Abstract
When the swine alphaherpesvirus pseudorabies virus (PRV) infects the rat retina, it replicates in retinal ganglion cells and invades the central nervous system (CNS) via anterograde transynaptic spread through axons in the optic nerve. Virus can also spread to the CNS via retrograde transport through the oculomotor nucleus that innervates extraocular muscles of the eye. Since retrograde infection of the CNS precedes anterograde transynaptic infection, the temporal sequence of infection of the CNS depends on the route of invasion. Thus, motor neurons are infected first (retrograde infection), followed by CNS neurons innervated by the optic nerve (anterograde transynaptic infection). This temporal separation in the appearance of virus in separate groups of neurons enabled us to compare the immune responses to different stages of CNS infection in the same animal. The data revealed focal trafficking of peripheral immune cells into areas of the CNS infected by retrograde or anterograde transport after PRV Becker was injected into the vitreous body of the eye. Cells expressing the leukocyte common antigen, CD45+, entered the area of infection from local capillaries prior to any overt expression of neuropathology, and quantitative analysis demonstrated that the number of cells increased in proportion to the number of infected neurons within a given region. Recruitment of cells of monocyte/macrophage lineage began prior to the appearance of CD8+ cytotoxic lymphocytes, which were, in turn, followed by CD4+ lymphocytes. These data demonstrate that PRV replication in CNS neurons stimulates the focal infiltration of specific classes of CD45+ cells in a time-dependent, temporally organized fashion that is correlated directly with the number of infected neurons and the time that a given region has been infected.
In recent years, it has become increasingly apparent that cells of hematopoietic origin play an integral role in the response of the nervous system to neurotropic viral infections (for recent reviews, see references 10, 23, and 52). The early work of Townsend (62) demonstrated a reduction in herpes simplex virus type 1 (HSV-1)-induced demyelinating central nervous system (CNS) lesions in nude mice compared to that in intact controls. Thereafter, Simmons and Tscharke (51) showed that HSV-1-induced neuronal destruction in the peripheral nervous system increased in mice depleted of CD8 lymphocytes, suggesting that these lymphocytes are essential in the clearance of virus. A number of subsequent studies demonstrated trafficking of peripheral immune cells into CNS in response to viral infection but reported differing roles for these cells. For example, Subak-Sharpe and colleagues (57) showed that the depletion of CD8+ cytotoxic lymphocytes does not alter the invasiveness or spread of Semliki Forest virus through the CNS but does prevent the demyelinating lesions that occur in intact animals. Similarly, Hudson and Streilein (27) demonstrated that accumulation of lymphocytes in HSV-infected regions of mouse cerebral cortex correlates with the appearance of focal lesions in the region of infection. In contrast, studies examining the role of T lymphocytes in replication, spread, and virus-induced pathogenesis of vesicular stomatitis virus (28) and mouse hepatitis virus (58, 74) demonstrated that CD4+ and CD8+ lymphocytes are important for viral clearance but play only a minor role in the generation of pathology. Some of these differences may be related to unique responses to different strains of virus or the models of infection used in the different analyses. Nevertheless, it remains clear that trafficking of immune cells into the brain is a fundamental response to neurotropic viral invasion.
In spite of the well-documented infiltration of immune cells into the brain, less is known regarding the time course of recruitment of different classes of leukocytes. Weinstein and colleagues (70) demonstrated focal infiltration of granulocytes, T lymphocytes, and monocytes in response to the spread of HSV through the CNS but did not determine the kinetics of appearance of different cell types. T-lymphocyte infiltration into HSV-infected brain tissue has also been reported by Lewandowski and colleagues, but their focus has been on major histocompatibility complex antigen and cytokine expression in response to infection by different HSV strains (34–36). Williamson and coworkers (75) isolated and characterized a heterogeneous population of mononuclear cells recovered from the brains of mice infected with mouse hepatitis virus and reported a temporal association between the peak incidence of CD8+ cells and reduction of the intracerebral concentration of virus. Similarly, an association between the directed infiltration of cells of monocytic lineage and the development of pathological changes in PRV-infected CNS neurons has also been demonstrated (6, 46). These observations suggest that the peripheral immune system mounts a complex multicellular response to viral infections of the CNS, but the mechanisms that lead to the directed recruitment of immune cells to the infection site in the CNS remain to be established. Furthermore, the way in which this response functions to limit virus dissemination through the CNS or to clear an infection requires a more detailed characterization of the kinetics of immune cell recruitment in relation to the extent of viral replication.
In the present investigation, we extended our prior analysis of the spatial and temporal response of resident glial and immune cells to PRV infection of the CNS (6, 46), using a well-characterized model of a viral infection in which virus enters the CNS through visual circuitry (8, 9, 15, 73). An important feature of this model is that viral infection of the CNS is achieved by peripheral inoculation rather than by direct injection of the brain parenchyma. Thus, the brain response to viral infection can be monitored over time and cellular responses to viral replication can be evaluated in the absence of nonspecific damage to the CNS that would result from direct injection. Our experiments demonstrate that the number of leukocytes entering the CNS after lethal infection with virulent PRV correlates with the number of infected neurons and that there are differences in the times when functionally distinct subclasses of immune cells appear in the area of infection.
MATERIALS AND METHODS
Virus.
The virulent Becker strain of PRV was grown in a porcine kidney cell line (PK15) in a biosafety level 2 containment facility. Cells infected with virus were scraped into medium, freeze-thawed, sonicated, centrifuged to clear cellular debris, and stored frozen at −70°C (14). The number of PFU in each viral stock was determined on PK15 cells and adjusted to 108 PFU/ml. Aliquots of virus were thawed immediately prior to use.
Animals and inoculations.
Twenty-three male Sprague-Dawley rats (Harlan Sprague Dawley, Indianapolis, Ind.) weighing 282 ± 11 g (mean ± standard error of the mean) at the time of injection were used in this study. The rats were housed in a biosafety level 2 containment laboratory that met specific safety regulations required for experiments using an NIH/CDC class II pathogen (7). The rats were housed two per cage in a constant photoperiod (12 h light–12 h dark; light on at 0700) and provided with unlimited access to food and water. All rats were allowed to acclimate to the animal facility for at least 3 days prior to any experimental procedure. Procedures used for the maintenance and use of experimental animals complied with the regulations described in the NIH Guide for the Care and Use of Laboratory Animals and were reviewed and approved by the University of Pittsburgh and Princeton University Animal Care and Use Committees.
Experimental animals (n = 19) were anesthetized with xylazine (7 mg/kg) and ketamine (60 mg/kg), and then 2 μl of PRV Becker was injected into the vitreous body of the right eye (at a rate of 1 μl/min) with a Hamilton microliter syringe fitted with a 26-gauge needle. The needle was left in place for 5 min following injection of the inoculum. Control rats received identical injections of either 2% fetal bovine serum (FBS) in Dulbecco’s modified Eagle’s medium (DMEM; n = 2 rats) or mock-infected medium (2% FBS in DMEM that was incubated with virus-free PK15 cells for 72 h; n = 2 rats). The rats were returned to their home cage, where they remained until the time of perfusion. All injections occurred during the light phase of the photoperiod.
Perfusion fixation and tissue preparation.
At various times after inoculation (for virus-injected rats, 51 to 52 h [n = 5], 70 to 80 h [n = 11], and 80 to 89 h [n = 3]; for control animals, 74 h [n = 4]), the rats were anesthetized with an overdose of ketamine and perfused transcardially with isotonic saline, followed by 4% paraformaldehyde containing lysine and sodium metaperiodate (39). The brains were removed, postfixed for 1 h at 4°C, and cryoprotected with phosphate-buffered 20% sucrose solution at 4°C for at least 24 h. Coronal sections of brain tissue (35-μm thick) were cut with a freezing microtome and stored in a cryoprotectant medium at −20°C (69) prior to immunohistochemical localization of viral antigen or leukocytes.
Antibodies.
Viral structural proteins were detected by using a rabbit polyvalent antiserum (Rb134) that was generated against acetone-inactivated PRV virions (7). Mononuclear lymphocytes were detected with a mouse monoclonal antibody to rat leukocyte common antigen (anti-CD45; Pharmingen, San Diego, Calif.); CD45 is expressed on all hematopoietic cells (except erythrocytes) as well as microglia. Mononuclear phagocytes were detected with a mouse monoclonal antibody (ED-1; Bioproducts for Science, Indianapolis, Ind.) that recognizes a cytoplasmic antigen expressed by rat monocytes and macrophages (13). Cytolytic T lymphocytes were detected with a mouse monoclonal antibody (anti-CD8β; Pharmingen) generated against the β chain of the rat CD8 antigen which is expressed on cytotoxic T lymphocytes but is absent on natural killer cells (61). T-helper lymphocytes were detected with a mouse monoclonal antibody (OX-38; Pharmingen) that recognizes rat CD4 antigen. OX-38 recognizes the same epitope of CD4 as W3/25, and these antibodies have been reported to also recognize CD4 expressed on rat macrophages and rat microglia (30, 43, 67).
Immunocytochemical localizations.
The avidin-biotin modification of the immunoperoxidase procedure (26) was used to localize all antigens. Sections at a minimum frequency of 280 μm were incubated free-floating in primary antibody overnight at 4°C at the following dilutions: Rb134, 1:10,000; anti-CD45, 1:1,000; ED-1, 1:1,000; anti-CD8β, 1:500; anti-CD4, 1:1,000. Species-specific affinity-purified secondary antibodies (Jackson ImmunoResearch Laboratories) and Vectastain reagents (Vectastain Elite Kit; Vector Laboratories) were used to complete the immunohistochemical processing in accordance with published procedures (5). Processed sections were mounted on gelatin-coated glass slides, dehydrated with a series of graded ethanols, and cleared in xylene, and cover- glasses were fixed with Cytoseal 60 (VWR Scientific, Cleveland, Ohio). Staining for CD4+ cells was not performed on brain tissue from 3 of the 19 virus-infected rats because there was no more tissue available from these brains.
Data analysis.
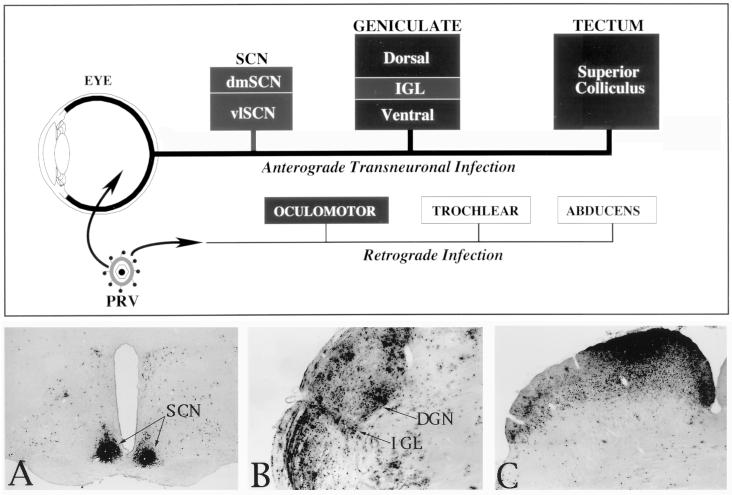
The organization of the neuronal circuitry analyzed in this study is shown in Fig. 1. Quantitative determinations of the number of infected neurons and cells immunopositive for CD45, ED-1, CD8β, or CD4 antigens were made for motor neurons in the oculomotor nucleus ipsilateral to the injected eye and two retinorecipient regions (the suprachiasmatic nucleus [SCN] and the dorsolateral geniculate nucleus [DGN]) contralateral to the injected eye. Immunoreactive cells in the SCN and DGN contralateral to the injected eye were counted because more than half of the rat retinal ganglion cell axons cross to innervate the opposite side of the brain. As a result, the number of infected neurons in visual centers such as the SCN, geniculate nuclei, and tectum are always greater in the side opposite to the injected eye (9). In contrast, the innervation of extraocular eye muscles originates from motor neurons ipsilateral to the eye.
FIG. 1.
A schematic illustration of the organization of the neuronal circuitry that was the subject of analysis in this investigation is shown at the top of the figure. Filled boxes represent areas that were included in the analysis, while open boxes represent motor nuclei that were virus infected but not subjected to analysis in this study. Injections of virus into the vitreous body of the eye produced an anterograde transynaptic infection of retinorecipient neurons in the diencephalon (SCN and geniculate complex) and midbrain (tectum). The distribution of infected neurons in these regions at long postinoculation intervals after injection of PRV Becker is illustrated in the photomicrographs of the SCN (A), the geniculate complex that includes the DGN and IGL (B), and the tectum (C). Leakage of virus into the eye also produced a retrograde infection of motor neurons innervating eye musculature (oculomotor, trochlear, and abducens nuclei). dmSCN, dorsomedial suprachiasmic nucleus; vlSCN, ventrolateral suprachiasmic nucleus.
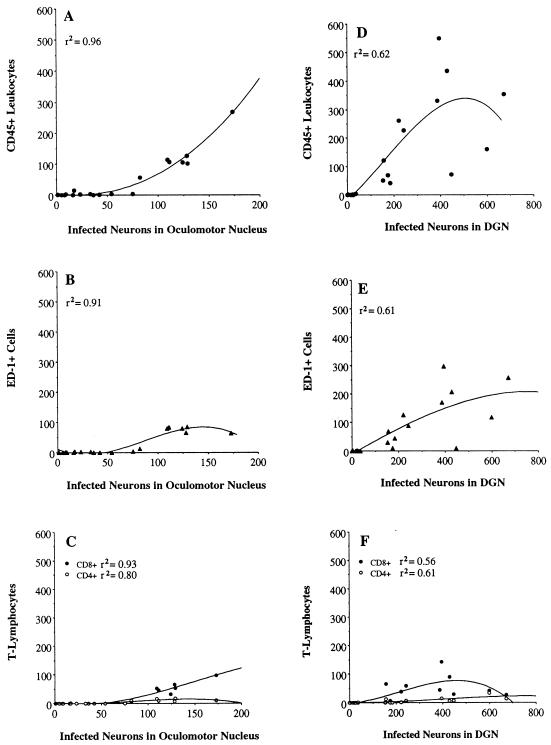
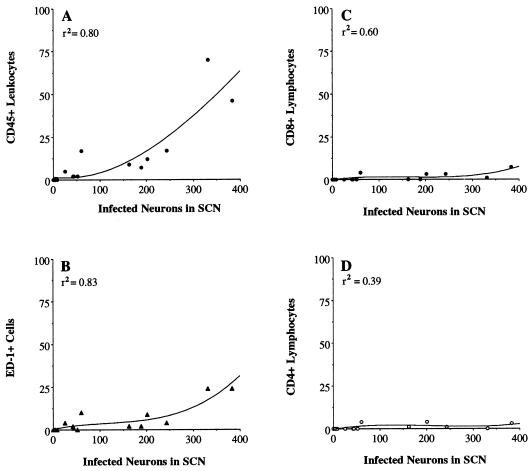
Cell counts were obtained by preparing camera lucida drawings that defined the number and distribution of immunopositive cells in each area. To ensure accurate comparisons between animals, we selected comparable coronal planes of a section midway through the linear extent of each nucleus. The numbers of virus-infected neurons and leukocytes in brain sections of every infected rat were then counted. For each virus-infected rat, and each defined area of infection, the number of CD45+, ED-1+, CD8β+, or CD4+ leukocytes was paired with the number of virus-infected neurons for that rat within that area of infection. Because leukocyte recruitment was the key variable about which predictions were to be made, the numbers of leukocytes were placed on the ordinate and the numbers of infected neurons were placed on the abscissa when graphs were made. These variable pairs were plotted as scatterplots. That is, the number of CD45+, ED-1+, CD4+, or CD8β+ cells counted within an area of infection were plotted as a function of the number of virus-infected neurons counted within that region, and the best-fitting line of the data was generated by using regression analysis. Thus, each data point in Fig. 6 and 7 represents the data used in separate analyses for each brain region and each leukocyte subtype. Plots of linear regression lines for these data indicated that the relation between leukocyte infiltration and viral infection was best described by fitting curvilinear regression lines generated with polynomial functions. The polynomial equation for each analysis was empirically determined by adding increasing powers of x (i.e., x2 and x3 for a cubic equation) and the associated regression coefficient (slope factor) to best fit each set of data (53). The corresponding polynomial curve was drawn by using these equations. The degree of association between leukocyte infiltration and viral infection was determined by using the coefficient of determination (r2); greater values of r2 indicate a higher degree of association between leukocyte infiltration and viral infection.
FIG. 6.
Quantitative analyses showing the relation between leukocyte infiltration and viral infection in the oculomotor nucleus and DGN after injection of PRV Becker into the vitreous body of the eye. CD45-, ED-1-, CD8β-, and CD4-immunopositive leukocytes and neurons containing immunoreactivity to viral structural proteins were counted in brain tissue from each virus-infected rat. The individual data points in these figures are the actual number of cells counted for each rat in each region of infection. These figures also show the best-fitting curvilinear line of the data. No inferences should be made about the shape of the end of each curve, which is based on only a few data points. r2 indicates the strength of the association between leukocyte infiltration and viral infection. Note that the range of values on the x axis changes according to the area of infection.
FIG. 7.
Quantitative analyses showing the relation between leukocyte infiltration and viral infection in the SCN after injection of PRV Becker into the vitreous body of the eye. CD45-, ED-1-, CD8β-, and CD4-immunopositive leukocytes and neurons containing immunoreactivity to viral structural proteins were counted in brain tissue from each virus-infected rat. The individual data points in these figures are the actual number of cells counted for each rat in each region of infection. These figures also show the best-fitting curvilinear line of the data. r2 indicates the strength of the association between leukocyte infiltration and viral infection.
RESULTS
PRV Becker produced a predictable course of CNS infection.
Consistent with previous studies (8, 9, 15, 73), injection of PRV Becker into the vitreous body of the eye infected different visual centers in the brain (Fig. 1) at two time points separated by approximately 24 h. The progression of infection through this circuitry was marked by the sequential infection of neurons involved in visual perception (DGN) and reflex movement of the eyes (tectum) approximately 50 h after inoculation, followed by areas of brain involved in the temporal (circadian) organization of behavior (SCN and intergeniculate leaflet [IGL]) at approximately 72 h. Motor neurons in the oculomotor nucleus were infected within 52 h of inoculation by the retrograde transport of PRV that had leaked into the orbit. The data also confirmed the cytopathic effects of this virulent PRV strain on CNS neurons (6, 9, 46). Neuropathological changes were noted shortly after the onset of viral replication and became increasingly pronounced with advancing survival.
Viral infection led to focal recruitment of immune cells into the CNS.
Analysis of CD45 immunoreactivity in control and PRV-infected brains revealed marked differences in the patterns of cellular staining. CD45+ leukocytes, identified as cells with a spherical morphology (5 to 10 μm in widest diameter) and no immunoreactive processes, were few in number (approximately 5 cells/brain) and were randomly distributed throughout the brain after injections of mock-infected medium or 2% FBS in DMEM into the vitreous body of the eye (data not shown). CD45+ microglia, identified by their characteristic small cell bodies and cytoplasmic processes, were lightly stained in control animals and widely distributed throughout the brain with no differential concentration within any region. These patterns of staining differed considerably from that which occurred in PRV-infected brain tissue. Although we did not analyze the glial cell response in the present study, the CD45+ microglia in densely infected areas displayed a reactive morphology with characteristic increases in the size of their perikarya and processes, consistent with our findings in autonomic circuitry (46).
Intensely labeled CD45+ leukocytes were differentially concentrated in areas of viral infection. The number of these cells correlated with the extent of infection in different retinorecipient regions of the CNS. At early stages of infection in each brain region (i.e., shortly after the onset of viral replication in neurons), the CD45+ cells were few in number and closely associated with blood vessels in the area of infection (Fig. 2B, 5B, and 5F). As the number of infected neurons increased within an area, the CD45+ cells also became more prevalent and were found throughout the parenchyma of the area of infection (Fig. 2F, 3B, and 4B). Figure 6A shows that there were few CD45+ leukocytes recruited when less than 100 oculomotor neurons were infected but there was a progressive increase in the number of CD45+ leukocytes as the number of infected cells approached 200. The numbers of CD45+ cells present in the oculomotor nucleus were strongly correlated with the numbers of infected neurons in this nucleus (r2 = 0.96). Similiarly, the numbers of CD45+ cells localized within the DGN and SCN were strongly associated with the numbers of infected neurons in these regions (Fig. 6D and 7A).
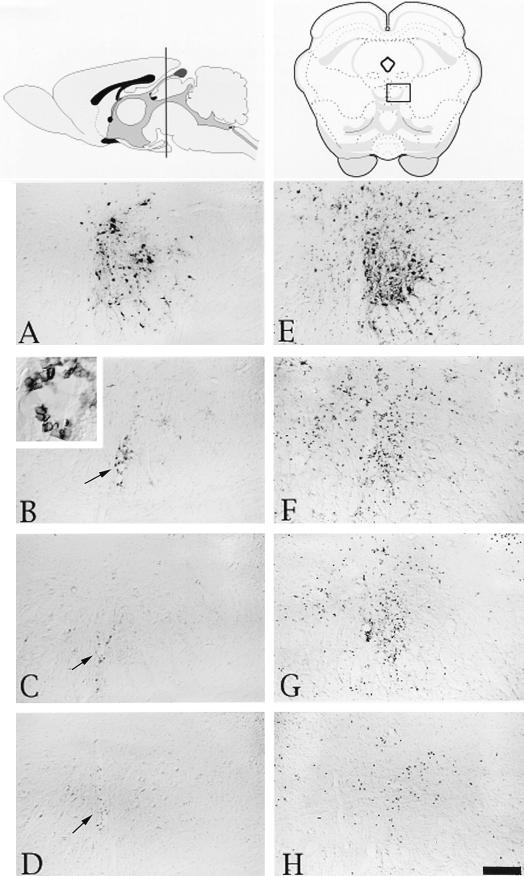
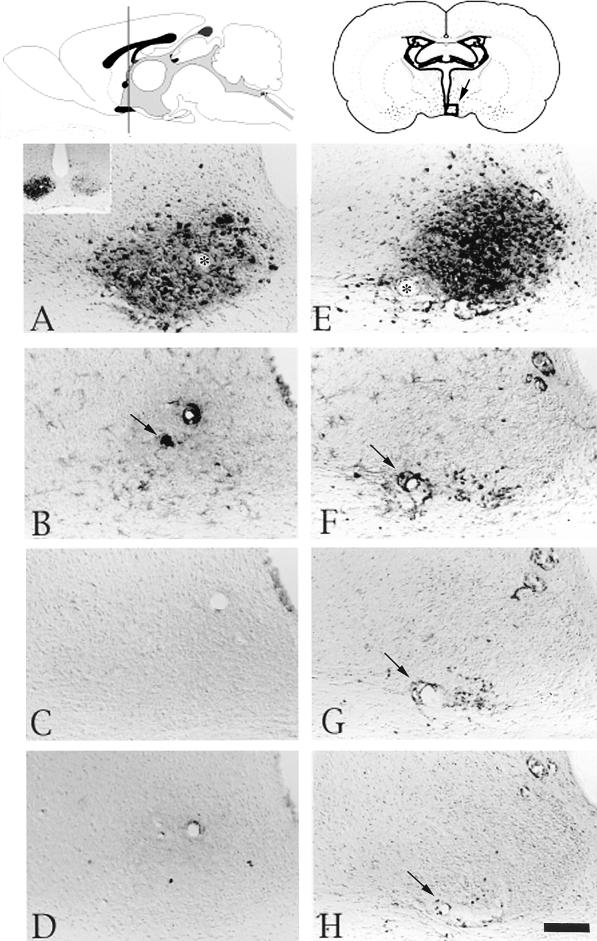
FIG. 2.
Localization of viral antigen and phenotypically distinct populations of leukocytes in the oculomotor nucleus after injection of PRV Becker into the vitreous body of the eye. At the top of the figure are schematic drawings (modified from Swanson’s brain map computer graphics files [59]) of a sagittal view of the brain (left) and a coronal section with an open square in the middle indicating the location of the oculomotor nucleus (right). Micrographs of adjacent sections of tissue that were processed with antibodies recognizing viral, CD45, ED-1, or CDβ+ antigen are shown below the drawings. (A to D) Micrographs of brain tissue from a representative rat with several virus-infected neurons. These pictures show viral immunoreactivity in the oculomotor nucleus (A), immunoreactive cells expressing the CD45 antigen and an enlarged view of these cells by a blood vessel (B), ED-1+ cells (C), and CD8β+ leukocytes (D). (E to H) Micrographs of brain tissue from a representative rat with many virus-infected neurons in the oculomotor nucleus. This series of micrographs show viral immunoreactivity (E), immunoreactive cells expressing the CD45 antigen (F), ED-1+ immunoreactive cells (G), and CD8β+ leukocytes (H). Scale bar, 100 μm for all figures. Arrows indicate blood vessels.
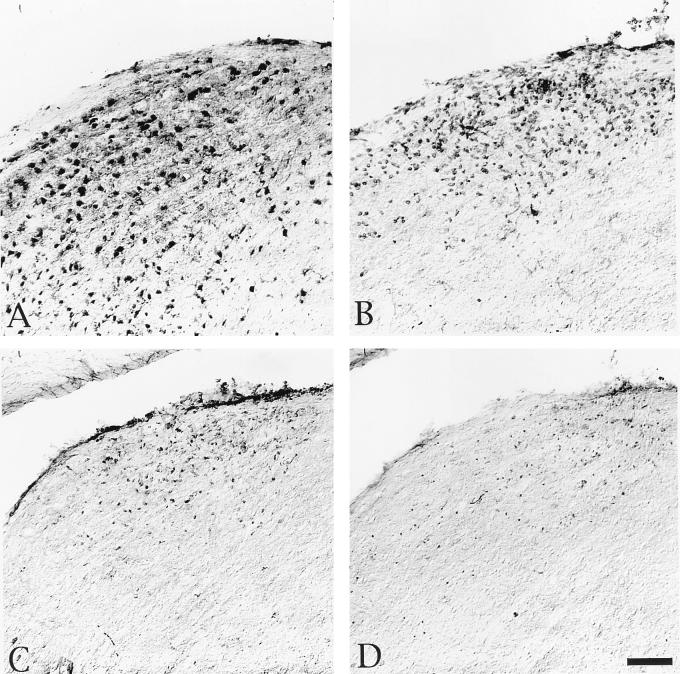
FIG. 5.
Localization of viral antigen and phenotypically distinct populations of leukocytes in the SCN after injection of PRV Becker into the vitreous body of the eye. At the top of the figure are schematic drawings of a sagittal view of the brain (left) and a coronal section with an open square indicating the location of the SCN (right). The micrographs are of adjacent sections of tissue from a representative animal with several infected neurons in the SCN (A to D) and from a representative animal with many more infected neurons in the SCN (E to H). They illustrate viral immunoreactivity (A and E), immunoreactivity of cells expressing the CD45 antigen (B and F), ED-1+ immunoreactive cells (C and G), immunoreactive CD8β+ leukocytes (D and H). Scale bar, 100 μm for all figures. Asterisks in panels A and B and arrows in panels B, F, G, and H indicate blood vessels.
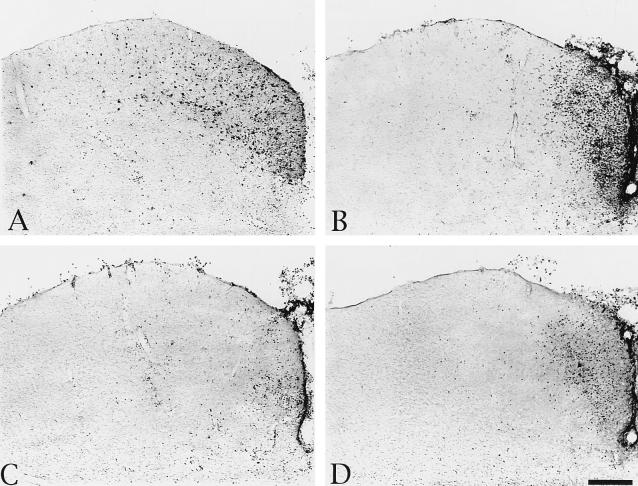
FIG. 3.
Localization of viral antigen and phenotypically distinct populations of leukocytes in the DGN after injection of PRV Becker into the vitreous body of the eye. The micrographs are of adjacent sections of tissue from a representative animal with numerous infected neurons in the DGN and illustrate viral immunoreactivity (A), immunoreactivity of cells expressing the CD45 antigen (B), ED-1+ immunoreactive cells (C), and immunoreactive CD8β+ leukocytes (D). Scale bar, 100 μm for all figures.
FIG. 4.
Localization of viral antigen and the virus-induced cellular infiltrate of phenotypically distinct populations of leukocytes in the superior colliculus after injection of PRV Becker into the vitreous body of the eye. The micrographs are of adjacent sections of tissue from a representative animal with numerous infected neurons in the superior colliculus and illustrate viral immunoreactivity (A), immunoreactivity of cells expressing the CD45 antigen (B), ED-1+ immunoreactive cells (C), and immunoreactive CD8β+ leukocytes (D). Scale bar, 100 μm for all figures.
The CD45+ cellular infiltrate in areas of viral infection contains distinct subclasses of immune cells.
Analysis of adjacent sections stained with antibodies recognizing CD4, CD8β, and monocytic cells revealed that each of these subclasses of CD45+ immune cells were present in the population of cells that entered the brain in response to PRV infection of neurons (Fig. 2 to 7). The numbers of cells varied directly with the number of infected neurons within a region; the largest number of each subclass of immune cell was always observed in areas with the most advanced infection (i.e., numerous infected neurons) (Fig. 2 to 7). The numbers of ED-1+ cells and CD8β+ cells correlated well with the number of virus-infected neurons in the oculomotor nucleus, DGN, and SCN (r2 ranging from 0.56 to 0.94) (Fig. 6 and 7). There were fewer CD4+ cells relative to the other populations of cells, with only scattered cells apparent in areas with the most advanced infection (data not shown). Almost all of the CD4+ cells were small and spherical in morphology, and these were counted as CD4+ lymphocytes. There was a high degree of association between the number of CD4+ lymphocytes and the number of infected neurons in the oculomotor nucleus and DGN (Fig. 6C and F); because there were so few CD4+ lymphocytes in the SCN, the association between the extent of CD4+ lymphocyte infiltration and viral infection in this area (Fig. 7D) was less strong than that in other regions. Lightly stained CD4+ microglia were also present in areas with numerous virus-infected neurons. Furthermore, the number of CD45+ leukocytes localized within the oculomotor nucleus, DGN, and SCN was always substantially larger than either the ED-1+, CD8β+, or CD4+ population of cells or the sum of these distinct population of leukocytes (Fig. 6 and 7).
There are timing differences in the recruitment of immune cell subclasses into infected areas.
In each case, ED-1+ cells were greater in number than the CD8β+ cells in adjacent sections from the same animal (Fig. 2 to 5 and compare Fig. 6B and C, Fig. 6E and F, and Fig. 7B and C). Likewise, as the number of infected neurons in each area increased, there was a greater number of CD8β+ cells than CD4+ cells localized within virus-infected areas (Fig. 6C and F and compare Fig. 7C and D).
The extent of leukocyte infiltration into specific virus-infected areas paralleled the time that specific areas became infected.
Comparison of the extent of infiltration into specific areas of infection indicated that the maximal numbers of CD45+, ED-1+, CD8β+, and CD4+ leukocytes localized in the SCN (Fig. 7) were less than those found in the oculomotor nucleus and DGN (Fig. 6). Thus, the lesser extent of leukocyte infiltration into the SCN corresponded with the later onset of viral replication in the SCN.
DISCUSSION
Our data add, in two important ways, to a large body of literature (see reference 23 for a recent review) demonstrating focal recruitment of a heterogeneous population of leukocytes to sites of CNS viral infection. First, we found that the magnitude of immune cell recruitment is dependent directly upon the extent of viral infection within a given region. Second, we observed a temporal segregation of functionally distinct populations of immune cells, thereby providing insight into the possible functional significance of different components of this response.
The trafficking of leukocytes into regions of infection occurred irrespective of the route of viral invasion (anterograde versus retrograde). However, there were differences in the onset of immune cell invasion in different regions. For example, the earliest trafficking was observed in the oculomotor nucleus, the DGN, and the tectum. Each of these regions is characterized by viral replication that begins approximately 48 h postinoculation and increases rapidly thereafter. This is in contrast to the onset of viral replication in neurons of the hypothalamic SCN and IGL, where PRV-infected neurons cannot be detected immunohistochemically until approximately 72 h postinoculation (8, 9, 15, 73). Thereafter, the number of infected neurons in each of these regions increases rapidly as virus moves transynaptically through the short-axon, local-circuit neurons that constitute these cell groups. The data showing a lesser extent of leukocyte infiltration in the SCN relative to that in the oculomotor nucleus, DGN, and tectum support the conclusion that the extent of leukocyte infiltration into the CNS correlated with the time that specific areas became infected. The data showing larger infiltrations of ED-1+ cells relative to CD8β+ T cells and the greater number of CD8β+ T cells relative to CD4+ T cells in each area of infection at each survival period suggested that ED-1+ cells entered infected areas earlier than CD8β+ T cells did and that CD4+ T cells followed CD8β+ T cells.
The focal, time-dependent recruitment of lymphocytes from the vasculature into virus-infected sites is consistent with prior investigations that have demonstrated directed recruitment of immune cells into the brain in response to neurotropic viral infections. However, the functional consequences of this recruitment appear to vary according to the type of viral infection and the route of viral invasion. Compelling evidence supports the conclusion that CD4+ and CD8+ lymphocytes participate in the clearance of mouse hepatitis virus and vesicular stomatitis virus from the nervous system (28, 74, 75). In contrast, T lymphocytes have been suggested to mediate immunopathology when the cornea or CNS is infected with an alphaherpesvirus (27, 33, 42, 47, 62–65). For example, in the corneal scarification model employed by Kristensson et al. (33) and Townsend (62–65), peripheral inoculation of the cornea with HSV produced a demyelinating lesion of the centrally projecting portion of trigeminal axons that was preceded by the sequential recruitment of macrophages and T cells to the trigeminal root zone. The extent of this pathology was reduced in athymic nude mice, leading Townsend to hypothesize that interactions of macrophages and T cells contribute to the pathological changes in the central trigeminal axons. This conclusion is supported by the demonstration that CD8+ T cells are associated with focal CNS temporal lobe lesions in natural killer cell-deficient, HSV-infected mice (27).
Data in support of immune cells participating in virus-induced pathology is evident in the present results as well as those of prior studies using PRV in this model. For example, the late recruitment of cells of monocyte/macrophage lineage to viral infection sites observed in the present study reproduces the previous demonstration of this temporal association (46). Subsequent ultrastructural analysis of that response demonstrated that these cells were intimately associated with neurons in advanced stages of degeneration and appear to contribute to the degeneration of the infected neurons (6). The present demonstration that the recruitment times of CD8+ and CD4+ T lymphocytes are similarly delayed argues that these cells also contribute to the killing of infected neurons. However, the mechanism through which this is achieved and the role of these leukocytes at PRV-infected brain sites require further investigation. The direct lytic actions of CD8+ T lymphocytes occur after their T-cell receptor recognizes immunogenic peptide bound to major histocompatibility complex (MHC) class I molecules on the surface of antigen-presenting cells (1, 22, 55, 58, 75, 78). The consensus is that cell surface expression of MHC molecules occurs on glial, endothelial, and ependymal cells (2, 17, 18, 41, 48, 68, 70) but not on neurons (31, 32, 36, 41, 70). Furthermore, most herpesviruses, including PRV, encode proteins that can inhibit MHC-viral peptide complex formation or transport in infected cells (4, 15a, 40). These findings suggest that CD8+ T lymphocytes may not mediate direct cytotoxicity against PRV-infected neurons; instead, these cells may participate in cytokine-mediated, nonlytic control of viral replication. Further studies are needed to examine this hypothesis and to determine whether other CNS cells serve as targets for the CD8+ T lymphocytes that localize to PRV-infected areas.
Few CD4-positive lymphocytes were found in infected areas of the brain even at the latest times after infection. Therefore, it is difficult to ascribe any role to these cells in the rather massive infiltration of CD8-positive T cells and the subsequent pathogenic events that ensue in the infected tissue. This observation is consistent with the results of Stohlman and colleagues that show few CD4+ lymphocytes in the parenchyma of mouse hepatitis virus-infected mouse brain (54).
T cells normally enter the CNS only after they are activated by contact with helper cells and viral antigen (24, 25, 72). In our experiments, it is not clear where the infiltrating CD8-positive cells were activated. It is unlikely that activation occurred at the injection site because the inner compartment of the eye, like the CNS, contains few lymphocytes and lacks lymphatic channels. Moreover, there is an effective blood-eye barrier that precludes simple diffusion of virus into the circulation (11, 44, 56). Only when this barrier is breached would we expect lymphocytes to contact virus (19, 49, 77). Even if T cells were to enter the eye, the widespread expression of Fas ligand on ocular tissue should effectively lead to Fas-mediated apoptosis of the invading lymphocytes (20, 21, 37). However, if virus in the inoculum leaked into the orbit of the eye during injection, it might become accessible to immune cells and activate them. Since we do find infection of oculomotor pathways that innervate external ocular muscles, such a route is certainly possible. Another possible site of T-cell activation may be in the deep cervical lymph nodes that receive antigens from cerebrospinal fluid and extracellular fluid of the brain (3, 12). Viral proteins released from infected cells in the brain could be carried to these deep lymph nodes, where T-cell activation could occur. Further work is necessary to test these ideas.
Lymphocytes may be directed towards PRV infection sites in the brain by signals from infected neurons or surrounding glial cells that become reactive in response to infection. This idea is supported by studies showing that the initial reactive response to PRV infection of autonomic circuitry consists of a focal reactive response of astrocytes and microglia at the infection site (6, 46). Astrocytes may play an important role in leukocyte recruitment because they express adhesion molecules and synthesize monocyte chemoattractant proteins (29, 45). Through their extensive array of processes, astrocytes are ideally situated to bridge the space between infected neurons and the surrounding vasculature. Reactive glial responses, and lymphocytes adhering to endothelial cells, may contribute to further emigration and influx by secreting cytokines that promote increased expression of adhesion molecules on the luminal surface of endothelial cells (16, 38, 50). Given the short time period involved before lymphocytes appear, the synergistic responses of glial and endothelial cells (66) and the innate immune response to infection are likely to be part of the signaling cascade that stimulates the homing and extravasation of monocytes and T lymphocytes from the vasculature into virus-infected sites.
There is a fundamental question of why the animals die several days following virus injection, given the rather circumscribed sites of viral pathology in the visual centers of the brain. Perhaps the influx of lymphocytes and the attendant more-global immune-induced pathology are responsible, rather than simply the loss of neurons. Another fundamental question concerns the molecular nature of the signals from the infected cells that ultimately call in the leukocytes. Less-virulent viruses will enable us to determine which viral or host gene products are involved. For example, we have noted that gE and gI, viral membrane proteins, play a role in virulence and neuropathology (8, 60, 73).
In summary, our experiments show that CD45+ leukocytes migrated through the vasculature and parenchyma in a focused response to a site-specific neurotropic virus infection in the CNS. Moreover, this recruitment into the CNS correlated with the extent of infection. The rapid and dynamic pattern of lymphocyte trafficking during viral infection of the CNS supports the conclusion that there is immunologic surveillance of the CNS (24, 25, 71, 72, 76). A better understanding of the temporal organization of the trafficking of other leukocytes into the CNS and the signaling cascade mediating leukocyte recruitment will provide further insight into the mechanisms of antiviral defense responses. Our ongoing studies employing this model and isogenic PRV strains that differ in virulence will provide further insight into the role of viral proteins in the neuroimmune response to infection.
ACKNOWLEDGMENTS
We gratefully acknowledge William Chambers for his helpful comments on this manuscript, Marlies Eldridge for growing and titering the stocks of PRV used in these studies, and Jared Marks for his expert technical help with the CD4 staining.
This research was supported by National Institute of Health grants MH01369 (S.R.), MH53574 (J.P.C.), NINDS33506 (L.W.E.), and MH484311 (A.F.S.).
REFERENCES
- 1.Berke G. Unlocking the secrets of CTL and NK cells. Immunol Today. 1995;16:343–346. doi: 10.1016/0167-5699(95)80152-9. [DOI] [PubMed] [Google Scholar]
- 2.Bi Z, Barna M, Komatsu T, Reiss C S. Vesticular stomatitis virus infection of the central nervous system activates both innate and acquired immunity. J Virol. 1995;69:6466–6472. doi: 10.1128/jvi.69.10.6466-6472.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bradbury M W B. Overview of passage routes of interstitial fluid to the lymphatics: history and current concepts. In: Johansson B B, Owman C, Widner H, editors. Pathophysiology of the brain-brain barrier. Oxford, United Kingdom: Elsevier; 1990. pp. 413–420. [Google Scholar]
- 4.Burgert H G. Subversion of the MHC class I antigen-presentation pathway by adenoviruses and herpes simplex viruses. Trends Microbiol. 1996;4:107–112. doi: 10.1016/0966-842X(96)81527-7. [DOI] [PubMed] [Google Scholar]
- 5.Card J P, Enquist L W. The use of pseudorabies virus for definition of synaptically linked populations of neurons. In: Adolph K W, editor. Methods in molecular genetics. Orlando, Fla: Academic Press Inc.; 1994. pp. 363–382. [Google Scholar]
- 6.Card J P, Rinaman L, Lynn R B, Lee B H, Meade R P, Miselis R R, Enquist L W. Pseudorabies virus infection of the rat central nervous system: ultrastructural characterization of viral replication, transport and pathogenesis. J Neurosci. 1993;13:2515–2539. doi: 10.1523/JNEUROSCI.13-06-02515.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Card J P, Rinaman L, Schwaber J S, Miselis R R, Whealy M E, Robbins A K, Enquist L W. Neurotropic properties of pseudorabies virus: uptake and transneuronal passage in the rat central nervous system. J Neurosci. 1990;10:1974–1994. doi: 10.1523/JNEUROSCI.10-06-01974.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Card J P, Whealy M E, Robbins A K, Enquist L W. Pseudorabies virus envelope glycoprotein gI influences both neurotropism and virulence during infection of the rat visual system. J Virol. 1992;66:3032–3041. doi: 10.1128/jvi.66.5.3032-3041.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Card J P, Whealy M E, Robbins A K, Moore R Y, Enquist L W. Two α-herpesvirus strains are transported differentially in the rodent visual system. Neuron. 1991;6:957–969. doi: 10.1016/0896-6273(91)90236-s. [DOI] [PubMed] [Google Scholar]
- 10.Cassady K, Whitley R. Pathogenesis and pathophysiology of viral infections of the central nervous system. In: Scheld W, Whitely R J, Durack D, editors. Infections of the central nervous system. Philadelphia, Pa: Lippincott-Raven; 1997. pp. 7–22. [Google Scholar]
- 11.Cousins S W, Dix R D. Immunology of the eye. In: Keane R W, Hickey W F, editors. Immunology of the nervous system. New York, N.Y: Oxford University Press; 1997. pp. 668–702. [Google Scholar]
- 12.Cserr H F, Knopf P M. Cervical lymphatics, the blood-brain barrier and the immunoreactivity of the brain: a new view. Immunol Today. 1992;13:507–512. doi: 10.1016/0167-5699(92)90027-5. [DOI] [PubMed] [Google Scholar]
- 13.Dijkstra C D, Dobb E A, Joling P, Kraal G. The heterogeneity of mononuclear phagocytes in lymphoid organs: distinct macrophage subpopulations in the rat recognized by monoclonal antibodies ED1, ED2 and ED3. Immunology. 1985;54:589–599. [PMC free article] [PubMed] [Google Scholar]
- 14.Enquist L W, Card J P. Pseudorabies virus: a tool for tracing neuronal connections. In: Lowenstein P R, Enquist L W, editors. Protocols for gene transfer in neuroscience: towards gene therapy of neurological disorders. New York, N.Y: John Wiley & Sons Ltd.; 1996. pp. 333–348. [Google Scholar]
- 15.Enquist L W, Dubin J, Whealy M E, Card J P. Complementation analysis of pseudorabies virus gE and gI mutants in retinal ganglion cell neurotropism. J Virol. 1994;68:5275–5279. doi: 10.1128/jvi.68.8.5275-5279.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15a.Enquist, L. W., and R. Sparks-Thissen. Unpublished observations.
- 16.Fabry Z, Raine C S, Hart M N. Nervous tissue as an immune compartment: the dialect of the immune response in the CNS. Immunol Today. 1994;15:218–224. doi: 10.1016/0167-5699(94)90247-X. [DOI] [PubMed] [Google Scholar]
- 17.Gairin J E, Joly E, Oldstone M B A. Persistent infection with lymphocytic choriomeningitis virus enhances expression of MHC class I glycoprotein on cultured mouse brain endothelial cells. J Immunol. 1991;146:3953–3957. [PubMed] [Google Scholar]
- 18.Gehrmann J, Matsumoto Y, Kreutzberg G W. Microglia: intrinsic immune effector cell of the brain. Brain Res Rev. 1995;20:269–287. doi: 10.1016/0165-0173(94)00015-h. [DOI] [PubMed] [Google Scholar]
- 19.Geiger K, Howes E, Sarvetnick N. Ectoptic expression of gamma interferon in the eye protects transgenic mice from intraocular herpes simplex virus type I infections. J Virol. 1994;68:5556–5567. doi: 10.1128/jvi.68.9.5556-5567.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Griffith T S, Brunner T, Fletcher S M, Green D R, Ferguson T A. Fas ligand-induced apoptosis as a mechanism of immune privilege. Science. 1995;270:1189–1192. doi: 10.1126/science.270.5239.1189. [DOI] [PubMed] [Google Scholar]
- 21.Griffith T S, Yu X, Herndon J M, Green D R, Ferguson T A. CD95-induced apoptosis of lymphocytes in an immune privileged site induces immunological tolerance. Immunity. 1996;5:7–16. doi: 10.1016/s1074-7613(00)80305-2. [DOI] [PubMed] [Google Scholar]
- 22.Griffiths G M. The cell biology of CTL killing. Curr Opin Immunol. 1995;17:343–348. doi: 10.1016/0952-7915(95)80108-1. [DOI] [PubMed] [Google Scholar]
- 23.Hickey W, Lassmann H, Cross A. Lymphocyte entry and initiation of inflammation in the central nervous system. In: Keane R W, Hickey W F, editors. Immunology of the nervous system. New York, N.Y: Oxford University Press; 1997. pp. 200–225. [Google Scholar]
- 24.Hickey W F, Gonatas N K, Kimura H, Wilson D B. Identification and quantitation of T lymphocyte subsets found in the spinal cord of the Lewis rat during acute experimental allergic encephalomyelitis. J Immunol. 1983;131:2805–2809. [PubMed] [Google Scholar]
- 25.Hickey W F, Hsu B L, Kiura H. T-lymphocyte entry into the central nervous system. J Neurosci Res. 1991;28:254–260. doi: 10.1002/jnr.490280213. [DOI] [PubMed] [Google Scholar]
- 26.Hsu S M, Raine L, Fanger H. The use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody procedures. J Histochem Cytochem. 1981;29:577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- 27.Hudson S, Streilein J. Functional cytotoxic T cells are associated with focal lesions in the brains of SJL mice with experimental herpes simplex encephalitis. J Immunol. 1994;152:5540–5547. [PubMed] [Google Scholar]
- 28.Huneycutt B S, Bi Z, Aoki C J, Reiss C S. Central neuropathogenesis of vesicular stomatitis virus infection of immunodeficient mice. J Virol. 1993;67:6698–6706. doi: 10.1128/jvi.67.11.6698-6706.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hurwitz A A, Lyman W D, Berman J W. Tumor necrosis factor alpha and transforming growth factor beta upregulate astrocyte expression of monocyte chemoattractant protein-1. J Neuroimmunol. 1995;57:193–198. doi: 10.1016/0165-5728(95)00011-p. [DOI] [PubMed] [Google Scholar]
- 30.Jefferies W A, Green J R, Williams A F. Authentic T helper CD4 (W3/25) antigen on rat peritoneal macrophages. J Exp Med. 1985;162:117–127. doi: 10.1084/jem.162.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Joly E, Mucke L, Oldstone M B A. Viral persistence in neurons explained by lack of major histocompatibility complex class I expression. Science. 1991;253:1283–1285. doi: 10.1126/science.1891717. [DOI] [PubMed] [Google Scholar]
- 32.Joly E, Oldstone M B A. Neuronal cells are deficient in loading peptides onto MHC class I molecules. Neuron. 1992;8:1185–1190. doi: 10.1016/0896-6273(92)90138-4. [DOI] [PubMed] [Google Scholar]
- 33.Kristensson K, Svennerholm B, Persson L, Vahlne A, Lycke E. Latent HSV trigeminal ganglionic infection in mice and demyelination in the central nervous system. J Neurol Sci. 1979;43:253–263. doi: 10.1016/0022-510x(79)90119-9. [DOI] [PubMed] [Google Scholar]
- 34.Lewandowski G, Hobbs M V. Evidence for deficiencies in intracerebral cytokine production, adhesion molecule induction, and T cell recruitment in herpes simplex virus type-2 infected mice. J Neuroimmunol. 1998;81:58–65. doi: 10.1016/s0165-5728(97)00159-8. [DOI] [PubMed] [Google Scholar]
- 35.Lewandowski G, Hobbs M V, Bloom F E. Alteration of intracerebral cytokine production in mice infected with herpes simplex virus types 1 and 2. J Neuroimmunol. 1994;55:23–34. doi: 10.1016/0165-5728(94)90143-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lewandowski G A, Lo D, Bloom F E. Interference with major histocompatability complex class II restricted antigen presentation in the brain by herpes simplex virus type I: a possible mechanism of evasion of the immune response. Proc Natl Acad Sci USA. 1993;90:2005–2009. doi: 10.1073/pnas.90.5.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lynch D H, Ransdell F, Alderson M R. Fas and FasL in the homeostatic regulation of immune responses. Immunol Today. 1995;16:569–574. doi: 10.1016/0167-5699(95)80079-4. [DOI] [PubMed] [Google Scholar]
- 38.Marker O, Scheynius A, Christensen J P, Thomsen A R. Virus-activated T cells regulate expression of adhesion molecules on endothelial cells in sites of infection. J Neuroimmunol. 1995;62:35–42. doi: 10.1016/0165-5728(95)00099-n. [DOI] [PubMed] [Google Scholar]
- 39.McLean I W, Nakane P K. Periodate-lysine-paraformaldehyde fixative. A new fixative for immunoelectron microscopy. J Histochem Cytochem. 1974;22:1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- 40.Mellencamp M W, O’Brien P C M, Stevenson J R. Pseudorabies virus-induced suppression of major histocampatibility complex class I antigen expression. J Virol. 1991;65:3365–3368. doi: 10.1128/jvi.65.6.3365-3368.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mucke L, Oldstone M B A. The expression of major histocompatibility complex (MHC) class I antigens in the brain differs markedly in acute and persistent infections with lymphocytic choriomeningitis virus (LCMV) J Neuroimmunol. 1992;36:193–198. doi: 10.1016/0165-5728(92)90050-U. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Niemialtowski M, Rouse B T. Phenotypic and functional studies on ocular T cells during herpetic infections of the eye. J Immunol. 1992;148:1864–1870. [PubMed] [Google Scholar]
- 43.Perry H V, Gordon S. Modulation of CD4 antigen on macrophages and microglia in rat brain. J Exp Med. 1987;166:1138–1143. doi: 10.1084/jem.166.4.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rahi A H S. Immunopathology of the eye. In: Behan P, Spreafico F, editors. Neuroimmunology. New York, N.Y: Raven Press; 1984. pp. 415–431. [Google Scholar]
- 45.Ransohoff R M, Hamilton T A, Tani M, Stoler M H, Shick H E, Major J A, Estes M L, Thomas D M, Tuohy V K. Astrocyte expression of mRNA encoding cytokines IP-10 and JE/MCP-1 in experimental autoimmune encephalomyelitis. FASEB J. 1993;7:592–600. doi: 10.1096/fasebj.7.6.8472896. [DOI] [PubMed] [Google Scholar]
- 46.Rinaman L, Card J P, Enquist L W. Spatiotemporal responses of astrocytes, ramified microglia, and brain macrophages to central neuronal infection with pseudorabies virus. J Neurosci. 1993;13:685–702. doi: 10.1523/JNEUROSCI.13-02-00685.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rouse B T. Virus-induced immunopathology. Adv Virus Res. 1996;47:353–376. doi: 10.1016/S0065-3527(08)60739-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sethna M P, Lampson L A. Immune modulation within the brain: recruitment of inflammatory cells and increased major histocompatibility antigen expression following intracerebral injection of interferon-gamma. J Neuroimmunol. 1991;34:121–132. doi: 10.1016/0165-5728(91)90121-m. [DOI] [PubMed] [Google Scholar]
- 49.Shimeld C, Whiteland J L, Nicholls S M, Easty D L, Hill T J. Immune cell infiltration in corneas of mice with recurrent herpes simplex virus disease. J Gen Virol. 1996;77:977–985. doi: 10.1099/0022-1317-77-5-977. [DOI] [PubMed] [Google Scholar]
- 50.Shimizu Y, Newman W, Tanaka Y, Shaw S. Lymphocyte interactions with endothelial cells. Immunol Today. 1992;13:106–112. doi: 10.1016/0167-5699(92)90151-V. [DOI] [PubMed] [Google Scholar]
- 51.Simmons A, Tscharke D C. Anti-CD8 impairs clearance of herpes simplex virus from the nervous system: implications for the fate of virally infected neurons. J Exp Med. 1992;175:1337–1344. doi: 10.1084/jem.175.5.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sloan D J, Wood M J, Charlton H W. Leukocyte recruitment and inflammation in the CNS. Trends Neurosci. 1992;15:276–278. doi: 10.1016/0166-2236(92)90075-j. [DOI] [PubMed] [Google Scholar]
- 53.Sokal R R, Rohlf F J. Multiple and curvilinear regression. In: Sokal R R, Rohlf F J, editors. Biometry principles and practice of statistics in biological research. W. H. New York, N.Y: Freeman & Co.; 1981. pp. 671–683. [Google Scholar]
- 54.Stohlman S A, Bergmann C C, Lin M T, Cua D J, Hinton D R. CTL effector function within the central nervous system requires CD4+ cells. J Immunol. 1998;160:2896–2904. [PubMed] [Google Scholar]
- 55.Stohlman S A, Kyuwa S, Polo J M, Brady D, Lai M M C, Bergmann C C. Characterization of mouse hepatitis virus-specific cytotoxic T cells derived from the central nervous system of mice infected with the JHM strain. J Virol. 1993;67:7050–7059. doi: 10.1128/jvi.67.12.7050-7059.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Streilein J W, Taylor A W. Immunologic principles related to the nervous system and the eye: concerning the existence of a neural-ocular immune system. In: Keane R W, Hickey W F, editors. Immunology of the nervous system. New York, N.Y: Oxford University Press; 1997. pp. 99–133. [Google Scholar]
- 57.Subak-Sharpe I, Dyson H, Fazakerley J. In vivo depletion of CD8+ T cells prevents lesions of demyelination in Semliki Forest virus infection. J Virol. 1993;67:7629–7633. doi: 10.1128/jvi.67.12.7629-7633.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sussman M A, Shubin R A, Kyuwa S, Stohlman S A. T-cell-mediated clearance of mouse hepatitis virus strain JHM from the central nervous system. J Virol. 1989;63:3051–3056. doi: 10.1128/jvi.63.7.3051-3056.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Swanson L W. Brain maps computer graphics files, 1.0 ed. New York, N.Y: Elsevier Science; 1994. [Google Scholar]
- 60.Tirabassi R S, Townley R A, Eldridge M G, Enquist L W. Characterization of pseudorabies virus mutants expressing carboxy-terminal truncations of gE: evidence for envelope incorporation, virulence, and neurotropism domains. J Virol. 1997;71:6455–6464. doi: 10.1128/jvi.71.9.6455-6464.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Torres-Nagel N, Kraus E, Brown M H, Tiefenthaler G, Mitnacht R, Williams A F, Hunig T. Differential thymus dependence of rat CD8 isoform expression. Eur J Immunol. 1992;22:2841–2848. doi: 10.1002/eji.1830221113. [DOI] [PubMed] [Google Scholar]
- 62.Townsend J J. The demyelinating effect of corneal HSV infections in normal and nude (athymic) mice. J Neurol Sci. 1981;50:435–441. doi: 10.1016/0022-510x(81)90155-6. [DOI] [PubMed] [Google Scholar]
- 63.Townsend J J. Macrophage response to herpes simplex encephalitis in immune competent and T cell-deficient mice. J Neuroimmunol. 1985;7:195–206. doi: 10.1016/s0165-5728(84)80019-3. [DOI] [PubMed] [Google Scholar]
- 64.Townsend J J. The relationship of astrocytes and macrophages to CNS demyelination after experimental herpes simplex virus infection. J Neuropathol Exp Neurol. 1981;40:369–379. doi: 10.1097/00005072-198107000-00002. [DOI] [PubMed] [Google Scholar]
- 65.Townsend J J. Schwann cell demyelination in experimental herpes simplex encephalitis at the trigeminal root entry zone. J Neuropathol Exp Neurol. 1983;42:529–538. doi: 10.1097/00005072-198309000-00005. [DOI] [PubMed] [Google Scholar]
- 66.Vitkovic L, Cunha A D, Tyor W R. Cytokine expression and pathogenesis in AIDS brain. In: Price R W, Perry S W, editors. HIV, AIDS and the brain. New York, N.Y: Raven Press; 1994. pp. 203–222. [PubMed] [Google Scholar]
- 67.Wallgren A C, Karlsson-Parra A, Korsgren O. The main infiltrating cell in xenograft rejection is a CD4+ macrophage and not a T lymphocyte. Transplantation. 1995;60:594–601. doi: 10.1097/00007890-199509270-00013. [DOI] [PubMed] [Google Scholar]
- 68.Wang Y, Calder V L, Lightman S L, Greenwood J. Antigen presentation by rat brain and retinal endothelial cells. J Neuroimmunol. 1995;61:231–239. doi: 10.1016/0165-5728(95)00096-k. [DOI] [PubMed] [Google Scholar]
- 69.Watson R E, Wiegand S T, Clough R W, Hoffman G E. Use of cryoprotectant to maintain long-term peptide immunoreactivity and tissue morphology. Peptides. 1986;7:155–159. doi: 10.1016/0196-9781(86)90076-8. [DOI] [PubMed] [Google Scholar]
- 70.Weinstein D L, Walker D G, Akiyama H, McGeer P L. Herpes simplex virus type I infection of the CNS induces major histocompatibility complex antigen expression on rat microglia. J Neurosci Res. 1990;26:55–65. doi: 10.1002/jnr.490260107. [DOI] [PubMed] [Google Scholar]
- 71.Wekerle H, Engelhar T B, Risau W, Meyermann R. Interaction of T lymphocytes with cerebral endothelial cells in vitro. Brain Pathol. 1991;1:107–114. doi: 10.1111/j.1750-3639.1991.tb00647.x. [DOI] [PubMed] [Google Scholar]
- 72.Wekerle H, Linington C, Lassmann H, Meyermann R. Cellular immune reactivity within the CNS. Trends Neurosci. 1986;9:271–277. [Google Scholar]
- 73.Whealy M E, Card J P, Robbins A K, Dubin J R, Rziha H J, Enquist L W. Specific pseudorabies virus infection of the rat visual system requires both gI and gp63 glycoproteins. J Virol. 1993;67:3786–3797. doi: 10.1128/jvi.67.7.3786-3797.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Williamson J S P, Stohlman S A. Effective clearance of mouse hepatitis virus from the central nervous system requires both CD4+ and CD8+ T cells. J Virol. 1990;64:4589–4592. doi: 10.1128/jvi.64.9.4589-4592.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Williamson J S P, Sykes K C, Stohlman S A. Characterization of brain-infiltrating mononuclear cells during infection with mouse hepatitis virus strain JHM. J Neuroimmunol. 1991;32:199–207. doi: 10.1016/0165-5728(91)90189-E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wood M J, Byrnes A P, Rabkin S D, Pfaff D W, Charlton H M. Immunological consequences of HSV-1 mediated gene transfer into the CNS. Gene Ther. 1994;1:S82. [PubMed] [Google Scholar]
- 77.Zaltas M M, Opremcak M, Hemady R, Foster C S. Immunohistopathologic findings in herpes simplex virus chorioretinitis in the von Szily model. Investig Opthalmol Vis Sci. 1992;33:68–77. [PubMed] [Google Scholar]
- 78.Zuckermann F A, Zsak L, Mettenleitter T C, Ben-Porat T. Pseudorabies virus glycoprotein gIII is a major target antigen for murine and swine virus-specific cytotoxic T lymphocytes. J Virol. 1990;64:802–812. doi: 10.1128/jvi.64.2.802-812.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]