TP: total protein; UN: urea nitrogen; WBC: white blood cell.
Figure 3.
Chest computed tomography images before antitubercular treatment.
Chest CT scan images showing: (a) inflammatory nodules (white arrow) and pleural thickening (black arrows) in the pulmonary apex regions, (a and b) emphysematous changes in the apical and middle lung fields. CT, computed tomography.
The patient was asymptomatic and had no abnormal physical findings, such as anemia or emaciation, at the time of diagnosis (Table 1). Blood examination revealed increased C-reactive protein (CRP) level and erythrocyte sedimentation rate (ESR), suggesting a chronic inflammatory state (Table 1). Therefore, the patient was required to undergo antitubercular treatment according to the international guidelines for tuberculosis10). The patient was treated with isoniazid, rifampicin, ethambutol, and pyrazinamide for 2 months, followed by isoniazid and rifampicin for an additional 4 months. During treatment, the patient was followed up every 2 weeks to assess any treatment-related adverse events. Two weeks after initiating antitubercular treatment, small skin eruptions with slight itchiness developed on his extremities and trunk. However, these eruptions improved following oral administration of an antihistamine. The patient completed the treatment regimen without additional adverse events. A follow-up colonoscopy revealed healing of the mucosal edema and erosions in the terminal ileum (Figure 1b). Biopsy specimens obtained from the scars showed fibrosis and slight infiltration of inflammatory cells into the stroma; however, no epithelioid granulomas or caseating necrosis were observed. In addition, a PCR test and a 6-week culture of the biopsy specimens confirmed the absence of Mycobacterium tuberculosis. The CRP levels and ESR returned to normal after antitubercular treatment. Intestinal tuberculosis did not recur during the 3 years of follow-up.
Discussion
Individuals with intestinal tuberculosis commonly present with nonspecific clinical manifestations, such as fever, abdominal pain, diarrhea, weight loss, and fatigue1, 4, 5); however, symptoms are occasionally absent1, 5,6,7). The diverse and nonspecific clinical presentations of intestinal tuberculosis make it difficult to diagnose11). Diagnosing asymptomatic intestinal tuberculosis is particularly challenging, leading to incorrect or missed diagnosis1, 5). Untreated intestinal tuberculosis can cause severe morbidity, leading to prolonged hospitalization and surgery11). Therefore, understanding the features of asymptomatic intestinal tuberculosis is essential for adequate disease management.
Fifteen asymptomatic intestinal tuberculosis cases, including this case, have been reported since 2004 (Table 2). A colonoscopy was performed on all patients. Twelve of the 15 patients (Cases 1–6 and 9–14) had lesions in the large intestine, and three (Cases 7, 8, and 15), including our patient, had lesions only in the terminal ileum (Table 3). In our patient (Case 15), the lesions were discovered after an incidental colonoscope insertion into the terminal ileum during the initial colonoscopy, leading to the diagnosis of intestinal tuberculosis. The terminal ileum is not routinely examined during colonoscopy5, 9); therefore, missed diagnoses may occur in patients with lesions localized to the terminal ileum. In Case 8, the lesions were confined to the terminal ileum and were overlooked during the initial colonoscopy5). Considering that the terminal ileum is one of the most frequent sites of intestinal tuberculosis8, 11), colonoscopy and observation of the terminal ileum are essential for diagnosing intestinal tuberculosis.
Table 2. Characteristics of the asymptomatic patients with intestinal tuberculosis (clinical profile).
| Author, year | Case no. | Age (years)/sex | Clinical symptoms | Reason for CS | Underlying disease | Refs. |
|---|---|---|---|---|---|---|
| Sato et al., 2004 | 1 | 64 F | None | FOBT positive | N/A | 5) |
| Sato et al., 2004 | 2 | 55 F | None | FOBT positive | N/A | 5) |
| Sato et al., 2004 | 3 | 69 F | None | FOBT positive | N/A | 5) |
| Sato et al., 2004 | 4 | 65 F | None | FOBT positive | N/A | 5) |
| Sato et al., 2004 | 5 | 58 F | None | FOBT positive | N/A | 5) |
| Sato et al., 2004 | 6 | 84 M | None | FOBT positive | N/A | 5) |
| Sato et al., 2004 | 7 | 74 M | None | Follow up of colon polyps | N/A | 5) |
| Sato et al., 2004 | 8 | 56 M | None | Postoperative surveillance | N/A | 5) |
| Yang et al., 2007 | 9 | 38 M | None | Medical checkup | None | 6) |
| Yamane et al., 2013 | 10 | 47 M | None | FOBT positive | None | 7) |
| Yamane et al., 2013 | 11 | 72 F | None | FOBT positive | None | 7) |
| Inoue et al., 2017 | 12 | 62 F | None | FOBT positive | None | 8) |
| Lin et al., 2022 | 13 | N/A | None | Medical checkup | N/A | 9) |
| Lin et al., 2022 | 14 | N/A | None | Medical checkup | N/A | 9) |
| Current study | 15 | 71 M | None | FOBT positive | Hypertension | N/A |
CS: colonoscopy; FOBT: fecal occult blood test; F: female; M: male; N/A: not applicable; No.: number; Refs.: references.
Table 3. Characteristics of the asymptomatic patients with intestinal tuberculosis (lesion site and endoscopic findings).
| Author, year | Case no. | Lesion site | Endoscopic findings | Refs. |
|---|---|---|---|---|
| Sato et al., 2004 | 1 | Transverse colon | Annular ulcer with flared surrounding nodules | 5) |
| Sato et al., 2004 | 2 | Ascending colon | Annular ulcer with flared surrounding nodules | 5) |
| Sato et al., 2004 | 3 | Ascending colon | Annular ulcer with flared surrounding nodules | 5) |
| Sato et al., 2004 | 4 | Transverse colon | Small ulcers without surrounding nodules | 5) |
| Sato et al., 2004 | 5 | Ascending colon | Small ulcers without surrounding nodules | 5) |
| Sato et al., 2004 | 6 | Sigmoid colon | Multiple erosions, mucosal edema | 5) |
| Sato et al., 2004 | 7 | Terminal ileum | Aphthous ulcer, erosions | 5) |
| Sato et al., 2004 | 8 | Terminal ileum | Aphthous ulcer, erosions | 5) |
| Yang et al., 2007 | 9 | Cecum | Annular ulcer with flared surrounding nodules | 6) |
| Yamane et al., 2013 | 10 | Terminal ileum–Ascending colon | Annular ulcer, scarring mucosa | 7) |
| Yamane et al., 2013 | 11 | Terminal ileum–Ascending colon | Annular ulcer, multiple erosions | 7) |
| Inoue et al., 2017 | 12 | Cecum–Ascending colon | Annular ulcer with scars | 8) |
| Lin et al., 2022 | 13 | Terminal ileum–Cecum | Small ulcers with irregular fold | 9) |
| Lin et al., 2022 | 14 | Terminal ileum– Cecum | Small ulcers with irregular fold | 9) |
| Current study | 15 | Terminal ileum | Multiple erosions, mucosal edema | N/A |
N/A: not applicable; No.: number; Refs.: references.
A positive FOBT result led to the diagnosis in 10 of the 15 cases of asymptomatic intestinal tuberculosis presented in Table 2. FOBT, particularly immunological FOBT (IFOBT), is widely used for colorectal cancer (CRC) screening because its sensitivity is higher than that of guaiac-based FOBT12, 13). IFOBT is specific for human hemoglobin and is more sensitive and specific for detecting occult bleeding from any intestinal lesion14). Therefore, IFOBT can show a positive reaction to lesions with occult lower gastrointestinal bleeding, including CRC. A prospective cross-sectional study involving 200 asymptomatic subjects in Bangladesh confirmed positive IFOBT results in 90 patients. Eighty of them underwent colonoscopy, and intestinal tuberculosis was identified in five patients (6%)14). This indicates that a positive FOBT result can lead to a diagnosis of intestinal tuberculosis in asymptomatic individuals, although the prevalence of intestinal tuberculosis differs by country and region11). Therefore, clinicians should consider intestinal tuberculosis in the differential diagnosis of patients with positive FOBT results without gastrointestinal symptoms.
Several diagnostic modalities, including pathological examination, acid-fast bacilli staining, culture, PCR of biopsy specimens, and IGRA, have been used to diagnose intestinal tuberculosis. The sensitivity and specificity of each modality have been reported to be 68% and 77.1% for pathological examination15), 17.3%–31% and 100% for acid-fast staining1), 9.3% and 100% for culture1), 42% and 97% for PCR16), and 74%–88% and 74%–87% for IGRA17,18,19,20). Except for IGRA, the sensitivity of these modalities is relatively low, and the risk of obtaining false-negative results is high. Therefore, a combination of diagnostic modalities is required for diagnose21). Several diagnostic modalities were used in all asymptomatic intestinal tuberculosis cases, as shown in Table 4, and a combination of these modalities helped diagnose intestinal tuberculosis in 14 of 15 cases (Cases 2–15). Case 1 had nonspecific findings on pathological or bacteriological tests for intestinal tuberculosis screening (Table 4). However, the patient was suspected to have the disease based on typical endoscopic findings (Table 3). Our patient also had nonspecific findings on pathological examination and acid-fast bacilli staining (Ziehl–Neelsen staining). However, the results of both culture and PCR of biopsy specimens facilitated the diagnosis of intestinal tuberculosis (Table 4). A recent report demonstrated that a combination of history-taking, physical examination, and several diagnostic modalities can improve diagnosis accuracy and prevent underdiagnosis1). Thus, clinicians should use a combination of diagnostic modalities for suspected intestinal tuberculosis.
Table 4. Characteristics of the asymptomatic patients with intestinal tuberculosis (pathological findings and bacteriological results).
| Author, year | Case no. | Caseating granuloma | Non-caseating granuloma | Ziehl–Neelsen staining | Culture of biopsy samples | PCR of biopsy samples | IGRA | Refs. |
|---|---|---|---|---|---|---|---|---|
| Sato et al., 2004 | 1 | − | + | − | − | N/A | N/A | 5) |
| Sato et al., 2004 | 2 | − | + | − | + (M. tuberculosis) | N/A | N/A | 5) |
| Sato et al., 2004 | 3 | − | + | − | + (M. tuberculosis) | N/A | N/A | 5) |
| Sato et al., 2004 | 4 | − | − | − | + (M. tuberculosis) | N/A | N/A | 5) |
| Sato et al., 2004 | 5 | − | − | − | + (M. tuberculosis) | N/A | N/A | 5) |
| Sato et al., 2004 | 6 | − | − | + | − | N/A | N/A | 5) |
| Sato et al., 2004 | 7 | + | − | − | − | N/A | N/A | 5) |
| Sato et al., 2004 | 8 | − | + | − | + (M. tuberculosis) | N/A | N/A | 5) |
| Yang et al., 2007 | 9 | + | − | − | + (M. tuberculosis) | + | N/A | 6) |
| Yamane et al., 2013 | 10 | − | + | − | − | − | + | 7) |
| Yamane et al., 2013 | 11 | − | − | − | − | − | + | 7) |
| Inoue et al., 2017 | 12 | − | − | − | − | − | + | 8) |
| Lin et al., 2022 | 13 | − | + | + | − | N/A | N/A | 9) |
| Lin et al., 2022 | 14 | − | − | − | − | N/A | + | 9) |
| Current study | 15 | − | + | − | + (M. tuberculosis) | + | + | N/A |
IGRA: Interferon gamma release assay; N/A: not applicable; No.: number; Refs.: references.
The primary treatment for intestinal tuberculosis is oral administration of antitubercular agents, including isoniazid, rifampin, ethambutol, and pyrazinamide10). International guidelines recommend 6 months of therapy with standard regimens for pulmonary tuberculosis10), which is sufficient to achieve a response. Prolonged treatment for more than 6 months showed no additional benefit22). A small proportion of patients may require surgery due to complications, such as intestinal strictures, despite the administration of oral antitubercular agents23, 24). None of the asymptomatic intestinal tuberculosis cases identified in the literature review required surgery. All patients were cured after receiving oral antitubercular agents, although the details of each treatment regimen and period were not fully reported for all patients5,6,7,8,9). Thus, the oral administration of antitubercular agents effectively treats asymptomatic intestinal tuberculosis.
Conclusion
This study describes a case of asymptomatic intestinal tuberculosis in which a colonoscope insertion into the terminal ileum was critical for diagnosis. Asymptomatic intestinal tuberculosis can occasionally be discovered by colonoscopy after a positive FOBT result, and the lesions are sometimes present only in the terminal ileum. Therefore, clinicians need to consider intestinal tuberculosis in the differential diagnosis of the causes of positive FOBT results and perform colonoscopies, including observation of the terminal ileum.
Conflict of interest
The authors declare that they have no conflict of interest.
Funding information
No funding was received.
Ethics approval and consent to participate
Ethical approval was obtained from the Ethics Committee of the Gifu University Graduate School of Medicine (approval number: 2023-S01). Consent was obtained from the patient in this study.
Consent for publication
Written informed consent was provided by the patient for the publication of this study.
Data availability statement
The anonymized patient data used in this study are all included in the text.
Author contributions
HS and MS contributed to the study conception and design. HS, HI, JT, MK, TI, and YS performed the case study and acquired the data and images. HS analyzed and interpreted the data and wrote the manuscript. HS, TI, YS, and MS revised the manuscript. MS supervised manuscript preparation. All the authors have read and approved the final version of the manuscript.
References
- 1.Maulahela H, Simadibrata M, Nelwan EJ, et al. Recent advances in the diagnosis of intestinal tuberculosis. BMC Gastroenterol 2022; 22: 89. doi: 10.1186/s12876-022-02171-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Global Tuberculosis Report 2022. 2022. https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2022. Accessed November 17, 2023.
- 3.Alvares JF, Devarbhavi H, Makhija P, et al. Clinical, colonoscopic, and histological profile of colonic tuberculosis in a tertiary hospital. Endoscopy 2005; 37: 351–356. doi: 10.1055/s-2005-861116 [DOI] [PubMed] [Google Scholar]
- 4.Marshall JB. Tuberculosis of the gastrointestinal tract and peritoneum. Am J Gastroenterol 1993; 88: 989–999. [PubMed] [Google Scholar]
- 5.Sato S, Yao K, Yao T, et al. Colonoscopy in the diagnosis of intestinal tuberculosis in asymptomatic patients. Gastrointest Endosc 2004; 59: 362–368. doi: 10.1016/S0016-5107(03)02716-0 [DOI] [PubMed] [Google Scholar]
- 6.Yang CC, Chen CH, Yan SL. Endoscopic detection of colonic tuberculosis in an asymptomatic patient. Endoscopy 2007; 39(Suppl 1): E40. doi: 10.1055/s-2006-945059 [DOI] [PubMed] [Google Scholar]
- 7.Yamane T, Umeda A, Shimao H. Analysis of recent cases of intestinal tuberculosis in Japan. Intern Med 2014; 53: 957–962. doi: 10.2169/internalmedicine.53.1862 [DOI] [PubMed] [Google Scholar]
- 8.Inoue I, Ichinose M, Maekita T, et al. A case of colonic tuberculosis in asymptomatic patients. J Wakayama Med Soc 2017; 68: 137–138(in Japanese, Abstract in English) [Google Scholar]
- 9.Lin YC, Liao SC, Chang CH, et al. Endoscopic features and clinical course of patients with asymptomatic cecal ulcers. BMC Gastroenterol 2022; 22: 309. doi: 10.1186/s12876-022-02383-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blumberg HM, Burman WJ, Chaisson RE, et al. American Thoracic Society, Centers for Disease Control and Prevention and the Infectious Diseases Society.American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: treatment of tuberculosis. Am J Respir Crit Care Med 2003; 167: 603–662. doi: 10.1164/rccm.167.4.603 [DOI] [PubMed] [Google Scholar]
- 11.Al-Zanbagi AB, Shariff MK. Gastrointestinal tuberculosis: a systematic review of epidemiology, presentation, diagnosis and treatment. Saudi J Gastroenterol 2021; 27: 261–274. doi: 10.4103/sjg.sjg_148_21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allison JE, Tekawa IS, Ransom LJ, et al. A comparison of fecal occult-blood tests for colorectal-cancer screening. N Engl J Med 1996; 334: 155–159. doi: 10.1056/NEJM199601183340304 [DOI] [PubMed] [Google Scholar]
- 13.Castiglione G, Zappa M, Grazzini G, et al. Immunochemical vs guaiac faecal occult blood tests in a population-based screening programme for colorectal cancer. Br J Cancer 1996; 74: 141–144. doi: 10.1038/bjc.1996.329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mollick SH, Roy PK, Bhuiyan MR, et al. Prevalence of colorectal diseases in immunological fecal occult blood test (I-FOBT) positive patients in a tertiary care hospital in Bangladesh. Mymensingh Med J 2014; 23: 764–769. [PubMed] [Google Scholar]
- 15.Mehta V, Desai D, Abraham P, et al. Making a positive diagnosis of intestinal tuberculosis with the aid of new biologic and histologic features: how far have we reached? Inflamm Intest Dis 2019; 3: 155–160. doi: 10.1159/000496482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jin T, Fei B, Zhang Y, et al. The diagnostic value of polymerase chain reaction for Mycobacterium tuberculosis to distinguish intestinal tuberculosis from crohn’s disease: a meta-analysis. Saudi J Gastroenterol 2017; 23: 3–10. doi: 10.4103/1319-3767.199135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen W, Fan JH, Luo W, et al. Effectiveness of interferon-gamma release assays for differentiating intestinal tuberculosis from Crohn’s disease: a meta-analysis. World J Gastroenterol 2013; 19: 8133–8140. doi: 10.3748/wjg.v19.i44.8133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ng SC, Hirai HW, Tsoi KK, et al. Systematic review with meta-analysis: accuracy of interferon-gamma releasing assay and anti-Saccharomyces cerevisiae antibody in differentiating intestinal tuberculosis from Crohn’s disease in Asians. J Gastroenterol Hepatol 2014; 29: 1664–1670. doi: 10.1111/jgh.12645 [DOI] [PubMed] [Google Scholar]
- 19.Limsrivilai J, Shreiner AB, Pongpaibul A, et al. Meta-analytic Bayesian model for differentiating intestinal tuberculosis from Crohn’s disease. Am J Gastroenterol 2017; 112: 415–427. doi: 10.1038/ajg.2016.529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao Y, Xu M, Chen L, et al. Levels of TB-IGRA may help to differentiate between intestinal tuberculosis and Crohn’s disease in patients with positive results. Therap Adv Gastroenterol 2020; 13: 1756284820922003. doi: 10.1177/1756284820922003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patel B, Yagnik VD. Clinical and laboratory features of intestinal tuberculosis. Clin Exp Gastroenterol 2018; 11: 97–103. doi: 10.2147/CEG.S154235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jullien S, Jain S, Ryan H, et al. Six-month therapy for abdominal tuberculosis. Cochrane Database Syst Rev 2016; 11: CD012163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pratap Mouli V, Munot K, Ananthakrishnan A, et al. Endoscopic and clinical responses to anti-tubercular therapy can differentiate intestinal tuberculosis from Crohn’s disease. Aliment Pharmacol Ther 2017; 45: 27–36. doi: 10.1111/apt.13840 [DOI] [PubMed] [Google Scholar]
- 24.Sharma V, Mandavdhare HS, Dutta U. Letter: mucosal response in discriminating intestinal tuberculosis from Crohn’s disease-when to look for it? Aliment Pharmacol Ther 2018; 47: 859–860. doi: 10.1111/apt.14495 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The anonymized patient data used in this study are all included in the text.