Abstract
Infective second-stage juveniles (J2) of Meloidogyne spp. migrate towards host roots, which depends on several factors, including root exudates and soil temperature. Although Meloidogyne enterolobii is a highly virulent nematode that affects major agricultural crops worldwide, there is limited ecological data about it. The objective of this study was to determine the J2 migration pattern vertically in 14-cm long segmented soil columns towards tomato (Solanum lycopersicum) and marigold (Tagetes patula) roots, each grown at two soil temperatures (20 or 26ºC). Bottomless cups with tomatoes or marigolds were attached to the top of each column; cups with no plants were used as untreated controls. Juveniles (1,000/column) were injected into a hole located 1 cm from the bottom of each column. The apparatuses were placed in growth chambers at 20 or 26ºC, and J2 were allowed to migrate for 3, 6, 9, or 12 days after injection (DAI). At each harvest, J2 were extracted from each ring of the columns and counted to compare their distribution, and root systems were stained to observe root penetration. M. enterolobii migrated over 13 cm vertically 3 DAI regardless of temperature, even without plant stimuli. The vertical migration was greater at 26ºC, where 60% of active J2 were found at distances >13 cm at 12 DAI. Temperature did not affect root penetration. Overall, a greater number of J2 was observed in tomato roots, and root penetration increased over time.
Keywords: Behavior, Meloidogyne enterolobii, migration, Solanum lycopersicum, Tagetes patula
Meloidogyne enterolobii (Yang and Eisenback, 1983) is one of the most damaging root-knot nematode (RKN) species because of its wide host range and ability to overcome RKN resistance genes in several agricultural crops (Carneiro et al., 2006; Brito et al., 2007; Cetintas et al., 2008; Kiewnick et al., 2009; Pinheiro et al., 2013; Gonçalves et al., 2014; Rosa et al., 2015). Since 2000, when the first outbreaks of M. enterolobii were observed in major guava-producing states in Brazil (Carneiro et al., 2001; Gomes et al., 2011; Pereira et al., 2009), reports on the occurrences of this species also damaging other economically important crops rapidly increased worldwide. Currently, M. enterolobii is considered the major pathogen and threat to guava production in tropical regions (Elling, 2013) and to sweet potato in the USA, where it has been reported to cause severe yield reductions and poor tuber quality (Rutter et al., 2019). As a result, M. enterolobii has been added to lists of pests recommended for regulation as quarantine organisms in the USA and Europe (Anonymous, 2017; Anonymous, 2018a, 2018b; EPPO, 2020).
After hatching from the eggs, second-stage juveniles (J2) of RKN migrate randomly through the porous spaces between soil particles in search of the roots of host plants (Abad et al., 2009; Bilgrami and Gaugler, 2004). Once a suitable host is found, it migrates coordinately toward its roots, penetrates root tips, moves within the cortex until finding an appropriate cell to establish a feeding site, and initiates parasitism (Elling, 2013; Warmerdam et al., 2018). During the host-finding phase, changes in environmental variables and soil attributes significantly influence the process of J2 migration (Carrillo and Hallem, 2015; Gallardo et al., 2015; Hunt et al., 2001; Wallace, 1968).
Temperature is a major factor for RKN J2 development, egg hatch, and movement (Curtis et al., 2009; Dávila-Negrón and Dickson, 2013; Leitão et al., 2021a). Prot and van Gundy (1981a) observed greater migration of M. incognita at temperatures between 18 and 22ºC, whereas Wallace (1966) noted that M. javanica migration was greater at 25ºC. The vertical migration of three individual populations of M. hapla, M. chitwoodi race 1, and M. chitwoodi race 2 was studied at 12, 18, and 24ºC; J2 of all three species were able to migrate greater distances at 18ºC (Pinkerton et al., 1987). These findings indicate that distinct RKN species migrate differently in specific temperature ranges.
In addition to temperature, J2 respond to root exudates released by host plants (Wang et al., 2018). Chemotaxis is the primary means by which J2 perceive chemical cues and start migrating coordinately towards host roots (Andaló et al., 2014; Rasmann et al., 2012; Reynolds, et al., 2011). Although several studies have evaluated the chemotactic response of Meloidogyne in agar plates, such bidimensional assays do not significantly represent the soil matrix tridimensional environment. Thus, soil column assays provide more realistic results to field conditions (Spence et al., 2008). Preferential migration of J2 towards good hosts has been observed (Dalzell et al., 2011; Dutta et al., 2011; Prot, 1976); however, Meloidogyne J2 can randomly migrate long distances even under plant-free conditions (Leitão et al., 2021b; Pinkerton et al., 1987; Oliveira et al., 2020). Despite the economic importance of M. enterolobii to agriculture and food security and the amount of information available focusing on this nematode species in the last 20 years, little information is available about its migratory behavior within different soils.
The objectives of this study were to determine i) the migratory behavior of J2 of M. enterolobii under host (tomato) and nonhost (marigold) plant stimuli; ii) whether J2 can migrate long distances even under host-free conditions; and iii) the effect of temperature on the migration and root penetration of J2.
Materials and Methods
Nematode Inoculum
An isolate of M. enterolobii (N01- 00514) obtained from the RKN collection, Division of Plant Industry – DPI/FDACS, Gainesville, FL, USA (Brito et al., 1994) was reared on tomato (Solanum lycopersicum ‘Cobra’) in a greenhouse at the University of Florida, Gainesville, FL, USA and used in this study. The J2 were obtained by extracting nematode eggs using 0.52% sodium hypochlorite (NaOCl) solution according to Hussey and Barker (1973), modified by Bonetti and Ferraz (1981). The egg suspension was then poured onto modified Baermann funnels at 27ºC containing 2-ply facial tissue paper (Rodríguez-Kábana and Pope, 1981). After the first 24 hr, the J2 collected were discarded to avoid using old J2 for the assays (Leitão et al., 2021b). Freshly hatched J2 were collected on a sieve with 25-µm openings daily for 4 days and stored at 4ºC until the beginning of the experiment.
Seedlings of tomato and marigold
Seeds of tomato ‘Cobra’ and marigold (Tagetes patula ‘Petite’) were sown into vermiculite in plastic seedling trays, one seed per cell, and placed in a greenhouse (28 ± 3ºC, 90–95% relative humidity) for germination. Four-week-old seedlings of both plant species were used in migration columns after their root systems were thoroughly washed with water to remove vermiculite debris.
Experimental apparatus
The migration of M. enterolobii was evaluated in polyvinyl chloride (PVC) soil columns (Fig. 1) based on the specifications by Pinkerton et al. (1987). The columns consisted of a Styrofoam cup attached to three 4-cm long rings and one 2-cm long ring (inoculation ring) with a 4.4 cm internal diameter. A hole was drilled into the inoculation ring, 1 cm above its base, for the injection of J2. The rings were taped together to provide a total internal volume of 213 cm3. Each column was filled with heat (82ºC) pasteurized Lamellic Quartzipsamments (96% sand, 2% silt, and 2% clay, 0.27% OM) (Soil Survey Staff, 2010) collected from a peanut field in Levy County, FL, USA (Fig. 2A). The soil was compacted to provide 1.2 kg dm−3 bulk density to simulate field conditions (Table 1).
Figure 1:
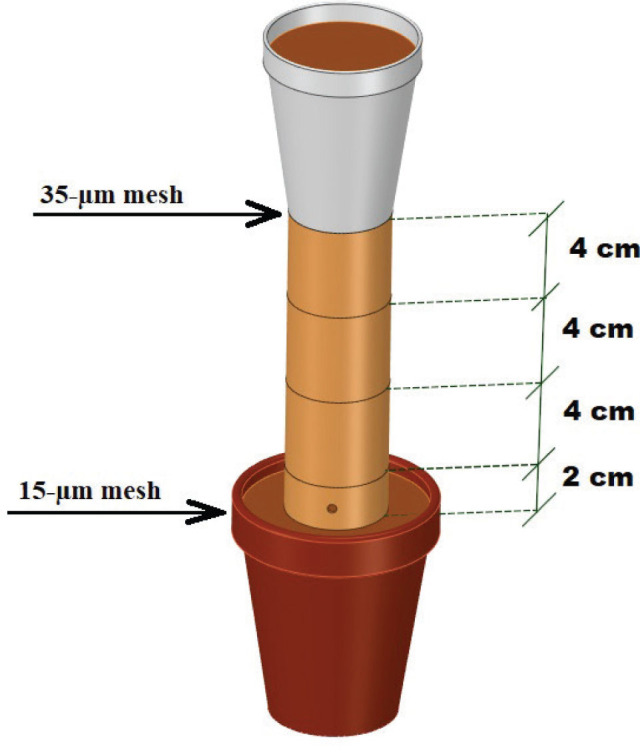
Diagram sketch of an experimental soil-filled column. Each column was comprised of PVC pipe cut into three 4-cm-long sections. Each section had an internal diameter of 4.4 cm and was taped tightly together. A 2-cm-long injection ring was placed at the bottom of each migration column with a hole through which nematode suspensions could be injected. A 15-µm mesh screen was attached to the bottom of each injection ring. A 300-cm3 Styrofoam cup with the bottom removed was taped to the top of each migration column. Each cup had a nylon mesh screen with 35-µm openings taped to the bottom. One-third of the columns had no plant, one-third contained tomato (Solanum lycopersicum), and one-third contained marigold (Tagetes patula) seedlings.
Figure 2:
Experiment step-by-step. A: Soil columns filled with sandy soil; B: Styrofoam cups with either no plant, tomato, or marigold were attached to the columns and then transferred to separate growth chambers, each set at either 20 or 26ºC; C: Injection of suspension of second-stage juveniles of Meloidogyne enterolobii into the hole in the injection ring; D: Dismantling of each column was performed with a spatula to separate the rings; E: The 35-µm mesh screen prevented roots from growing into the columns; F: An example of a tomato plant root system at 12 DAI; G: Soil from each ring was placed in separate cups for centrifugal-flotation extraction of second-stage juveniles; H: Root staining with acid fuchsin was used for determining nematode penetration numbers.
Table 1:
Chemical attributes characterization of the soil used to fill the columns.
| Chemical attributes | Unit | Depth (m) 0.00–0.40 |
|---|---|---|
| pH | (1:2.5) | 5.6 |
| Extractable P | mg Kg−1 | >135 |
| Extractable K | mg Kg−1 | 13 |
| Extractable Mg | mg Kg−1 | 0 |
| Extractable Ca | mg Kg−1 | 164 |
| Organic Matter | % | 0.27 |
Notes: P: Phosphorus; K: Potassium; Mg: Magnesium; Ca: Calcium.
Bottomless Styrofoam cups with 300 g of soil were taped to the top of PVC columns and had a single tomato or marigold seedling transplanted or no seedling (control). To avoid roots from growing into the system, a nylon mesh with 35-μm openings was taped between the cups and the columns (Prot, 1976). In contrast, a nylon mesh with 15-μm openings (smaller than J2 body diameter) was attached to the bottom of the inoculation ring to prevent J2 from moving out of the ring (Pudasaini et al., 2007). The assembled columns were placed in environmental chambers under a completely randomized blocks design with four replicates, where half of the columns were kept at 20ºC and the other half at 26ºC, each with 16 hr light/8 hr dark photoperiod (Fig. 2B).
Soil water content was maintained at 10% by weight throughout the experiment by replacing the amount of water lost by evapotranspiration through daily top irrigation. To mitigate the effect of temperature oscillations while watering the columns, water bottles were kept inside each environmental chamber.
Approximately 1 ml of nematode suspension containing 1,000 ± 100 M. enterolobii J2 was injected into the inoculation hole (Fig. 2C). Columns were dismantled across each one of the five individual sections (Styrofoam cup, three middle rings, and inoculation ring) at 3, 6, 9, and 12 days after inoculation (DAI) (Fig. 2D). At each interval, one column of each stimulus treatment – tomato, marigold, and control – was randomly selected and plants were carefully uprooted. A total of 24 columns (three stimuli × four intervals × two temperatures) were dismantled per replicate, providing a grand total of 120 sections (24 columns × 5 sections) and 480 experimental units (120 sections × four replicates). Nylon meshes (35 and 15 µm) were checked under a stereomicroscope for trapped J2 at each sampling time and whether the 35-µm mesh screen prevented root growth into the columns (Fig. 2E). An example of a root system of a tomato seedling at 12 DAI is shown (Fig. 2F). J2 were extracted from the soil in each separate ring (Fig. 2G) by the centrifugal-flotation technique (Jenkins, 1964). The number of J2 retrieved from the soil of each ring and Styrofoam cup was counted and recorded. J2 were grouped as recovered (total number of nematodes) and active (J2 that showed movement regardless of intensity). During column sampling, the roots of tomatoes and marigolds were washed, weighed, and stained with acid fuchsin (Byrd et al., 1983) to compare root penetration between plants and over time (Fig. 2H). Additionally, fresh shoots and roots were weighed to record potential correlations with J2 migration.
Statistical analysis
Nematode data were subjected to transformation √x + 0.05 before statistical analyses to homogenize variances. Repeated measures MANOVA was used to test the effects of temperature, plant stimuli, distance migrated, and time on the migration of J2 of M. enterolobii. A chi-square test was further performed on significant results to compare J2 distribution within the columns, and the least squared difference (LSD) mean comparison test was used for J2 inside roots at 5% probability. All the analyses were performed on the statistical software RStudio (RStudio, Boston, MA, 2015).
Results
At each sampling period, no M. enterolobii J2 were found on the PVC ring walls or trapped within the meshes. There was an influence of temperature on recovered J2 (P < 0.0001), and there was an interaction between stimulus and distance (P < 0.05) and time and distance (P < 0.0001, Table 2). The migration of active J2 along the columns was influenced by the effects of each factor separately (P < 0.01), and there was a triple interaction among time, temperature, and distance (P < 0.05, Table 2).
Table 2:
Repeated measure MANOVA summary of the effects of temperature, plant stimulus, section, and time on second-stage juveniles (J2) of Meloidogyne enterolobii vertical migration in PVC columns filled with sandy soil.
| Source | Recovered J2 | Active J2 | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| df | SS | MS | F | p-value | SS | MS | F | p-value | |
| Block | 3 | 16.00 | 5.50 | 1.04 | 0.3755 | 86.91 | 28.97 | 8.37 | <0.0001 |
| Temperature (Temp) | 1 | 129.20 | 129.20 | 24.62 | <0.0001 | 194.18 | 194.18 | 56.13 | <0.0001 |
| Stimulus (Stim) | 2 | 78.40 | 39.20 | 7.47 | 0.0007 | 47.94 | 23.97 | 6.93 | 0.0011 |
| Section (Sec) | 4 | 3884.40 | 971.10 | 184.97 | <0.0001 | 628.20 | 157.05 | 45.40 | <0.0001 |
| Temp×Stim | 2 | 10.80 | 5.40 | 1.02 | 0.3603 | 1.30 | 0.65 | 0.19 | 0.8276 |
| Temp×Sec | 4 | 4.40 | 1.10 | 0.20 | 0.9380 | 8.60 | 2.15 | 0.62 | 0.6469 |
| Stim×Sec | 8 | 96.00 | 12.00 | 2.29 | 0.0210 | 41.12 | 5.14 | 1.48 | 0.1614 |
| Temp×Stim×Sec | 8 | 78.40 | 9.80 | 1.86 | 0.0653 | 35.44 | 4.43 | 1.28 | 0.2526 |
| Time | 3 | 142.20 | 47.40 | 9.03 | <0.0001 | 146.28 | 48.76 | 14.09 | <0.0001 |
| Time×Temp | 3 | 3.00 | 1.00 | 0.19 | 0.9065 | 16.98 | 5.66 | 1.64 | 0.1805 |
| Time×Stim | 6 | 45.00 | 7.50 | 1.43 | 0.2021 | 15.00 | 2.50 | 0.72 | 0.6322 |
| Time×Sec | 12 | 730.80 | 60.90 | 11.60 | <0.0001 | 493.68 | 41.14 | 11.89 | <0.0001 |
| Time×Temp×Stim | 6 | 22.80 | 3.80 | 0.71 | 0.6383 | 15.72 | 2.62 | 0.76 | 0.6034 |
| Time×Temp×Sec | 12 | 55.20 | 4.60 | 0.88 | 0.5640 | 82.20 | 6.85 | 2.01 | 0.0228 |
| Time×Stim×Sec | 24 | 193.00 | 8.04 | 1.53 | 0.0547 | 106.80 | 4.45 | 1.29 | 0.1682 |
| Time×Temp×Stim×Sec | 24 | 64.80 | 2.70 | 0.52 | 0.9711 | 44.64 | 1.86 | 0.54 | 0.9652 |
Notes: df: degree of freedom; SS: Sum of squares; MS: Mean square.
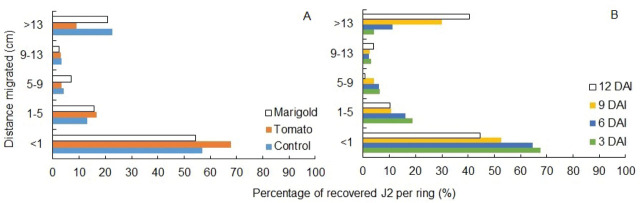
More than 50% (range of 3053 to 3825 specimens) of recovered J2 were found in the inoculation ring regardless of plant stimuli (Fig. 3A). A migration tendency was observed along the columns; however, at distances greater than 13 cm, greater percentages of recovered J2 were observed for control and marigold columns (1523 and 1164 J2s, representing more than 20%). In contrast, only 9% (435) of recovered J2 were found at >13 cm under tomato (Fig. 3A). Regarding J2 migration over time, a steady reduction in the number of recovered J2 found in the inoculation ring occurred, as opposed to an increase in the number of J2 found at distances >13 cm. Almost 70% (3247) of recovered J2 were extracted from the inoculation ring at 3 DAI, whereas 44% (1502) were observed at 12 DAI (Fig. 3B). On the other hand, there was an increase in the percentage of J2 that were able to migrate more than 13 cm, where 4 (240), 11 (489), 30 (1025), and 40% (1368) were observed at 3, 6, 9, and 12 DAI, respectively (Fig. 3B).
Figure 3:
Distribution of recovered second-stage juveniles (J2) of Meloidogyne enterolobii extracted from PVC column sections each 4-cm long × 4.4-cm internal diameter. A. The percentages of second-stage juveniles (J2) migrating in columns containing either tomato, marigold, or no plant untreated control, where bars represent average data pooled from all temperatures and days after inoculation (DAI) (n = 32). B. The percentages of J2 migrating in columns sampled at four dates 3, 6, 9, and 12 DAI, where bars represent average data pooled from all temperatures and stimuli (n = 24). The bars among the different sampling periods are statistically different according to X2 test (P < 0.01).
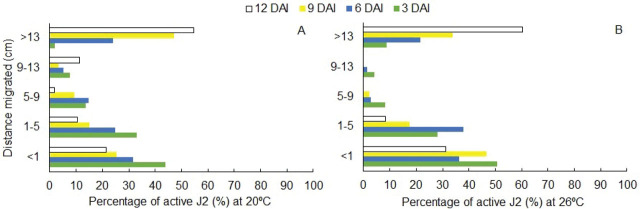
At 3 DAI, irrespectively of temperature, the greater percentages of active J2 were found at the inoculation ring (Fig. 4). Nonetheless, some nematodes migrated 13 cm in the same period, a behavior that was even more evident at 26ºC, where around 9% (100) of active J2 was recovered near the rhizosphere of the plant (Fig. 4B). At 20ºC, active J2 percentage decreased with time in the inoculation ring, from 44% (713) to 22% (207) at 3 and 12 DAI, respectively (Fig. 4A); however, in the last section, there was an increase of active J2 over time at both temperatures (Fig. 4).
Figure 4:
Distribution of active second-stage juveniles (J2) of Meloidogyne enterolobii in PVC soil columns (sections were 4-cm long and 4.4-cm internal diameter) over distance migrated (cm) and sampling time (3, 6, 9, and 12 days after inoculation [DAI]) at two different temperatures: 20ºC (A) and 26ºC (B). Each bar represents the average data pooled from all plant stimuli (n = 12). Distribution percentages were statistically different according to X2 test (P < 0.01).
Active J2 between the second and fourth sections (1–13 cm) showed a different distribution pattern in relation to temperature. At 20ºC, the J2 were more evenly distributed over time, whereas at 26ºC, the number of active J2 recovered from 5 to 9 and 9 to 13 cm distances decreased (Fig. 4).
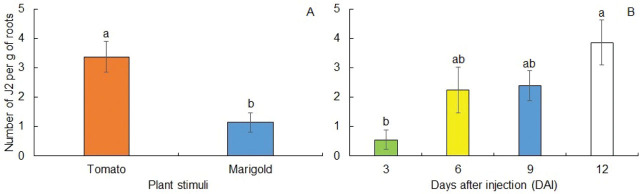
The J2 of M. enterolobii inside roots after migrating through the column were influenced by stimulus and time (P < 0.0001, Table 3). The number of J2 that were able to penetrate roots was always higher in tomato roots throughout the experiment (Fig. 5A). Even though nematodes were found at distances greater than 13 cm at 3 DAI in the soil from the Styrofoam cups regardless of stimuli (Fig. 3), J2 were only observed inside tomato roots (Supplementary Fig. 1).
Table 3:
Repeated measure MANOVA summary of second-stage juveniles (J2) of Meloidogyne enterolobii inside roots after migrating through sandy soil-filled PVC columns.
| Source | df | J2 inside roots | |||
|---|---|---|---|---|---|
|
| |||||
| SS | MS | F | p-value | ||
| Block | 3 | 2.96 | 0.99 | 2.99 | 0.0409 |
| Temperature (Temp) | 1 | 0.02 | 0.02 | 0.05 | 0.8333 |
| Stimulus (Stim) | 1 | 7.48 | 7.48 | 22.69 | <0.0001 |
| Temp×Stim | 1 | 0.02 | 0.02 | 0.06 | 0.8067 |
| Time | 3 | 9.01 | 3.00 | 9.10 | <0.0001 |
| Time×Temp | 3 | 0.22 | 0.07 | 0.22 | 0.8841 |
| Time×Stim | 3 | 2.74 | 0.91 | 2.76 | 0.0528 |
| Time×Temp×Stim | 3 | 0.07 | 0.02 | 0.07 | 0.9748 |
Notes: df: degree of freedom; SS: Sum of squares; MS: Mean square.
Figure 5:
Penetration of second-stage juveniles (J2) of Meloidogyne enterolobii into tomato and marigold roots (A) and their rates over time for both plant species (B). Bars represent means of J2 compared by LSD test. Different letters indicate statistically different penetration rates at 5% of probability.
Shoot and root weight were significantly influenced by time (data not shown). Plant growth data suggest that the experimental period was not sufficient to cause damage to the plants due to M. enterolobii infection, except for root weight at 26ºC, which was nearly the same over time and even lower when compared to 20ºC at 12 DAI. This behavior might be due to the greater migration of J2 at 26ºC (Fig. 3). Since there was no significant interaction between stimuli and time, data from different DAI were combined in Fig. 5A, and data from both plant stimuli were combined in Fig. 5B. At 3 DAI, less than 1 J2/g of roots were able to penetrate plant roots. The number of J2 inside the roots of both plant species was higher at 12 DAI (P < 0.05) with approximately 4 J2/g of roots (Fig. 5B). It is important to mention that there was no penetration of M. enterolobii in T. patula at 3 DAI (Supplementary Fig. 1).
Discussion
Nematode migration is defined as a coordinated movement of J2 towards host roots due to a gradient of root exudates (Bilgrami and Gaugler, 2004; Robinson and Perry, 2006); however, the literature on the behavior of J2 of M. enterolobii within the soil is scarce, especially in soil column migration assays.
Initially, most of the recovered J2 remained in the inoculation ring, but this decreased as the percentage of J2 moved away from the ring over time. Other researchers have noticed that the majority of injected J2 remained at or close to the inoculation ring in the short term (Francilino et al., 2017; Fujimoto et al., 2010; Nježić et al., 2014; Prot and van Gundy, 1981b; Pudasaini et al., 2007).
The presence of J2 at the top of the column at 3 DAI indicates that the texture of the Candler soil did not hinder nematode migration, which is directly related to soil pore space (Wallace, 1958a, 1958b). Nematodes can only migrate through pores wider than their body diameter (Wallace, 1968); thus, sandy soils most likely improve J2 migration because such soils are reported to have larger pore spacing (Rinaldi et al., 2014). The migration of J2 in soils with more than 30% clay content was negative (van Gundy, 1985).
The main approach for plant-parasitic nematodes to recognize plant hosts is chemotaxis (Reynolds et al., 2011). It is proposed that freshly hatched J2 migrate in relation to the attractiveness of root exudates (Ferraz and Brown, 2016).
After J2 hatch from eggs, they migrate within the soil without feeding (Wallace, 1966). They depend on the content of lipid reserves within their bodies to be motile until they find host roots (Das et al., 2011); therefore, the residence time in the soil is crucial for their viability and, consequently, their infection potential (Rocha et al., 2010, 2016). Reynolds et al. (2011) reported that nematodes tend to choose the shortest route to reach a good host plant, thereby saving energy reserves by remaining less time in soil.
The presence of a stimulus from a good host plant positively affected the vertical and horizontal migration of M. javanica (Prot, 1976) and M. incognita (Dalzell et al., 2011). On the other hand, vertical migration of M. chitwoodi occurred regardless of the presence of tomato plants in sandy-loam-filled columns (Pinkerton et al., 1987). The different results may be explained by the random root-knot nematode movement in the soil (Feltham et al., 2002) or varying traits among different Meloidogyne spp. The greater migration rate of M. enterolobii J2 observed at 26ºC during the first few days (3–6 DAI) may be attributed to the thermophilic trait of this species (Karssen et al., 2013). M. hapla and M. chitwoodi, which are considered cryophiles, showed a greater motility rate at 18ºC than at 24ºC (Pinkerton et al., 1987). In addition, J2 of M. enterolobii were found to be more mobile than M. incognita at 20ºC (Oliveira et al., 2020).
At 26ºC, the number of active J2 recovered from 5–9 and 9–13 cm decreased, suggesting a rapid movement of J2 towards plant roots. This behavior might suggest a rapid movement of the J2 towards the roots contained within the Styrofoam cup, which may be explained by an increased metabolism of Meloidogyne spp. J2, since they are poikilothermic organisms (Pudasaini et al., 2007), and promotion of root exudation changes on the cell membrane permeability and diffusion processes of the exudates (Neumann and Römheld, 2001) at higher temperatures.
Prot (1976) reported a high number of J2 of M. javanica inside tomato roots, 200 and 150 J2, in 25 and 50 cm columns, respectively, at 9 DAI. A 1.2-cm wide column was used, which might have favored J2 vertical migration. Columns with smaller diameters may not reflect field conditions since they favor the vertical migration of nematodes due to the small internal volume of the column and the lack of opportunity for lateral displacement of J2 (Spence et al., 2008). In fact, the higher percentage of M. enterolobii at the top of the control columns observed in our experiment might be a result of J2 random movement (Leitão et al., 2021a, 2021b), in addition to restricted lateral movement due to the volume limitation imposed by the PVC rings.
To our knowledge, there is no literature on the host status of Tagetes spp. to M. enterolobii. Marigold plants have been widely studied for suppressing plant-parasitic nematode population densities, especially Meloidogyne spp. (Kalaiselvam and Devaraj, 2011). There are records that certain types are resistant to M. incognita (Buena et al., 2008); however, data for M. enterolobii response is lacking. Rich et al. (2009) compiled information on the parasitism of different Meloidogyne spp. in several weed plants. No M. enterolobii parasitizing species were observed from the family Asteraceae, a botanical family to which Tagetes spp. belongs. Evaluating the host status of marigolds was not the objective of the present study; therefore, future studies with different species of marigolds must be performed to confirm their host status.
Extracts from parts of Tagetes spp. are used to manage RKN because of their repellent properties (Wang et al., 2018) that decrease J2 mobility of species such as M. incognita, M. javanica, and M. paranaensis (Munhoz et al., 2017). Marahatta et al. (2012) state that the suppressive effect is more evident when roots are actively developing. The results from our experiment indicate that T. patula may be useful in managing M. enterolobii since J2 take longer to penetrate its roots. The presence of J2 inside T. patula roots only at 9 and 12 DAI may indicate that it provides a less attractive stimulus to M. enterolobii.
In summary, M. enterolobii was able to migrate distances of more than 13 cm as soon as 3 DAI. Temperature influenced the vertical migration of M. enterolobii, which migrated faster at 26ºC, irrespective of the stimulus. Root penetration was always greater in tomato roots, whereas T. patula plants delayed the penetration of M. enterolobii.
Acknowledgments
This study was supported by a Scholarship Grant (PDSE - 88881.135122/2016-01) from the Brazilian Federal Agency for Support and Evaluation of Graduate Education (CAPES). The authors wish to thank Sai Qiu for her assistance in rearing the nematode isolate.
Supplementary Figure
Supplementary Figure 1:
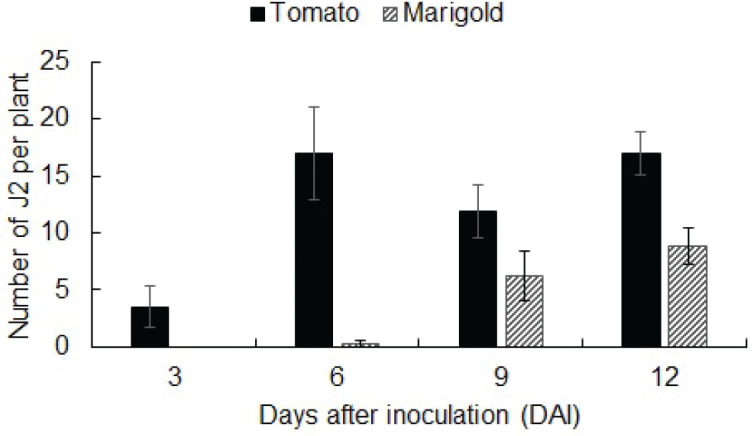
Penetration of second-stage juveniles (J2) of Meloidogyne enterolobii into tomato and marigold roots across 3, 6, 9, and 12 days after inoculation (DAI). Bars represent the average of J2 per plant for both temperatures (n=8), and error bars represent the standard errors of the mean. There was no interaction between stimulus and time; therefore, no statistical analysis was performed for this data.
Literature Cited
- Abad P., Castagnone-Sereno P., Rosso M., Engler J. A., Favery B. Perry R. N., Moens M., Starr J. L. Root-knot Nematodes. Wallingford: CAB International; 2009. Invasion, feeding and development; pp. 163–181. [Google Scholar]
- Andaló V., Moreira G. F., Moino A. Junior. Heterorhabditis amazonensis RSC5 (Rhabditida: Heterorhabditidae) displacement and host recognition. Revista Colombiana de Entomologia. 2014;40:91–97. [Google Scholar]
- Anonymous. Agricultural review. Raleigh, NC: North Carolina Department of Agriculture and Consumer Services, Public Affairs Division; 2017. Available at: https://www.ncagr.gov/paffairs/AgReview/articles/2017/June/NCDACS-warns-of-emerging-nematode.htm. (accessed July 16, 2021). [Google Scholar]
- Anonymous. New crop pest identified in Louisiana. Baton Rouge, LA: Department of Agriculture and Forestry, State of Louisiana; 2018a. available at: http://www.ldaf.state.la.us/news/new-crop-pest-identified-in-louisiana/ (accessed July 16, 2021). [Google Scholar]
- Anonymous. Declaration of emergency. Baton Rouge, LA: Office of Agriculture and Environmental Sciences Horticulture and Quarantine, Department of Agriculture and Forestry, State of Louisiana; 2018b. Available at: https://www.doa.la.gov/osr/EMR/2019/1906EMR020.pdf. (accessed July 16, 2021). [Google Scholar]
- Bilgrami A. L., Gaugler R. Gaugler R., Bilgrami A. L. Nematode Behaviour. Wallingford: CAB International; 2004. Feeding behavior; pp. 63–90. [Google Scholar]
- Bonetti J. I. S., Ferraz S.. Modificação do método de Hussey and Barker para extração de ovos de Meloidogyne exigua em cafeeiro. Fitopatologia Brasileira. 1981;6:553. [Google Scholar]
- Brito J. A., Powers T., Mullin P. G., Inserra R. N., Dickson D. W.. Morphological and molecular characterization of Meloidogyne mayaguensis isolates from Florida. Journal of Nematology. 2004;36:232–240. [PMC free article] [PubMed] [Google Scholar]
- Brito J. A., Stanley J. D., Kaur K., Cetintas R., Di Vito M., Thies J. A., Dickson D. W.. Effects of the Mi-1, N, and Tabasco genes on infection and reproduction of Meloidogyne mayaguensis on tomato and pepper genotypes. Journal of Nematology. 2007;39:327–332. [PMC free article] [PubMed] [Google Scholar]
- Buena A. P., Díez-Rojo M. A., López-Pérez J. A., Robertson L., Escuer M., Bello A.. Screening of Tagetes patula L. on different populations of Meloidogyne. Crop Protection. 2008;27:96–100. [Google Scholar]
- Byrd D. W. Jr, Kirkpatrick T., Barker K. R.. An improved technique for clearing and staining plant tissues for detection of nematodes. Journal of Nematology. 1983;15:142–143. [PMC free article] [PubMed] [Google Scholar]
- Carneiro R. M. D. G., Almeida M. R. A., Braga R. S., Almeida C. A., Gioria R.. First record of Meloidogyne mayaguensis parasitising resistant root-knot nematode pepper and tomato plants in São Paulo State, Brazil. Nematologia Brasileira. 2006;30:81–6. [Google Scholar]
- Carneiro R. M. D. G., Moreira W. A., Almeida M. R. A., Gomes A. C. M. M.. Primeiro registro de Meloidogyne mayaguensis em goiabeira no Brasil. Nematologia Brasileira. 2001;25:223–228. [Google Scholar]
- Carrillo M. A., Hallem E. A.. Gas sensing in nematodes. Molecular Neurobiology. 2015;51:919–931. doi: 10.1007/s12035-014-8748-z. [DOI] [PubMed] [Google Scholar]
- Cetintas R., Brito J. A., Dickson D. W.. Virulence of four Florida isolates of Meloidogyne mayaguensis to selected soybean genotypes. Nematropica. 2008;38:127–135. [Google Scholar]
- Curtis R. H. C., Robinson A. F., Perry R. N. Perry R. N., Moens M., Starr J. L. Root-knot Nematodes. Wallingford: CAB International; 2009. Hatch and host location; pp. 139–162. [Google Scholar]
- Dalzell J. J., Kerr R., Corbett M. D., Fleming C. C., Maule A.G.. Novel bioassays to examine the host-finding ability of plant-parasitic nematodes. Nematology. 2011;13:211–220. [Google Scholar]
- Das S., Wesemael W. M. L., Perry R. N.. Effect of temperature and time on the survival and energy reserves of juveniles of Meloidogyne spp. Agricultural Science Research Journal. 2011;1:102–112. [Google Scholar]
- Dávila-Negrón M., Dickson D. W.. Comparative thermal-time requirements for development of Meloidogyne arenaria, M. incognita, and M. javanica at constant temperature. Nematropica. 2013;43:152–163. [Google Scholar]
- Dutta T. K., Powers S. J., Kerry B. R., Gaur H. S., Curtis R. H. C.. Comparison of host recognition, invasion, development and reproduction of Meloidogyne graminicola and M. incognita on rice and tomato. Nematology. 2011;13:509–520. [Google Scholar]
- Elling A. A.. Major emerging problems with minor Meloidogyne species. Phytopathology. 2013;103:1092–1102. doi: 10.1094/PHYTO-01-13-0019-RVW. [DOI] [PubMed] [Google Scholar]
- EPPO - European and Mediterranean Plant Protection Organization. 2020. Available at: https://www.eppo.int/ACTIVITIES/plant_quarantine/A2_list .
- Feltham D. L., Chaplain M. A. J., Young I. M., Crawford J. W.. A mathematical analysis of a minimal model of nematode migration in soil. Journal of Biological Systems. 2002;10:15–32. [Google Scholar]
- Ferraz L. C. C. B., Brown D. J. F. Nematologia de Plantas: Fundamentos e Importância. first ed. Manaus: Norma Editora; 2016. [Google Scholar]
- Francilino A. H., Pedrosa E. M. R., Silva E. F. F., Rolim M. M., Cardoso M. S. O., Maranhão S. R. V. L.. Efeito do fluxo de água, isca vegetal e volume de poros do solo na mobilidade de Pratylenchus coffeae. Nematropica. 2017;47:63–73. [Google Scholar]
- Fujimoto T., Hasegawa S., Otobe K., Mizukubo T.. The effect of soil water flow and soil properties on the motility of second-stage juveniles of the root-knot nematode (Meloidogyne incognita) Soil Biology and Biochemistry. 2010;42:1065–1072. [Google Scholar]
- Gallardo J. Á. M., Valdés T. D., Ruvalcaba L. P., Molar R. A., Torres J. V., Fasio J. A. C.. Nematodos fitoparasitos y su relación com factores edáficos de papaya em Colima, México. Revista Mexicana de Ciências Agrícolas. 2015;6:251–257. [Google Scholar]
- Gomes V. M., Souza R. M., Mussi-Dias V., Silveira S. F., Dolinski C.. Guava decline: A complex disease involving Meloidogyne mayaguensis and Fusarium solani. Journal of Phytopathology. 2011;159:45–50. [Google Scholar]
- Gonçalves L. S. A., Gomes V. M., Robaina R. R., Valim R. H., Rodrigues R., Aranha F. M.. Resistance to root-knot nematode (Meloidogyne enterolobii) in Capsicum spp. accessions. Revista Brasileira de Ciências Agrárias. 2014;9:49–52. [Google Scholar]
- Hunt H. W., Wall D. H., DeCrappeo N. M., Brenner J.. A model for nematode locomotion in soil. Nematology. 2001;3:705–716. [Google Scholar]
- Hussey R. S., Barker K. R.. Comparison of methods collecting inocula of Meloidogyne spp. including a new technique. Plant Disease Reporter. 1973;57:1025–1028. [Google Scholar]
- Jenkins W. R.. A rapid centrifugal-flotation technique for separating nematodes from soil. Plant Disease Reporter. 1964;48:692. [Google Scholar]
- Kalaiselvam I., Devaraj A.. Effect of root exudates of Tagetes sp. on egg hatching behavior of Meloidogyne incognita. International Research Journal of Pharmacy. 2011;2:93–96. [Google Scholar]
- Karssen G., Weseamel W., Moens M. Perry R. N., Moens M. Plant Nematology. Wallingford: CAB International; 2013. Root-knot nematodes; pp. 73–108. [Google Scholar]
- Kiewnick S., Dessimoz M., Franck L.. Effects of the Mi-1 and the N root-knot nematode-resistance gene on infection and reproduction of Meloidogyne enterolobii on tomato and pepper cultivars. Journal of Nematology. 2009;41:134–139. [PMC free article] [PubMed] [Google Scholar]
- Leitão D. A. H. S., Pedrosa E. M. R., Dickson D. W., Oliveira A. K. S., Rolim M. M.. Temperature: a driving factor for Meloidogyne floridensis migration toward different hosts. Journal of Nematology. 2021a;53:e2021–74. doi: 10.21307/jofnem-2021-074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitão D. A. H. S., Pedrosa E. M. R., Dickson D. W., Brito J. A., Oliveira A. K. S., Rolim M. M.. Upward migration of second-stage juveniles of Meloidogyne floridensis and M. incognita under different plant stimuli. European Journal of Plant Pathology. 2021b;161:301–311. [Google Scholar]
- Marahatta S. P., Wang K., Sipes B. S., Hooks C. R. R.. Effects of Tagetes patula on active and inactive stages of root-knot nematodes. Journal of Nematology. 2012;44:26–30. [PMC free article] [PubMed] [Google Scholar]
- Munhoz V. M., Baida F. C., Lopes G. C., Santiago D. C., Souza J. R. P., Mello J. C. P.. Extract and semi-purified fractions of Tagetes patula flowers in the control of root-knot nematodes. Semina: Ciências Agrárias. 2017;38:3529–3538. [Google Scholar]
- Neumann G., Römheld V. Pinton R., Varanini Z., Nannipieri P. The Rhizosphere: Biochemistry and Organic Substances at the Soil-Plant Interface. New York: Marcel Dekker; 2011. The release of root exudates as affected by plant’s physiological status; pp. 41–93. [Google Scholar]
- Nježić B., Sutter N., Moens M.. Interaction of Tagetes patula cv. Single Gold with the life cycle of the plant-parasitic nematodes Meloidogyne chitwoodi and Pratylenchus penetrans. Russian Journal of Nematology. 2014;22:101–108. [Google Scholar]
- Oliveira A. K. S., Pedrosa E. M. R., Dickson D. W., Vau S. J. S. S. O., Leitão D. A. H. S., Silva E. F. F.. Migration and penetration of Meloidogyne enterolobii and M. incognita in soil columns with tomato and marigold. European Journal of Plant Pathology. 2020;158:591–598. [Google Scholar]
- Pereira F. M., Souza R. M., Souza P. M., Dolinski C., Santos G. K.. Estimativa do impacto econômico e social direto de Meloidogyne mayaguensis na cultura da goiaba no Brasil. Nematologia Brasileira. 2009;33:176–181. [Google Scholar]
- Pinheiro J. B., Reifschneider F. J. B., Pereira R. B., Moita A. W.. Reprodução de Meloidogyne spp. em Capsicum spp. Nematologia Brasileira. 2013;37:20–25. [Google Scholar]
- Pinkerton J. N., Mojtahedi H., Santo G. S., O’Bannon J. H.. Vertical migration of Meloidogyne chitwoodi and M. hapla under controlled temperature. Journal of Nematology. 1987;19:152–157. [PMC free article] [PubMed] [Google Scholar]
- Prot J.. Amplitude et cinétique des migrations du nématode Meloidogyne javanica sous l’influence d’um plant de tomate. Cahiers – ORSTOM. Série biologie. 1976;6:157–166. [Google Scholar]
- Prot J., van Gundy S. D.. Influence of photoperiod and temperature on migrations of Meloidogyne juveniles. Journal of Nematology. 1981a;13:217–220. [PMC free article] [PubMed] [Google Scholar]
- Prot J., van Gundy S. D.. Effect of soil texture and the clay component on migration of Meloidogyne incognita second-stage juveniles. Journal of Nematology. 1981b;13:213–217. [PMC free article] [PubMed] [Google Scholar]
- Pudasaini M. P., Viaene N., Moens M.. The influence of host and temperature on the vertical migration of Pratylenchus penetrans. Nematology. 2007;9:437–447. [Google Scholar]
- Rasmann S., Ali J. G., Helder J., van der Putten W. H.. Ecology and evolution of soil nematode chemotaxis. Journal of Chemical Ecology. 2012;38:615–628. doi: 10.1007/s10886-012-0118-6. [DOI] [PubMed] [Google Scholar]
- Reynolds A. M., Dutta T. K., Curtis R. H. C., Powers S. J., Gaur H. S., Kerry B. R.. Chemotaxis can take plant-parasitic nematodes to the source of a chemo-attractant via the shortest possible routes. Journal of the Royal Society Interface. 2011;8:568–577. doi: 10.1098/rsif.2010.0417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich J. R., Brito J. A., Kaur R., Ferrell J. A.. Weed species as hosts of Meloidogyne: A review. Nematropica. 2009;39:157–185. [Google Scholar]
- Rinaldi L. K., Nunes J., Montecelli T. D. N.. Efeito de texturas do solo sobre populações de Meloidogyne javanica e Meloidogyne incognita em soja. Cultivando Saber. 2014;7:83–101. [Google Scholar]
- Robinson A. F., Perry R. N. Perry R. N., Moens M. Plant Nematology. Wallingford: CAB International; 2006. Behaviour and sensory perception; pp. 210–233. [Google Scholar]
- Rocha F. S., Campos V. P., Souza J. T.. Variation in lipid reserves of second-stage juveniles of Meloidogyne exigua in a coffee field and its relationship with infectivity. Nematology. 2010;12:365–371. [Google Scholar]
- Rocha F. S., Campos V. P., Fernandes M. F. G., Muniz M. F. S.. Migration and reproduction of Meloidogyne incognita in two soil textures. Nematropica. 2016;46:162–171. [Google Scholar]
- Rodríguez-Kábana R., Pope M. H.. A simple incubation method for the extraction of nematodes from soil. Nematropica. 1981;11:175–186. [Google Scholar]
- Rosa J. M. O., Westerich J. N., Wilcken S. R. S.. Reprodução de Meloidogyne enterolobii em olerícolas e plantas utilizadas na adubação verde. Revista Ciencia Agronomica. 2015;46:826–835. [Google Scholar]
- Rutter W. B., Skantar A. M., Handoo Z. A., Muller J. D., Aultman S. P., Agudelo P.. Meloidogyne enterolobii found infecting root-knot nematode resistant sweetpotato in South Carolina, United States. Plant Disease. 2019;103:775. [Google Scholar]
- Soil Survey Staff. Keys to Soil Taxonomy. 11th ed. Washington, DC: USDA-Natural Resources Conservation Service; 2010. [Google Scholar]
- Spence K. O., Lewis E. E., Perry R. N.. Host-finding and invasion by entomopathogenic and plant-parasitic nematodes: Evaluating the ability of laboratory bioassays to predict field results. Journal of Nematology. 2008;40:95–98. [PMC free article] [PubMed] [Google Scholar]
- van Gundy S. D. Sasser J. N., Carter C. C. An Advanced Treatise on Meloidogyne. Raleigh: North Carolina University Graphics; 1985. Ecology of Meloidogyne spp. - emphases on environmental factors affecting survival and pathogenicity; pp. 177–182. [Google Scholar]
- Wallace H. R.. Movement of eelworms. I. The influence of pore size and moisture content of the soil on the migration of larvae of the beet eelworm, Heterodera schachtii Schmidt. Annals of Applied Biology. 1958a;46:74–85. [Google Scholar]
- Wallace H. R.. Movement of eelworms. II. A comparative study of the movement in soil of Heterodera schachtii Schmidt and of Ditylenchus dipsaci (Kuhn) Filipjev. Annals of Applied Biology. 1958b;46:86–94. [Google Scholar]
- Wallace H. R.. Factors influencing the infectivity of plant parasitic nematodes. Proceedings of the Royal Society B: Biological Sciences. 1966;164:592–614. [Google Scholar]
- Wallace H. R.. The dynamics of nematode movement. Annual Review of Phytopathology. 1968;6:91–114. [Google Scholar]
- Wang C., Masler E. P., Rogers S. T.. Response of Heterodera glycines and Meloidogyne incognita infective juveniles to root tissues, root exudates, and root extracts from three plant species. Plant Disease. 2018;102:1733–1740. doi: 10.1094/PDIS-09-17-1445-RE. [DOI] [PubMed] [Google Scholar]
- Warmerdam S., Sterken M. G., van Schaik C., Oortwijn M. E. P., Sukarta O. C. A., Lozano-Torres J. L., Dicke M., Helder J., Kammenga J. E., Goverse A., Bakker J., Smant G.. Genome-wide association mapping of the architecture of susceptibility to the root-knot nematode Meloidogyne incognita in Arabidopsis thaliana. New Phytologist. 2018;218:724–737. doi: 10.1111/nph.15034. [DOI] [PMC free article] [PubMed] [Google Scholar]