Abstract
Children with sex chromosome trisomy (SCT) are at increased risk for developing language difficulties. Earlier studies have reported that as many as 70–80% of individuals with SCT show some form of language difficulties. Language develops rapidly in the first years of life; knowledge about language development at an early age is needed. The present study aims to identify the language abilities of young children with SCT across multiple language domains and to identify the percentage of children that, according to clinical guidelines, have language difficulties. Children between the ages of 1–6-years (NSCT = 103, Ncontrols = 102) were included. Nonverbal communication, early vocabulary, semantic, syntax, and phonological skills were assessed. Language difficulties were already present in 1-year-old children with SCT and across the age range in various language domains. Clinical classification showed that, depending on the assessed domain, 14.8–50.0% of the children scored below the 16th percentile. There was no effect of time of diagnosis, ascertainment bias, research site, nor SCT specific karyotype (XXX, XXY, XYY) on language outcomes. Overall, language difficulties can already be present in very young children with SCT within various language domains. These findings appear to be robust within the SCT group. These results highlight the importance of monitoring both receptive and expressive language development already at the earliest stages of nonverbal communication. Finally, as early language skills are the building blocks for later social communication, literacy, and self-expression, studies that investigate the effect of early interventions on later language outcomes are warranted.
Keywords: Sex chromosome trisomy, language development, receptive language, expressive language, early childhood
Sex chromosome trisomy (SCT), the presence of an extra X or Y chromosome leading to an XXX, XXY, or XYY karyotype, is caused by a spontaneous error during early cell division (Leggett et al., 2010). With an estimated prevalence of 1:650 to 1:1000 live births (Bojesen et al., 2003; Groth et al., 2013; Morris et al., 2008), SCT is one of the most common chromosomal duplications in humans. The presence of an extra X or Y chromosome can impact neurocognitive development in children (for a review see Urbanus et al., 2019), and previous studies have shown that individuals with SCT have an increased risk for neurodevelopmental disorders (for a review see Van Rijn, 2019), and behavioral problems (Urbanus et al., 2020).
One of the most distinctive traits of SCT is the impact the extra chromosome may have on language development. Previous studies have reported that as many as 70–80% of included individuals with SCT have some form of language difficulty (for a review see e.g., Boada et al., 2009; Leggett et al., 2010; Robinson et al., 1983). Most of these studies have included school-aged children or adolescents, with only a handful of studies including small samples of children under the age of 4 years, a time when language develops rapidly. Language development plays an important role in cognitive and social development (Simms, 2007), and is required for communication of one’s needs, thoughts, and emotions. In addition, language is needed for learning and evaluation, for example, in helping us reflect upon what we experience, and helping us understand the world around us. Language is also critical for reading and literacy. If language develops poorly, this can have severe consequences for other developmental domains (e.g., cognitive and emotional development), consequently also affecting one’s ability to participate in society, or the experienced quality of life.
Typically, before young children are able to use spoken language, children use gestures to communicate with others (i.e., early nonverbal communication). With increasing age, children start to understand the meaning of perceived words, sentences, and conversations (i.e., the development of receptive language), and then they start to use spoken language (i.e., expressive language) to convey meaning and thoughts through the production of words and sentences, as they engage in conversation (Levey, 2019). Children need to develop certain language skills to acquire adequate linguistic competence. The distinction between the following skills can be made: 1) Phonology (how sounds form a word), 2) morphology (how words are formed), 3) syntax (how words are combined to form sentences), 4) semantics (specific meaning of words, phrases, and sentences; including lexicon or vocabulary), and 5) pragmatics (use of language in a social setting; Owens Jr., 2011) .
Although not much is known about the first few years of language development in SCT, review studies, which cover results from both prospective newborn screening studies and more recent research, and include individuals regardless of time of diagnosis (i.e., prenatal or postnatal), generally report difficulties in one or more of the language domains. Overall, within the SCT group as a whole, studies report difficulties with language already at a young age. Language difficulties are both reported by parents as well as demonstrated in task performances of included children. Generally, studies report large effect sizes, ranging from .96 to 2.18 (Cohen’s d), indicating high clinical significance of language difficulties (Urbanus et al., 2019). For school-aged girls with XXX, the results overall show an increased risk for early developmental speech and language difficulties (Leggett et al., 2010), with expressive language possibly more affected than receptive language (Tartaglia et al., 2010). Fifty to 75% of girls show compromised receptive and expressive language (Otter et al., 2010). Language problems often continue in adolescence and young adulthood, and therefore continue to interfere with overall functioning (Otter et al., 2010; Tartaglia et al., 2010). For school-aged boys with XXY, the results overall show compromised speech and language development (Boada et al., 2009), with language difficulties occurring in 70–80% of the children (Boada et al., 2009; Geschwind et al., 2000). Similar to girls with XXX, expressive language appears to be more severely affected than receptive language in boys with XXY (Leggett et al., 2010; Visootsak & Graham, 2006). There is evidence for general language impairments of a persistent nature (Hong & Reiss, 2014; Verri et al., 2010), with difficulties becoming more prominent with increasing age (Geschwind et al., 2000; Mandoki et al., 1991). For boys with XYY, information is limited. Re and Birkhoff (2015) report compromised speech and language development in childhood, and Leggett et al. (2010) report mixed findings, indicating that more research is needed.
Collectively, the studies included in the reviews demonstrate that atypical language development is common in individuals with SCT, and that persistent language impairment may influence quality of life. However, most of these findings are based on studies including school-aged children, adolescents, or adults, and both the number of the included individuals and the recruitment strategy (e.g., prospective follow-up, clinical-, or research groups) of the group varied from study to study, making it difficult to generalize results. Only a few previous studies have focused on very young children with SCT (Zampini et al., 2020, 2017, 2018). To understand the emergence and trajectory of developmental language problems, it is important to assess language abilities in infancy and toddlerhood at the early stages of rapid development and to assess multiple language domains at different developmental stages. This stresses the need for studies focusing on the first years of life, in order to identify children at risk for language difficulties and to detect early markers of aberrant language development. The present study focuses on the first 6 years of life; a time when several important milestones within child development occur, starting from a period where children mostly rely on nonverbal communication and start to use words to a period where children start learning in school.
It is important to explore if signs of difficulties in language development can already be identified in very young children with SCT. As there is a significant brain growth in the first three years of life and language difficulties have shown to be persistent across the life span, early detection of risk in language development could support the need for the development of tailored support programs and early preventive intervention to mitigate worse outcomes later in life.
This study evaluates a range of language outcomes in children with SCT. More specifically, this study focuses on the use of early non-verbal communication (i.e., gestures), early vocabulary, semantics, syntax, and phonological processing skills. Factors that could contribute to individual differences in language abilities in the SCT population were assessed, this included specific SCT karyotype (i.e., XXX vs XXY, vs XXY), timing of diagnosis, ascertainment bias, and research site. Recognizing that language develops dynamically during early childhood, the core goal of this study is to investigate the role of age in the language abilities of children with SCT. Specifically, this study aims to identify the language abilities of children with SCT at different developmental stages; to describe the variability within these abilities; and to identify the proportion of children who, according to clinical guidelines, have language difficulties.
Materials and methods
Participants
The present study is part of a larger ongoing project (TRIXY Early Childhood Study). The TRIXY Early Childhood Study is a longitudinal study that included children with and without SCT aged 1–7 years, and aims to identify neurodevelopmental risk in young children with an extra X or Y chromosome. For the present study, children aged 1–6 years were included; only results from the first visit are reported.
In total, 205 children participated in the present study, 103 children with SCT and 102 children without SCT. Ages ranged from 11 months to 6 years and 11 months (see Table 1 for descriptives of the groups). Of the 103 children with SCT, 70 children received a prenatal diagnosis with genetic testing performed due to routine prenatal screening or advanced maternal age. Of the 33 children who received a postnatal diagnosis, 14 received the diagnosis because of a developmental delay (including language delays), 10 because of physical and/or growth problems (e.g., small testes), and nine because of medical concerns or suspicion of other genetic syndromes. Within the XXY-group, 24 children (49%) had received early testosterone supplements.
Table 1.
Descriptives SCT versus controls.
| SCT | XXX | XXY | XYY | Control | XX | XY | p (SCT vs control) | SCT comparisons | |
|---|---|---|---|---|---|---|---|---|---|
| Total N | 103 | 32 | 49 | 22 | 102 | 58 | 44 | ||
| Age | 3.54 (1.83) |
4.17 (1.69) |
3.16 (1.85) |
3.47 (1.80) |
3.60 (1.62) |
3.63 (1.62) |
3.56 (1.63) |
.785 | XXX = XXY = XYY |
| GIFa | 97.45 (17.01) |
94.90 (16.56) |
100.42 (16.65) |
94.26 (18.24) |
105.70 (14.34) |
104.19 (13.57) |
107.68 (15.23) |
<.001 | XXX = XXY = XYY |
| SESb | 5.93 (.94) |
5.94 (1.03) |
6.05 (.88) |
5.66 (.92) |
5.43 (1.40) |
5.24 (1.33) |
5.68 (1.47) |
.003 | XXX = XXY = XYY |
Scores represent Means (SD).
SCT = Sex Chromosome Trisomy; SCT comparisons = XXX versus XXY versus XYY; GIF = level of global intellectual functioning; SES = socioeconomic status.
Data for 6 children with SCT was incomplete.
Data for 1 child with SCT was missing.
Recruitment and assessment took place at the Trisomy of the X and Y chromosomes (TRIXY) Expert Center in the Netherlands and at the eXtraordinarY Kids Clinic in Developmental Pediatrics at Children’s Hospital Colorado in the USA. With the help of clinical genetics departments (from the Netherlands, the Dutch speaking parts in Belgium, and Colorado), pediatricians, and national advocacy or support groups for individuals with SCT children in the SCT group were recruited by sending out recruitment flyers and with postings on the internet (e.g., TRIXY website and the eXtraordinarY Kids Facebook page). In order to assess ascertainment bias in the SCT group three subgroups were identified: (A) “active prospective follow-up” included families that were actively followed after a prenatal diagnosis (51.5% of the SCT group), (B) “Information seeking parents” included families who enrolled in the study because they wanted more information about SCT, but did not have specific concerns about the development of their child (27.2% of the SCT group), and (C) “Clinically referred cases” included families who enrolled after receiving professional help because of specific concerns about the development of their child (21.4% of the SCT group). Non-clinical controls were recruited from the western part of the Netherlands. In collaboration with public sites, such as public daycare centers and public schools, and with the help of government institutions, researchers had access to the civil registry. Via these public sites, information brochures were distributed to parents with children of eligible age. If parents were interested in the study, they were able to contact the researchers to receive further information about the study and to discuss enrollment.
For all participants, both the child and parent had to speak Dutch or English. Exclusion criteria included a history of traumatic brain injury, severely impaired hearing or sight, neurological illnesses, or colorblindness. Specific for the non-clinical control group, children with a previous diagnosis according to the Diagnostic and Statistical Manual of Mental Disorders (American Psychiatric Association, 2013) were excluded. SCT was defined by trisomy in at least 80% of the cells, and was confirmed by standard karyotyping. For ethical reasons, genetic screening was not performed in the control group. As the prevalence of SCT ranges from 1:650 to 1:1000, the risk of inclusion of a child with SCT in the control group was considered minimal and acceptable.
Background information of participants
Global intellectual functioning (GIF) was assessed with the Bayley Scales of Infant and Toddler Development (NSCT = 34; Ncontrol = 31; Bayley, 2006) in the one-year olds, and the short-version of the Wechsler Preschool and Primary Scale of Intelligence third-edition (NSCT = 61; Ncontrol = 71; WPPSI; Wechsler, 2002) or the Wechsler Nonverbal Scale of Ability (NSCT = 2; Wechsler & Naglieri, 2006) in children aged 3 years or older. GIF scores for six children in the SCT group were missing. There was a significant difference in average full-scale intelligence scores between the SCT and control group, t(197) = −3.70, p < .001, d = .53. Although both groups on average scored within the average range, the SCT group scored lower (M = 97.45, SD = 17.01) than the control group (M = 105.69, 14.34). For the children assessed with the WPPSI, non-verbal reasoning scores were also available; children in the SCT group scored significantly lower (N = 62, M = 96.48, SD = 17.16) than the control group (N = 71, M = 106.35, SD = 14.56, p < .001, d = .62).
As a marker for socioeconomic status (SES), parents were asked to indicate the highest level of education they had received. Data was collected for both caregivers. To be able to compare data from participants from all countries, parental education was converted to a global scale with the criteria of Hollingshead (Hollingshead, 1975). The Hollingshead scale ranges from 0 (no formal education) to 7 (graduate/professional training). The highest level of education according to the Hollingshead criterion was then averaged for both caregivers. If no second caregiver was present (3.9% of the participants), the level of education for only one parent was used. SES for one child in the SCT group was unknown. There was a significant difference in average SES between the SCT and the control group, t(176.70) = 2.99, p = .003, d = .42. On average, the SES of the SCT group was higher (M = 5.93, SD = .94) than the SES of the control group (M = 5.43, SD = 1.40).
Lastly, we looked at average parental age, where age of both caregivers was averaged. Parental age for one child in the SCT group was missing. Parental age was significantly higher in the SCT group (M = 39.21, SD = 4.99) than in the control group (M = 36.02, SD = 5.19), t(202) = 4.46, p < .001 d = .63.
As there were significant differences between the SCT and control group on global intellectual functioning, SES, and average parental age, correlations were calculated between these variables and all outcome measures for each age group and for the SCT and control group separately. All correlations can be found in the Supplementary materials part A.
Age groups
To test for age-dependent differences, participants were divided into the following age groups: (1) the 1-year-old group (aged 11–23 months; NSCT = 35, Ncontrols = 31), (2) the 3–4-year-old group (aged 35–59 months; NSCT = 42, Ncontrols = 45), and (3) the 5–6-year-old group (aged 60–83 months; NSCT = 26, Ncontrols = 26). Descriptives per age group can be found in Table 2. The ratio of SCT karyotypes was assessed across age groups, there were no significant differences (p = .093) indicating that the distribution of karyotypes was similar in each age group.
Table 2.
SCT versus controls per age group.
| 1-year-olds |
3–4-year-olds |
5–6-year olds |
SCT Comparisons by age groups | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SCT | Control | p | SCT | Control | p | SCT | Control | p | ||
| Total N | 35 | 31 | 42 | 45 | 26 | 26 | ||||
| Age | 1.39 (.36) |
1.53 (.28) |
.073 | 3.87 (.60) |
3.87 (.60) |
.981 | 5.90 (.59) |
5.62 (.40) |
.044 | |
| GIF | 99.32 (13.41) |
99.71 (13.98) |
.910 | 102.36 (19.15) |
105.73 (14.70) |
.364 | 86.83 (13.44) |
112.77 (10.96) |
<.001 | 1 = 3–4 > 5–6 |
| Verbal IQa | 102.74 (18.67) |
106.82 (17.43) |
.306 | 85.83 (12.30) |
114.58 (16.10) |
<.001 | ||||
| Nonverbal IQb | 100.26 (17.60) |
104.02 (14.82) |
.290 | 90.09 (14.62) |
110.38 (13.44) |
<.001 | ||||
| Ratio Karyotypes (XXX/XXY/XYY) | 6/21/8 | 13/20/9 | 13/8/5 | XXX = XXY = XYY | ||||||
Scores represent Means (SD).
SCT = Sex Chromosome Trisomy; SCT comparisons by age group = comparison of outcome in 1-year-olds with SCT versus 3–4-year-olds with SCT versus 5–6 year-olds with SCT; GIF = level of global intellectual functioning.
Verbal IQ was missing for 4 SCT children in the 3–4 year-old group and 3 SCT children in the 5–6 year-old group.
Nonverbal IQ was missing for 3 SCT children in the 3–4 year-old group and 3 SCT children in the 5–6 year-old group.
Procedure
This study was approved by the Ethical Committee of Leiden University Medical Center, the Netherlands and the Colorado Multiple Institutional Review Board (COMIRB) in Colorado, USA. After providing a description of the study to the parent(s) of the child, written informed consent according to the declaration of Helsinki was obtained.
Assessment took place at various sites (Colorado USA, the Netherlands, Belgium) either in a quiet room at the university or at home. To standardize the testing environment, the testing set-up and research protocols were identical on all sites. Researchers from Leiden University were responsible for project and data-management (i.e., training and supervision of researchers, processing and scoring of data).
Due to the inclusion of participants from various sites, tasks and questionnaires were administered in either Dutch or English. With the exception of one task, all tasks and questionnaires were available in both languages. The Dutch and English versions of the tasks and questionnaires are very similar, with sufficient psychometric properties, and can be used interchangeably. Both versions come with language-specific norms based on population samples. For one questionnaire, the number of items differed between the Dutch and English versions; adjustments in the scores were made when applicable. As the task to assess phonological processing skills was not available in Dutch, this task was administered in the USA group only. All tests and questionnaires were administered and interpreted according to the standardized procedure as specified in the instruments manual.
Instruments
Early non-verbal communication and early vocabulary
Within the youngest age group (1-year-olds), parents were asked to complete the age-appropriate version of the MacArthur-Bates Communicative Development Inventories (CDI), either in English (Fenson et al., 2007) or in Dutch (Zink & Lejaegere, 2014). For children aged 11–16 months, parents filled out the Words and Gestures (CDI W&G) form. For children aged 17–23 months, parents filled out the Words and Sentences (CDI W&S) form. The CDI was completed by the primary caregiving parent (92.1% mother) of the child.
Words and gestures – early non-verbal communication.
Early forms of communication for children aged 11–16 months were assessed with the CDI Words and Gestures part II: Actions and gestures, which consists of five subsections. Subsections A and B together measure “early gestures,” and address questions regarding the first communicative gestures as a measure of the onset of intentional communication (subsection A) and games and routines as a measure of the early social interactive basis for communicative development (subsection B). Subsections C through E measure “later gestures,” and address questions regarding actions with objects and imitating other adult actions as a measure of understanding of the world of objects and the use of things (subsections C and D), and pretending to be a parent as a measure of true symbolic gestures (subsection E). Depending on the form used (USA versus Dutch form, respectively), 17/18 early gestures and 45/48 later gestures were assessed.
Words and gestures – early vocabulary.
Early vocabulary of children aged 11–16 months was assessed with the CDI Words and Gestures part I – subsection D: Vocabulary checklist. Within the vocabulary checklist, parents can indicate which of the words a child understands (receptive early vocabulary) and which of the words a child understands and says (expressive early vocabulary). The number of items included in the vocabulary checklist depends on the used form, with 396 items in the USA form, and 434 items in the Dutch form.
Words and sentences – early vocabulary.
Early vocabulary of children aged 17–23 months was assessed with the CDI Words and Sentences part I – subsection A: Vocabulary checklist. The administration of the Dutch version of the vocabulary checklist is similar to the CDI W&G vocabulary, with a total number of 702 items. The USA version of the checklist, however, only requires parents to indicate which of the words a child says (expressive early vocabulary), with a total of 680 items.
Semantic language skills
Semantic language skills were assessed with the Bayley Scales of Infant and Toddler Development (Bayley, 2006) in the 1-year-olds, and with the Clinical Evaluation of Language Fundamentals Preschool (CELF-P; Wiig et al., 2004, 2012) and the Peabody Picture Vocabulary Test (PPVT; Dunn & Dunn, 1997, 2005) in the 3–6-year-olds.
One-year-olds.
The Bayley Scales were used as an indicator for the development of children aged 1–42 months in five developmental domains. For this study, only the language scale was used. The Bayley Language Scale consists of separate subtests for receptive and expressive communication. The receptive communication subtest assesses pre-verbal behavior, ability to identify objects and pictures, and understanding of verbal messages. The expressive communication subtest assesses pre-verbal communication, ability to name objects and pictures, and the ability to use multiple-word sentences.
Three-to-six-year-olds.
The CELF-P was used to assess several elements of language in children aged 3–7 years. For this study, the CELF-P subtest Expressive Vocabulary was used. This subtest assesses the ability to label people, objects, and actions based on colored images. Higher scores indicate better expressive vocabulary skills.
The PPVT was used to assess receptive vocabulary in individuals aged 2–90+ years. This test measures listening comprehension of spoken words. For each item, the participant is shown four black and white pictures, and the participant has to identify the picture that illustrates the stimulus word that is orally presented by the researcher. Higher scores indicate better receptive vocabulary skills.
Syntax and phonological processing
Within the 3–4 and 5–6 year old children, the subtest Sentence Structure from the CELF-P was used as an indication of syntactic development. This subtest assesses the ability to interpret sentences that increase in length and structural complexity. The child was presented four colored pictures on one page and had to select the picture that illustrated the sentence that was orally presented by the researcher. Higher scores indicate better syntactic understanding.
In the USA 3–4 and 5–6 year old groups, phonological processing skills were assessed with the NEPSY-II phonological processing subtest (Korkman et al., 2007a, 2007b). In the 3–4 year old group, phonological processing was assessed using word segment recognition. This subtest assesses a child’s ability to identify a word when given an orally presented word segment (e.g., “-og” for dog). In the 5–6 year old group, elision at the syllable and phoneme level was also used in addition to the word segment recognition task.
Statistical analyses
Raw scores, clinical risk assessment, and Z-scores
Three types of scores were used. First, raw scores were used to compare the children in the SCT versus the control group. Raw scores (scores unadjusted for age) were preferred over standardized scores to examine the relation between age and language skills for each age group separately. Secondly, raw scores were converted into percentile scores based on age and country-specific norms. Percentile scores were then divided into categories to assess variability of scores within the SCT group based on the psychometric conversion table for neuropsychological tests (Lezak et al., 2004). This resulted in the following seven categories: 1) Severely impaired (percentile score of 1.99 or lower), 2) mildly impaired (percentile scores between 2 and 8), 3) low average (percentile scores between 9 and 24), 4) average (percentile scores between 25 and 75), 5) high average (percentile scores between 76 and 91), 6) superior (percentile scores between 92 and 97), and 7) very superior (percentile score of 98 or higher). In addition, clinical risk was assessed; when a child scored below the 16th percentile (i.e., 1 SD below mean), this child was considered as having “language difficulties.” Finally, standard and scaled scores were converted into Z-scores with the same psychometric conversion table in order to compare outcomes on language domains independent of type of (age appropriate) test.
Analyses
Karyotype and boy/girl specific outcomes were compared with nonparametric Kruskal Wallis tests or ANCOVA in case of age differences between groups. SCT versus control group differences were analyzed with one-way-between subjects ANOVA, with the language scores as dependent variables and research group as independent variable. ANOVA was run for each age group separately. To assess the impact of SCT specific characteristics (i.e., time of diagnosis, ascertainment bias, research site), one-way ANOVA was used as well. When applicable, post-hoc analyses were used to identify significant group effects. Effect sizes were calculated with Cohen’s d when applicable, where . Clinical risk assessment was done with descriptive frequencies and as an indication of effect size, odds ratio was calculated.
Data were analyzed using the Statistical Package for the Social Sciences (SPSS) Version 25. Level of significance was set at p ≤ .05, two-tailed. Analyses were run initially without covariates, and to account for differences in nonverbal abilities run again in the 3–4 and 5–6 year-olds with nonverbal IQ as covariate. Due to the number of statistical analyses, a correction of alpha (i.e., the Benjamini–Hochberg Procedure) was conducted to control the false discovery rate.
Results
Karyotype specific language outcomes
First, as boys and girls may develop language at a different pace (Eriksson et al., 2012), we compared language outcomes of boys and girls in our control sample for all language outcomes with the nonparametric Kruskal Wallis test due to sample sizes. Within the 1-year old control group, there was a significant age difference between boys and girls, with the average age slightly higher in the girls. For that reason, group comparisons in this group were analyzed with ANCOVA with age as covariate. Within the 3–4 and 5–6-year-olds, age was not statistically different between boys and girls. For all of the included language outcomes, results were not statistically different between boys and girls (p ranging from .118 to .998). For that reason, we did not expect sex-differences within our SCT group, and karyotype-specific outcomes (i.e., XXX, XXY, and XYY) were compared.
Explorative, karyotype-specific outcomes for the language domains were assessed. First, with ANOVA, receptive semantic and expressive semantic language skills were compared. Z-scores were used to compare scores regardless of used instrument. No significant differences between the three karyotypes were found for receptive (p = .493) or expressive semantic language skills (p = .106). Next, the nonparametric Kruskal Wallis test was used to assess karyotype-specific outcomes within each age group. Average age was compared between the three karyotypes in each age group and no significant differences were found. The nonparametric Kruskal Wallis test did not yield significant differences for the language outcomes (p ranging from .118 to .966). For each of the language outcomes, number of included children per karyotype, average outcomes, and the results of the ANOVA and Kruskal Wallis tests are shown in Table 3.
Table 3.
Karyotype-specific outcomes on the language domains: Overall and per age group.
| N (XXX/XXY/XYY) | XXX | XXY | XYY | p | ||
|---|---|---|---|---|---|---|
| Overall | Receptive semantic skills | 30/49/20 | −.09 (.91) | .02 (.94) | −.28 (1.01) | .493 |
| 1-year-olds | Expressive semantic skills | 31/49/19 | −.55 (1.10) | −.21 (.84) | −.72 (1.07) | .106 |
| Early receptive vocabulary | 4/12/5 | 36.25 (36.90) | 37.25 (24.17) | 43.60 (32.07) | .966 | |
| Early expressive vocabulary | 6/19/8 | 22.67 (16.84) | 40.58 (20.66) | 34.00 (22.59) | .167 | |
| Receptive semantic skills | 6/21/7 | 18.50 (5.24) | 17.48 (5.42) | 18.43 (4.39) | .694 | |
| Expressive semantic skills | 6/21/7 | 18.67 (7.29) | 18.76 (5.64) | 18.71 (5.96) | .757 | |
| 3–4-year-olds | Receptive semantic skills | 12/20/9 | 56.75 (16.67) | 54.35 (16.39) | 46.44 (17.66) | .401 |
| Expressive semantic skills | 12/20/8 | 16.58 (7.69) | 15.45 (5.88) | 11.88 (9.99) | .490 | |
| Syntax | 12/20/8 | 12.75 (5.23) | 10.70 (4.44) | 11.13 (4.91) | .407 | |
| 5–6-year-olds | Receptive semantic skills | 12/8/4 | 72.75 (10.67) | 86.38 (13.15) | 75.00 (18.49) | .312 |
| Expressive semantic skills | 13/8/4 | 24.54 (6.28) | 28.75 (7.07) | 27.75 (7.14) | .118 | |
| Syntax | 12/8/4 | 15.33 (4.44) | 17.50 (2.67) | 15.25 (4.27) | .661 |
Scores represent means (SD) based on raw scores. p-values for the age groups represent outcomes from the nonparametric Kruskal–Wallis test based on mean ranks.
For each karyotype separately, clinical classification was conducted by calculating the percentage of children with “language difficulties” (i.e., a score at or below the 16th percentile). Due to the small sample size for some karyotypes in the age groups, age groups were collapsed in this analysis. For girls with an extra X chromosome, 23.3% (7/30) had difficulties with receptive semantic skills, and 35.5% (11/31) had difficulties with expressive semantic skills. For boys with an extra X chromosome, 14.3% (7/49) had difficulties with receptive semantic skills, and 18.4% (9/49) had difficulties with expressive semantic skills. For boys with an extra Y chromosome, 20.0% (4/20) had difficulties with receptive semantic skills, and 36.8% (7/19) had difficulties with expressive semantic skills. A visualization of results can be found in Figure 1.
Figure 1.
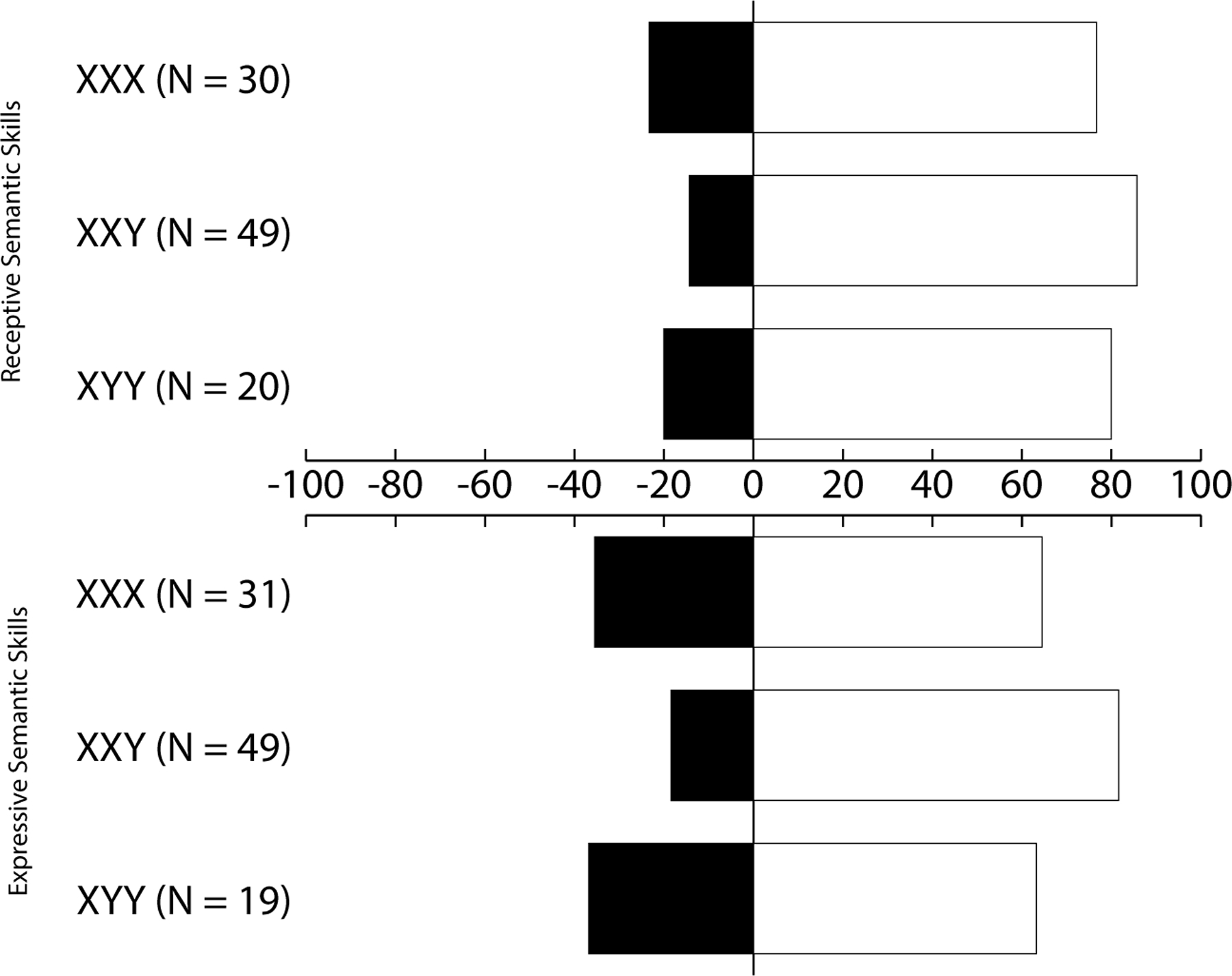
Percentage of SCT children with language difficulties (i.e., scores at or below the 16th percentile) on receptive and expressive semantic skills per karyotype.
As there were no significant differences between the SCT karyotypes on the language outcomes, the three SCT karyotypes were collapsed into one group (SCT group) for subsequent analyses.
Language difficulties at different developmental stages
One-year-old children
There was missing data for one or more of the assessments for three children in the SCT group and one child in the control group. Mean results and effect sizes per language domain can be found in Table 4.
Table 4.
Mean results and effect sizes for each language domain per age group: SCT versus control.
| 1-year-olds |
3–4-year-olds |
5–6-year-olds |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| SCT | Control | d | SCT | Control | d | SCT | Control | d | |
| Early receptive vocabulary | 19.83 (18.81) |
48.42 (29.10) |
1.13*** | N/A | N/A | N/A | N/A | ||
| Early expressive vocabulary | 5.07 (7.87) |
18.04 (23.03) |
.77** | N/A | N/A | N/A | N/A | ||
| Receptive semantic skills | 17.85 (5.07) |
22.32 (4.13) |
.96*** | 53.32 (16.77) |
58.37 (13.42) |
.33 | 77.67 (13.85) |
89.50 (6.64) |
1.10*** |
| Expressive semantic skills | 18.74 (5.81) |
23.32 (5.45) |
.81** | 15.08 (7.37) |
23.27 (6.11) |
1.22*** | 26.40 (6.68) |
33.77 (3.20) |
1.42*** |
| Syntax | N/A | N/A | 11.40 (4.74) |
13.14 (5.10) |
.35 | 16.04 (3.88) |
19.96 (1.80) |
1.31*** | |
Note: scores represent Means (SD)
SCT = Sex Chromosome Trisomy; N/A = not applicable – test was not administered in this age group Significance:
p < .05;
p < .01;
p < .001
Cohen’s d effect size SCT versus controls
Early receptive and expressive vocabulary
A one-way between-subjects ANOVA compared the mean raw scores of receptive and expressive vocabulary for children with SCT and controls. For receptive vocabulary, there was a significant difference between children with and without SCT, Welch’s F(1, 48.76) = 18.12, p < .001. On average, children with SCT had a smaller receptive vocabulary than the control group. For expressive vocabulary, there was also a significant difference between children with and without SCT, Welch’s F(1, 35.13) = 8.60, p = .006. On average, children with SCT had a smaller expressive vocabulary than the control group. Effect sizes for both receptive and expressive vocabulary for one-year-old children indicate large deviations.
Semantic language skills
A one-way between-subjects ANOVA compared the mean raw scores of receptive and expressive semantic skills for children with SCT and controls. For receptive semantic skills, there was a significant difference between the SCT and the control group, F(1,63) = 15.02, p < .001. On average, children with SCT had lower receptive semantic skills than controls. For expressive semantic skills, there was also a significant difference between the SCT and the control group, F(1,63) = 10.72, p = .002. On average, children with SCT had lower expressive semantic skills than controls. Effect sizes for both receptive and expressive semantic skills indicate large deviations.
Three-and-four-year-old children
There was missing data for one or more of the assessments for three children in the SCT group and two children in the control group. Mean results and effect sizes per language domain can be found in Table 4.
Semantic language skills
A one-way between-subjects ANOVA compared the mean raw scores of receptive and expressive semantic skills for children with SCT and controls. For receptive semantic skills, there was no significant difference between the SCT and the control group, F(1,82) = 2.34, p = .130. Children with SCT on average performed similarly to controls. For expressive semantic skills, there was a significant difference between the SCT and the control group, F(1,82) = 31.01, p < .001. On average, children with SCT had significantly lower expressive semantic scores than controls. Effect sizes for expressive semantic skills indicate large deviations.
Syntax
A one-way between subjects ANOVA compared the mean raw scores of syntactic language skills for children with SCT and controls. There was no significant difference between the SCT and control group, F(1,82) = 2.60, p = .111. Children with SCT in this age group had similar syntactic skills as controls.
Five-and-six-year-old children
There was missing data for one or more of the assessments for two children in the SCT group. Mean results and effect sizes per language domain can be found in Table 4.
Semantic language skills
A one-way between-subjects ANOVA compared the mean raw scores of receptive and expressive semantic skills for children with SCT and controls. For receptive semantic skills, there was a significant difference between the SCT and the control group, Welch’s F(1,32.45) = 14.45, p = .001. On average, the children with SCT had lower receptive semantic skills than the controls. For expressive semantic skills, there was also a significant difference between the SCT and the control group, Welch’s F(1,34.18) = 24.89, p < .001. On average, children with SCT had significantly lower expressive semantic scores than controls. Effect sizes for both receptive and expressive semantic skills indicate large deviations.
Syntax
A one-way between subjects ANOVA compared the mean raw scores of syntactic language skills for children with SCT and controls. There was a significant difference between the SCT and control group, Welch’s F(1,31.87) = 20.40, p < .001. Children with SCT had lower syntactic language skills than the controls. Effect sizes indicate large deviations.
The effect of non-verbal intelligence on language outcomes and corrective analyses
To assess the effect of non-verbal intelligence on language outcomes, all statistical analyses in the 3–4 and 5–6 year-olds were run with non-verbal intelligence as covariate. For all analyses, the pattern of findings was the same as without the correction for nonverbal intelligence. This indicates that the differences between children with and without SCT on language outcomes remain significant, irrespective whether or not a deficit in nonverbal IQ was present.
Due to the multiple statistical analyses, a Benjamini–Hochberg Procedure was run to control the false discovery rate. Results after the procedure followed the same pattern of findings, indicating that significant results represent true findings rather than false discoveries.
Affected language domains in children with SCT: Variability and clinical classifications
When applicable, raw data were converted to percentile scores and classified based on a psychometric conversion table. Children who scored below the 16th percentile were considered as having “language difficulties”; the percentage of children on each of the language outcomes is described below. Table 5 displays the variability in outcomes (i.e., percentage of children per clinical classification), the percentages of children with “language difficulties” and the odds ratio (i.e., the change of having language difficulties in the SCT group compared to the control group) for each language domain. A visual representation of the percentage of children with language difficulties can be found in Figure 2.
Table 5.
Clinical classification of children with language difficulties and variability per language domain for 1–6-year-old children with SCT.
| Clinical classification: language difficulties | Clinical classification: variability |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Severely impaired | Mildly impaired | Low average | Average | High average | Superior | Very superior | ||
| Early gestures | 16 | 31.3% OR = 7.17 |
6.3% | 31.3% | 31.3% | 18.8% | 12.5% | ||
| Later gestures | 16 | 43.8% OR = 1.94 |
6.3% | 56.3% | 31.3% | 6.3% | |||
| Total gestures | 16 | 50.0% OR = 6.00 |
18.8% | 31.3% | 43.8% | 6.3% | |||
| Receptive vocabulary | 21 | 19.0% OR = 2.12 |
4.8% | 9.5% | 28.6% | 52.4% | 4.8% | ||
| Expressive vocabulary | 33 | 27.3% OR = 1.88 |
6.1% | 6.1% | 18.2% | 69.7% | |||
| Receptive semantic skills | 99 | 18.2% OR = 5.33 |
1.0% | 8.1% | 14.1% | 52.5% | 18.2% | 6.1% | |
| Expressive semantic skills | 99 | 27.3% OR = 5.04 |
5.1% | 8.1% | 14.1% | 65.7% | 5.1% | 2.0% | |
| Syntax | 64 | 32.8% OR = 2.93 |
3.1% | 15.6% | 14.1% | 56.3% | 9.4% | 1.6% | |
| Phonological processing | 27 | 14.8% OR = N/Aa |
14.8% | 77.8% | 7.4% | ||||
Language difficulties: Children who scored at or below the 16th percentile; OR = odds ratio.
There was no control group available for the phonological processing task; the OR could not be calculated.
Figure 2.
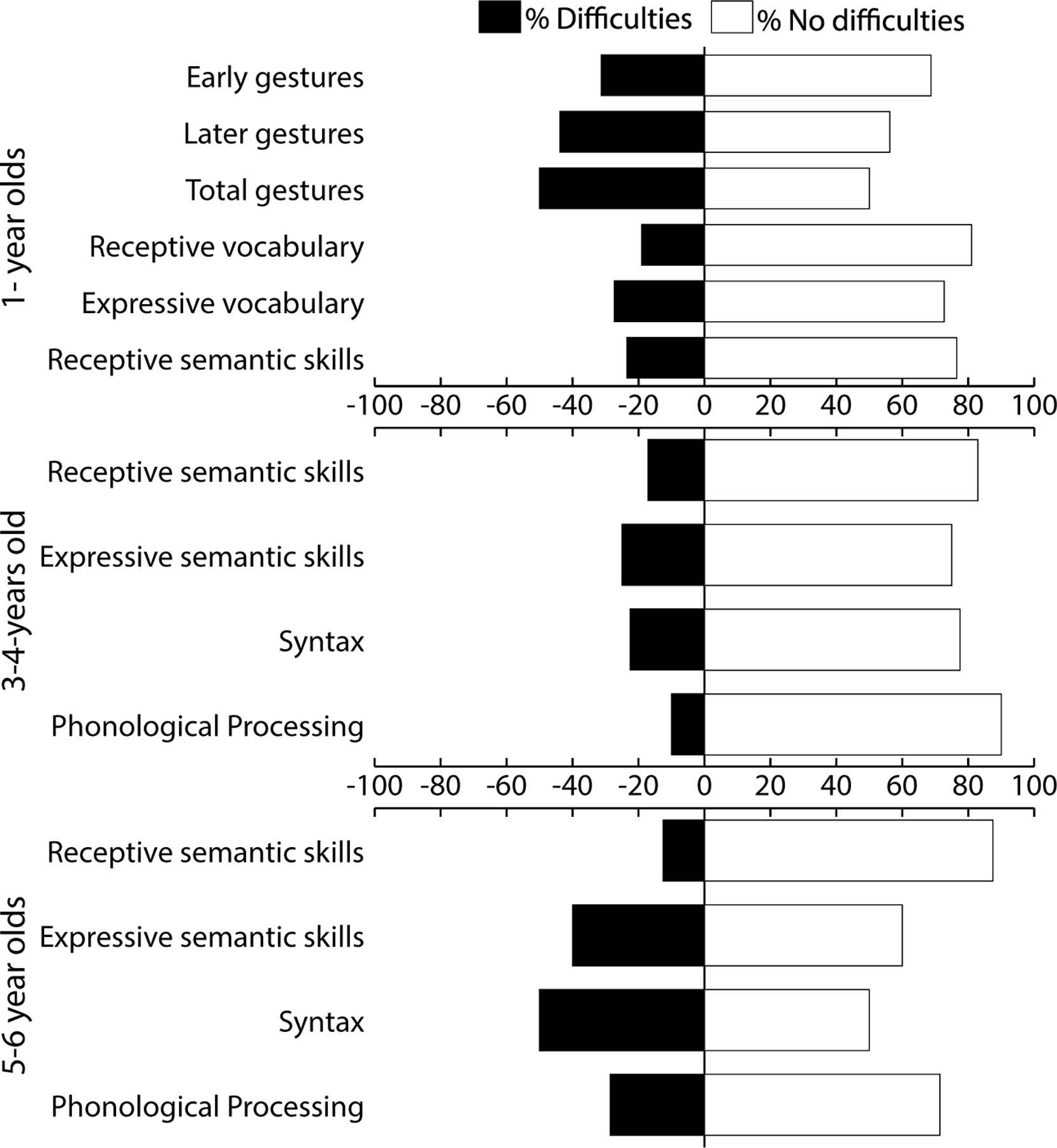
Visual representation of language difficulties per age group for SCT children only. The dark bars represent the percentage of children with scores at or below the 16th percentile on each of the language domains.
Early non-verbal communication skills: Actions and gestures
Within the youngest age group (11–15 months; Mage = 12.6 months, SD = 1.22 months) of children with SCT (N = 16) the CDI questionnaire asks parents about the number of gestures their child uses. Already with these earliest forms of communication, up to half of the children with SCT had difficulties.
Early receptive and expressive vocabulary
Parents of all one-year-olds with SCT were asked to indicate how many words their child understood and/or produced (Nunderstood = 21; Nproduced = 33). Classification of these results shows that already at this age, receptive and/or expressive vocabulary skills can be (severely) impaired in children with SCT when their performance is compared to children from the same country and the same age. Within this sample of one-year-old children with SCT, 19.0% had difficulties with understanding words (or receptive vocabulary), and 27.3% with producing words (or expressive vocabulary).
Semantic language skills
Receptive and expressive semantic skills were assessed in children of all ages (Nreceptive = 99; Nexpressive = 99). Overall, for receptive semantic skills, 18.2% of the children performed below what is expected at their age (N = 23) and 27.3% of the children had expressive semantic skills below what is expected (N = 27).
The large sample size allowed clinical classification per age group, which showed that in one-year-old-children, 23.5% had difficulties with receptive semantic skills (N = 34), and 20.6% had difficulties with expressive semantic skills (N = 34). In the 3–4-year-old group, receptive semantic skills were assessed in 41 children and expressive semantic skills in 40 children. In this group of 3–4-year-olds, 17.1% had difficulties with receptive semantic skills, and 25.0% had difficulties with expressive semantic skills. Finally, in the 5–6-year-old group, receptive semantic skills were assessed in 24 children and expressive semantic skills were assessed in 25 children. In this group of 5–6-year-olds, 12.5% of the children had difficulties with receptive semantic skills and 40.0% had difficulties with expressive semantic skills.
Syntax and phonological processing
As syntax and phonological processing were only assessed in 3–6-year-old children, age groups were collapsed for maximum statistical power. For syntactic development (N = 64), 32.8% of the children performed below what is expected at their age. Phonological processing skills were only assessed in the USA group, resulting in a smaller group of participants (N = 27). Overall, phonological processing skills appear to be affected in a smaller group of children with SCT.
Semantic language outcomes: Impact of SCT characteristics
The impact of time of diagnosis (prenatal versus postnatal), ascertainment bias (prospective follow up, information seeking, and clinically referred), and research site on language outcomes was assessed with ANOVA. Only measures for receptive semantic and expressive semantic skills were included, as these outcomes were available for participants of all ages. To allow for comparisons regardless of used instrument, standardized and scaled scores were converted into Z-scores based on the psychometric table. There were no significant differences in either receptive or expressive semantic outcomes for prenatal versus postnatal diagnosis, for ascertainment bias, or for research site. Results can be found in Table 6.
Table 6.
SCT characteristics and average scores for expressive and receptive semantic skills.
| Time of diagnosis |
Ascertainment biasa |
Recruitment site |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Prenatal | Postnatal | p | A | B | C | p | USA | NL/BE | p | |
| N | 67 | 31 | 51 | 26 | 21 | 56/55 | 43/44 | |||
| Receptive semantic skills | −.05 (.94) |
−.19 (.90) |
.513 | −.09 (.94) |
−.06 (.95) |
−.15 (.92) |
.941 | −.17 (.97) |
.04 (.91) | .280 |
| Expressive semantic skills | −.33 (1.03) |
−.60 (.89) |
.205 | −.42 (.86) |
−.31 (1.02) |
−.54 (1.26) |
.731 | −.43 (1.02) | −.39 (.96) | .854 |
Scores represent Means (SD).
Ascertainment bias: A = active prospective follow up; B = information seeking parents; C = clinically referred cases.
Discussion
The goal of this cross-sectional study was to describe the language profile of a large group of young children with SCT at an age when language is undergoing rapid growth, and by assessing multiple language domains to pinpoint on which of the language outcomes children experience difficulties. For that reason, this study aimed to answer the following key questions: First, to identify the language profiles in children with SCT at different developmental stages within the 1 to 6 year age range. Second, to identify the proportion of children with difficulties in language development and to describe the variability of language development within the SCT group. Finally, in addition to these key questions, this study aimed to evaluate factors that could impact language outcomes (i.e., SCT karyotype, time of diagnosis, ascertainment bias, and research site).
Several factors that could affect language outcomes were assessed: SCT karyotypes, time of diagnosis, ascertainment bias, and research site. Regarding karyotype-specific outcomes, we first compared receptive and expressive semantic skills between XXX, XXY, and XYY for children of all ages. In line with earlier studies, our results indicated that there were no significant differences between the three SCT karyotypes on these two language outcomes (e.g., Bishop et al., 2018; Lee et al., 2012). Next, exploratively, we looked at karyotype-specific differences within each age group and did not find differences between the three groups on the included language outcomes. However, due to small sample sizes, results should interpreted with caution. In addition – when controlling for age-dependent factors – we did not find differences in semantic outcomes between children with a prenatal diagnosis or postnatal diagnosis, nor were outcomes related to ascertainment bias (i.e., the way participants enrolled in the study), or research site. Overall, it appears that language outcomes are very robust within the SCT group. As we did not find evidence for significant differences between the three SCT karyotypes and as previous MRI studies have implied homologous effects of an extra X or Y chromosome on development of the brain (Raznahan et al., 2016), we considered the children with SCT as one group for further analyses to explore age-dependent effects in more detail.
With regard to the first aim, the results indicated that children with SCT on average have poorer language skills than children without SCT. In one-year-old children, children with SCT produced and understood fewer words than their peers without SCT, according to parent report. Within this same age group, poorer receptive and expressive semantic skills were also found with neuropsychological assessment. When ranking the effect sizes within the one-year old group, the largest deviations from the control group were found for early receptive vocabulary. In 3–4-year-old children, children with SCT on average had poorer expressive semantic skills than their peers without SCT. Within this age group, however, we found similar receptive semantic skills and (receptive) syntactic language skills in children with SCT and contro. In the 5–6-year-olds, children with SCT had poorer receptive and expressive semantic skills as well as poorer (receptive) syntactic skills than their peers without SCT. When ranking the effect sizes for this age group, the largest deviations from the control group were found for expressive semantic skills. There was a slight difference in the average age and age distribution (not reported) between the SCT and control group in the 5–6-year-old group, with relatively more older children in the SCT group. As this difference was not in favor of the SCT children (i.e., due to the higher age, higher scores could have been expected), the results presented here might be a slight underestimation.
Collectively, these results imply that the increased risk for language problems starts at a very early age, and that poorer skills compared to children without SCT are a robust finding across developmental stages and the various language domains. Such early language difficulties in the SCT population fit with the idea that language impairments are anchored in early brain development. Studies have shown that both the X and Y chromosomes contain genes that are important for neural development and related cognitive functions (Lenroot et al., 2014; Raznahan et al., 2016). Neuroimaging studies have been conducted to research the consequences of the extra sex chromosome on both the structure and the functioning of the brain. Although studies that provide evidence of a direct link between structural differences and language outcomes in individuals with SCT are lacking (for a review see Skakkebaek et al., 2020), structural differences between children with and without SCT have been found in brain regions that are anatomically consistent with areas that are important for language and/or play a role in language-based learning difficulties (Bryant et al., 2012; Giedd et al., 2007; Lenroot et al., 2014). Only a handful of studies have used functional neuroimaging to test differences in brain activation during a language task, and most of these studies focused on differences in language lateralization (Van Rijn et al., 2008; Wilson & Bishop, 2018). Results of these studies are somewhat mixed; a study of school-aged children did not find differences in language lateralization (Wilson & Bishop, 2018), whereas a study of adults did find differences (Van Rijn et al., 2008). Given that compromised language development is anchored very early in development, longitudinal studies are needed to model to what degree early markers of language difficulties predict cognitive and behavioral outcomes, as well as risk for psychopathology later in life.
Second, outcomes were categorized according to clinical guidelines and compared to performance expected at each child’s chronological age. Results showed that there is much variability within the SCT group. While some children score in the average or above average range, a group of children with SCT performs below what is expected for their age. Based on the classification of language difficulties (i.e., children who scored at or below the 16th percentile), children in the 1-year-old-group showed increased risk for difficulties not only with spoken language, but also with nonverbal communication, such as using gestures for intentional communication and imitating adult actions. The percentage of children that experiences difficulties ranged from 31.3% to 50%. In addition to these difficulties with early nonverbal communication, a group of 1-year-old children with SCT has difficulties with early receptive (19.4%) and/or expressive (27.3%) vocabulary skills. Regarding semantics, 23.5% of the one-year-olds children has difficulties with receptive semantic skills, and 20.6% of the children has difficulties with expressive semantic skills. Within the 3–4 year-old-group, we found that 17.1% of our group experiences difficulties with receptive semantic skills and a quarter of the children with expressive semantic skills. Within the 5–6-year-olds, this was the case for 12.5% of the children with receptive semantic skills, and for expressive semantic skills, 40.0% of the children experienced difficulties. Odds ratio indicated that the risk of language difficulties was 2–7 times higher in the SCT group as compared to the control group, depending on the language function. Collectively, these results show that a large group of children with SCT already has a disadvantage from an early age. When ranking the odds ratio, the SCT group, compared to the control group, had the highest odds for clinical scores on the domain of early nonverbal communication, followed by receptive semantics and expressive semantics. We speculate that these domains are affected the most in the SCT group, taking into account that sample sizes differ between language outcomes. Although this is a cross-sectional sample, these results indicate that difficulties on some domains may become more prominent with increasing age, warranting early support and preventive intervention.
To our knowledge, this is one of the first studies that included a large group (N = 103) of children with SCT at a very young age when language is developing rapidly, and that studied several language domains. There are three other recent studies focusing on language skills in very young children with SCT. These studies include groups of children that participated in a clinical monitoring program in Italy. Similar to our results, these three studies also indicated that compromised language is evident in very young children with SCT. Regarding early communication skills, in contrast to our results, Zampini et al. (2018) found no differences in the number of gestures used by 18-month-old boys with XXY (N = 13) compared to typically developing boys. A second study by this group, with 24-month-old children (8 XXY and 7 XXX), however, found that children with an extra X chromosome used more gestures than children without the extra chromosome (Zampini et al., 2017). As the children included in this study were older (24 months of age), it is possible that as age increases, children start to compensate for their verbal difficulties by using more gestures, a finding that has also been established in other clinical populations, such as children with specific language impairment, down syndrome, or autism (Capone & McGregor, 2004). It should also be noted that the findings by Zampini et al. (2017) and Zampini et al. (2018) are based on observed spontaneous communicative acts during an unstructured play session, whereas our findings are based on parent report; therefore, the studied gestures may differ between studies. Early receptive and expressive vocabulary has also been assessed by the research group of Zampini and colleagues. In a group of 8-month-old children (9 XXY, 10 XXX, 7 XYY), no significant differences in receptive vocabulary were found between children with and without SCT (Zampini et al., 2020). Expressive vocabulary was assessed in the 13 boys with XXY at 18 months and the boys and girls with an extra X at 24 months. These results show that compared to typically developing peers, children with an extra X chromosome at 18 and 24 months have significantly lower expressive vocabularies (Zampini et al., 2017, 2018). Similar to our findings, Zampini et al. (2017) and Zampini et al. (2020) found no differences between the SCT groups. Finally, differences in outcomes between the Italian studies and the current study could also be due to differences in recruitment (i.e., a clinical sample versus a research sample).
With regard to studies assessing receptive and expressive vocabulary skills in broader age groups (up to 18 years), studies report mixed findings. One study with 4–18 year old XXY boys found age-appropriate receptive and expressive vocabulary scores when comparing the boys to the norming sample (Ross et al., 2008). A second study by the same research group with 4–18 year old boys (XXY and XYY) compared outcomes to typically developing boys (Ross et al., 2009). For both the XXY and the XYY group separately, authors reported lower receptive and expressive vocabulary scores compared to the typically developing controls. In addition, the authors compared outcomes between XXY and XYY boys. For expressive vocabulary, no differences were found, whereas for receptive vocabulary, the XYY boys had worse outcomes than the XXY boys.
With regard to studies that included assessments of semantics and syntax, in children with SCT up to 18 years, studies generally report impairments on these language domains. A study by St John et al. (2019) similarly to our findings reported lower overall receptive and expressive language skills in a group of boys (N = 22) with XXY aged 1–17 years. In addition, Ross et al. (2008) found that 4–18 year old boys with XXY (N = 50) performed below age expectations compared to the norming sample on tests assessing semantic and syntactic language skills. In addition, when comparing the younger boys (4–10-year-olds) with the older boys (10–18-year-olds), the authors found significantly more problems in the older boys. Similar to our cross-sectional findings, these findings could imply that language problems become more substantial over time. Lastly, a study by Ross et al. (2009) showed impaired semantic and syntactic skills in boys with XXY (N = 93, aged 4–18 years) and XYY (N = 21, aged 4–14;4 years), with no differences in performance between these two groups. Reported outcomes for phonological processing skills are mixed, with some studies reporting impairments (e.g., Ross et al., 2009), whereas other studies report age-appropriate phonological processing skills (e.g., Ross et al., 2008), similar to our findings. As phonological processing has been shown in many studies to be a predictor of later literacy skills, and there are a large number of children with SCT to have later reading problems, it is important to learn more about the phonological development in very young children with SCT and to identify whether targeting phonological processing early may decrease risk for later challenges.
In our study, depending on the studied language domain, we found rates of clinically relevant difficulties ranging from 12% to 50%. These percentages are lower than reported percentages in other studies (for reviews see Boada et al., 2009; Leggett et al., 2010; Robinson et al., 1983). It is possible that the percentage found in the current study is representative for children in this young age group, and that the percentage of children that experience difficulties depends on the included (age)group. Although not longitudinally studied, results of this study indicate that problems, especially with expressive language, could intensify over time. This phenomenon is also known as “growing into a deficit,” and occurs when a child stays behind on what is expected with increasing age, resulting in a growing deviation of performance compared to peers (Rourke et al., 1983). Another explanation could be the method to examine language development. This study included both parent reports and neuropsychological testing; it is possible that percentages vary across studies depending on the included measurements. Some studies included in the reviews (e.g., by Boada et al., 2009; Leggett et al., 2010; Robinson et al., 1983) for example, included not only specific language measures, but also speech assessments, auditory processing skills, verbal intelligence, or school reports. Also, some studies included verbal academic skills (e.g., reading, writing, spelling), language-based learning problems such as dyslexia, or (only) reported the number of children that have received speech- or language therapy. This study included a young group of children, regardless of time of diagnosis or ascertainment bias, and from multiple research sites to represent the SCT population. This study used valid and reliable standardized assessment in addition to parent report to assess language outcomes and to identify the percentage of children with language difficulties. Our results stress the importance of early assessment of language performance. Already from a young age, there are children with SCT who fall behind age-expectations on various language domains. If the number of children who experience language difficulties increases with age, clinicians should closely monitor the language development of children with SCT, and intervene early when needed.
From a clinical perspective, our results highlight the importance of monitoring language development in children with SCT very early in development, at the earliest stages of nonverbal communication. As our results show, large deviations from the control group were found on nearly all language domains. This stresses the importance that not only expressive, but also receptive language skills should be assessed on a regular basis. Language affects every day functioning. If language skills are compromised, this could affect outcomes in other domains, including academic achievement, and quality of life. Current findings stress the need for screening and close monitoring of language development in this group of children from an early age onwards, for example, during routine child-monitoring programs. Through early intervention, parents should be supported to stimulate the language development of their child, which is important for all children, but could possibly be even more crucial for children with SCT. When a child does not meet language milestones, we recommend standard neuropsychological screening, which should include nonverbal communication, as well as receptive and expressive language skills. With neuropsychological screening, children who are at-risk for suboptimal language development could be identified and the outcomes of the screening could serve as a guide for a tailored treatment and/or intervention plan (e.g., speech therapy). Finally, studies should evaluate to what degree existing intervention programs are beneficial for children with SCT, and if needed, specific interventions tailored to the needs of children with SCT should be developed.
Although our study included a large group of young children with SCT, there were also limitations to this study accompanied by suggestions for future research. First, it is possible that by dividing the group into smaller subgroups based on age, power to detect clinically relevant differences may have been lost. Also, within this study we included children within three age groups (i.e., 1-year old, 3–4-year-old, and 5–6-year-old children). As language develops rapidly in early childhood, further exploration regarding age-specific language abilities within smaller age groups is warranted. Second, we have looked at karyotype specific differences on language outcomes for each age group separately. Due to the sample sizes, our methods were explorative. To gain more insight in language profiles for each karyotype, future studies should include large samples to study both age-specific and karyotype-specific outcomes. Third, we included children with XXY syndrome regardless whether children had received testosterone supplements. To our knowledge, there is only one randomized controlled trial (RCT) that assessed the outcome of androgen treatment (oral oxadrolone) on cognitive functioning in children (Ross et al., 2017). Although the RCT by Ross et al. (2017) reported no effect of early androgen treatment on language outcomes, a large group of children (49%) in the present study had received testosterone replacement therapy. More RCTs on the effect of testosterone replacement treatment on neurocognitive outcomes in young children with SCT are needed before conclusions about potential risks or benefits can be made. Fourth, it should be noted that some of the included children were unable to participate in one or more of the tasks; it cannot be precluded that reported results are slightly underestimated. However, the various ascertainment strategies and the lack of impact of ascertainment strategies on outcomes, contributes to the generalization of results to the population of diagnosed children with SCT. Fifth, although we were able to look at several aspect of language development, it is important to gain more insight into the overall neurocognitive profile of children with SCT, including the broader communication domain (i.e., pragmatic language abilities, or language in an academic setting), but also for example, social cognitive abilities and executive functioning. Although we did not find differences between the three karyotypes on the included language outcomes in this study, it is possible that there are karyotype specific differences on other domains, a question that should be addressed in future studies. It should also be noted that we found lower average nonverbal IQ in our 5–6 year olds with SCT compared to controls, a finding that was not observed in our 3–4 year-olds. When exploring the neurocognitive profile of children with SCT it is important to also take nonverbal IQ into account, as children with SCT may have a nonverbal deficit in addition to other neurocognitive difficulties. Another aspect that should be taken into account in future studies are environmental factors; factors that could possibly moderate outcomes. In our study for example, we found a difference in SES between the SCT and control group, in favor for the SCT group. Although we did not find substantial correlations between SES and language outcomes in either the control or SCT group, we cannot preclude that SES could indirectly impact other mediating factors, such as services received. Finally, as this was a cross-sectional study, our interpretation of age effects is based on different children, and language development over time should be assessed with longitudinal studies. Within these longitudinal studies, other possible confounding factors, such as familial learning difficulties and services received, should also be taken into account. Recently, two studies have been designed to provide in this by studying trajectories of neurodevelopment and behavioral outcomes in the first few years of life, and by looking into predictors of positive and negative outcomes; the TRIXY Early Childhood study, Leiden University, the Netherlands, and the eXtraordinarY babies study, Denver, USA (Tartaglia et al., 2020).
To conclude, our results show that already at a young age, language is a vulnerable domain in children with SCT. Both receptive and expressive language can be affected and should be monitored closely. More longitudinal studies are needed to investigate the impact of early language interventions on later language outcomes. Finally, interventions should be implemented as soon as needed, to prevent more severe problems in later life.
Supplementary Material
Acknowledgments
The authors thank the families that participated in our study, and the research assistants and students for their help with data collection and processing.
Funding
This work was supported by the Nederlandse Organisatie voor Wetenschappelijk Onderzoek (NWO) [number 016.165.397]; work in Colorado was partially supported by infrastructure of NIH/NCATS Colorado CTSA [number UL1 TR002535]. Contents are the authors’ sole responsibility and do not necessarily represent official NIH views. The authors report no conflicts of interest.
Footnotes
Supplemental data for this article can be accessed at https://doi.org/10.1080/09297049.2021.1960959.
Disclosure statement
No conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding author (SvR) upon reasonable request.
References
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). [Text Revision]. 10.1176/appi.books.9780890425596 [DOI] [Google Scholar]
- Bayley N (2006). Bayley scales of infant and toddler development - (third ed.). Harcourt Assessment. [Google Scholar]
- Bishop DVM, Brookman-Byrne A, Gratton N, Gray E, Holt G, Morgan L, Morris S, Paine E, Thornton H, & Thompson PA (2018). Language phenotypes in children with sex chromosome trisomies. Wellcome Open Research, 3, 143. 10.12688/wellcomeopenres.14904.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boada R, Janusz J, Hutaff-Lee C, & Tartaglia N (2009). The cognitive phenotype in Klinefelter syndrome: A review of the literature including genetic and hormonal factors. Developmental Disabilities Research Reviews, 15(4), 284–294. 10.1002/ddrr.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bojesen A, Juul S, & Gravholt CH (2003). Prenatal and postnatal prevalence of Klinefelter syndrome: A national registry study. The Journal of Clinical Endocrinology & Metabolism, 88(2), 622–626. 10.1210/jc.2002-021491 [DOI] [PubMed] [Google Scholar]
- Bryant DM, Hoeft F, Lai S, Lackey J, Roeltgen D, Ross J, & Reiss AL (2012). Sex chromosomes and the brain: A study of neuroanatomy in XYY syndrome. Developmental Medicine and Child Neurology, 54(12), 1149–1156. 10.1111/j.1469-8749.2012.04418.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capone NC, & McGregor KK (2004). Gesture development: A review for clinical and research practices. Journal of Speech, Language, and Hearing Research, 47(1), 173–186. 10.1044/1092-4388(2004/015) [DOI] [PubMed] [Google Scholar]
- Dunn LM, & Dunn LM (1997). Peabody picture vocabulary test - Third Edition manual. American Guidance Service. [Google Scholar]
- Dunn LM, & Dunn LM (2005). Peabody picture vocabulary test-III-NL. Handleiding (Schlichting L, Trans.). Harcourt Test Publishers. [Google Scholar]
- Eriksson M, Marschik PB, Tulviste T, Almgren M, Pérez Pereira M, Wehberg S, Marjanoviĉ-Umek L, Gayraud F, Kovacevic M, & Gallego C (2012). Differences between girls and boys in emerging language skills: Evidence from 10 language communities. British Journal of Developmental Psychology, 30(2), 326–343. 10.1111/j.2044-835X.2011.02042.x [DOI] [PubMed] [Google Scholar]
- Fenson L, Marchman VA, Thal DJ, Dale PS, Reznick JS, & Bates E (2007). MacArthur-bates communicative development inventories: User’s guide and technical manual (2nd ed.). Brookes. [Google Scholar]
- Geschwind DH, Boone K, Miller BL, & Swerdloff RS (2000). Neurobehavioral phenotype of Klinefelter syndrome. Mental Retardation and Developmental Disabilities Research Reviews, 6 (2), 107–116. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Clasen LS, Wallace GL, Lenroot RK, Lerch JP, Wells EM, Blumenthal JD, Nelson JE, Tossell JW, Stayer C, Evans AC, & Samango-Sprouse CA (2007). XXY (Klinefelter syndrome): A pediatric quantitative brain magnetic resonance imaging case-control study. Pediatrics, 119(1), e232–240. 10.1542/peds.2005-2969 [DOI] [PubMed] [Google Scholar]
- Groth KA, Skakkebaek A, Høst C, Gravholt CH, & Bojesen A (2013). Klinefelter syndrome - a clinical update. The Journal of Clinical Endocrinology & Metabolism, 98(1), 20–30. 10.1210/jc.2012-2382 [DOI] [PubMed] [Google Scholar]
- Hollingshead AB (1975). Four factor index of social status [Unpublished Manuscript]. Yale University. [Google Scholar]
- Hong DS, & Reiss AL (2014). Cognitive and neurological aspects of sex chromosome aneuploidies. The Lancet Neurology, 13(3), 306–318. 10.1016/S1474-4422(13)70302-8 [DOI] [PubMed] [Google Scholar]
- Korkman M, Kirk U, & Kemp SL (2007a). NEPSY II. Administrative manual. Psychological Corporation. [Google Scholar]
- Korkman M, Kirk U, & Kemp SL (2007b). NEPSY II. Clinical and Interpretative manual. Psychological Corporation. [Google Scholar]
- Lee NR, Wallace GL, Adeyemi EI, Lopez KC, Blumenthal JD, Clasen LS, & Giedd JN (2012). Dosage effects of X and Y chromosomes on language and social functioning in children with supernumerary sex chromosome aneuploidies: Implications for idiopathic language impairment and autism spectrum disorders. Journal of Child Psychology and Psychiatry, 53(10), 1072–1081. 10.1111/j.1469-7610.2012.02573.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leggett V, Jacobs P, Nation K, Scerif G, & Bishop DV (2010). Neurocognitive outcomes of individuals with a sex chromosome trisomy: XXX, XYY, or XXY: A systematic review. Developmental Medicine & Child Neurology, 52(2), 119–129. 10.1111/j.1469-8749.2009.03545.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot RK, Blumenthal JD, Wallace GL, Clasen LS, Lee NR, & Giedd JN (2014). A case-control study of brain structure and behavioral characteristics in 47,XXX syndrome. Genes Brain and Behavior, 13(8), 841–849. 10.1111/gbb.12180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levey S (2019). An introduction to language development (Second ed.). Plural Publishing, Inc. [Google Scholar]
- Lezak MD, Howieson D, Loring D, & Hannay H (2004). Neuropsychological assessment (4th ed.). Oxford University Press. [Google Scholar]
- Mandoki MW, Sumner GS, Hoffman RP, & Riconda DL (1991). A review of Klinefelter’s syndrome in children and adolescents. Journal of the American Academy of Child and Adolescent Psychiatry, 30(2), 167–172. 10.1097/00004583-199103000-00001 [DOI] [PubMed] [Google Scholar]
- Morris JK, Alberman E, Scott C, & Jacobs P (2008). Is the prevalence of Klinefelter syndrome increasing? European Journal of Human Genetics, 16(2), 163–170. 10.1038/sj.ejhg.5201956 [DOI] [PubMed] [Google Scholar]
- Otter M, Schrander-Stumpel CT, & Curfs LM (2010). Triple X syndrome: A review of the literature. European Journal of Human Genetics, 18(3), 265–271. 10.1038/ejhg.2009.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens RE Jr. (2011). Language development: An introduction. (8th ed., International ed ed.). Pearson Education [Google Scholar]
- Raznahan A, Lee NR, Greenstein D, Wallace GL, Blumenthal JD, Clasen LS, & Giedd JN (2016). Globally divergent but locally convergent X- and Y-chromosome influences on cortical development. Cerebral Cortex, 26(1), 70–79. 10.1093/cercor/bhu174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Re L, & Birkhoff JM (2015). The 47,XYY syndrome, 50 years of certainties and doubts: A systematic review. Aggression and Violent Behavior, 22, 9–17. 10.1016/j.avb.2015.02.003 [DOI] [Google Scholar]
- Robinson A, Bender B, Borelli J, Puck M, & Salbenblatt J (1983). Sex chromosomal anomalies: Prospective studies in children. Behavior Genetics, 13(4), 321–329. 10.1007/BF01065770 [DOI] [PubMed] [Google Scholar]
- Ross JL, Kushner H, Kowal K, Bardsley M, Davis S, Reiss AL, Tartaglia N, & Roeltgen D (2017). Androgen treatment effects on motor function, cognition, and behavior in boys with Klinefelter syndrome. The Journal of Pediatrics, 185, 193–199.e194. 10.1016/j.jpeds.2017.02.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross JL, Roeltgen DP, Stefanatos G, Benecke R, Zeger MPD, Kushner H, Ramos P, Elder FF, & Zinn AR (2008). Cognitive and motor development during childhood in boys with Klinefelter syndrome. American Journal of Medical Genetics Part A, 146A(6), 708–719. 10.1002/ajmg.a.32232 [DOI] [PubMed] [Google Scholar]
- Ross JL, Zeger MP, Kushner H, Zinn AR, & Roeltgen DP (2009). An extra X or Y chromosome: Contrasting the cognitive and motor phenotypes in childhood in boys with 47, XYY syndrome or 47,XXY Klinefelter syndrome. Developmental Disabilities Research Reviews, 15(4), 309–317. 10.1002/ddrr.85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rourke BP, Bakker DJ, Fisk JL, & Strang JD (1983). Child neuropsychology: Introduction to theory, research, and clinical practice. Guilford Press. [Google Scholar]
- Simms MD (2007). Language disorders in children: Classification and clinical syndromes. Pediatric Clinics of North America, 54(3), 437–467. 10.1016/j.pcl.2007.02.014 [DOI] [PubMed] [Google Scholar]
- Skakkebaek A, Gravholt CH, Chang S, Moore PJ, & Wallentin M (2020). Psychological functioning, brain morphology, and functional neuroimaging in Klinefelter syndrome. American Journal of Medical Genetics Part C, 184(2), 506–517. 10.1002/ajmg.c.31806 [DOI] [PubMed] [Google Scholar]
- St John M, Ponchard C, van Reyk O, Mei C, Pigdon L, Amor DJ, & Morgan AT (2019). Speech and language in children with Klinefelter syndrome. Journal of Communication Disorders, 78, 84–96. 10.1016/j.jcomdis.2019.02.003 [DOI] [PubMed] [Google Scholar]
- Tartaglia N, Howell S, Davis S, Kowal K, Tanda T, Brown M, Boada C, Alston A, Crawford L, Thompson T, Van Rijn S, Wilson R, Janusz J, & Ross J (2020). Early neurodevelopmental and medical profile in children with sex chromosome trisomies: Background for the prospective eXtraordinarY babies study to identify early risk factors and targets for intervention. American Journal of Medical Genetics Part C: Seminars in Medical Genetics, 184(2), 428–443. 10.1002/ajmg.c.31807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartaglia N, Howell S, Sutherland A, Wilson R, & Wilson L (2010). A review of trisomy X (47,XXX). Orphanet Journal of Rare Diseases, 5(1), 8. 10.1186/1750-1172-5-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanus E, Swaab H, Tartaglia N, Cordeiro L, & Van Rijn S (2020). The behavioral profile of children aged 1–5 years with sex chromosome trisomy (47,XXX, 47,XXY, 47,XYY). American Journal of Medical Genetics Part C, 184C(2), 444–455. 10.1002/ajmg.c.31788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanus E, Van Rijn S, & Swaab H (2019). A review of neurocognitive functioning of children with sex chromosome trisomies: Identifying targets for early intervention. Clinical Genetics, 97 (1), 156–167. 10.1111/cge.13586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Rijn S (2019). A review of neurocognitive functioning and risk for psychopathology in sex chromosome trisomy (47,XXY, 47,XXX, 47,XYY). Current Opinion in Psychiatry, 32(2), 79–84. 10.1097/yco.0000000000000471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rijn S, Aleman A, Swaab H, Vink M, Sommer I, & Kahn RS (2008). Effects of an extra X chromosome on language lateralization: An fMRI study with Klinefelter men (47,XXY). Schizophrenia Research, 101(1–3), 17–25. 10.1016/j.schres.2008.02.001 [DOI] [PubMed] [Google Scholar]
- Verri A, Cremante A, Clerici F, Destefani V, & Radicioni A (2010). Klinefelter’s syndrome and psychoneurologic function. Molecular Human Reproduction, 16(6), 425–433. 10.1093/molehr/gaq018 [DOI] [PubMed] [Google Scholar]
- Visootsak J, & Graham JM Jr. (2006). Klinefelter syndrome and other sex chromosomal aneuploidies. Orphanet Journal of Rare Diseases, 1(1), 42. 10.1186/1750-1172-1-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D (2002). WPPSI-III: Technical and interpretative manual. The Psychological Corporation. [Google Scholar]
- Wechsler D, & Naglieri JA (2006). Wechsler nonverbal scale of ability. Harcourt Assessment. [Google Scholar]
- Wiig EH, Secord WA, & Semel E (2004). Clinical evaluation of language fundamentals - Preschool (2nd ed.). Harcourt Assessment. [Google Scholar]
- Wiig EH, Secord WA, & Semel E (2012). Clinical evaluation of language fundamentals preschool (CELF-2NL) - Nederlandse Versie (de Jong J, Trans.). Pearson Assessment and Information. [Google Scholar]
- Wilson AC, & Bishop DVM (2018). Sex chromosome trisomies are not associated with atypical lateralization for language. Developmental Medicine and Child Neurology, 60(11), 1132–1139. 10.1111/dmcn.13929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zampini L, Burla T, Silibello G, Capelli E, Dall’Ara F, Rigamonti C, Ajmone PF, Monti F, Zanchi P, Lalatta F, Costantino MA, & Vizziello PG (2020). Preverbal skills in 8-month-old children with sex chromosome trisomies. First Language, 41(2), 200–217. 10.1177/0142723720962944 [DOI] [Google Scholar]
- Zampini L, Burla T, Silibello G, Dall’Ara F, Rigamonti C, Lalatta F, & Vizziello P (2017). Early communicative skills of children with Klinefelter syndrome. Clinical Linguistics & Phonetics, 32(7), 577–586. 10.1080/02699206.2017.1384853 [DOI] [PubMed] [Google Scholar]
- Zampini L, Draghi L, Silibello G, Dall’Ara F, Rigamonti C, Suttora C, Zanchi P, Salerni N, Lalatta F, & Vizziello P (2018). Vocal and gestural productions of 24-month-old children with sex chromosome trisomies. International Journal of Language & Communication Disorders, 53 (1), 171–181. 10.1111/1460-6984.12334 [DOI] [PubMed] [Google Scholar]
- Zink I, & Lejaegere M (2014). N-CDIs: Lijsten voor communicatieve Ontwikkeling, aanpassing en Hervorming van de MacArthur CDIs van Fenson et al. Vlaamse Vereniging voor Logopedisten. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author (SvR) upon reasonable request.


