Abstract
Objective:
Sex-specific differences are observed in various liver diseases, but the influence of sex on the outcomes of hepatocellular carcinoma (HCC) after liver transplantation (LT) remains to be determined. This study is the first Chinese nationwide investigation of the role of sex in post-LT outcomes in patients with HCC.
Methods:
Data for recipients with HCC registered in the China Liver Transplant Registry between January 2015 and December 2020 were analyzed. The associations between donor, recipient, or donor-recipient transplant patterns by sex and the post-LT outcomes were studied with propensity score matching (PSM). The survival associated with different sex-based donor-recipient transplant patterns was further studied.
Results:
Among 3,769 patients enrolled in this study, the 1-, 3-, and 5-year overall survival (OS) rates of patients with HCC after LT were 96.1%, 86.4%, and 78.5%, respectively, in female recipients, and 95.8%, 79.0%, and 70.7%, respectively, in male recipients after PSM (P = 0.009). However, the OS was comparable between recipients with female donors and male donors. Multivariate analysis indicated that male recipient sex was a risk factor for post-LT survival (HR = 1.381, P = 0.046). Among the donor-recipient transplant patterns, the male-male donor-recipient transplant pattern was associated with the poorest post-LT survival (P < 0.05).
Conclusions:
Our findings highlighted that the post-LT outcomes of female recipients were significantly superior to those of male recipients, and the male-male donor-recipient transplant pattern was associated with the poorest post-LT survival. Livers from male donors may provide the most benefit to female recipients. Our results indicate that sex should be considered as a critical factor in organ allocation.
Keywords: Sex, liver transplantation, hepatocellular carcinoma, outcome, recipient, donor
Introduction
Liver transplantation (LT) is an effective therapeutic option for patients with early stage hepatocellular carcinoma (HCC), because it simultaneously removes intrahepatic tumors as well as the pathogenic liver background. In China, from 2015 to 2020, approximately 45% of adult LTs were performed for malignant liver tumors1–3. Because of a shortage of available donor grafts, not all patients with HCC waiting for liver grafts can receive LT4. Therefore, selecting the best candidates who would benefit from LT for HCC and identifying factors that should be considered in organ allocation are critical.
Sex is an important factor that markedly influences the occurrence of liver diseases, including viral hepatitis, liver cirrhosis, and HCC5–7. Epidemiological studies have shown a higher incidence of HCC in males than females, and comparable results have also been observed in mouse hepatocarcinogenesis models8–10. Studies have yielded contradictory conclusions regarding the influence of sex on the outcomes of LT for HCC11–14. A study from Korea has found higher recurrence risk in LT recipients with HCC with male rather than female donors12. However, this conclusion has been challenged by a study demonstrating that donor sex does not affect post-LT recurrence of HCC in a Japanese cohort13. In another study from the United States, the sex of recipients was found to have a significant impact on post-LT HCC recurrence14. The differences in the results might have arisen from differences in tumor etiology and race. Hence, the influence of sex on survival after LT for HCC remains to be determined, particularly for HCC caused by etiological factors such as hepatitis B infection, in diverse cohorts. Moreover, a larger scale clinical study is also needed to further uncover the roles of sex in the outcomes of LT for HCC. However, no prior studies have been performed in China to investigate the influence of sex on post-transplant outcomes of patients with HCC.
Herein, we present the first large-scale study in China exploring the correlation between sex and post-LT outcomes in patients with HCC through propensity score matching (PSM) analysis. Our results revealed that the sex of the recipient, rather than that of the donor, is associated with post-LT survival in HCC. Of note, donor-recipient matching patterns based on sex were also associated with post-LT survival in recipients with HCC. This study highlights sex-specific differences in post-LT outcomes in HCC, and provides evidence that modifications are needed to achieve liver transplant allocation by sex.
Methods
Patients and informed consent
This study retrospectively analyzed LTs performed between January 2015 and December 2020, with clinicopathological data registered in the China Liver Transplant Registry (CLTR). The study was performed in accordance with the Declaration of Helsinki and was approved by the CLTR. The Institutional Review Board of the China Liver Transplantation Registration Scientific Committee and the ethics committee approved the study, under approval number 20220023. The civilian organ donation has been the sole source for organ transplantation in China since January 201515. No organs in this study were procured from executed prisoners, and informed consent was obtained for the organs in all LTs. All patients with HCC scheduled for LT were evaluated preoperatively through ultrasound, Doppler ultrasound, computed tomography (CT), magnetic resonance imaging, positron emission tomography-CT, bone scintigraphy, and colonoscopy to rule out extrahepatic diseases that were contraindications for LT. Preoperative loco-regional therapies, such as radiofrequency ablation (RFA), transcatheter arterial chemoembolization (TACE), and percutaneous ethanol injection, were performed to control or decrease tumor burden. Radiological information was acquired from the most recent CT or magnetic resonance imaging examination before LT. HCC recurrence was diagnosed according to the Guidelines for the Diagnosis and Treatment of Primary Liver Cancer in China.
Inclusion and exclusion criteria
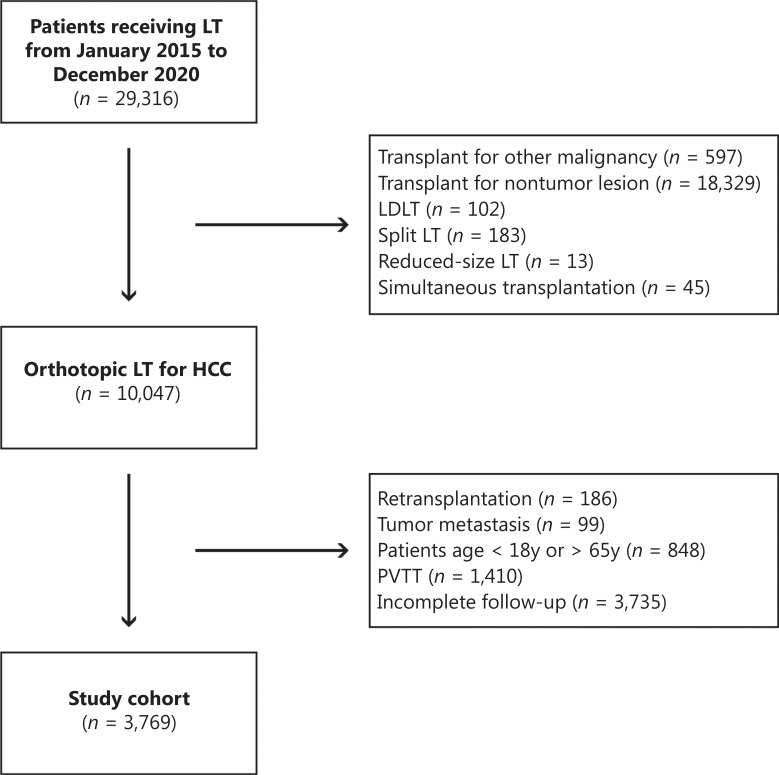
A total of 3,769 patients were studied. Patients with the following features were excluded: other concurrent malignancies; pathologically confirmed tumor types other than HCC, such as intrahepatic cholangiocarcinoma, combined hepatocellular-cholangiocarcinoma, fibrolamellar hepatocellular carcinoma, or secondary metastatic tumor; and lack of histological confirmation of HCC for LT. Patients who received living donor LT, split LT, reduced-size LT, or simultaneous transplantation were excluded. Pediatric patients (< 18 years) and older patients (> 65 years) were excluded. Furthermore, patients with type 1 and type 2 portal vein tumor thrombus or tumor metastasis were excluded. All enrolled patients were followed up at least 6 months after LT, and those with questionable data or a lack of essential data for analysis were also excluded. The flowchart of this study is shown in Figure 1.
Figure 1.
Flowchart of the study.
Baseline characteristics and postoperative follow-up
The baseline clinical characteristics of the patients were collected. Demographic characteristics included age, sex, body mass index (BMI), and donation after brain death donor (DBD) and donation after cardiac death donor (DCD), and age, sex, and BMI for recipients. The collected laboratory variables and clinicopathologic characteristics included matched or mismatched ABO blood type, hepatitis B virus (HBV) infection, Child-Pugh grade, model for end-stage liver disease (MELD) score, preoperative loco-regional therapy, largest tumor size, tumor number, presence of satellite lesions, hypertension, diabetes, whether the tumor met the Milan criteria and Hangzhou criteria for LT, cold ischemia time, operative time, perioperative bleeding, and use of salvage LT. The data for tumor size, tumor number, and presence of satellite lesions were based on the postoperative pathological examination. All patients received regular follow-up after discharge from the hospital. Any patient who did not attend a follow-up appointment was contacted by a research nurse via telephone. Liver function tests; serum tumor marker assays; abdominal ultrasound; and chest, abdomen, and bone CT scans were routinely performed every 6 months for the first 2 or 3 years, then annually thereafter.
PSM analysis
PSM analysis was performed to decrease selection bias and make the groups comparable. Three PSM comparisons were made: 1) PSM with a 1:2 ratio of female vs. male recipients (382 and 764, respectively); 2) PSM with a 1:2 ratio of recipients receiving grafts from female donors vs. recipients receiving grafts from male donors (621 and 1,242, respectively); and 3) PSM with a 1:2:2:2 ratio of female donor-female recipient pattern (F-F) vs. male donor-male recipient pattern (M-M) vs. male donor-female recipient pattern (M-F) vs. female donor-male recipient pattern (F-M) (84, 168, 168, and 168, respectively). The standardized mean difference (SMD) was used to evaluate the balance of clinical parameters among groups.
Statistical analysis
Continuous variables with a normal distribution are expressed as mean ± standard deviation (SD) or median (interquartile range). Categorical variables are expressed as numbers (n) or proportions (%). Student’s t-test was used for comparison of continuous variables when applicable; otherwise, the Mann-Whitney U test was applied. Categorical variables were compared with the χ2 test or Fisher’s exact test, as appropriate. The Kaplan-Meier method, based on the log-rank test, was used to compare disease-free survival (DFS) and overall survival (OS) rates among groups by sex. For time-to-event data in which the proportional hazards assumption was violated, restricted mean survival time (RMST) analysis was used to evaluate between-group differences. Furthermore, the survival associated with different donor-recipient match patterns was compared to demonstrate the correlation between sex-based donor-recipient patterns and the outcomes of LT for HCC. Statistical analysis was performed in SAS, version 9.4, software (SAS Institute Inc, Cary, NC). The statistical significance threshold was set to P < 0.05 in all analyses. Univariable and multivariable Cox proportional hazards models were used to identify independent risk factors of post-LT outcomes in patients with HCC. The hazard ratio (HR) and its 95% confidence interval (CI) were estimated in the univariable and multivariable Cox regression analyses. In multivariate analysis, factors with P < 0.05 in the univariate analysis were finally tested, and P < 0.05 in the Cox model was considered statistically significant.
Results
PSM analysis to balance baseline characteristics
We performed PSM to eliminate confounders and make the clinical characteristics comparable between the male and female recipients who underwent LT for HCC. Because the number of male recipients exceeded that of female recipients, we conducted 1:2 nearest neighbor matching (382:764 female to male recipients) based on the propensity score. Likewise, because a substantial sex difference was observed among the LT donors, 1:2 matching (621:1,242 female to male donors) was used to investigate the influence of donor sex on the outcomes of recipients who underwent LT for HCC. To study the correlation between post-LT outcomes and the donor-recipient match patterns based on sex, 1:2:2:2 matching (84:168:168:168 female donor-female recipient vs. male donor-male recipient vs. male donor-female recipient vs. female donor-male recipient) was performed. Various variables were considered for PSM. The recipient characteristics included age, common diseases, Child-Pugh classification, and the MELD score, among other parameters. The donor characteristics included age, sex, BMI, and donor type (DBD, DCD, and DBCD). The tumor biology characteristics included tumor size; tumor number; and transplant criteria such as the Milan and Hangzhou criteria. Intraoperative parameters, such as operative time, operative bleeding, and cold ischemia time, were also used for PSM.
Demographic and clinical parameters of male and female recipients after matching
After PSM, all variables were comparable between groups (Table 1). Most patients with HCC who underwent LT in this study were older than 50 years (64.5% of male recipients vs. 63.6% of female recipients) and had a history of hepatitis B viral infection, (78.4% of male recipients and 78.0% of female recipients; P = 0.88). A substantial fraction of recipients (58.1% of male recipients and 57.6% of female recipients) received grafts from young donors (below 50 years of age; P = 0.87). Most recipients did not receive neoadjuvant TACE (85.5% of male recipients vs. 85.3% of female recipients) or RFA (94.2% of male recipients vs. 95.0% of female recipients) before LT. Analysis of the tumor biology characteristics of the LT recipients revealed that most of the tumors were solitary (64.9% of male recipients vs. 64.9% of female recipients) and ≤ 5 cm in size (82.7% of male recipients vs. 81.9% of female recipients). The median follow-up time was 29.1 months in male recipients and 30.1 months in female recipients (P = 0.43).
Table 1.
Clinical characteristics of liver transplant recipients with HCC by recipient sex after PSM
| Variables | Male recipient n = 764 |
Female recipient n = 382 |
P value | SMD |
|---|---|---|---|---|
| Donor age | 0.87 | −0.01 | ||
| < 50 y | 444 (58.12%) | 220 (57.59%) | ||
| ≥ 50 y | 320 (41.88%) | 162 (42.41%) | ||
| Donor sex | 0.80 | −0.01 | ||
| Male | 601 (78.66%) | 298 (78.01%) | ||
| Female | 163 (21.34%) | 84 (21.99%) | ||
| Donor BMI | 22.8 (20.8–24.4) | 22.9 (20.8–24.5) | 0.62 | −0.03 |
| Donor type | 0.87 | −0.01 | ||
| DBD | 432 (56.54%) | 214 (56.02%) | ||
| DCD | 332 (43.46%) | 168 (43.98%) | ||
| ABO blood incompatibility | 0.64 | −0.03 | ||
| Incompatible | 13 (1.70%) | 8 (2.09%) | ||
| Compatible | 751 (98.30%) | 374 (97.91%) | ||
| Recipient age | 0.76 | 0.02 | ||
| < 50 y | 271 (35.47%) | 139 (36.39%) | ||
| ≥ 50 y | 493 (64.53%) | 243 (63.61%) | ||
| Hypertension | 0.88 | −0.01 | ||
| No | 698 (91.36%) | 348 (91.10%) | ||
| Yes | 66 (8.64%) | 34 (8.90%) | ||
| Diabetes | 0.83 | −0.02 | ||
| No | 734 (96.07%) | 366 (95.81%) | ||
| Yes | 30 (3.93%) | 16 (4.19%) | ||
| HBsAg | 0.88 | −0.01 | ||
| Negative | 165 (21.60%) | 84 (21.99%) | ||
| Positive | 599 (78.40%) | 298 (78.01%) | ||
| Child-Pugh classification | 0.99 | 0.01 | ||
| A | 157 (20.55%) | 80(20.94%) | ||
| B | 287 (37.57%) | 143 (37.43%) | ||
| C | 320 (41.88%) | 159(41.62%) | ||
| Any TACE | 0.95 | −0.01 | ||
| No | 653 (85.47%) | 326 (85.34%) | ||
| Yes | 111 (14.53%) | 56 (14.66%) | ||
| Any RFA | 0.58 | 0.04 | ||
| No | 720 (94.24%) | 363 (95.03%) | ||
| Yes | 44 (5.76%) | 19 (4.97%) | ||
| MELD | 14 (9–29) | 14 (9–30) | 0.72 | −0.02 |
| Beyond Milan criteria | 0.69 | 0.03 | ||
| No | 495 (64.79%) | 243 (63.61%) | ||
| Yes | 269 (35.21%) | 139 (36.39%) | ||
| Beyond Hangzhou criteria | 0.62 | 0.03 | ||
| No | 670 (87.70%) | 331 (86.65%) | ||
| Yes | 94 (12.30%) | 51 (13.35%) | ||
| Largest tumor size | 0.59 | −0.05 | ||
| < 3 cm | 318(41.62%) | 147 (38.48%) | ||
| 3–5 cm | 314 (41.10%) | 166 (43.46%) | ||
| > 5 cm | 132 (17.28%) | 69 (18.06%) | ||
| Tumor number | 1.00 | 0 | ||
| Single | 496 (64.92%) | 248 (64.92%) | ||
| Multiple | 268 (35.08%) | 134 (35.08%) | ||
| Microsatellite | 1.00 | 0 | ||
| No | 706 (92.41%) | 353 (92.41%) | ||
| Yes | 58 (7.59%) | 29 (7.59%) | ||
| Cold ischemia time (h) | 6.0 (4.4–7.5) | 6.0 (4.5–7.8) | 0.43 | −0.05 |
| Operative time (h) | 6.9 (6.0–8.0) | 6.9 (5.8–8.1) | 0.91 | −0.002 |
| Operative bleeding (mL) | 800 (500–1500) | 900 (500–1500) | 0.77 | −0.02 |
| Salvage LT | 0.53 | 0.04 | ||
| No | 664 (86.91%) | 337 (88.22%) | ||
| Yes | 100 (13.09%) | 45 (11.78%) | ||
| Follow-up time (months) | 29.1 (18.0–41.6) | 30.1 (18.4–42.7) | 0.43 | −0.05 |
PSM, propensity score matching; LT, liver transplantation; BMI, body mass index; MELD, model for end-stage liver disease; CHILD, Child-Pugh; DBD, donation after brain death; DCD, donation after circulatory death; DBCD, donation after brain and cardiac death; RFA, radiofrequency ablation; TACE, transcatheter arterial chemoembolization; SMD, standardized mean difference.
Significantly lower rates of post-LT OS in male than female recipients
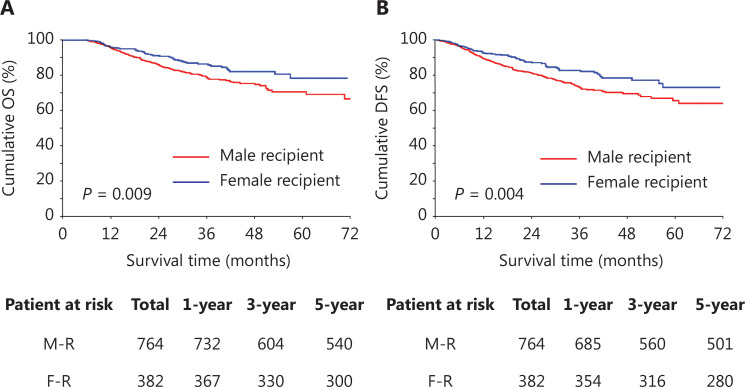
The outcomes of recipients with LT for HCC were compared between the male and female recipients after PSM. significant differences in post-transplant OS and DFS were observed between male and female recipients. The OS of the female recipients was significantly superior to that of the male recipients (Figure 2A). The 1-, 3-, and 5-year OS rates were 96.1%, 86.4%, and 78.5%, respectively, in female recipients, and 95.8%, 79.0%, and 70.7%, respectively, in male recipients (P = 0.009). The DFS at 1 year, 3 years, and 5 years was 92.6%, 82.7%, and 73.2%, respectively, in female recipients, whereas the estimated DFS rates of male recipients at 1 year, 3 years, and 5 years were 89.6%, 73.2%, and 65.5%, respectively (P = 0.004; Figure 2B).
Figure 2.
Overall survival (OS) and disease-free survival (DFS) of male recipients and female recipients after PSM. (A) OS of male recipients and female recipients. (B) DFS of male recipients and female recipients. M-R, male recipients; F-R, female recipients.
Risk factors of outcomes after LT for HCC
Univariate and multivariate analysis were applied to determine the risk factors associated with OS in recipients who underwent LT for HCC (Table 2). The univariate analysis revealed several factors significantly associated with recipients’ outcomes after LT. Male recipient sex (HR = 1.376, 95% CI 1.003–1.883), ABO blood incompatibility (HR = 1.918, 95% CI 1.106–3.327), preoperative TACE (HR = 1.254, 95% CI 1.049–1.498), large tumor size (≥ 5 cm; HR = 2.591, 95% CI 2.175–3.086), multiple tumors (HR = 1.502, 95% CI 1.268–1.778), tumors exceeding the Milan criteria (HR = 2.766, 95% CI 2.320–3.297), and long operation times (≥ 6 h; HR = 1.269, 95% CI 1.061–1.515) were identified as risk factors for post-LT outcomes (P < 0.05). In the multivariate analysis, male recipient sex was significantly associated with OS after LT for HCC (HR = 1.381, 95% CI 1.006–1.895, P = 0.046). Additionally, large tumor size (≥ 5 cm; HR = 1.627, 95% CI 1.288–2.055), tumors exceeding the Milan criteria (HR = 1.986, 95% CI 1.552–2.540), and long operation times (≥ 6 h; HR = 1.279, 95% CI 1.069–1.529) were also independent risk factors associated with OS after LT for HCC (P < 0.05).
Table 2.
Univariate and multivariate analysis of post-transplant HCC overall survival
| Variable | Univariable |
Multivariable |
||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Donor age | 1.138 (0.958–1.352) | 0.142 | ||
| > 50 | ||||
| Donor sex | 1.145 (0.905–1.451) | 0.259 | ||
| Male | ||||
| Recipient sex | 1.376 (1.003–1.883) | 0.048 | 1.381 (1.006–1.895) | 0.046 |
| Male | ||||
| Donor BMI | 1.075 (0.901–1.284) | 0.424 | ||
| ≤ 23.9 | ||||
| ABO blood incompatibility | 1.918 (1.106–3.327) | 0.020 | 1.544 (0.889–2.683) | 0.123 |
| MELD score | 1.062 (0.888–1.269) | 0.512 | ||
| > 20 | ||||
| HBV (+) | 1.040 (0.816–1.326) | 0.749 | ||
| Child-Pugh A | 0.966 (0.866–1.076) | 0.529 | ||
| Largest tumor size | 2.591 (2.175–3.086) | < 0.001 | 1.627 (1.288–2.055) | < 0.001 |
| > 5 cm | ||||
| Cold ischemia time | 1.008 (0.822–1.237) | 0.939 | ||
| > 8 h | ||||
| Preoperative TACE | 1.254 (1.049–1.498) | 0.013 | 1.127 (0.942–1.348) | 0.190 |
| Operation time | 1.269 (1.061–1.515) | 0.009 | 1.279 (1.069–1.529) | 0.007 |
| ≥ 6 h | ||||
| Tumor number | 1.502 (1.268–1.778) | < 0.001 | 1.190 (0.978–1.446) | 0.082 |
| Multiple | ||||
| Donor type | 1.166 (0.984–1.383) | 0.076 | 1.172 (0.989–1.390) | 0.067 |
| DCD | ||||
| Beyond Milan criteria | 2.766 (2.320–3.297) | < 0.001 | 1.986 (1.552–2.540) | < 0.001 |
OS, overall survival; HR, hazard ratio; CI, confidence interval; BMI, body mass index; MELD, model for end-stage liver disease; CHILD, Child-Pugh; HBV, hepatitis B virus; TACE, transcatheter arterial chemoembolization.
Comparable OS in recipients with female or male donors
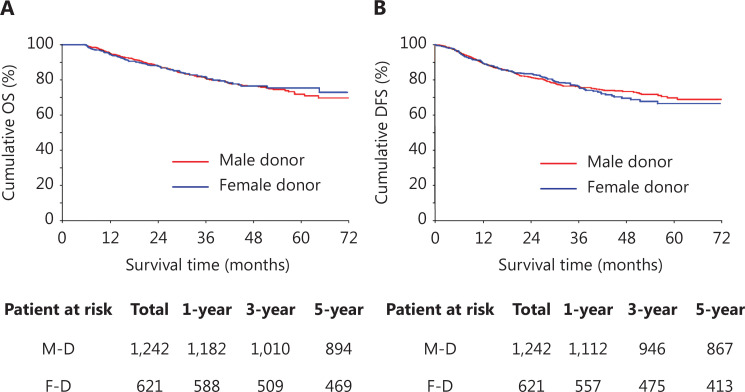
To further investigate whether donor sex might also be associated with the outcomes of recipients receiving LT for HCC, we compared the OS and DFS between recipients who received grafts from male donors and female donors. We also performed PSM to eliminate confounders between male donors and female donors. The matching variables included the recipient and donor characteristics, tumor biology characteristics, and intraoperative parameters, as described previously (Table 3). After PSM, recipients who received grafts from male donors showed OS and DFS comparable to those of recipients who received grafts from female donors (Figure 3A and 3B). The 1-, 3-, and 5-year OS rates were 95.1%, 81.3%, and 72.0% in recipients with male donors and 94.7%, 81.9%, and 75.6% in recipients with female donors (P > 0.05; Figure 3A). The DFS at 1 year, 3 years, and 5 years was 89.6%, 76.2%, and 69.8%, respectively, for recipients with male donors and 89.7%, 76.4%, and 66.6%, respectively, for recipients with female donors (P > 0.05; Figure 3B).
Table 3.
Clinical characteristics of liver transplant recipients with HCC by donor sex after PSM
| Variables | Male donor n = 1242 |
Female donor n = 621 |
P value | SMD |
|---|---|---|---|---|
| Donor age | 0.89 | 0.01 | ||
| < 50 y | 718 (57.81%) | 361 (58.13%) | ||
| ≥ 50 y | 524 (42.19%) | 260 (41.87%) | ||
| Recipient sex | 0.14 | 0.07 | ||
| Male | 1061 (85.43%) | 546 (87.92%) | ||
| Female | 181 (14.57%) | 75 (12.08%) | ||
| Donor BMI | 22.8 (20.8–24.5) | 22.2 (20.2–24.7) | 0.38 | 0.05 |
| Donor type | 0.43 | −0.04 | ||
| DBD | 724 (58.29%) | 350 (56.36%) | ||
| DCD | 518 (41.71%) | 271 (43.64%) | ||
| ABO blood incompatibility | 0.51 | 0.03 | ||
| Incompatible | 30 (2.42%) | 12 (1.93%) | ||
| Compatible | 1212 (97.58%) | 609 (98.07%) | ||
| Recipient age | 0.71 | 0.02 | ||
| < 50 y | 495 (39.86%) | 253 (40.74%) | ||
| ≥ 50 y | 747 (60.14%) | 368 (59.26%) | ||
| Hypertension | 0.95 | −0.01 | ||
| No | 1137 (91.55%) | 568 (91.47%) | ||
| Yes | 105 (8.45%) | 53 (8.53%) | ||
| Diabetes | 0.92 | −0.01 | ||
| No | 1100 (88.57%) | 551 (88.73%) | ||
| Yes | 142 (11.43%) | 70 (11.27%) | ||
| HBsAg | 1.00 | 0 | ||
| Negative | 176 (14.17%) | 88 (14.17%) | ||
| Positive | 1066 (85.83%) | 533 (85.83%) | ||
| Child-Pugh classification | 0.74 | −0.02 | ||
| A | 266 (21.42%) | 134 (21.58%) | ||
| B | 480 (38.65%) | 229 (36.88%) | ||
| C | 496 (39.94%) | 258 (41.55%) | ||
| Any TACE | 0.56 | 0.03 | ||
| No | 1045 (84.14%) | 529 (85.19%) | ||
| Yes | 197 (15.86%) | 92 (14.81%) | ||
| Any RFA | 0.78 | −0.01 | ||
| No | 1170 (94.20%) | 583 (93.88%) | ||
| Yes | 72 (5.80%) | 38 (6.12%) | ||
| MELD | 14 (9–28) | 14 (9–30) | 0.67 | −0.02 |
| Beyond Milan criteria | 0.89 | 0.01 | ||
| No | 732 (58.94%) | 243 (58.62%) | ||
| Yes | 510 (41.06%) | 139 (41.38%) | ||
| Beyond Hangzhou criteria | 1.00 | 0 | ||
| No | 1056 (85.02%) | 528 (85.02%) | ||
| Yes | 186 (14.98%) | 93 (14.98%) | ||
| Largest tumor size | 0.83 | 0.01 | ||
| < 3 cm | 474 (38.16%) | 236 (38.00%) | ||
| 3–5 cm | 515 (41.47%) | 265 (42.67%) | ||
| > 5 cm | 253 (20.37%) | 120 (19.32%) | ||
| Tumor number | 0.97 | 2.64 | ||
| Single | 721 (58.05%) | 361 (58.13%) | ||
| Multiple | 521 (41.95%) | 260 (41.87%) | ||
| Microsatellite | 0.64 | 0.03 | ||
| No | 1097 (88.33%) | 553 (89.05%) | ||
| Yes | 145 (11.67%) | 68 (10.95%) | ||
| Cold ischemia time (h) | 6.0 (4.7–8.0) | 6.0 (4.7–8.0) | 0.59 | 0.03 |
| Operative time (h) | 7.2 (6.0–8.3) | 7.0 (6.0–8.3) | 0.50 | 0.05 |
| Operative bleeding (mL) | 900 (500–1500) | 1000 (500–1500) | 0.49 | 0.03 |
| Salvage LT | 0.79 | −0.01 | ||
| No | 1048 (84.38%) | 521 (83.90%) | ||
| Yes | 194 (15.62%) | 100 (16.10%) | ||
| Follow-up time (months) | 29.9 (18.0–42.5) | 28.9 (17.3–41.3) | 0.15 | 0.07 |
PSM, propensity score matching; LT, liver transplantation; BMI, body mass index; MELD, model for end-stage liver disease; CHILD, Child-Pugh; DBD, donation after brain death; DCD, donation after circulatory death; DBCD, donation after brain and cardiac death; RFA, radiofrequency ablation; TACE, transcatheter arterial chemoembolization; SMD, standardized mean difference.
Figure 3.
Overall survival (OS) and disease-free survival (DFS) of recipients with male donors and female donors after PSM. (A) OS of recipients with male donors and female donors. (B) DFS of recipients with male donors and female donors. M-D, male donors; F-D, female donors.
Sex based donor-recipient match patterns and clinical characteristics of different groups after PSM
To study the post-transplant outcomes associated with different donor-recipient match patterns based on sex, we divided the donors and recipients into 4 patterns: male donor to male recipient (M-M), male donor to female recipient (M-F), female donor to male recipient (F-M), and female donor to female recipient (F-F). Given that fewer patients had an F-F transplant pattern than the other 3 patterns, we performed 1:2:2:2 (F-F:M-M:M-F:F-M) nearest neighbor matching based on the propensity score to make the clinical characteristics comparable across groups. After PSM, the balance across the 4 groups significantly improved, and no significant differences were found in multiple variables, including recipient and donor demographics, tumor biology characteristics, and intraoperative parameters (Table 4).
Table 4.
Clinical characteristics of liver transplant recipients with HCC according to different donor-recipient matched-patterns based on sex after PSM
| Variables | Female donor and female recipient (F-F) n = 84 |
Male donor and male recipient (M-M) n = 168 |
Male donor and female recipient (M-F) n = 168 |
Female donor and male recipient (F-M) n = 168 |
P value |
|---|---|---|---|---|---|
| Donor age | 0.49 | ||||
| < 50 y | 48 (57.14%) | 87 (51.79%) | 93 (55.36%) | 101 (60.12%) | |
| ≥ 50 y | 36 (42.86%) | 81 (48.21%) | 75 (44.64%) | 67 (39.88%) | |
| Donor type | 0.57 | ||||
| DBD | 48 (57.14%) | 96 (57.14%) | 97 (63.69%) | 107 (57.74%) | |
| DCD | 36 (42.86%) | 72 (42.86%) | 71 (36.31%) | 61 (42.26%) | |
| ABO blood incompatibility | 0.46 | ||||
| Incompatible | 2 (2.38%) | 7 (4.17%) | 3 (1.79%) | 3 (1.79%) | |
| Compatible | 82 (97.62%) | 161 (95.83%) | 165 (98.21%) | 165 (98.21%) | |
| Recipient age | 0.45 | ||||
| < 50 y | 29 (34.52%) | 53 (31.55%) | 67 (39.88%) | 61 (36.31%) | |
| ≥ 50 y | 55 (65.48%) | 115 (68.45%) | 101 (60.12%) | 107 (63.69%) | |
| Hypertension | 0.62 | ||||
| No | 80 (95.24%) | 153 (91.07%) | 154 (91.67%) | 157 (93.45%) | |
| Yes | 4 (4.76%) | 15 (8.93%) | 14 (8.33%) | 11 (6.55%) | |
| Diabetes | 0.29 | ||||
| No | 78 (92.86%) | 153 (91.07%) | 160 (95.24%) | 151 (89.88%) | |
| Yes | 6 (7.14%) | 15 (8.93%) | 8 (4.76%) | 17 (10.12%) | |
| HBsAg | 0.99 | ||||
| Negative | 16 (19.05%) | 33 (19.64%) | 33 (19.64%) | 33 (19.64%) | |
| Positive | 68 (80.95%) | 135 (80.36%) | 135 (80.36%) | 135 (80.36%) | |
| Child-Pugh classification | 0.69 | ||||
| A | 18 (21.43%) | 26 (15.48%) | 33 (19.64%) | 36 (21.43%) | |
| B | 27 (32.14%) | 71 (42.26%) | 64 (38.10%) | 64 (38.10%) | |
| C | 39 (46.43%) | 71 (42.26%) | 71 (42.26%) | 68 (40.48%) | |
| Any TACE | 0.98 | ||||
| No | 74 (88.10%) | 148 (88.10%) | 146 (86.90%) | 148 (88.10%) | |
| Yes | 10 (11.90%) | 20 (11.90%) | 22 (13.10%) | 20 (11.90%) | |
| Any RFA | 0.97 | ||||
| No | 79 (94.05%) | 160 (95.24%) | 160 (95.24%) | 159 (94.64%) | |
| Yes | 5 (5.95%) | 8 (4.76%) | 8 (4.76%) | 9 (5.36%) | |
| MELD | 14 (9–31) | 13 (9–30) | 14.5 (9–30) | 15 (9–30.5) | 0.98 |
| Beyond Milan criteria | 0.77 | ||||
| No | 54 (64.29%) | 104 (61.90%) | 112 (66.67%) | 104 (61.90%) | |
| Yes | 30 (35.71%) | 64 (38.10%) | 56 (33.33%) | 64 (38.10%) | |
| Beyond Hangzhou criteria | 0.74 | ||||
| No | 71 (84.52%) | 143 (85.12%) | 149 (88.69%) | 145 (86.31%) | |
| Yes | 13 (15.48%) | 25 (14.88%) | 19 (11.31%) | 23 (13.69%) | |
| Largest tumor size | 0.49 | ||||
| < 3 cm | 32 (38.10%) | 57 (33.93%) | 70 (41.67%) | 72 (42.86%) | |
| 3–5 cm | 37 (44.05%) | 70 (41.67%) | 70 (41.67%) | 64 (38.10%) | |
| > 5 cm | 15 (17.86%) | 41 (24.40%) | 28 (16.67%) | 32 (19.05%) | |
| Tumor number | 0.97 | ||||
| Single | 54 (64.29%) | 113 (67.26%) | 111 (66.07%) | 112 (66.67%) | |
| Multiple | 30 (35.71%) | 55 (32.74%) | 57 (33.93%) | 56 (33.33%) | |
| Microsatellite | 0.65 | ||||
| No | 77 (91.67%) | 156 (92.86%) | 152 (90.48%) | 158 (94.05%) | |
| Yes | 7 (8.33%) | 12 (7.14%) | 16 (9.52%) | 10 (5.95%) | |
| Cold ischemia time (h) | 6.5 (4.6–8.0) | 6.0 (4.4–7.4) | 6.0 (4.5–7.7) | 6.0 (4.6–7.5) | 0.57 |
| Operative time (h) | 6.8 (5.6–8.2) | 6.8 (5.6–8.0) | 6.9 (5.9–8.2) | 7.0 (6.0–8.0) | 0.89 |
| Operative bleeding (mL) | 750 (400–1500) | 800 (500–1500) | 1000 (600–1500) | 1000 (500–1500) | 0.27 |
| Salvage LT | 0.85 | ||||
| No | 73 (86.90%) | 150 (89.29%) | 151 (89.88%) | 152 (90.48%) | |
| Yes | 11 (13.10%) | 18 (10.71%) | 17 (10.12%) | 16 (9.52%) |
PSM, propensity score matching; LT, liver transplantation; BMI, body mass index; MELD, model for end-stage liver disease; CHILD, Child-Pugh; DBD, donation after brain death; DCD, donation after circulatory death; DBCD, donation after brain and cardiac death; RFA, radiofrequency ablation; TACE, transcatheter arterial chemoembolization.
The male-to-male LT transplant pattern has the poorest outcomes after LT for HCC
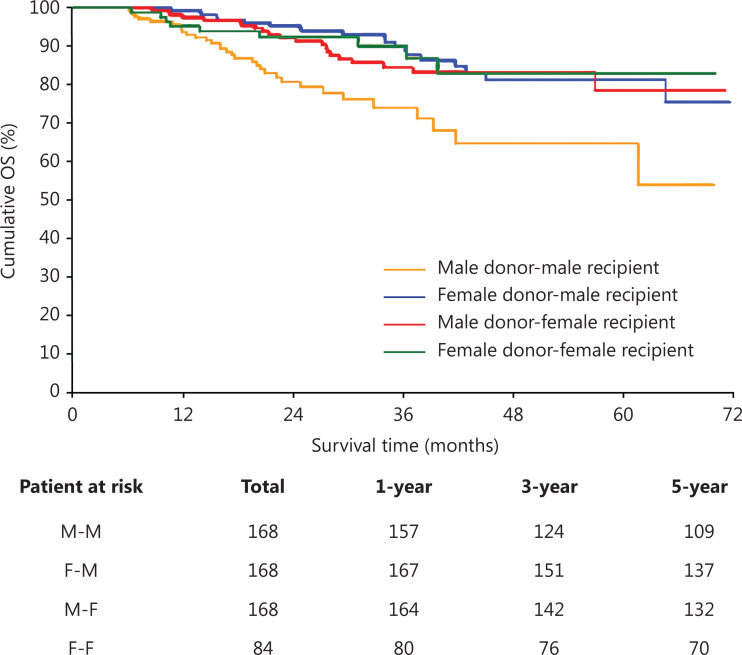
After matching, we compared the OS across the M-M, M-F, F-M, and F-F groups. Among these patterns, M-F, F-M, and F-F, had similar OS, and the F-F group had the best 5-year OS. However, the M-M pattern had the poorest OS. Significant differences in 5-year OS were observed for the M-M pattern compared with the other 3 patterns [RMST ratio (M-M to M-F) = 0.882, 95% CI (0.803–0.968), P = 0.008; RMST ratio (M-M to F-M) = 0.866, 95% CI (0.791–0.948), P = 0.002; RMST ratio (M-M to F-F) = 0.875, 95% CI (0.788–0.972), P = 0.012; Figure 4]. The 1-, 3-, and 5-year OS rates were 93.7%, 74.0%, and 64.8%, respectively, for the M-M pattern; 97.6%, 84.5%, and 78.6%, respectively, for the M-F pattern; 99.4%, 90.0%, and 81.3%, respectively, for the F-M pattern; and 95.2%, 90.2%, and 83.0%, respectively, for the F-F pattern (Figure 4). These results demonstrated a significant difference in long-term post-transplant outcomes when a male graft is assigned to a male recipient vs. a female recipient (M-M vs. M-F P = 0.008), but not in the allocation of a female graft (F-M vs. F-F, P = 0.790).
Figure 4.
Overall survival (OS) of different donor-recipient match patterns based on sex after PSM. OS of different transplant patterns based on sex. M-M, male donor transplant to male recipient; F-F, female donor transplant to female recipient; M-F, male donor transplant to female recipient; F-M, female donor transplant to male recipient.
Discussion
LT is a definitive treatment option for HCC, but the favorable prognosis of LT for HCC relies on the precise selection of good candidates16,17. Since Mazzaferro and colleagues18–23 proposed the Milan criteria for LT in patients with HCC, several studies have suggested various other patient selection criteria to achieve optimal outcomes in these patients. Sex differences are universally observed in many liver diseases, including HCC. Although various studies have focused on the selection criteria for LT by taking various parameters into account, few have considered sex. In this study, the correlation between sex and post-LT outcomes of patients with HCC was investigated in Chinese nationwide data. Female recipients had better outcomes than their male counterparts. However, no clear effects of donor sex on the outcomes of HCC after LT were observed. Our findings highlight the influence of sex on the post-LT outcomes of patients with HCC, thus providing evidence that recipient sex should be carefully considered as an important parameter in outcome evaluation and organ allocation in LT for HCC.
Biological sex influences HCC
Sex differences in HCC have long been known: women have a 50%–75% lower risk of developing HCC than men, among people with end-stage liver disease or viral hepatitis5. Moreover, in the United States, women are 2 times less likely to receive LT than men24–26. Recently, the role of sex in LT for HCC has attracted substantial attention. In this study, women were found to be less likely than men to receive deceased donor LT for HCC in China. Given the large difference in the numbers of male vs. female LT recipients, we performed 1:2 matching to analyze the difference in post-transplant outcomes between female recipients and male recipients. HCC is a digestive malignancy with complex heterogeneity, and sex hormones are widely accepted to account for the sex-specific differences in HCC occurrence27–31. Androgens promote HCC tumorigenesis, whereas estrogen plays an opposite role32–35. Some studies have sought to identify the mechanism underlying the sex differences in HCC occurrence by using animal models. Immune factors, such as Kupffer cells and IL-6 signaling, as well as adiponectin, have been suggested to account for the sex differences in HCC occurrence9,36. Altogether, prior studies have indicated that high levels of estrogen tend to prevent hepatocarcinogenesis. In our study, we simultaneously compared the effects of donor and recipient sex differences on post-LT survival in patients with HCC. Recipient sex was significantly associated with post-LT survival, whereas donor sex had no effect. Female recipients are generally accepted to have higher levels of estrogen than male recipients, and our results provided further support that high levels of estrogen may play protective roles in patients with HCC after LT. Interestingly, our study also revealed that donor sex was not significantly associated with post-LT survival, thus suggesting that the high levels of estrogen in female recipients, rather than an increase in estrogen receptors in the donor liver, might account for the better post-LT outcomes. Nevertheless, the underlying mechanism will require further basic studies in the future.
Sex differences in LT for HCC
Previous studies have investigated the correlation between sex and HCC recurrence post-LT by using various databases, such as the United Network for Organ Sharing/Organ Procurement and Transplantation Network, and the Japanese Liver Transplantation Society13,14. However, the influence of sex on the outcomes of LT for HCC may differ by tumor etiology (e.g., alcohol, HCV, NASH, or HBV) and race. We performed the first study in China investigating the role of sex in post-LT outcomes by analyzing registry data for LT recipients with HCC caused primarily by hepatitis B viral infection. In contrast to analysis of living donor LT for HCC, this study focused on the outcomes after deceased donor LT for HCC. Our findings demonstrated that sex-based donor-recipient match patterns were associated with recipient outcomes, and the pattern of male donor to male recipient was associated with the poorest post-LT survival. However, no a significant difference in post-LT survival was observed between the female donor to female recipient pattern and the female donor to male recipient pattern. These findings may have clinical applications. For instance, our data may indicate that the liver from a male donor may be of greater benefit to a female than a male recipient, whereas the liver from a female donor may be transplanted to either a male or a female recipient without resulting in a survival difference.
Limitations of the study
This study has several limitations. First, several patients receiving LT were lost to long-term follow-up. Another limitation is that most patients with HCC in our study had hepatitis B viral infection as the etiological factor; consequently, our results might differ from those for HCC caused by other etiological factors, such as alcohol, hepatitis C viral infection, or fatty liver disease. Therefore, more studies are required to elucidate the sex-based differences in post-LT outcomes in patients with HCC with various HCC etiological causes. Furthermore, the results in this study pertain to recipients who underwent deceased donor LT and might potentially differ from those for living donor LT or split LT. Given that a central effect might have existed in this multicenter cohort study, we took several measures to decrease such a central effect. The number of patients enrolled in the study from different centers was similar in the initial study design. We also used the electronic data capture systems to improve the accuracy of data acquisition. Moreover, the researchers in each research center were required to evaluate the data by using uniform standards to decrease the central effect.
Conclusions
This study is the first multicenter cohort investigation in China providing evidence of the role of sex in LT for HCC. Male recipient sex was associated with poorer post-LT survival than female recipient sex; moreover, the male-male donor-recipient transplant pattern was associated with unfavorable post-LT outcomes. Collectively, our findings highlight that sex should be considered a critical parameter in organ allocation.
Acknowledgments
The authors thank the China Liver Transplant Registry and the National Center for Healthcare Quality Management in Liver Transplant for accepting the request to conduct this study.
Funding Statement
This work was supported by funding from the National Key Research and Development Program of China (Grant No. 2021YFA1100500); The Major Research Plan of the National Natural Science Foundation of China (Grant No. 92159202); Key Program, National Natural Science Foundation of China (Grant No. 81930016); National Natural Science Foundation of China (Grant No. 82300743); Zhejiang Provincial Natural Science Foundation of China (Grant No. LQ23H160044); and Key Research & Development Program of Zhejiang Province (Grant Nos. 2019C03050, 2022C03108, and 2021C03118).
Conflict of interest statement
No potential conflicts of interest are disclosed.
Author contributions
Planned and supervised the study: Xiao Xu, Shusen Zheng, Jiayin Yang, Jinzhen Cai.
Collected the data: Zhengxin Wang, Wenzhi Guo, Guoyue Lyu, Haizhi Qi, Meihua Sui.
Performed statistical analysis and prepared the graphics: Zhisheng Zhou, Jian Chen.
Interpreted the data and wrote the manuscript: Jian Chen, Zhe Yang, Fengqiang Gao.
Revised the manuscript: Jian Chen, Kai Wang, Di Lu, Junli Chen.
Data availability statement
Data were generated by the authors and available on request.
References
- 1.Ling S, Jiang G, Que Q, Xu S, Chen J, Xu X. Liver transplantation in patients with liver failure: twenty years of experience from China. Liver Int. 2022;42:2110–6. doi: 10.1111/liv.15288. [DOI] [PubMed] [Google Scholar]
- 2.Liu Z, Liu Y, Zhang W, Hong Y, Meng J, Wang J, et al. Deep learning for prediction of hepatocellular carcinoma recurrence after resection or liver transplantation: a discovery and validation study. Hepatol Int. 2022;16:577–89. doi: 10.1007/s12072-022-10321-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cao M, Li H, Sun D, He S, Yan X, Yang F, et al. Current cancer burden in China: epidemiology, etiology, and prevention. Cancer Biol Med. 2022;19:1121–38. doi: 10.20892/j.issn.2095-3941.2022.0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Briceño J, Calleja R, Hervás C. Artificial intelligence and liver transplantation: looking for the best donor-recipient pairing. Hepatobiliary Pancreat Dis Int. 2022;21:347–53. doi: 10.1016/j.hbpd.2022.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Sarkar M, Watt KD, Terrault N, Berenguer M. Outcomes in liver transplantation: does sex matter? J Hepatol. 2015;62:946–55. doi: 10.1016/j.jhep.2014.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang H, Han J, Xing H, Li ZL, Schwartz ME, Zhou YH, et al. Sex difference in recurrence and survival after liver resection for hepatocellular carcinoma: a multicenter study. Surgery. 2019;165:516–24. doi: 10.1016/j.surg.2018.08.031. [DOI] [PubMed] [Google Scholar]
- 7.Ji X, Wang J, Li Z, Shen Q, Tuo J, Bi J, et al. Dietary fat intake and liver cancer risk: a prospective cohort study in Chinese women. Cancer Biol Med. 2021;19:370–83. doi: 10.20892/j.issn.2095-3941.2020.0633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gamaev L, Mizrahi L, Friehmann T, Rosenberg N, Pappo O, Olam D, et al. The pro-oncogenic effect of the lncRNA H19 in the development of chronic inflammation-mediated hepatocellular carcinoma. Oncogene. 2021;40:127–39. doi: 10.1038/s41388-020-01513-7. [DOI] [PubMed] [Google Scholar]
- 9.Naugler WE, Sakurai T, Kim S, Maeda S, Kim K, Elsharkawy AM, et al. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317:121–4. doi: 10.1126/science.1140485. [DOI] [PubMed] [Google Scholar]
- 10.Zheng B, Zhu YJ, Wang HY, Chen L. Gender disparity in hepatocellular carcinoma (HCC): multiple underlying mechanisms. Sci China Life Sci. 2017;60:575–84. doi: 10.1007/s11427-016-9043-9. [DOI] [PubMed] [Google Scholar]
- 11.Serrano MT, Sabroso S, Esteban LM, Berenguer M, Fondevila C, Lorente S, et al. Mortality and causes of death after liver transplantation: analysis of sex differences in a large nationwide cohort. Transpl Int. 2022;35:10263. doi: 10.3389/ti.2022.10263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han S, Yang JD, Sinn DH, Kim JM, Choi GS, Jung G, et al. Risk of post-transplant hepatocellular carcinoma recurrence is higher in recipients of livers from male than female living donors. Ann Surg. 2018;268:1043–50. doi: 10.1097/SLA.0000000000002318. [DOI] [PubMed] [Google Scholar]
- 13.Taura K, Shimamura T, Akamatsu N, Umeshita K, Fujiyoshi M, Abe H, et al. No impact of donor sex on the recurrence of hepatocellular carcinoma after liver transplantation. J Hepatobiliary Pancreat Sci. 2022;29:570–84. doi: 10.1002/jhbp.1134. [DOI] [PubMed] [Google Scholar]
- 14.Cullaro G, Rubin J, Mehta N, Yao F, Verna EC, Lai JC. Sex-based disparities in hepatocellular carcinoma recurrence after liver transplantation. Transplantation. 2021;105:2420–6. doi: 10.1097/TP.0000000000003575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang J, Millis JM, Mao Y, Millis MA, Sang X, Zhong S. Voluntary organ donation system adapted to Chinese cultural values and social reality. Liver Transpl. 2015;21:419–22. doi: 10.1002/lt.24069. [DOI] [PubMed] [Google Scholar]
- 16.Moeckli B, Majno P, Orci LA, Peloso A, Toso C. Liver transplantation selection and allocation criteria for hepatocellular carcinoma: a European perspective. Semin Liver Dis. 2021;41:172–81. doi: 10.1055/s-0041-1723032. [DOI] [PubMed] [Google Scholar]
- 17.Zhan QF, Ling SB, Deng YN, Shan QN, Ye QW, Xu SJ, et al. Hangzhou criteria as downstaging criteria in hepatocellular carcinoma before liver transplantation: a multicenter study from China. Hepatobiliary Pancreat Dis Int. 2020;19:349–57. doi: 10.1016/j.hbpd.2020.06.011. [DOI] [PubMed] [Google Scholar]
- 18.Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–9. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 19.Xu X, Lu D, Ling Q, Wei X, Wu J, Zhou L, et al. Liver transplantation for hepatocellular carcinoma beyond the Milan criteria. Gut. 2016;65:1035–41. doi: 10.1136/gutjnl-2014-308513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yao FY, Xiao L, Bass NM, Kerlan R, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: validation of the UCSF-expanded criteria based on preoperative imaging. Am J Transplant. 2007;7:2587–96. doi: 10.1111/j.1600-6143.2007.01965.x. [DOI] [PubMed] [Google Scholar]
- 21.Mazzaferro V, Llovet JM, Miceli R, Bhoori S, Schiavo M, Mariani L, et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10:35–43. doi: 10.1016/S1470-2045(08)70284-5. [DOI] [PubMed] [Google Scholar]
- 22.Sapisochin G, Goldaracena N, Laurence JM, Dib M, Barbas A, Ghanekar A, et al. The extended Toronto criteria for liver transplantation in patients with hepatocellular carcinoma: a prospective validation study. Hepatology. 2016;64:2077–88. doi: 10.1002/hep.28643. [DOI] [PubMed] [Google Scholar]
- 23.Kaido T, Ogawa K, Mori A, Fujimoto Y, Ito T, Tomiyama K, et al. Usefulness of the Kyoto criteria as expanded selection criteria for liver transplantation for hepatocellular carcinoma. Surgery. 2013;154:1053–60. doi: 10.1016/j.surg.2013.04.056. [DOI] [PubMed] [Google Scholar]
- 24.Bryce CL, Chang CCH, Angus DC, Arnold RM, Farrell M, Roberts MS. The effect of race, sex, and insurance status on time-to-listing decisions for liver transplantation. J Transplant. 2010;2010:467976. doi: 10.1155/2010/467976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thuluvath PJ, Guidinger MK, Fung JJ, Johnson LB, Rayhill SC, Pelletier SJ. Liver transplantation in the United States, 1999-2008. Am J Transplant. 2010;10:1003–19. doi: 10.1111/j.1600-6143.2010.03037.x. [DOI] [PubMed] [Google Scholar]
- 26.Hermann HC, Klapp BF, Danzer G, Papachristou C. Gender-specific differences associated with living donor liver transplantation: a review study. Liver Transpl. 2010;16:375–86. doi: 10.1002/lt.22002. [DOI] [PubMed] [Google Scholar]
- 27.Kur P, Kolasa-Wołosiuk A, Misiakiewicz-Has K, Wiszniewska B. Sex hormone-dependent physiology and diseases of liver. Int J Environ Res Public Health. 2020;17:E2620. doi: 10.3390/ijerph17082620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee SR, Lee YH, Yang H, Lee HW, Lee GS, An BS, et al. Sex hormone-binding globulin suppresses NAFLD-triggered hepatocarcinogenesis after menopause. Carcinogenesis. 2019;40:1031–41. doi: 10.1093/carcin/bgz107. [DOI] [PubMed] [Google Scholar]
- 29.El Mahdy Korah T, Abd Elfatah Badr E, Mohamed Emara M, Ahmed Samy Kohla M, Gamal Saad Michael G. Relation between sex hormones and hepatocellular carcinoma. Andrologia. 2016;48:948–55. doi: 10.1111/and.12536. [DOI] [PubMed] [Google Scholar]
- 30.Lee SR, Jeong SH, Heo JH, Jo SL, Ko JW, Kwun HJ, et al. Dietary intake of 17α-ethinylestradiol promotes HCC progression in humanized male mice expressing sex hormone-binding globulin. Int J Mol Sci. 2021;22:12557. doi: 10.3390/ijms222212557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen J, Li Z, Jia X, Song W, Wu H, Zhu H, et al. Targeting anillin inhibits tumorigenesis and tumor growth in hepatocellular carcinoma via impairing cytokinesis fidelity. Oncogene. 2022;41:3118–30. doi: 10.1038/s41388-022-02274-1. [DOI] [PubMed] [Google Scholar]
- 32.Han Q, Yang D, Yin C, Zhang J. Androgen receptor (AR)-TLR4 crosstalk mediates gender disparities in hepatocellular carcinoma incidence and progression. J Cancer. 2020;11:1094–103. doi: 10.7150/jca.30682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O’Brien MH, Pitot HC, Chung SH, Lambert PF, Drinkwater NR, Bilger A. Estrogen receptor-α suppresses liver carcinogenesis and establishes sex-specific gene expression. Cancers (Basel) 2021;13:2355. doi: 10.3390/cancers13102355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sukocheva OA. Estrogen, estrogen receptors, and hepatocellular carcinoma: are we there yet? World J Gastroenterol. 2018;24:1–4. doi: 10.3748/wjg.v24.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meng X, Liu X. Therapeutic value of estrogen receptor α in hepatocellular carcinoma based on molecular mechanisms. J Clin Transl Hepatol. 2022;10:140–6. doi: 10.14218/JCTH.2021.00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manieri E, Herrera-Melle L, Mora A, Tomás-Loba A, Leiva-Vega L, Fernández DI, et al. Adiponectin accounts for gender differences in hepatocellular carcinoma incidence. J Exp Med. 2019;216:1108–19. doi: 10.1084/jem.20181288. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data were generated by the authors and available on request.